Peridotite, a dense ultramafic igneous rock composed mainly of olivine and pyroxene, is gaining renewed interest as a sustainable material in the age of decarbonization. Known for its role in Earth’s mantle dynamics, peridotite has now entered the spotlight for its natural ability to sequester carbon dioxide via mineral carbonation. Beyond geology, its properties are being explored for applications in carbon capture and storage (CCS), green construction, and materials engineering. This blog unpacks the mineralogical composition, industrial-grade classifications, performance characteristics, and emerging innovations of peridotite with technical insights tailored for professionals in energy, sustainability, and advanced materials sectors through PatSnap Eureka AI Agent.
What Is Peridotite and Why It Matters
Peridotite is a coarse-grained, ultramafic rock rich in magnesium and iron silicates. Its major mineral constituents include:
- Olivine (Mg,Fe)2SiO4
- Orthopyroxene (Enstatite or Hypersthene)
- Clinopyroxene (Augite or Diopside)
- Chromite, Spinel, and Garnet (in trace amounts)
Peridotite originates in the upper mantle and is exposed through tectonic uplift or obduction. It serves as the parent rock for serpentinite and is a primary source of magnesian silicates used in CO2 mineralization.
Material Composition and Grade Data
Peridotite grades vary based on mineralogical content and industrial end use. Below are key grade categories and typical data:
| Grade Type | Olivine Content | MgO (%) | SiO2 (%) | Suppliers |
|---|---|---|---|---|
| High-Olivine Grade | >85% | 45–49 | 38–42 | Sibelco (MagSil 45), Thermolith SA |
| Industrial Grade | 60–80% | 40–45 | 35–40 | LKAB Minerals (UltraMag), Imerys |
| Refractory Grade | 70–90% (low Fe) | 46–48 | 38–40 | Steinsvik Olivine, Tata Chemicals |
These grades are used in metallurgical flux, refractory lining, and carbon capture technologies, depending on their MgO content and reactivity.
Key Properties That Define Peridotite
- High Magnesium Oxide Content: Makes it chemically reactive with CO2 in mineral carbonation.
- Thermal Stability: Useful in refractory applications up to 1,800°C.
- Density & Hardness: Provides mechanical strength in construction fill and cementitious materials.
- CO2 Sequestration Potential: Can permanently fix CO2 as magnesite and silica.
- Low Alkalinity: Unlike serpentine, it does not pose leaching hazards.

Core Applications Across Industries
1. Carbon Capture & Storage (CCS)
Peridotite exhibits exceptional reactivity with atmospheric or pressurized CO₂, enabling natural mineral carbonation that forms stable magnesium carbonates such as magnesite. This geochemical process not only offers a permanent carbon sink but also occurs spontaneously in certain geological settings, such as the Semail Ophiolite in Oman. Engineered carbonation processes are being deployed in pilot CCS projects, such as CarbFix in Iceland, where in-situ injection into ultramafic formations accelerates CO₂ mineralization rates. Researchers are optimizing peridotite grain size, fracture networks, and fluid injection strategies to enhance reactivity and scalability.
2. Green Cement & Low-Carbon Aggregates
Finely ground peridotite serves as a pozzolanic or supplementary cementitious material (SCM), partially replacing Portland clinker in cement formulations. This reduces both CO₂ process emissions and energy intensity, while enhancing the mechanical durability and chemical resistance of the resulting concrete. Companies like Blue Planet Ltd. integrate mineralized CO₂ with peridotite-derived aggregates to produce carbon-negative building materials, while CarbFix explores its role in CO₂-solidified aggregates. Additionally, peridotite’s silicate phase contributes to alkali-silica reaction (ASR) resistance in concrete applications.
3. Refractory and Metallurgical Applications
Due to its high melting point, low porosity, and chemical inertness, high-purity peridotite is employed in refractory linings for steel ladles, kilns, and metallurgical furnaces. The material’s MgO-rich phase improves thermal shock resistance and stability under reducing atmospheres. In steel production, peridotite can act as a fluxing agent that facilitates slag formation and impurity removal. Its use in ferroalloy smelting and non-ferrous metallurgy is also gaining traction, particularly in magnesite-substitute systems.
4. Soil Conditioning & Agricultural Inputs
Peridotite’s magnesium and trace element content makes it suitable for soil remineralization, especially in acidic, weathered, or nutrient-depleted soils. When ground into rock dust, it contributes to pH buffering, improves cation exchange capacity (CEC), and releases plant-accessible Mg, Ni, and Si. This approach is aligned with regenerative agriculture and is under study for enhanced weathering applications—where silicate minerals react with atmospheric CO₂ to form bicarbonates, supporting both carbon sequestration and soil fertility.
5. Additive Manufacturing & Advanced Ceramics
Emerging research explores peridotite as a feedstock for sintered ceramics, 3D printed refractories, and Mg–Si–O based composite materials. Its controlled mineralogy and thermal properties allow it to serve as a reinforcing phase in advanced oxide ceramics, especially for high-temperature structural components. The development of synthetic or doped peridotite analogs is enabling tailored material behaviors, such as improved thermal conductivity, abrasion resistance, and compatibility with binder jetting or stereolithography-based additive processes.
Comparative Advantages and Limitations
Peridotite, as an ultramafic igneous rock rich in olivine and pyroxenes, exhibits unique geochemical and geomechanical characteristics that make it particularly advantageous in certain industrial and environmental contexts. However, its usage also presents technical and logistical constraints. The following table summarizes the material’s comparative profile:
| Dimension | Advantages | Limitations | Comparison with Similar Materials |
|---|---|---|---|
| CO₂ Sequestration Potential | High natural reactivity with CO₂ enables accelerated mineral carbonation, locking carbon in stable magnesite and calcite phases. | Reaction rates under natural conditions are slow; engineered enhancement (e.g., fracturing, CO₂ pressurization) is required. | Outperforms basalts in carbonation rate due to higher MgO content and porosity, but less accessible than some basalts. |
| Mechanical Strength | High compressive strength; can support deep subsurface operations and is suitable for construction aggregates. | Heterogeneity in grain structure can lead to localized weakness; difficult to standardize as a structural material. | Comparable to gabbro and dunite, but less uniform than engineered concrete blends. |
| Thermal Stability | Resistant to high-temperature degradation (>1200°C), useful for high-temperature refractory applications. | Contains trace minerals that may become chemically unstable in oxidizing atmospheres or acid leachate conditions. | Surpasses sandstone or limestone in thermal resistance; similar to serpentinite. |
| Availability & Cost | Widely present in ophiolite belts and mantle-derived massifs; relatively low extraction cost. | Geographic clustering limits global availability; transportation from remote ultramafic regions adds to cost. | More abundant and lower-cost than chromitite, but less refined in purity. |
| Environmental Benefits | Naturally contributes to alkaline buffering, reduces heavy metal mobility, and enables passive carbon capture ecosystems. | Ecological disturbance during mining, particularly in fragile alpine or oceanic terrains; requires responsible land reclamation. | More eco-beneficial than inert silicate rocks like granite or diorite. |
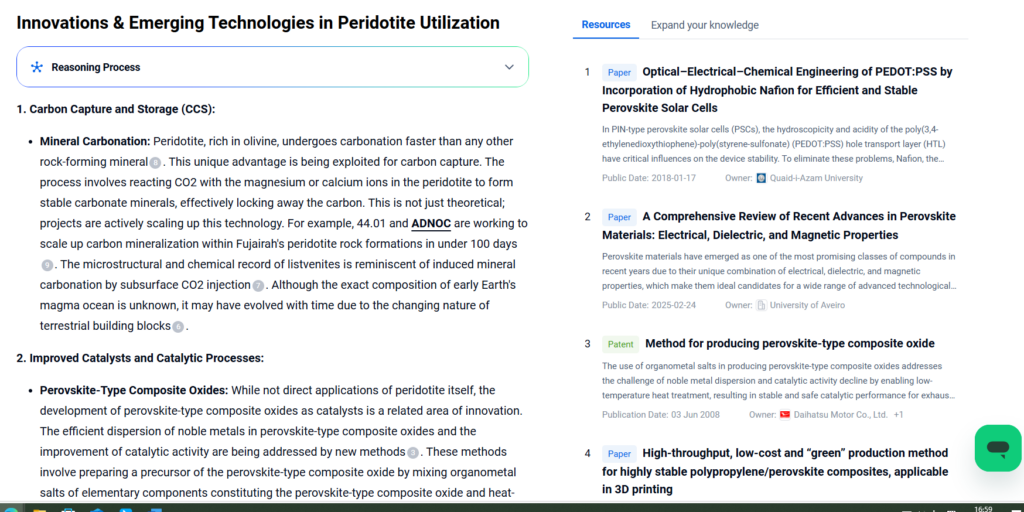
Innovations & Emerging Technologies in Peridotite Utilization
Cutting-edge research and industrial applications are leveraging the intrinsic mineralogy and geochemical reactivity of peridotite to tackle pressing global challenges—especially in climate mitigation, resource circularity, and geotechnical engineering.
Enhanced Weathering for Gigaton-Scale Carbon Removal
Recent field trials in Oman and Iceland have explored crushed peridotite spreading to accelerate weathering reactions at surface temperatures. With the addition of water and microbial catalysts, olivine-rich zones can capture up to 1–2 kg CO₂ per kg of rock over a few decades. Research is ongoing into optimizing particle size, hydration cycles, and CO₂ delivery methods to scale this process efficiently.
Subsurface CO₂ Injection with Reactive Reservoirs
Projects such as CarbFix and DEEP-C investigate the feasibility of injecting CO₂ into fractured peridotite formations to induce in situ mineralization. This method offers a secure, long-term storage solution for CO₂ as solid carbonate minerals, with monitoring supported by geophysical sensors and isotopic tracers. Innovations include supercritical CO₂ flow modeling and nano-fluid stimulation to increase reaction surface area.
Geopolymer Binders Derived from Olivine Phases
Peridotite residues are being chemically activated with alkaline solutions to form low-carbon geopolymers, an emerging class of sustainable construction materials. These binders display favorable compressive strength and reduced curing times compared to conventional Portland cement. They also demonstrate excellent durability under acidic and saline conditions, making them suitable for coastal infrastructure.
Selective Leaching for Metal Recovery
Ongoing developments in hydrometallurgical extraction are targeting the recovery of nickel, chromium, and rare earth elements (REEs) from peridotite matrices using bioleaching or acid heap methods. This adds value to waste rock from mining operations, aligning with circular economy principles and reducing the demand for primary ore extraction.
Petrological Engineering for Subsurface Stability
Advanced rock mechanics simulations are being used to understand the deformation and failure mechanisms of peridotite in deep geological repositories. This includes modeling anisotropic fracture propagation, permeability evolution during serpentinization, and coupling of thermal-mechanical effects—vital for both carbon storage and nuclear waste containment strategies.
Artificial Peridotite Synthesis and Functional Mimics
Some labs are exploring the synthetic reproduction of peridotite microstructures to create engineered geomaterials with tailored properties, such as controlled porosity or catalytic reactivity. These materials aim to replicate carbonation reactions at faster rates while maintaining mechanical stability, potentially serving as novel filters, CO₂ scrubbers, or reactive barriers.
Challenges and What’s Next for Peridotite
Despite its promise, the widespread adoption of peridotite technologies is hindered by:
- Lack of process standardization for mineral carbonation.
- Need for techno-economic validation at industrial scale.
- Slow kinetics of natural carbonation without pressure/temp catalysts.
- Environmental scrutiny over mining in ecologically sensitive regions.
Ongoing R&D is focused on improving carbonation efficiency, modular reactor designs, and integrating peridotite into circular industrial ecosystems.
Conclusion
Peridotite is transitioning from a geologic curiosity to a material of the future. With its extraordinary CO2 absorption potential, thermal endurance, and mineral versatility, it is well-positioned to support global decarbonization strategies. As technologies evolve and pilot projects scale, sourcing professionals, engineers, and sustainability leaders should monitor peridotite’s role in the carbon-neutral material value chain.
FAQs
A1: Generally, peridotite is non-toxic. However, if contaminated with asbestos-like chrysotile or processed without dust controls, it may pose inhalation risks.
A2: Its high MgO content reacts with CO2 to form stable carbonates like magnesite, making the sequestration permanent and environmentally safe.
A3: Yes. MagSil 45, UltraMag, and CarbFix materials incorporate peridotite or its derivatives in CCS, cement, and soil conditioning applications.
A4: While natural peridotite is abundant, synthetic analogues can be engineered for controlled compositions, particularly in ceramics and refractory composites.
Want to explore patents, supplier trends, or the latest innovations in peridotite-based materials?
👉 Explore deeper insights using PatSnap Eureka AI Agent to uncover deep technical insights, forecast material performance, and benchmark innovation leaders—faster and smarter.