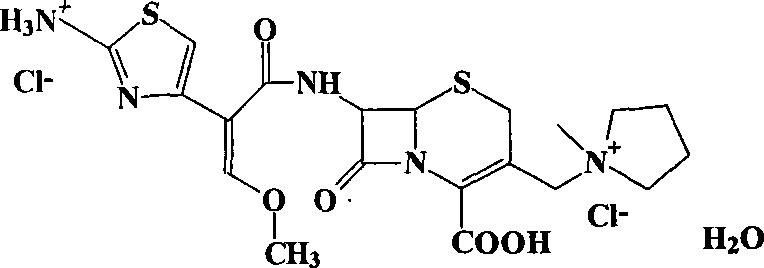
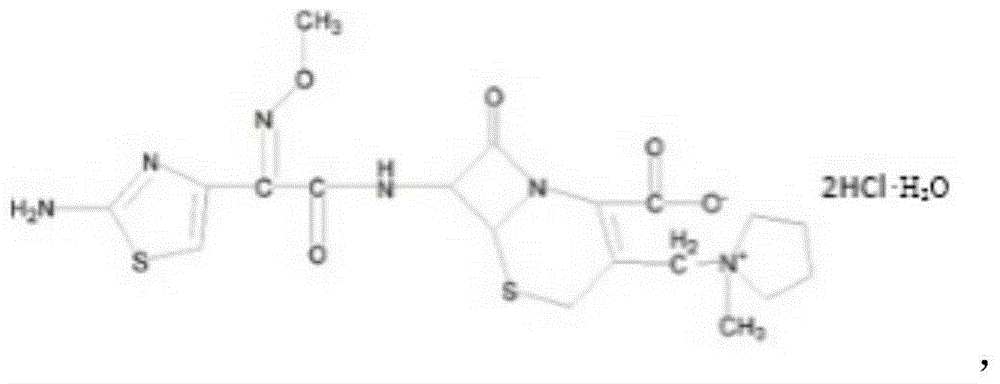
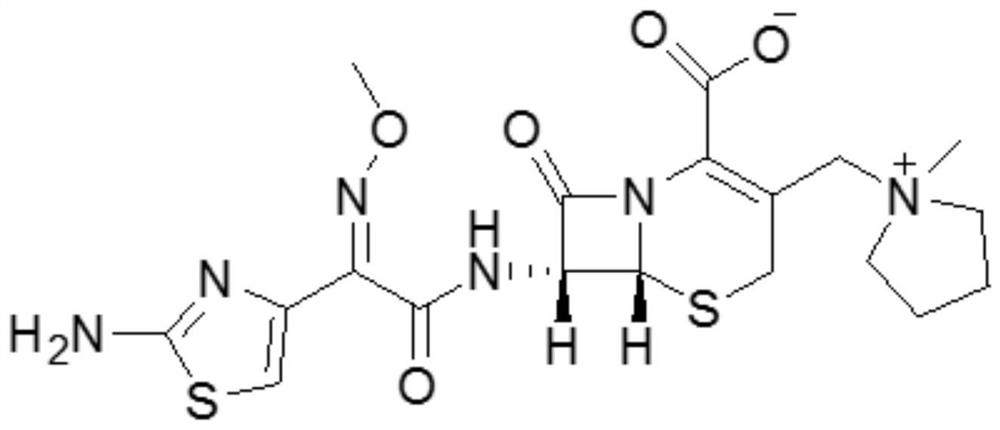
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
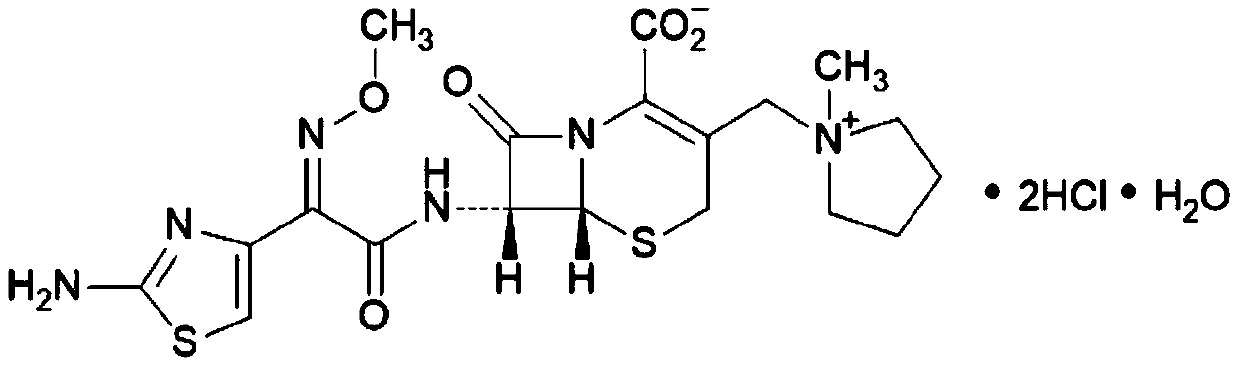
56 results about "Cefepime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
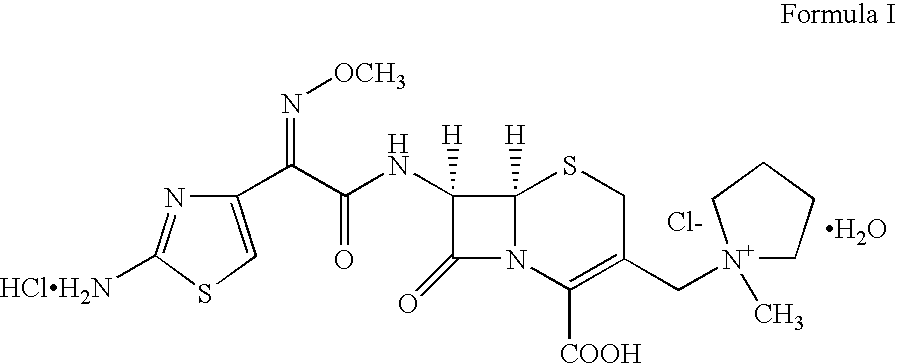
Cefepime is used to treat a wide variety of bacterial infections.
Therapy for Treating Resistant Bacterial Infections
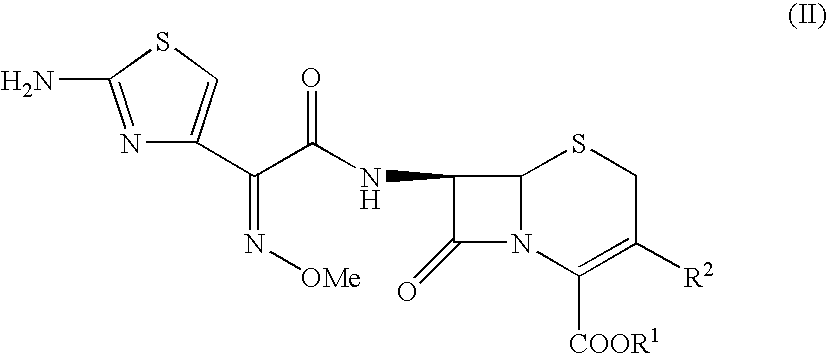
The invention relates to an improved therapy for treating resistant bacterial infections caused by extended-spectrum β-lactamase (ESBLs)-producing strains in a warm-blooded animal, adjuvant step down therapy, and pharmaceutical compositions for such therapies. The invention also relates to a method for inhibiting bacterial resistance in ESBLs-producing strains so as to have better control over the therapy; achieve reduced hospital stay and adjuvant step down therapy so as to avoid recrudescence. In particular, the therapy includes antibacterial combination of cefepime with sulbactam via parenteral route, followed by oral third generation cephalosporin with a suitable β lactamase inhibitor.
Owner:PATEL MAHESH VITHALBHAI +5
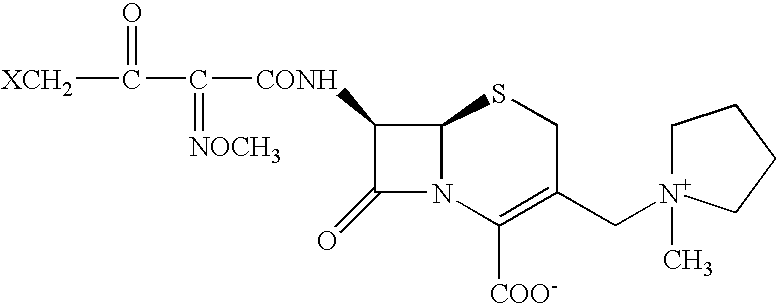
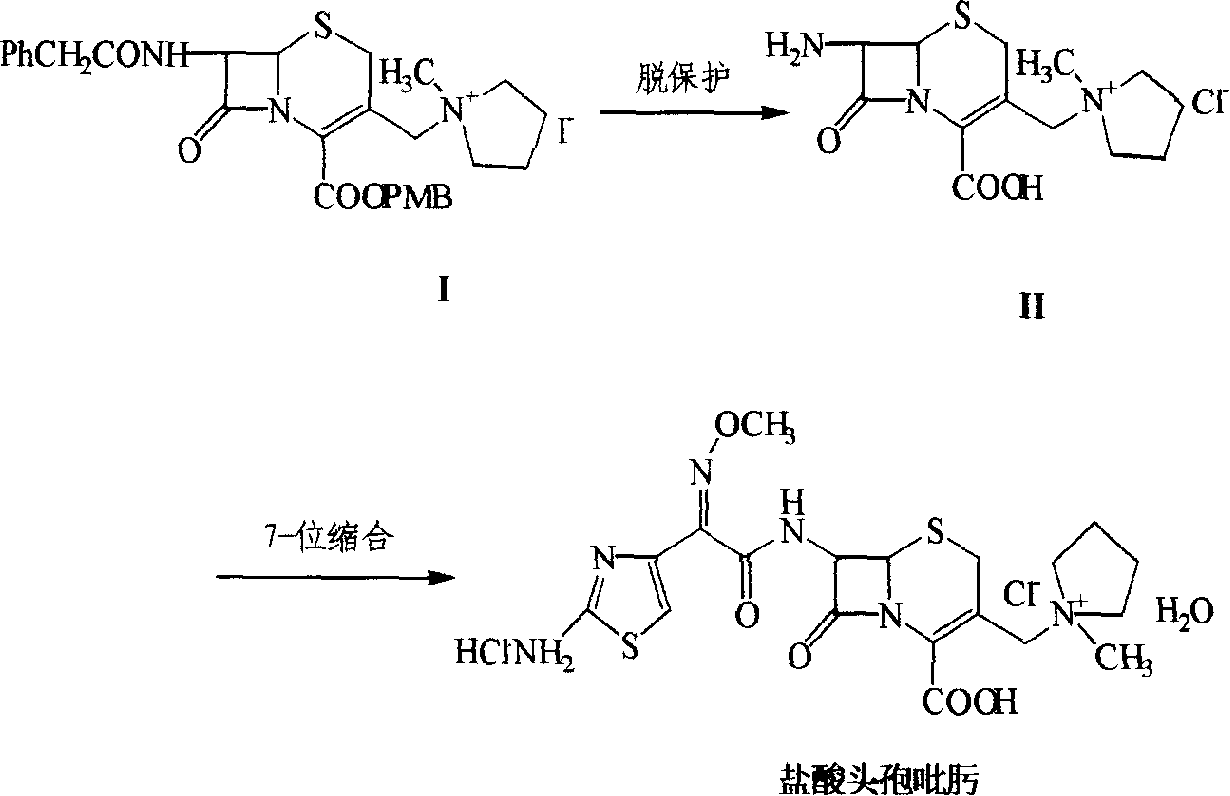
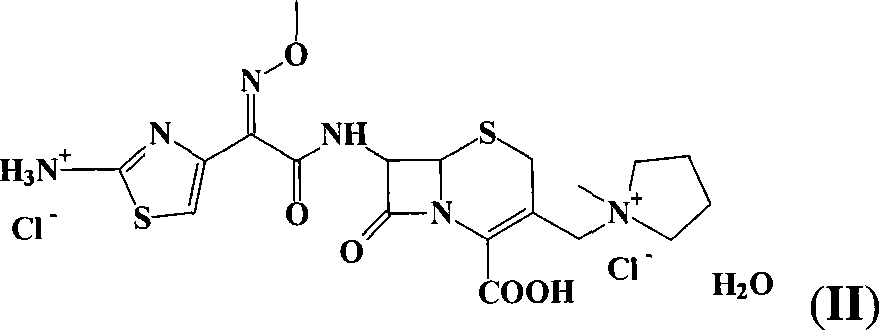
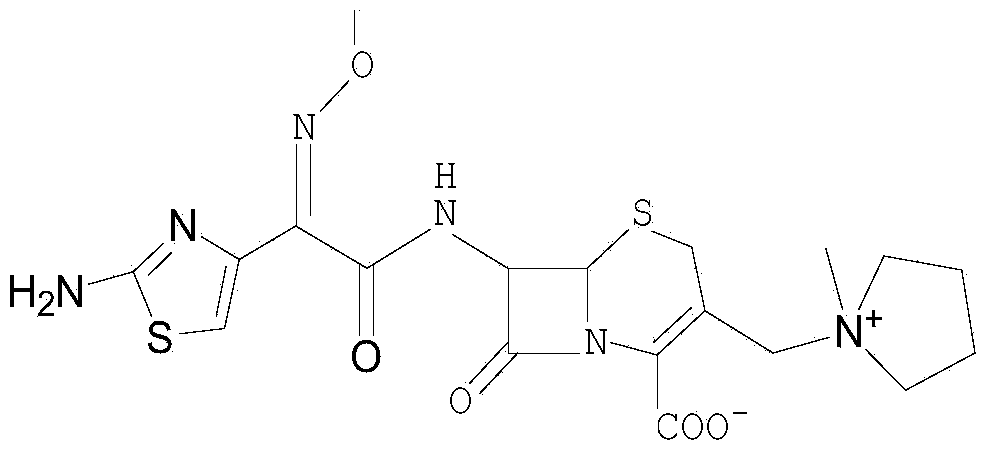
Synthesis method of cefepime hydrochloride
InactiveCN102408440AReduce the impactReduce degradationOrganic chemistryCefepime hydrochlorideSynthesis methods
The invention belongs to the field of medical intermediate and particularly relates to a synthesis method of cefepime hydrochloride. The method comprises the following steps: adding (6R,7R)-7-amino-3-[(1-methyl-1-pyrrolidine)methyl]cef-3-ene-4-carboxylic acid hydrochloride and AE active ester in a mixed solvent of water and a water-soluble organic solvent; adjusting the pH value to 5.5-7.5, and performing an acylation reaction while keeping the temperature; extracting after the reaction, and recycling the organic phase through reduced pressure distillation; and adjusting the pH value of the water phase with hydrochloric acid, and crystallizing to prepare cefepime hydrochloride. The reaction is milder and adopts an acidic or nearly neutral environment, the influence of the reaction on cefepime is lower, the reaction is easier to control, the degradation probability and ring opening probability can be reduced, the purity of the finished product can be increased, the yield of cefepime can be increased, the quality of cefepime can be increased and the purity is above 99.5% through high performance liquid chromatography (HPLC) detection.
Owner:YIYUAN XINQUAN CHEM
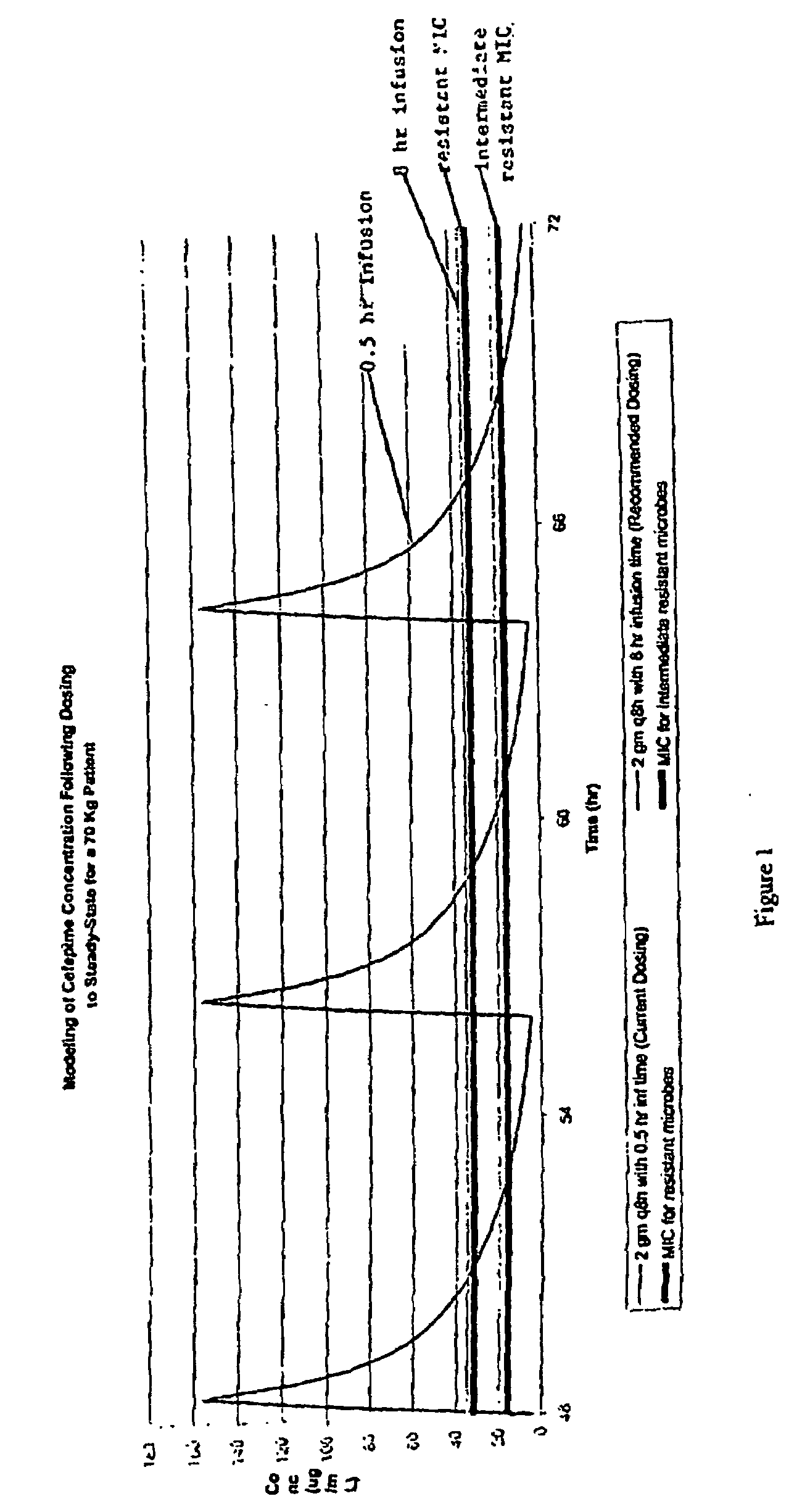
Stable liquid formulations of Anti-infective agents and adjusted Anti-infective agent dosing regimens
InactiveUS20090227554A1Efficient infectionAntibacterial agentsOrganic active ingredientsRegimenMedicine
Provided are methods of determining a resistance-adjusted dosage regimen of an anti-infective agent for treatment of an infection of a mammal by a resistant infective organism, wherein an effective dosage regimen of the anti-infective agent is known for treatment of an infection of the mammal by a susceptible strain of the infective organism. Methods of treating a cefepime resistant bacterial infection in a patient are also provided.
Owner:ELAN PHRMA INT LTD
Process for producing Cefepime and cephalosporin analogues
Process for producing Cefepime, Cefpirome and Cefquinome, whereby a cephalosporin containing a quaternary ammonium group is reacted with thiourea to provide the aforesaid cephalosporins.
Owner:ACS DOBFAR SPA
Process for preparing cefepime
A novel process is disclosed for the preparation of Cefepime, a cephalosporin antibiotic, using novel new intermediates of the general Formula, where X represents Bromine or Chlorine atom This process comprises the step of cyclizing the bromo or chloro intermediate with thiourea to produce Cefepime of high purity. A process to prepare bromo or chloro intermediate comprising the acylation of 7-Amino-3-[(1-methyl-1-pyrrolidinium) methyl]-3-cephem-4-carboxylate with 4-halo-2-methoxyimino-3-oxobutyric acid halide is also described.
Owner:HANDA VIJAY KUMAR +2
Synthesis method of cefepime hydrochloride
ActiveCN107201391AReduce usageLow costOrganic chemistryFermentationCefepime hydrochlorideSynthesis methods
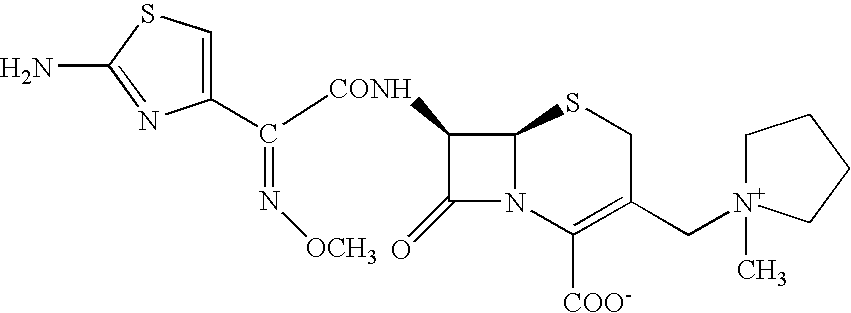
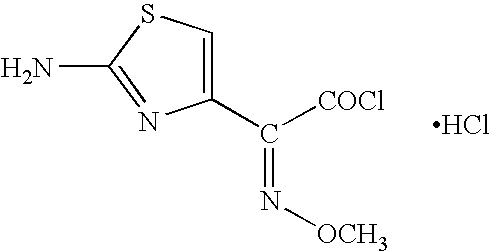
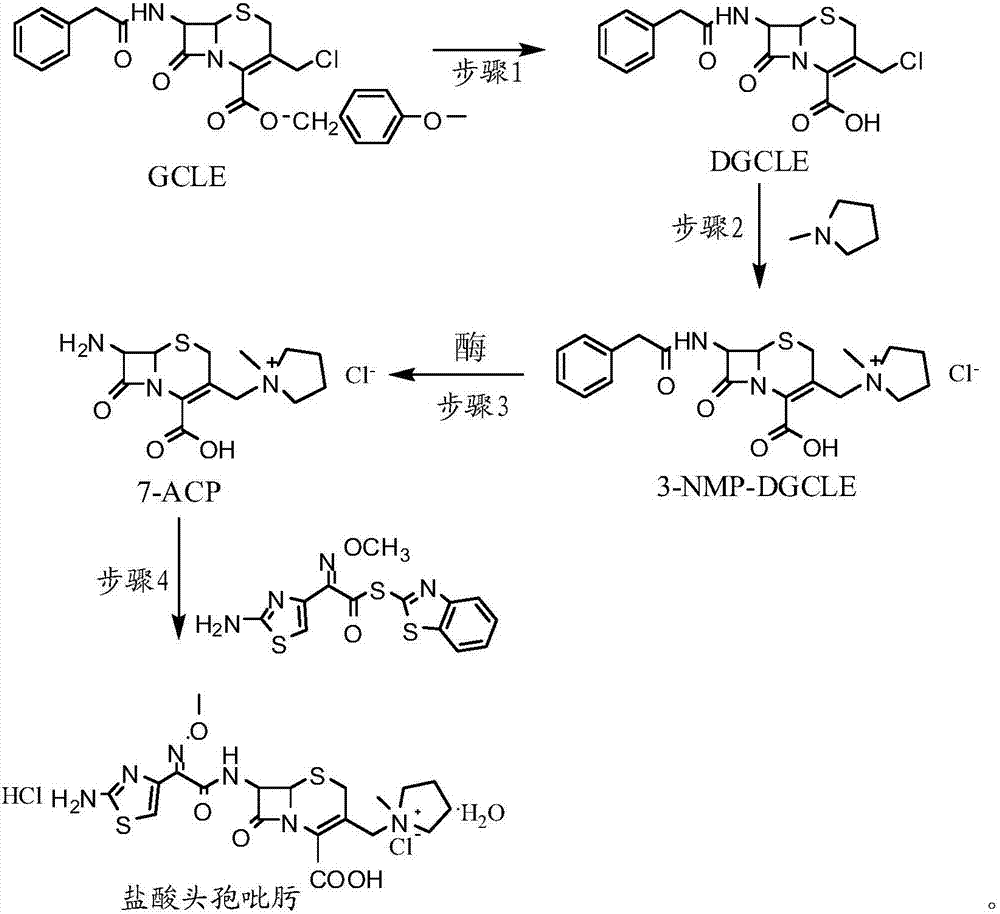
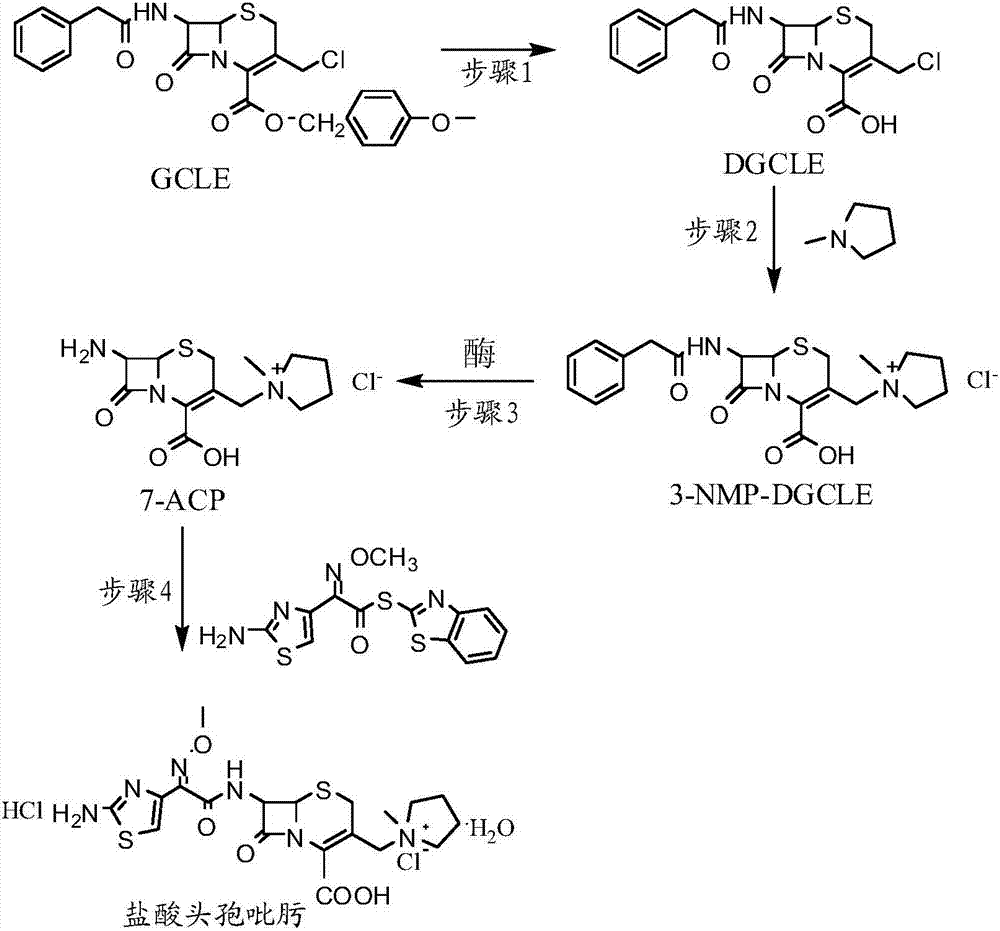
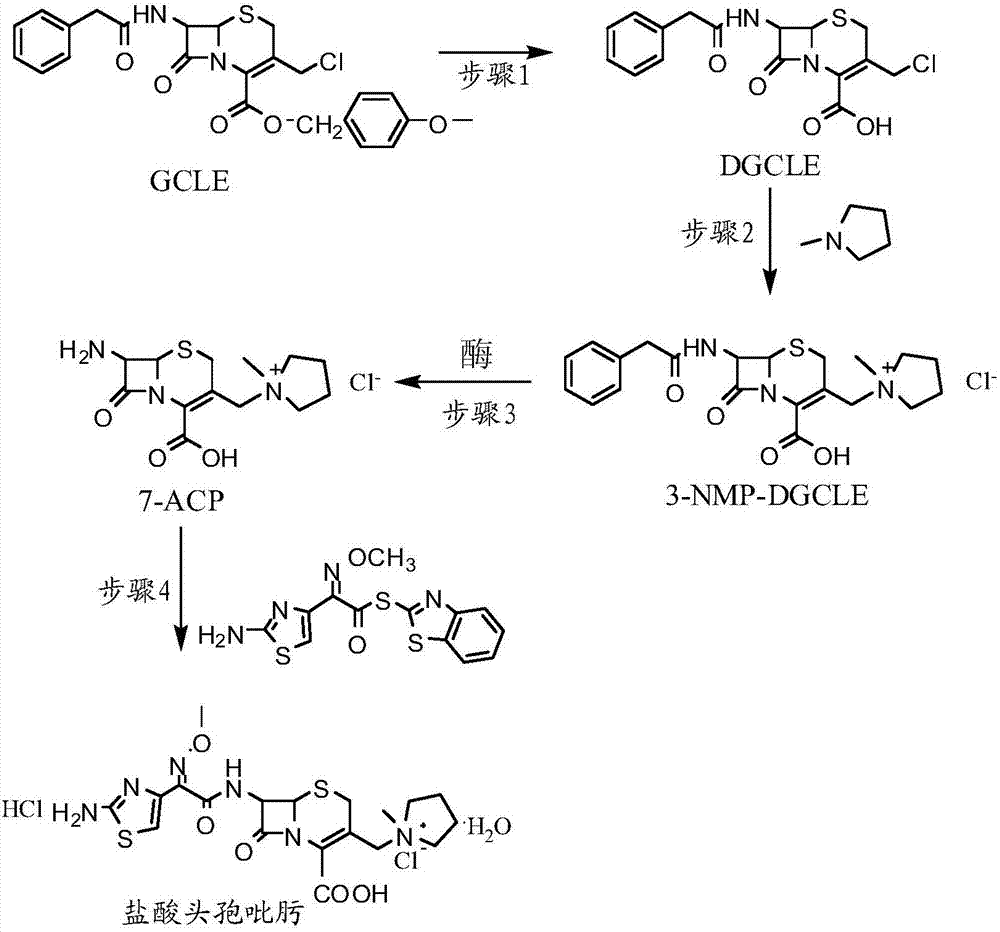
The invention provides a synthesis method of cefepime hydrochloride. The synthesis method comprises the following steps: taking GCLE (7-phenylacetamide-3-chloromethyl-3-cepham-4-carboxylic acid p-methyl-oxybenzyl ester) as a raw material; cutting a 4-site protecting group (p-methoxybenzyl) and enabling the 4-site protecting group to react with N-methylpyrrolidine (NMP); then cutting a 7-site protecting group through an enzyme method to obtain an immediate 7-amino-3-(1-methylpyrrolidine)methyl)-3-cepham-4-carboxylic acid hydrochloride (7-ACP); taking cheap and available methoxyiminoacetic acid mercaptobenzothiazole active ester (AE-active ester) and the 7-ACP to be subjected to 7-site acylation reaction, so as to finally prepare the cefepime hydrochloride. A route provided by the invention can be used for obtaining the high-yield and high-quality cefepime hydrochloride without a delta2 isomer. The synthesis method provided by the invention has the advantages of simple process, no harsh reaction conditions and the like and is very suitable for industrial production.
Owner:吉林省爱诺德生物工程有限公司
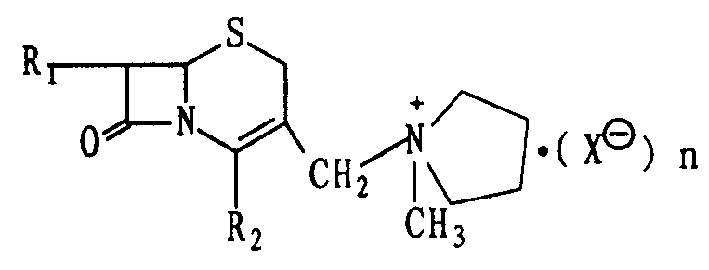
Cephe alkene onium salt compound and its preparation and use in preparation of cefepime
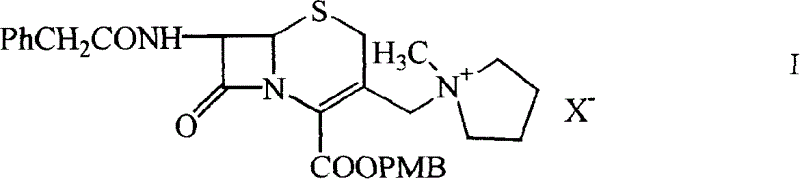
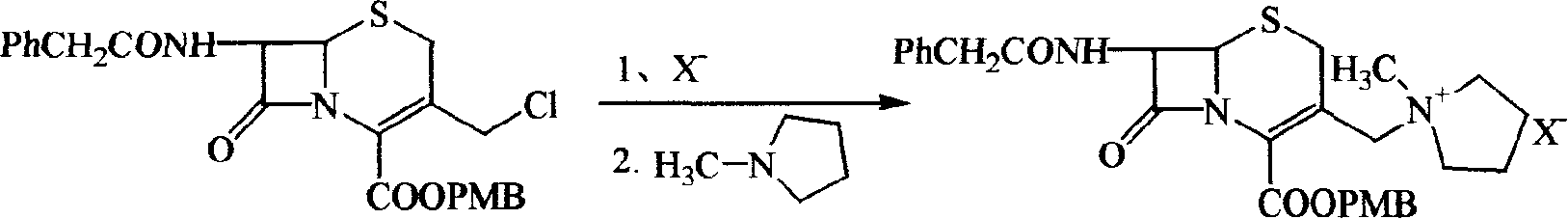
ActiveCN1644583AFew reaction stepsControl process costAntibacterial agentsOrganic chemistrySide chainAlkene
A cepha alkene onium salt compound, its production and use are disclosed. The constitutional formula and surface features of 7-phenylacetyl amino-group-3-(1-methyl-1-pentazane onium group ) methyl-3-cepha alkane-4-carboxylic acid methoxyl carbobenzoxy group are also disclosed. The production is carried out by taking GCLE original material, nucleophilic substituting reacting, removing blocking group and thiaoxamidine side chain active ester reacting. It achieves low cost, stable product quality, and no environmental pollution.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Cephalo olefine onium salt compound and its preparing method, and method for synthesizing cephalo pyoxime with said compound
InactiveCN1392149AMild reaction conditionsReduce manufacturing costOrganic chemistryCefepime hydrochlorideMedicinal chemistry
The present invention relates to 7beta-alkylamido-3-(1-methyl-1-pyrrolidyl onium) methyl-3-cephalo olefine-4-carboxylate and its preparation process and the synthesizing process of cephalo pyroxime hydrochloride with the said intermediate. The present invention has mild reaction condition, no need of expensive reagent and benzyl penicillin acylase, low cost, high product purity, and simple and practical reaction path.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
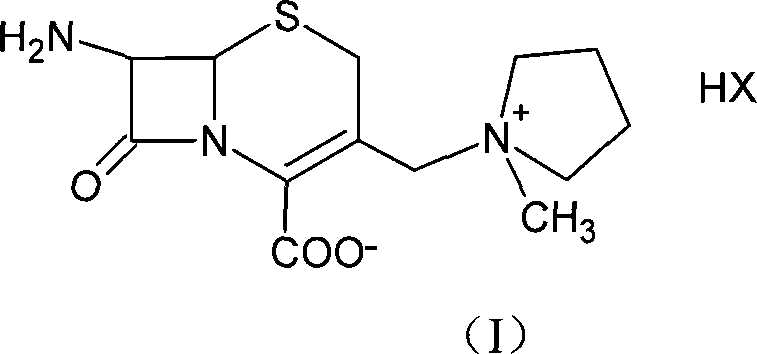
Method for preparing cefepime dihydrochloride
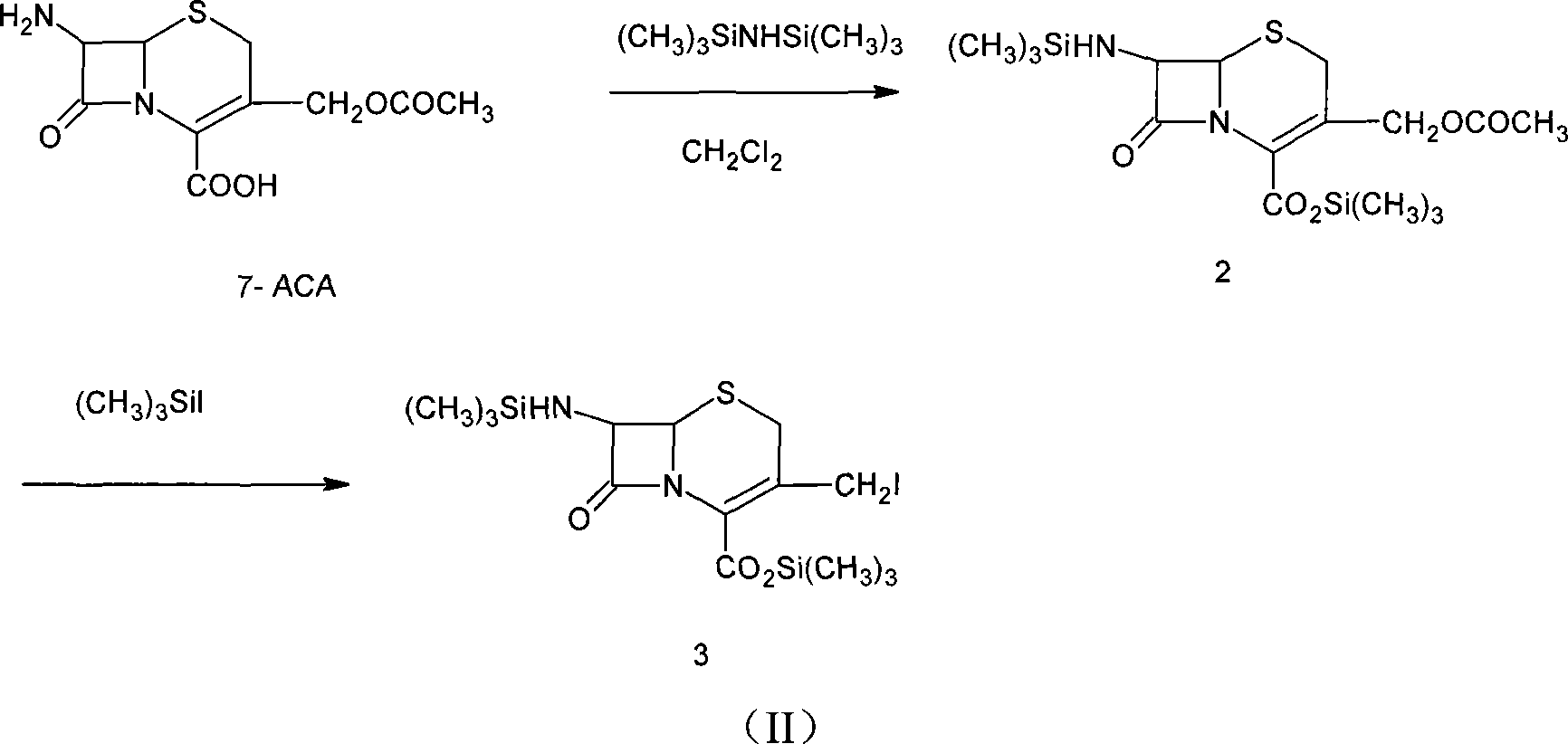
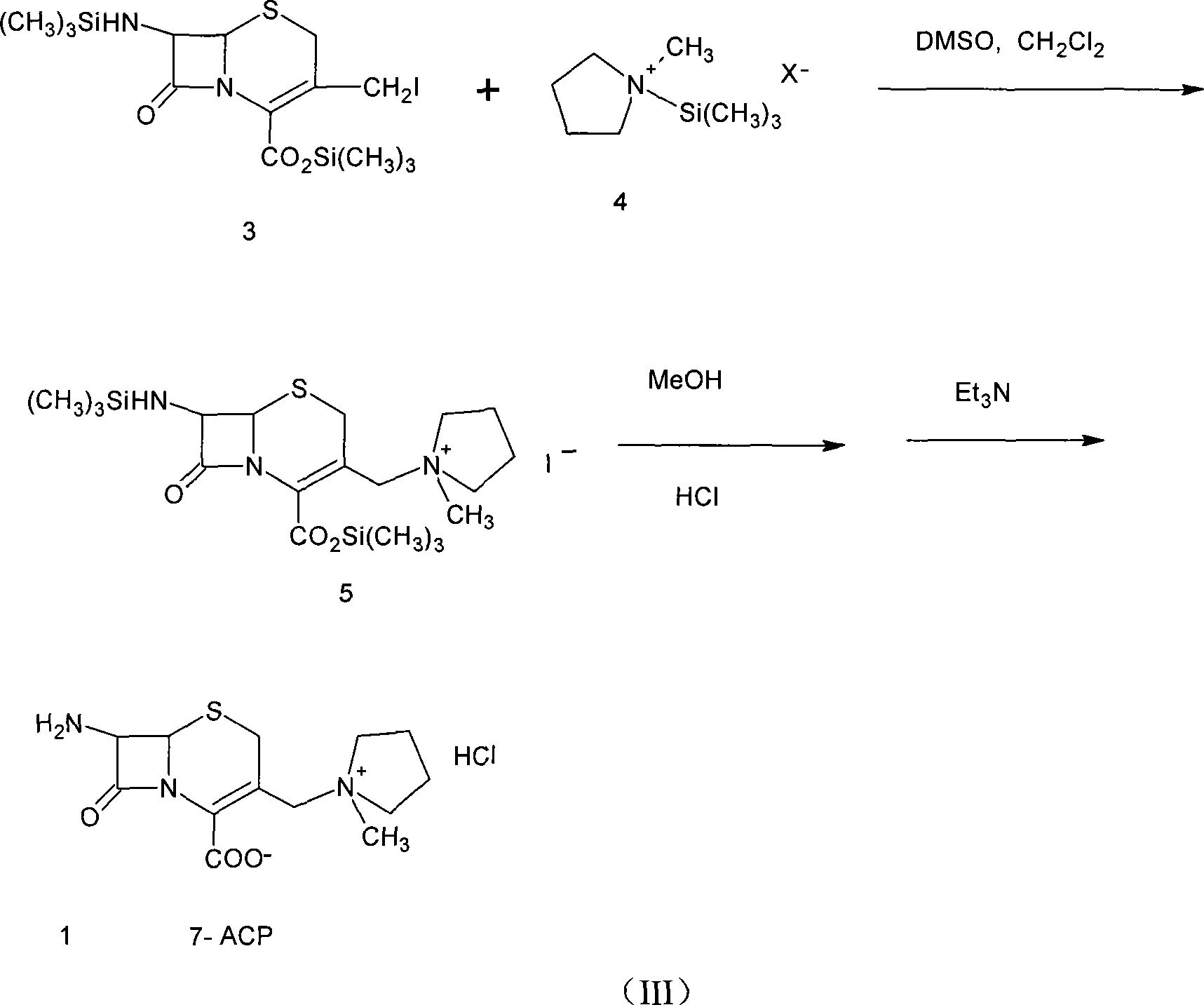
The invention provides a method for preparing cefepime dihydrochloride. The method employs the raw materials of 7-ACA, HMDS, TMSI, NMP and CH3OH, and a catalyst is hydrochloric acid having 98% of concentration. According to the invention, the method employs a one kettle way, a cefepime dihydrochloride crude product is prepared, then the cefepime dihydrochloride crude product is purified, and finally cefepime dihydrochloride is obtained. The purity of the cefepime dihydrochloride is 97%, yield can reach 31.3%, compared with the current synthesis technology, the yield is increased by 3.3-10% (by metering with 7-ACA), and the purity is increased by 4%. The preparation method has the advantages that the industrial production requirements of short reaction period, easily available raw materials and low cost can be satisfied, and has industrial application.
Owner:CHANGCHUN UNIV OF TECH
Method of synthesizing cefepime intermediate in mixed solvent
InactiveCN101054385AHigh yieldQuality improvementOrganic chemistryBulk chemical production7-ACACarboxylic salt
The present invention provides a method for synthesizing cefepime intermidiate in mixed solvent. The method is : using 7-ACA as material, generating 3-iodo intermediate and reacting with N-methyl pyrrolidine in mixed solution of methylene chloride and N,N-dimethyl formamide etc, high quality, high yield, delta2 isomer-free cefepime intermidiate 7-amido-3-[(1-methyldihydropyrrole)methyl]-3-cepha-4- carboxylate hydrochloride (7-ACP) 7-ACP yield amounts to 80% (mol yield) and is higher than existing technology. Tone grade of 7-ACP is less than 3,and purity is larger than 99.5%.
Owner:UNIV OF JINAN
Method for preparing cefepime dihydrochloride monohydrate crystal
InactiveCN101200473AReduce dosageEffective decolorization effectOrganic chemistryPurification methodsCrystallization
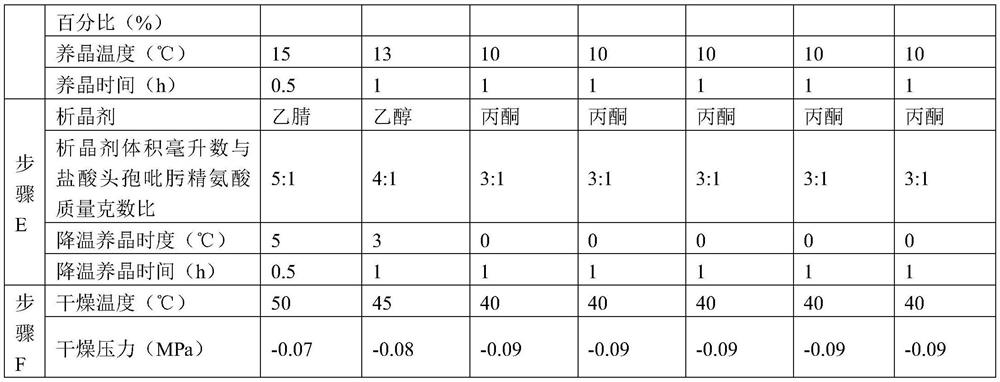
The invention relates to a preparation or purification method of cefepime dihydrochloride monohydrate. The invention is characterized in that acetone, ethanol or the mixed solution of acetone and ethanol at the random portion is added into the methanol solution containing cefepime dihydrochloride and then cefepime dihydrochloride monohydrate crystal is precipitated. The method can effectively avoid the formation of cefepime dihydrochloride dihydrate.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
Preparation of cefepime hydrochlorice by sodium salt precipitation method
This invention relates to a preparation method of cefepime muriaficus. It uses water solubility basicity sodium salt ( sodium acetate, sodium lactate isooctanoid acid sodium, sodium carbonate, sodium bicarbonate, sodium hydroxide or rand om hybrid) to regulate sulfuric acid cefepime solution to subacidity or neutrality, add organic solvent to precipitate out sodium sulfate, sodium bisulfate or their hydrate. Then add hydrochloric acid for salification, and obtain cefepime muriaficus. This invention effectively avoide degradation of efepime, advance yield and purity; greatly cut erosion of acidity feed liquid to equipments; significant reduce dosage of organic solvent, advance production efficiency.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Refining method of high-purity cefepime dihydrochloride monohydrate
The invention belongs to the field of medicines and in particular relates to a refining method of high-purity cefepime dihydrochloride monohydrate. The refining method comprises the following steps: dissolving cefepime inner salt, concentrated hydrochloric acid, sodium hydrogen sulfite and EDTA-Na2 (ethylene diamine tetraacetic acid-sodium 2) in methanol, adding active carbon, mixing, and filtering; adding acetone into filter liquor, crystallizing, filtering again, and washing a filter cake to obtain cefepime dihydrochloride monohydrate. According to the refining method, the conditions are mild, cefepime is less influenced, and the process is easy to operate; the cefepime loss is low in the reaction process, cefepime dihydrochloride monohydrate is obtained by the method, cefepime dihydrochloride dihydrate is prevented from being generated, the purity of cefepime is improved, and the quality of cefepime is greatly improved.
Owner:YIYUAN XINQUAN CHEM
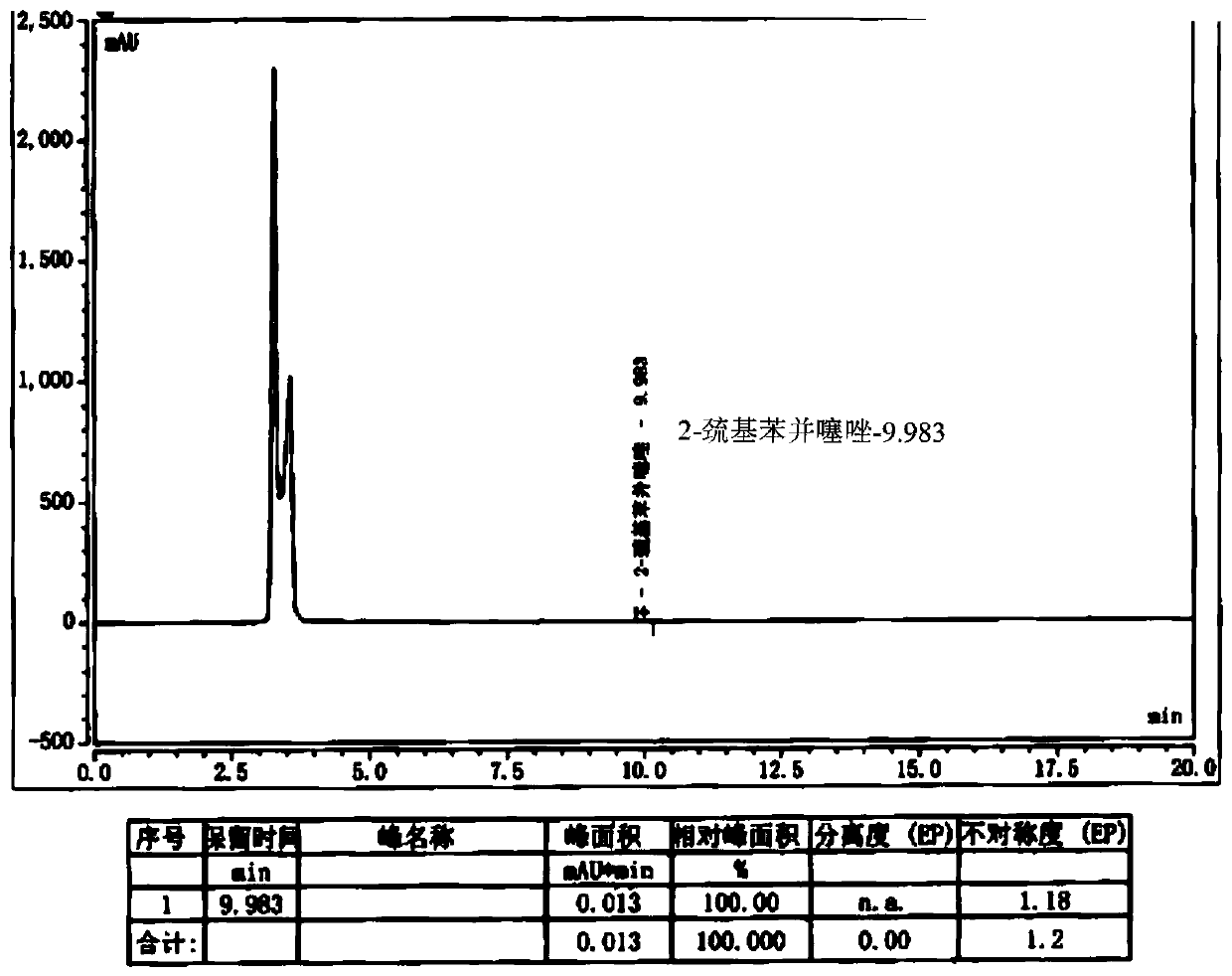
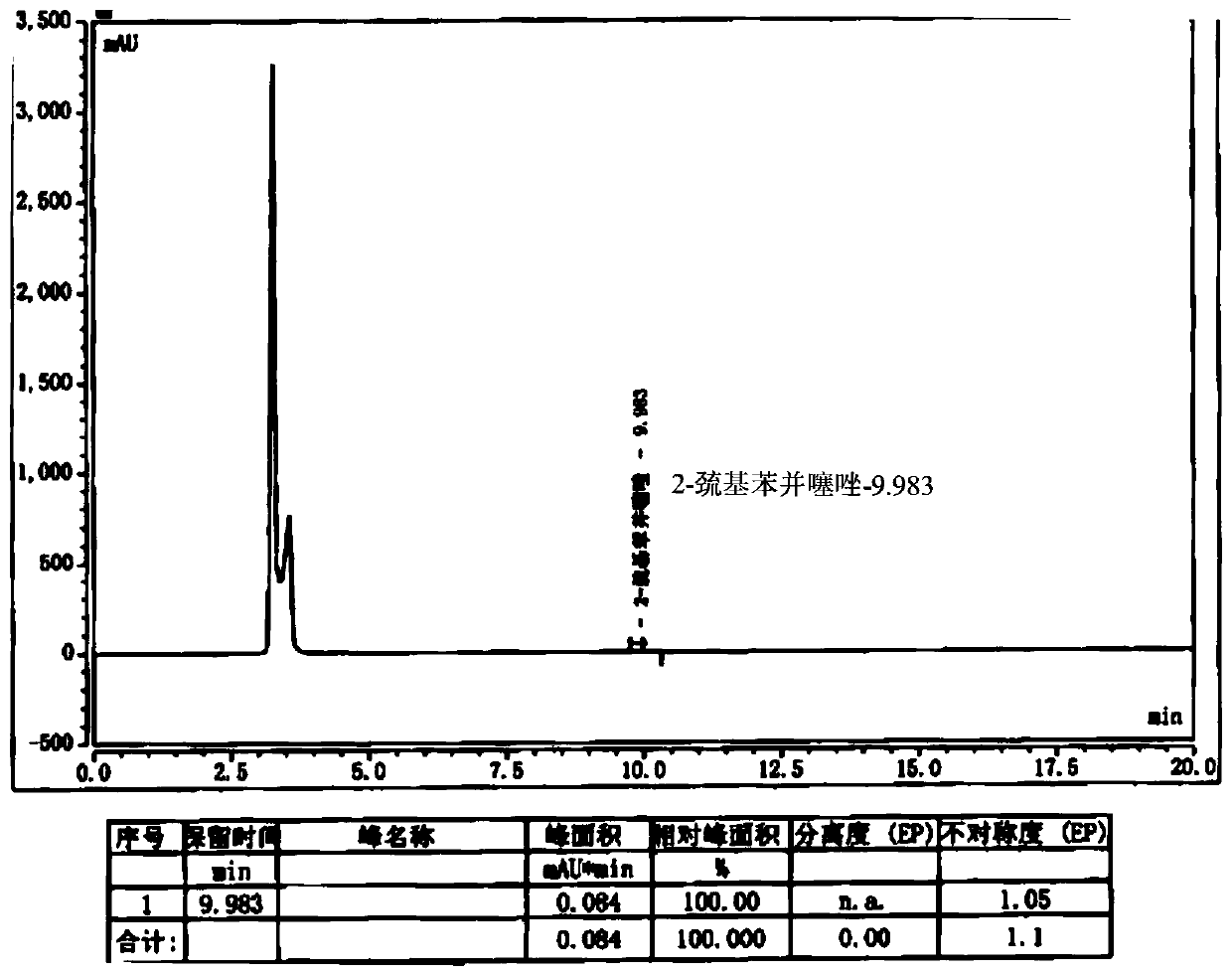
Preparation method of cefepime hydrochloride having content of genotoxic impurity 2-mercaptobenzothiazole reduced
ActiveCN110655528AThe purpose of reducing residueImprove securityOrganic chemistryCefepime hydrochlorideSide chain
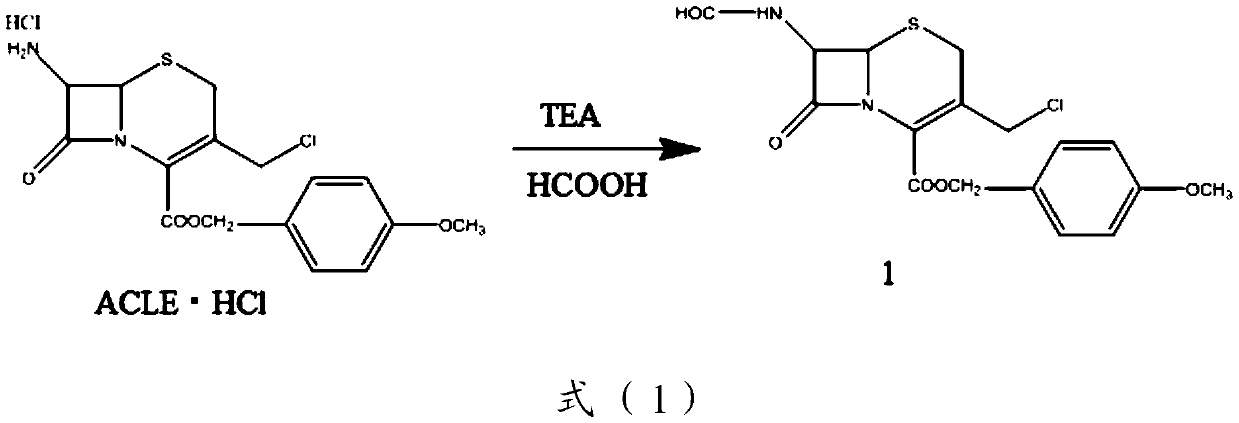
The invention belongs to the field of medicinal chemistry, and relates to a preparation method of cefepime hydrochloride. The preparation method comprises the steps: in a dichloromethane solvent, in the presence of triethylamine, carrying out acylation reaction on a cefepime side chain compound and AE active ester; after the reaction, adding water into the reaction liquid, and extracting to obtaina water phase; adding hydrochloric acid into the water phase to adjust the pH value of the water phase to 2.0-2.5, then adding a water-insoluble organic solvent, extracting, standing for layering, and separating out the water phase; adjusting the pH value of the water phase to 1.0-1.5 with hydrochloric acid after activated carbon decoloration, and adding a crystallization solvent to obtain cefepime hydrochloride. According to the cefepime hydrochloride prepared by the preparation method disclosed by the invention, the content of a genotoxic impurity 2-mercaptobenzothiazole in the cefepime hydrochloride is reduced to 1-5 mmp, even 1-3 mmp, so that the safety of a cefepime hydrochloride medicine is improved.
Owner:GUANGZHOU HC PHARM CO LTD
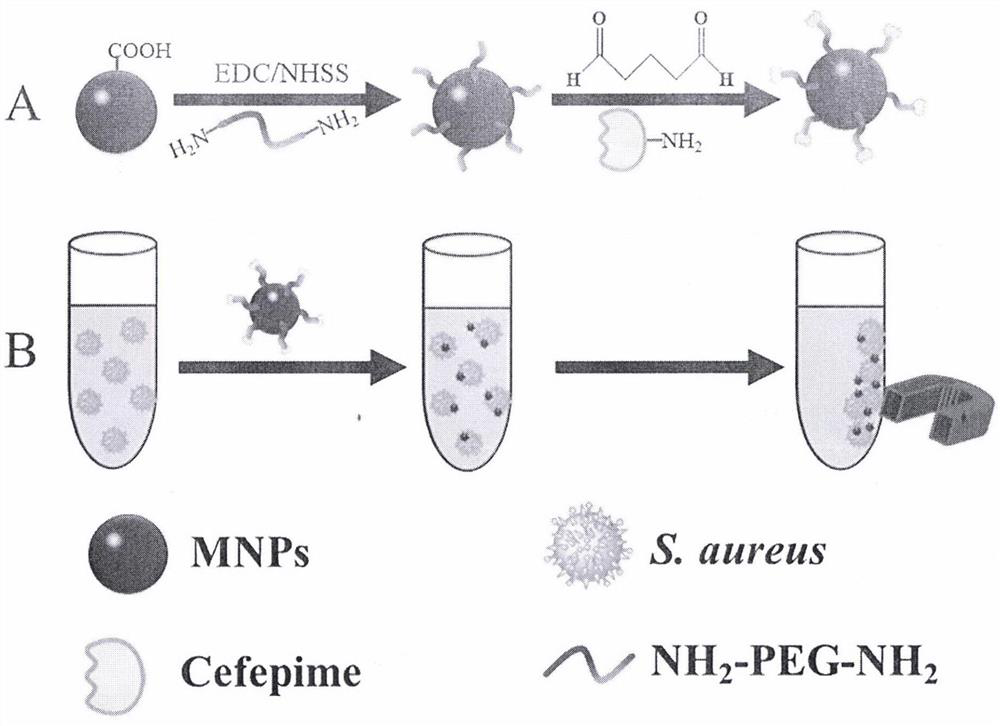
Method for enriching staphylococcus aureus by magnetic beads
PendingCN114774508AStrong magnetic responseLarge specific surface areaMicrobiological testing/measurementBiological material analysisMagnetite NanoparticlesStaphylococcus aureus
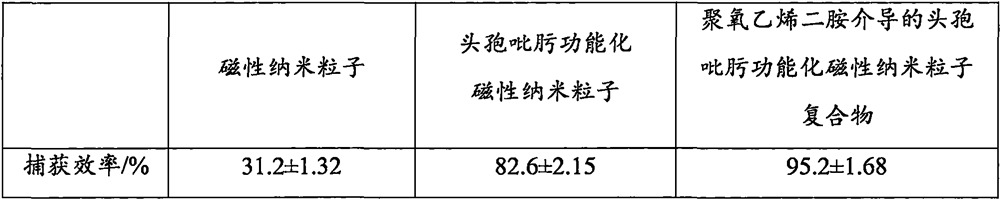
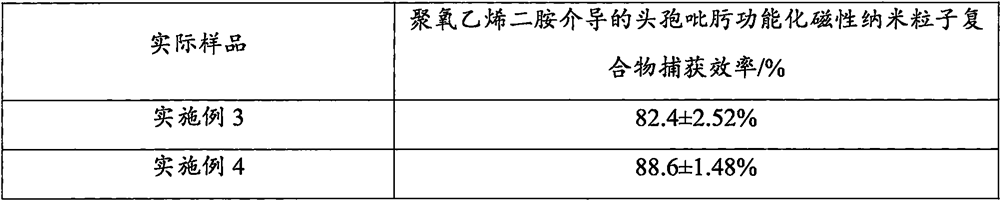
The invention discloses a method for enriching staphylococcus aureus by magnetic beads, and belongs to the technical field of biology. Comprising the following steps: washing and resuspending magnetic nanoparticles; activating the magnetic nanoparticles to obtain activated magnetic nanoparticles; carrying out reaction on polyoxyethylene diamine and the activated magnetic nanoparticles to obtain a polyoxyethylene diamine modified magnetic nanoparticle compound; the preparation method comprises the following steps: reacting glutaraldehyde with a polyoxyethylene diamine modified magnetic nanoparticle compound to obtain a substance A; the method comprises the following steps: reacting cefepime with a substance A to obtain a polyoxyethylene diamine mediated cefepime functionalized magnetic nanoparticle compound; and enriching and capturing. According to the present invention, the polyoxyethylene diamine is adopted as the mediator so as to reduce the steric hindrance, the cefepime is adopted as the recognition element so as to improve the binding force with bacteria, and the nano-scale magnetic beads with the large specific surface area are adopted to improve the loading capacity of the recognition element so as to improve the bacteria capture efficiency. Compared with the traditional method, the method has the advantages of short incubation time, less material consumption, low cost and wide adaptability.
Owner:南昌大学第一附属医院
Process for producing Cefepime and cephalosporin analogues
Process for producing Cefepime, Cefpirome and Cefquinome, whereby a cephalosporin containing a quaternary ammonium group is reacted with thiourea to provide the aforesaid cephalosporins.
Owner:ACS DOBFAR SPA
Cefepime hydrochloride medicine composition, powder-injection thereof and preparation method thereof
InactiveCN102743390AGood pH stabilityUniform product qualityAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSide effect
The invention relates to a cefepime hydrochloride medicine composition, a powder-injection thereof and a preparation method thereof. The medicine composition comprises the following ingredients: cefepime hydrochloride, L-arginine and hydroxypropyl-beta-cyclodextrin, wherein the weight of the L-arginine is 40% of that of the cefepime hydrochloride, and the weight of the hydroxypropyl-beta-cyclodextrin is 10% of that of the cefepime hydrochloride. The medicine composition is good in mixing uniformity, has small pH value change in subpackaging and transportation processes, is small in gastrointestinal tract side effect, and greatly improves the stability and safety of the medicine.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
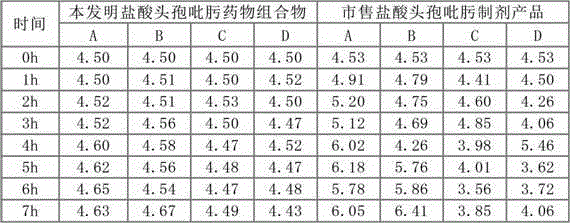
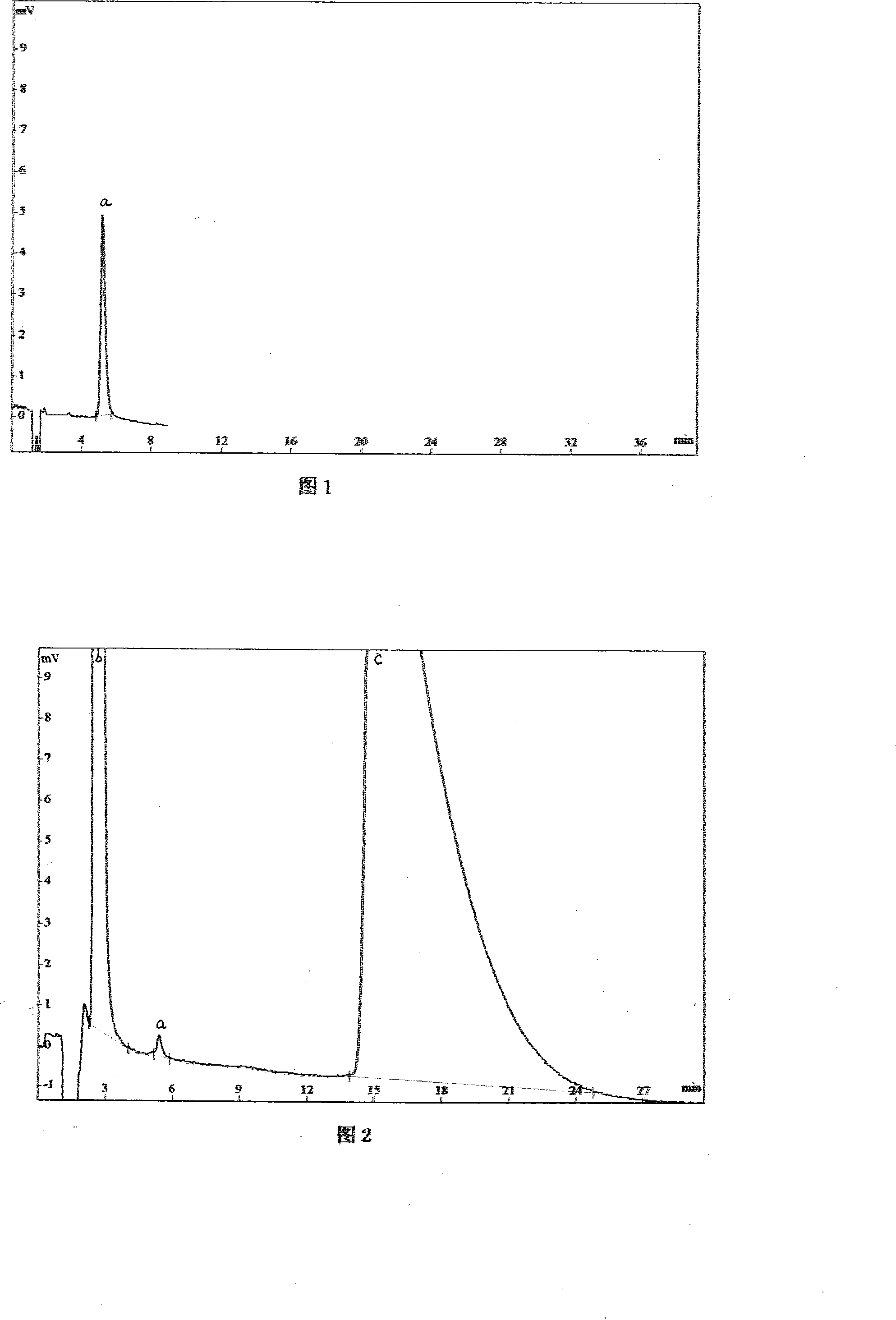
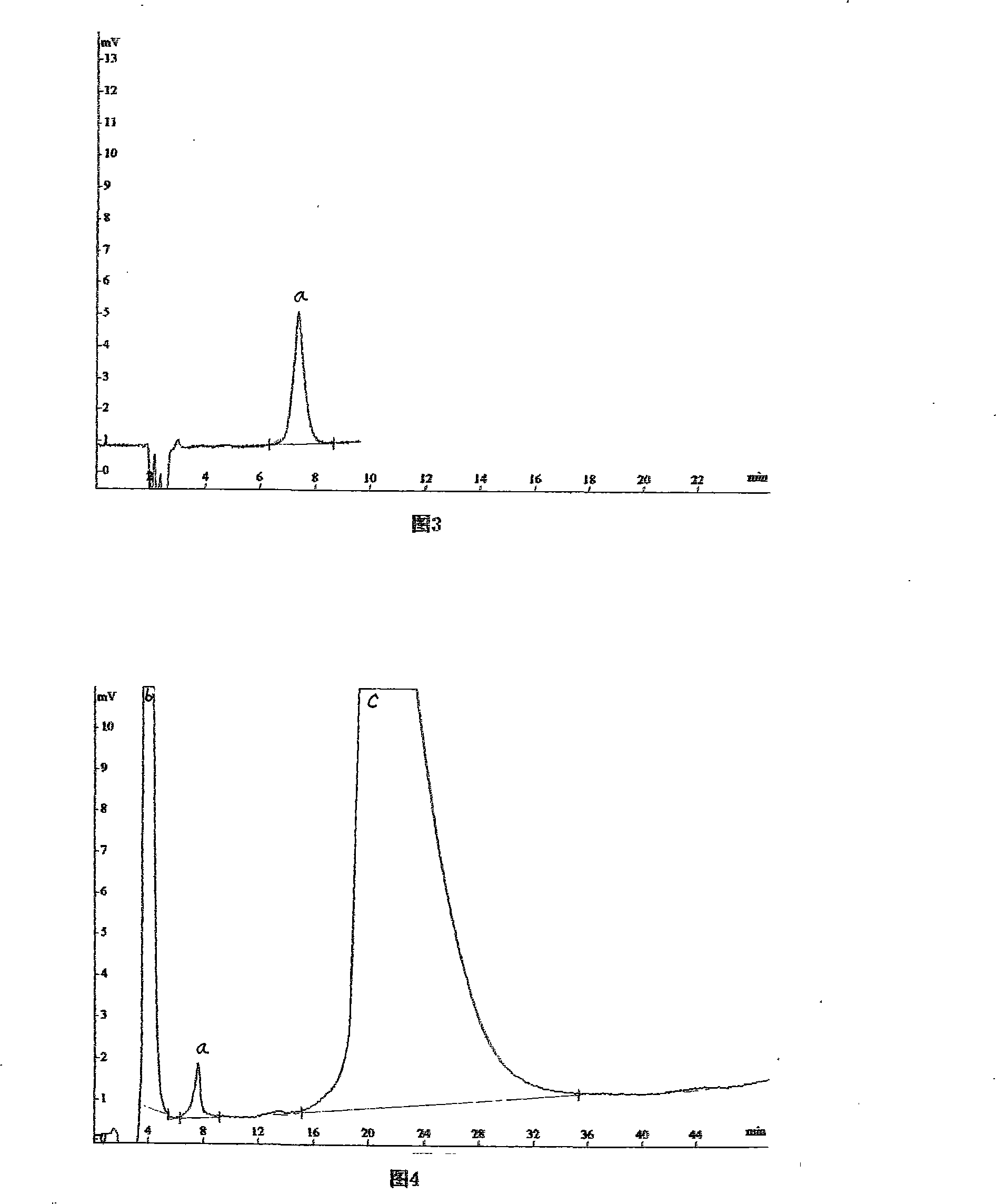
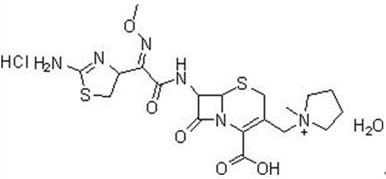
Hydrochloric acid cefepime raw material and method for measuring content of N-methyl pyrrolidine in preparation thereof
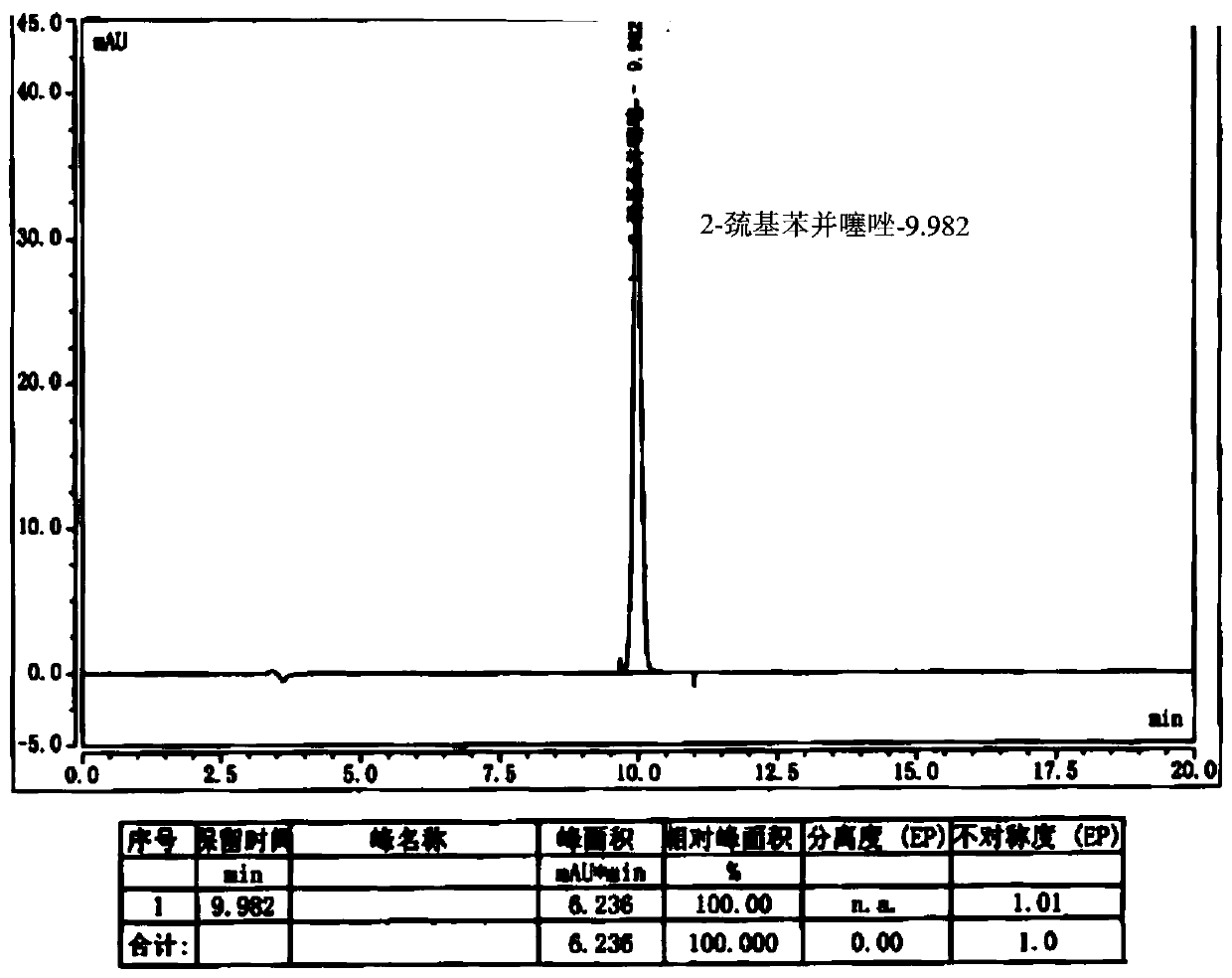
ActiveCN101226174AImprove balanceShort measurement timeComponent separationCefepime hydrochlorideRetention time
The invention relates to a measurement method of N-methylpyrrolidine content of cefepime dihydrochloride material and relative agent, for evaluating the quality of cefepime dihydrochloride material and relative agent. The invention discloses a liquid chromatograph which comprises using carboxylic cationic column as analysis column, uses a conductive detector to check, preparing flow phase, preparing sample solution and reference substance solution, using test method to respectively inject the sample solution and reference substance solution into a liquid chromatograph, recording high pressure liquid chromatographs, and calculating the N-methylpyrrolidine content of the sample. The inventive chromatograph system has easy balance, short sample test time, better repeatability in preserved time, stable baseline, durable column, high quality of chromatograph peak of N-methylpyrrolidine, better repeatability, accurate, simple and reliable process.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Preparation of cefepime halogen acid salt by calcium salt precipitation process
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
Liposome vitamin A acid aerosol for treating chronic obstructive pulmonary disease
InactiveCN100441187CGood curative effectLong lastingAerosol deliveryRespiratory disorderAdditive ingredientCholesterol
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
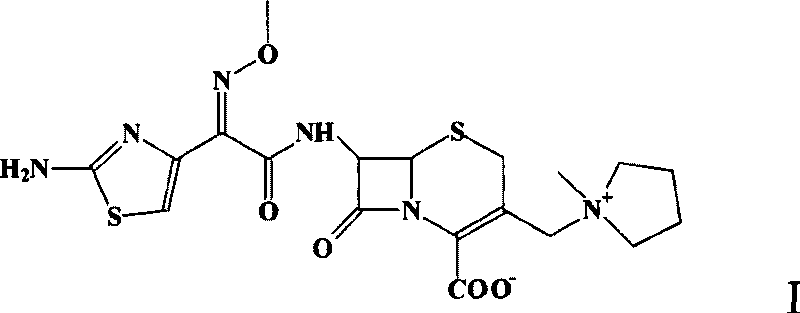
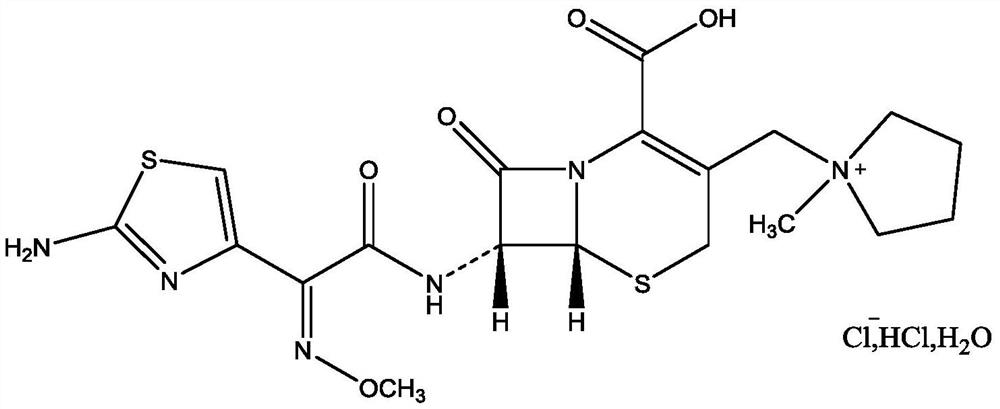
Cefepime dihydrochloride compound and pharmaceutical composition thereof
ActiveCN104610283AHigh purityIncrease contentAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSide effect
The invention discloses a cefepime dihydrochloride compound. The cefepime dihydrochloride compound is prepared by a following method comprising the following steps: (1) dissolving a cefepime dihydrochloride crude product into water and performing membrane separation and impurity removal by selecting a separating membrane with the molecular weight cut off of 500-3000; (2) adding the impurity-removed solution into an organic solvent, then adding active carbon into the obtained solution, stirring, filtering the solution to remove charcoal, and collecting the filter solution; (3) separating and purifying the filter solution with a chromatographic column, wherein a mobile phase is a mixed solvent of isopropanol and acetonitrile, and a stationary phase filler is silica gel or aluminum oxide; and (4) concentrating the separated and purified filter solution, and performing spray-drying to obtain the cefepime dihydrochloride compound. The invention also discloses a pharmaceutical composition containing the cefepime dihydrochloride compound, wherein the pharmaceutical composition is sterile powder for injection. The cefepime dihydrochloride compound and the pharmaceutical composition disclosed by the invention have low impurity content and few toxic and side effects, so that the medication safety of the patient is greatly improved.
Owner:YOUCARE PHARMA GROUP
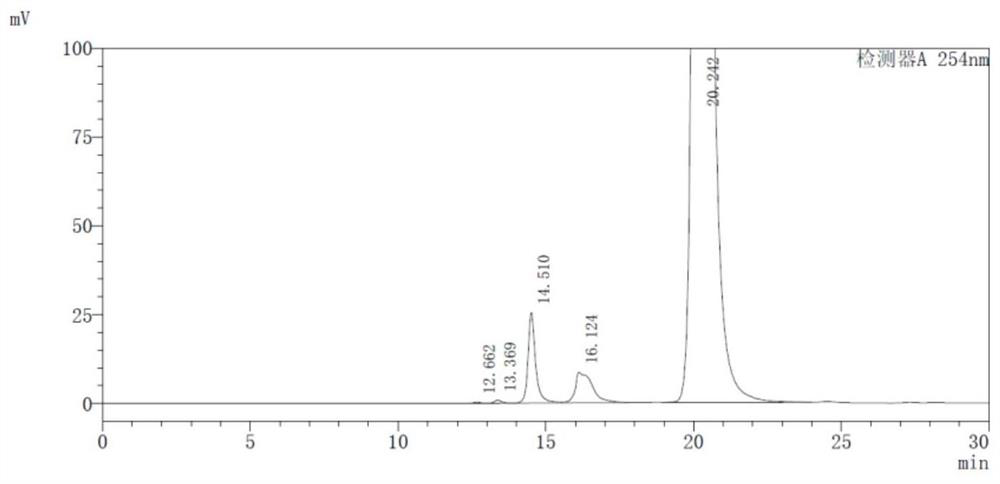
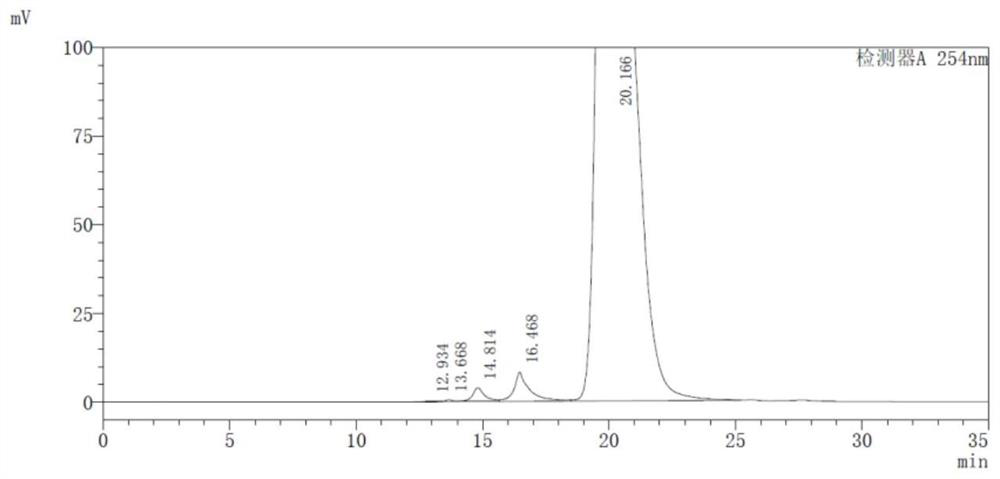
HPLC detection method for high-molecular impurities in cefepime and preparation thereof
The invention discloses an HPLC detection method for high-molecular impurities in cefepime and a preparation thereof. The method comprises the steps of separating and measuring polymer impurities in the cefepime and the preparation thereof by using a high performance liquid chromatograph and an isocratic elution method, wherein a chromatographic column with the detection wavelength of 254 + / -2nm takes globular protein chromatographic hydrophilic silica gel as a filler, and a mixed solution consisting of a phosphate buffer solution with the concentration of 0.005 mol / L and the volume percentage of 87.5 to 92.5% and an organic phase with the volume percentage of 7.5 to 12.5% is used as a mobile phase. According to the method, the quality of the high-molecular impurities in cefepime and the preparation thereof can be controlled, and the product quality and medication safety are guaranteed.
Owner:TIANSHENG PHARMA GROUP
Liposome vitamin A acid aerosol for treating chronic obstructive pulmonary disease
InactiveCN1927207AImprove stabilityProlong the action timeAerosol deliveryRespiratory disorderAdditive ingredientCholesterol
The invention relates to a bangosome retinoic acid aerosol which can prevent chronic obstructive pulmonary. The ingredients of the aerosol include: retinoic acid bangosome, cefepime or ceftazidime, fluormone or cetacort and distilled water. The mentioned retinoic acid bangosome is made of the following materials: 1-2 share (by weight) retinoic acid, 10-15 shares (by weight) cholesterol, 10-15 shares (by weight) lecithin and 0.01-0.2 VE. The producing procedures go as follows: mix 2-3 shares (by weight) retinoic acid bangosome with 0.1-0.2 cefepime or ceftazidime, 0.0005-0.0025 fluormone or 0.01-0.05 cetacort; add distilled water to the ingredients at the ratio of 1:15-1:20; fill the material into clean jars to get the aerosol.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Synthesis method of cefepime hydrochloride
InactiveCN113024581ALow costEasy to handleOrganic chemistryBulk chemical productionCefepime hydrochlorideCarboxylic acid
The invention relates to a synthesis method of cefepime hydrochloride, which comprises the following steps: by taking 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid diphenylmethyl ester hydrochloride as a raw material, protecting amino by using di-tert-butyl dicarbonate ester, reacting with N-methylpyrrolidine, and removing 4-site and 7-site protecting groups by using acid to obtain an intermediate 7-amino-3-(1-methyltetrahydropyrrole)methyl)-3-cephem-4-carboxylic acid hydrochloride (7-ACP); and then carrying out an acylation reaction with aminothiazide sulfhydryl benzothiazole active ester (AE-active ester) to obtain the product. The route has the advantages of simple reaction treatment, harsh reaction conditions, less isomer generation, high yield, high purity and simple process, and the method is suitable for industrial production.
Owner:刀鹏
Method for preparing cefepime dihydrochloride monohydrate crystal
InactiveCN101200473BReduce dosageEffective decolorization effectOrganic chemistryPurification methodsCrystallization
The invention relates to a preparation or purification method of cefepime dihydrochloride monohydrate. The invention is characterized in that acetone, ethanol or the mixed solution of acetone and ethanol at the random portion is added into the methanol solution containing cefepime dihydrochloride and then cefepime dihydrochloride monohydrate crystal is precipitated. The method can effectively avoid the formation of cefepime dihydrochloride dihydrate.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
A kind of purification method of cefepime hydrochloride
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
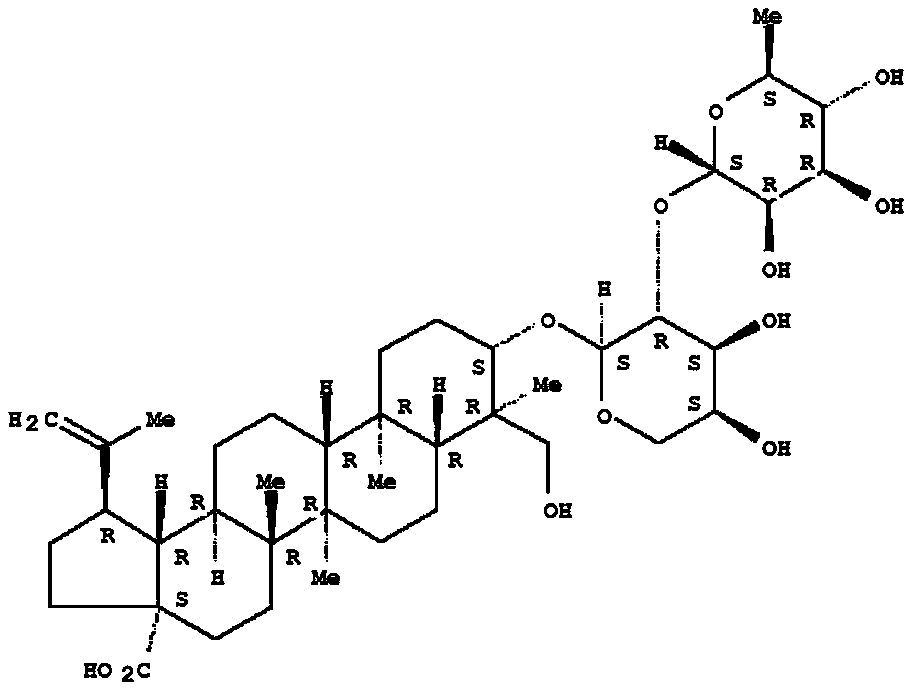
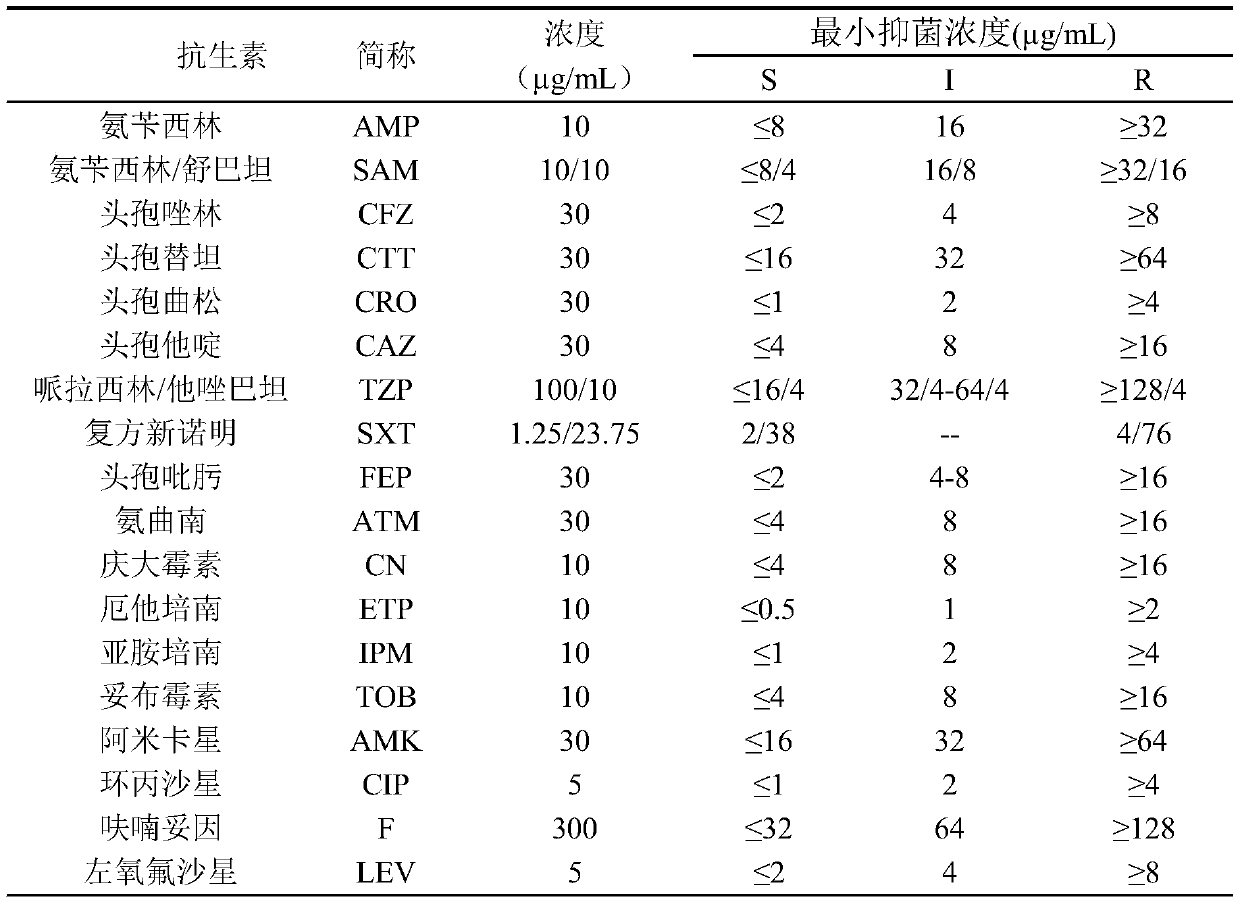
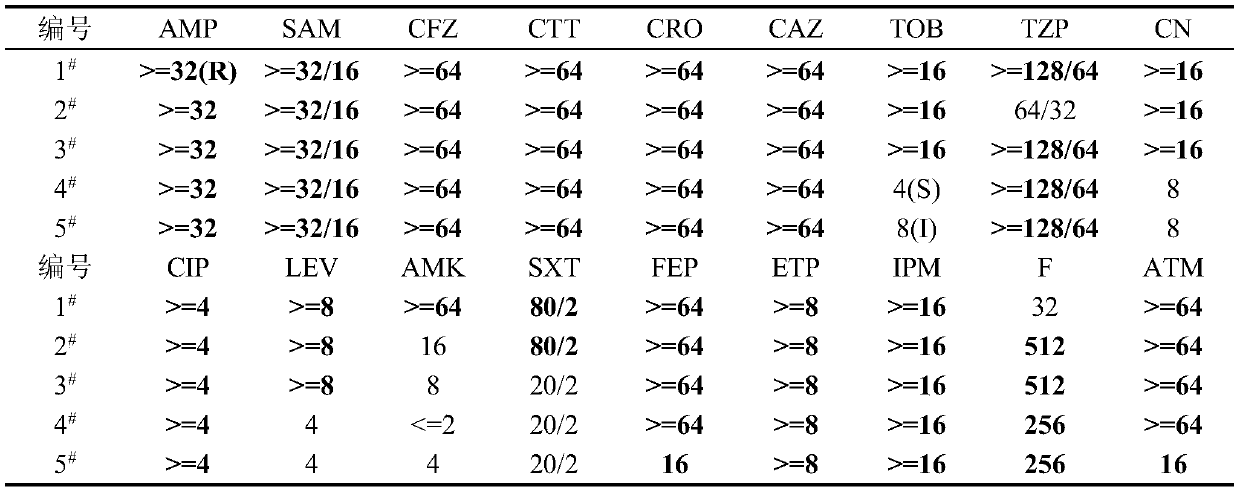
Application of pulsatilla saponin A3 in inhibiting growth of multi-drug-resistant Providencia rettgeri
Owner:SHAANXI UNIV OF SCI & TECH
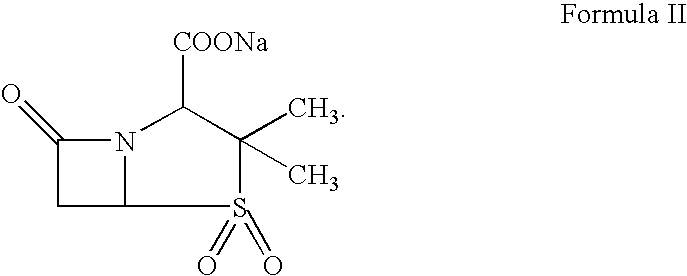
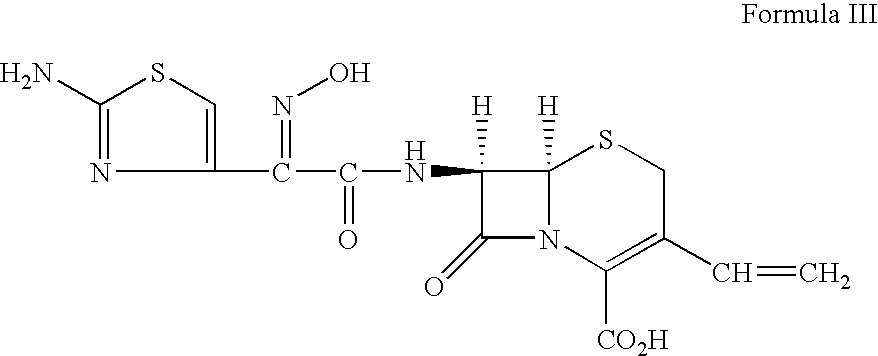
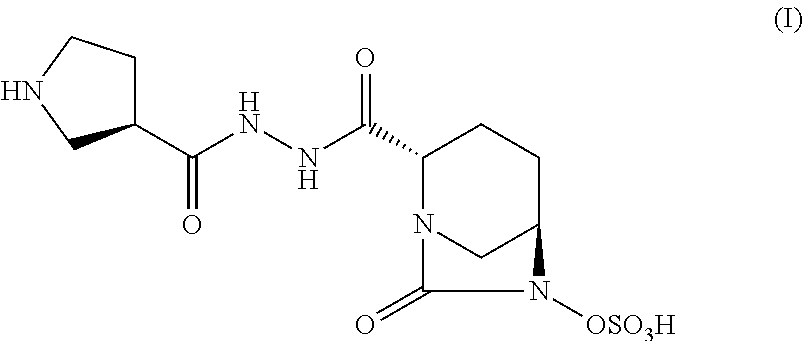
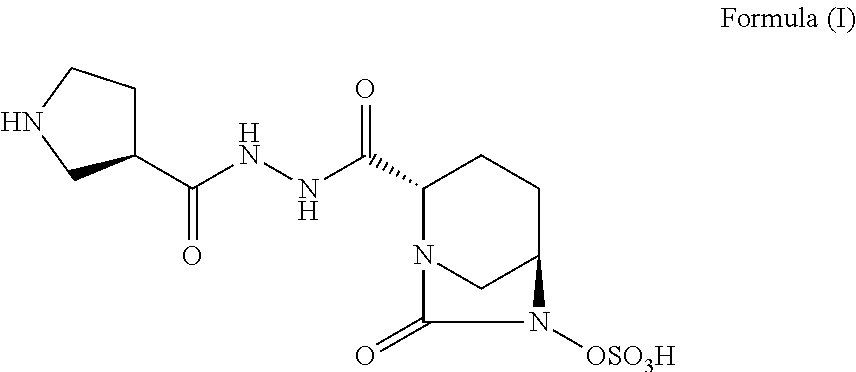
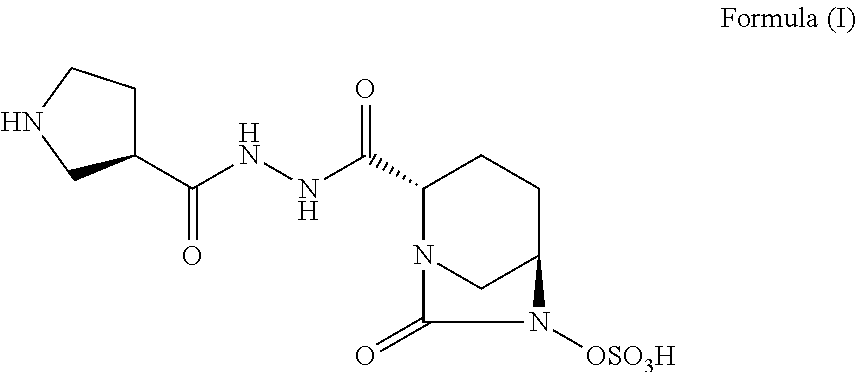
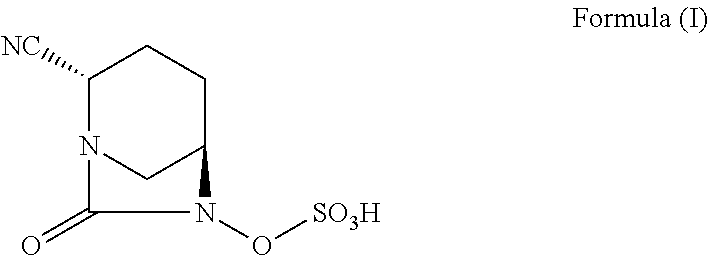
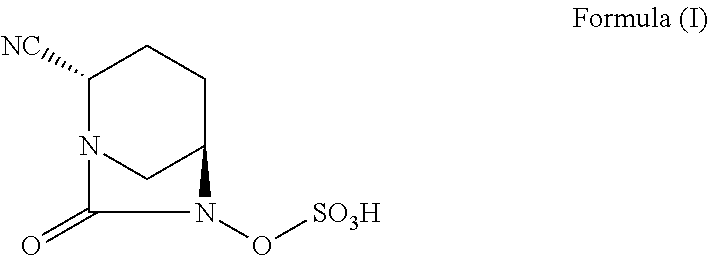
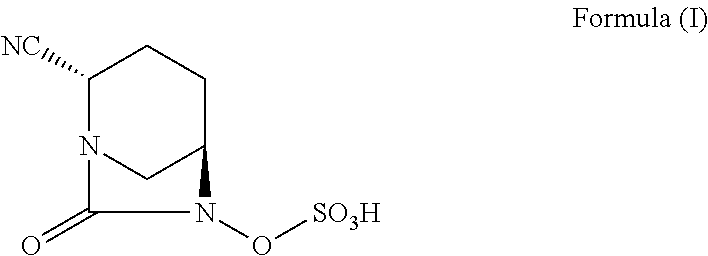
Pharmaceutical combinations comprising antibacterial agents
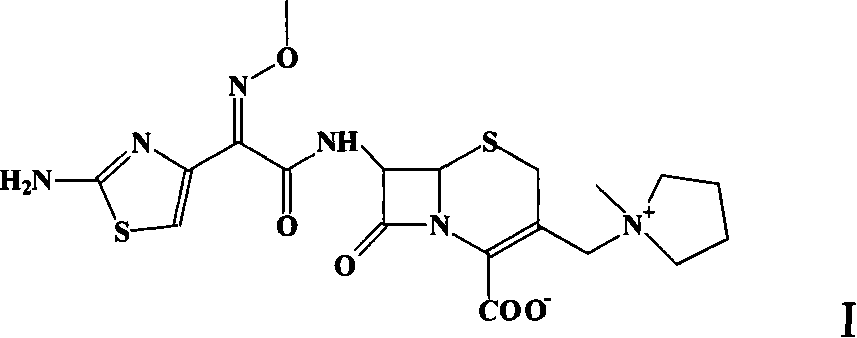
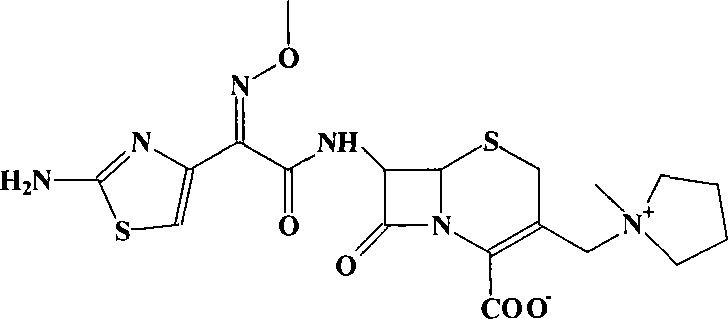
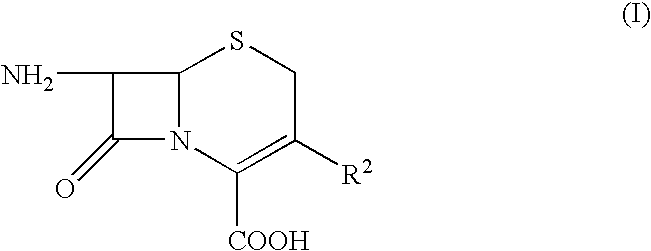
Pharmaceutical compositions comprising antibacterial agents selected from cefepime, cefpirome or a pharmaceutically acceptable derivative thereof, and compound of Formula (I) or a stereoisomer or a pharmaceutical acceptable derivative thereof, are disclosed.
Owner:WOCKHARDT LTD
Cefepime hydrochloride medicinal preparation for treating new indications of tympanitis
InactiveCN111560028AIncrease contentImprove clinical treatment effectOrganic active ingredientsSenses disorderCefepime hydrochloridePharmaceutical drug
The invention relates to the technical field of medicinal preparation, and discloses cefepime hydrochloride, a preparation, a preparation method and a new indication for treating tympanitis. The cefepime hydrochloride provided by the specific preparation method is low in impurity content, high in stability and remarkable in medicine effect, the quality of a preparation product is improved, the safety and effectiveness of the preparation product are guaranteed, and the cefepime hydrochloride has the application in the aspect of preparing a medicine for treating tympanitis.
Owner:广东赛法洛药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com