How to Address Hydrochloric Acid Spills Effectively?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Spill Response Background and Objectives
Hydrochloric acid (HCl) spills represent a significant hazard in various industrial and laboratory settings. The effective management of such incidents is crucial for ensuring workplace safety, environmental protection, and regulatory compliance. Over the past decades, the approach to handling HCl spills has evolved significantly, driven by advancements in chemical safety protocols and the development of specialized neutralization and containment technologies.
The primary objective of HCl spill response is to quickly and safely neutralize the acid, contain its spread, and mitigate potential harm to personnel, equipment, and the environment. This involves a multi-faceted approach that combines immediate action protocols, proper use of personal protective equipment (PPE), and the application of appropriate neutralizing agents.
Historically, the management of acid spills relied heavily on basic neutralization techniques and rudimentary containment methods. However, the increasing scale of industrial operations and the growing awareness of environmental impacts have necessitated more sophisticated approaches. Modern HCl spill response strategies now incorporate advanced absorbent materials, specialized neutralizing agents, and engineered containment systems designed to handle large-scale incidents.
The evolution of HCl spill response techniques has been largely influenced by regulatory frameworks such as OSHA's Hazardous Waste Operations and Emergency Response (HAZWOPER) standards in the United States and similar regulations worldwide. These guidelines have established comprehensive protocols for spill prevention, response planning, and personnel training, significantly improving the overall safety landscape.
Current technological trends in HCl spill management focus on developing more efficient neutralization methods, improving personal protective equipment, and enhancing real-time monitoring capabilities. Innovations in this field aim to reduce response times, minimize the volume of neutralizing agents required, and provide better protection for response personnel.
The goals of modern HCl spill response extend beyond immediate containment and neutralization. They encompass the entire lifecycle of spill management, including prevention, early detection, rapid response, thorough cleanup, and comprehensive post-incident analysis. This holistic approach aims to not only address immediate safety concerns but also to prevent future incidents and continuously improve response strategies.
As industrial processes become more complex and environmental regulations more stringent, the field of HCl spill response continues to evolve. Future objectives in this area include the development of smart sensors for early leak detection, the integration of robotics and automation in spill response to minimize human exposure, and the creation of more environmentally friendly neutralization agents that leave minimal residual impact.
The primary objective of HCl spill response is to quickly and safely neutralize the acid, contain its spread, and mitigate potential harm to personnel, equipment, and the environment. This involves a multi-faceted approach that combines immediate action protocols, proper use of personal protective equipment (PPE), and the application of appropriate neutralizing agents.
Historically, the management of acid spills relied heavily on basic neutralization techniques and rudimentary containment methods. However, the increasing scale of industrial operations and the growing awareness of environmental impacts have necessitated more sophisticated approaches. Modern HCl spill response strategies now incorporate advanced absorbent materials, specialized neutralizing agents, and engineered containment systems designed to handle large-scale incidents.
The evolution of HCl spill response techniques has been largely influenced by regulatory frameworks such as OSHA's Hazardous Waste Operations and Emergency Response (HAZWOPER) standards in the United States and similar regulations worldwide. These guidelines have established comprehensive protocols for spill prevention, response planning, and personnel training, significantly improving the overall safety landscape.
Current technological trends in HCl spill management focus on developing more efficient neutralization methods, improving personal protective equipment, and enhancing real-time monitoring capabilities. Innovations in this field aim to reduce response times, minimize the volume of neutralizing agents required, and provide better protection for response personnel.
The goals of modern HCl spill response extend beyond immediate containment and neutralization. They encompass the entire lifecycle of spill management, including prevention, early detection, rapid response, thorough cleanup, and comprehensive post-incident analysis. This holistic approach aims to not only address immediate safety concerns but also to prevent future incidents and continuously improve response strategies.
As industrial processes become more complex and environmental regulations more stringent, the field of HCl spill response continues to evolve. Future objectives in this area include the development of smart sensors for early leak detection, the integration of robotics and automation in spill response to minimize human exposure, and the creation of more environmentally friendly neutralization agents that leave minimal residual impact.
Industrial Demand for HCl Spill Management
The industrial demand for effective hydrochloric acid (HCl) spill management has been steadily increasing due to the widespread use of HCl in various sectors. Chemical manufacturing, metal processing, oil and gas industries, and water treatment facilities are among the primary consumers of HCl, creating a significant need for robust spill management solutions.
In the chemical manufacturing sector, HCl is a crucial component in the production of various chemicals, including PVC, pharmaceuticals, and agrochemicals. The large-scale use of HCl in these processes necessitates comprehensive spill management strategies to ensure worker safety and environmental protection. Metal processing industries utilize HCl for pickling and descaling operations, where the risk of spills is inherent to the process, driving the demand for efficient containment and neutralization methods.
The oil and gas industry employs HCl in well stimulation and acidizing processes, creating a unique set of challenges for spill management in remote and environmentally sensitive locations. As exploration and production activities expand, the need for specialized HCl spill management solutions tailored to these environments has grown substantially.
Water treatment facilities use HCl for pH adjustment and chlorine production, requiring reliable spill management systems to prevent environmental contamination and ensure operational continuity. The increasing focus on water quality and stringent regulations has further amplified the demand for advanced HCl spill management technologies in this sector.
The growing emphasis on workplace safety and environmental regulations has been a significant driver for the HCl spill management market. Industries are increasingly investing in preventive measures, including improved storage and handling systems, as well as rapid response equipment for spill containment and neutralization. This trend has led to the development of innovative products such as specialized absorbents, neutralizing agents, and personal protective equipment designed specifically for HCl spills.
Furthermore, the globalization of supply chains and the expansion of chemical industries in emerging economies have created new markets for HCl spill management solutions. As these regions adopt stricter safety and environmental standards, the demand for advanced spill management technologies is expected to surge.
The industrial demand for HCl spill management is also driven by the potential financial and reputational risks associated with inadequate spill response. Companies are recognizing the importance of investing in comprehensive spill management strategies to mitigate these risks and maintain their competitive edge in the market.
In the chemical manufacturing sector, HCl is a crucial component in the production of various chemicals, including PVC, pharmaceuticals, and agrochemicals. The large-scale use of HCl in these processes necessitates comprehensive spill management strategies to ensure worker safety and environmental protection. Metal processing industries utilize HCl for pickling and descaling operations, where the risk of spills is inherent to the process, driving the demand for efficient containment and neutralization methods.
The oil and gas industry employs HCl in well stimulation and acidizing processes, creating a unique set of challenges for spill management in remote and environmentally sensitive locations. As exploration and production activities expand, the need for specialized HCl spill management solutions tailored to these environments has grown substantially.
Water treatment facilities use HCl for pH adjustment and chlorine production, requiring reliable spill management systems to prevent environmental contamination and ensure operational continuity. The increasing focus on water quality and stringent regulations has further amplified the demand for advanced HCl spill management technologies in this sector.
The growing emphasis on workplace safety and environmental regulations has been a significant driver for the HCl spill management market. Industries are increasingly investing in preventive measures, including improved storage and handling systems, as well as rapid response equipment for spill containment and neutralization. This trend has led to the development of innovative products such as specialized absorbents, neutralizing agents, and personal protective equipment designed specifically for HCl spills.
Furthermore, the globalization of supply chains and the expansion of chemical industries in emerging economies have created new markets for HCl spill management solutions. As these regions adopt stricter safety and environmental standards, the demand for advanced spill management technologies is expected to surge.
The industrial demand for HCl spill management is also driven by the potential financial and reputational risks associated with inadequate spill response. Companies are recognizing the importance of investing in comprehensive spill management strategies to mitigate these risks and maintain their competitive edge in the market.
Current Challenges in HCl Spill Containment
The containment of hydrochloric acid (HCl) spills presents several significant challenges that require immediate attention and specialized approaches. One of the primary difficulties lies in the highly corrosive nature of HCl, which can rapidly damage various materials and surfaces upon contact. This corrosivity not only poses risks to infrastructure and equipment but also complicates the selection of appropriate containment materials and personal protective equipment (PPE) for responders.
Another major challenge is the potential for HCl to release toxic fumes, particularly when it comes into contact with certain metals or other reactive substances. These fumes can create hazardous atmospheric conditions, necessitating the use of specialized respiratory protection and ventilation systems. The risk of inhalation injuries to both responders and nearby personnel adds an extra layer of complexity to containment efforts.
The volatility of HCl also presents difficulties in terms of rapid spread and potential for secondary reactions. In liquid form, HCl can quickly flow into cracks, crevices, and drainage systems, making complete containment challenging. Moreover, its ability to react with a wide range of substances can lead to unexpected chemical reactions, potentially exacerbating the spill situation.
Environmental concerns further complicate HCl spill containment. The acid's potential to contaminate soil and water sources necessitates swift and comprehensive containment measures to prevent ecological damage. This often requires a delicate balance between rapid response and careful, methodical containment procedures to minimize environmental impact.
The scale of HCl spills can vary significantly, from small laboratory incidents to large industrial accidents. This variability demands flexible and scalable containment strategies, as well as the availability of sufficient quantities of neutralizing agents and absorbent materials. Ensuring an adequate supply of these resources, especially for large-scale spills, can be logistically challenging.
Training and preparedness pose ongoing challenges in HCl spill containment. Responders must be well-versed in the specific hazards of HCl, proper containment techniques, and the use of specialized equipment. Regular drills and updated training protocols are essential to maintain readiness, but implementing comprehensive training programs across diverse industrial and laboratory settings can be resource-intensive.
Lastly, the time-sensitive nature of HCl spill response adds pressure to containment efforts. The rapid corrosive action of HCl means that even small delays in response can lead to significant damage and increased risks. This necessitates not only quick reaction times but also well-coordinated response plans that can be executed swiftly and efficiently under high-stress conditions.
Another major challenge is the potential for HCl to release toxic fumes, particularly when it comes into contact with certain metals or other reactive substances. These fumes can create hazardous atmospheric conditions, necessitating the use of specialized respiratory protection and ventilation systems. The risk of inhalation injuries to both responders and nearby personnel adds an extra layer of complexity to containment efforts.
The volatility of HCl also presents difficulties in terms of rapid spread and potential for secondary reactions. In liquid form, HCl can quickly flow into cracks, crevices, and drainage systems, making complete containment challenging. Moreover, its ability to react with a wide range of substances can lead to unexpected chemical reactions, potentially exacerbating the spill situation.
Environmental concerns further complicate HCl spill containment. The acid's potential to contaminate soil and water sources necessitates swift and comprehensive containment measures to prevent ecological damage. This often requires a delicate balance between rapid response and careful, methodical containment procedures to minimize environmental impact.
The scale of HCl spills can vary significantly, from small laboratory incidents to large industrial accidents. This variability demands flexible and scalable containment strategies, as well as the availability of sufficient quantities of neutralizing agents and absorbent materials. Ensuring an adequate supply of these resources, especially for large-scale spills, can be logistically challenging.
Training and preparedness pose ongoing challenges in HCl spill containment. Responders must be well-versed in the specific hazards of HCl, proper containment techniques, and the use of specialized equipment. Regular drills and updated training protocols are essential to maintain readiness, but implementing comprehensive training programs across diverse industrial and laboratory settings can be resource-intensive.
Lastly, the time-sensitive nature of HCl spill response adds pressure to containment efforts. The rapid corrosive action of HCl means that even small delays in response can lead to significant damage and increased risks. This necessitates not only quick reaction times but also well-coordinated response plans that can be executed swiftly and efficiently under high-stress conditions.
Existing HCl Neutralization Methods
01 Neutralization and absorption methods
Effective handling of hydrochloric acid spills involves neutralization using alkaline substances and absorption with appropriate materials. This approach helps to reduce the acidity and contain the spill, minimizing environmental impact and safety risks.- Neutralization and absorption methods: Effective handling of hydrochloric acid spills involves neutralization and absorption techniques. Common neutralizing agents include sodium bicarbonate or calcium carbonate. After neutralization, absorbent materials such as sand or specialized chemical absorbents are used to contain and remove the spill. This approach helps to minimize environmental impact and safety hazards.
- Specialized equipment for acid spill containment: Various specialized equipment and devices are designed for containing and managing hydrochloric acid spills. These may include acid-resistant containers, spill berms, and specialized pumps for safe removal of the spilled acid. Such equipment is crucial for quick and effective response to acid spills, reducing potential damage and ensuring worker safety.
- Personal protective equipment (PPE) for acid spill response: Proper personal protective equipment is essential for personnel dealing with hydrochloric acid spills. This includes acid-resistant suits, gloves, boots, and respiratory protection. The effectiveness of the spill response is greatly enhanced when responders are adequately protected, allowing them to work safely and efficiently in containing and cleaning up the spill.
- Environmental remediation techniques: After initial containment and neutralization, environmental remediation techniques are employed to address any residual contamination from hydrochloric acid spills. This may involve soil treatment, groundwater remediation, or specialized cleaning of affected surfaces. The goal is to restore the affected area to its pre-spill condition and prevent long-term environmental damage.
- Training and emergency response protocols: Effective management of hydrochloric acid spills relies heavily on proper training and well-established emergency response protocols. This includes regular drills, up-to-date safety procedures, and clear communication channels. Well-trained personnel who understand the risks and proper handling techniques can significantly improve the effectiveness of spill response and minimize potential harm.
02 Specialized equipment for acid spill containment
Utilizing specialized equipment designed for acid spill containment can significantly improve the effectiveness of managing hydrochloric acid spills. This may include acid-resistant containers, pumps, and personal protective equipment tailored for handling corrosive substances.Expand Specific Solutions03 Chemical treatment and conversion processes
Advanced chemical treatment methods can be employed to convert hydrochloric acid into less harmful substances. These processes may involve the use of specific reagents or catalysts to transform the acid, reducing its corrosive properties and environmental impact.Expand Specific Solutions04 Monitoring and detection systems
Implementing sophisticated monitoring and detection systems can enhance the effectiveness of managing hydrochloric acid spills. These systems can provide early warnings, real-time data on spill progression, and assist in coordinating response efforts.Expand Specific Solutions05 Environmental remediation techniques
Employing specific environmental remediation techniques can improve the long-term effectiveness of addressing hydrochloric acid spills. These may include soil treatment methods, groundwater purification processes, and ecosystem restoration approaches to mitigate the impact of acid contamination.Expand Specific Solutions
Key Players in Chemical Safety Industry
The hydrochloric acid spill management market is in a growth phase, driven by increasing industrial safety regulations and environmental concerns. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is moderately mature, with ongoing innovations in spill containment and neutralization methods. Key players like Baker Hughes Co., Halliburton Energy Services, Inc., and Schlumberger Technologies, Inc. are leveraging their expertise in chemical handling to develop advanced solutions. Companies such as DuPont de Nemours, Inc. and Wacker Chemie AG are focusing on eco-friendly neutralization agents, while Fluid Energy Group Ltd. is pioneering safer acid alternatives. The competitive landscape is characterized by a mix of established industrial giants and specialized chemical companies, all striving to enhance safety and efficiency in acid spill management.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed an innovative approach to hydrochloric acid spill management, focusing on rapid containment and neutralization. Their system utilizes a proprietary blend of fast-acting neutralizing agents that can handle high-volume spills effectively. The company has also invested in developing portable, high-capacity pumping systems that can quickly transfer large amounts of spilled acid to containment vessels[2]. Baker Hughes' approach includes the use of advanced geomembrane liners for secondary containment, which are highly resistant to acid corrosion and can prevent ground contamination[4]. Additionally, they have implemented a comprehensive digital monitoring system that uses IoT sensors to detect leaks and spills in real-time, allowing for immediate response[6]. The company also offers specialized training programs for industrial clients, focusing on proper acid handling and emergency response procedures[8].
Strengths: Rapid and effective neutralization capabilities; advanced containment solutions; real-time digital monitoring; comprehensive training programs. Weaknesses: May require significant initial investment; system complexity could necessitate specialized personnel for operation and maintenance.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has developed a multi-layered approach to addressing hydrochloric acid spills. Their system begins with prevention, utilizing advanced coating technologies for storage tanks and transport containers that significantly reduce the risk of corrosion-induced leaks. For spill response, Akzo Nobel has created a range of specialized neutralizing agents that are not only highly effective but also environmentally benign[1]. These agents are designed to work in conjunction with their proprietary spill containment systems, which use innovative materials to rapidly contain and control the spread of acid[3]. Akzo Nobel has also invested in developing smart sensor networks that can detect minute changes in air quality and surface pH, providing early warning of potential spills[5]. Additionally, the company offers comprehensive training programs for industrial clients, covering everything from proper handling procedures to advanced emergency response techniques[7].
Strengths: Strong focus on prevention through advanced coatings; environmentally friendly neutralizing agents; innovative containment systems; advanced early detection technology. Weaknesses: Potentially high initial implementation costs; may require significant changes to existing infrastructure and procedures.
Innovative HCl Spill Control Technologies
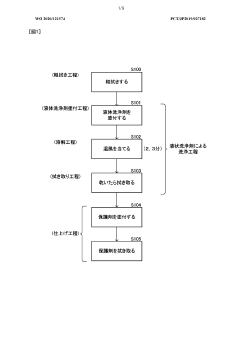
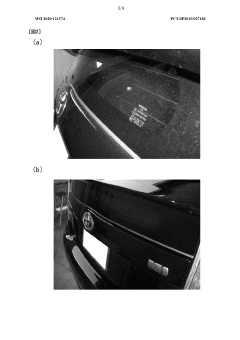
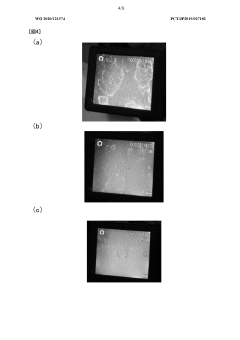
Method for washing exposed surfaces, liquid detergent, and detergent set
PatentWO2020121574A1
Innovation
- A method involving a liquid cleaning agent containing hydrochloric acid and ammonium fluoride, applied with hot air to dissolve dirt, followed by wiping, and a protective coating to prevent future staining, without using abrasives.
Improvements relating to hypochlorous acid solutions
PatentActiveUS20180282160A1
Innovation
- A process involving the treatment of an aqueous hypochlorite solution with a precipitation agent to form a counter-ion precipitate, followed by separation and acidification with a strong inorganic acid to create a stable aqueous solution of hypochlorous acid with low chloride content and controlled pH, allowing for the production of solutions suitable for both surface disinfection and water chlorination.
Environmental Impact of HCl Spills
Hydrochloric acid (HCl) spills pose significant environmental risks due to their corrosive nature and potential for widespread damage. When HCl is released into the environment, it can have severe impacts on soil, water bodies, and ecosystems. The immediate effect is a rapid decrease in pH levels, which can be lethal to aquatic life and vegetation. In water bodies, even small amounts of HCl can drastically alter the chemical balance, leading to fish kills and long-term ecological disruption.
Soil contamination is another critical concern. HCl spills can render soil infertile by altering its chemical composition and killing beneficial microorganisms. This not only affects plant growth but can also lead to the mobilization of heavy metals, potentially contaminating groundwater sources. The acid's reaction with soil minerals can release toxic compounds, further exacerbating environmental damage.
Air quality is also impacted by HCl spills. The vapors released can form an acidic mist, causing respiratory issues in humans and animals. This mist can also contribute to acid rain, affecting areas far beyond the initial spill site. In urban environments, HCl spills can corrode infrastructure, including metal structures and concrete, leading to potential safety hazards and costly repairs.
The long-term environmental effects of HCl spills can persist for years. Ecosystems may take significant time to recover, with some species potentially facing local extinction. The acid can leach into groundwater, contaminating drinking water sources and posing health risks to communities. Remediation efforts are often complex and expensive, involving neutralization techniques and extensive soil and water treatment.
Moreover, HCl spills can have cascading effects on food chains. Contamination of agricultural land can lead to crop failures and bioaccumulation of toxins in livestock, potentially entering the human food supply. In marine environments, HCl spills can damage coral reefs and disrupt fisheries, impacting both biodiversity and local economies dependent on these resources.
Given these severe environmental impacts, effective prevention and rapid response strategies are crucial. This includes implementing robust containment measures in industrial settings, developing comprehensive emergency response plans, and investing in training for handling and transporting HCl. Advanced monitoring systems and improved spill detection technologies are also essential in minimizing the environmental footprint of potential HCl incidents.
Soil contamination is another critical concern. HCl spills can render soil infertile by altering its chemical composition and killing beneficial microorganisms. This not only affects plant growth but can also lead to the mobilization of heavy metals, potentially contaminating groundwater sources. The acid's reaction with soil minerals can release toxic compounds, further exacerbating environmental damage.
Air quality is also impacted by HCl spills. The vapors released can form an acidic mist, causing respiratory issues in humans and animals. This mist can also contribute to acid rain, affecting areas far beyond the initial spill site. In urban environments, HCl spills can corrode infrastructure, including metal structures and concrete, leading to potential safety hazards and costly repairs.
The long-term environmental effects of HCl spills can persist for years. Ecosystems may take significant time to recover, with some species potentially facing local extinction. The acid can leach into groundwater, contaminating drinking water sources and posing health risks to communities. Remediation efforts are often complex and expensive, involving neutralization techniques and extensive soil and water treatment.
Moreover, HCl spills can have cascading effects on food chains. Contamination of agricultural land can lead to crop failures and bioaccumulation of toxins in livestock, potentially entering the human food supply. In marine environments, HCl spills can damage coral reefs and disrupt fisheries, impacting both biodiversity and local economies dependent on these resources.
Given these severe environmental impacts, effective prevention and rapid response strategies are crucial. This includes implementing robust containment measures in industrial settings, developing comprehensive emergency response plans, and investing in training for handling and transporting HCl. Advanced monitoring systems and improved spill detection technologies are also essential in minimizing the environmental footprint of potential HCl incidents.
Safety Regulations for Acid Handling
Safety regulations for acid handling are crucial in effectively addressing hydrochloric acid spills. These regulations are designed to protect workers, the environment, and the public from the hazards associated with acid exposure. The Occupational Safety and Health Administration (OSHA) in the United States has established comprehensive guidelines for handling hazardous chemicals, including hydrochloric acid.
OSHA's Hazard Communication Standard (HCS) requires employers to provide information about chemical hazards and associated protective measures to their workers. This includes proper labeling of containers, safety data sheets, and training programs. For hydrochloric acid specifically, containers must be clearly labeled with the chemical name, hazard warnings, and precautionary statements.
Personal protective equipment (PPE) is a critical component of acid handling safety. Regulations mandate the use of appropriate PPE, including chemical-resistant gloves, goggles or face shields, and protective clothing. Respiratory protection may also be required depending on the concentration and potential for vapor exposure.
Workplace design and engineering controls are essential aspects of acid handling safety regulations. These include proper ventilation systems, emergency eyewash stations, and safety showers. Storage areas for hydrochloric acid must be properly designed with secondary containment to prevent spills from spreading.
Emergency response planning is another key element of safety regulations. Facilities handling hydrochloric acid are required to have written emergency action plans that outline procedures for spill containment, evacuation, and decontamination. Regular drills and training exercises are mandated to ensure workers are prepared to respond effectively to acid spills.
Transportation of hydrochloric acid is regulated by the Department of Transportation (DOT) in the United States. These regulations cover proper packaging, labeling, and documentation for shipments of hazardous materials. Vehicles transporting hydrochloric acid must display appropriate placards and follow specific routing requirements.
Regular inspections and audits are required to ensure compliance with safety regulations. This includes routine checks of storage areas, PPE, and emergency response equipment. Documentation of these inspections, as well as any incidents or near-misses, must be maintained and reviewed to identify areas for improvement in safety protocols.
Employee training is a cornerstone of acid handling safety regulations. Workers must receive comprehensive training on the hazards of hydrochloric acid, proper handling techniques, use of PPE, and emergency response procedures. This training must be documented and refreshed periodically to ensure ongoing compliance and safety awareness.
OSHA's Hazard Communication Standard (HCS) requires employers to provide information about chemical hazards and associated protective measures to their workers. This includes proper labeling of containers, safety data sheets, and training programs. For hydrochloric acid specifically, containers must be clearly labeled with the chemical name, hazard warnings, and precautionary statements.
Personal protective equipment (PPE) is a critical component of acid handling safety. Regulations mandate the use of appropriate PPE, including chemical-resistant gloves, goggles or face shields, and protective clothing. Respiratory protection may also be required depending on the concentration and potential for vapor exposure.
Workplace design and engineering controls are essential aspects of acid handling safety regulations. These include proper ventilation systems, emergency eyewash stations, and safety showers. Storage areas for hydrochloric acid must be properly designed with secondary containment to prevent spills from spreading.
Emergency response planning is another key element of safety regulations. Facilities handling hydrochloric acid are required to have written emergency action plans that outline procedures for spill containment, evacuation, and decontamination. Regular drills and training exercises are mandated to ensure workers are prepared to respond effectively to acid spills.
Transportation of hydrochloric acid is regulated by the Department of Transportation (DOT) in the United States. These regulations cover proper packaging, labeling, and documentation for shipments of hazardous materials. Vehicles transporting hydrochloric acid must display appropriate placards and follow specific routing requirements.
Regular inspections and audits are required to ensure compliance with safety regulations. This includes routine checks of storage areas, PPE, and emergency response equipment. Documentation of these inspections, as well as any incidents or near-misses, must be maintained and reviewed to identify areas for improvement in safety protocols.
Employee training is a cornerstone of acid handling safety regulations. Workers must receive comprehensive training on the hazards of hydrochloric acid, proper handling techniques, use of PPE, and emergency response procedures. This training must be documented and refreshed periodically to ensure ongoing compliance and safety awareness.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!