Overview
The US market has 1 drug approved as L-methylfolate, known by its chemical name Levomefolate calcium. Originally developed by Merck & Co., Inc., this compound is primarily used as a dietary supplement and also has applications in treating Angelman Syndrome. The drug has received orphan drug designation for Megaloblastic Anemia, highlighting its importance in treating specific rare conditions. L-methylfolate represents an important option in the folate supplement market, with its bioavailable form of folate being particularly valuable for individuals with genetic variations affecting folate metabolism, such as those with MTHFR gene polymorphisms.
Detailed Description
Drug Approval of L-Methylfolate
Levomefolate calcium was originally developed by Merck & Co., Inc. and has been granted special approval as follows:
| Organization | Disease Name | Special Review | Country | Approval Date |
|---|---|---|---|---|
| Cox Biosciences LLC | Anemia, Megaloblastic | Orphan Drug | United States | 2015-02-10 |
The drug is currently approved for use as a dietary supplement and for therapeutic applications related to Angelman Syndrome and megaloblastic anemia.
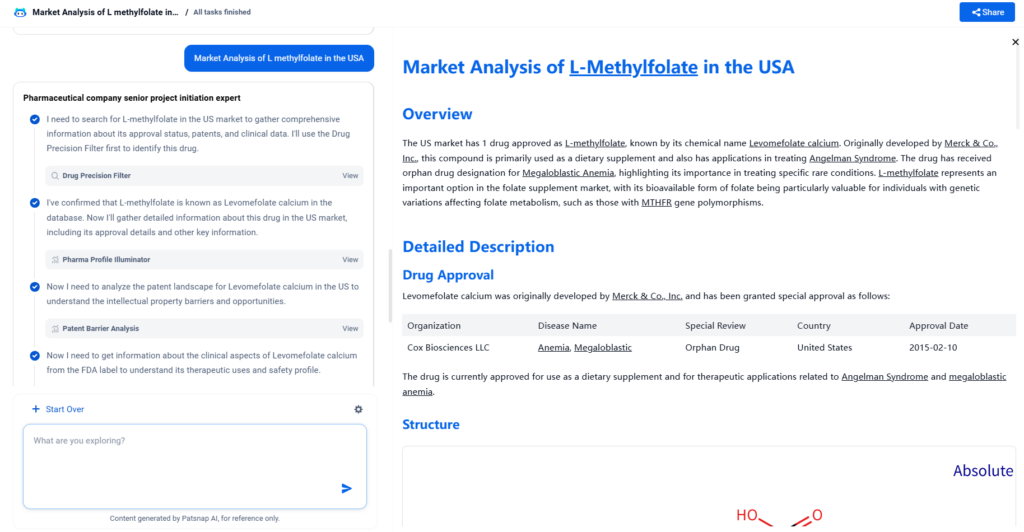
Structure
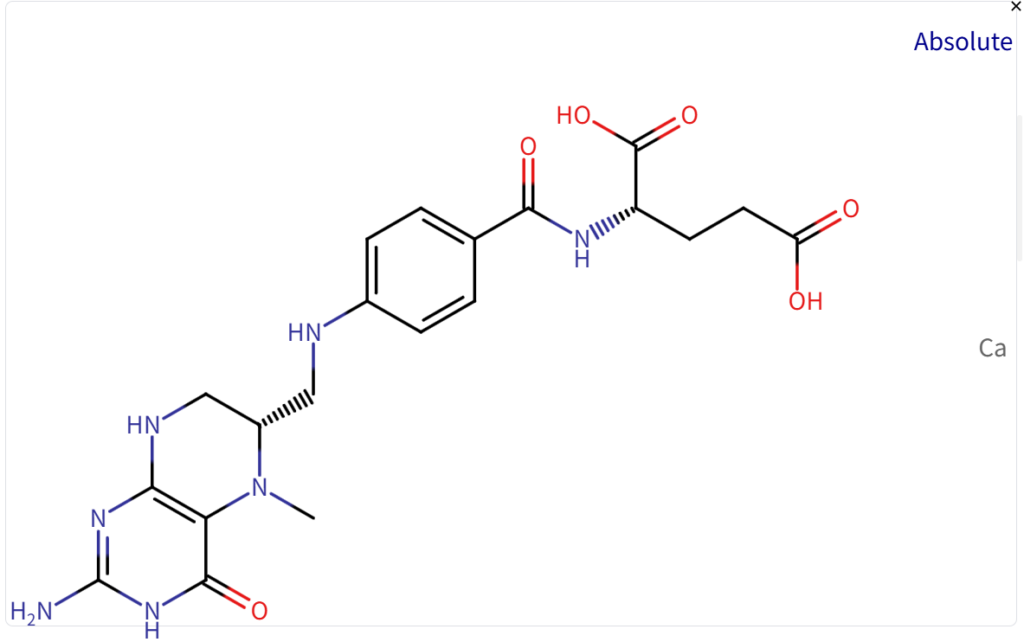
Patent Barrier of L-Methylfolate
Registration Patent Analysis
No core patents were found for Levomefolate calcium in the FDA Orange Book, indicating that the basic compound itself may not be under patent protection.
Other Patent Barrier Analysis
While there are no core patents listed, there are several non-original patents related to Levomefolate calcium, including formulation, process, crystal form, and new use patents. Some key active patents include:
Several companies have patents on different aspects of L-methylfolate, including formulations, crystal forms, manufacturing processes, and new uses. The earliest active patent is set to expire in 2026 (US20160095860A1), while the latest extends to 2038 (US20210107902A1).
Clinical Results of L-Methylfolate
Based on the FDA Label Clinical Insight, the following information about L-methylfolate (Levomefolate calcium) was found:
Nonclinical Toxicology Studies
- Mutagenicity Testing:
- Both in vitro and in vivo mutagenesis studies indicated no evidence of mutagenic activity associated with Levomefolate calcium.
- Carcinogenicity Studies:
- Long-term animal studies have not been conducted to evaluate the carcinogenic potential of Levomefolate calcium specifically 1.
Pharmacodynamic/Pharmacokinetic Considerations
- Folate compounds like Levomefolate calcium have the potential to modify the pharmacokinetics or pharmacodynamics of certain antifolate drugs (e.g., antiepileptics, methotrexate).
- Some drugs such as methotrexate and sulfasalazine have been reported to reduce folate concentrations, highlighting the importance of considering drug-drug interactions.
Infringement Cases
No infringement cases related to L-methylfolate (Levomefolate calcium) were found.
Policy and Regulatory Risk Warning
After a comprehensive search, no specific market exclusivity or data protection period was identified for L-methylfolate in the USA beyond the orphan drug designation for Megaloblastic Anemia (granted in 2015). Orphan drug designation typically provides 7 years of market exclusivity, which would expire in 2022 if the drug received approval for this indication.
L-methylfolate is available as both a prescription medical food and as an over-the-counter dietary supplement, creating a complex regulatory landscape. As a medical food, it’s regulated differently from traditional pharmaceuticals but must still comply with FDA requirements for safety and manufacturing.
Market Entry Assessment & Recommendations of L-Methylfolate
- Patent Landscape Assessment:
- While there are no core patents in the FDA Orange Book, there are multiple active patents covering various aspects of L-methylfolate that extend until 2038.
- Companies interested in entering this market should consider:
- Developing non-infringing formulations or crystal forms
- Exploring licensing opportunities with current patent holders
- Focusing on applications not covered by existing patents
- Evaluating the strength and scope of existing patents, as some may be narrow or potentially challengeable
- Market Positioning Strategies:
- Differentiation through improved formulations, stability, or bioavailability
- Targeting specific patient populations, particularly those with MTHFR gene variants who cannot efficiently convert folic acid to its active form
- Exploring combination products with other vitamins or minerals for synergistic effects
- Developing novel delivery systems or dosage forms that improve patient compliance or absorption
- Regulatory Pathway Considerations:
- For dietary supplement applications, follow FDA guidelines for structure/function claims
- For pharmaceutical applications, consider the 505(b)(2) pathway leveraging existing safety data
- For medical food applications, ensure compliance with specific FDA requirements for this category
- Explore potential for additional orphan drug designations for rare diseases where folate metabolism plays a role
- Commercial Opportunities:
- Growing consumer awareness of folate’s importance in pregnancy and genetic variations in folate metabolism
- Increasing interest in personalized nutrition based on genetic testing
- Potential applications in psychiatric conditions, cardiovascular disease, and other areas where folate metabolism is implicated
- Rising demand for bioavailable forms of nutrients, especially among health-conscious consumers
- Manufacturing Considerations:
- Develop cost-effective and scalable manufacturing processes, as several patents cover specific production methods
- Consider strategic partnerships with companies holding process patents
- Invest in quality control measures to ensure consistent purity and potency, as this can be a differentiating factor
- Market Expansion Opportunities:
- Explore international markets with different patent landscapes
- Investigate new therapeutic areas where L-methylfolate may provide benefits
- Consider direct-to-consumer genetic testing partnerships to identify individuals who would benefit from L-methylfolate
- Develop educational programs for healthcare providers about the differences between folic acid and L-methylfolate
The market for L-methylfolate presents diverse opportunities across pharmaceutical, medical food, and dietary supplement categories. With careful navigation of the patent landscape and strategic positioning, there are significant opportunities for both innovative products and generic entries in specific segments of this market.
For more detailed information of L-methylfolate, try PatSnap Eureka AI Agent.