Isocyanate Research: Progress in Green Chemistry Applications
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isocyanate Evolution and Research Objectives
Isocyanates have played a pivotal role in the chemical industry since their discovery in the mid-19th century. Initially synthesized by Wurtz in 1848, these compounds have undergone significant evolution in terms of production methods, applications, and environmental considerations. The journey of isocyanates from laboratory curiosities to industrial staples has been marked by continuous innovation and adaptation to changing market demands and regulatory landscapes.
The primary objective of current isocyanate research is to address the environmental and health concerns associated with traditional isocyanate chemistry while maintaining or enhancing their valuable properties. This goal aligns with the broader principles of green chemistry, which seek to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances. Researchers are focusing on developing sustainable alternatives to conventional isocyanates, exploring bio-based precursors, and investigating novel synthesis routes that minimize environmental impact.
One of the key research objectives is the development of non-toxic or less toxic isocyanate alternatives. This includes the exploration of blocked isocyanates, which are less reactive at room temperature and release the active isocyanate only under specific conditions, thereby reducing exposure risks. Another area of focus is the creation of isocyanate-free systems that can provide similar performance characteristics in applications such as polyurethane production.
The quest for greener isocyanate chemistry has also led to increased interest in renewable resources as starting materials. Researchers are investigating the potential of bio-based polyols and other natural compounds as precursors for isocyanate production. This approach not only addresses sustainability concerns but also opens up new possibilities for biodegradable and environmentally friendly products.
Additionally, there is a growing emphasis on improving the efficiency of isocyanate production processes. This includes the development of catalysts that enable lower reaction temperatures and pressures, as well as the design of continuous flow reactors that optimize yield and reduce waste. These advancements aim to decrease the energy consumption and carbon footprint associated with isocyanate manufacturing.
The evolution of isocyanate research is also driven by regulatory pressures and market demands for safer, more sustainable materials. As such, a significant portion of current research is dedicated to understanding and mitigating the potential health and environmental risks associated with isocyanate exposure. This includes studies on toxicology, occupational safety, and the development of improved personal protective equipment.
The primary objective of current isocyanate research is to address the environmental and health concerns associated with traditional isocyanate chemistry while maintaining or enhancing their valuable properties. This goal aligns with the broader principles of green chemistry, which seek to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances. Researchers are focusing on developing sustainable alternatives to conventional isocyanates, exploring bio-based precursors, and investigating novel synthesis routes that minimize environmental impact.
One of the key research objectives is the development of non-toxic or less toxic isocyanate alternatives. This includes the exploration of blocked isocyanates, which are less reactive at room temperature and release the active isocyanate only under specific conditions, thereby reducing exposure risks. Another area of focus is the creation of isocyanate-free systems that can provide similar performance characteristics in applications such as polyurethane production.
The quest for greener isocyanate chemistry has also led to increased interest in renewable resources as starting materials. Researchers are investigating the potential of bio-based polyols and other natural compounds as precursors for isocyanate production. This approach not only addresses sustainability concerns but also opens up new possibilities for biodegradable and environmentally friendly products.
Additionally, there is a growing emphasis on improving the efficiency of isocyanate production processes. This includes the development of catalysts that enable lower reaction temperatures and pressures, as well as the design of continuous flow reactors that optimize yield and reduce waste. These advancements aim to decrease the energy consumption and carbon footprint associated with isocyanate manufacturing.
The evolution of isocyanate research is also driven by regulatory pressures and market demands for safer, more sustainable materials. As such, a significant portion of current research is dedicated to understanding and mitigating the potential health and environmental risks associated with isocyanate exposure. This includes studies on toxicology, occupational safety, and the development of improved personal protective equipment.
Green Chemistry Market Demand Analysis
The green chemistry market has witnessed significant growth in recent years, driven by increasing environmental concerns and stringent regulations. The demand for sustainable and eco-friendly chemical processes and products has created a substantial market opportunity for isocyanate research in green chemistry applications. This market segment is expected to continue its upward trajectory due to several key factors.
Firstly, there is a growing awareness among consumers and industries about the environmental impact of traditional chemical processes. This has led to a shift towards more sustainable alternatives, including green chemistry applications. Isocyanates, being versatile compounds used in various industries, have become a focal point for research and development in this area.
The automotive and construction industries are major drivers of the green chemistry market demand for isocyanates. These sectors are increasingly adopting eco-friendly materials and processes to reduce their carbon footprint and meet regulatory requirements. Isocyanate-based green chemistry applications, such as bio-based polyurethanes and low-VOC coatings, are gaining traction in these industries.
Furthermore, the packaging industry is experiencing a surge in demand for sustainable solutions. Isocyanate research in green chemistry is addressing this need by developing biodegradable and recyclable packaging materials. This trend is expected to contribute significantly to market growth in the coming years.
The healthcare and pharmaceutical sectors are also showing increased interest in green chemistry applications of isocyanates. The development of biocompatible materials and drug delivery systems using environmentally friendly isocyanate-based compounds is driving market demand in these areas.
Additionally, the textile industry is embracing green chemistry solutions to reduce its environmental impact. Isocyanate research is contributing to the development of sustainable textile finishes and coatings, further expanding the market potential.
Government initiatives and regulations promoting sustainable practices are playing a crucial role in shaping market demand. Many countries have implemented policies to encourage the adoption of green chemistry principles, creating a favorable environment for isocyanate research and applications.
The Asia-Pacific region is emerging as a key market for green chemistry applications, driven by rapid industrialization and increasing environmental awareness. This presents significant opportunities for isocyanate research and development in the region.
In conclusion, the green chemistry market demand for isocyanate research and applications is robust and diverse, spanning multiple industries and regions. As sustainability continues to be a priority for businesses and consumers alike, the market is poised for further growth and innovation in the coming years.
Firstly, there is a growing awareness among consumers and industries about the environmental impact of traditional chemical processes. This has led to a shift towards more sustainable alternatives, including green chemistry applications. Isocyanates, being versatile compounds used in various industries, have become a focal point for research and development in this area.
The automotive and construction industries are major drivers of the green chemistry market demand for isocyanates. These sectors are increasingly adopting eco-friendly materials and processes to reduce their carbon footprint and meet regulatory requirements. Isocyanate-based green chemistry applications, such as bio-based polyurethanes and low-VOC coatings, are gaining traction in these industries.
Furthermore, the packaging industry is experiencing a surge in demand for sustainable solutions. Isocyanate research in green chemistry is addressing this need by developing biodegradable and recyclable packaging materials. This trend is expected to contribute significantly to market growth in the coming years.
The healthcare and pharmaceutical sectors are also showing increased interest in green chemistry applications of isocyanates. The development of biocompatible materials and drug delivery systems using environmentally friendly isocyanate-based compounds is driving market demand in these areas.
Additionally, the textile industry is embracing green chemistry solutions to reduce its environmental impact. Isocyanate research is contributing to the development of sustainable textile finishes and coatings, further expanding the market potential.
Government initiatives and regulations promoting sustainable practices are playing a crucial role in shaping market demand. Many countries have implemented policies to encourage the adoption of green chemistry principles, creating a favorable environment for isocyanate research and applications.
The Asia-Pacific region is emerging as a key market for green chemistry applications, driven by rapid industrialization and increasing environmental awareness. This presents significant opportunities for isocyanate research and development in the region.
In conclusion, the green chemistry market demand for isocyanate research and applications is robust and diverse, spanning multiple industries and regions. As sustainability continues to be a priority for businesses and consumers alike, the market is poised for further growth and innovation in the coming years.
Current Challenges in Isocyanate Green Synthesis
The synthesis of isocyanates using green chemistry principles faces several significant challenges. One of the primary obstacles is the continued reliance on phosgene as a key reagent in traditional isocyanate production. Phosgene is highly toxic and corrosive, posing severe health and environmental risks. Developing alternative, safer routes that eliminate the need for phosgene remains a major focus of research efforts.
Another challenge lies in finding suitable catalysts that can facilitate isocyanate formation under milder conditions. Current processes often require high temperatures and pressures, leading to increased energy consumption and potential safety hazards. The search for efficient, selective catalysts that can operate at lower temperatures and pressures is crucial for improving the sustainability of isocyanate synthesis.
The use of renewable feedstocks in isocyanate production presents both opportunities and challenges. While bio-based raw materials offer a more sustainable alternative to petrochemical-derived precursors, their chemical diversity and variability can complicate process control and product consistency. Developing robust methods to handle and convert these renewable resources into high-quality isocyanates is an ongoing area of research.
Solvent selection and reduction pose additional challenges in green isocyanate synthesis. Many current processes rely on organic solvents that may be harmful to the environment or human health. Finding greener solvent alternatives or developing solvent-free methodologies is essential for improving the overall sustainability of isocyanate production.
The formation of by-products and waste streams in isocyanate synthesis also presents significant environmental concerns. Improving reaction selectivity and developing efficient separation and purification techniques are critical for minimizing waste generation and maximizing atom economy. This challenge is particularly important when considering the principles of green chemistry and the need for more sustainable industrial processes.
Scaling up green synthesis methods from laboratory to industrial scale remains a significant hurdle. Many promising green chemistry approaches for isocyanate production have been demonstrated on a small scale, but translating these methods to commercial production volumes while maintaining efficiency and cost-effectiveness is a complex task that requires further research and development.
Lastly, the economic viability of green isocyanate synthesis methods compared to traditional processes presents a challenge for widespread adoption. Developing cost-competitive green alternatives that can compete with established production methods is crucial for driving industry transition towards more sustainable practices in isocyanate manufacturing.
Another challenge lies in finding suitable catalysts that can facilitate isocyanate formation under milder conditions. Current processes often require high temperatures and pressures, leading to increased energy consumption and potential safety hazards. The search for efficient, selective catalysts that can operate at lower temperatures and pressures is crucial for improving the sustainability of isocyanate synthesis.
The use of renewable feedstocks in isocyanate production presents both opportunities and challenges. While bio-based raw materials offer a more sustainable alternative to petrochemical-derived precursors, their chemical diversity and variability can complicate process control and product consistency. Developing robust methods to handle and convert these renewable resources into high-quality isocyanates is an ongoing area of research.
Solvent selection and reduction pose additional challenges in green isocyanate synthesis. Many current processes rely on organic solvents that may be harmful to the environment or human health. Finding greener solvent alternatives or developing solvent-free methodologies is essential for improving the overall sustainability of isocyanate production.
The formation of by-products and waste streams in isocyanate synthesis also presents significant environmental concerns. Improving reaction selectivity and developing efficient separation and purification techniques are critical for minimizing waste generation and maximizing atom economy. This challenge is particularly important when considering the principles of green chemistry and the need for more sustainable industrial processes.
Scaling up green synthesis methods from laboratory to industrial scale remains a significant hurdle. Many promising green chemistry approaches for isocyanate production have been demonstrated on a small scale, but translating these methods to commercial production volumes while maintaining efficiency and cost-effectiveness is a complex task that requires further research and development.
Lastly, the economic viability of green isocyanate synthesis methods compared to traditional processes presents a challenge for widespread adoption. Developing cost-competitive green alternatives that can compete with established production methods is crucial for driving industry transition towards more sustainable practices in isocyanate manufacturing.
Existing Green Isocyanate Production Methods
01 Synthesis and production of isocyanates
Various methods and processes for synthesizing and producing isocyanates are described. These include novel reaction pathways, catalysts, and production techniques to improve yield, purity, and efficiency in isocyanate manufacturing.- Synthesis and production of isocyanates: Various methods and processes for synthesizing and producing isocyanates are described. These include novel catalysts, reaction conditions, and precursor materials to improve yield, purity, and efficiency in isocyanate production.
- Applications of isocyanates in polymer chemistry: Isocyanates are widely used in polymer chemistry, particularly in the production of polyurethanes. The patents describe various applications, including coatings, adhesives, foams, and elastomers, as well as novel formulations and processing techniques.
- Isocyanate-based catalysts and additives: Several patents focus on the development of isocyanate-based catalysts and additives for various chemical processes. These include novel catalyst systems, stabilizers, and modifiers that enhance reaction rates, selectivity, or product properties.
- Safety and handling of isocyanates: Given the reactive nature of isocyanates, patents address safety concerns and handling procedures. This includes methods for reducing toxicity, improving storage stability, and developing safer alternatives or modified isocyanates with reduced health risks.
- Isocyanate-free alternatives and substitutes: Some patents explore alternatives to traditional isocyanates, aiming to address environmental and health concerns. These include bio-based substitutes, non-isocyanate polyurethanes, and alternative chemistries that provide similar functionalities without the use of isocyanates.
02 Applications of isocyanates in polymer chemistry
Isocyanates are widely used in polymer chemistry, particularly in the production of polyurethanes. The patents discuss various applications, including coatings, adhesives, foams, and elastomers, as well as novel formulations and processing techniques.Expand Specific Solutions03 Isocyanate-based catalysts and reaction modifiers
Several patents focus on the development of isocyanate-based catalysts and reaction modifiers. These compounds are used to enhance chemical reactions, improve product properties, or facilitate specific industrial processes.Expand Specific Solutions04 Safety and handling of isocyanates
Given the reactive nature of isocyanates, patents in this category address safety concerns and handling procedures. This includes methods for reducing toxicity, improving storage stability, and developing safer formulations for various applications.Expand Specific Solutions05 Isocyanate-free alternatives and substitutes
Some patents explore alternatives to traditional isocyanates, aiming to develop more environmentally friendly or less hazardous options. These include novel compounds, formulations, or processes that can replace isocyanates in certain applications while maintaining desired properties.Expand Specific Solutions
Key Players in Green Isocyanate Research
The isocyanate research field, focusing on green chemistry applications, is in a transitional phase, moving from early-stage development to more mature applications. The market size is expanding, driven by increasing demand for sustainable chemical processes. Technological maturity varies across companies, with industry leaders like Covestro, BASF, and Wanhua Chemical Group making significant advancements. Emerging players such as Evonik and Asahi Kasei are also contributing to innovation. The competitive landscape is characterized by a mix of established chemical giants and specialized firms, with research institutions like the Chinese Academy of Sciences playing crucial roles in driving fundamental breakthroughs.
Covestro Deutschland AG
Technical Solution: Covestro has developed a novel approach to produce isocyanates using carbon dioxide as a raw material, reducing the reliance on fossil fuels. Their CO2 technology, known as cardyon®, incorporates up to 20% CO2 into polyols used for polyurethane production[1]. This process not only reduces the carbon footprint but also improves the sustainability of isocyanate production. Covestro has also invested in developing bio-based isocyanates, utilizing plant-based raw materials to replace petroleum-derived precursors[2]. Their research focuses on improving the efficiency of these green chemistry processes to make them economically viable on an industrial scale.
Strengths: Innovative use of CO2 as a raw material, reducing carbon footprint. Commitment to bio-based alternatives. Weaknesses: Higher production costs compared to traditional methods, potential scalability challenges for bio-based processes.
BASF Corp.
Technical Solution: BASF has made significant strides in green chemistry applications for isocyanate production. They have developed a novel process called "gas-phase technology" for the production of TDI (toluene diisocyanate), which reduces energy consumption by up to 80% compared to conventional methods[3]. BASF has also focused on developing non-isocyanate polyurethanes (NIPUs) as an alternative to traditional isocyanate-based systems. Their research includes the use of cyclic carbonates and amines to produce polyhydroxyurethanes, which offer improved environmental and health profiles[4]. Additionally, BASF is exploring bio-based raw materials for isocyanate production, aiming to reduce dependence on fossil resources.
Strengths: Energy-efficient production processes, development of non-isocyanate alternatives, focus on bio-based raw materials. Weaknesses: Potential performance trade-offs with NIPUs, higher costs associated with bio-based raw materials.
Breakthrough Green Isocyanate Technologies
Sustainable preparation of organic amino compounds for the production of organic isocyanates
PatentWO2024017890A2
Innovation
- A process using green hydrogen and green ammonia to produce organic amino compounds, which are then used to synthesize isocyanates, incorporating renewable energy sources and reducing waste through closed-loop recycling of materials.
Catalytic synthesis of free isocyanates
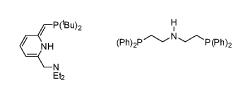
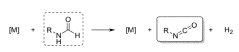
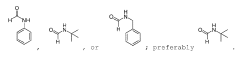
PatentWO2023274492A1
Innovation
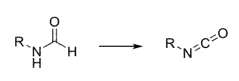
- A catalytic dehydrogenation process using a Group VII, VIII, or IX transition metal complex to convert formamides into free isocyanates, employing homogeneous catalysis to improve yield and selectivity, and releasing hydrogen gas as a by-product.
Environmental Impact Assessment
The environmental impact of isocyanates has been a significant concern in the chemical industry, prompting extensive research into green chemistry applications. Traditional isocyanate production and usage have been associated with various environmental issues, including air and water pollution, as well as potential health risks for workers and surrounding communities.
Recent advancements in green chemistry have led to the development of more environmentally friendly isocyanate production methods. These include the use of renewable feedstocks, such as plant-based oils, to replace petroleum-derived raw materials. This shift not only reduces the carbon footprint of isocyanate production but also decreases reliance on non-renewable resources.
Water-based polyurethane systems have emerged as a promising alternative to solvent-based systems, significantly reducing volatile organic compound (VOC) emissions. These systems have shown comparable performance to traditional isocyanate-based products while minimizing environmental impact and improving worker safety.
Biodegradable isocyanates have also been a focus of recent research, addressing the end-of-life environmental concerns associated with conventional isocyanate-based products. These novel materials are designed to break down naturally in the environment, reducing long-term ecological impact and waste accumulation.
Life cycle assessments (LCAs) have been conducted to evaluate the overall environmental impact of green isocyanate technologies compared to traditional methods. These studies have generally shown significant reductions in greenhouse gas emissions, energy consumption, and water usage across the product lifecycle.
Efforts to improve the energy efficiency of isocyanate production processes have led to the development of novel catalysts and reaction conditions. These innovations have resulted in lower energy requirements and reduced waste generation, further minimizing the environmental footprint of isocyanate manufacturing.
The implementation of closed-loop systems and improved recycling technologies has also contributed to reducing the environmental impact of isocyanate production and use. These systems aim to recover and reuse solvents, unreacted monomers, and by-products, minimizing waste and resource consumption.
Regulatory frameworks, such as REACH in the European Union, have played a crucial role in driving the adoption of greener isocyanate technologies. These regulations have encouraged industry players to invest in research and development of more sustainable alternatives, leading to a gradual shift towards environmentally friendly practices in the isocyanate sector.
Recent advancements in green chemistry have led to the development of more environmentally friendly isocyanate production methods. These include the use of renewable feedstocks, such as plant-based oils, to replace petroleum-derived raw materials. This shift not only reduces the carbon footprint of isocyanate production but also decreases reliance on non-renewable resources.
Water-based polyurethane systems have emerged as a promising alternative to solvent-based systems, significantly reducing volatile organic compound (VOC) emissions. These systems have shown comparable performance to traditional isocyanate-based products while minimizing environmental impact and improving worker safety.
Biodegradable isocyanates have also been a focus of recent research, addressing the end-of-life environmental concerns associated with conventional isocyanate-based products. These novel materials are designed to break down naturally in the environment, reducing long-term ecological impact and waste accumulation.
Life cycle assessments (LCAs) have been conducted to evaluate the overall environmental impact of green isocyanate technologies compared to traditional methods. These studies have generally shown significant reductions in greenhouse gas emissions, energy consumption, and water usage across the product lifecycle.
Efforts to improve the energy efficiency of isocyanate production processes have led to the development of novel catalysts and reaction conditions. These innovations have resulted in lower energy requirements and reduced waste generation, further minimizing the environmental footprint of isocyanate manufacturing.
The implementation of closed-loop systems and improved recycling technologies has also contributed to reducing the environmental impact of isocyanate production and use. These systems aim to recover and reuse solvents, unreacted monomers, and by-products, minimizing waste and resource consumption.
Regulatory frameworks, such as REACH in the European Union, have played a crucial role in driving the adoption of greener isocyanate technologies. These regulations have encouraged industry players to invest in research and development of more sustainable alternatives, leading to a gradual shift towards environmentally friendly practices in the isocyanate sector.
Regulatory Framework for Green Isocyanates
The regulatory framework for green isocyanates is evolving rapidly as governments and industry stakeholders recognize the need for more sustainable and environmentally friendly chemical processes. This framework encompasses a range of policies, standards, and guidelines aimed at promoting the development and adoption of green isocyanate technologies while mitigating potential risks to human health and the environment.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for green chemistry and sustainable chemical management. These guidelines provide a foundation for national and regional regulatory approaches to green isocyanates.
In the European Union, the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation plays a crucial role in governing the use of isocyanates and promoting greener alternatives. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals, including isocyanates, and to provide safety information to users.
The United States Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA) to regulate chemical substances, including isocyanates. The EPA's Green Chemistry Program encourages the development of innovative chemical technologies that reduce or eliminate the use of hazardous substances in chemical products and processes.
Many countries have introduced specific regulations targeting isocyanates due to their potential health hazards. For instance, Germany has implemented strict occupational exposure limits for isocyanates and mandates the use of less hazardous substitutes where possible. Similarly, Canada has established regulations under the Canadian Environmental Protection Act to control the use and disposal of certain isocyanates.
Industry associations, such as the American Chemistry Council and the European Diisocyanate and Polyol Producers Association, have developed voluntary initiatives and best practices for the safe handling and use of isocyanates. These self-regulatory efforts complement government regulations and promote responsible product stewardship.
As research in green isocyanate technologies progresses, regulatory frameworks are expected to adapt to accommodate new innovations. This may include incentives for companies developing green isocyanate alternatives, such as tax credits or expedited approval processes for environmentally friendly products. Additionally, regulatory bodies are likely to increase their focus on life cycle assessments and circular economy principles in evaluating the environmental impact of isocyanate-based products.
The ongoing development of international standards for green chemistry and sustainable chemical processes will further shape the regulatory landscape for green isocyanates. These standards will help harmonize global approaches to regulating and promoting environmentally friendly isocyanate technologies, facilitating international trade and collaboration in this rapidly evolving field.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for green chemistry and sustainable chemical management. These guidelines provide a foundation for national and regional regulatory approaches to green isocyanates.
In the European Union, the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation plays a crucial role in governing the use of isocyanates and promoting greener alternatives. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals, including isocyanates, and to provide safety information to users.
The United States Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA) to regulate chemical substances, including isocyanates. The EPA's Green Chemistry Program encourages the development of innovative chemical technologies that reduce or eliminate the use of hazardous substances in chemical products and processes.
Many countries have introduced specific regulations targeting isocyanates due to their potential health hazards. For instance, Germany has implemented strict occupational exposure limits for isocyanates and mandates the use of less hazardous substitutes where possible. Similarly, Canada has established regulations under the Canadian Environmental Protection Act to control the use and disposal of certain isocyanates.
Industry associations, such as the American Chemistry Council and the European Diisocyanate and Polyol Producers Association, have developed voluntary initiatives and best practices for the safe handling and use of isocyanates. These self-regulatory efforts complement government regulations and promote responsible product stewardship.
As research in green isocyanate technologies progresses, regulatory frameworks are expected to adapt to accommodate new innovations. This may include incentives for companies developing green isocyanate alternatives, such as tax credits or expedited approval processes for environmentally friendly products. Additionally, regulatory bodies are likely to increase their focus on life cycle assessments and circular economy principles in evaluating the environmental impact of isocyanate-based products.
The ongoing development of international standards for green chemistry and sustainable chemical processes will further shape the regulatory landscape for green isocyanates. These standards will help harmonize global approaches to regulating and promoting environmentally friendly isocyanate technologies, facilitating international trade and collaboration in this rapidly evolving field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!