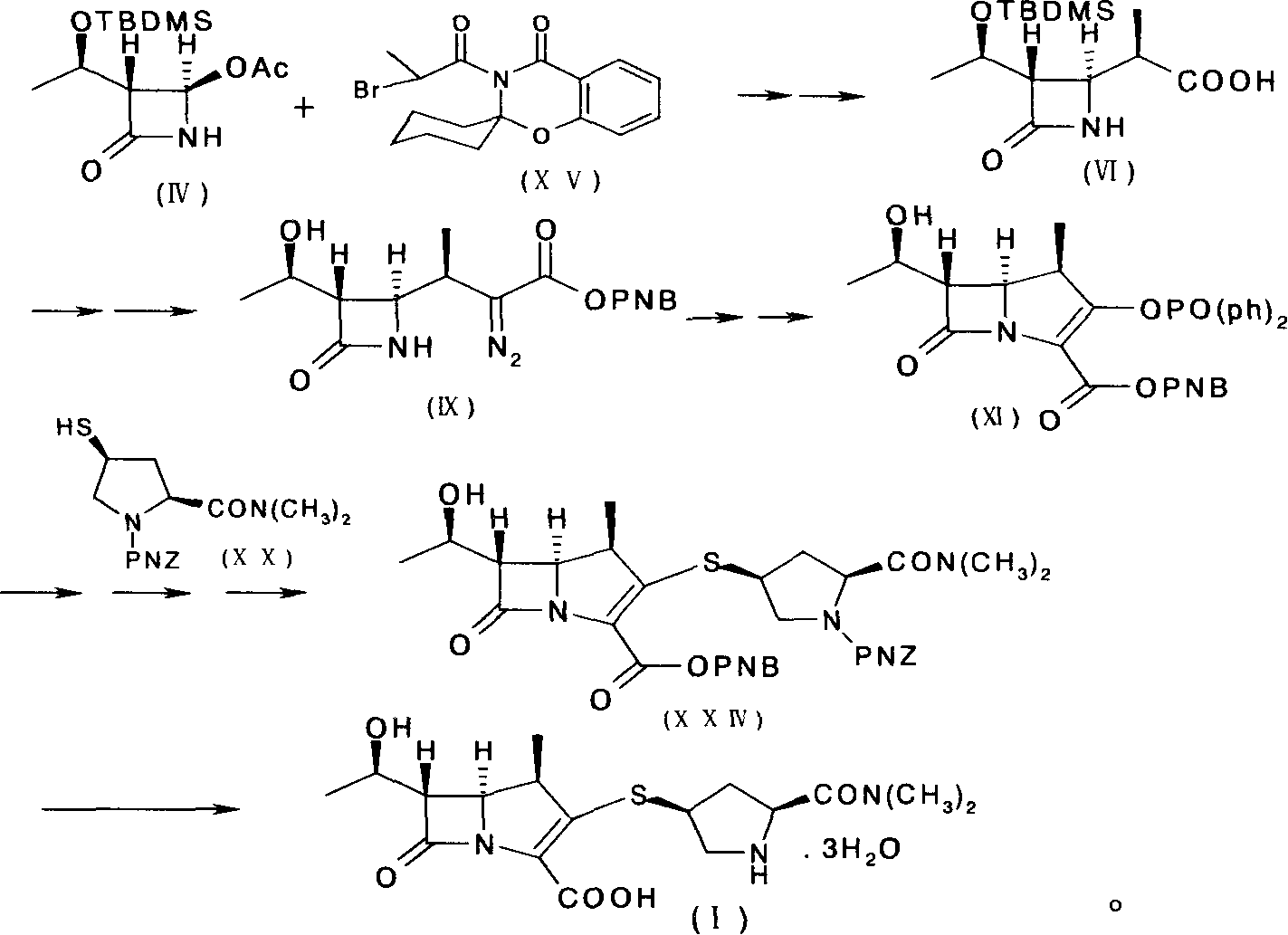
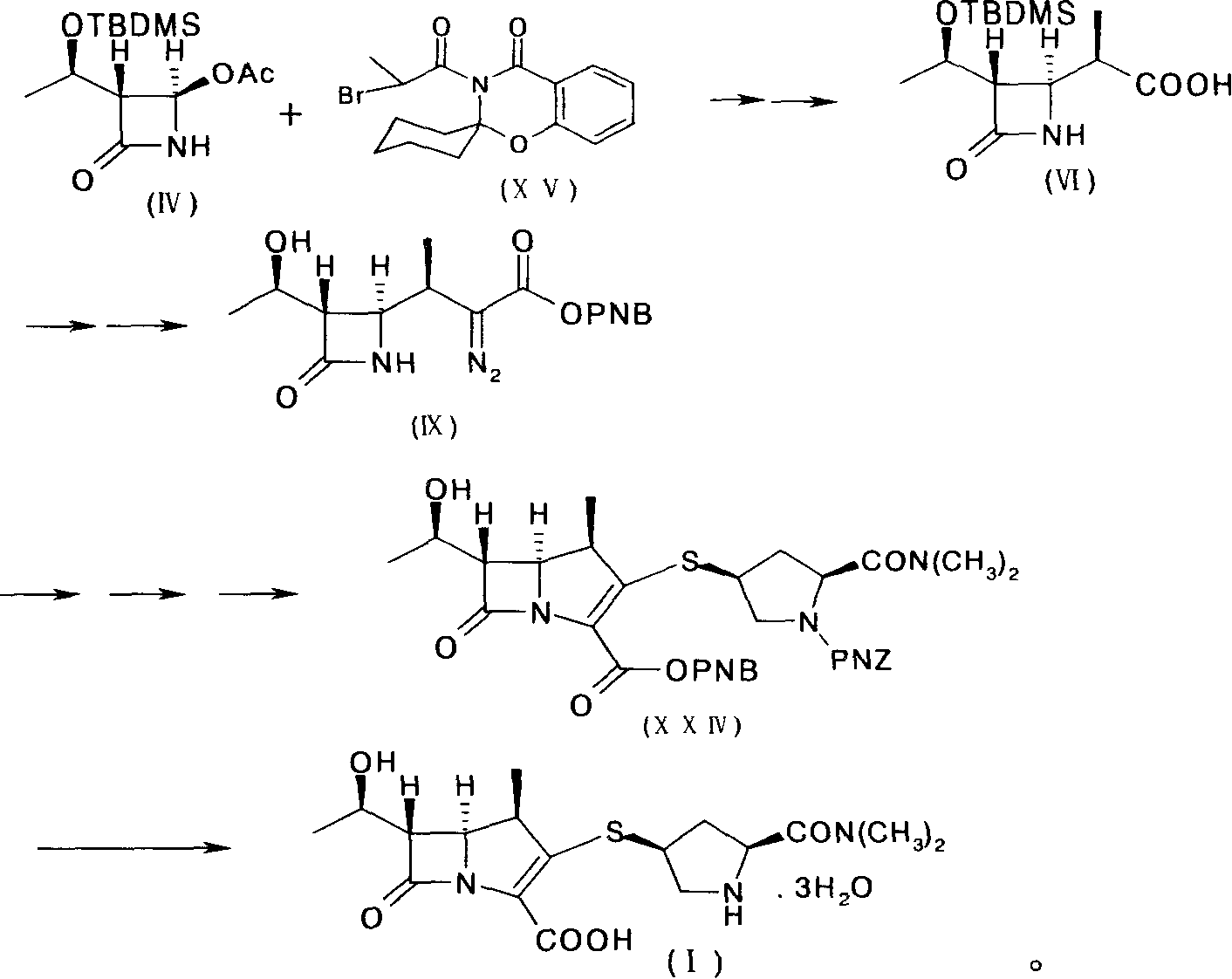
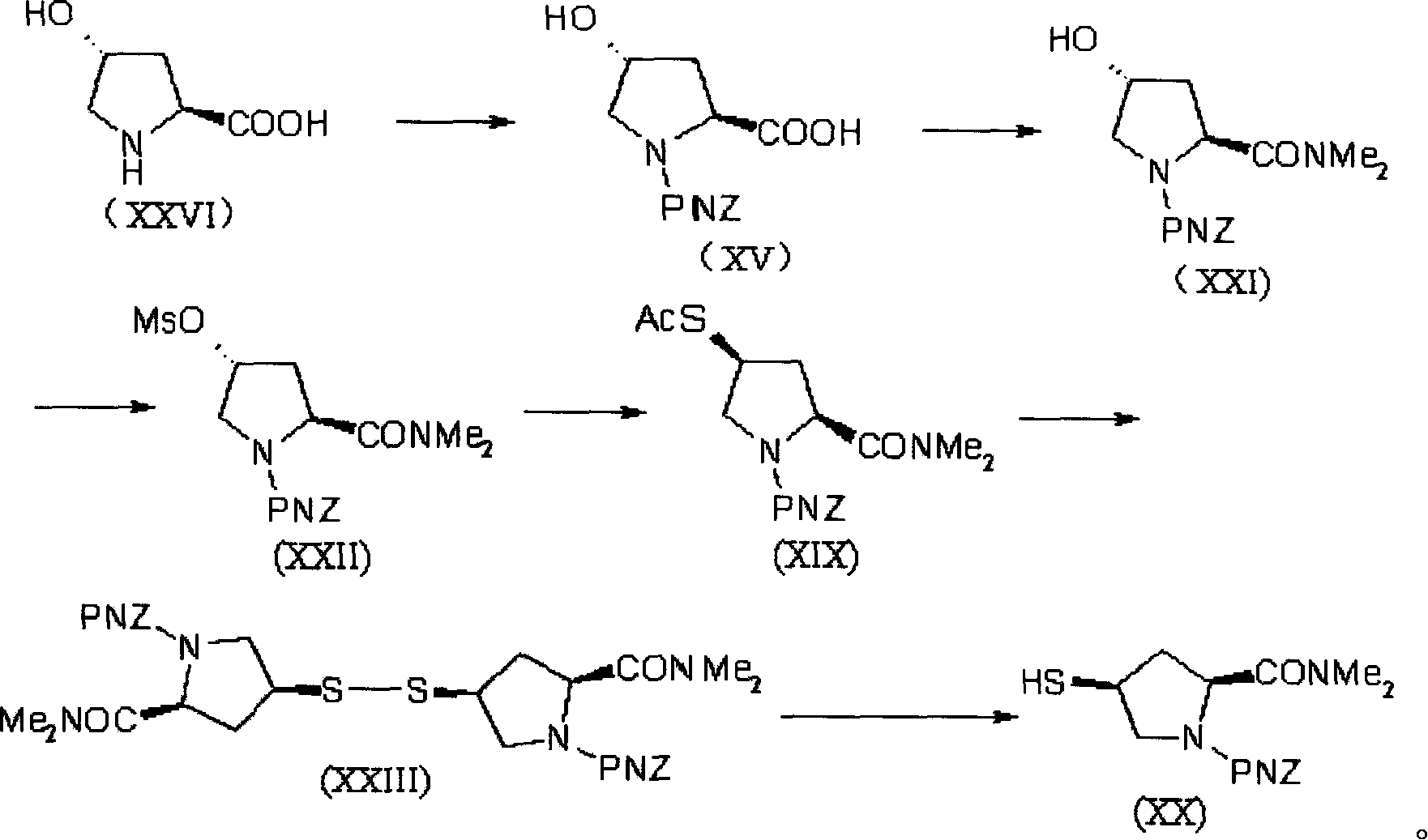
Preparation method of meluopeinan
A technology of meropenem and separation method, applied in the field of preparation of meropenem, can solve problems such as affecting product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] [Example 1] (5R, 6S, 8R, 2'S, 4'S)-p-nitrobenzyl-3-[4-(1-p-nitrobenzyloxycarbonyl-1-dimethylaminocarbonyl)pyrrolidine Thio]-6-(1-p-nitrobenzyloxycarbonylethoxy)-1-azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (XXIV) Synthesis
[0085] In a bottle equipped with a reflux cooler, add 40 g (0.102 mol) of diazoketone ester (IX) and 200 ml of ethyl acetate, stir and heat the mixture to 60 ° C, add 140 mg of rhodium octanoate, and stir vigorously at the same temperature 30 minutes, until the reaction of the diazoketoester (IX) was completed, a solution of 1-β-methylbicyclic ketoester (X) was obtained. The reaction solution was directly carried on to the next step without separation.
[0086] Take the solution of the above-mentioned 1-β-methylbicyclic ketone ester (X), add 300ml of acetonitrile (dry product), cool in an ice-salt bath to -10~-15°C and protect with nitrogen, then add 14.57g (0.113mol) of Diisopropylethylamine and 27.54 g (0.102 mol) of diphenyl chlorophospha...
Embodiment 2
[0091] [Example 2] (5R, 6S, 8R, 2'S, 4'S)-3-[4-(2-dimethylaminocarbonyl)pyrrolidinylthio]-6-(1-hydroxyethyl)-1-nitrogen Synthesis of Heterobicyclo[3.2.0]-hept-2-en-7-one-2-carboxylic acid (I)
[0092](5R, 6S, 8R, 2'S, 4'S)-p-nitrobenzyl-3-[4-(1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl)-pyrrolidinylthio] -6-(1-p-nitrobenzyloxycarbonylethoxy)-1-azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (XXIV) (ie One-step concentrated oil) 15g (HPLC quantification: 12.5g) was dissolved in 250ml tetrahydrofuran, and 200ml water was added under stirring. The solution was placed in a 1 L autoclave, and then 5.5 g of 2,6-lutidine and 2 g of 10% palladium on carbon were added with stirring. The resulting mixture was hydrogenated for 1 hour under a hydrogen pressure of 1.8 MPa. Remove the catalyst by filtration, dilute the filtrate with 800ml of acetone, then add seed crystals at 5-15°C, after 30 minutes a large amount of crystals will separate out, then add 400ml of acetone dropwise ...
Embodiment 3
[0097] [Example 3] (5R, 6S, 8R, 2'S, 4'S)-3-[4-(2-dimethylaminocarbonyl)pyrrolidinylthio]-6-(1-hydroxyethyl)-1-nitrogen Synthesis of Heterobicyclo[3.2.0]-hept-2-en-7-one-2-carboxylic acid (I)
[0098] (5R, 6S, 8R, 2'S, 4'S)-p-nitrobenzyl-3-[4-(1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl)-pyrrolidinylthio] -6-(1-p-nitrobenzyloxycarbonylethoxy)-1-azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (XXIV) 10g (crystal matter) was dissolved in 250ml tetrahydrofuran, and 200ml water was added under stirring. The solution was placed in a 1 L autoclave, and then 5.5 g of 2,6-lutidine and 2 g of 10% palladium on carbon were added with stirring. The resulting mixture was hydrogenated for 1 hour under a hydrogen pressure of 1.8 MPa. Remove the catalyst by filtration, dilute the filtrate with 800ml of acetone, then add seed crystals at 5-15°C, after 30 minutes a large amount of crystals will separate out, then add 400ml of acetone dropwise at the same temperature, stir for 30 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com