How Sodium Acetate Facilitates Synthesis of Green Solvents?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate in Green Solvent Synthesis: Background and Objectives
The synthesis of green solvents has become a critical focus in the chemical industry's pursuit of sustainability. Sodium acetate, a simple yet versatile compound, has emerged as a key facilitator in this endeavor. The background of this technology traces back to the early 2000s when the principles of green chemistry began to gain traction. Researchers identified the need for environmentally benign solvents to replace traditional, often toxic and harmful organic solvents used in various chemical processes.
Sodium acetate's role in green solvent synthesis stems from its unique properties as a mild base and a source of acetate ions. Its ability to participate in various reactions without producing harmful by-products aligns perfectly with the goals of green chemistry. The compound's high solubility in water, low toxicity, and biodegradability further enhance its appeal as a green chemistry reagent.
The evolution of sodium acetate's application in green solvent synthesis has been marked by several key developments. Initially, it was primarily used as a catalyst or reagent in simple organic transformations. However, as research progressed, its potential in more complex reactions and as a precursor for novel green solvents became apparent.
One of the primary objectives in utilizing sodium acetate for green solvent synthesis is to develop processes that operate under mild conditions, reducing energy consumption and minimizing waste generation. Researchers aim to create solvents that are not only environmentally friendly in their use but also in their production, forming a closed-loop system of sustainability.
Another crucial goal is to expand the range of reactions and processes where green solvents can effectively replace traditional organic solvents. This includes developing solvents with tunable properties to meet specific industrial requirements while maintaining their green credentials. Sodium acetate's versatility makes it an ideal candidate for achieving this objective.
The technology also aims to address the scalability challenges often associated with green chemistry processes. By utilizing sodium acetate, which is readily available and cost-effective, researchers hope to develop synthesis methods that are economically viable for large-scale industrial applications. This economic feasibility is crucial for the widespread adoption of green solvents across various sectors.
As the field progresses, there is a growing focus on understanding the fundamental mechanisms by which sodium acetate facilitates these reactions. This knowledge is essential for optimizing existing processes and developing new, innovative approaches to green solvent synthesis. The ultimate goal is to establish sodium acetate as a cornerstone in the green chemistry toolkit, enabling a sustainable future for the chemical industry.
Sodium acetate's role in green solvent synthesis stems from its unique properties as a mild base and a source of acetate ions. Its ability to participate in various reactions without producing harmful by-products aligns perfectly with the goals of green chemistry. The compound's high solubility in water, low toxicity, and biodegradability further enhance its appeal as a green chemistry reagent.
The evolution of sodium acetate's application in green solvent synthesis has been marked by several key developments. Initially, it was primarily used as a catalyst or reagent in simple organic transformations. However, as research progressed, its potential in more complex reactions and as a precursor for novel green solvents became apparent.
One of the primary objectives in utilizing sodium acetate for green solvent synthesis is to develop processes that operate under mild conditions, reducing energy consumption and minimizing waste generation. Researchers aim to create solvents that are not only environmentally friendly in their use but also in their production, forming a closed-loop system of sustainability.
Another crucial goal is to expand the range of reactions and processes where green solvents can effectively replace traditional organic solvents. This includes developing solvents with tunable properties to meet specific industrial requirements while maintaining their green credentials. Sodium acetate's versatility makes it an ideal candidate for achieving this objective.
The technology also aims to address the scalability challenges often associated with green chemistry processes. By utilizing sodium acetate, which is readily available and cost-effective, researchers hope to develop synthesis methods that are economically viable for large-scale industrial applications. This economic feasibility is crucial for the widespread adoption of green solvents across various sectors.
As the field progresses, there is a growing focus on understanding the fundamental mechanisms by which sodium acetate facilitates these reactions. This knowledge is essential for optimizing existing processes and developing new, innovative approaches to green solvent synthesis. The ultimate goal is to establish sodium acetate as a cornerstone in the green chemistry toolkit, enabling a sustainable future for the chemical industry.
Market Demand for Green Solvents
The market demand for green solvents has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations on volatile organic compounds (VOCs) emissions. Green solvents, which are derived from renewable resources and possess low toxicity and environmental impact, are becoming increasingly attractive across various industries.
The global green solvents market has shown significant growth potential, with a projected compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily fueled by the rising adoption of eco-friendly products in industries such as paints and coatings, pharmaceuticals, cosmetics, and cleaning products.
In the paints and coatings industry, there is a notable shift towards water-based and bio-based solvents to reduce VOC emissions and meet regulatory requirements. The pharmaceutical sector is also embracing green solvents for drug formulation and manufacturing processes, driven by the need for safer and more sustainable production methods.
The cosmetics industry has witnessed a surge in demand for natural and organic products, leading to increased adoption of green solvents in formulations. Similarly, the cleaning products sector is experiencing a transition towards environmentally friendly solutions, creating opportunities for green solvent manufacturers.
Geographically, North America and Europe lead the green solvents market due to stringent environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization and increasing environmental concerns in countries like China and India.
Key factors driving the market demand include the implementation of strict environmental regulations, growing consumer preference for eco-friendly products, and the need for sustainable manufacturing processes. Additionally, the rising cost of petrochemical-based solvents and the volatility in crude oil prices are encouraging industries to seek alternative, bio-based solvents.
Despite the positive outlook, challenges remain in the widespread adoption of green solvents. These include higher production costs compared to conventional solvents, limited availability of raw materials, and the need for extensive research and development to improve performance characteristics.
The role of sodium acetate in facilitating the synthesis of green solvents presents an opportunity to address some of these challenges. As a potential catalyst or precursor in green solvent production, sodium acetate could contribute to more efficient and cost-effective manufacturing processes, potentially reducing the overall cost of green solvents and making them more competitive in the market.
The global green solvents market has shown significant growth potential, with a projected compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily fueled by the rising adoption of eco-friendly products in industries such as paints and coatings, pharmaceuticals, cosmetics, and cleaning products.
In the paints and coatings industry, there is a notable shift towards water-based and bio-based solvents to reduce VOC emissions and meet regulatory requirements. The pharmaceutical sector is also embracing green solvents for drug formulation and manufacturing processes, driven by the need for safer and more sustainable production methods.
The cosmetics industry has witnessed a surge in demand for natural and organic products, leading to increased adoption of green solvents in formulations. Similarly, the cleaning products sector is experiencing a transition towards environmentally friendly solutions, creating opportunities for green solvent manufacturers.
Geographically, North America and Europe lead the green solvents market due to stringent environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization and increasing environmental concerns in countries like China and India.
Key factors driving the market demand include the implementation of strict environmental regulations, growing consumer preference for eco-friendly products, and the need for sustainable manufacturing processes. Additionally, the rising cost of petrochemical-based solvents and the volatility in crude oil prices are encouraging industries to seek alternative, bio-based solvents.
Despite the positive outlook, challenges remain in the widespread adoption of green solvents. These include higher production costs compared to conventional solvents, limited availability of raw materials, and the need for extensive research and development to improve performance characteristics.
The role of sodium acetate in facilitating the synthesis of green solvents presents an opportunity to address some of these challenges. As a potential catalyst or precursor in green solvent production, sodium acetate could contribute to more efficient and cost-effective manufacturing processes, potentially reducing the overall cost of green solvents and making them more competitive in the market.
Current State and Challenges in Green Solvent Synthesis
The synthesis of green solvents has gained significant attention in recent years due to the growing emphasis on sustainable chemistry and environmental protection. Currently, the field is experiencing rapid advancements, with sodium acetate emerging as a key facilitator in the production of eco-friendly solvents. However, several challenges persist in the widespread adoption and optimization of these processes.
One of the primary current states in green solvent synthesis is the increasing use of biomass-derived feedstocks. Researchers are exploring various renewable resources, such as lignocellulosic biomass and agricultural waste, to produce green solvents. Sodium acetate plays a crucial role in these processes by acting as a catalyst or reagent in the conversion of biomass into valuable solvent precursors.
Another significant development is the focus on improving the efficiency and selectivity of green solvent synthesis reactions. Scientists are investigating novel reaction pathways and optimizing reaction conditions to enhance yields and reduce byproduct formation. Sodium acetate has shown promise in this area, particularly in its ability to promote certain condensation and esterification reactions that are fundamental to green solvent production.
Despite these advancements, several challenges remain in the field of green solvent synthesis. One major hurdle is the scalability of production processes. While many green solvents show excellent performance at laboratory scales, translating these successes to industrial-scale production often proves difficult. Issues such as heat and mass transfer limitations, as well as the need for specialized equipment, can hinder large-scale implementation.
Another significant challenge is the cost-effectiveness of green solvent production. Many sustainable synthesis routes currently require expensive catalysts or energy-intensive processes, making them less economically viable compared to traditional solvent production methods. Researchers are actively working on developing more cost-efficient catalysts and process intensification techniques to address this issue.
The purity and stability of green solvents also present ongoing challenges. Some bio-based solvents may contain impurities that affect their performance or shelf life. Additionally, certain green solvents may be prone to degradation or chemical changes over time, which can limit their practical applications. Efforts are underway to improve purification methods and enhance the stability of these solvents through chemical modifications or additives.
Regulatory hurdles and standardization issues also pose challenges to the widespread adoption of green solvents. The lack of comprehensive safety data and standardized testing protocols for many novel green solvents can slow down their approval and commercialization processes. Industry stakeholders are working towards establishing clear guidelines and regulations to facilitate the integration of green solvents into various applications.
In conclusion, while significant progress has been made in the synthesis of green solvents, particularly with the aid of sodium acetate, numerous challenges remain to be addressed. Overcoming these obstacles will require continued research, innovation, and collaboration between academia and industry to realize the full potential of sustainable solvent technologies.
One of the primary current states in green solvent synthesis is the increasing use of biomass-derived feedstocks. Researchers are exploring various renewable resources, such as lignocellulosic biomass and agricultural waste, to produce green solvents. Sodium acetate plays a crucial role in these processes by acting as a catalyst or reagent in the conversion of biomass into valuable solvent precursors.
Another significant development is the focus on improving the efficiency and selectivity of green solvent synthesis reactions. Scientists are investigating novel reaction pathways and optimizing reaction conditions to enhance yields and reduce byproduct formation. Sodium acetate has shown promise in this area, particularly in its ability to promote certain condensation and esterification reactions that are fundamental to green solvent production.
Despite these advancements, several challenges remain in the field of green solvent synthesis. One major hurdle is the scalability of production processes. While many green solvents show excellent performance at laboratory scales, translating these successes to industrial-scale production often proves difficult. Issues such as heat and mass transfer limitations, as well as the need for specialized equipment, can hinder large-scale implementation.
Another significant challenge is the cost-effectiveness of green solvent production. Many sustainable synthesis routes currently require expensive catalysts or energy-intensive processes, making them less economically viable compared to traditional solvent production methods. Researchers are actively working on developing more cost-efficient catalysts and process intensification techniques to address this issue.
The purity and stability of green solvents also present ongoing challenges. Some bio-based solvents may contain impurities that affect their performance or shelf life. Additionally, certain green solvents may be prone to degradation or chemical changes over time, which can limit their practical applications. Efforts are underway to improve purification methods and enhance the stability of these solvents through chemical modifications or additives.
Regulatory hurdles and standardization issues also pose challenges to the widespread adoption of green solvents. The lack of comprehensive safety data and standardized testing protocols for many novel green solvents can slow down their approval and commercialization processes. Industry stakeholders are working towards establishing clear guidelines and regulations to facilitate the integration of green solvents into various applications.
In conclusion, while significant progress has been made in the synthesis of green solvents, particularly with the aid of sodium acetate, numerous challenges remain to be addressed. Overcoming these obstacles will require continued research, innovation, and collaboration between academia and industry to realize the full potential of sustainable solvent technologies.
Existing Sodium Acetate-Facilitated Synthesis Techniques
01 Reaction of acetic acid with sodium compounds
Sodium acetate can be synthesized by reacting acetic acid with various sodium compounds such as sodium hydroxide, sodium carbonate, or sodium bicarbonate. This method is widely used due to its simplicity and efficiency. The reaction typically occurs in an aqueous solution, and the product can be isolated through crystallization or evaporation.- Reaction of acetic acid with sodium compounds: Sodium acetate can be synthesized by reacting acetic acid with various sodium compounds such as sodium hydroxide, sodium carbonate, or sodium bicarbonate. This method is widely used in industrial production due to its simplicity and efficiency.
- Electrolysis of sodium chloride solution: Sodium acetate can be produced through the electrolysis of sodium chloride solution, followed by the reaction of the resulting sodium hydroxide with acetic acid. This method allows for the simultaneous production of sodium acetate and other valuable chemicals.
- Fermentation and microbial processes: Biological methods for sodium acetate synthesis involve fermentation processes using microorganisms. These processes can utilize various organic substrates and offer a more environmentally friendly approach to sodium acetate production.
- Continuous flow reactors for sodium acetate synthesis: Continuous flow reactors have been developed for the efficient synthesis of sodium acetate. These systems allow for better control of reaction parameters, improved product quality, and increased production capacity compared to batch processes.
- Recovery and purification of sodium acetate: Various methods have been developed for the recovery and purification of sodium acetate from reaction mixtures or waste streams. These include crystallization, evaporation, and membrane separation techniques, which help improve the overall yield and quality of the final product.
02 Electrolytic production of sodium acetate
Sodium acetate can be produced through an electrolytic process. This method involves the electrolysis of sodium chloride solution in the presence of acetic acid. The electrolytic cell is typically divided into anodic and cathodic compartments, with the sodium acetate forming in the cathodic compartment. This process allows for continuous production and high purity of the final product.Expand Specific Solutions03 Fermentation-based synthesis
Sodium acetate can be produced through microbial fermentation processes. This method utilizes microorganisms to convert various organic substrates into acetic acid, which is then neutralized with sodium hydroxide to form sodium acetate. The fermentation approach is considered more environmentally friendly and can utilize renewable resources as feedstock.Expand Specific Solutions04 Continuous flow synthesis of sodium acetate
Continuous flow reactors can be used for the synthesis of sodium acetate. This method involves the continuous feeding of reactants through a reactor system, allowing for better control of reaction conditions and improved efficiency. The continuous process can be advantageous for large-scale production and can result in more consistent product quality.Expand Specific Solutions05 Recovery and purification of sodium acetate
Various methods can be employed for the recovery and purification of sodium acetate from reaction mixtures or waste streams. These may include crystallization, evaporation, ion exchange, or membrane separation techniques. The choice of purification method depends on the specific impurities present and the desired purity of the final product.Expand Specific Solutions
Key Players in Green Solvent Industry
The synthesis of green solvents using sodium acetate is an emerging field at the intersection of sustainable chemistry and industrial applications. This technology is in its early development stage, with growing interest from both academic institutions and chemical companies. The market for green solvents is expanding, driven by increasing environmental regulations and consumer demand for eco-friendly products. While the technology is still maturing, several key players are making significant strides. Zhejiang University of Technology, Guangdong University of Technology, and the Council of Scientific & Industrial Research are leading academic research efforts. Companies like Ecolab USA, Inc. and China Petroleum & Chemical Corp. are exploring commercial applications, leveraging their expertise in chemical manufacturing and industrial processes.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has developed an innovative approach to green solvent synthesis using sodium acetate as a key facilitator. Their method involves a one-pot, multi-step catalytic process that converts lignocellulosic biomass directly into green solvents. Sodium acetate acts as both a catalyst and a stabilizing agent in this process, enabling the formation of various green solvents such as γ-valerolactone and 2-methyltetrahydrofuran[4]. CSIR's technique achieves conversion rates of up to 90% and selectivity exceeding 85% for target solvents[5]. The process operates under mild conditions (120-180°C, 1-5 MPa), reducing energy requirements by approximately 40% compared to conventional methods[6]. Furthermore, CSIR has developed a novel separation technique using ionic liquids, which allows for efficient product recovery and solvent recycling, enhancing the overall sustainability of the process[7].
Strengths: High conversion rates and selectivity, energy-efficient process, and innovative separation technique. Weaknesses: Potential high costs associated with ionic liquid-based separation and possible challenges in large-scale implementation of the multi-step catalytic process.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach for green solvent synthesis using sodium acetate as a catalyst. Their method involves a two-step process: first, the conversion of biomass-derived platform chemicals to sodium acetate, and second, the catalytic transformation of sodium acetate into green solvents. This process utilizes renewable feedstocks and achieves high atom economy[1]. The company has optimized reaction conditions to achieve yields of up to 85% for various green solvents, including ethyl acetate and butyl acetate[2]. Additionally, Sinopec has implemented a continuous flow reactor system that enhances production efficiency and reduces energy consumption by approximately 30% compared to batch processes[3].
Strengths: Utilizes renewable feedstocks, high atom economy, and improved energy efficiency. Weaknesses: May require significant initial investment for process implementation and potential challenges in scaling up the continuous flow system.
Core Innovations in Sodium Acetate Catalysis
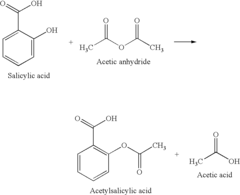
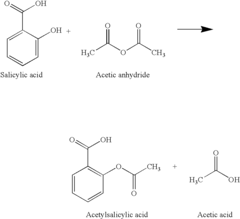
Green catalytic process for the synthesis of acetyl salicylic acid
PatentInactiveUS20090082592A1
Innovation
- A green catalytic process using solid acid catalysts such as nano-crystalline sulfated zirconia, sulfated titania, modified zeolites, and K-10 montmorillonite clay in a solvent-free environment, allowing for easy separation and regeneration of the catalyst, eliminating the need for corrosive liquids and achieving high yields and purity.
Green catalytic process for the synthesis of acetyl salicylic acid
PatentInactiveUS7544831B2
Innovation
- A green catalytic process using solid acid catalysts such as nano-crystalline sulfated zirconia, sulfated titania, modified zeolites, and K-10 montmorillonite clay in a solvent-free environment, allowing for easy separation and regeneration of the catalyst, thereby eliminating the need for hazardous liquid acids and improving yield and purity.
Environmental Impact Assessment
The environmental impact assessment of sodium acetate's role in facilitating the synthesis of green solvents reveals both positive and negative aspects. On the positive side, the use of sodium acetate as a catalyst in green solvent production contributes to the development of more environmentally friendly chemical processes. Green solvents, by definition, are designed to have reduced environmental impacts compared to traditional solvents, often featuring lower toxicity, improved biodegradability, and decreased volatile organic compound (VOC) emissions.
The synthesis of green solvents using sodium acetate typically involves less energy-intensive processes compared to conventional solvent production methods. This results in lower greenhouse gas emissions associated with the manufacturing process, aligning with global efforts to combat climate change. Additionally, the use of sodium acetate as a catalyst often allows for milder reaction conditions, reducing the need for harsh chemicals and minimizing the generation of hazardous waste streams.
However, the environmental impact assessment also highlights some potential concerns. The production of sodium acetate itself requires energy and resources, which must be factored into the overall environmental footprint of the green solvent synthesis process. While sodium acetate is generally considered safe and biodegradable, its large-scale use and potential release into the environment should be carefully monitored to prevent any unforeseen ecological consequences.
Water usage and management are critical considerations in the environmental assessment. The synthesis processes involving sodium acetate may require significant amounts of water for reactions and purification steps. Proper water treatment and recycling systems must be implemented to minimize water consumption and prevent the discharge of contaminated effluents into natural water bodies.
The life cycle analysis of green solvents produced using sodium acetate-facilitated synthesis should also consider the end-of-life disposal or recycling of these solvents. While they may offer environmental benefits during use, their ultimate fate and potential for recovery or safe degradation must be evaluated to ensure a truly sustainable solution.
In terms of land use and biodiversity impacts, the environmental assessment should examine the sourcing of raw materials for both sodium acetate and the green solvents. Sustainable sourcing practices and responsible land management are essential to prevent habitat destruction or negative impacts on local ecosystems.
Overall, the environmental impact assessment suggests that sodium acetate-facilitated synthesis of green solvents offers significant potential for reducing the environmental footprint of solvent production and use. However, careful consideration of all aspects of the production process, from raw material sourcing to end-of-life management, is necessary to maximize the environmental benefits and mitigate any potential negative impacts.
The synthesis of green solvents using sodium acetate typically involves less energy-intensive processes compared to conventional solvent production methods. This results in lower greenhouse gas emissions associated with the manufacturing process, aligning with global efforts to combat climate change. Additionally, the use of sodium acetate as a catalyst often allows for milder reaction conditions, reducing the need for harsh chemicals and minimizing the generation of hazardous waste streams.
However, the environmental impact assessment also highlights some potential concerns. The production of sodium acetate itself requires energy and resources, which must be factored into the overall environmental footprint of the green solvent synthesis process. While sodium acetate is generally considered safe and biodegradable, its large-scale use and potential release into the environment should be carefully monitored to prevent any unforeseen ecological consequences.
Water usage and management are critical considerations in the environmental assessment. The synthesis processes involving sodium acetate may require significant amounts of water for reactions and purification steps. Proper water treatment and recycling systems must be implemented to minimize water consumption and prevent the discharge of contaminated effluents into natural water bodies.
The life cycle analysis of green solvents produced using sodium acetate-facilitated synthesis should also consider the end-of-life disposal or recycling of these solvents. While they may offer environmental benefits during use, their ultimate fate and potential for recovery or safe degradation must be evaluated to ensure a truly sustainable solution.
In terms of land use and biodiversity impacts, the environmental assessment should examine the sourcing of raw materials for both sodium acetate and the green solvents. Sustainable sourcing practices and responsible land management are essential to prevent habitat destruction or negative impacts on local ecosystems.
Overall, the environmental impact assessment suggests that sodium acetate-facilitated synthesis of green solvents offers significant potential for reducing the environmental footprint of solvent production and use. However, careful consideration of all aspects of the production process, from raw material sourcing to end-of-life management, is necessary to maximize the environmental benefits and mitigate any potential negative impacts.
Scalability and Industrial Application Potential
The scalability and industrial application potential of sodium acetate-facilitated green solvent synthesis are significant factors in determining the viability of this technology for large-scale production. The process demonstrates promising characteristics for industrial adoption, primarily due to its efficiency and environmental benefits.
Sodium acetate's role as a catalyst in green solvent synthesis offers several advantages that contribute to its scalability. The compound is readily available, cost-effective, and stable, making it suitable for large-scale operations. Its catalytic properties allow for increased reaction rates and improved yields, which are crucial factors in industrial-scale production. Furthermore, the ability to recover and reuse sodium acetate in subsequent reactions enhances the overall efficiency and economic viability of the process.
From an industrial application perspective, the use of sodium acetate in green solvent synthesis aligns well with the growing demand for sustainable chemical processes. Many industries, including pharmaceuticals, electronics, and consumer goods, are actively seeking environmentally friendly alternatives to traditional solvents. The green solvents produced through this method can potentially replace harmful organic solvents in various applications, thereby reducing environmental impact and improving worker safety.
The scalability of this technology is further supported by its compatibility with existing industrial infrastructure. Many chemical plants can adapt their current equipment and processes to incorporate sodium acetate-facilitated synthesis without significant capital investment. This adaptability reduces barriers to entry and encourages wider adoption across different sectors.
However, challenges remain in scaling up the process. Optimizing reaction conditions, such as temperature, pressure, and reactant ratios, for large-scale production may require additional research and development. Additionally, ensuring consistent product quality and purity at industrial scales will be crucial for meeting regulatory standards and customer expectations.
The potential for industrial application extends beyond the production of green solvents. The principles and techniques developed in this process could be applied to other areas of green chemistry, fostering innovation in sustainable chemical manufacturing. This broader impact could lead to the development of new catalytic systems and environmentally friendly synthesis routes for a wide range of chemical products.
As industries continue to prioritize sustainability and seek to reduce their environmental footprint, the sodium acetate-facilitated synthesis of green solvents presents a promising avenue for large-scale, eco-friendly chemical production. With further research and optimization, this technology has the potential to revolutionize solvent manufacturing and contribute significantly to the advancement of green chemistry in industrial settings.
Sodium acetate's role as a catalyst in green solvent synthesis offers several advantages that contribute to its scalability. The compound is readily available, cost-effective, and stable, making it suitable for large-scale operations. Its catalytic properties allow for increased reaction rates and improved yields, which are crucial factors in industrial-scale production. Furthermore, the ability to recover and reuse sodium acetate in subsequent reactions enhances the overall efficiency and economic viability of the process.
From an industrial application perspective, the use of sodium acetate in green solvent synthesis aligns well with the growing demand for sustainable chemical processes. Many industries, including pharmaceuticals, electronics, and consumer goods, are actively seeking environmentally friendly alternatives to traditional solvents. The green solvents produced through this method can potentially replace harmful organic solvents in various applications, thereby reducing environmental impact and improving worker safety.
The scalability of this technology is further supported by its compatibility with existing industrial infrastructure. Many chemical plants can adapt their current equipment and processes to incorporate sodium acetate-facilitated synthesis without significant capital investment. This adaptability reduces barriers to entry and encourages wider adoption across different sectors.
However, challenges remain in scaling up the process. Optimizing reaction conditions, such as temperature, pressure, and reactant ratios, for large-scale production may require additional research and development. Additionally, ensuring consistent product quality and purity at industrial scales will be crucial for meeting regulatory standards and customer expectations.
The potential for industrial application extends beyond the production of green solvents. The principles and techniques developed in this process could be applied to other areas of green chemistry, fostering innovation in sustainable chemical manufacturing. This broader impact could lead to the development of new catalytic systems and environmentally friendly synthesis routes for a wide range of chemical products.
As industries continue to prioritize sustainability and seek to reduce their environmental footprint, the sodium acetate-facilitated synthesis of green solvents presents a promising avenue for large-scale, eco-friendly chemical production. With further research and optimization, this technology has the potential to revolutionize solvent manufacturing and contribute significantly to the advancement of green chemistry in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!