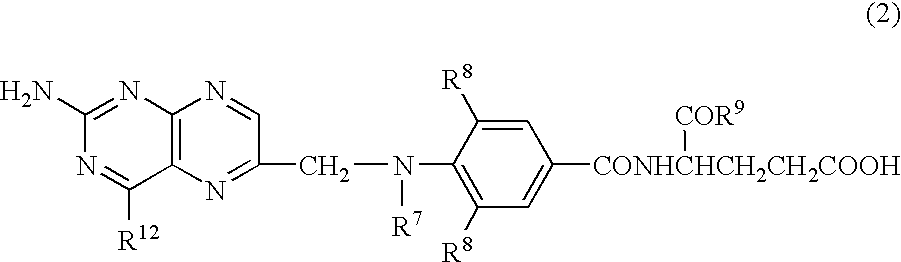
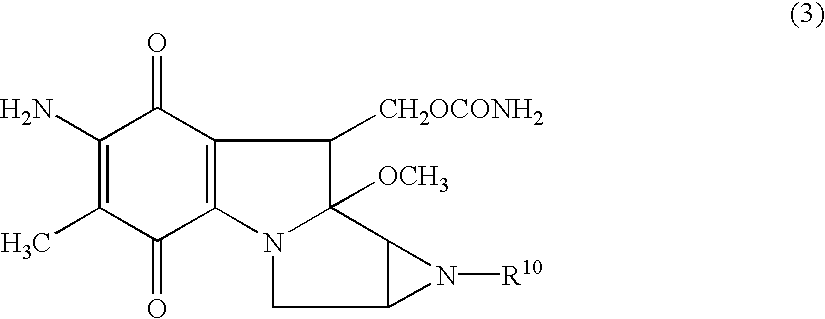
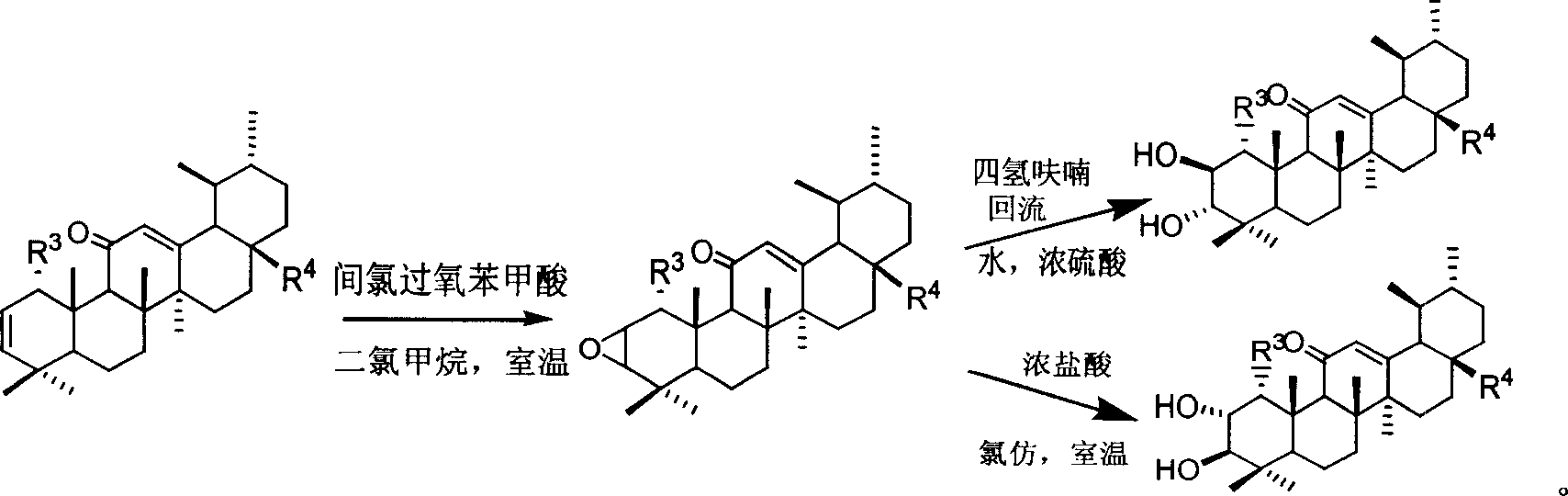
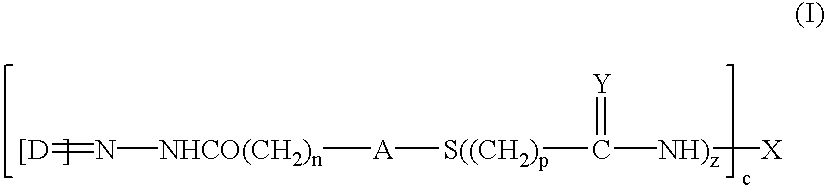Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
940 results about "Carcinoma Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
[edit on Wikidata] Carcinoma is a type of cancer that develops from epithelial cells. Specifically, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that arises from cells originating in the endodermal, mesodermal or ectodermal germ layer during embryogenesis.
Methods and reagents for the rapid and efficient isolation of circulating cancer cells
InactiveUS7332288B2Efficient ConcentrationEasy to detectNanomagnetismMicrobiological testing/measurementCirculating cancer cellCell sensitivity
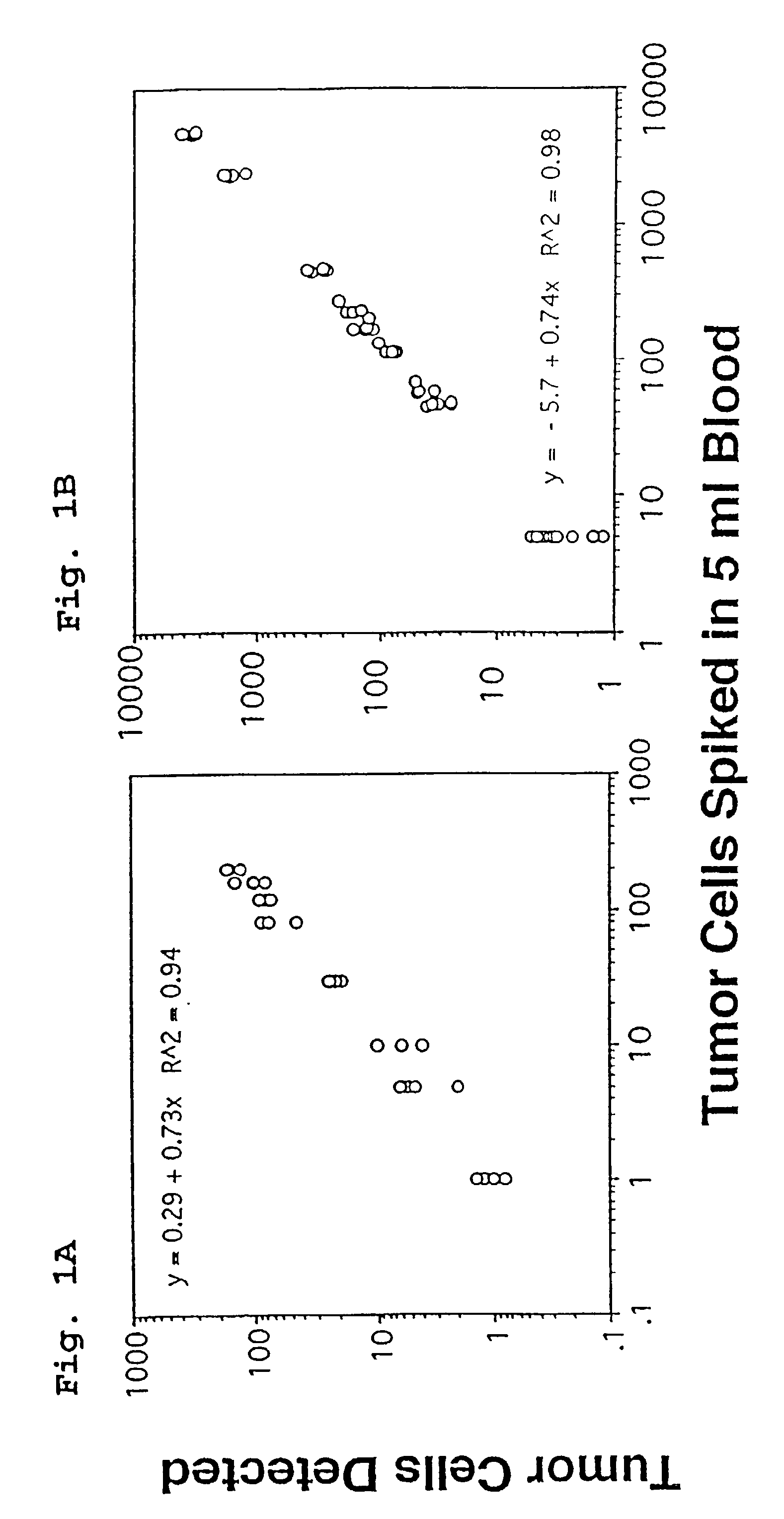
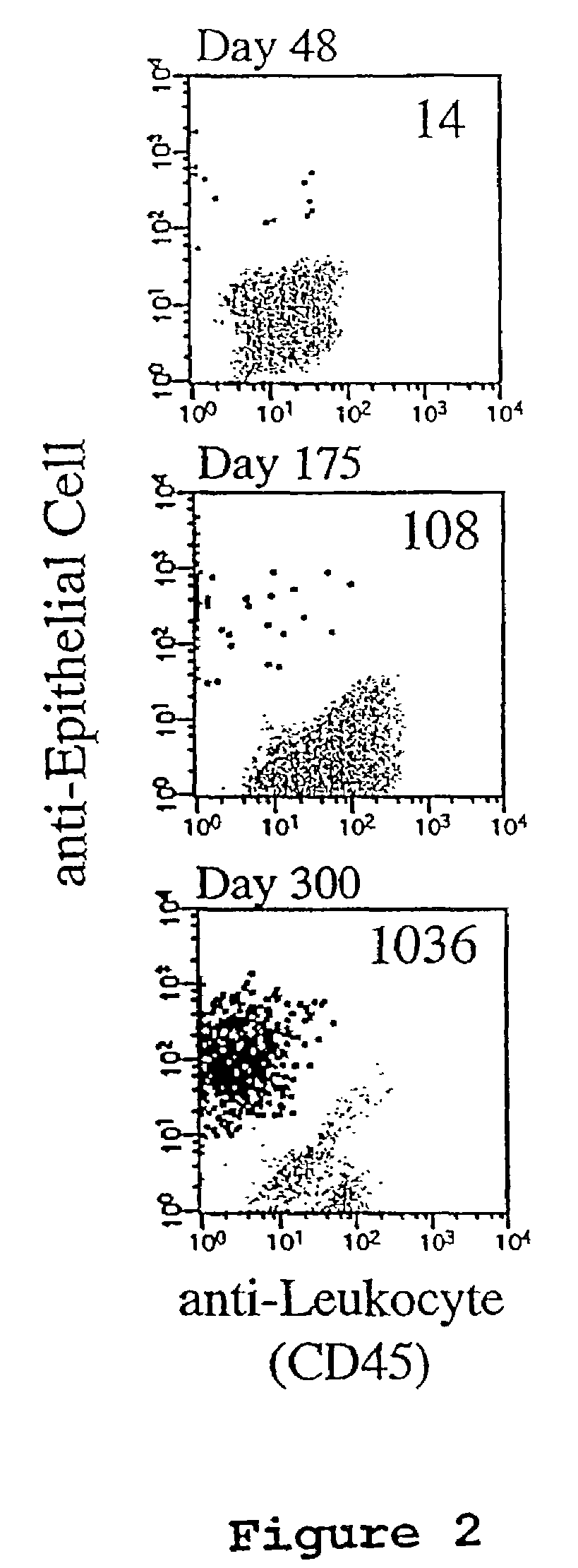
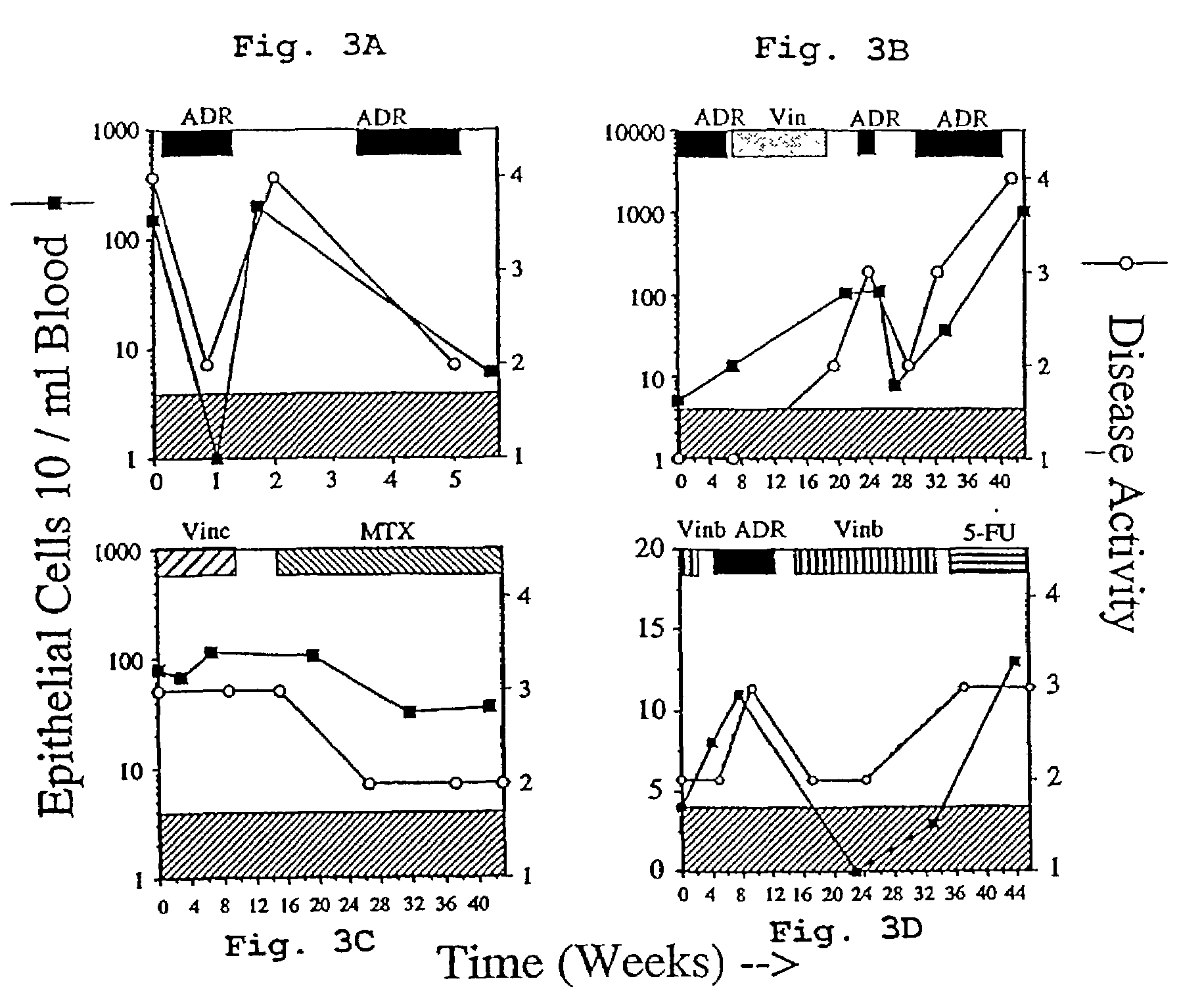
A highly sensitive assay is disclosed which combines immunomagnetic enrichment with multiparameter flow cytometric and immunocytochemical analysis to detect, enumerate and characterize carcinoma cells in the blood. The assay can detect one epithelial cell or less in 1 ml of blood and has a greater sensitivity than conventional PCR or immunohistochemistry by 1-2 orders of magnitude. In addition, the assay facilitates the biological characterization and staging of carcinoma cells.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Breast biopsy system and methods
InactiveUS20050004492A1Raise the possibilityMinimizing risk of migrationSurgical needlesVaccination/ovulation diagnosticsCancer cellSurgical department
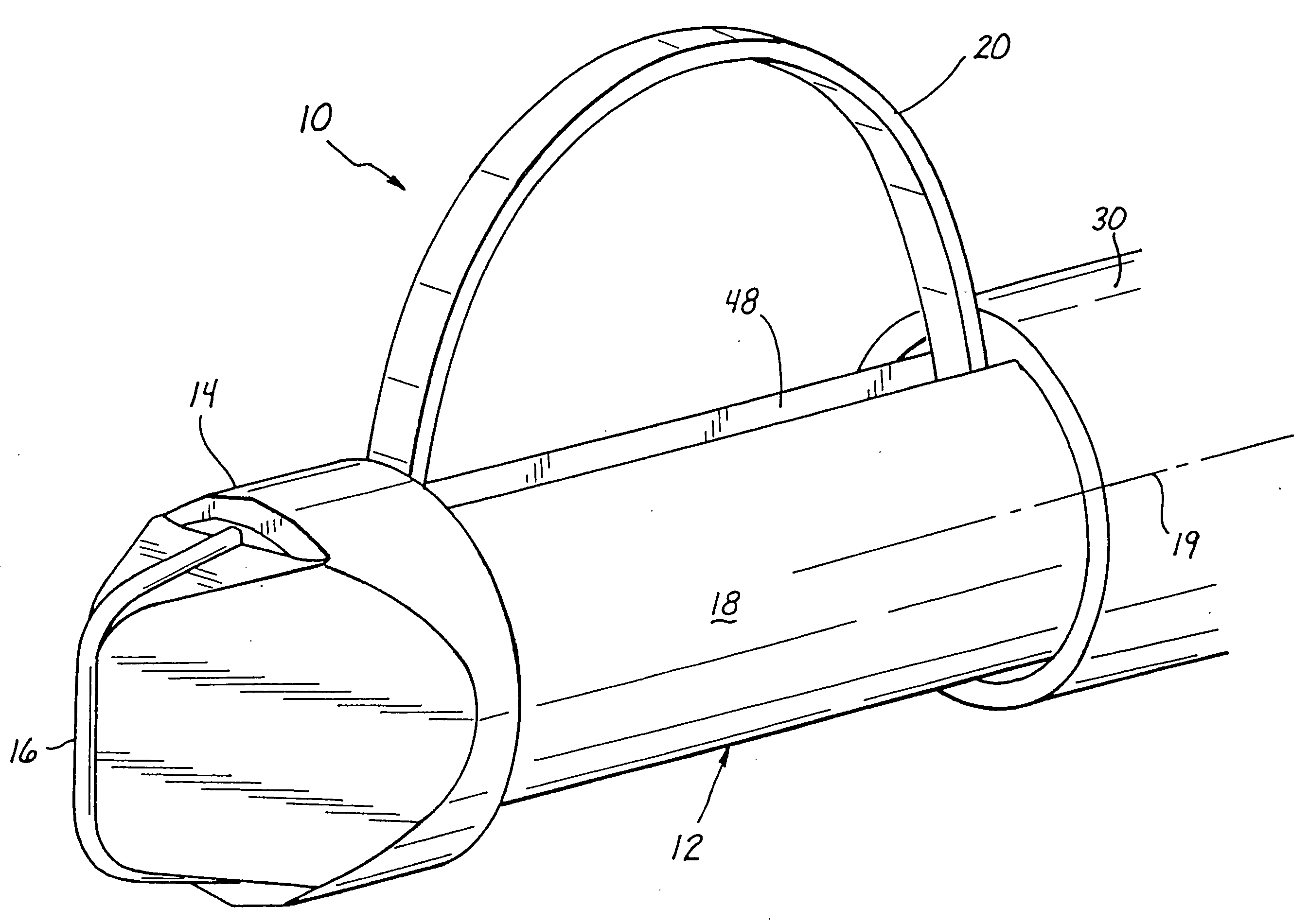
An apparatus and method are provided for precisely isolating a target lesion in a patient's body tissue, resulting in a high likelihood of “clean” margins about the lesion when it is removed for diagnosis and / or therapy. This approach advantageously will often result in the ability to both diagnose and treat a malignant lesion with only a single percutaneous procedure, with no follow-up percutaneous or surgical procedure required, while minimizing the risk of migration of possibly cancerous cells from the lesion to surrounding tissue or the bloodstream. In particular, the apparatus comprises a biopsy instrument having a distal end adapted for entry into the patient's body, a longitudinal shaft, and a cutting element disposed along the shaft. The cutting element is actuatable between a radially retracted position and a radially extended position. Advantageously, the instrument is rotatable about its axis in the radially extended position to isolate a desired tissue specimen from surrounding tissue by defining a peripheral margin about the tissue specimen. Once the tissue specimen is isolated, it may be segmented by further manipulation of the cutting element, after which the tissue segments are preferably individually removed from the patient's body through a cannula or the like. Alternatively, the specimen may be encapsulated and removed as an intact piece.
Owner:SENORX
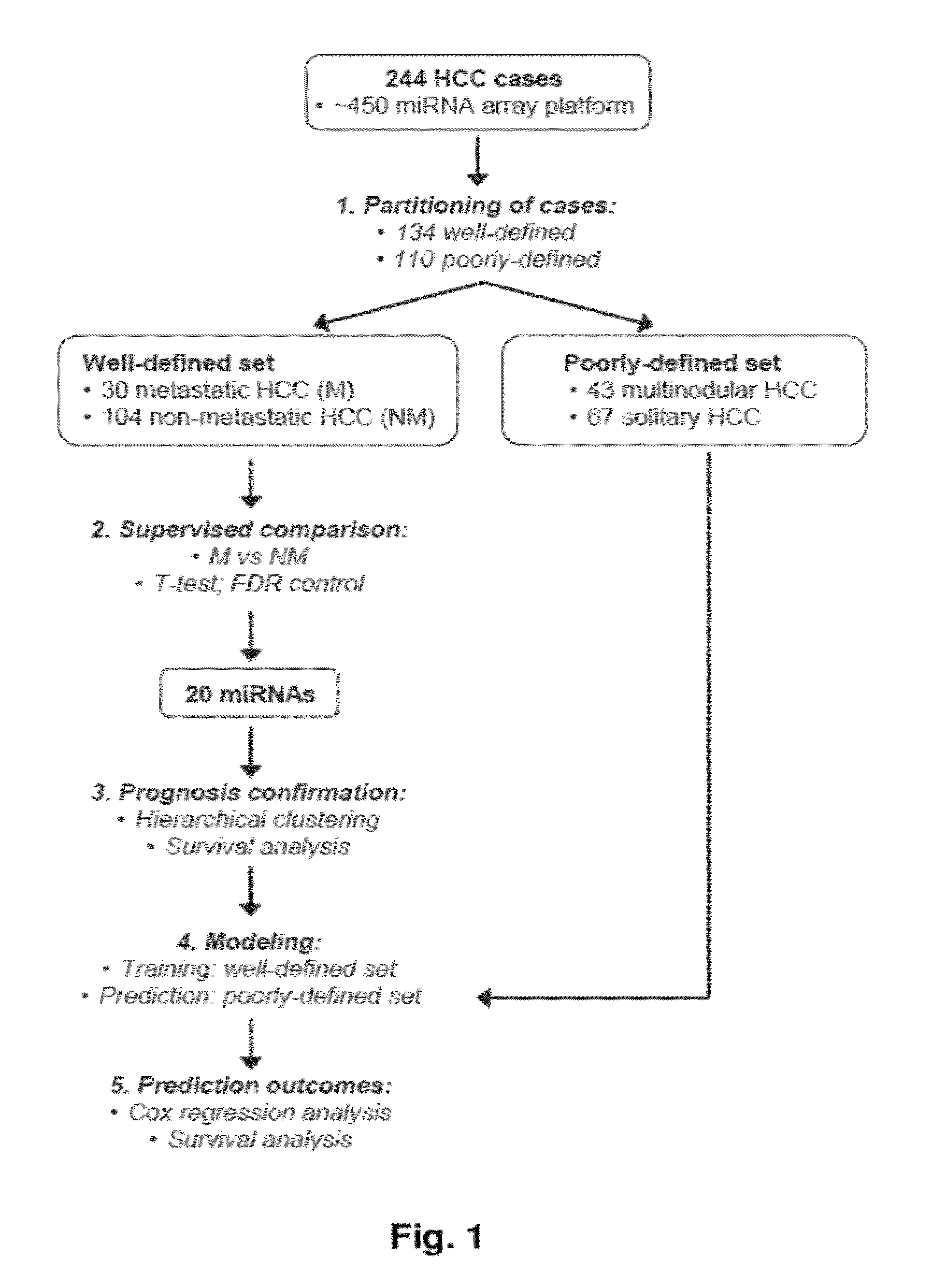
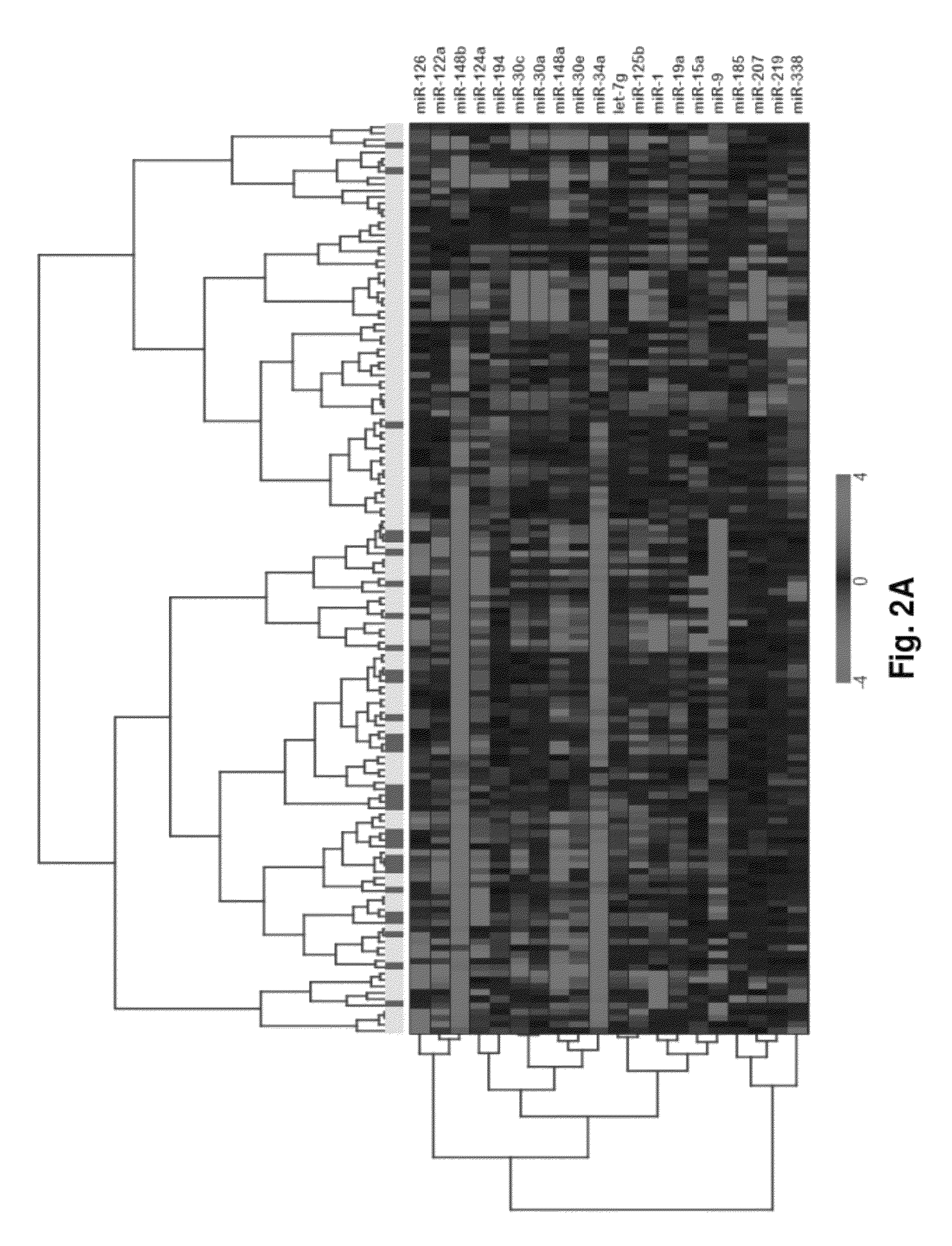
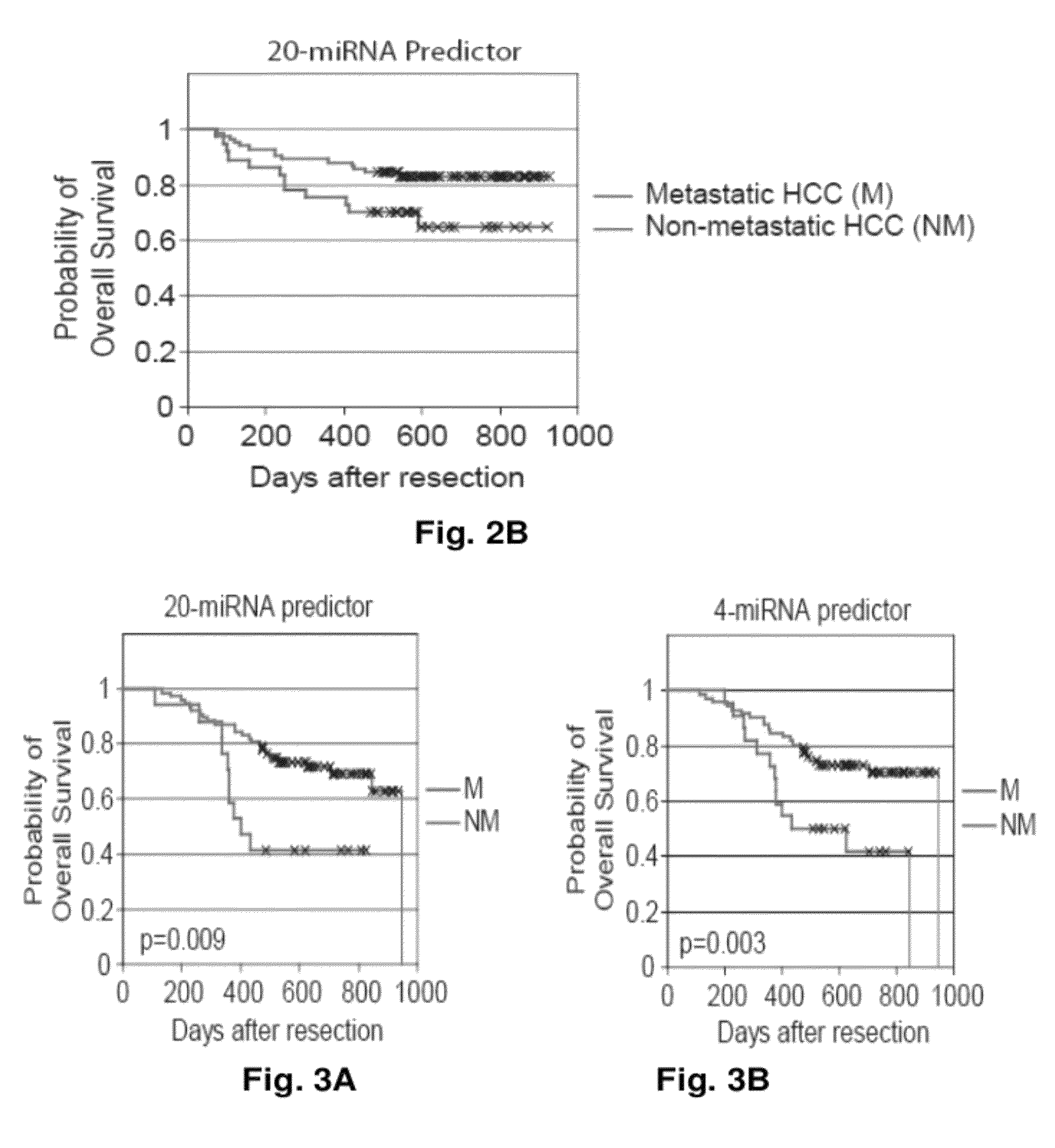
MicroRNA expression signature for predicting survival and metastases in hepatocellular carcinoma
InactiveUS8252538B2Independent and significant predictor of patient prognosis and relapseEnable prognosisSugar derivativesNucleotide librariesLymphatic SpreadHepatocellular carcinoma
Provided herein are methods and compositions for the diagnosis, prognosis and treatment of Hepatocellular carcinoma (HCC). Also provided are methods of identifying anti-HCC agents.
Owner:THE OHIO STATES UNIV +2
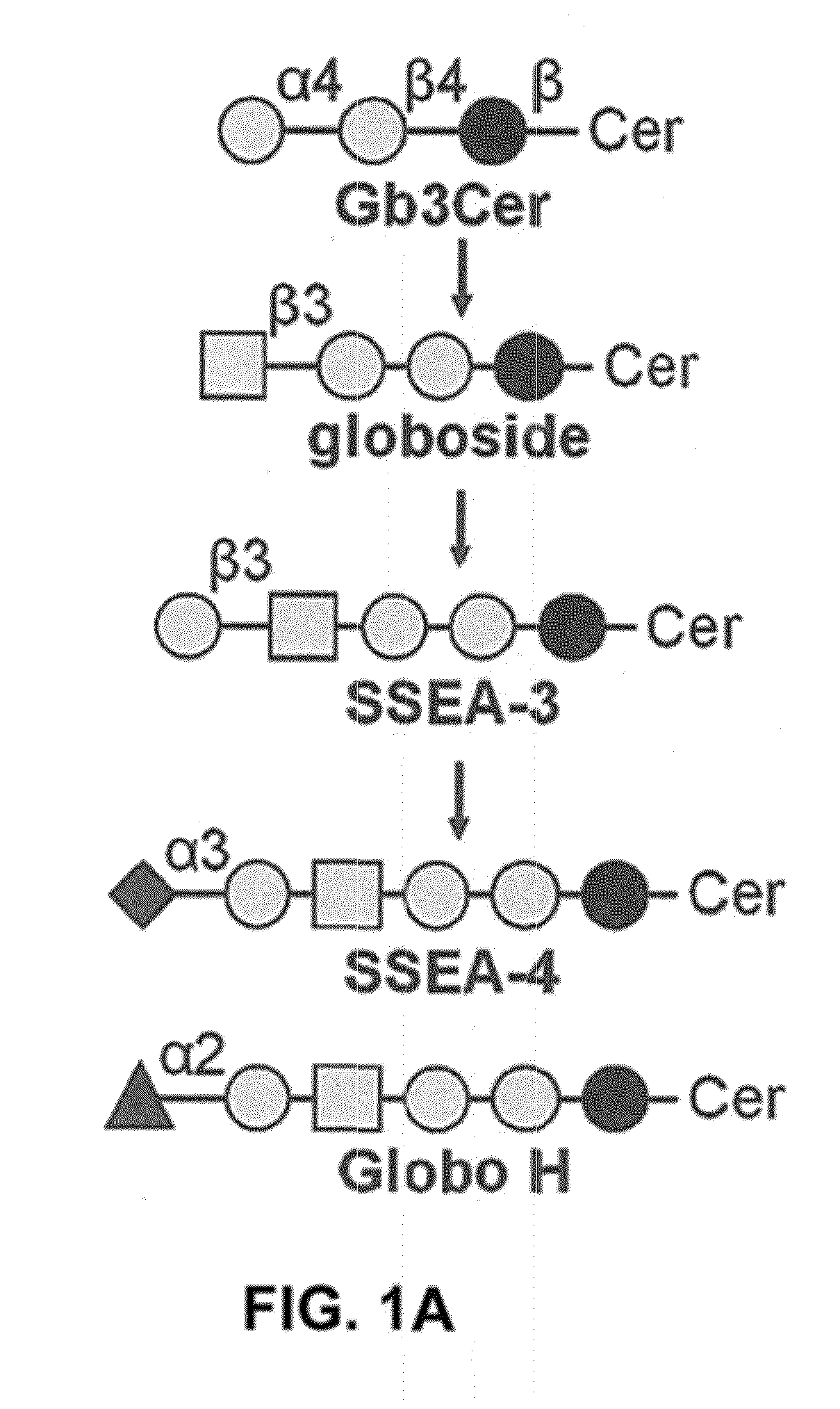
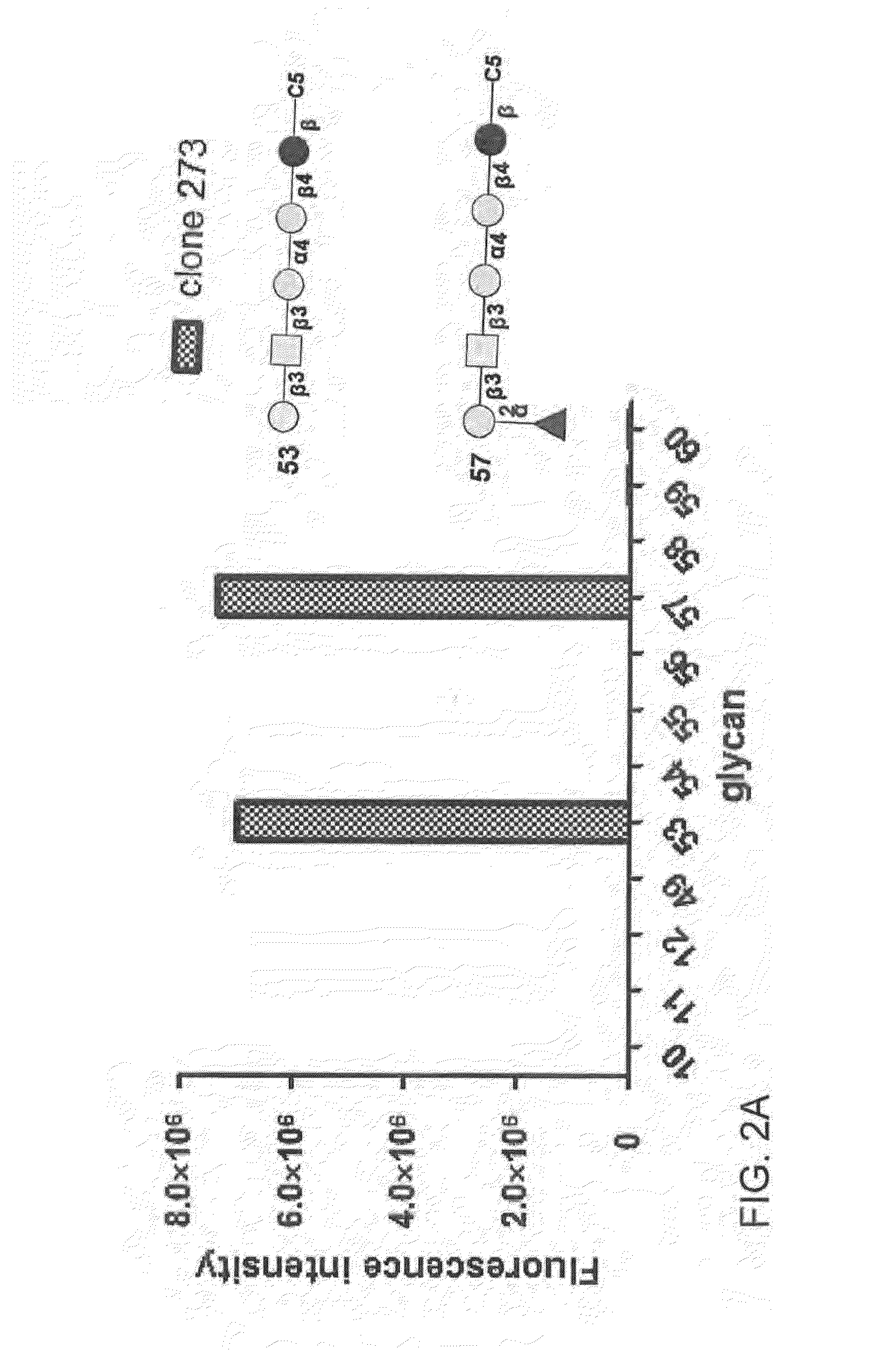
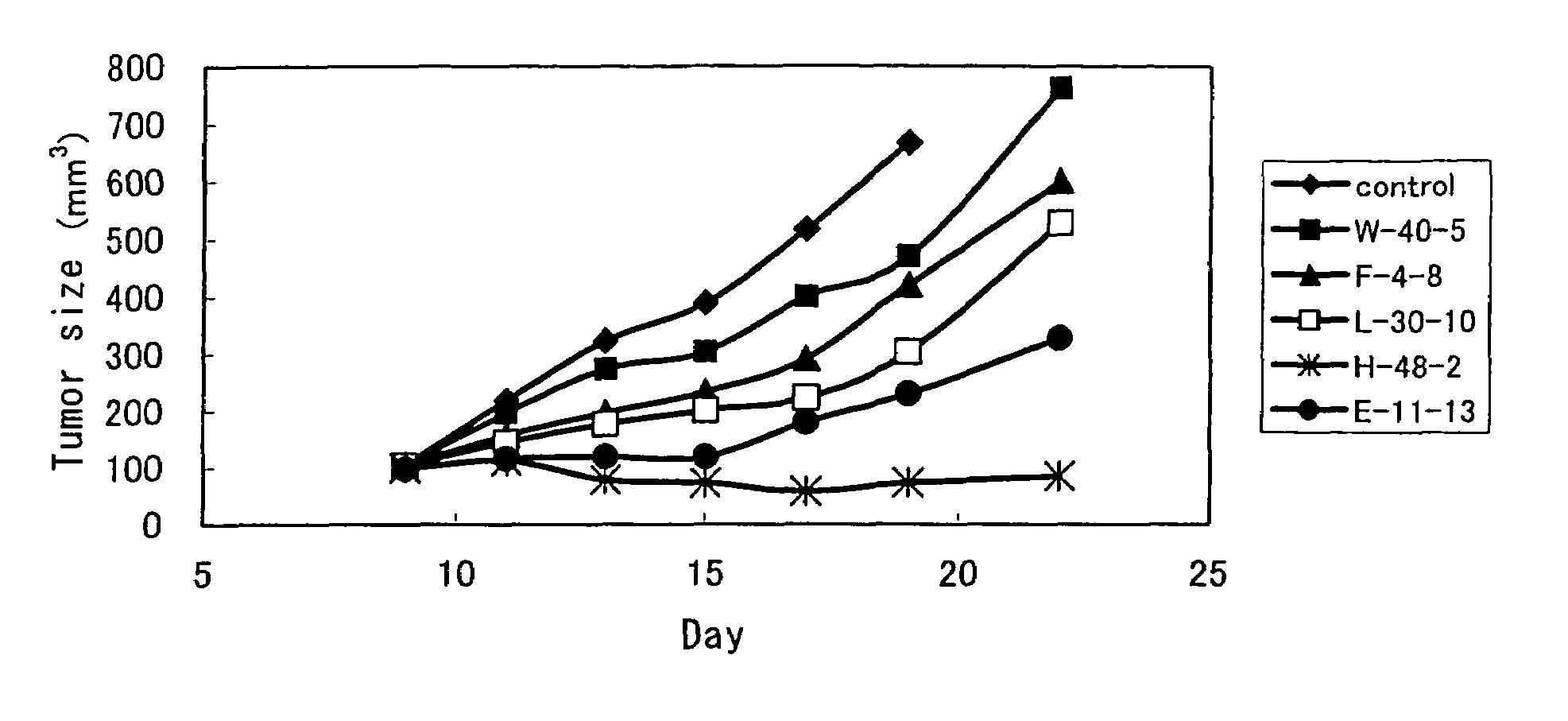
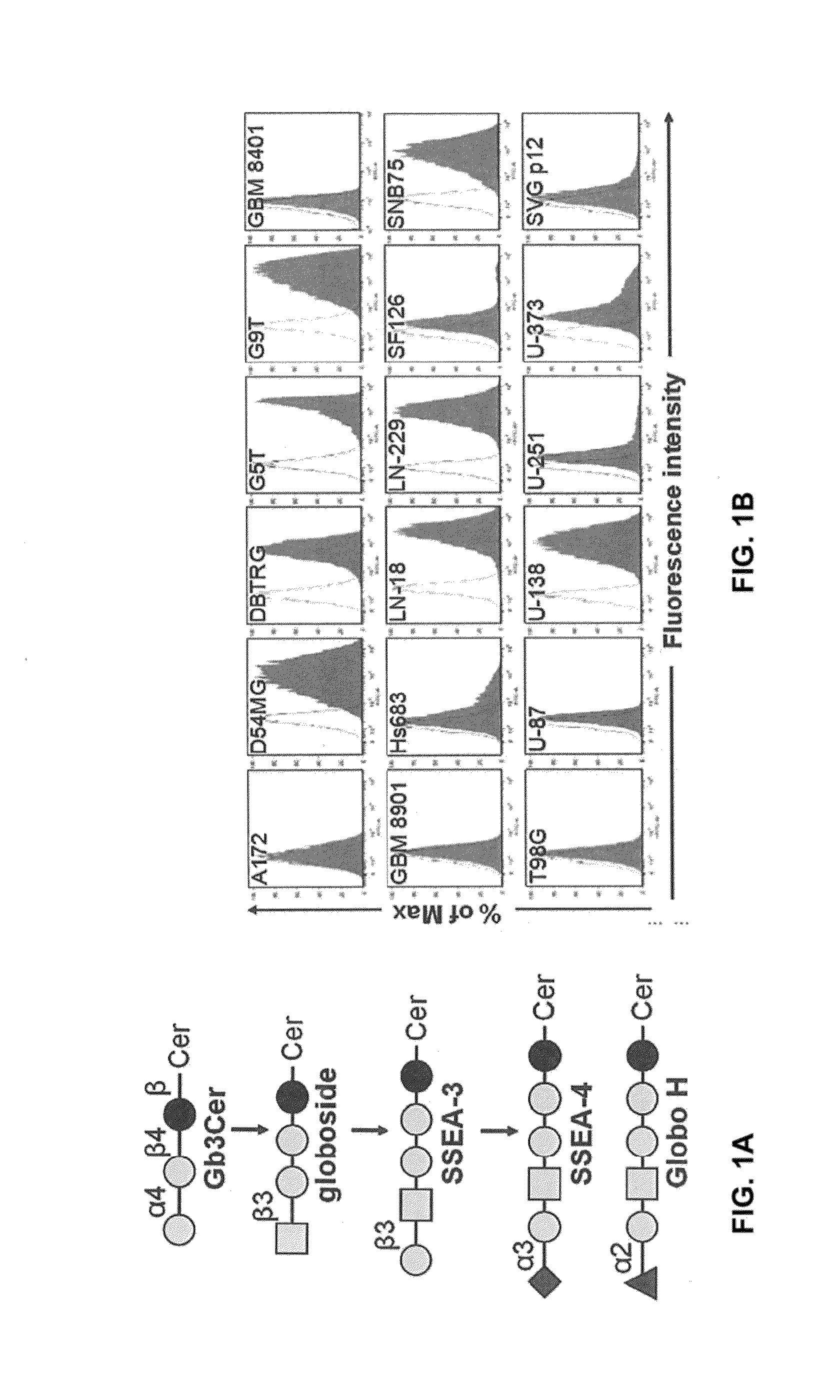
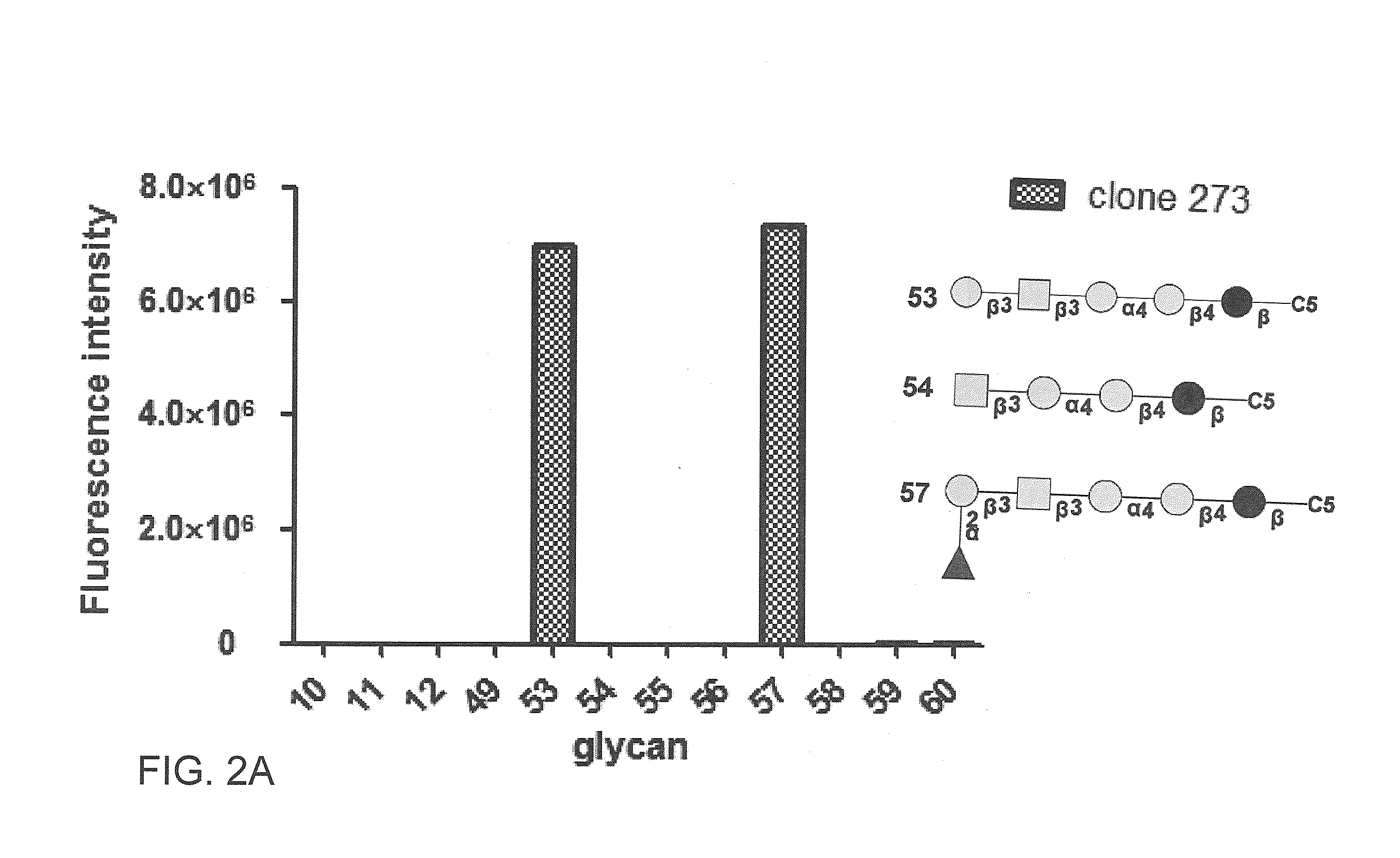
Compositions and methods for treatment and detection of cancers
ActiveUS20160102151A1Shrink tumorElimination of malignant cellLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen Binding Fragment
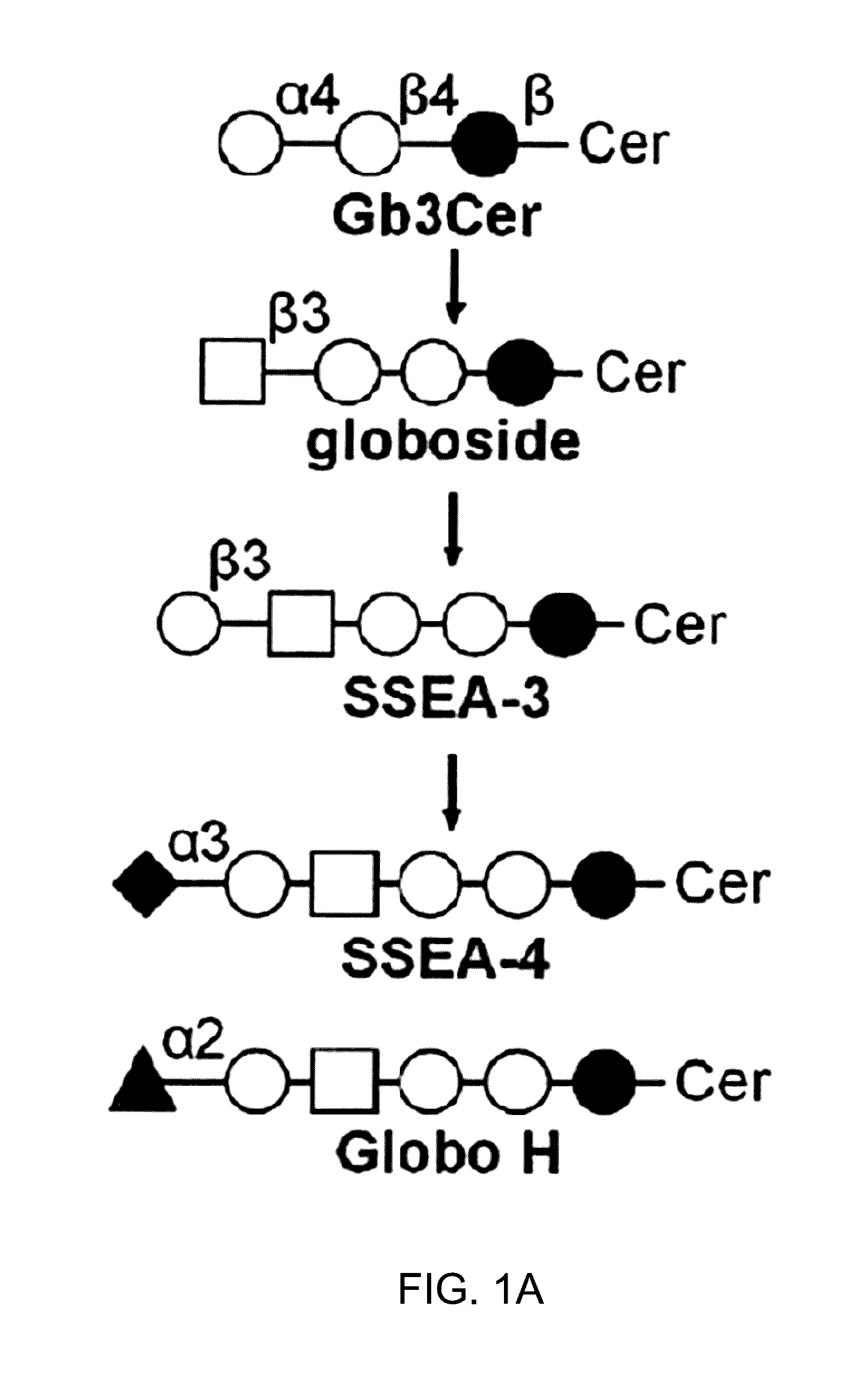
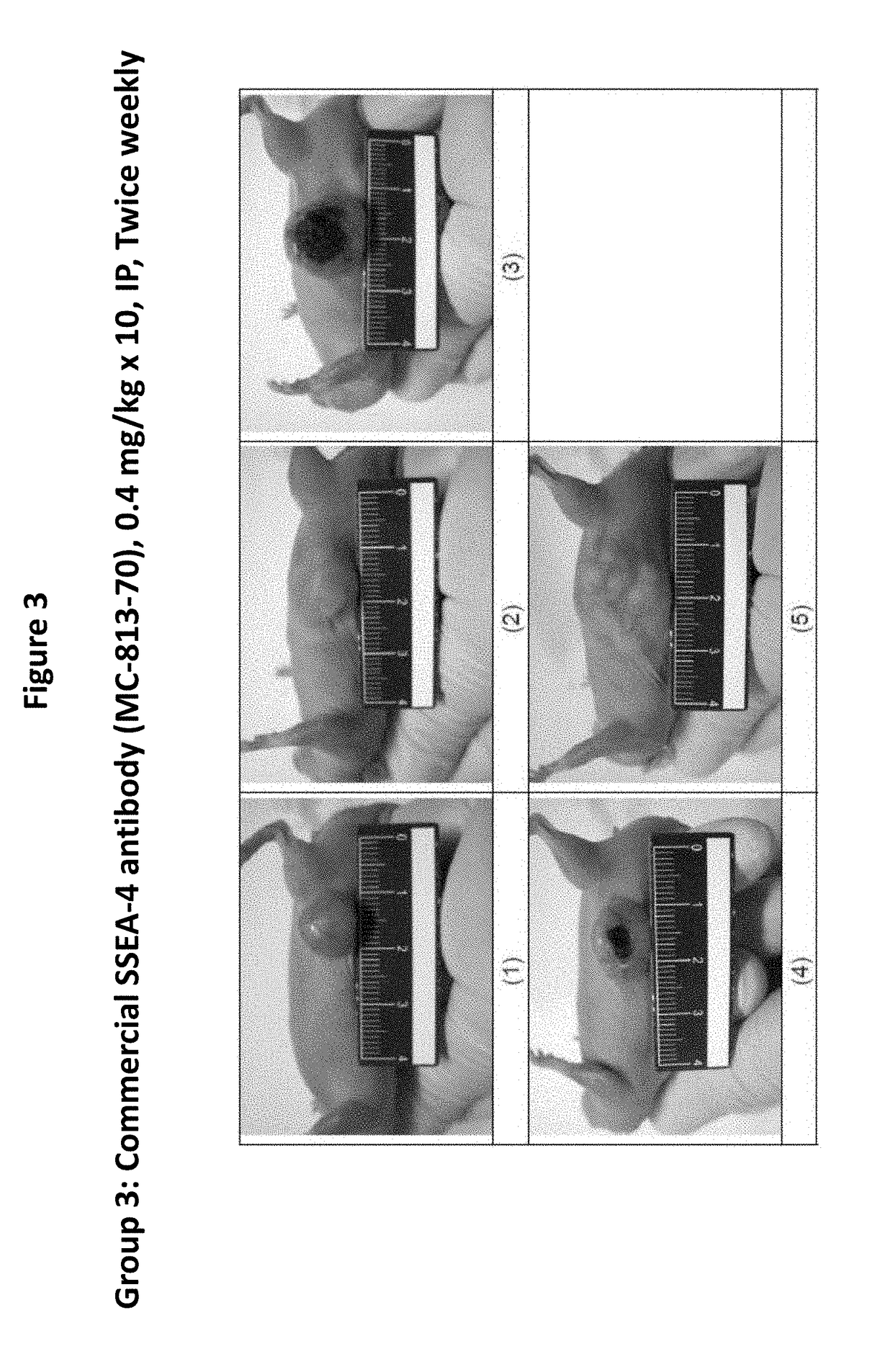
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
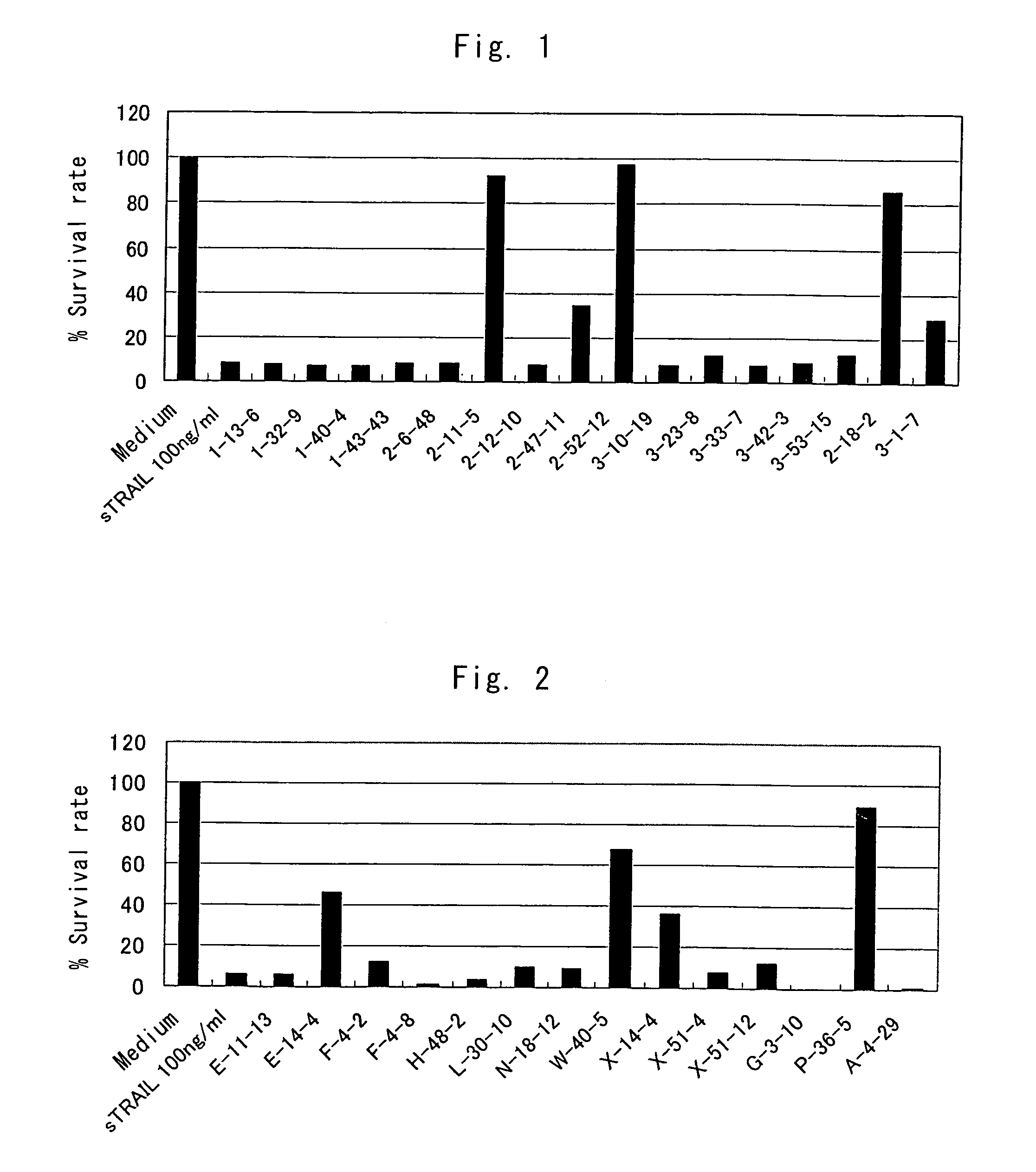
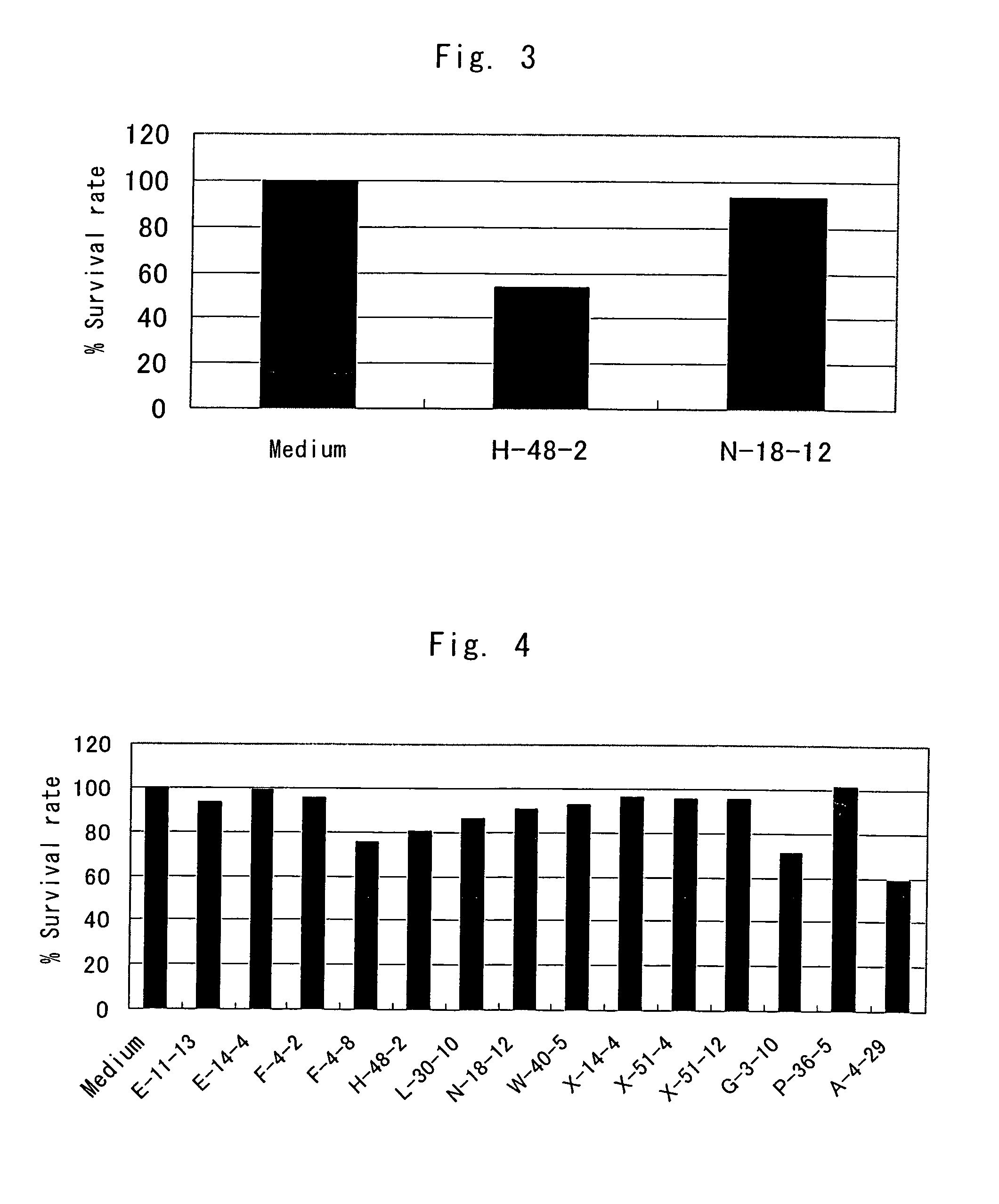
Anti-TRAIL-R antibodies
Anti-TRAIL-R1 and R2 antibodies or functional fragments thereof, having at least one property selected from the following (a) to (c) of:(a) having activity to induce apoptosis in carcinoma cells expressing TRAIL-R1 and / or TRAIL-R2;(b) not having effect on normal human cells expressing TRAIL-R1 and / or TRAIL-R2; and(c) not inducing human hepatocyte toxicity.
Owner:KIRIN BEER KABUSHIKI KAISHI KAISHA
Compositions and methods for treatment and detection of cancers
ActiveUS20150344551A1Shrink tumorEliminate the problemImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, lung, breast, mouse, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervix, ovary, and / or prostate cancer.
Owner:ACAD SINIC
Methods for detecting hepatocellular carcinoma
InactiveUS8357489B2Microbiological testing/measurementDisease diagnosisHepatocellular carcinomaMedicine
A method for evaluating hepatocellular carcinoma in a subject is provided. In certain embodiments, the method comprises: a) obtaining a hepatocellular carcinoma protein marker profile for a sample obtained from the subject; and b) comparing the protein marker profile to a control profile.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Method for diagnosing hepatocellular carcinomas
InactiveUS20070054849A1Relieve symptomsInhibit expressionCompound screeningApoptosis detectionHepatocellular carcinomaNormal cell
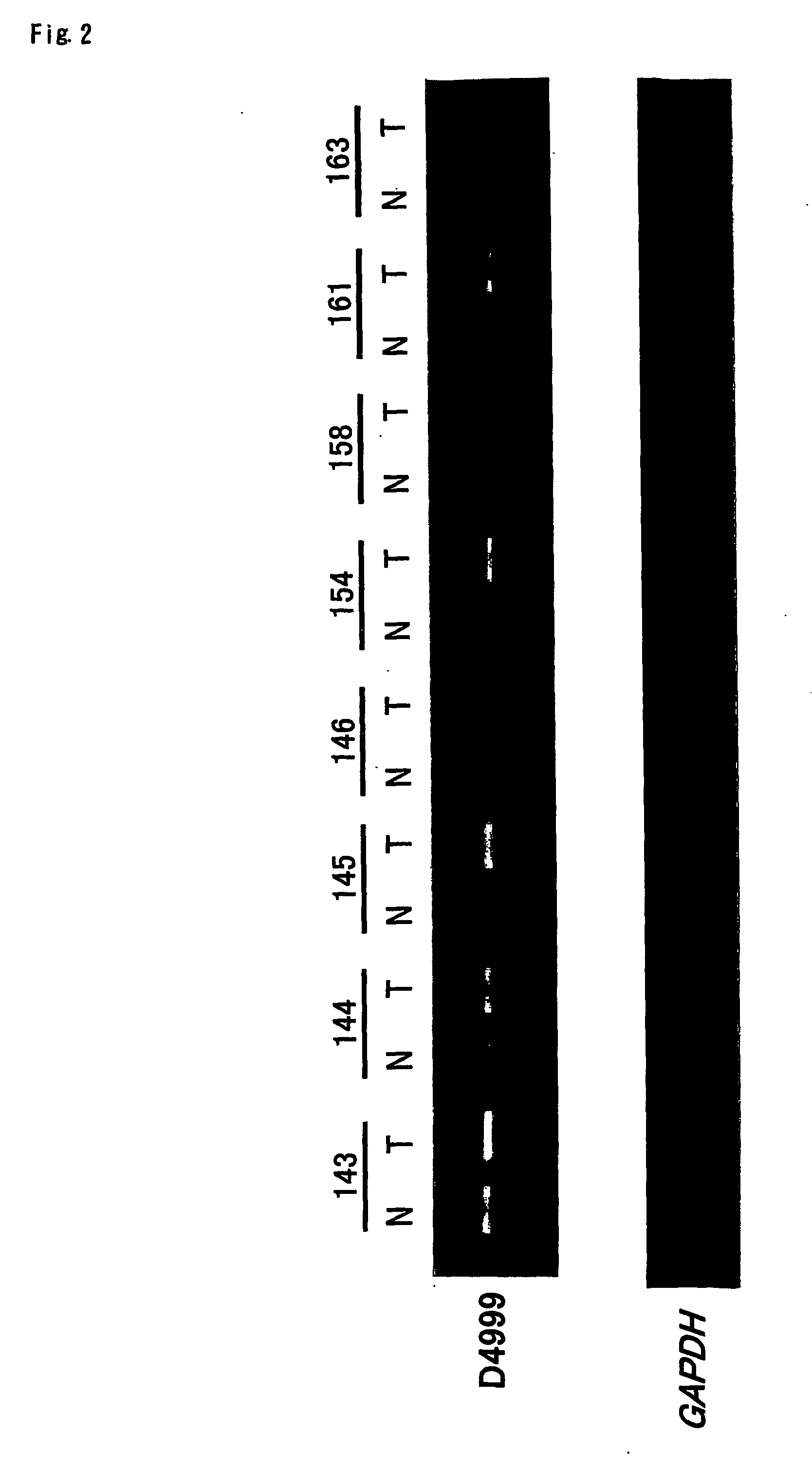
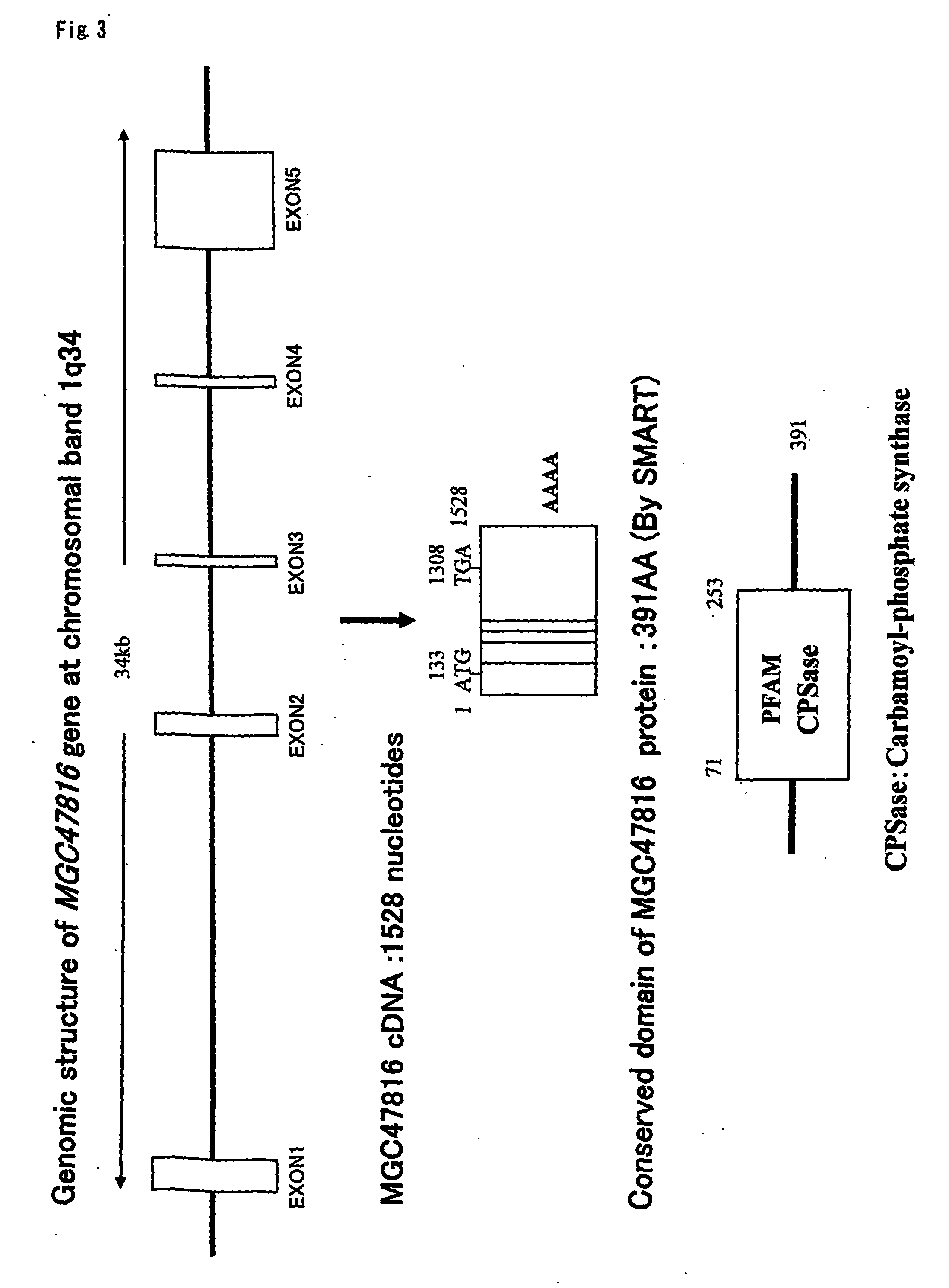
Objective methods for detecting, diagnosing, treating and preventing hepatocellular carcinoma (HCC) are described herein. In particular, the present invention relates to two genes up-regulated in HCC cells as compared to normal cells, referred to herein as MGC47816 and HES6. In one embodiment, the diagnostic method involves the determining the expression level of MGC47816 or HES6 that discriminate between hepatocellular carcinoma cells and normal cells. The present invention further provides methods of screening for therapeutic agents useful in the treatment of HCC, methods of treating HCC, and methods for vaccinating a subject against HCC.
Owner:ONCOTHERAPY SCI INC
Methods and kits for the detection of circulating tumor cells in pancreatic patients using polyspecific capture and cocktail detection reagents
InactiveUS20120094275A1Easy to detectSufficient binding capacityMicrobiological testing/measurementBiological testingAbnormal tissue growthAntigen
A highly sensitive assay is disclosed which combines immunomagnetic enrichment with multiparameter flow cytometric or image cytometry to detect, enumerate and characterize carcinoma cells in the blood. The present invention incorporates the conjugation of different antibodies to the same ferrofluid. This has the effect of making the ferrofluid polyspecific with respect to the antigens that the ferrofluid will bind. The multiple antibodies present on the same ferrofluid do not appear to block or otherwise interfere with each other. Such ferrofluids have the highly desirable effect of being able to bind specifically to more than one type of cell. The assay is especially useful to enable the capture of CTCs that have low EpCAM expression, but high expression of other tumor markers; Accordingly, the assay facilitates the biological characterization and staging of carcinoma cells.
Owner:JANSSEN DIAGNOSTICS LLC
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents
InactiveUS6127415APreventing and controlling photoinducedPreventing and controlling and chronologic agingBiocideCosmetic preparationsDiseaseAnticarcinogen
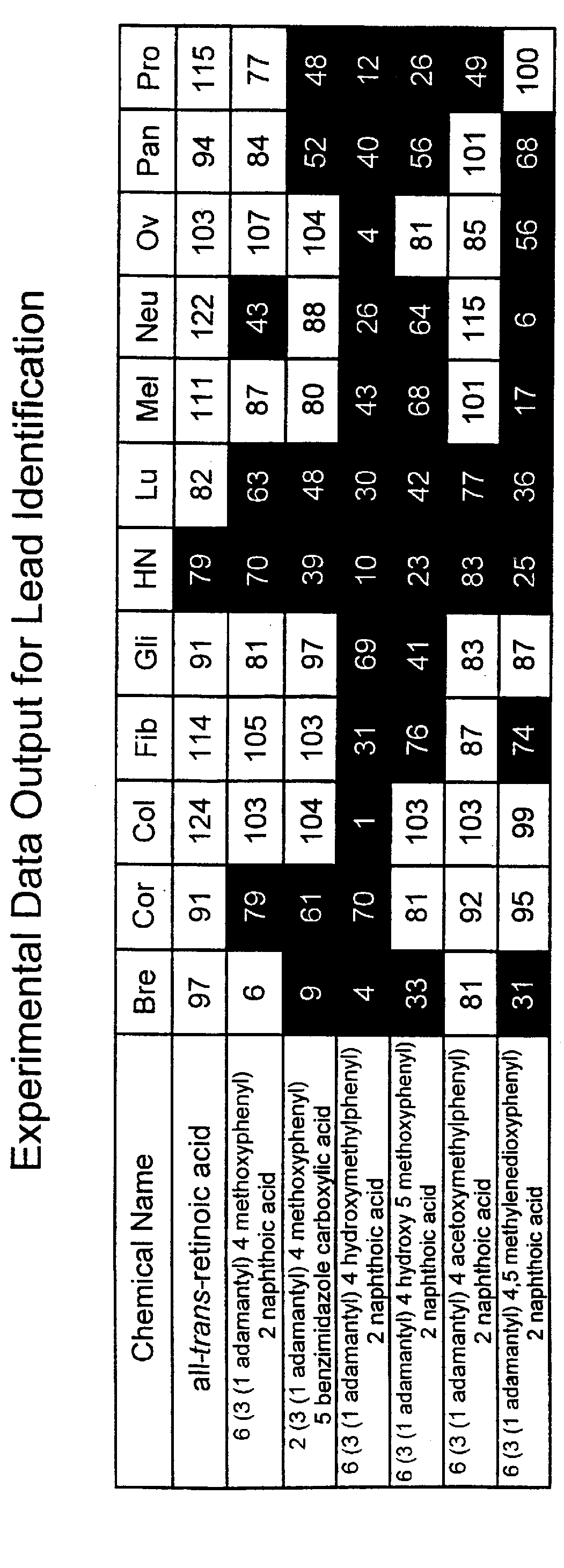
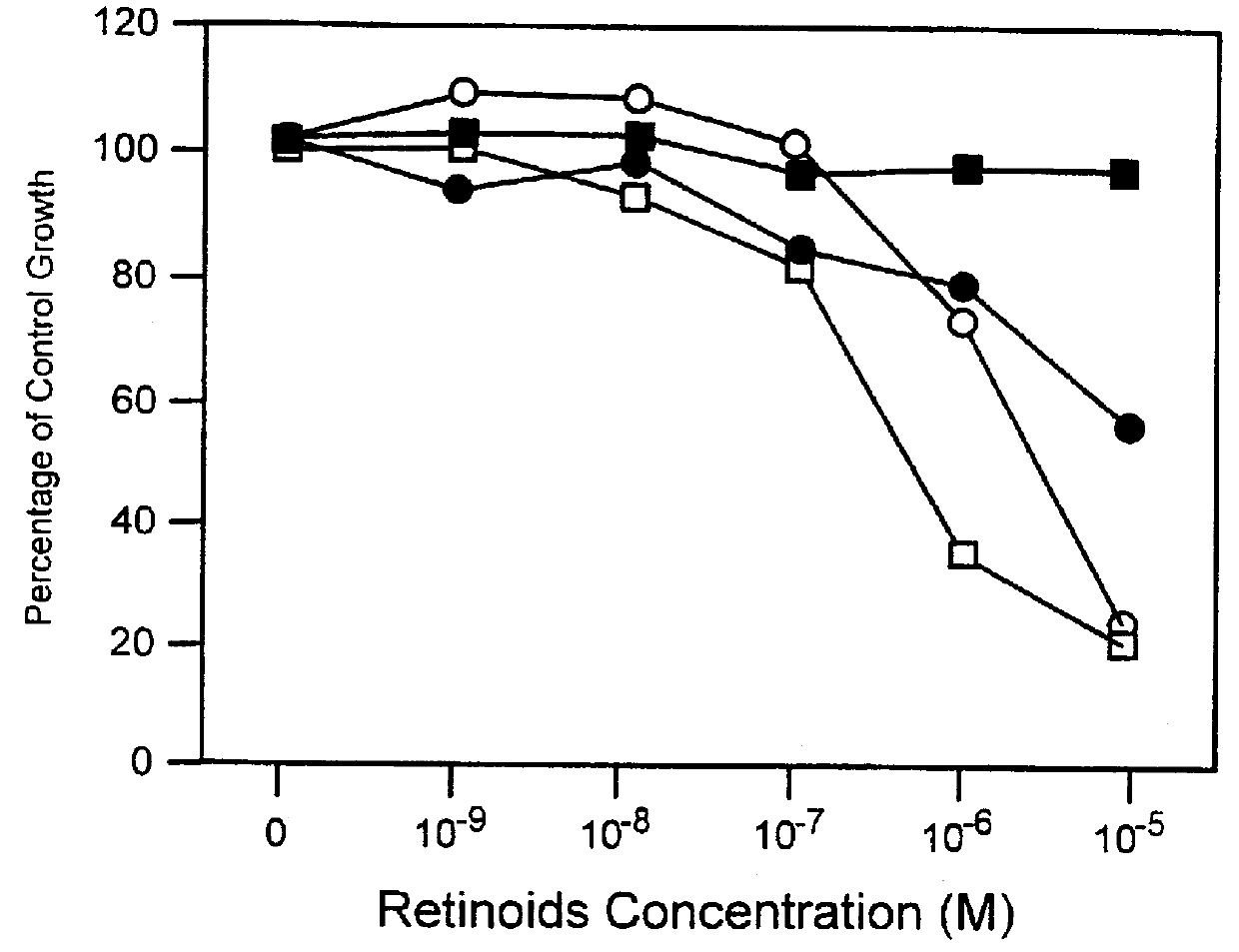
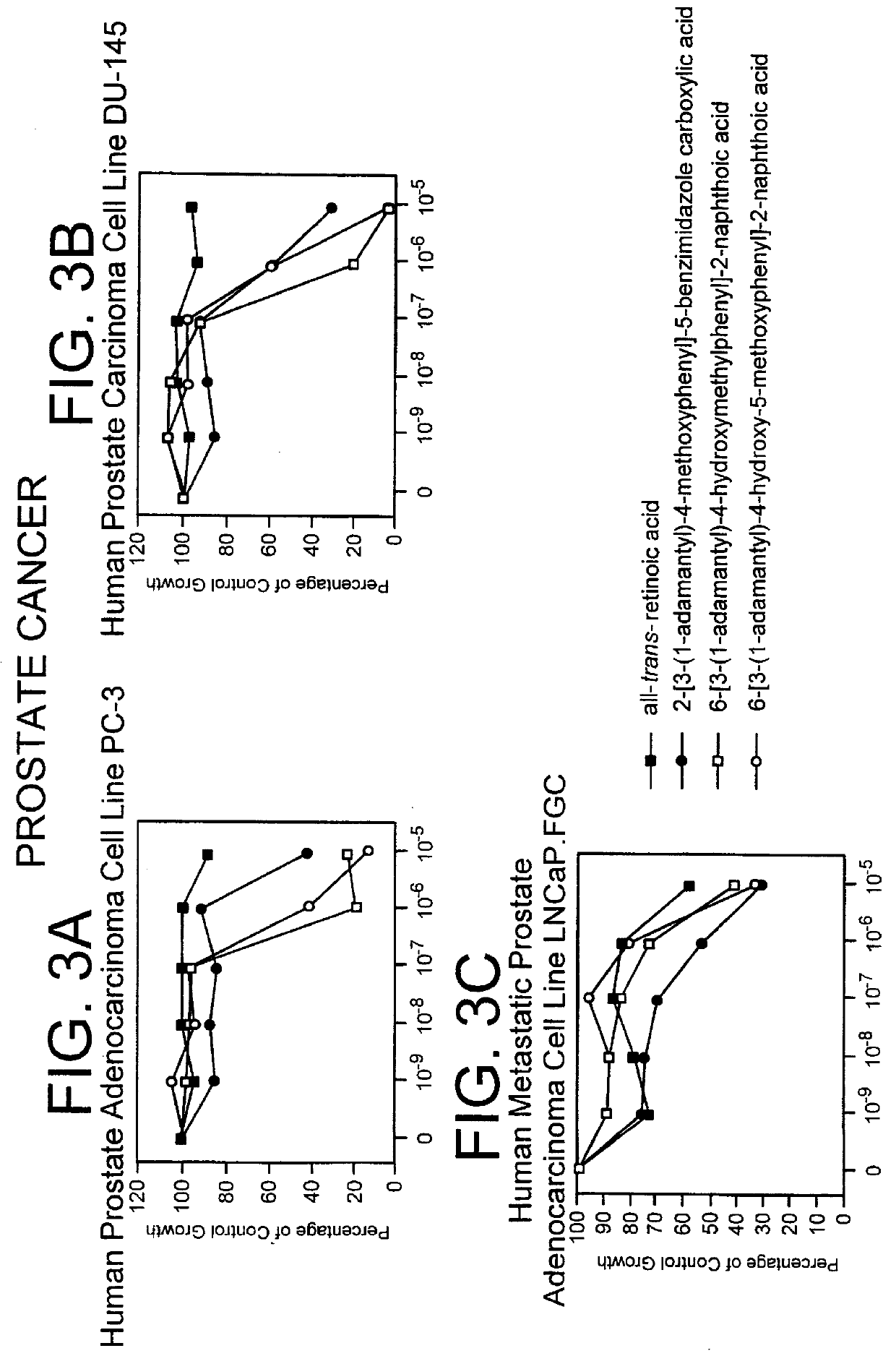
PCT No. PCT / US97 / 11564 Sec. 371 Date Apr. 14, 1999 Sec. 102(e) Date Apr. 14, 1999 PCT Filed Jul. 8, 1997 PCT Pub. No. WO98 / 01132 PCT Pub. Date Jan. 15, 1998The present invention relates to specific adamantyl or adamantyl group derivative containing retinoid compounds induce apoptosis of cancer cells. These adamantyl retinoid derivatives are useful for the treatment of many cancers and solid tumors, especially androgen-independent prostate cancer, skin cancer, pancreatic carcinomas, colon cancer, melanoma, ovarian cancer, liver cancer, small cell lung carcinoma, non-small cell lung carcinoma, cervical carcinoma, brain cancer, bladder cancer, breast cancer, neuroblastoma / glioblastoma, and leukemia. Also, the invention relates to novel adamantyl or adamantyl group derivative compounds which are useful as active agents for the treatment or prevention of keratinization disorders and other dermatological conditions, and other diseases.
Owner:GALDERMA RES & DEV SNC
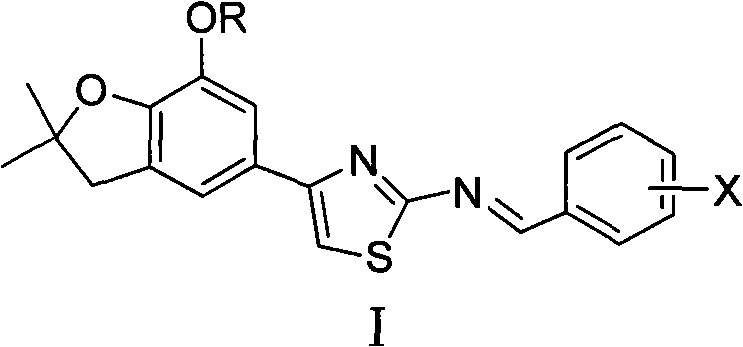
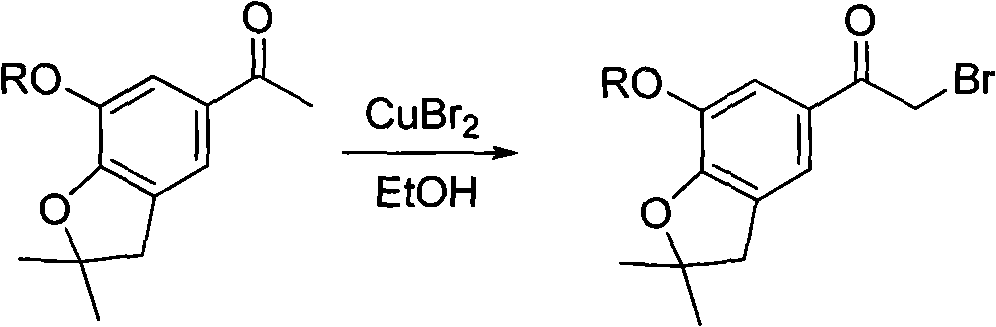
4-(benzofuran-5-yl)-2-benzal aminothiazole and application of 4-(benzofuran-5-base)-2-benzal aminothiazole as antineoplastic agent
The invention discloses 4-(benzofuran-5-yl)-2-benzal aminothiazole as shown in a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-benzal aminothiazole is as follows: 1-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl) butanone is subject to bromination and reacts with thiourea to obtain 4-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-2-aminothiazole which reacts with aromatic aldehyde to prepare the 4-(benzofuran-5-yl)-2-benzal aminothiazole. The 4-(benzofuran-5-yl)-2-benzal aminothiazole has good activity inhabiting activity on Hela cells, human liver cancer cells (Bel 7402 cells) and lung carcinoma cells (A549 cells) and can be used for preparing the antineoplastic agent.
Owner:HUNAN UNIV
Methods for Determining Heptocellular Carcinoma Subtype and Detecting Hepatic Cancer Stem Cells
InactiveUS20100197770A1Increased and decreased expressionOrganic active ingredientsElectrolysis componentsBiomarker (petroleum)Hepatic Cancers
The invention provides a method of determining an HCC subtype in a subject comprising a) obtaining a sample from the subject, b) assaying the sample to detect the expression of 1 or more biomarkers, and c) correlating the expression of the biomarkers with an HCC subtype in a subject. The invention further provides methods of detecting HCC stem cells in a sample. Additionally, the invention provides methods and compositions for treating subjects with HCC that take advantage of the biomarkers associated with HCC stem cells.
Owner:THE OHIO STATE UNIV RES FOUND +2
Quinoline derivatives as Anti-cancer agents
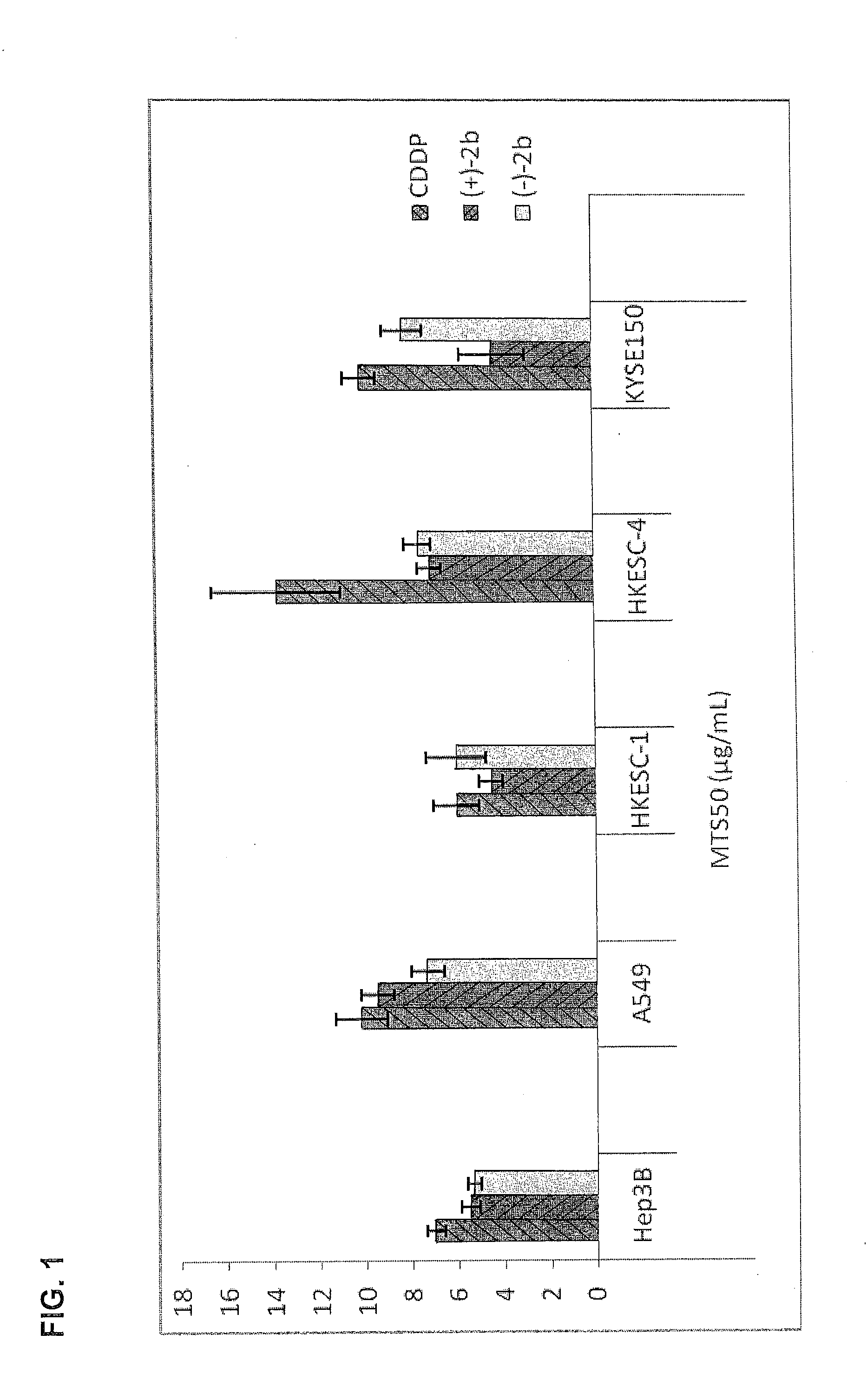
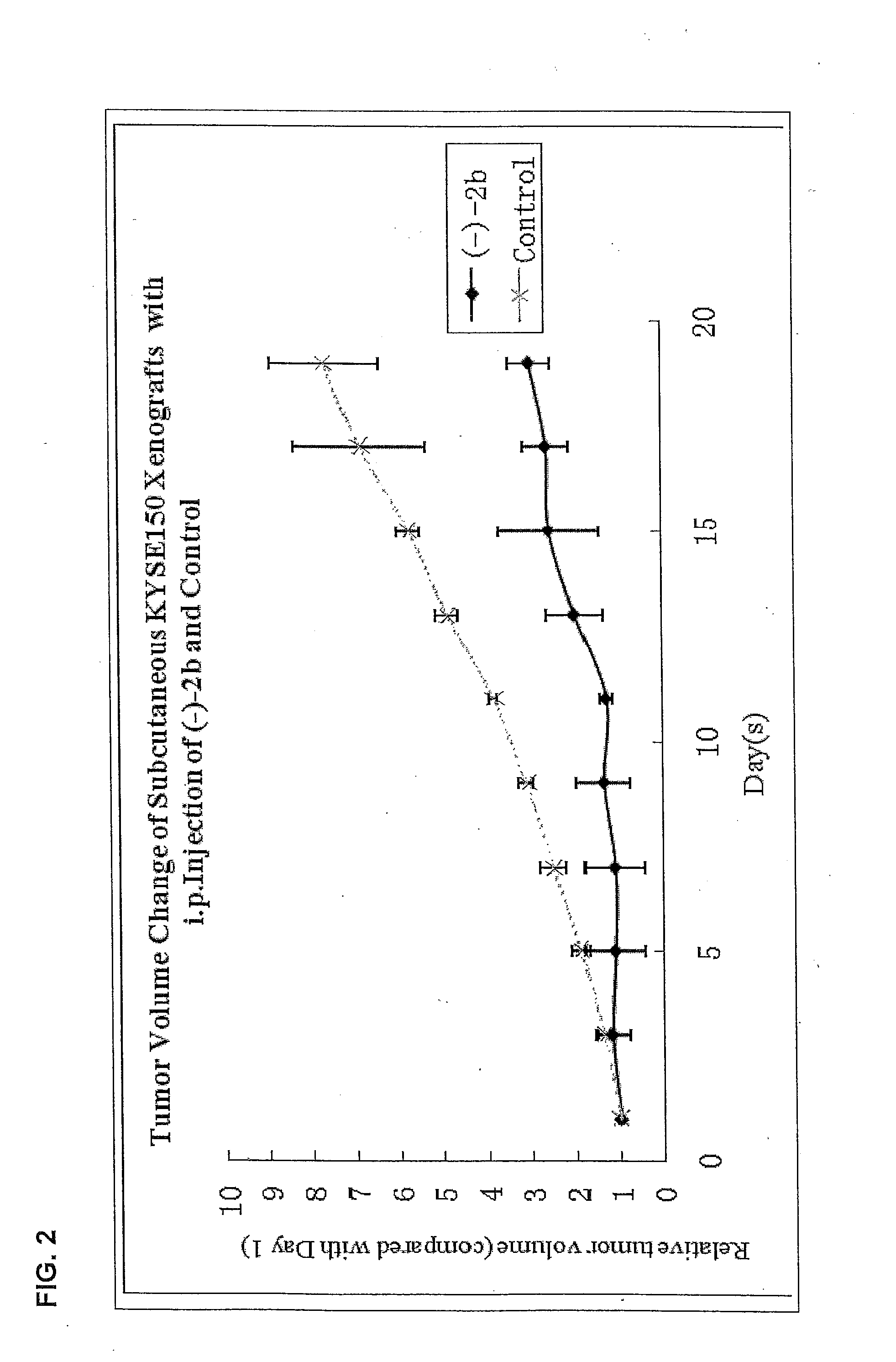
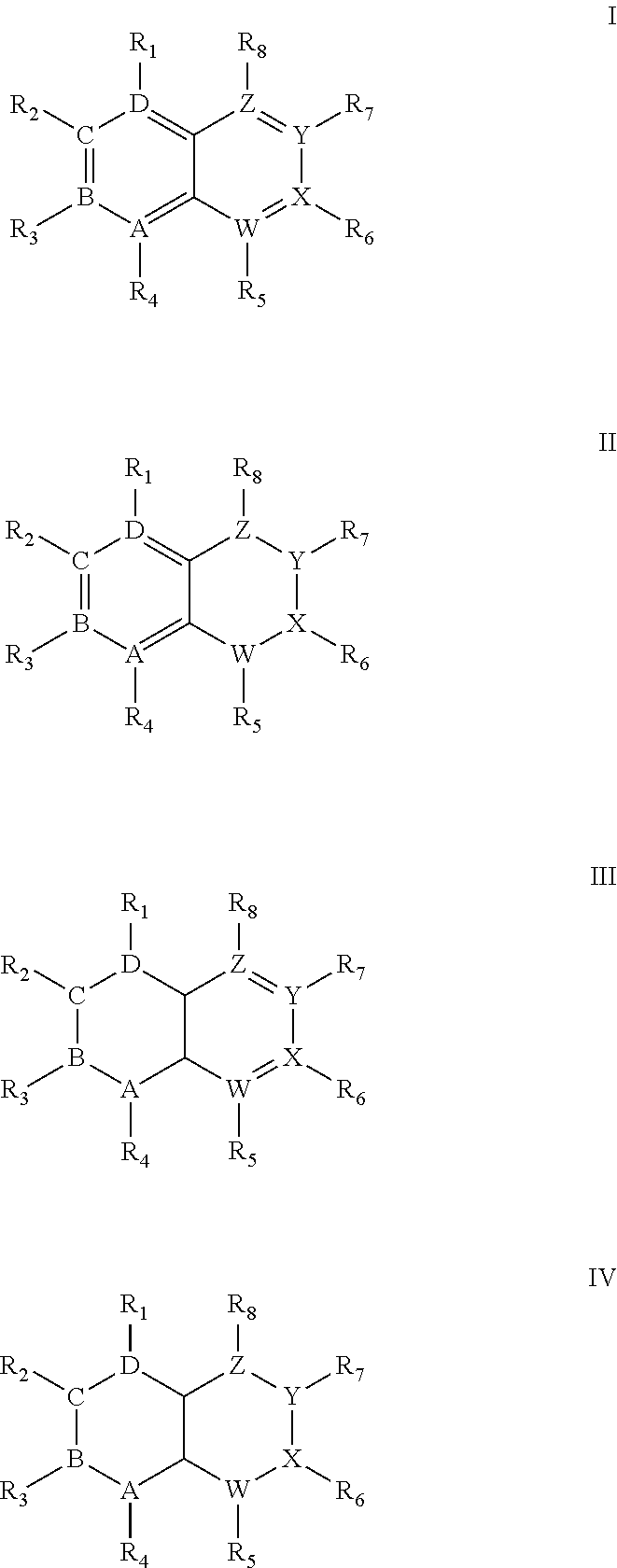
Quinoline derivatives showing anticancer activities against cancer cell lines of hepatocellular carcinoma (Hep3B), lung carcinoma (A549), esophageal squamous cell carcinoma (HKESC-1, HKESC-4 and KYSE150). The quinoline derivatives have a backbone structure of the following formulas:
Owner:THE HONG KONG POLYTECHNIC UNIV
Compositions and methods for treatment and detection of cancers
ActiveUS20160289340A1Useful in treatmentEnhanced ADCC activityImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
American cockroaches effective fraction for anti-tumor prepared by macroporous adsorption resin and use
ActiveCN101214262AAnthropod material medical ingredientsPharmaceutical delivery mechanismSolventChemistry
The present invention relates to a periplaneta americana extract effective part with cytotoxic activity, a preparation method and a medical purpose thereof. The periplaneta americana extract effective part of the present invention is refined from fresh or dry blattidae periplaneta americana body which is extracted by alcohol water, processed for macroporous resin column chromatography and is eluted by alcohol solvent. The periplaneta americana extract effective part obtained by the present invention has obvious cytotoxicity and internal anticancer efficacy towards oral epidermoid carcinoma (KB) cell, poorly differentiated gastric abenocarcinoma tumour (BGC-823) cell, human chronic myelogonium leukaemia cancer (K562) cell and human myelogonium leukaemia cancer cell (HL-60) and can be used for preparing for a drug and a health care product for remedying the tumours.
Owner:KUNMING SINOWAY NATURAL PHARMA
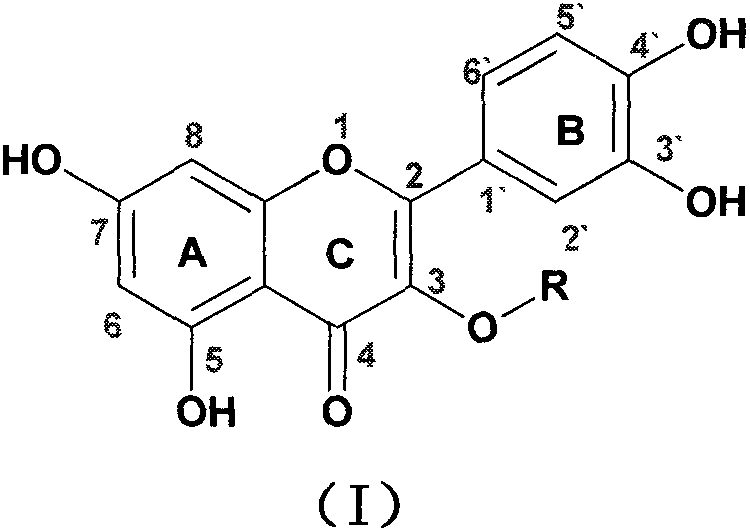
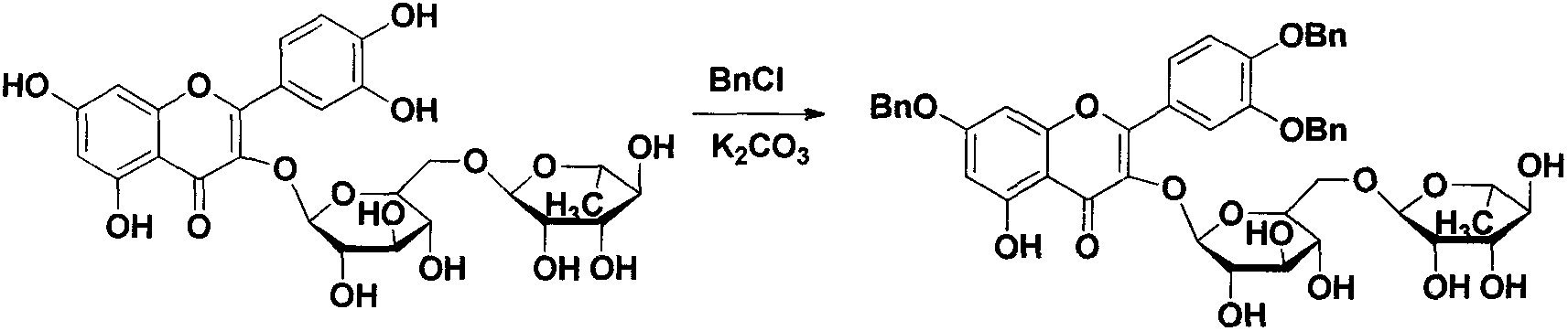
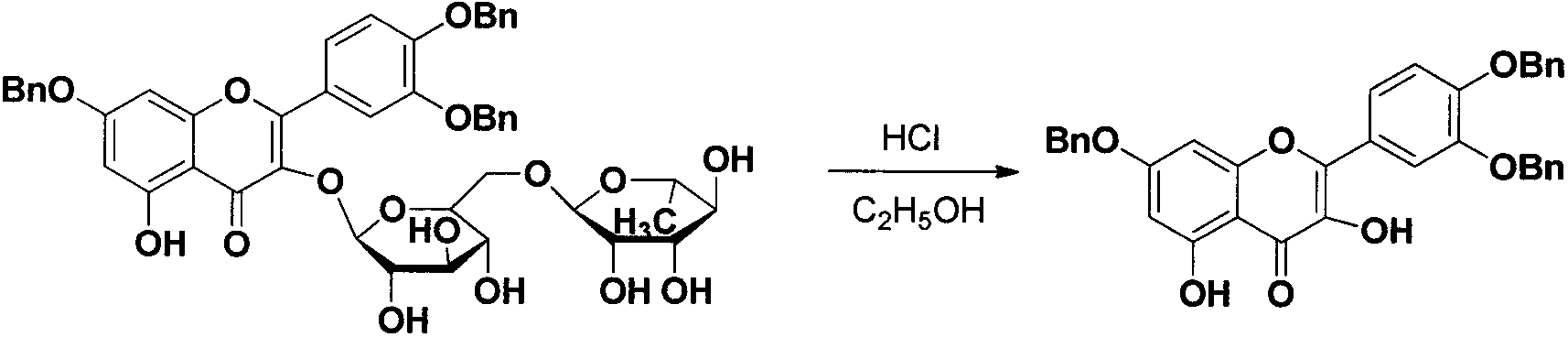
Quercetin-3-O-acyl ester and preparation method thereof
InactiveCN102659735AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryRutinHydrolysis
The invention relates to quercetin-3-O-acyl ester and a preparation method thereof and belongs to the technical field of pharmaceutical chemistry. The preparation method comprises the following steps: taking cheap rutin as a starting raw material and preparing a quercetin-3-O-acyl ester compound through benzylation, hydrolysis under the acidic conditions, esterification, catalytic hydrogenolysis and other reactions. The method has the characteristics of good selectivity, mild reaction condition, high yield, low cost, simplicity and convenience in operation, easiness in industrial production and the like. In addition, the inhibitory effect of the obtained quercetin-3-O-acyl ester on human esophageal squamous carcinoma cells EC9706, human prostatic cancer cells PC-3 and human gastric cancer cells MGC-803 is remarkably superior to that of quercetin; and the quercetin-3-O-acyl ester can be used in the field of foods and medicines and is possible to develop into a new candidate drug for treating tumor.
Owner:ZHENGZHOU UNIV
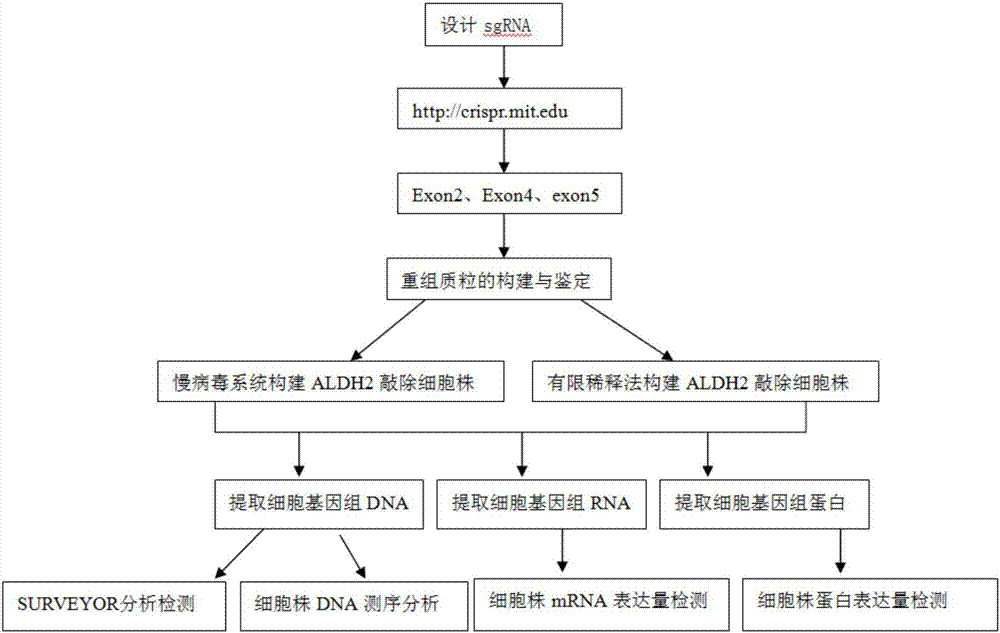
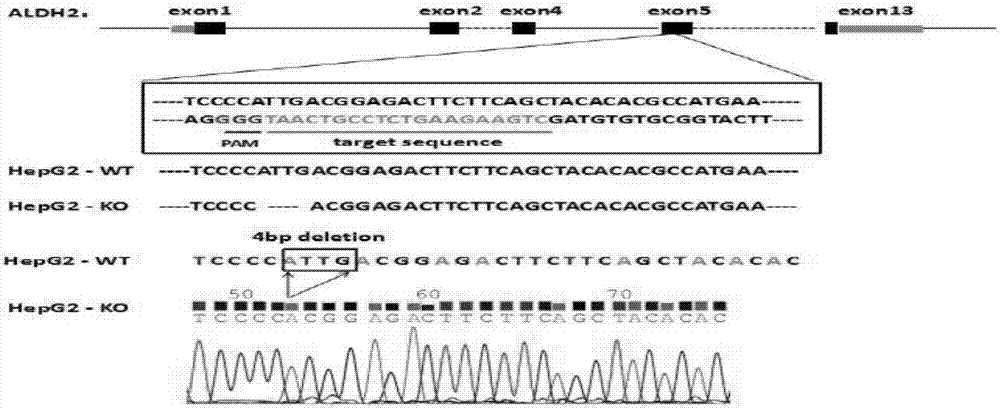
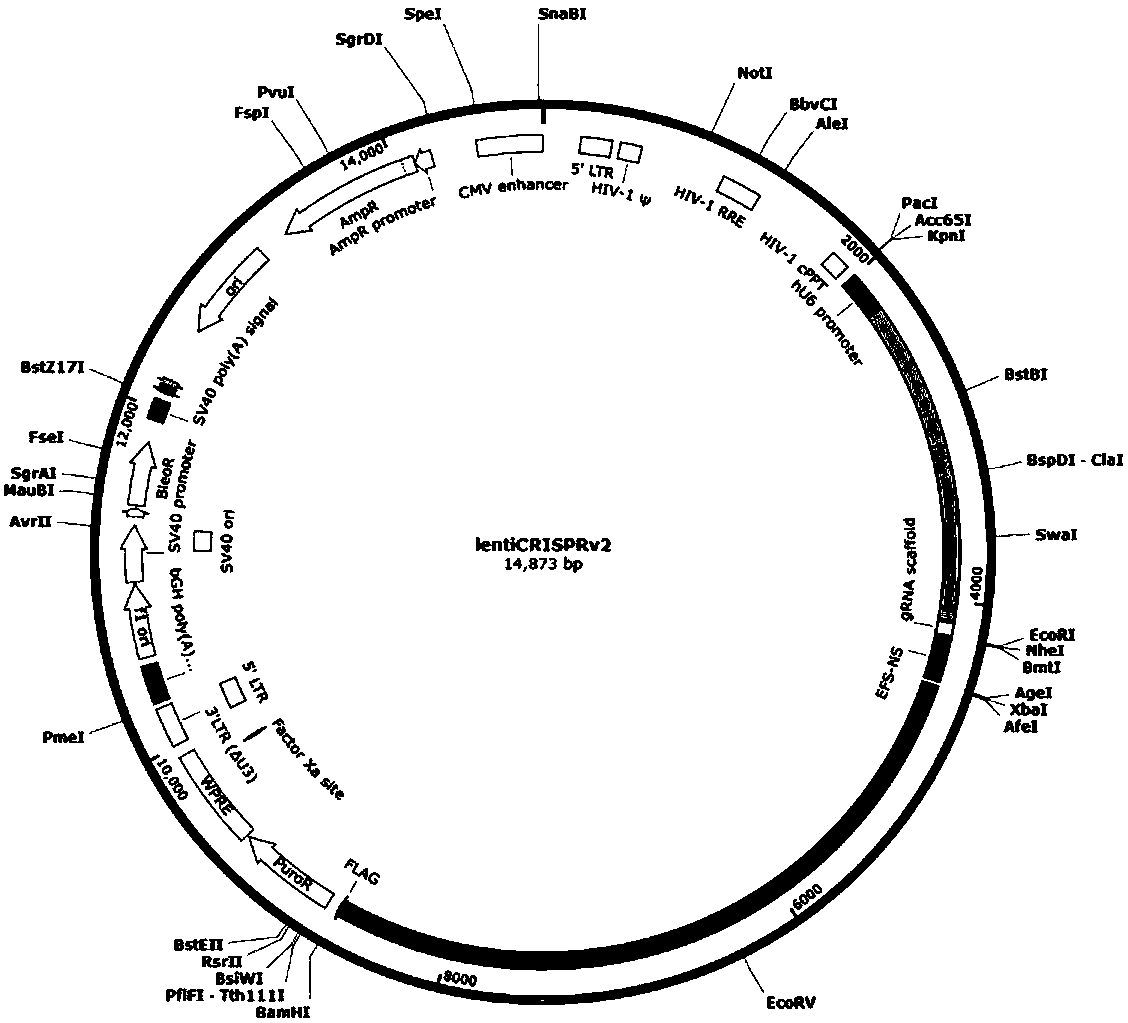
Construction method and application of sg RNA and ALDH2 gene deletion cell strains used for knocking out human ALDH2 gene
ActiveCN107502608AAchieve silencingImproved silence is not completeHydrolasesStable introduction of DNAIn vivoWilms' tumor
The invention provides an sg RNA sequence used for knocking out a human ALDH2 gene;,a target DNA sequence of the sg RNA is at least one of sequences as shown in SEQ ID NO:1, SEQ ID NO:2 and SEQ ID NO:3. The invention further provides a method for knocking out a human hepatoma carcinoma cell ALDH2 gene; the method utilizes a CRISPR / Cas system to modify the ALDH2 gene in a human hepatoma carcinoma cell. The invention further provides two ALDH2 gene deletion cell strains; ALDH2 participates in an important metabolic function of a body. The ALDH2 gene deletion cell strains provided by the invention provide an effective platform for metabolism study, in vivo, of exogenous chemicals or exogenous poisons, so that powerful means are provided for researching chronic diseases (such as alcoholic liver diseases and diabetes) as well as tumor-associated diseases.
Owner:SUN YAT SEN UNIV
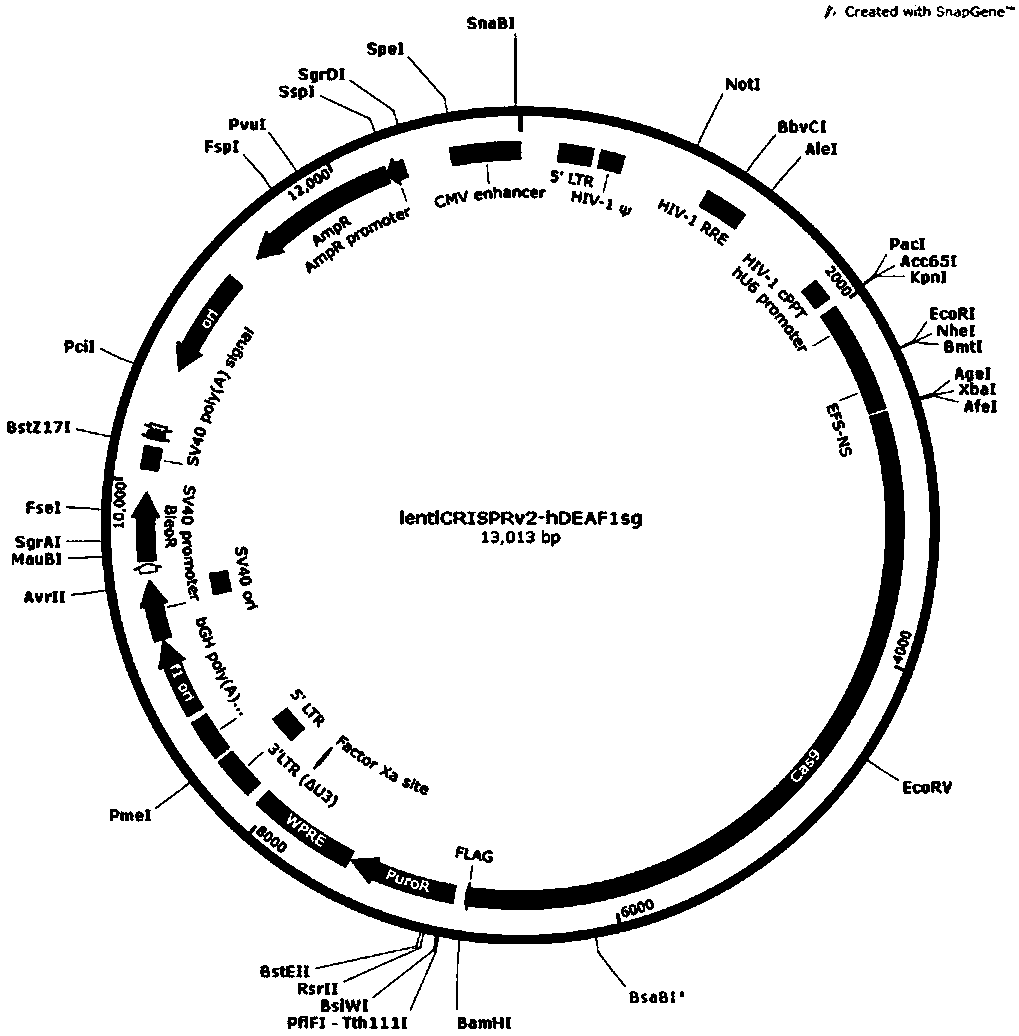
CRISPR-Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and specific sgRNA thereof
InactiveCN108396027AConvenient researchGenetically modified cellsStable introduction of DNAVector systemLentivirus
The invention discloses a CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and specific sgRNA thereof. The CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and the specific sgRNA thereof are characterized in that: firstly, sgRNA of a second exon of the specific targeted DEAF1 gene is obtained and the base sequence of the sgRNA is shown as SEQ ID NO.1; secondly, the sgRNA of the DEAF1 gene is constructed into a lentiviral vector system, which contains Cas9 protein; finally, the CRISPR / Cas9 lentivirus containing the sgRNA is infected with human colorectal carcinoma cell HT-29 cell, so that a cell strain of which DEAF1 protein expression level is obviously reduced is obtained. The CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene disclosed by the invention has the advantages of simple operation steps, good sgRNA target ability and high cutting efficiency for the DEAF1 gene; in addition, the constructed CRISPR / Cas9 lentiviral vector system has the advantage of high knockout efficiency and can specifically knock out the DEAF1 gene to obtain the human colorectal carcinoma cells knocking out the DEAF1 gene, and therebya powerful tool is provided for further studying an action mechanism of DEAF1 in the colorectal carcinoma cells.
Owner:OBIO TECH SHANGHAI CORP LTD
Replicating viral vectors for gene therapy
InactiveUS20120283318A1MaintenanceAntibacterial agentsOrganic active ingredientsGene deliveryHeterologous
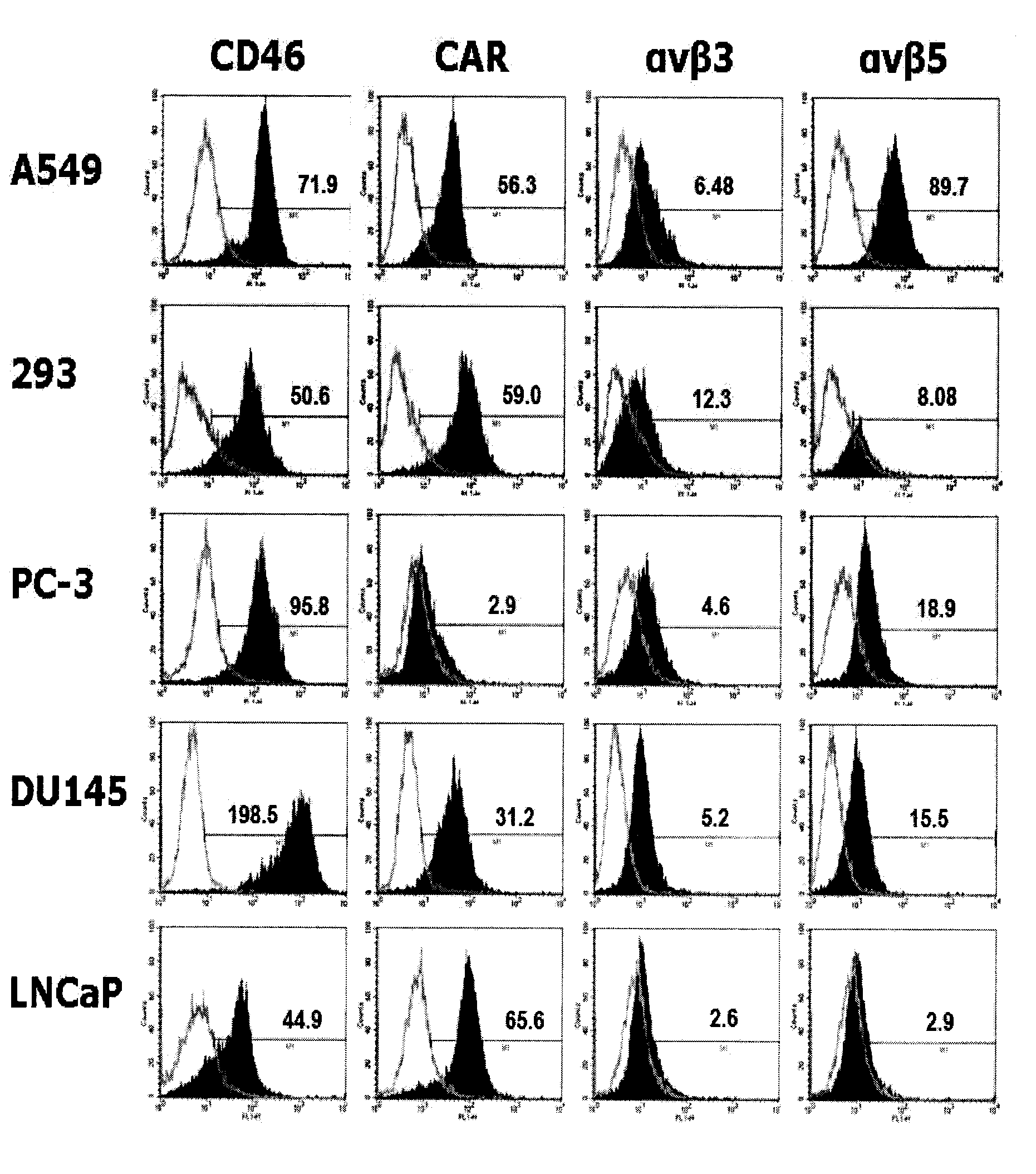
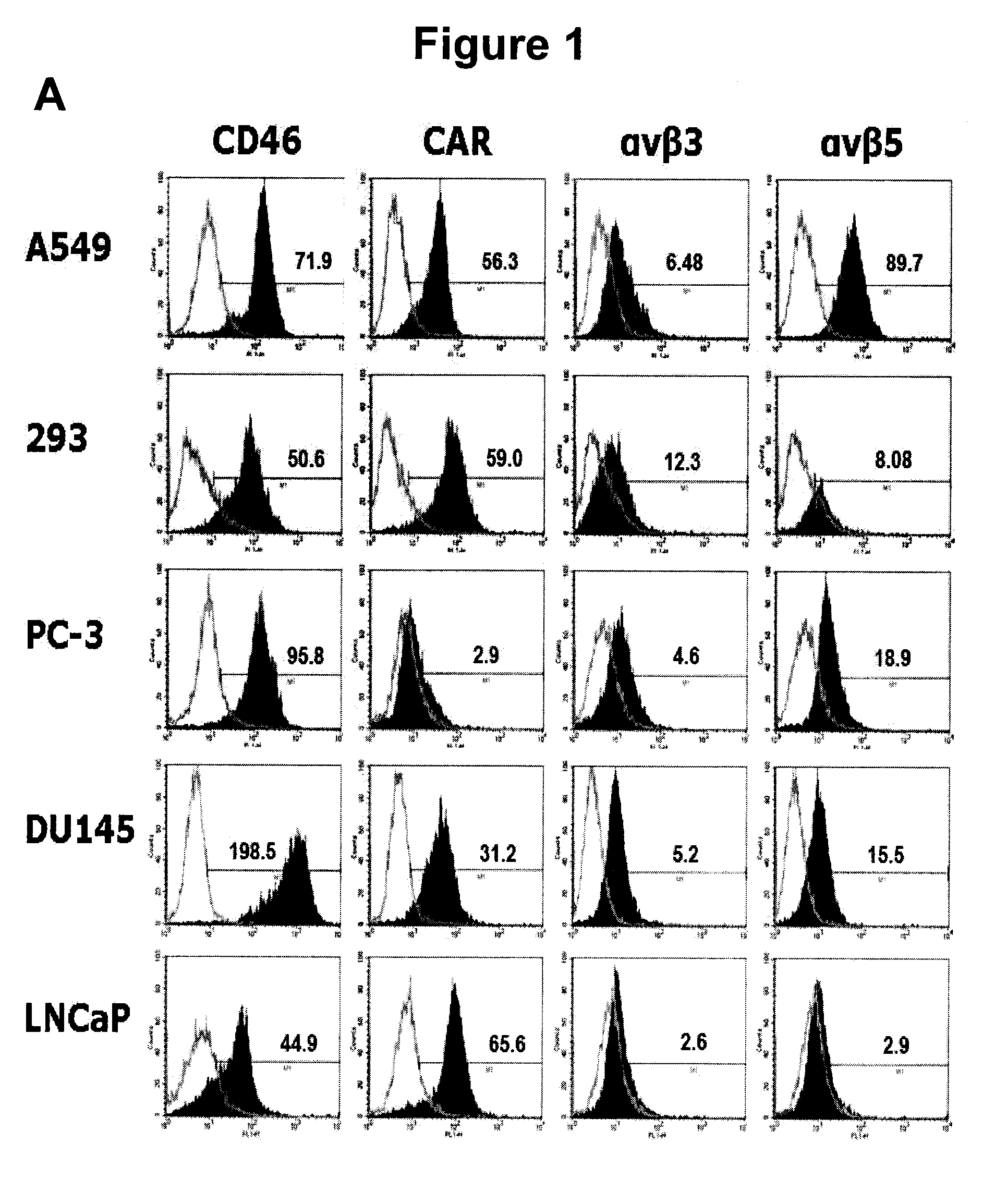
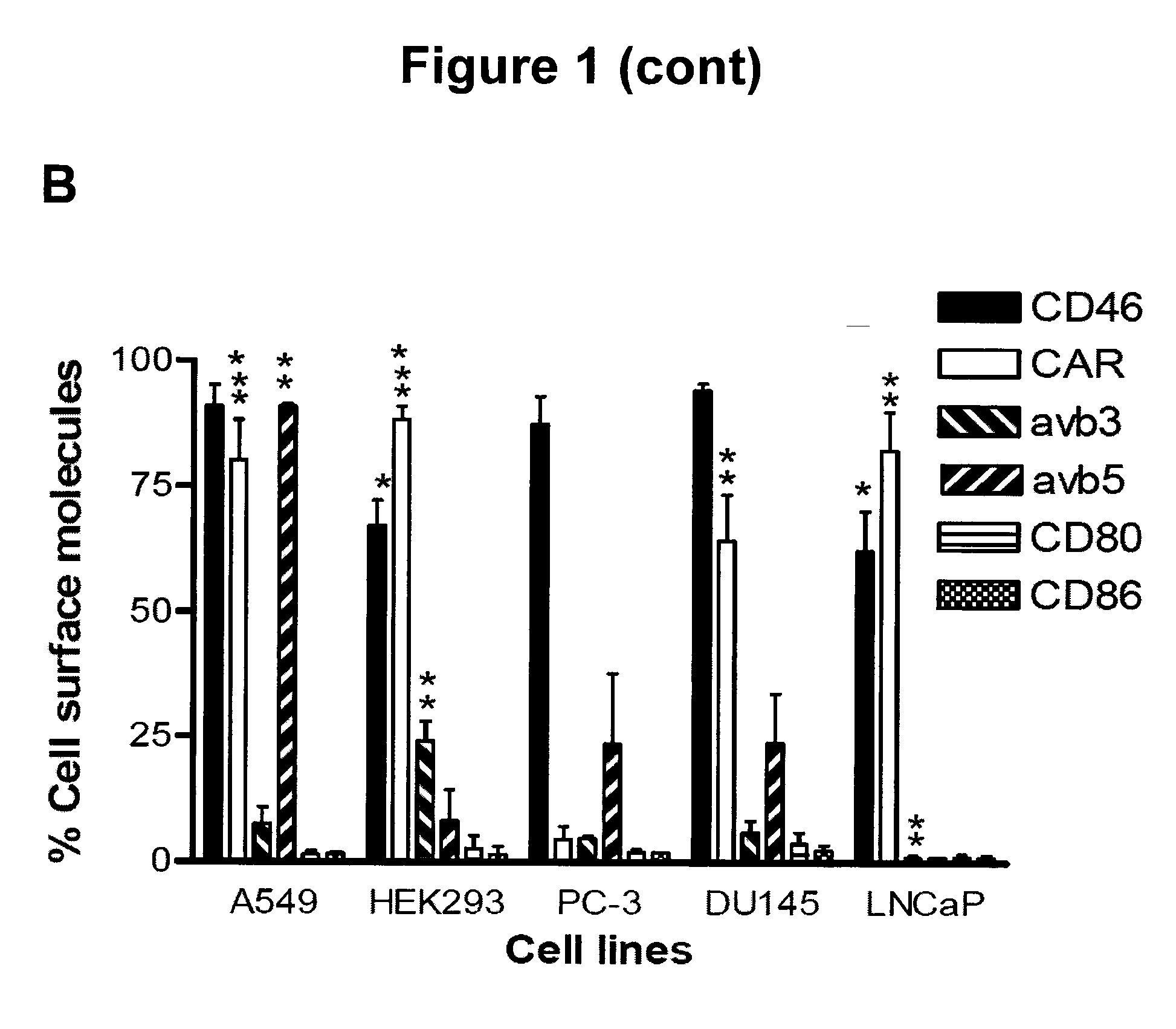
The present invention concerns the field of gene therapy and in particular the use of specific adenoviral vector systems for gene therapy, said vector systems offering enhanced efficiency and specificity for gene delivery. More specifically, the present invention provides replicating-competent adenoviral vector systems carrying one or more inserted heterologous gene. The adenoviral vectors system according to the invention are characterized by high binding efficiency and infectivity to cells of neural origin, endothelial cells, carcinoma cells and dendritic cells.
Owner:MEI YA FANG +1
Novel antibodies reactive with human carcinomas
InactiveUS20040043029A1Low immunogenicityImprove antibody based therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsAntibody fragmentsAntibody conjugate
The present invention relates to novel antibodies, antibody fragments and antibody conjugates and single-chain immunotoxins reactive with human carcinoma cells. More particularly, the antibodies, conjugates and single-chain immunotoxins of the invention include: a murine monoclonal antibody, BR96; a human / murine chimeric antibody, ChiBR96; a F(ab')2 fragment of BR96; ChiBR96-PE, ChiBR96-LysPE40, ChiBR96 F(ab')2-LysPE40 and ChiBR96 Fab'-LysPE40 conjugates and recombinant BR96 sFv-PE40 immunotoxin. These molecules are reactive with a cell membrane antigen on the surface of human carcinomas. The BR96 antibody and its functional equivalents, displays a high degree of selectivity for carcinoma cells and possess the ability to mediate antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity activity. In addition, the antibodies of the invention internalize within the carcinoma cells to which they bind and are therefore particularly useful for therapeutic applications, for example, as the antibody component of antibody-drug or antibody-toxin conjugates. The antibodies also have a unique feature in that they are cytotoxic when used in the unmodified form, at specified concentrations.
Owner:BRISTOL MYERS SQUIBB CO
A macrocyclic oxidation substituted pentacyclic triterpanoids derivative and preparation method and use thereof
InactiveCN101117349AStrong inhibitory activityOrganic active ingredientsMetabolism disorderAlgluceraseAnti-Tumor Drugs
The present invention relates to a pentacyclic triterpanoid derivative of multiple-oxide substitution of the A ring and the medicine salt or solvate of the derivative, and the present invention also relates to the preparation method, the drug combination, and medical use of the derivative. The compound of the present invention has the functions of inhibiting the activity of six human tumor cell strains in vitro, such as human prostate cancer cell (PC-3), nasopharyngeal carcinoma cells (CNE), oral squamous carcinoma cell(KB), human lung cancer cell (A549), human hepatoma cell (BEL-7404), and human cervix cancer cell (Hela), and the function of the invention is at the same magnitude of the positive control of cisplatin, thereby the compound can be used as expected antitumor drug. The compound of the present invention also inhibits the alpha glucosidase strongly, and the inhibiting effect is greater than the positive control of acarbose, thereby the compound can be used as expected medicine for preventing and treating diabetes and the treatment of the virus diseases.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Methods of treating cancer
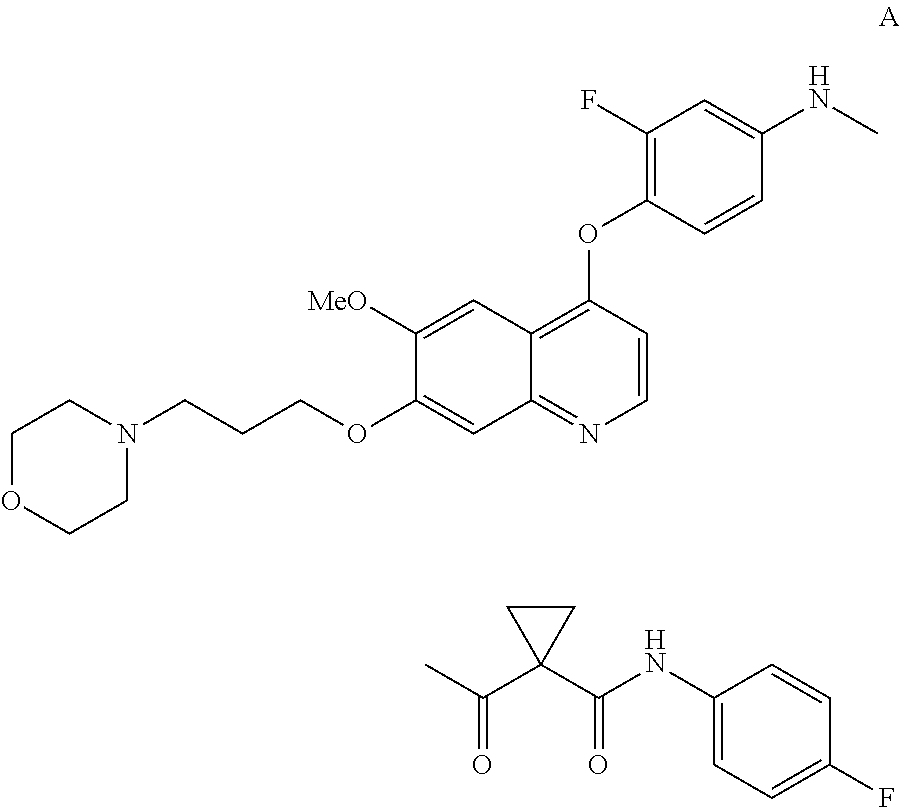
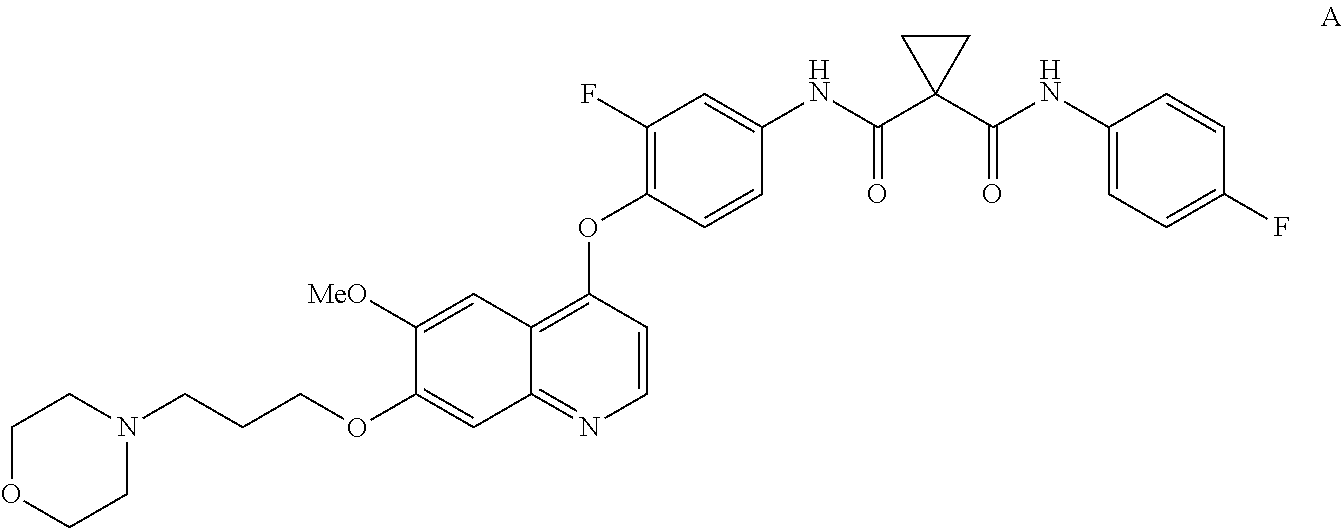
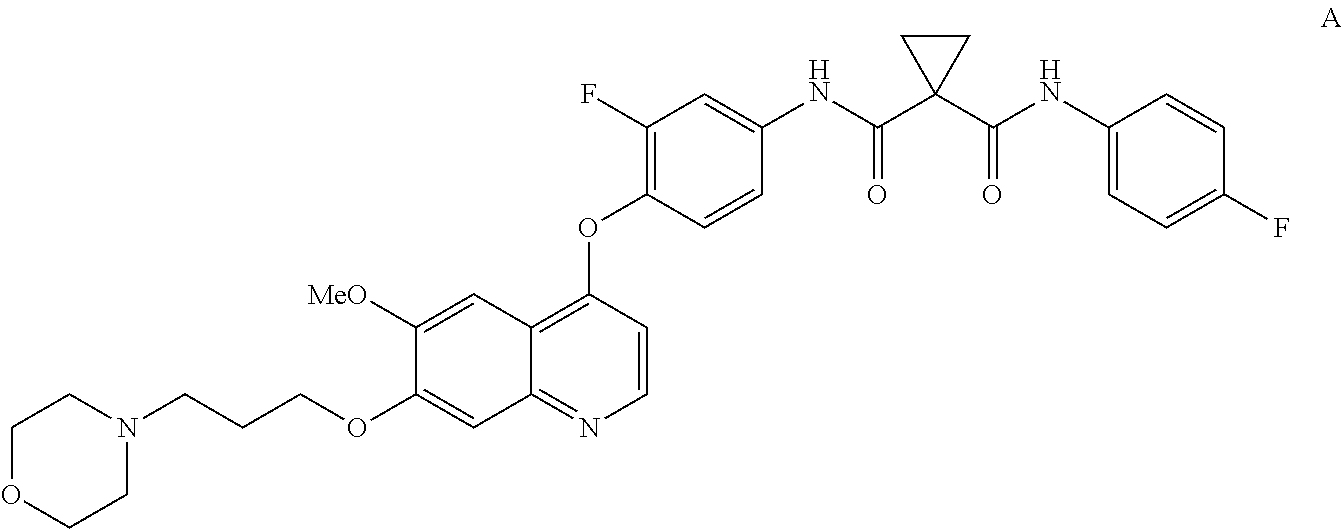
InactiveUS20130252956A1Organic active ingredientsAntineoplastic agentsCompound aHepatocellular carcinoma
Methods for treating a human having heptocellular carcinoma comprising administering to the human a therapeutically effective amount of Compound A:or a pharmaceutically acceptable salt thereof, wherein the human has a complete response or partial response.
Owner:EXELIXIS INC +1
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
Preparation method for chlorella polypeptide microcapsule
InactiveCN102847134APromote development and utilizationHas inhibitory effectHydrolysed protein ingredientsDigestive systemUltrafiltrationIon exchange
The invention provides a preparation method for a chlorella polypeptide microcapsule. The method comprises the following steps: extracting chlorella protein by using a low temperature ultrahigh pressure continuous flow cell crusher; hydrolyzing the chlorella protein with papain; filtering the hydrolyzed chlorella protein with an ultrafiltration centrifuge tube; then carrying out separation and purification by using ion exchange chromatography DEAE-52 and gel chromatography dextrangel G-25 columns; and finally utilizing complex coacervation to prepare the chlorella polypeptide microcapsule. The chlorella polypeptide microcapsule prepared in the invention has antitumor activity, e.g., the chlorella polypeptide microcapsule has inhibitory effects on in vitro growth of human hepatoma carcinoma cells HepG2 and the inhibition rate of the chlorella polypeptide microcapsule reaches 38% when concentration is 400 mu g / mL. Therefore, the chlorella polypeptide microcapsule prepared in the invention is beneficial for development and utilization of antitumor health food and medicinal products.
Owner:SOUTH CHINA UNIV OF TECH
Novel anti-tumor application of penicillium enol A1 from penicillium citrinum
InactiveCN103865808AGood antitumor activityOrganic active ingredientsFungiHuman gastric carcinomaPenicillium cainii
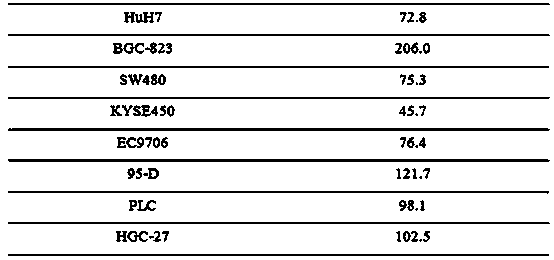
The invention relates to novel anti-tumor application of an alkaloid compound penicillium enol A1 from penicillium citrinum. The penicillium citrinum IBPT-5 is collected in the China Center for Type Culture Collection (CCTCC), which is located in Wuhan University and has the collection number of CCTCC NO:M2013713, on December 25, 2013. Experiments prove that the compound has better anti-tumor activity on various tumor cells, and can be used for preparing cell proliferation inhibition medicines or anti-tumor medicines for anti-tumor study, wherein tumor cells comprise human colon cancer cells SW620, human hepatoma cells Huh7, human gastric carcinoma cells BGC-823, human colon cancer cells SW480, human esophageal squamous carcinoma cells KYSE450, human esophageal cancer cells EC9706, human highly metastatic lung carcinoma cells 95-D, human hepatoma cells PLC and human gastric carcinoma cells HGC-27.
Owner:FUZHOU UNIV
2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose and preparation method and application thereof
ActiveCN104817605AGood anti-rectal cancer activitySugar derivativesSugar derivatives preparationTriazole derivativesPharmaceutical Substances
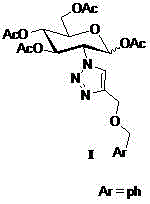
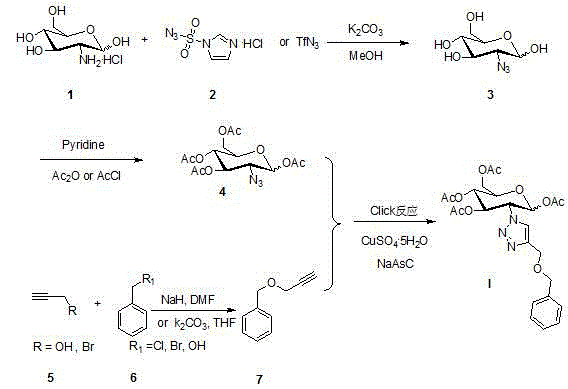
The core structure of 2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose with an anti-colorectal carcinoma activity is that a 1,2,4-triazole derivative substitutes 2-site of 1,3,4,6-O- acetyl-D-glucose. The above compound has a good activity of inhibiting colorectal carcinoma cells, and can be used as an anti-colorectal carcinoma medicine. A synthesis method of the above compound comprises the following steps: 2-amino-D-glucose hydrochloride and an azidation reagent are used as raw materials to generate a 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate under an alkaline condition; 3-propargyl bromide and phenylcarbinol react under the action of sodium hydride to generate phenyl propargyl ether; and the 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate and phenyl propargyl ether undergo a click reaction in a solvent under the catalysis of monovalent copper to generate 2-(1',2',3'-triazole-4'-benzyloxy)-1,3,4,6-O-acetyl-D-glucose.
Owner:HUBEI ENG UNIV
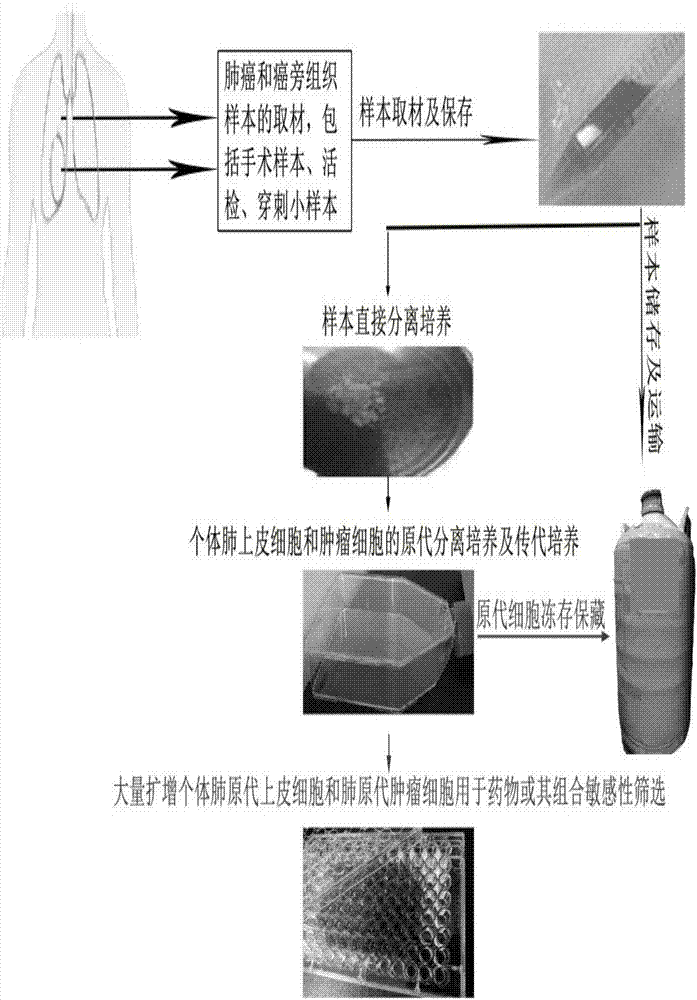
In-vitro individualized medicine test method for lung cancer and culture medium
InactiveCN107151645AProlong survival timeLow toxicityCell dissociation methodsCompound screeningProcess systemsIndividualized treatment
The invention discloses an in-vitro individualized medicine test method for lung cancer and a culture medium, and belongs to the field of cell biology and pharmacology. The in-vitro individualized medicine test method and the culture medium have the advantages that the culture medium M can be applied to normal epithelial cells and primary tumor cells of human or mammals, accordingly, in-vitro culture systems for tumor cells and para-carcinoma cells of lung cancer patients can be established, and primary cells, with continuous passage and quick proliferation functions, of patient individuals can be obtained; the culture systems can be used for detecting the sensitivity and the safety of medicines or combination groups of the medicines, and accordingly stable, accurate and reliable lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be established; individualized sensitive chemotherapy medicines or compositions screened by the aid of the lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be combined with clinical medicine or relevant subjects, accordingly, clinical individualized treatment schemes can be formulated, and the in-vitro individualized medicine test method and the culture medium can ultimately serve for clinical application.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Ribonucleases and methods of making them recombinantly
Methods for recombinantly producing new RNases, as well as previously-known RNases, are disclosed. The new RNases are active against human carcinoma cells.
Owner:ALFACELL +1
Single-stranded deoxyribonucleic acid (ssDNA) aptamer targeting human highly metastatic hepatoma carcinoma cell and application thereof
ActiveCN102766634AAvoid troubleOrganic active ingredientsMicrobiological testing/measurementLymphatic SpreadNucleotide
The invention discloses a single-stranded deoxyribonucleic acid (ssDNA) aptamer targeting human highly metastatic hepatoma carcinoma cell and an application of the ssDNA aptamer, and relates to the field of diagnosis and treatment of the recurrence and metastasis of the hepatocellular carcinoma, so that the high-efficient novel specific molecules are provided for the diagnosis and treatment of the recurrence and metastasis of the hepatocellular carcinoma. According to the invention, a subtractive cell-SELEX technology is applied, two hepatoma carcinoma cell systems with same genetic background and obviously different metastatic potentials are a subtractive target and a screening target, respectively, and the hepatoma carcinoma cell systems are MHCC97-L (low metastasis) and HCCLM9 (high metastasis), so that the ssDNA aptamer is capable of specifically recognizing the highly metastatic potential hepatoma carcinoma cell HCCLM9. The aptamer is a single-stranded DNA molecular probe capableof specially combining with a surface of a human highly metastatic hepatoma carcinoma cell membrane, and a nucleotide sequence of aptamer is represented by SEQ ID No.1. The aptamer is expected to play an important role in preparation of a reagent for diagnosing or treating the hepatocellular carcinoma.
Owner:WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com