Thiol-containing compounds for the removal of elements from contaminated milieu and methods of use
A technology of compounds and polymers, applied in the field of compounds of metals and main group elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
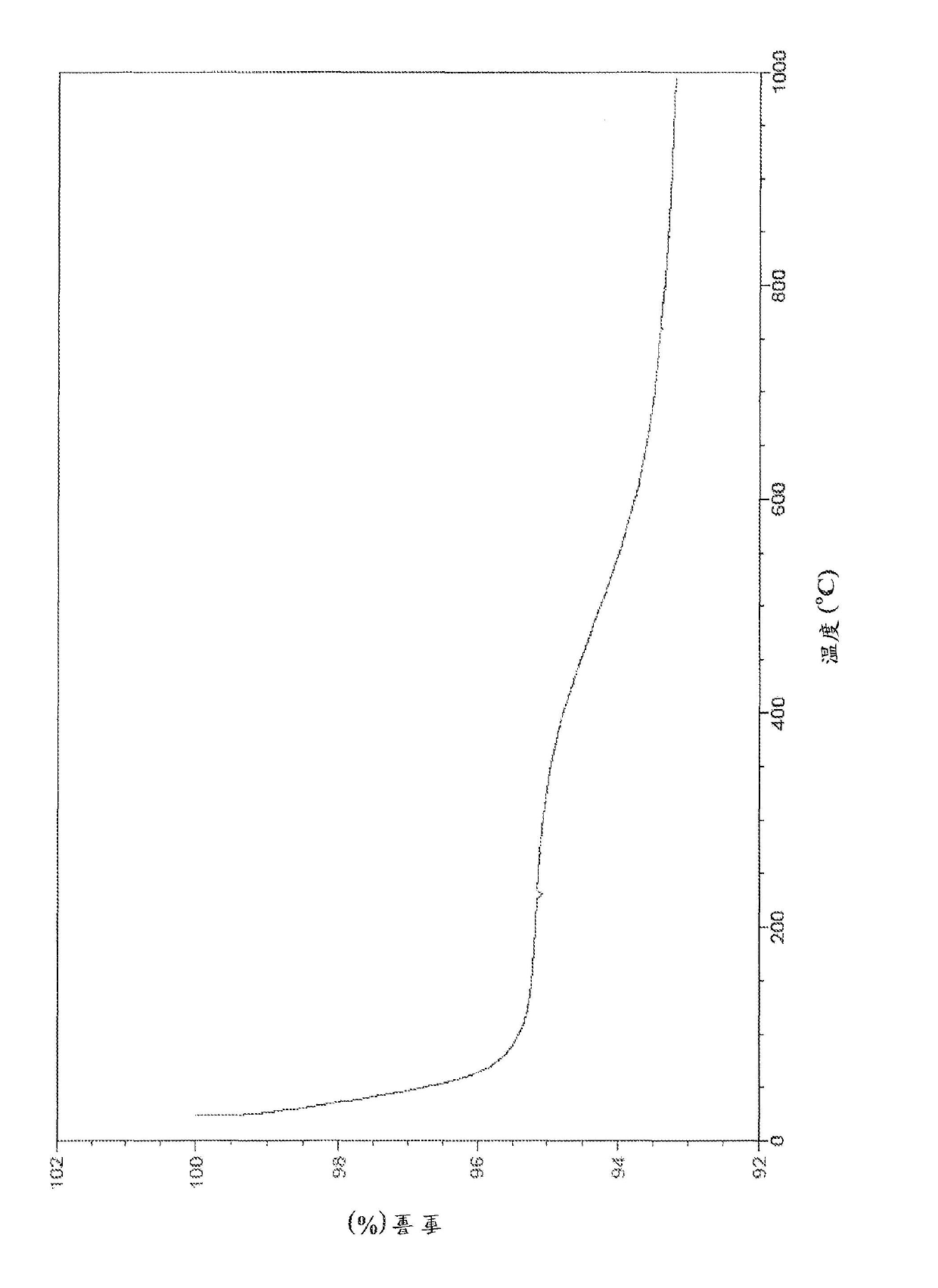
[0156] This example details the synthesis of one non-limiting embodiment of AB9, the scheme is as follows:
[0157]
[0158] / or will be obtained from Sigma- 0.78 g of L-cysteine hydrochloride (5.0 mmol) was dissolved in 100 ml of deionized water. 1.02 g of triethylamine (10 mmol, 1.4 ml) (hereinafter referred to as "TEA"), and obtained from 0.5 g (2.5 mmol) of isophthaloyl chloride were each dissolved in Acros 20 ml of tetrahydrofuran (hereinafter referred to as "THF"). TEA dissolved in THF was slowly added to the stirred deionized water solution of L-cysteine hydrochloride in the flask under nitrogen flow. After stirring for 5-10 minutes, isophthaloyl chloride dissolved in THF was slowly added to the flask. As the reaction proceeded, the reaction mixture turned pale yellow. The reaction mixture was stirred for an additional 16-18 hours. After 16-18 hours, the aqueous layer was extracted with 100 mL of ethyl acetate. Subsequently, the ethyl acetate layer was...
Embodiment 2
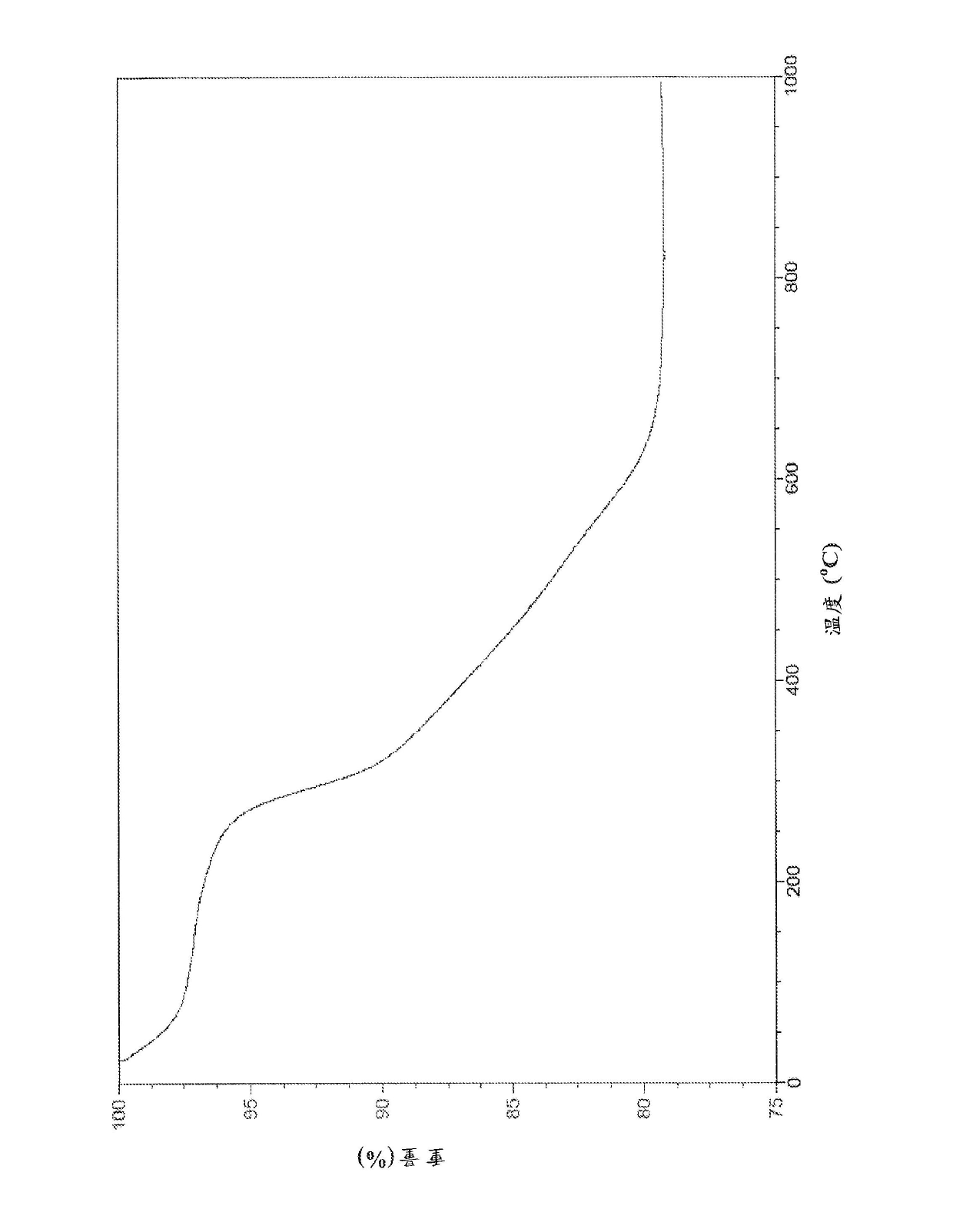
[0160] This example details the synthesis of a non-limiting embodiment of MEAB9, the scheme is as follows:
[0161]
[0162] 2.57 g of L-cysteine methyl ester hydrochloride (15 mmol) was dissolved in 150 ml of chloroform. 1.52 g of TEA (15 mmol, 2.07 mL) was dissolved in 25 mL of chloroform, and 1 g of isophthaloyl chloride (5 mmol) was dissolved in 40 mL of chloroform. The TEA solution and the isophthaloyl chloride solution were slowly added to the L-cysteine methyl ester hydrochloride solution. The reaction was stirred for 24 hours. Subsequently, the reaction solution was filtered, and the filtrate was washed with 200 ml of 10% Wash with hydrochloric acid three times. After washing, the chloroform layer was filtered again and washed with anhydrous Na 2 SO 4 dry. Chloroform was removed under vacuum to obtain a highly viscous oily liquid product. The oily liquid was redissolved in chloroform, and the chloroform was removed under vacuum. This step was repeated t...
Embodiment 3
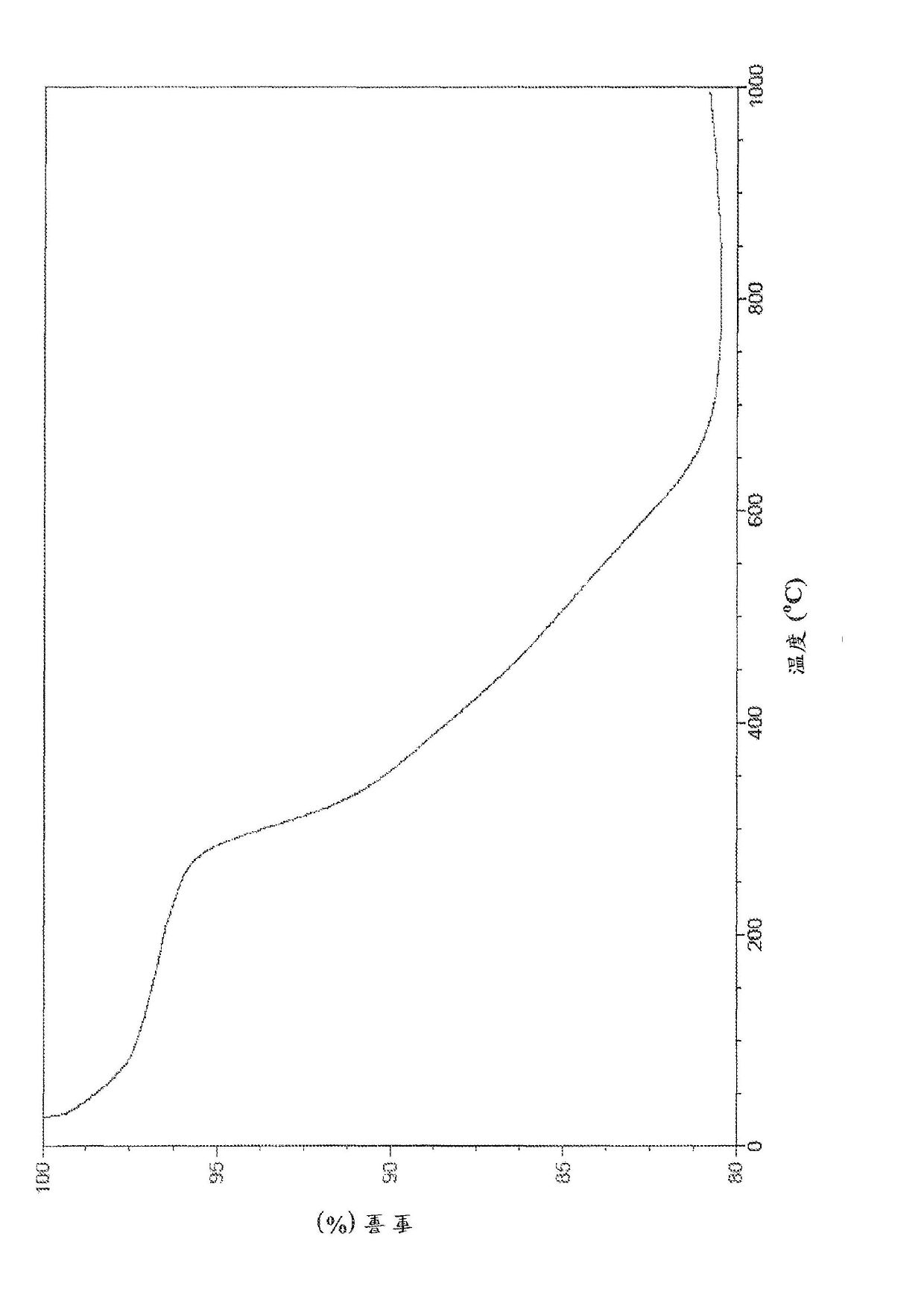
[0164] This example details the synthesis of a non-limiting embodiment of EEAB9 as follows:
[0165]
[0166] 2.72 g of L-cysteine ethyl ester hydrochloride (15 mmol) was dissolved in 150 ml of chloroform. 1.48 g of TEA (15 mmol, 2.02 mL) was dissolved in 25 mL of chloroform, and 1 g of isophthaloyl chloride (5 mmol) was dissolved in 40 mL of chloroform. The TEA solution and the isophthaloyl chloride solution were slowly added to the L-cysteine ethyl ester hydrochloride solution. The reaction was stirred for 24 hours. Then filter the reaction solution, the filtrate with 1.5 liters of 20% Wash with hydrochloric acid three times. After washing, the chloroform layer was filtered again and washed with anhydrous Na 2 SO 4 dry. Chloroform was subsequently removed under vacuum to obtain a highly viscous oily liquid product. The oily liquid was redissolved in chloroform, which was then removed under vacuum. This step was repeated twice and the resulting white solid was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com