Multi-index protein chip inspection reagent unit of cardiovascular disease diagnosis and prediction
A cardiovascular and kit technology, applied in the field of in vitro clinical tests and biochips, can solve problems such as time-consuming and labor-intensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of detection reaction plate
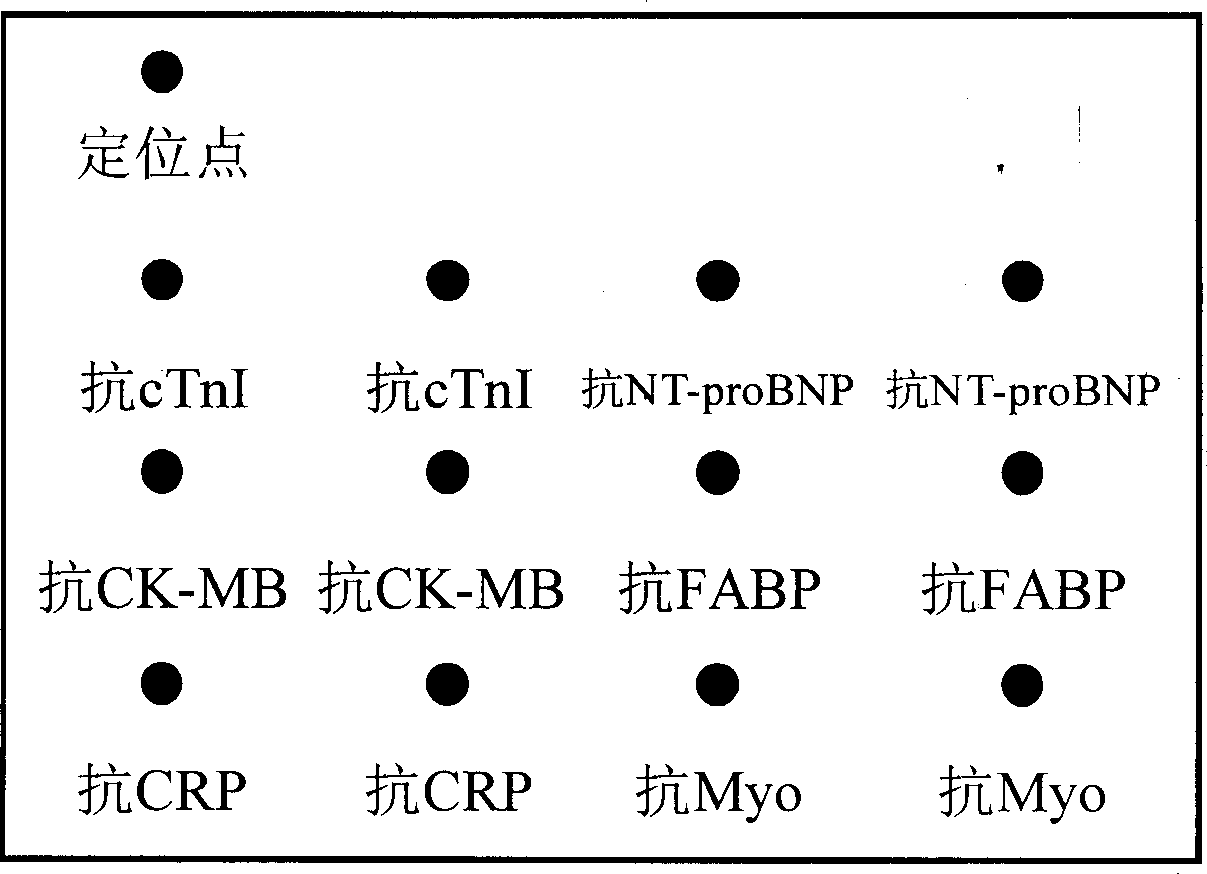
[0037]Take a 48-well plate, set 42 sample wells and 6 standard wells. Place a solid phase support (NC membrane or PDVF membrane) at the bottom of each reaction well, and the solid phase support is coated with anti-CRP, anti-Myoglobin, anti-cTnI, anti-cTnT, anti-NT-proBNP, anti-CK-MB , protein microarrays of eight antibodies including anti-FABP and anti-GPBB ( figure 1 ).
Embodiment 2
[0039] Kit preparation
[0040] Prepare a test kit containing eight indicators of cardiovascular disease integrated detection, which contains a reaction well plate (24-48 people) of embodiment 1, 1 bottle of washing concentrate, 1 bottle of enzyme-labeled working solution, and 1 bottle of detection solution A , a bottle of detection solution B, and a standard bottle.
[0041] Washing Concentrate, Enzyme Label Working Solution, Detection Solution A, Detection Solution B
Embodiment 3
[0043] Integrated detection of eight indicators of cardiovascular disease
[0044] Test each blood sample according to the following method:
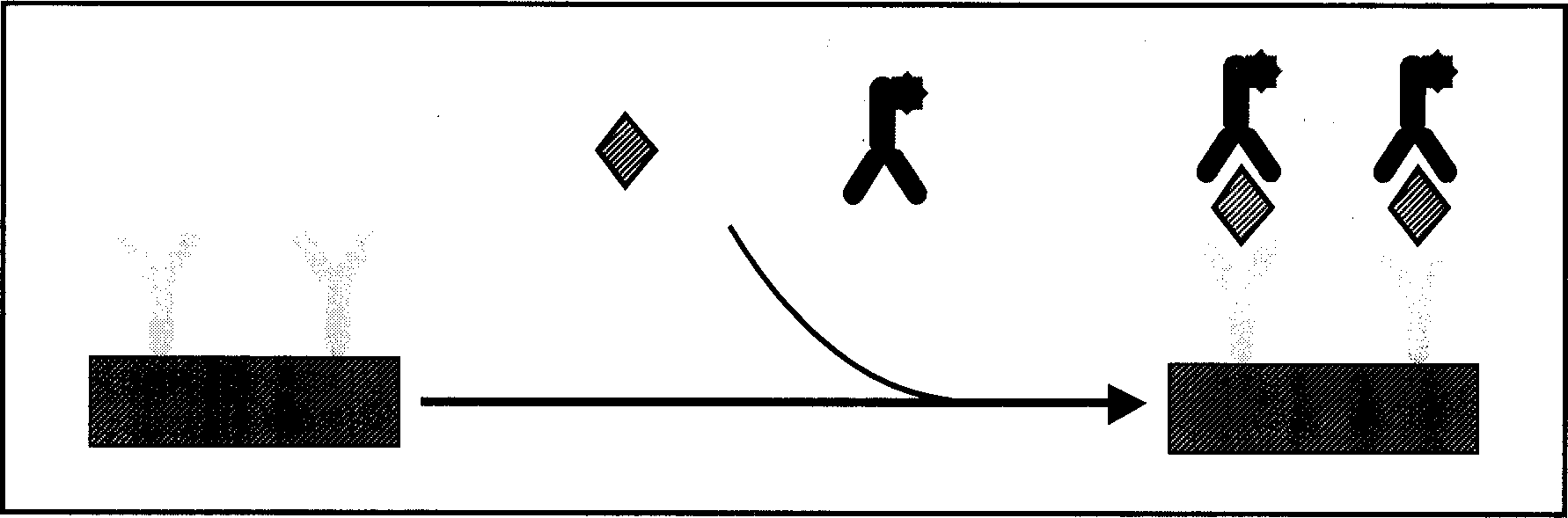
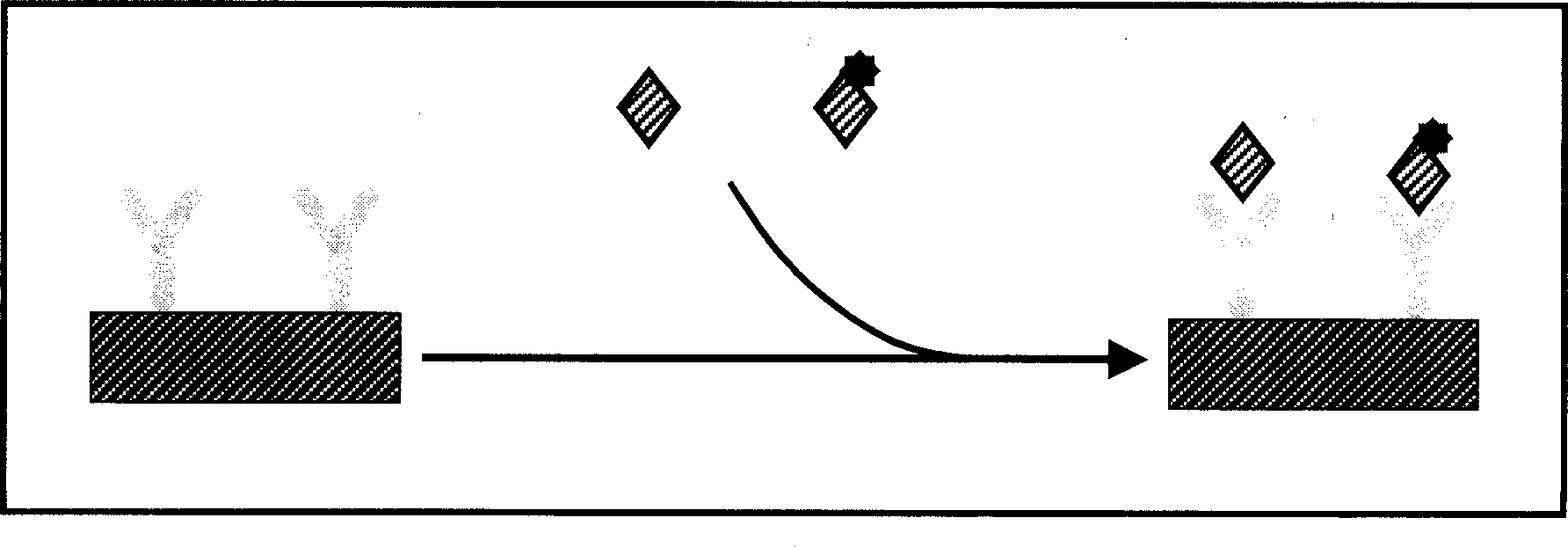
[0045] 1. Add test blood or standard substance to the corresponding reaction well, 75ul / well.
[0046] 2. Add enzyme-labeled working solution, 75ul / well.
[0047] 3. Incubate and shake at 37°C for 60 minutes.
[0048] 4. Wash 4-6 times with lotion, 2 minutes each time, vibrate.
[0049] 5. Add detection solution 20ul / well, so that the detection solution is evenly distributed at the bottom of the well.
[0050] 6. CCD detection, exposure for 30-60 seconds depending on the strength of the luminescent signal.
[0051] Result judgment:
[0052] According to the CutOff value of different indicators, refer to the gradient curve of the standard to determine the negative or positive of the sample serum.
[0053] At the same time, the serum samples tested were routinely tested by conventional methods. The result shows that the test result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com