
Overview
Prolia (Denosumab) is an established biologic in the US market, with 1 approved drug available. Developed by Amgen, Inc., it was first approved in 2010 and has maintained a significant market presence. As a RANKL inhibitor, Prolia has shown effectiveness in treating osteoporosis and related bone disorders. The drug has attracted considerable interest from competitors, with 32 biosimilars in development, highlighting the market’s attractiveness. Prolia’s patent portfolio is extensive, with numerous patents extending beyond 2030, providing Amgen with significant market protection for this valuable asset.
Detailed Description
Drug Information
Prolia (Denosumab) was developed by Amgen, Inc. and received FDA approval in the United States on June 1, 2010.

Bio-similars
There are 32 bio-similars, for example: Denosumab biosimilar (Biocon).
Special Review
| Organization | Indication | Special Review | Country | Approval Date |
|---|---|---|---|---|
| Amgen, Inc. | Giant Cell Tumors | Orphan Drug | United States | 20101220 |
| Amgen, Inc. | Hypercalcemia | Orphan Drug | United States | 20130911 |
Registration Patent Barrier Analysis
FDA Purple Book patents from Amgen, Inc. provide extensive protection for Prolia (Denosumab), with many patents extending into the 2030s.
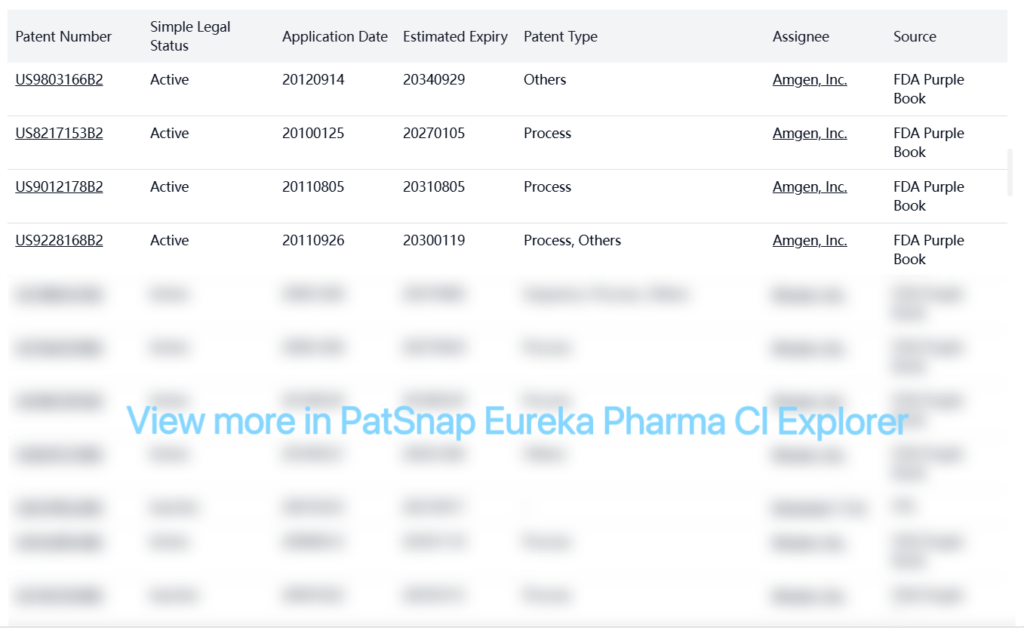
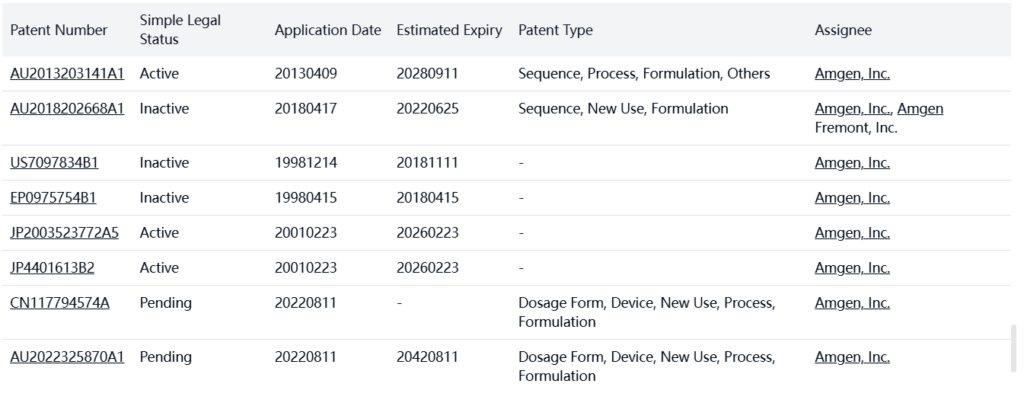
Other Patent Barrier Analysis
Amgen, Inc. holds additional patents in other jurisdictions, providing global protection for Denosumab.
There are also several patents from other companies related to Denosumab:
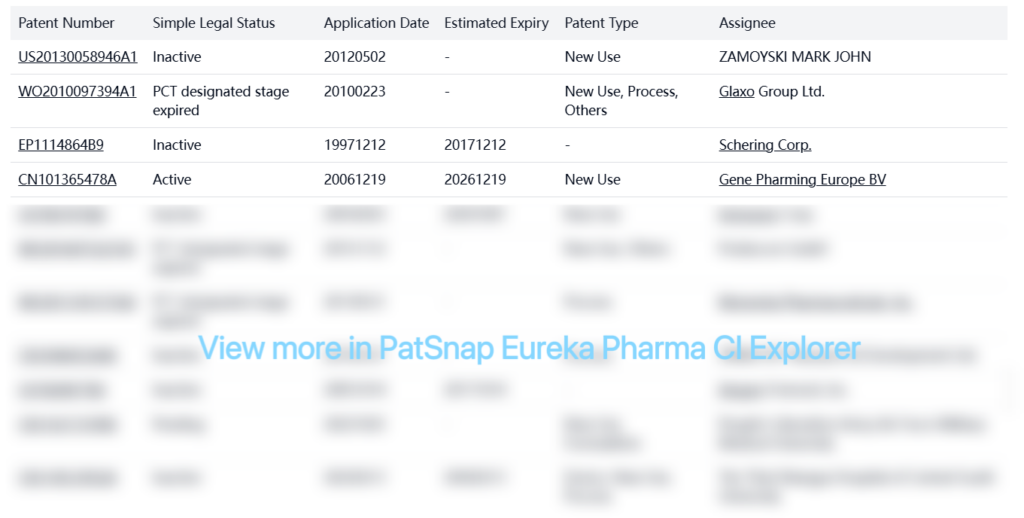
Patent Statement/Paragraph IV
No patent statement/Paragraph IV information found for US.
Clinical Results
- Mechanism of Action and Pharmacodynamics Studies:
In vitro and early in vivo studies characterized denosumab’s mechanism of action by demonstrating its binding affinity and specificity for RANKL, a protein essential for osteoclast formation, function, and survival. These studies established that by binding to RANKL, denosumab prevents its interaction with the receptor RANK on osteoclasts and their precursors—thereby reducing bone resorption and increasing bone mass and strength [1].Biomarker and dose-response experiments evaluated the pharmacodynamics of denosumab by measuring the reduction in bone resorption biomarkers. For example, after a 60 mg subcutaneous dose of Prolia, serum type 1 C-telopeptide (CTX) levels were reduced by approximately 85% at 3 days, with maximal reductions by one month. These effects were reversible, as partial attenuation of CTX suppression was observed by the end of each dosing interval and then re-established upon reinitiation of therapy.
2. Immunogenicity Studies:
To evaluate the immunogenic potential of denosumab, an electrochemiluminescent bridging immunoassay was used to detect binding antibodies, and a chemiluminescent cell-based assay was applied to assess neutralizing antibody formation. These studies, conducted over up to 5 years of treatment with Prolia, demonstrated that less than 1% of patients developed binding antibodies, and no patients were found to have neutralizing antibodies. Importantly, the presence of anti-drug antibodies had no clinically significant impact on the pharmacokinetics, pharmacodynamics, safety, or effectiveness of the drug.
3. Clinical Efficacy and Safety Studies:
Multiple clinical trials in postmenopausal women have been performed to assess both the efficacy and safety of denosumab. These studies not only quantified improvements in bone mineral density (BMD) but also monitored the reduction of fractures and the overall incidence of adverse reactions such as back pain, musculoskeletal pain, and other common side effects [5].A randomized, double-blind, placebo-controlled study was conducted in men with osteoporosis. In this trial, 120 men received Prolia while 120 received a placebo, with all participants supplemented with at least 1000 mg of calcium and 800 IU of vitamin D daily. The study assessed safety outcomes (like incidences of serious infections, adverse events leading to withdrawal, and common side effects such as back pain, arthralgia, and nasopharyngitis) as well as pharmacodynamic endpoints [3].A further clinical trial was executed in men with nonmetastatic prostate cancer receiving ADT. In a three-year, randomized, double-blind, placebo-controlled multinational study, 1,468 men were randomized to either placebo or a 60 mg dose of Prolia administered subcutaneously every six months. The primary endpoint was the percent change in lumbar spine BMD at 24 months, with new vertebral fractures through 36 months being evaluated as a key secondary outcome. The study demonstrated significant improvements in BMD in the Prolia-treated group relative to placebo, with a treatment difference of 6.7% (95% CI: 6.2, 7.1; p < 0.0001).
4. Additional Safety and Adverse Reaction Evaluations:
The safety profile of denosumab has been further explored across multiple patient populations. Adverse reactions have been monitored in clinical studies assessing postmenopausal osteoporosis, glucocorticoid-induced osteoporosis, and patients receiving cancer therapies (such as androgen deprivation therapy or aromatase inhibitors).
- Common adverse reactions include back pain, pain in the extremity, and musculoskeletal pain.
- Serious adverse reactions have been noted in relation to hypocalcemia, serious infections, dermatologic reactions, and rare events like osteonecrosis of the jaw or atypical femoral fractures. These evaluations are crucial in establishing risk management and patient education strategies.
Infringement Cases
No infringement cases were found for Prolia (Denosumab).
Policy and Regulatory Risk Warning
After a comprehensive search, Prolia (Denosumab) as a biologic drug in the US has 12 years of data exclusivity from its first approval date in 2010, which would have expired in 2022. However, the extensive patent protection extending into the 2030s and 2040s provides Amgen with significant market protection beyond the regulatory exclusivity period.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis of Prolia (Denosumab) in the US market, the following recommendations are provided:
- For Innovator (Amgen):
- Leverage the extensive patent portfolio extending into the 2030s and 2040s to maintain market exclusivity
- Focus on lifecycle management strategies, including new indications, formulations, or delivery devices to extend product lifecycle
- Develop patient support programs to enhance adherence and maintain market share
- Consider authorized biosimilar partnerships as patents begin to expire to maintain revenue streams
- Monitor and potentially challenge any biosimilar applications that may emerge
- For Biosimilar Developers:
- Conduct thorough freedom-to-operate analyses given the complex patent landscape
- Focus on developing differentiated administration devices or formulations that may avoid certain device patents
- Consider patent challenges or design-arounds for process patents where possible
- Prepare for entry after key patents expire in the 2030s
- Explore licensing opportunities with Amgen for earlier market entry
- Strategic Considerations:
- The extensive patent portfolio, particularly around manufacturing processes and devices, creates significant barriers to biosimilar entry
- The biologic nature of Prolia (Denosumab) makes exact replication challenging, providing additional protection beyond patents
- Market growth potential exists in expanding indications and reaching underserved patient populations
- Value-based contracting with payers could help maintain market share as competition eventually increases
- Real-world evidence generation should be prioritized to demonstrate long-term safety and efficacy advantages
The overall assessment indicates that Prolia will likely maintain strong market protection in the US through the mid-2030s due to its robust patent estate, with biosimilar competition becoming more viable thereafter. Strategic focus should be on expanding indications, improving administration, and enhancing patient experience to maximize value during the protected period.
For more scientific and detailed information of Prolia, try PatSnap Eureka Pharma CI Explorer.
Only takes 4 steps to unlock the detailed result 👇

