
Overview
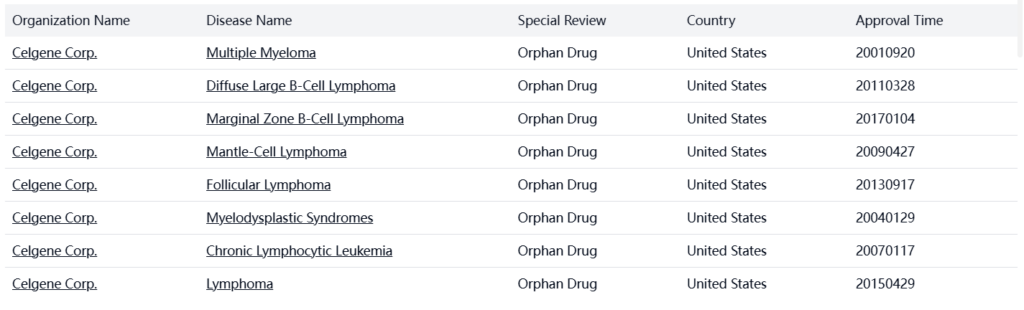
The US market currently has 1 approved drug containing lenalidomide. Lenalidomide, marketed under the brand name Revlimid, was originally developed by Celgene Corporation (now part of Bristol Myers Squibb) and has become a cornerstone therapy for multiple hematological malignancies. The drug has received multiple orphan drug designations in the US for various indications including Multiple Myeloma (2001), Myelodysplastic Syndromes (2004), Mantle-Cell Lymphoma (2009), Follicular Lymphoma (2013), and Marginal Zone B-Cell Lymphoma (2017), highlighting its significant therapeutic value in treating rare diseases. The drug’s market performance has been substantial due to its efficacy across multiple indications and its use in combination therapies, particularly with dexamethasone and rituximab, which have shown enhanced therapeutic effects.
Detailed Description
Drug Information
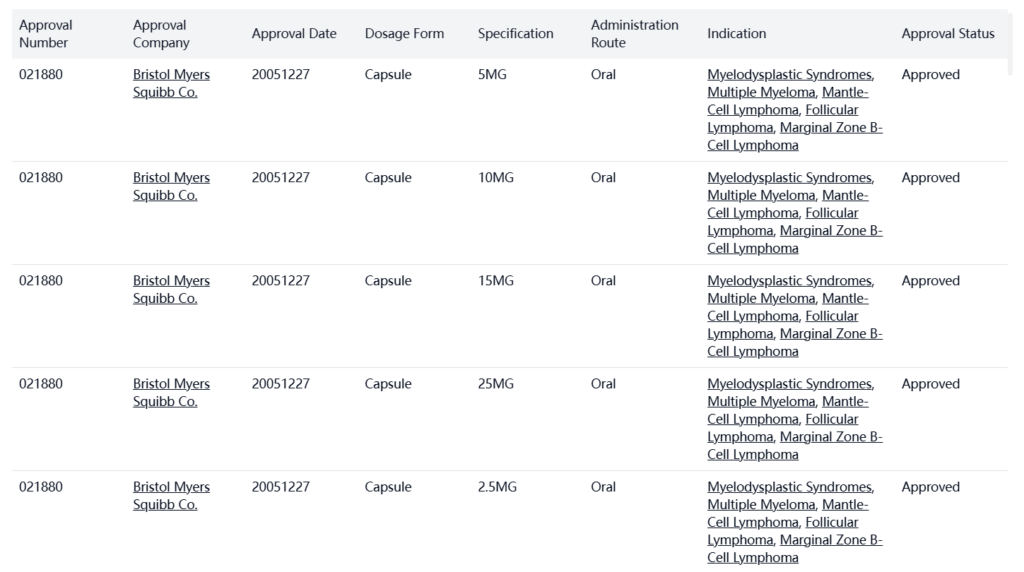
Lenalidomide was developed by Celgene Corp. and approved in the USA on December 27, 2005.
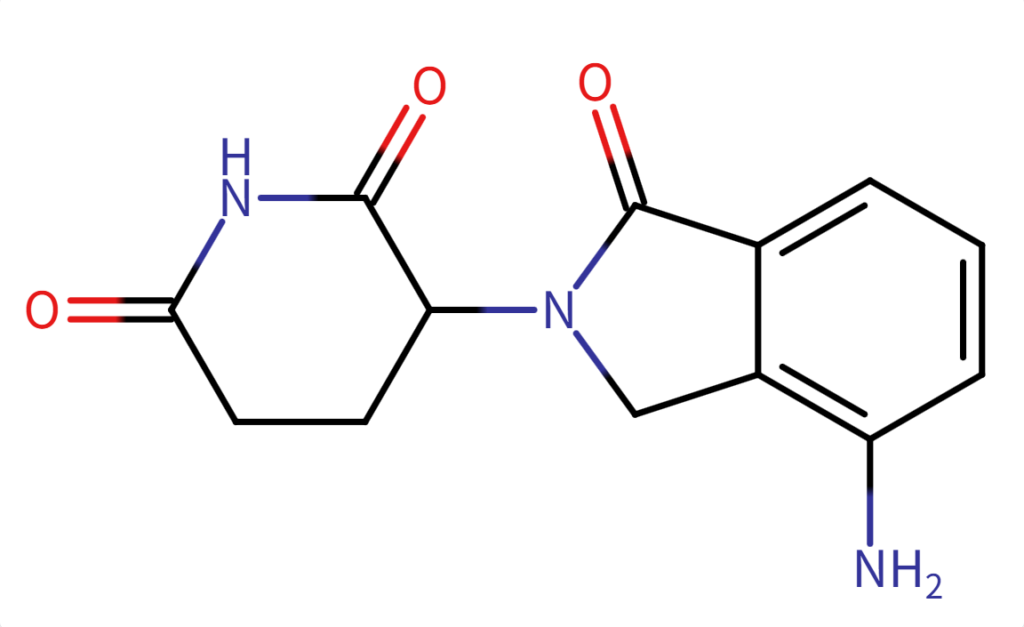
Structure
Special Review
Registration Patent Barrier Analysis
The FDA Orange Book lists numerous patents for lenalidomide, with most now inactive. Key patents include:
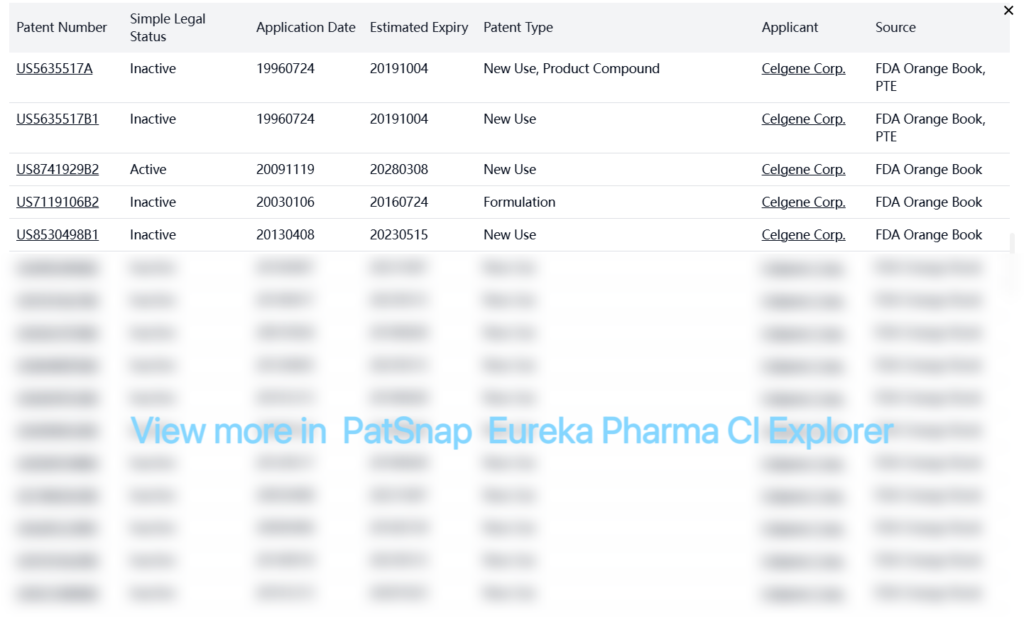
PTE patent:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant | Source |
|---|---|---|---|---|---|---|
| US5635517A | Inactive | 19960724 | 20191004 | New Use, Product Compound | Celgene Corp. | FDA Orange Book, PTE |
| US5635517B1 | Inactive | 19960724 | 20191004 | New Use | Celgene Corp. | FDA Orange Book, PTE |
Other Patent Barrier Analysis
Celgene Corporation holds numerous other patents related to lenalidomide, including:
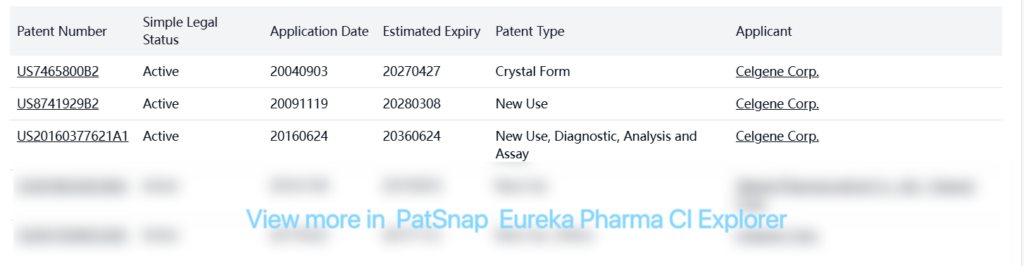
Additionally, there are numerous other patents from various companies related to lenalidomide, including process patents, crystal form patents, formulation patents, and combination therapy patents. Many of these patents are inactive or in the PCT designated stage expired status, suggesting limited current patent protection beyond the key patents listed in the FDA Orange Book.
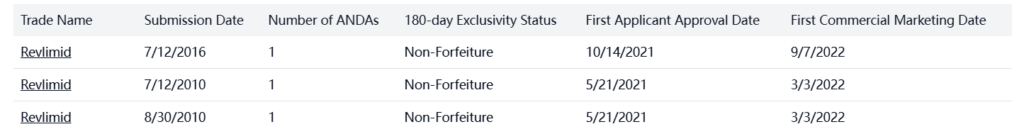
Patent Statement/Paragraph IV
There are multiple patent statements for lenalidomide in the US:
Clinical Results
- Mechanism of Action Studies:
Lenalidomide’s activities are mediated through cereblon, a component of the cullin ring E3 ubiquitin ligase complex. In the presence of lenalidomide, substrate proteins such as avolos, Ikaros (IKZF1/IKZF3), and CKI(Q) are ubiquitinated and subsequently degraded. This results in direct cytotoxicity and immunomodulatory effects on hematopoietic tumor cells [1]. - Anti-Proliferative and Apoptotic Effects:
In vitro experiments have shown that lenalidomide inhibits cell proliferation and induces apoptosis in various hematopoietic tumor cell lines, including multiple myeloma (MM), mantle cell lymphoma, del(5q) myelodysplastic syndromes, follicular lymphoma, and marginal zone lymphoma. - Combination Studies:
- With Dexamethasone: Research has shown that combining lenalidomide with dexamethasone synergistically increases the inhibition of cell proliferation and promotes apoptosis in multiple myeloma cells [1].
- With Rituximab: In vitro studies have demonstrated that when lenalidomide is combined with rituximab, there is an enhancement in antibody-dependent cell-mediated cytotoxicity (ADCC) and induction of apoptosis in follicular lymphoma cells, as well as potentiated ADCC in marginal zone lymphoma cells, compared with rituximab alone [1].
- Clinical Studies in Multiple Myeloma:
Multiple randomized, double-blind, placebo-controlled studies have been conducted in patients with multiple myeloma who had received at least one prior therapy. These studies compared a combination of lenalidomide with oral pulse high-dose dexamethasone versus dexamethasone alone. Patients in the lenalidomide/dexamethasone group received lenalidomide 25 mg once daily on Days 1 to 21 of a 28-day cycle, alongside dexamethasone. - Drug Interaction Studies:
- Studies have shown that single or multiple doses of dexamethasone (40 mg) do not exert a clinically relevant effect on the pharmacokinetics of lenalidomide when administered at 25 mg.
- Lenalidomide is a substrate of P-glycoprotein (P-gp), but studies with P-gp inhibitors showed no significant increase in lenalidomide concentration .
- In vitro studies have determined that lenalidomide is not a substrate for several key transporters and does not inhibit or induce cytochrome P450 isoenzymes, supporting its low potential for drug-drug interactions.
Infringement Cases
Based on the available data, no specific patent infringement incidents or related litigation involving lenalidomide were identified.
Policy and Regulatory Risk Warning
After a comprehensive search, it appears that most of lenalidomide’s key patents have expired or will expire soon. The compound patent (US5635517A) with patent term extension expired in October 2019. Several use patents expire between 2023-2024, with only a few active patents remaining until the late 2020s or 2030s. The product has already faced generic entry, with the first generic versions receiving approval in 2021 and entering the market in 2022 as evidenced by the patent statement information.
Market Entry Assessment & Recommendations
Lenalidomide’s patent landscape shows that most key patents have expired or will expire soon, with generic competition already in the market since 2022. This creates both challenges and opportunities:
- For Innovator (Bristol Myers Squibb):
- Focus on lifecycle management through combination therapies with newer agents
- Leverage remaining patent protection for specific indications or formulations
- Develop next-generation cereblon modulators with improved efficacy/safety profiles
- Emphasize brand loyalty and patient support programs to maintain market share
- Consider authorized generic strategies to capture part of the generic market
- For Generic Manufacturers:
- The market is already open to generics, with first entrants having launched in 2022
- Opportunities exist for additional generic entrants as volume restrictions on early entrants expire
- Differentiation through improved formulations, patient services, or cost advantages will be key
- Consider partnerships with payers for preferred formulary status
- Target international markets where patent expiration may occur later
- Market Considerations:
- Pricing pressure will increase as more generic competitors enter
- The drug remains important across multiple hematological malignancies with strong clinical data
- Combination therapy use will likely continue to grow, maintaining demand
- The orphan drug designations for multiple indications highlight the continued medical importance of the molecule
- Reimbursement policies will significantly impact market dynamics as generics proliferate
- Regulatory Strategy:
- Monitor any potential regulatory exclusivities for specific indications
- Evaluate opportunities for pediatric extensions or new formulations
- Consider opportunities in markets with different patent expiration timelines
- Monitor post-marketing safety requirements that may impact generic entry
In conclusion, lenalidomide represents a market in transition from branded exclusivity to generic competition. While key compound patents have expired, some use patents remain active until the mid-2020s, and a few specialized patents extend into the 2030s. Strategic positioning will be essential for both the innovator company to maintain value and for generic entrants to capture market share in this evolving landscape.
For more scientific and detailed information of lenalidomide, try PatSnap Eureka Pharma CI Explorer.

Only takes 4 steps to unlock the detailed result 👇

