How to Improve Safety in Hydrochloric Acid Transportation?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Transport Safety Background and Objectives
Hydrochloric acid (HCl) transportation has been a critical concern in the chemical industry for decades. As a highly corrosive and potentially hazardous substance, HCl poses significant risks during transport, necessitating stringent safety measures and continuous improvement efforts. The evolution of HCl transport safety has been marked by technological advancements, regulatory changes, and industry-wide collaborations aimed at minimizing accidents and environmental impacts.
The primary objective in improving HCl transportation safety is to prevent leaks, spills, and accidents that could harm human health, damage infrastructure, or contaminate the environment. This goal encompasses several key areas of focus, including the development of more robust containment systems, enhanced monitoring and control technologies, and improved emergency response protocols.
Over the years, the industry has witnessed a shift from basic safety measures to more sophisticated, integrated approaches. Early efforts focused on strengthening containers and improving handling procedures. However, as understanding of chemical properties and risk factors advanced, so did the complexity of safety strategies. Modern approaches now incorporate real-time monitoring systems, predictive analytics, and smart logistics to preemptively address potential hazards.
The current landscape of HCl transport safety is shaped by a combination of regulatory requirements, industry best practices, and technological innovations. Regulatory bodies worldwide have established strict guidelines for the packaging, labeling, and transportation of hydrochloric acid, often requiring specialized equipment and trained personnel. These regulations continue to evolve, driven by lessons learned from incidents and advancements in safety technologies.
Technological progress has played a crucial role in enhancing safety measures. The development of corrosion-resistant materials has significantly improved container durability, while the integration of sensors and IoT devices allows for real-time monitoring of temperature, pressure, and structural integrity during transport. These innovations not only help prevent accidents but also enable rapid response in case of emergencies.
Looking ahead, the objectives for further improving HCl transport safety are multifaceted. There is a growing emphasis on developing more sustainable and environmentally friendly transportation methods, such as exploring alternative packaging materials that reduce the environmental impact of potential spills. Additionally, there is a push towards greater standardization of safety protocols across different regions and modes of transport to ensure consistent high-level safety practices globally.
Another key objective is the integration of advanced data analytics and artificial intelligence to enhance risk prediction and management. By leveraging big data from various sources, including historical incident reports, weather patterns, and traffic conditions, the industry aims to create more accurate risk models and optimize transportation routes and schedules for maximum safety.
The primary objective in improving HCl transportation safety is to prevent leaks, spills, and accidents that could harm human health, damage infrastructure, or contaminate the environment. This goal encompasses several key areas of focus, including the development of more robust containment systems, enhanced monitoring and control technologies, and improved emergency response protocols.
Over the years, the industry has witnessed a shift from basic safety measures to more sophisticated, integrated approaches. Early efforts focused on strengthening containers and improving handling procedures. However, as understanding of chemical properties and risk factors advanced, so did the complexity of safety strategies. Modern approaches now incorporate real-time monitoring systems, predictive analytics, and smart logistics to preemptively address potential hazards.
The current landscape of HCl transport safety is shaped by a combination of regulatory requirements, industry best practices, and technological innovations. Regulatory bodies worldwide have established strict guidelines for the packaging, labeling, and transportation of hydrochloric acid, often requiring specialized equipment and trained personnel. These regulations continue to evolve, driven by lessons learned from incidents and advancements in safety technologies.
Technological progress has played a crucial role in enhancing safety measures. The development of corrosion-resistant materials has significantly improved container durability, while the integration of sensors and IoT devices allows for real-time monitoring of temperature, pressure, and structural integrity during transport. These innovations not only help prevent accidents but also enable rapid response in case of emergencies.
Looking ahead, the objectives for further improving HCl transport safety are multifaceted. There is a growing emphasis on developing more sustainable and environmentally friendly transportation methods, such as exploring alternative packaging materials that reduce the environmental impact of potential spills. Additionally, there is a push towards greater standardization of safety protocols across different regions and modes of transport to ensure consistent high-level safety practices globally.
Another key objective is the integration of advanced data analytics and artificial intelligence to enhance risk prediction and management. By leveraging big data from various sources, including historical incident reports, weather patterns, and traffic conditions, the industry aims to create more accurate risk models and optimize transportation routes and schedules for maximum safety.
Market Analysis for Safe HCl Transportation
The market for safe hydrochloric acid (HCl) transportation is experiencing significant growth due to increasing industrial applications and stringent safety regulations. The global HCl market is projected to reach $7.8 billion by 2027, with a compound annual growth rate (CAGR) of 6.2% from 2020 to 2027. This growth is primarily driven by the rising demand for HCl in various industries, including chemical manufacturing, steel pickling, and water treatment.
The demand for safe transportation solutions is particularly high in regions with robust chemical industries, such as North America, Europe, and Asia-Pacific. These regions account for the majority of HCl consumption and production, necessitating efficient and secure transportation methods. The United States, China, and Germany are among the top consumers and producers of HCl, creating substantial opportunities for transportation service providers and safety equipment manufacturers.
Safety concerns in HCl transportation have led to a growing market for specialized equipment and services. This includes corrosion-resistant tankers, advanced monitoring systems, and personal protective equipment (PPE) for handlers. The market for these safety-related products is expected to grow at a CAGR of 5.8% from 2021 to 2026, reaching a value of $1.2 billion by the end of the forecast period.
Regulatory bodies, such as the U.S. Department of Transportation (DOT) and the European Chemical Industry Council (CEFIC), have implemented strict guidelines for the transportation of hazardous materials, including HCl. These regulations have created a demand for compliance services, safety training programs, and advanced tracking systems. The market for regulatory compliance services in chemical transportation is estimated to grow at a CAGR of 7.5% from 2022 to 2027.
The adoption of digital technologies in HCl transportation safety is another emerging trend. IoT-enabled sensors, real-time monitoring systems, and blockchain-based tracking solutions are gaining traction. The market for these smart transportation solutions in the chemical industry is projected to reach $3.5 billion by 2025, with a CAGR of 14.2% from 2020 to 2025.
Environmental concerns are also shaping the market for safe HCl transportation. There is a growing demand for eco-friendly packaging materials and spill containment solutions. The market for sustainable packaging in chemical transportation is expected to grow at a CAGR of 8.3% from 2023 to 2028, driven by both regulatory pressures and corporate sustainability initiatives.
The demand for safe transportation solutions is particularly high in regions with robust chemical industries, such as North America, Europe, and Asia-Pacific. These regions account for the majority of HCl consumption and production, necessitating efficient and secure transportation methods. The United States, China, and Germany are among the top consumers and producers of HCl, creating substantial opportunities for transportation service providers and safety equipment manufacturers.
Safety concerns in HCl transportation have led to a growing market for specialized equipment and services. This includes corrosion-resistant tankers, advanced monitoring systems, and personal protective equipment (PPE) for handlers. The market for these safety-related products is expected to grow at a CAGR of 5.8% from 2021 to 2026, reaching a value of $1.2 billion by the end of the forecast period.
Regulatory bodies, such as the U.S. Department of Transportation (DOT) and the European Chemical Industry Council (CEFIC), have implemented strict guidelines for the transportation of hazardous materials, including HCl. These regulations have created a demand for compliance services, safety training programs, and advanced tracking systems. The market for regulatory compliance services in chemical transportation is estimated to grow at a CAGR of 7.5% from 2022 to 2027.
The adoption of digital technologies in HCl transportation safety is another emerging trend. IoT-enabled sensors, real-time monitoring systems, and blockchain-based tracking solutions are gaining traction. The market for these smart transportation solutions in the chemical industry is projected to reach $3.5 billion by 2025, with a CAGR of 14.2% from 2020 to 2025.
Environmental concerns are also shaping the market for safe HCl transportation. There is a growing demand for eco-friendly packaging materials and spill containment solutions. The market for sustainable packaging in chemical transportation is expected to grow at a CAGR of 8.3% from 2023 to 2028, driven by both regulatory pressures and corporate sustainability initiatives.
Current Challenges in HCl Transport Safety
The transportation of hydrochloric acid (HCl) presents significant safety challenges due to its corrosive and hazardous nature. One of the primary concerns is the risk of leaks or spills during transit, which can lead to severe environmental damage and pose serious health risks to workers and nearby communities. The highly reactive nature of HCl makes it particularly dangerous when exposed to other chemicals or materials, potentially causing violent reactions or the release of toxic fumes.
Container integrity is a critical issue in HCl transport. The corrosive properties of the acid can gradually degrade storage tanks and transport vessels, leading to weakened structures and increased likelihood of failures. This necessitates regular inspections and maintenance of transport equipment, which can be both time-consuming and costly for transportation companies.
Another significant challenge is the proper handling and transfer of HCl during loading and unloading processes. These operations present heightened risks of exposure to workers and require stringent safety protocols and specialized equipment. The potential for human error during these procedures remains a persistent concern in the industry.
Temperature control during transportation is also a crucial factor. Extreme temperatures can affect the stability of HCl, potentially leading to increased pressure within containers or changes in the acid's concentration. This requires careful monitoring and control systems to maintain safe conditions throughout the journey.
The regulatory landscape surrounding HCl transport adds another layer of complexity. Compliance with various national and international regulations, including proper labeling, documentation, and route planning, can be challenging for transporters. These regulations are often subject to changes, requiring constant vigilance and adaptation from companies involved in HCl transportation.
Emergency response preparedness is a critical aspect of HCl transport safety. In the event of an accident or spill, rapid and effective response is essential to minimize damage and protect public safety. This requires specialized training for transport personnel and coordination with local emergency services along transport routes.
The increasing demand for HCl in various industries has led to larger volumes being transported over longer distances, exacerbating existing safety challenges. This trend necessitates the development of more robust safety measures and technologies to mitigate risks associated with long-distance transportation of large quantities of this hazardous material.
Addressing these challenges requires a multifaceted approach, involving technological innovations, improved safety protocols, enhanced training programs, and stronger regulatory frameworks. The ongoing efforts to improve safety in HCl transportation reflect the industry's commitment to minimizing risks and ensuring the secure movement of this essential but dangerous chemical.
Container integrity is a critical issue in HCl transport. The corrosive properties of the acid can gradually degrade storage tanks and transport vessels, leading to weakened structures and increased likelihood of failures. This necessitates regular inspections and maintenance of transport equipment, which can be both time-consuming and costly for transportation companies.
Another significant challenge is the proper handling and transfer of HCl during loading and unloading processes. These operations present heightened risks of exposure to workers and require stringent safety protocols and specialized equipment. The potential for human error during these procedures remains a persistent concern in the industry.
Temperature control during transportation is also a crucial factor. Extreme temperatures can affect the stability of HCl, potentially leading to increased pressure within containers or changes in the acid's concentration. This requires careful monitoring and control systems to maintain safe conditions throughout the journey.
The regulatory landscape surrounding HCl transport adds another layer of complexity. Compliance with various national and international regulations, including proper labeling, documentation, and route planning, can be challenging for transporters. These regulations are often subject to changes, requiring constant vigilance and adaptation from companies involved in HCl transportation.
Emergency response preparedness is a critical aspect of HCl transport safety. In the event of an accident or spill, rapid and effective response is essential to minimize damage and protect public safety. This requires specialized training for transport personnel and coordination with local emergency services along transport routes.
The increasing demand for HCl in various industries has led to larger volumes being transported over longer distances, exacerbating existing safety challenges. This trend necessitates the development of more robust safety measures and technologies to mitigate risks associated with long-distance transportation of large quantities of this hazardous material.
Addressing these challenges requires a multifaceted approach, involving technological innovations, improved safety protocols, enhanced training programs, and stronger regulatory frameworks. The ongoing efforts to improve safety in HCl transportation reflect the industry's commitment to minimizing risks and ensuring the secure movement of this essential but dangerous chemical.
Existing HCl Transport Safety Solutions
01 Personal protective equipment for handling hydrochloric acid
Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, protective eyewear, face shields, and chemical-resistant clothing. The use of appropriate PPE helps prevent skin contact, eye injuries, and inhalation of acid fumes, ensuring the safety of workers handling this corrosive substance.- Personal protective equipment for handling hydrochloric acid: Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, goggles or face shields, and protective clothing. Specialized safety equipment such as acid-resistant suits and respiratory protection may be necessary for certain applications or in case of potential exposure to fumes.
- Storage and containment systems for hydrochloric acid: Safe storage of hydrochloric acid requires specialized containment systems. These may include corrosion-resistant tanks, secondary containment measures, and proper ventilation systems. Leak detection and spill control mechanisms are also important components of a comprehensive storage solution for this hazardous chemical.
- Neutralization and disposal methods for hydrochloric acid: Proper neutralization and disposal of hydrochloric acid are essential for safety and environmental protection. This may involve using alkaline substances to neutralize the acid before disposal, or specialized treatment processes to render the acid safe. Proper disposal methods must comply with local regulations and environmental standards.
- Emergency response and first aid for hydrochloric acid exposure: Effective emergency response protocols are crucial for dealing with hydrochloric acid incidents. This includes immediate flushing with water for skin or eye contact, proper ventilation in case of fume inhalation, and rapid medical attention. Specialized first aid kits and emergency shower/eyewash stations should be readily available in areas where hydrochloric acid is used or stored.
- Safe handling and transportation of hydrochloric acid: Safe handling and transportation of hydrochloric acid require specific procedures and equipment. This includes using corrosion-resistant materials for containers and pipelines, proper labeling and documentation, and specialized transport vehicles. Training for personnel involved in handling and transporting the acid is also crucial to ensure safety and prevent accidents.
02 Storage and containment systems for hydrochloric acid
Specialized storage and containment systems are essential for the safe handling of hydrochloric acid. These systems include corrosion-resistant tanks, secondary containment measures, and proper ventilation to prevent the accumulation of fumes. Implementing these storage solutions helps minimize the risk of leaks, spills, and potential environmental contamination.Expand Specific Solutions03 Neutralization and disposal methods for hydrochloric acid
Safe neutralization and disposal of hydrochloric acid are critical for environmental protection and worker safety. This involves using appropriate neutralizing agents, such as sodium hydroxide or calcium carbonate, to reduce the acid's corrosivity before disposal. Proper disposal methods ensure compliance with environmental regulations and prevent harm to ecosystems.Expand Specific Solutions04 Emergency response and first aid procedures
Establishing comprehensive emergency response and first aid procedures is vital when working with hydrochloric acid. This includes having readily available eyewash stations, safety showers, and spill control equipment. Training personnel in proper response techniques and immediate first aid measures helps minimize the severity of potential accidents and injuries.Expand Specific Solutions05 Monitoring and detection systems for hydrochloric acid
Implementing monitoring and detection systems is crucial for maintaining a safe working environment when handling hydrochloric acid. These systems include gas detectors, pH monitors, and leak detection equipment. Regular monitoring helps identify potential hazards early, allowing for prompt corrective actions and preventing dangerous situations from escalating.Expand Specific Solutions
Key Players in HCl Transport Industry
The hydrochloric acid transportation safety market is in a growth phase, driven by increasing industrial demand and stringent safety regulations. The market size is expanding, with a focus on developing advanced containment and monitoring solutions. Technologically, the field is moderately mature, with ongoing innovations in materials and safety systems. Companies like BASF Corp., FMC Corp., and China Petroleum & Chemical Corp. are leading players, investing in research and development to enhance transportation safety. Fluid Energy Group Ltd. and Dorf Ketal Chemicals FZE are also making significant contributions, particularly in developing environmentally friendly and safer acid formulations. The competitive landscape is characterized by a mix of established chemical giants and specialized firms, all striving to improve safety standards and efficiency in hydrochloric acid transportation.
BASF Corp.
Technical Solution: BASF has developed advanced corrosion-resistant materials and coatings for hydrochloric acid transportation. Their Elastocoat C spray technology provides a seamless, highly chemical-resistant lining for tank trucks and rail cars[1]. This polyurea-based coating system offers excellent adhesion to steel and significantly extends the service life of transport vessels. BASF has also introduced smart sensor technologies for real-time monitoring of tank integrity and acid concentration levels during transit[3]. Their integrated safety management system includes route optimization algorithms to minimize transport risks in populated areas.
Strengths: Comprehensive solution combining material science and digital technologies. Weaknesses: Higher initial implementation costs compared to traditional methods.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has implemented a multi-layered safety approach for hydrochloric acid transportation. They utilize double-walled tanker trucks with advanced leak detection systems and automatic shut-off valves[2]. Their proprietary corrosion-resistant alloy, developed specifically for HCl transport, shows a 40% increase in lifespan compared to standard materials[4]. Sinopec has also deployed a fleet-wide GPS tracking system integrated with a centralized control center for real-time monitoring and emergency response coordination. Additionally, they have implemented a comprehensive driver training program focusing on hazardous material handling and emergency procedures.
Strengths: Integrated approach combining material innovation, technology, and human factors. Weaknesses: High capital investment required for fleet-wide implementation.
Innovative HCl Containment Technologies
Treatment systems and methods using encapsulated corrosive fluids
PatentInactiveUS20210148212A1
Innovation
- Particle-encapsulated fluids are created using high-speed blending processes, where corrosive fluids are encapsulated in nanoparticles or silica particles, forming a dry or powdered form that can be safely handled and transported, with controlled release mechanisms for timed delivery.
Trailer and method for transporting peracetic acid
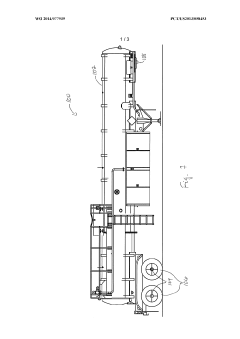
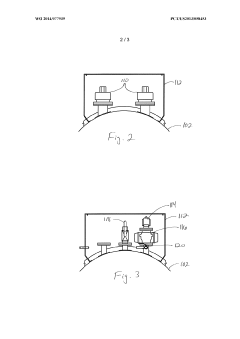
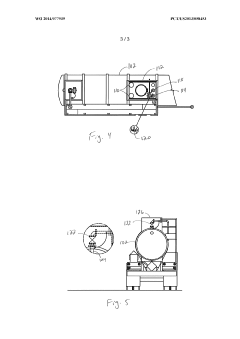
PatentWO2014077939A1
Innovation
- A modified trailer with a stainless steel tank equipped with external spill containment and pressure relief valves configured to provide relief at 20%-50% of the maximum allowable working pressure, featuring two recloseable 4" diameter pressure relief valves with a total pressure relief area of at least 11.94 in², designed to prevent over-pressurization during decomposition or fire events.
Regulatory Framework for HCl Transportation
The regulatory framework for hydrochloric acid (HCl) transportation is a complex and multi-layered system designed to ensure the safe handling and movement of this hazardous substance. At the international level, the United Nations' Recommendations on the Transport of Dangerous Goods serve as a foundational guideline, providing a global standard for the classification, packaging, and labeling of hazardous materials, including HCl.
In the United States, the Department of Transportation (DOT) oversees the transportation of hazardous materials through its Pipeline and Hazardous Materials Safety Administration (PHMSA). The Hazardous Materials Regulations (HMR), codified in Title 49 of the Code of Federal Regulations (49 CFR), parts 171-180, provide detailed requirements for HCl transportation. These regulations cover aspects such as proper packaging, labeling, placarding, and documentation.
The Occupational Safety and Health Administration (OSHA) also plays a crucial role in regulating HCl transportation safety. OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates that all employees involved in handling hazardous chemicals, including during transportation, must be properly trained and informed about the associated risks and safety procedures.
At the state level, additional regulations may apply, often building upon federal standards to address local concerns or specific transportation routes. For instance, some states impose stricter requirements for transporting hazardous materials through densely populated areas or environmentally sensitive regions.
The Environmental Protection Agency (EPA) contributes to the regulatory framework through its Resource Conservation and Recovery Act (RCRA) regulations, which govern the transportation of hazardous waste. While not all HCl transportation falls under RCRA, these regulations become relevant when the acid is being transported for disposal or recycling.
International shipments of HCl must comply with the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These codes align with the UN recommendations but provide more specific guidelines for their respective modes of transportation.
Compliance with these regulations requires extensive documentation, including shipping papers, safety data sheets (SDS), and emergency response information. Carriers and shippers must also adhere to specific training requirements, ensuring that all personnel involved in HCl transportation are knowledgeable about proper handling procedures and emergency protocols.
Regular updates to these regulations reflect evolving safety standards and technological advancements. For instance, recent amendments have focused on enhancing the traceability of hazardous materials shipments and improving emergency response capabilities through the use of electronic tracking systems and real-time monitoring technologies.
In the United States, the Department of Transportation (DOT) oversees the transportation of hazardous materials through its Pipeline and Hazardous Materials Safety Administration (PHMSA). The Hazardous Materials Regulations (HMR), codified in Title 49 of the Code of Federal Regulations (49 CFR), parts 171-180, provide detailed requirements for HCl transportation. These regulations cover aspects such as proper packaging, labeling, placarding, and documentation.
The Occupational Safety and Health Administration (OSHA) also plays a crucial role in regulating HCl transportation safety. OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates that all employees involved in handling hazardous chemicals, including during transportation, must be properly trained and informed about the associated risks and safety procedures.
At the state level, additional regulations may apply, often building upon federal standards to address local concerns or specific transportation routes. For instance, some states impose stricter requirements for transporting hazardous materials through densely populated areas or environmentally sensitive regions.
The Environmental Protection Agency (EPA) contributes to the regulatory framework through its Resource Conservation and Recovery Act (RCRA) regulations, which govern the transportation of hazardous waste. While not all HCl transportation falls under RCRA, these regulations become relevant when the acid is being transported for disposal or recycling.
International shipments of HCl must comply with the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These codes align with the UN recommendations but provide more specific guidelines for their respective modes of transportation.
Compliance with these regulations requires extensive documentation, including shipping papers, safety data sheets (SDS), and emergency response information. Carriers and shippers must also adhere to specific training requirements, ensuring that all personnel involved in HCl transportation are knowledgeable about proper handling procedures and emergency protocols.
Regular updates to these regulations reflect evolving safety standards and technological advancements. For instance, recent amendments have focused on enhancing the traceability of hazardous materials shipments and improving emergency response capabilities through the use of electronic tracking systems and real-time monitoring technologies.
Environmental Impact of HCl Transport
The transportation of hydrochloric acid (HCl) poses significant environmental risks that require careful consideration and management. HCl is a highly corrosive substance that can cause severe damage to ecosystems if released into the environment during transport. Accidental spills or leaks can lead to soil and water contamination, affecting plant and animal life in the surrounding areas.
One of the primary environmental concerns is the potential for HCl to alter the pH levels of soil and water bodies. Even small releases can cause localized acidification, disrupting the delicate balance of ecosystems and potentially leading to the death of aquatic organisms and vegetation. The impact can be particularly severe in freshwater environments, where sudden changes in acidity can have far-reaching consequences for the entire food chain.
Air pollution is another significant environmental risk associated with HCl transport. In the event of a spill or leak, HCl can vaporize and form a toxic cloud that can be harmful to both human health and the environment. These vapors can contribute to the formation of acid rain, which has long-term detrimental effects on forests, soil quality, and water bodies far from the original incident site.
The potential for long-term environmental damage from HCl transport accidents necessitates stringent safety measures and emergency response protocols. Proper containment systems, regular equipment maintenance, and the use of corrosion-resistant materials are essential to minimize the risk of environmental contamination. Additionally, transport routes should be carefully planned to avoid sensitive ecological areas and water sources.
Environmental monitoring along transport routes is crucial for early detection of potential leaks or spills. This can include regular soil and water testing, as well as air quality assessments in areas where HCl is frequently transported. Implementing such monitoring systems can help in rapid response to any incidents, thereby limiting environmental damage.
In the event of an HCl spill, immediate neutralization and containment are critical to mitigating environmental impact. Emergency response teams must be equipped with the necessary tools and materials to quickly neutralize the acid and prevent its spread into the surrounding environment. This may involve the use of alkaline substances to neutralize the acid and absorbent materials to contain the spill.
Sustainable practices in HCl transport also include the development and implementation of more environmentally friendly packaging and containment solutions. Innovations in this area can significantly reduce the risk of leaks and spills, thereby minimizing potential environmental harm. Furthermore, exploring alternative routes or transportation methods that reduce the overall environmental footprint of HCl transport should be a priority for companies involved in its distribution.
One of the primary environmental concerns is the potential for HCl to alter the pH levels of soil and water bodies. Even small releases can cause localized acidification, disrupting the delicate balance of ecosystems and potentially leading to the death of aquatic organisms and vegetation. The impact can be particularly severe in freshwater environments, where sudden changes in acidity can have far-reaching consequences for the entire food chain.
Air pollution is another significant environmental risk associated with HCl transport. In the event of a spill or leak, HCl can vaporize and form a toxic cloud that can be harmful to both human health and the environment. These vapors can contribute to the formation of acid rain, which has long-term detrimental effects on forests, soil quality, and water bodies far from the original incident site.
The potential for long-term environmental damage from HCl transport accidents necessitates stringent safety measures and emergency response protocols. Proper containment systems, regular equipment maintenance, and the use of corrosion-resistant materials are essential to minimize the risk of environmental contamination. Additionally, transport routes should be carefully planned to avoid sensitive ecological areas and water sources.
Environmental monitoring along transport routes is crucial for early detection of potential leaks or spills. This can include regular soil and water testing, as well as air quality assessments in areas where HCl is frequently transported. Implementing such monitoring systems can help in rapid response to any incidents, thereby limiting environmental damage.
In the event of an HCl spill, immediate neutralization and containment are critical to mitigating environmental impact. Emergency response teams must be equipped with the necessary tools and materials to quickly neutralize the acid and prevent its spread into the surrounding environment. This may involve the use of alkaline substances to neutralize the acid and absorbent materials to contain the spill.
Sustainable practices in HCl transport also include the development and implementation of more environmentally friendly packaging and containment solutions. Innovations in this area can significantly reduce the risk of leaks and spills, thereby minimizing potential environmental harm. Furthermore, exploring alternative routes or transportation methods that reduce the overall environmental footprint of HCl transport should be a priority for companies involved in its distribution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!