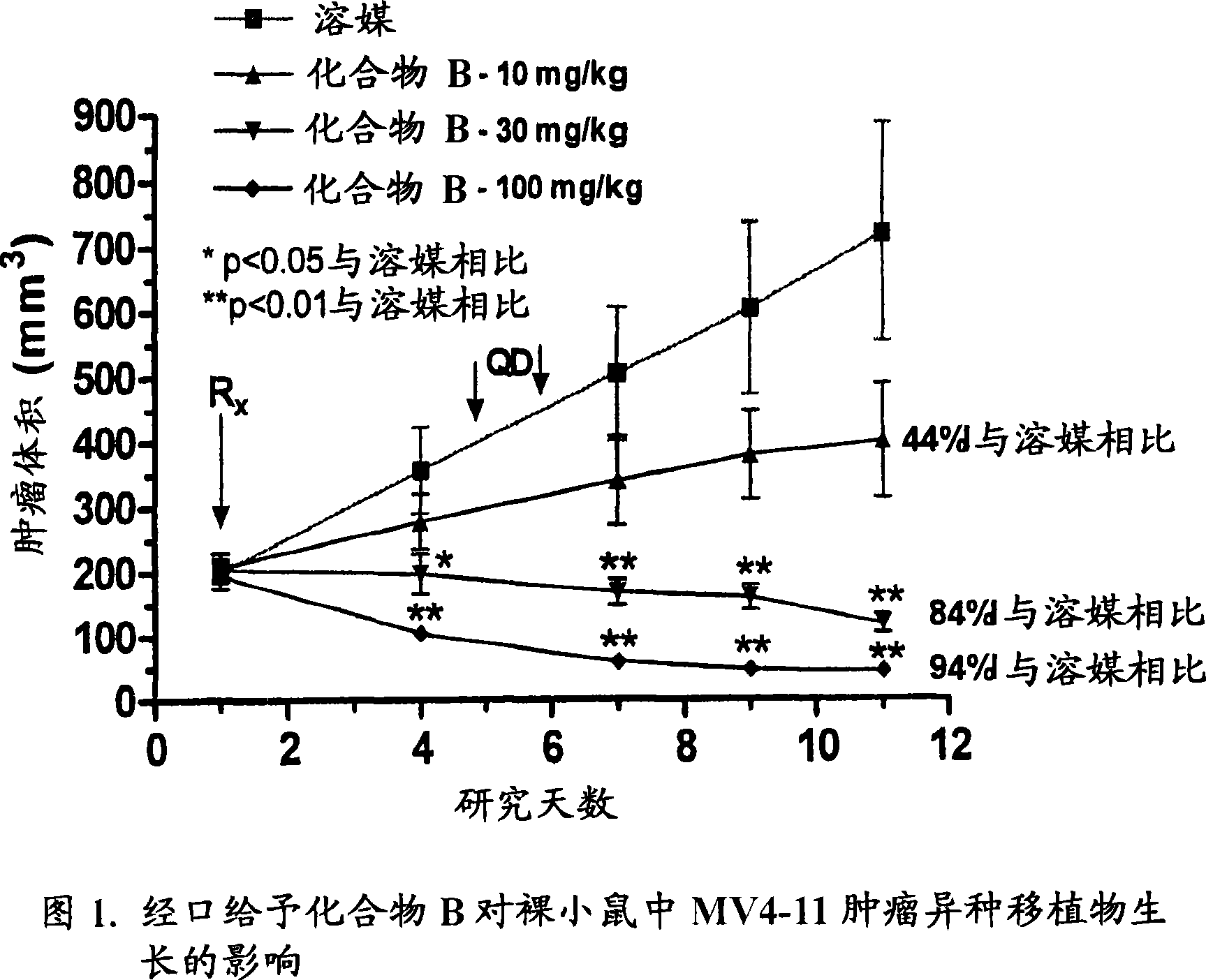
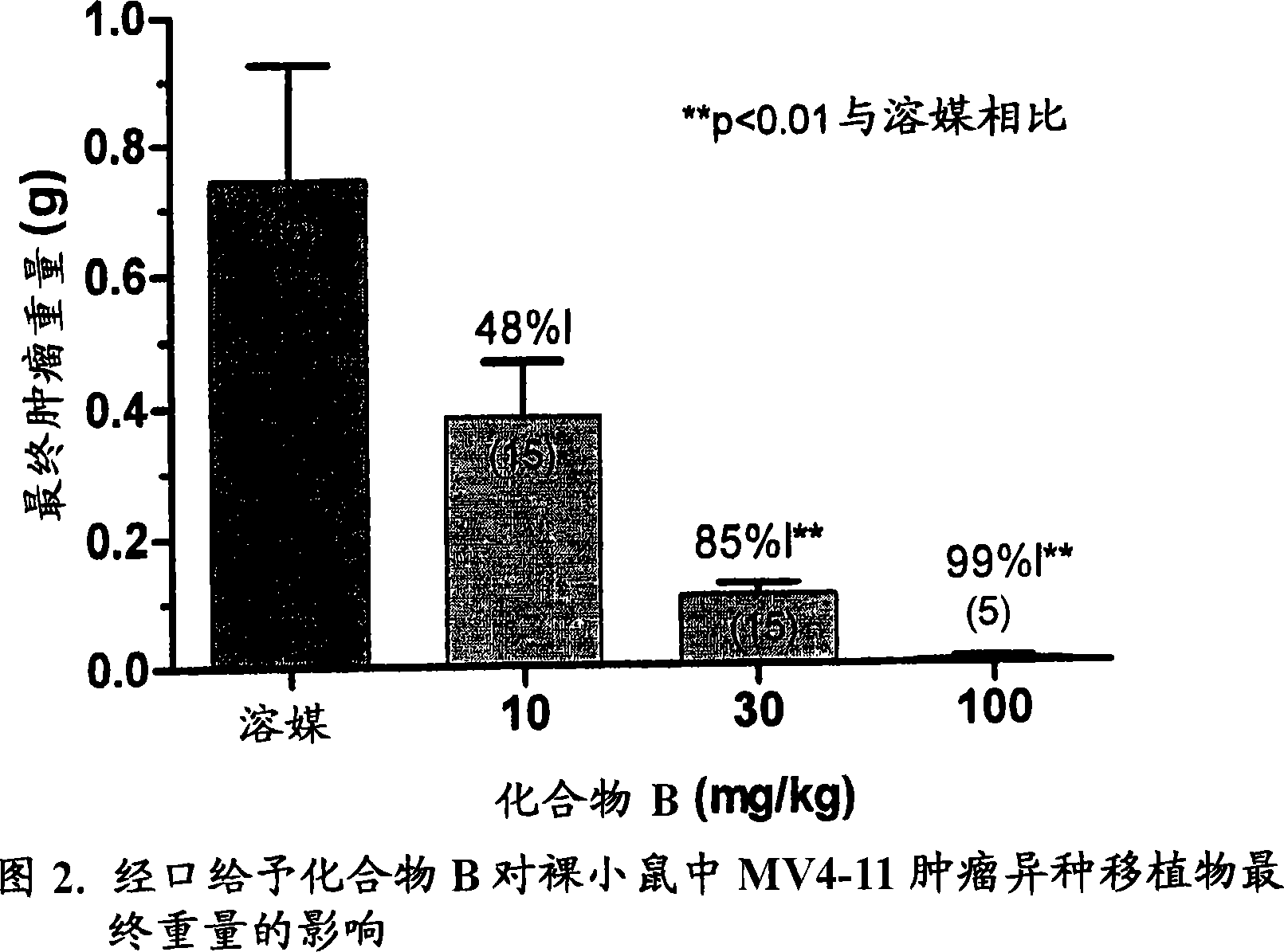
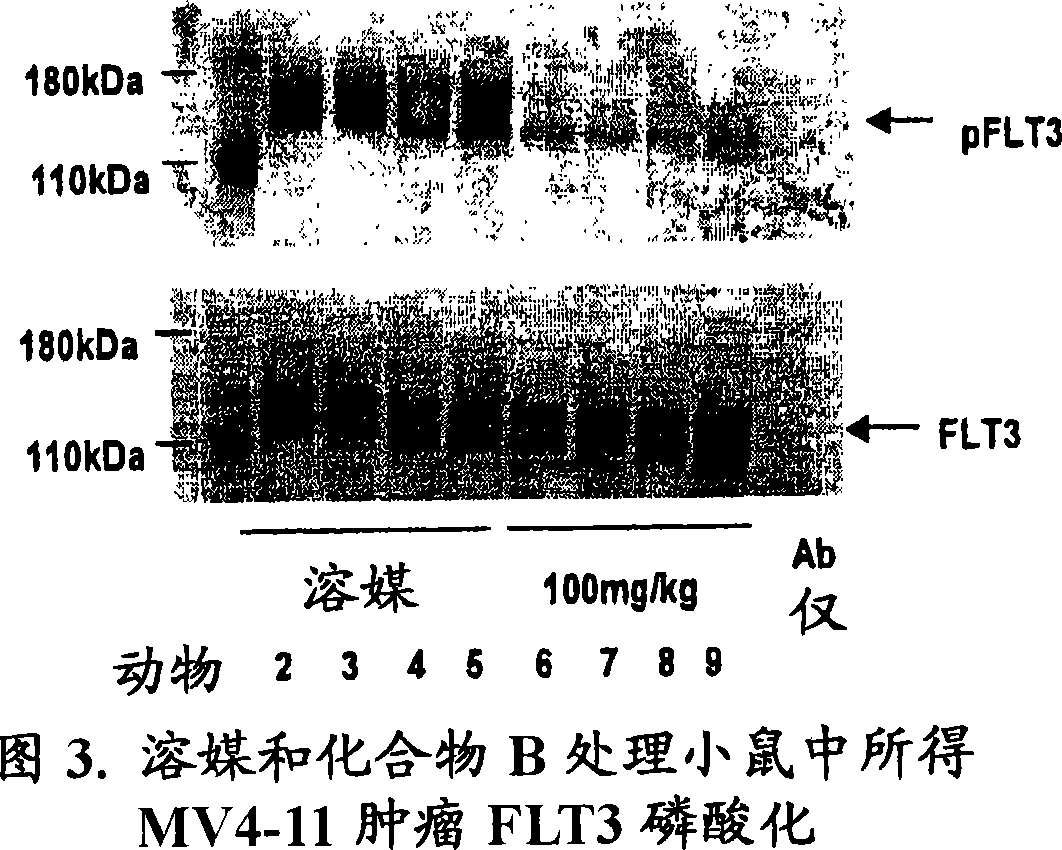
Synergistic modulation of FLT3 kinase using aminoquinoline and aminoquinazoline kinase modulators
A technology of kinase inhibitor and tyrosine kinase, which is used in medical preparations containing active ingredients, pharmaceutical formulations, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0771] (4-Isopropyl-phenyl)-carbamic acid 1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-yl ester (Compound No. 1)
[0772]
[0773] Add 1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-ol (29 mg, 0.1 mmol) prepared in Example 3a, 4- Cumyl phenyl ester (20 mg, 0.12 mmol) and dichloroethane (1 mL). After the mixture was stirred at 60°C for 16 hours, the contents were subjected to aqueous workup and purification by TLC to obtain the desired product in 65% yield.
[0774] 1 H NMR (300MHz, CDCl 3 )δ8.67(s, 1H), 7.33-7.25(m, 3H), 7.18(d, J=7.6Hz, 2H), 7.09(s, 1H), 6.64(s, 1H), 5.08(m, 1H ), 4; 02(s, 3H), 3.99(s, 3H), 3.95-3.89(m, 2H), 3.55-3.48(m, 2H), 2.88(sept, J=6.1Hz, 1H), 2.22- 2.14(m, 2H), 2.04-1.91(m, 2H), 1.23(d, J=6.1Hz, 6H);
[0775] LC / MS (ESI): theoretical mass 450.2, found 451.6 (M+H) + .
Embodiment 2
[0777] (4-Isopropyl-phenyl)-carbamic acid 1-(6,7-dimethoxy-quinazolin-4-yl)-pyrrolidin-3-yl ester (Compound No. 2)
[0778]
[0779] a. (4-isopropyl-phenyl)-carbamic acid 4-nitro-phenyl ester
[0780]
[0781] To a solution of 4-isopropylaniline (3.02 g, 22.3 mmol) in DCM (40 mL) and pyridine (10 mL) was added portionwise 4-nitrochloroformic acid with stirring under brief ice bath cooling over ~30 seconds phenyl phenyl ester (4.09 g, 20.3 mmol). After stirring at room temperature for 1 h, the homogeneous solution was diluted with DCM (100 mL), washed with 0.6M HCl (1×250 mL), 0.025M HCl (1×400 mL), water (1×100 mL) and 1M NaHCO 3 (1 x 100 mL) wash. The organic layer was dried (Na 2 8O 4 ), concentrated to afford the title compound as a pale pink solid (5.80 g, 95%).
[0782] 1 H NMR (300MHz, CDCl 3 )δ8.28(m, 2H), 7.42-7.32(m, 4H), 7.23(m, 2H), 6.93(brs, 1H), 2.90(h, J=6.9Hz, 1H), 1.24(d, J =6.9Hz, 6H).
[0783] LC / MS (ESI): theoretical mass 300.1, found 601.3 (2...
Embodiment 3
[0790] (4-Isopropoxy-phenyl)-carbamic acid 1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-yl ester (compound No. 3)
[0791]
[0792] a.1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-ol
[0793]
[0794] A solution of 4-hydroxypiperidine (40.4 mg, 0.400 mmol) in isopropanol (1 mL) was treated with 4-chloro-6,7-dimethoxy-quinazoline (89.9 mg, 0.401 mmol). After stirring overnight at 100 °C, the reaction was cooled to room temperature and dissolved in DCM (10 mL) and H 2 O (10mL). Na 2 SO 4 Dry and concentrate in vacuo to afford the title compound as a solid (60 mg, 52%).
[0795] b. (4-Isopropoxy-phenyl)-carbamic acid 1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-yl ester
[0796]
[0797] Add 1-(6,7-dimethoxy-quinazolin-4-yl)-piperidin-4-ol (29 mg, 0.1 mmol) prepared substantially according to Example 3a, chloroformic acid p-nitrogen to the bottle phenyl phenyl ester (24 mg, 0.12 mmol), triethylamine (20 mg, 0.2 mmol) and dichloroethane (1 mL). After the mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com