How to Improve Quantitative Analysis in Gel Electrophoresis?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Quantification Background and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology for decades, enabling the separation and analysis of DNA, RNA, and proteins based on their size and charge. The quantitative analysis of gel electrophoresis results has become increasingly important in various fields, including genomics, proteomics, and diagnostics. As researchers strive for more precise and reliable data, the need for improved quantification methods has grown significantly.
The evolution of gel electrophoresis quantification techniques has been driven by advancements in imaging technologies, software algorithms, and data analysis methods. Early approaches relied on visual estimation or densitometry of stained gels, which were subjective and limited in accuracy. The introduction of digital imaging systems and fluorescent labeling techniques marked a significant leap forward, allowing for more sensitive and reproducible measurements.
Current objectives in improving quantitative analysis in gel electrophoresis focus on enhancing accuracy, sensitivity, and reproducibility while expanding the dynamic range of detection. Researchers aim to develop methods that can reliably quantify both high and low abundance molecules within the same sample, a challenge that has long plagued traditional techniques. Additionally, there is a push towards standardization of quantification protocols to ensure consistency across different laboratories and experiments.
Another key goal is to integrate advanced image processing and machine learning algorithms into gel analysis workflows. These technologies have the potential to automate band detection, reduce background noise, and improve the overall precision of quantification. Furthermore, there is a growing interest in developing real-time quantification methods that can provide instant analysis during the electrophoresis process, potentially revolutionizing high-throughput applications.
The field is also exploring novel approaches to overcome limitations in traditional gel-based quantification. This includes the development of microfluidic devices for electrophoresis, which offer the promise of faster separation times, reduced sample volumes, and improved quantification accuracy. Additionally, researchers are investigating alternative detection methods, such as label-free techniques, to expand the range of molecules that can be accurately quantified.
As the demand for more sensitive and accurate molecular analysis continues to grow across various scientific disciplines, improving quantitative analysis in gel electrophoresis remains a critical area of research. The ultimate aim is to transform this classic technique into a cutting-edge tool capable of meeting the exacting standards of modern molecular biology and clinical diagnostics.
The evolution of gel electrophoresis quantification techniques has been driven by advancements in imaging technologies, software algorithms, and data analysis methods. Early approaches relied on visual estimation or densitometry of stained gels, which were subjective and limited in accuracy. The introduction of digital imaging systems and fluorescent labeling techniques marked a significant leap forward, allowing for more sensitive and reproducible measurements.
Current objectives in improving quantitative analysis in gel electrophoresis focus on enhancing accuracy, sensitivity, and reproducibility while expanding the dynamic range of detection. Researchers aim to develop methods that can reliably quantify both high and low abundance molecules within the same sample, a challenge that has long plagued traditional techniques. Additionally, there is a push towards standardization of quantification protocols to ensure consistency across different laboratories and experiments.
Another key goal is to integrate advanced image processing and machine learning algorithms into gel analysis workflows. These technologies have the potential to automate band detection, reduce background noise, and improve the overall precision of quantification. Furthermore, there is a growing interest in developing real-time quantification methods that can provide instant analysis during the electrophoresis process, potentially revolutionizing high-throughput applications.
The field is also exploring novel approaches to overcome limitations in traditional gel-based quantification. This includes the development of microfluidic devices for electrophoresis, which offer the promise of faster separation times, reduced sample volumes, and improved quantification accuracy. Additionally, researchers are investigating alternative detection methods, such as label-free techniques, to expand the range of molecules that can be accurately quantified.
As the demand for more sensitive and accurate molecular analysis continues to grow across various scientific disciplines, improving quantitative analysis in gel electrophoresis remains a critical area of research. The ultimate aim is to transform this classic technique into a cutting-edge tool capable of meeting the exacting standards of modern molecular biology and clinical diagnostics.
Market Demand for Precise Gel Analysis
The market demand for precise gel analysis in electrophoresis has been steadily growing, driven by the increasing need for accurate quantification in various fields of life sciences and biotechnology. Researchers, pharmaceutical companies, and diagnostic laboratories are constantly seeking more reliable and efficient methods to analyze complex biological samples.
In recent years, the global market for gel electrophoresis has experienced significant expansion, with a particular emphasis on quantitative analysis capabilities. This growth is primarily fueled by the rising prevalence of proteomics and genomics research, as well as the expanding applications in drug discovery and personalized medicine.
The pharmaceutical and biotechnology sectors have emerged as major contributors to the demand for precise gel analysis. These industries require highly accurate quantification of proteins, nucleic acids, and other biomolecules for drug development, quality control, and regulatory compliance. The ability to precisely measure and compare sample concentrations is crucial for ensuring the efficacy and safety of new therapeutics.
Academic and research institutions also play a significant role in driving the market demand. As scientific investigations become more complex and data-driven, there is an increasing need for sophisticated quantitative analysis tools in gel electrophoresis. Researchers are seeking solutions that can provide reproducible and statistically significant results, enabling them to draw more robust conclusions from their experiments.
The clinical diagnostics field has shown a growing interest in precise gel analysis techniques. With the advent of personalized medicine and the need for more accurate disease biomarkers, there is a rising demand for quantitative electrophoresis methods that can detect and measure specific proteins or nucleic acids in patient samples with high sensitivity and specificity.
Environmental and food safety sectors have also contributed to the market demand for precise gel analysis. These industries require accurate quantification of contaminants, allergens, and genetically modified organisms in various samples, driving the need for more advanced electrophoresis techniques and analysis tools.
As the demand for precise gel analysis continues to grow, there is an increasing focus on developing automated systems and software solutions that can streamline the quantification process and reduce human error. This trend is expected to further expand the market, as it addresses the need for high-throughput analysis in large-scale research and industrial applications.
In response to these market demands, manufacturers are investing in research and development to improve the accuracy, sensitivity, and reproducibility of gel electrophoresis systems. There is a particular emphasis on enhancing image acquisition technologies, developing more sophisticated analysis algorithms, and integrating machine learning capabilities to improve quantitative results.
In recent years, the global market for gel electrophoresis has experienced significant expansion, with a particular emphasis on quantitative analysis capabilities. This growth is primarily fueled by the rising prevalence of proteomics and genomics research, as well as the expanding applications in drug discovery and personalized medicine.
The pharmaceutical and biotechnology sectors have emerged as major contributors to the demand for precise gel analysis. These industries require highly accurate quantification of proteins, nucleic acids, and other biomolecules for drug development, quality control, and regulatory compliance. The ability to precisely measure and compare sample concentrations is crucial for ensuring the efficacy and safety of new therapeutics.
Academic and research institutions also play a significant role in driving the market demand. As scientific investigations become more complex and data-driven, there is an increasing need for sophisticated quantitative analysis tools in gel electrophoresis. Researchers are seeking solutions that can provide reproducible and statistically significant results, enabling them to draw more robust conclusions from their experiments.
The clinical diagnostics field has shown a growing interest in precise gel analysis techniques. With the advent of personalized medicine and the need for more accurate disease biomarkers, there is a rising demand for quantitative electrophoresis methods that can detect and measure specific proteins or nucleic acids in patient samples with high sensitivity and specificity.
Environmental and food safety sectors have also contributed to the market demand for precise gel analysis. These industries require accurate quantification of contaminants, allergens, and genetically modified organisms in various samples, driving the need for more advanced electrophoresis techniques and analysis tools.
As the demand for precise gel analysis continues to grow, there is an increasing focus on developing automated systems and software solutions that can streamline the quantification process and reduce human error. This trend is expected to further expand the market, as it addresses the need for high-throughput analysis in large-scale research and industrial applications.
In response to these market demands, manufacturers are investing in research and development to improve the accuracy, sensitivity, and reproducibility of gel electrophoresis systems. There is a particular emphasis on enhancing image acquisition technologies, developing more sophisticated analysis algorithms, and integrating machine learning capabilities to improve quantitative results.
Current Challenges in Gel Electrophoresis Quantification
Gel electrophoresis remains a cornerstone technique in molecular biology, yet its quantitative analysis faces several significant challenges. One of the primary issues is the inherent variability in gel preparation and running conditions, which can lead to inconsistencies in band intensity and migration patterns. This variability makes it difficult to achieve reproducible results across different experiments or laboratories.
Another major challenge is the limited dynamic range of traditional staining methods. Common dyes like ethidium bromide or SYBR Green have a narrow linear range for quantification, often leading to saturation of high-intensity bands and poor detection of low-abundance species. This limitation can result in inaccurate quantification, especially when dealing with samples that have a wide range of concentrations.
The presence of background noise and non-specific staining further complicates quantitative analysis. Impurities in the gel matrix, uneven illumination during imaging, and non-specific binding of dyes can all contribute to background signals that interfere with accurate band intensity measurements. This noise can be particularly problematic when analyzing faint bands or attempting to detect subtle differences between samples.
Gel-to-gel variations pose another significant hurdle in quantitative analysis. Even when using standardized protocols, slight differences in gel composition, electrophoresis conditions, or staining procedures can lead to variations in band intensity and migration. This variability makes it challenging to compare results across different gels or experiments, limiting the reliability of quantitative comparisons.
The lack of standardized methods for image acquisition and analysis also contributes to the challenges in gel electrophoresis quantification. Different imaging systems and software packages may use varying algorithms for background subtraction, band detection, and intensity measurement. This lack of standardization can lead to inconsistencies in data interpretation and make it difficult to compare results between different research groups or publications.
Furthermore, the manual nature of many gel analysis workflows introduces the potential for human error and subjectivity. Tasks such as lane and band selection, background correction, and normalization often rely on user judgment, which can introduce bias and reduce the reproducibility of results.
Lastly, the increasing demand for high-throughput analysis in modern molecular biology research presents a challenge for traditional gel electrophoresis techniques. The time-consuming nature of gel preparation, running, and analysis limits the number of samples that can be processed efficiently, making it difficult to meet the needs of large-scale studies or clinical applications.
Another major challenge is the limited dynamic range of traditional staining methods. Common dyes like ethidium bromide or SYBR Green have a narrow linear range for quantification, often leading to saturation of high-intensity bands and poor detection of low-abundance species. This limitation can result in inaccurate quantification, especially when dealing with samples that have a wide range of concentrations.
The presence of background noise and non-specific staining further complicates quantitative analysis. Impurities in the gel matrix, uneven illumination during imaging, and non-specific binding of dyes can all contribute to background signals that interfere with accurate band intensity measurements. This noise can be particularly problematic when analyzing faint bands or attempting to detect subtle differences between samples.
Gel-to-gel variations pose another significant hurdle in quantitative analysis. Even when using standardized protocols, slight differences in gel composition, electrophoresis conditions, or staining procedures can lead to variations in band intensity and migration. This variability makes it challenging to compare results across different gels or experiments, limiting the reliability of quantitative comparisons.
The lack of standardized methods for image acquisition and analysis also contributes to the challenges in gel electrophoresis quantification. Different imaging systems and software packages may use varying algorithms for background subtraction, band detection, and intensity measurement. This lack of standardization can lead to inconsistencies in data interpretation and make it difficult to compare results between different research groups or publications.
Furthermore, the manual nature of many gel analysis workflows introduces the potential for human error and subjectivity. Tasks such as lane and band selection, background correction, and normalization often rely on user judgment, which can introduce bias and reduce the reproducibility of results.
Lastly, the increasing demand for high-throughput analysis in modern molecular biology research presents a challenge for traditional gel electrophoresis techniques. The time-consuming nature of gel preparation, running, and analysis limits the number of samples that can be processed efficiently, making it difficult to meet the needs of large-scale studies or clinical applications.
Existing Quantitative Gel Analysis Methods
01 Quantitative analysis methods in gel electrophoresis
Various methods are employed for quantitative analysis in gel electrophoresis, including image analysis software, densitometry, and fluorescence-based techniques. These methods allow for accurate measurement of band intensity, protein concentration, and DNA/RNA quantity in electrophoresis gels.- Quantitative analysis methods in gel electrophoresis: Various methods are employed for quantitative analysis in gel electrophoresis, including image analysis software, densitometry, and fluorescence-based techniques. These methods allow for accurate measurement of band intensity, protein or DNA concentration, and molecular weight determination.
- Automated gel electrophoresis systems for quantitative analysis: Automated systems have been developed to improve the accuracy and efficiency of quantitative analysis in gel electrophoresis. These systems often incorporate advanced imaging technologies, automated sample loading, and integrated data analysis software to provide more reliable and reproducible results.
- Specialized gel compositions for enhanced quantitative analysis: Novel gel compositions have been formulated to improve the resolution and quantitative accuracy of gel electrophoresis. These specialized gels may incorporate specific polymers, buffer systems, or additives that enhance separation and facilitate more precise quantification of biomolecules.
- Integration of gel electrophoresis with other analytical techniques: Combining gel electrophoresis with other analytical techniques, such as mass spectrometry or chromatography, has led to more comprehensive and accurate quantitative analysis. These integrated approaches allow for improved identification and quantification of complex biological samples.
- Data analysis and interpretation tools for quantitative gel electrophoresis: Advanced software tools and algorithms have been developed to improve data analysis and interpretation in quantitative gel electrophoresis. These tools often incorporate machine learning techniques, statistical analysis, and database integration to enhance the accuracy and reliability of quantitative results.
02 Automated gel electrophoresis systems for quantitative analysis
Automated systems have been developed to improve the accuracy and efficiency of quantitative analysis in gel electrophoresis. These systems often incorporate advanced imaging technologies, automated sample loading, and integrated data analysis software to provide precise and reproducible results.Expand Specific Solutions03 Calibration and standardization techniques for quantitative gel electrophoresis
To ensure accurate quantitative analysis, various calibration and standardization techniques are employed in gel electrophoresis. These may include the use of internal standards, reference markers, and calibration curves to normalize data and account for variations between gels and experiments.Expand Specific Solutions04 Novel gel compositions for improved quantitative analysis
Researchers have developed novel gel compositions to enhance the resolution and quantitative accuracy of gel electrophoresis. These may include specialized polymers, buffer systems, or additives that improve band separation, reduce background noise, or enhance detection sensitivity for more precise quantification.Expand Specific Solutions05 Integration of gel electrophoresis with other analytical techniques
To improve quantitative analysis capabilities, gel electrophoresis is often integrated with other analytical techniques such as mass spectrometry, Western blotting, or capillary electrophoresis. These combined approaches allow for more comprehensive and accurate quantification of biomolecules separated by gel electrophoresis.Expand Specific Solutions
Key Players in Gel Analysis Technology
The gel electrophoresis quantitative analysis market is in a mature stage, with established players and well-defined technologies. The global market size is estimated to be in the billions, driven by increasing demand in life sciences research and diagnostics. Technological maturity is high, with leading companies like Agilent Technologies, Life Technologies, and Beckman Coulter offering advanced solutions. These firms have developed sophisticated instruments and software for precise quantification. However, there's ongoing innovation in areas such as high-throughput analysis and integration with other analytical techniques. Emerging players like AmberGen are introducing novel approaches, indicating potential for further market growth and technological advancements in specialized applications.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced gel electrophoresis systems with integrated quantitative analysis capabilities. Their 2100 Bioanalyzer system utilizes microfluidic technology for automated electrophoresis and precise quantification of nucleic acids and proteins[1]. The system employs laser-induced fluorescence detection and sophisticated software algorithms to provide high-resolution digital data for accurate quantitation. Agilent has also introduced the TapeStation systems, which use precast gel cassettes and automated sample loading for rapid, reproducible analysis[2]. These systems incorporate internal standards and calibration curves for reliable quantification across a wide dynamic range.
Strengths: High precision, automation, and reproducibility. Weaknesses: Higher cost compared to traditional gel systems, limited to specific proprietary consumables.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed the iBright Imaging Systems for advanced gel documentation and quantitative analysis. These systems utilize high-resolution CCD cameras and powerful LED illumination to capture detailed images of gels[3]. The iBright analysis software employs advanced algorithms for automated lane and band detection, background subtraction, and normalization. The system can perform relative and absolute quantification using various normalization methods. Life Technologies has also introduced fluorescent protein stains and DNA ladders optimized for quantitative analysis, improving sensitivity and dynamic range compared to traditional staining methods[4].
Strengths: User-friendly interface, versatile analysis options, and compatibility with various gel types. Weaknesses: Requires specialized imaging equipment, which may be costly for smaller labs.
Innovative Approaches in Gel Quantification
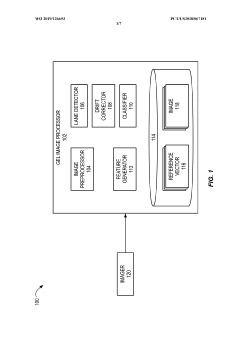
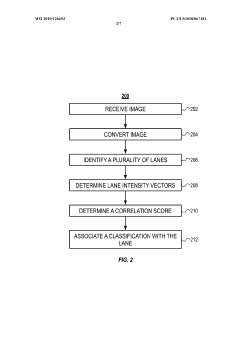
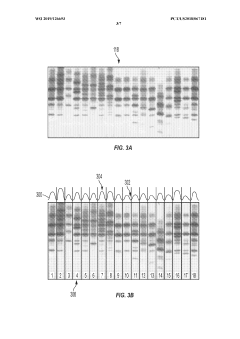
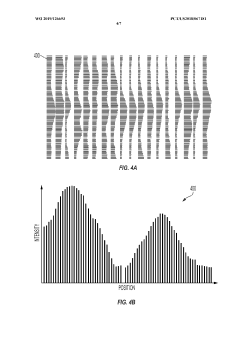
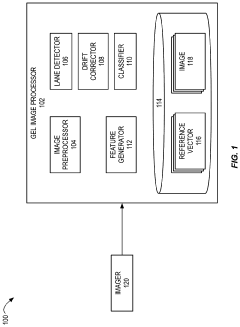
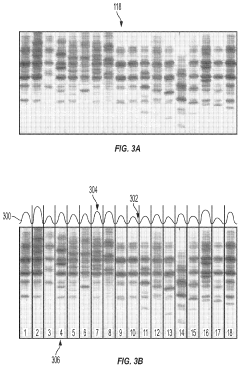
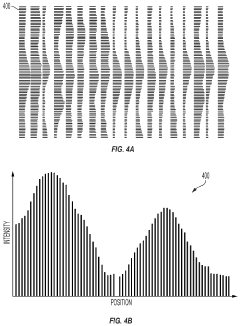
Automated analysis of analytical GELS and blots
PatentWO2019126693A1
Innovation
- An automated system that detects lanes in gels or blots, generates feature vectors, and classifies samples using a classifier based on reference datasets, significantly reducing analysis time and subjectivity through image processing and machine learning techniques.
Automated analysis of analytical gels and blots
PatentActiveUS20230417700A1
Innovation
- An automated system that detects lanes in gels or blots, generates feature vectors, and classifies samples using a classifier based on reference datasets, significantly reducing analysis time and subjectivity through image processing and machine learning techniques.
Standardization of Gel Quantification Protocols
Standardization of gel quantification protocols is crucial for improving the accuracy and reproducibility of quantitative analysis in gel electrophoresis. The development of standardized protocols involves several key components that address the variability inherent in gel-based quantification methods.
One of the primary aspects of standardization is the establishment of consistent sample preparation techniques. This includes defining precise methods for sample collection, storage, and processing to minimize pre-analytical variations. Standardized protocols should specify the optimal sample volumes, concentrations, and loading techniques to ensure uniform distribution across gel lanes.
The gel preparation process itself requires standardization to reduce inter-gel variability. This encompasses the selection of appropriate gel composition, concentration, and polymerization conditions. Standardized protocols should detail the exact formulations for gel solutions, including the type and concentration of acrylamide, cross-linkers, and buffer systems. Additionally, they should specify optimal running conditions, such as voltage, current, and duration, to achieve consistent separation of biomolecules.
Staining and imaging procedures are critical areas for standardization in gel quantification. Protocols should define the most suitable staining methods for specific biomolecules, including dye selection, incubation times, and destaining procedures. Imaging standardization involves specifying the equipment settings, exposure times, and image acquisition parameters to ensure consistent and comparable results across different experiments and laboratories.
Data analysis and interpretation represent another crucial aspect of standardization. Protocols should outline step-by-step procedures for image processing, background subtraction, and band intensity quantification. This includes the selection of appropriate software tools and algorithms for densitometric analysis, as well as guidelines for data normalization and statistical analysis.
Quality control measures are essential components of standardized gel quantification protocols. These should include the use of internal standards, calibration curves, and positive and negative controls to validate the accuracy and reliability of quantification results. Protocols should also specify acceptance criteria for gel quality and data integrity to ensure that only valid results are included in subsequent analyses.
Lastly, the standardization of gel quantification protocols should address the reporting and documentation of results. This involves establishing uniform formats for data presentation, including the reporting of raw data, normalized values, and statistical analyses. Standardized protocols should also emphasize the importance of thorough documentation of all experimental conditions and procedures to facilitate reproducibility and inter-laboratory comparisons.
One of the primary aspects of standardization is the establishment of consistent sample preparation techniques. This includes defining precise methods for sample collection, storage, and processing to minimize pre-analytical variations. Standardized protocols should specify the optimal sample volumes, concentrations, and loading techniques to ensure uniform distribution across gel lanes.
The gel preparation process itself requires standardization to reduce inter-gel variability. This encompasses the selection of appropriate gel composition, concentration, and polymerization conditions. Standardized protocols should detail the exact formulations for gel solutions, including the type and concentration of acrylamide, cross-linkers, and buffer systems. Additionally, they should specify optimal running conditions, such as voltage, current, and duration, to achieve consistent separation of biomolecules.
Staining and imaging procedures are critical areas for standardization in gel quantification. Protocols should define the most suitable staining methods for specific biomolecules, including dye selection, incubation times, and destaining procedures. Imaging standardization involves specifying the equipment settings, exposure times, and image acquisition parameters to ensure consistent and comparable results across different experiments and laboratories.
Data analysis and interpretation represent another crucial aspect of standardization. Protocols should outline step-by-step procedures for image processing, background subtraction, and band intensity quantification. This includes the selection of appropriate software tools and algorithms for densitometric analysis, as well as guidelines for data normalization and statistical analysis.
Quality control measures are essential components of standardized gel quantification protocols. These should include the use of internal standards, calibration curves, and positive and negative controls to validate the accuracy and reliability of quantification results. Protocols should also specify acceptance criteria for gel quality and data integrity to ensure that only valid results are included in subsequent analyses.
Lastly, the standardization of gel quantification protocols should address the reporting and documentation of results. This involves establishing uniform formats for data presentation, including the reporting of raw data, normalized values, and statistical analyses. Standardized protocols should also emphasize the importance of thorough documentation of all experimental conditions and procedures to facilitate reproducibility and inter-laboratory comparisons.
Data Management and Integration in Gel Analysis
Data management and integration play a crucial role in improving quantitative analysis in gel electrophoresis. As the volume and complexity of gel analysis data continue to grow, efficient data handling and integration become increasingly important for accurate and reproducible results.
One of the primary challenges in gel analysis is the management of large datasets generated from multiple experiments. Implementing robust data storage systems and standardized file formats can significantly enhance data organization and accessibility. Cloud-based storage solutions offer scalability and facilitate collaboration among researchers, enabling seamless sharing of gel images and analysis results.
Integration of gel analysis data with other experimental data types is essential for comprehensive interpretation. Developing data integration platforms that can combine gel electrophoresis results with proteomics, genomics, and other relevant data sources can provide a more holistic view of biological systems. These platforms should support data normalization and harmonization to ensure compatibility across different experimental techniques.
Automation of data processing workflows is another key aspect of improving quantitative analysis in gel electrophoresis. Implementing automated image analysis pipelines can reduce manual intervention, minimize human error, and increase throughput. These pipelines should incorporate advanced image processing algorithms for lane and band detection, background subtraction, and intensity quantification.
To enhance data quality and reliability, implementing robust quality control measures is crucial. This includes automated checks for gel image quality, detection of artifacts, and flagging of anomalous results. Incorporating metadata management systems can help track experimental conditions, sample information, and analysis parameters, ensuring full traceability and reproducibility of results.
Machine learning and artificial intelligence techniques can be leveraged to improve data analysis and interpretation. Developing ML models for pattern recognition in gel images, automated band identification, and prediction of protein properties based on migration patterns can significantly enhance the accuracy and speed of quantitative analysis.
Lastly, the development of user-friendly software interfaces that integrate all aspects of data management, analysis, and visualization is essential. These interfaces should provide intuitive tools for data exploration, statistical analysis, and generation of publication-quality figures, making complex gel analysis accessible to researchers with varying levels of computational expertise.
One of the primary challenges in gel analysis is the management of large datasets generated from multiple experiments. Implementing robust data storage systems and standardized file formats can significantly enhance data organization and accessibility. Cloud-based storage solutions offer scalability and facilitate collaboration among researchers, enabling seamless sharing of gel images and analysis results.
Integration of gel analysis data with other experimental data types is essential for comprehensive interpretation. Developing data integration platforms that can combine gel electrophoresis results with proteomics, genomics, and other relevant data sources can provide a more holistic view of biological systems. These platforms should support data normalization and harmonization to ensure compatibility across different experimental techniques.
Automation of data processing workflows is another key aspect of improving quantitative analysis in gel electrophoresis. Implementing automated image analysis pipelines can reduce manual intervention, minimize human error, and increase throughput. These pipelines should incorporate advanced image processing algorithms for lane and band detection, background subtraction, and intensity quantification.
To enhance data quality and reliability, implementing robust quality control measures is crucial. This includes automated checks for gel image quality, detection of artifacts, and flagging of anomalous results. Incorporating metadata management systems can help track experimental conditions, sample information, and analysis parameters, ensuring full traceability and reproducibility of results.
Machine learning and artificial intelligence techniques can be leveraged to improve data analysis and interpretation. Developing ML models for pattern recognition in gel images, automated band identification, and prediction of protein properties based on migration patterns can significantly enhance the accuracy and speed of quantitative analysis.
Lastly, the development of user-friendly software interfaces that integrate all aspects of data management, analysis, and visualization is essential. These interfaces should provide intuitive tools for data exploration, statistical analysis, and generation of publication-quality figures, making complex gel analysis accessible to researchers with varying levels of computational expertise.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!