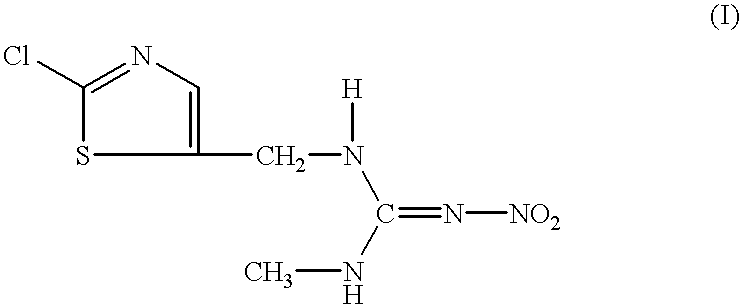
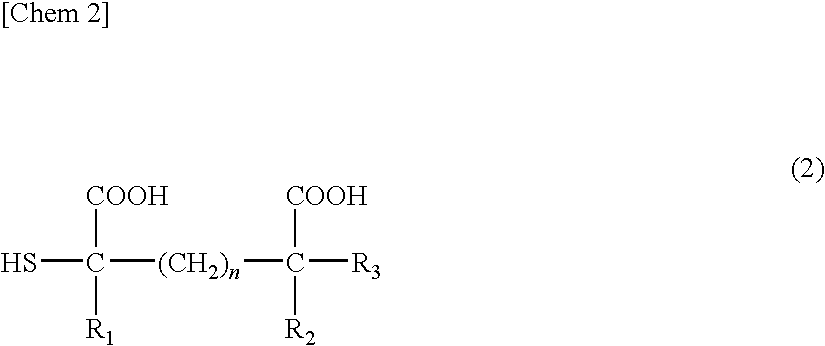
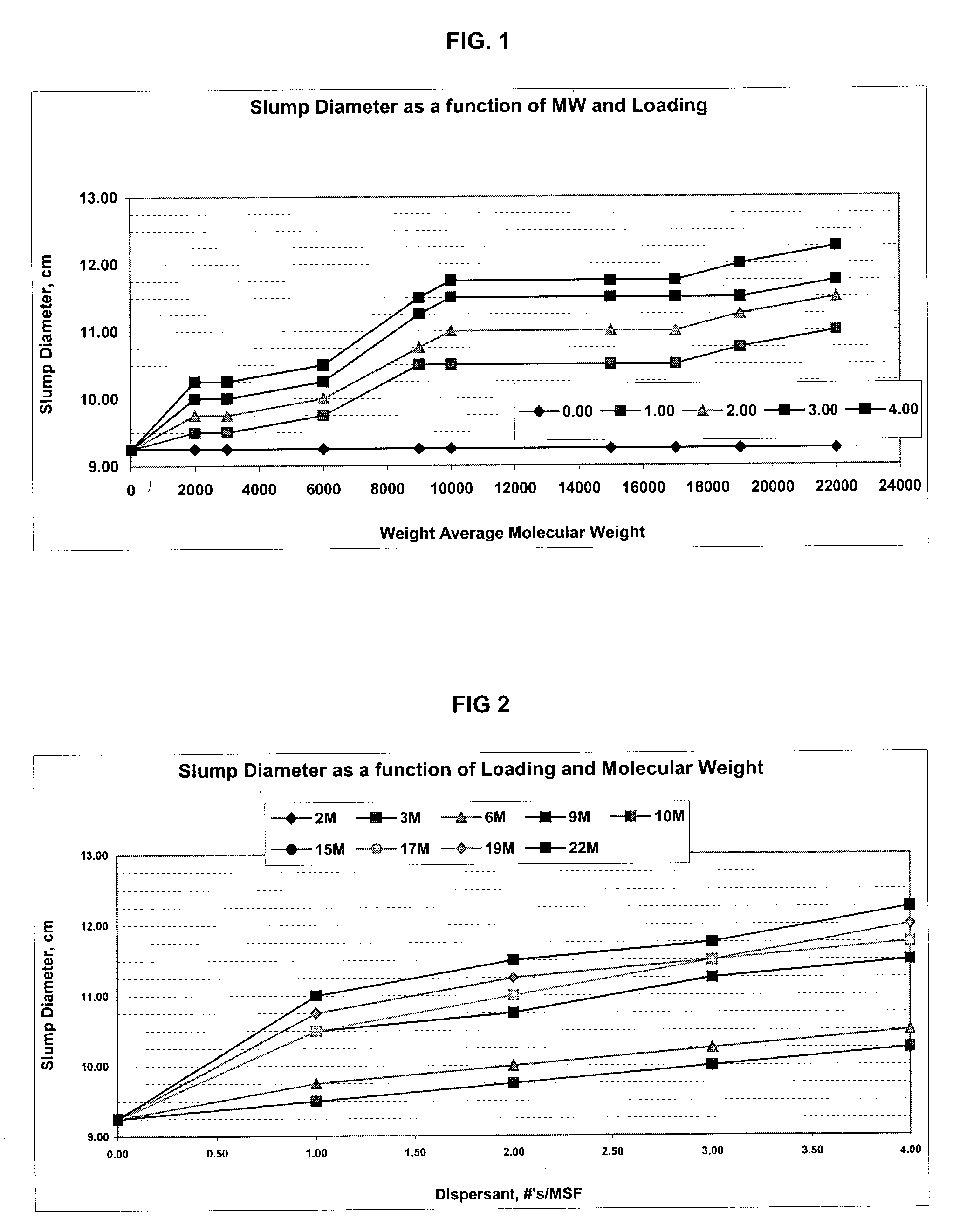
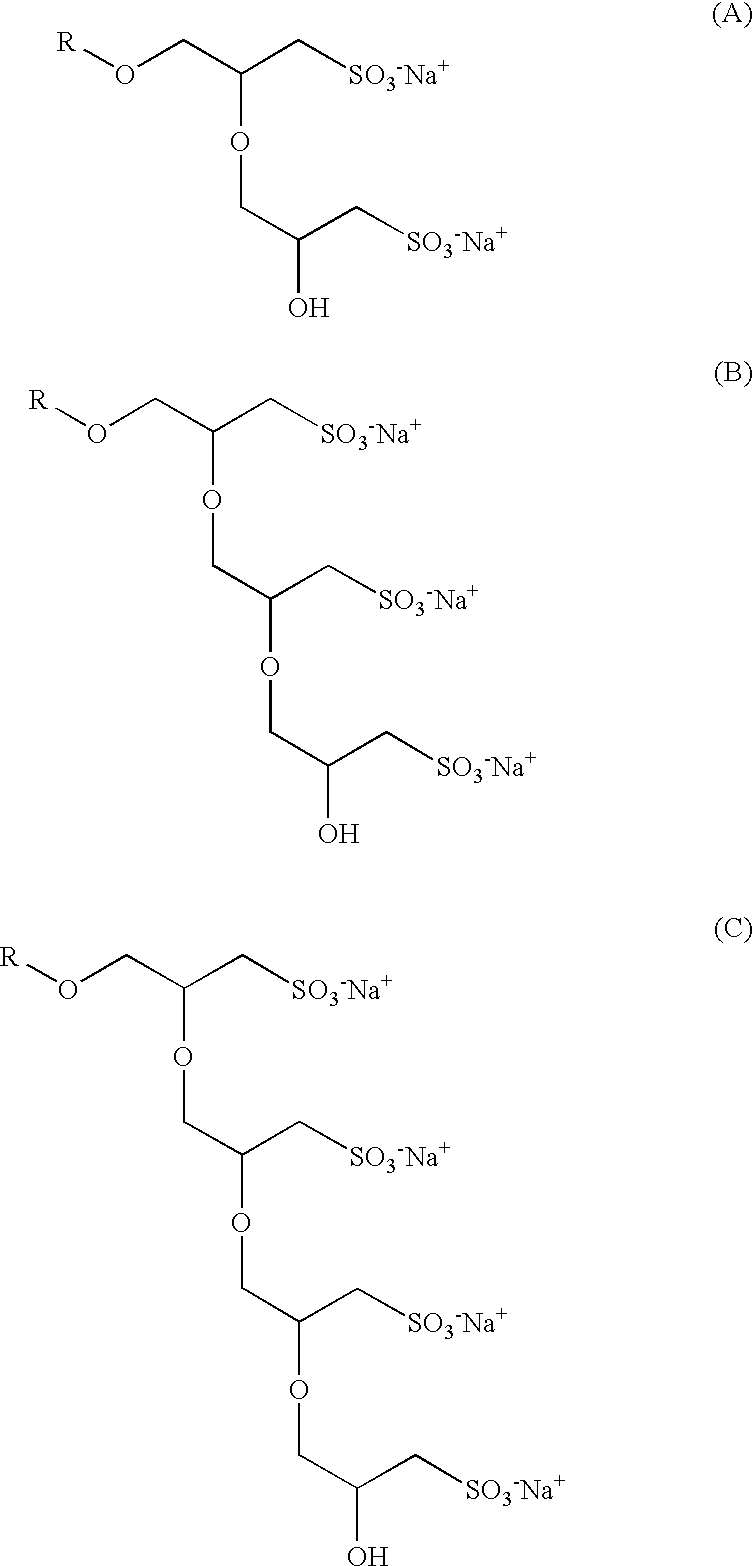
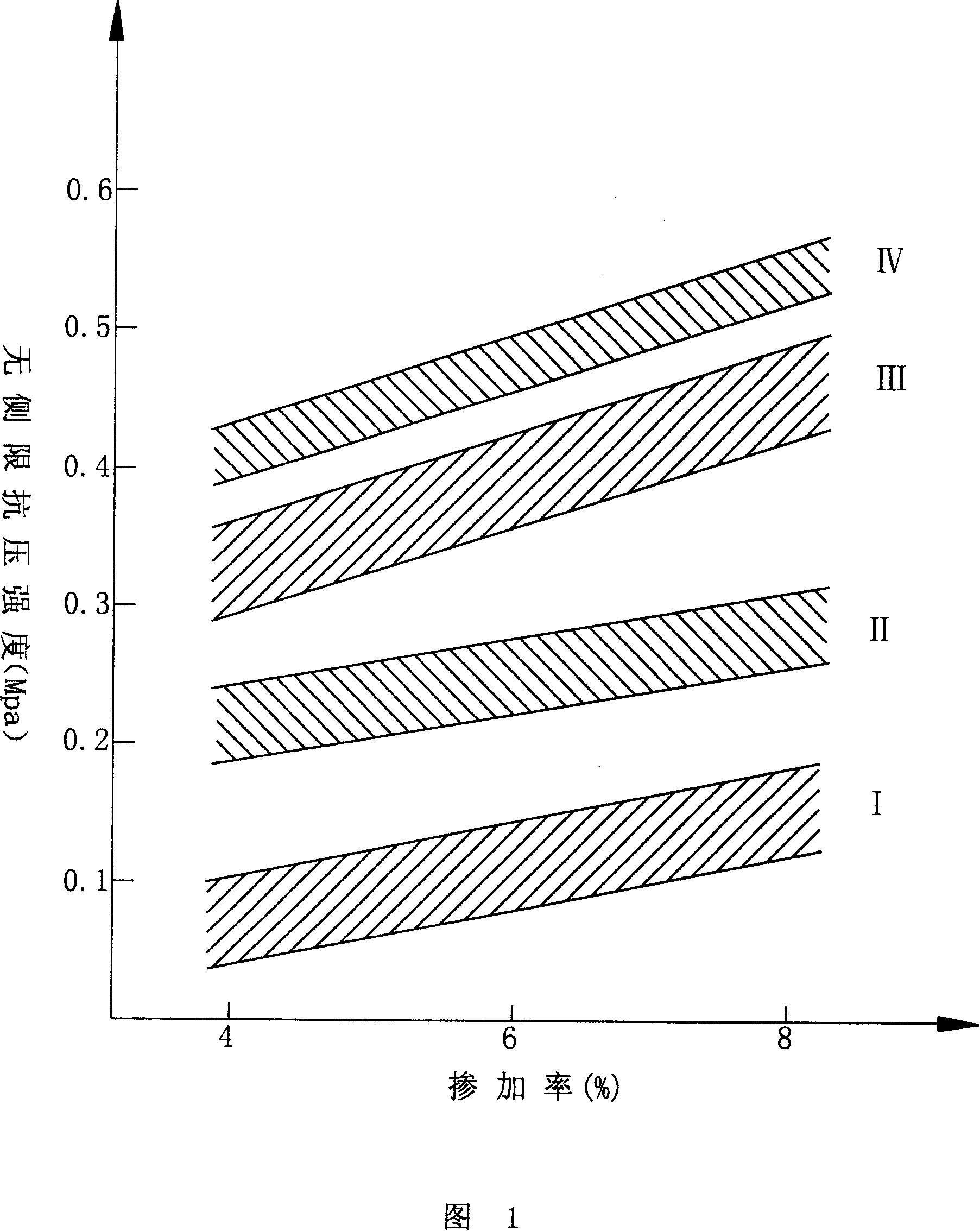
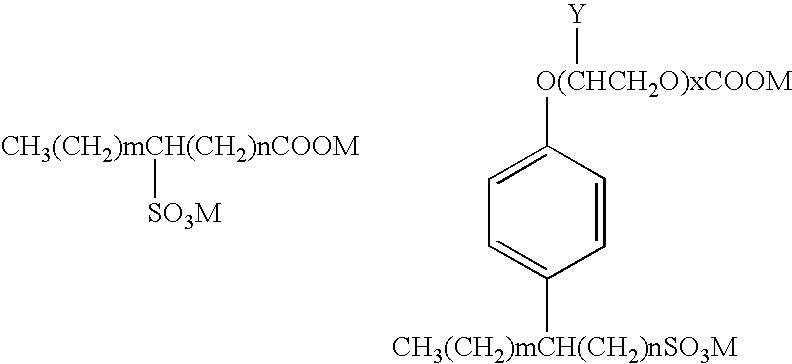
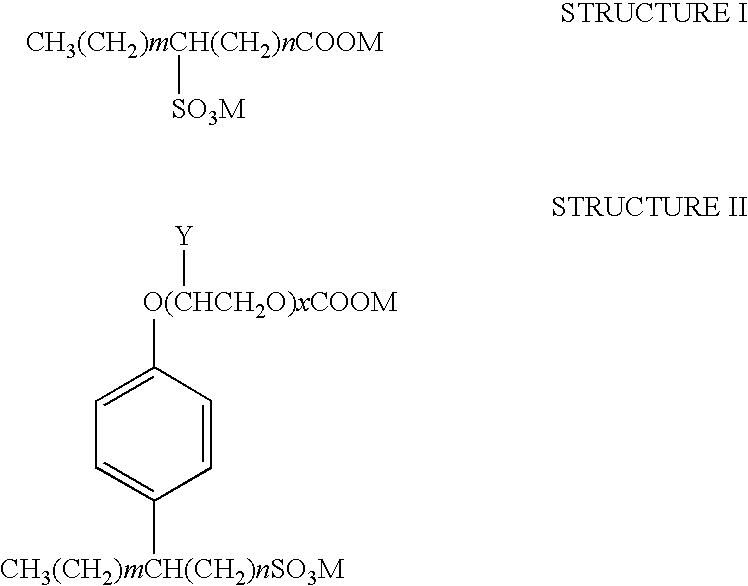
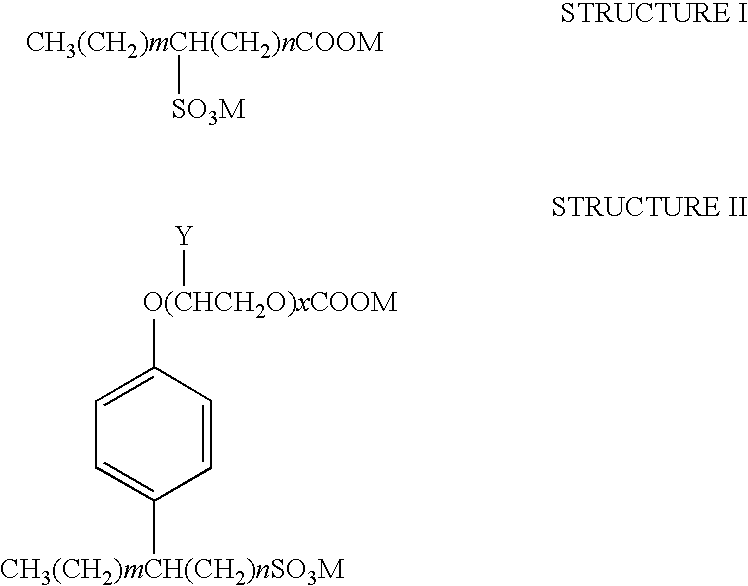
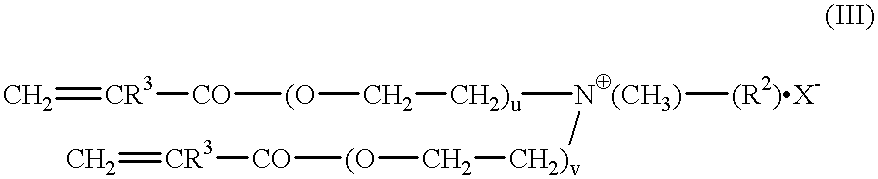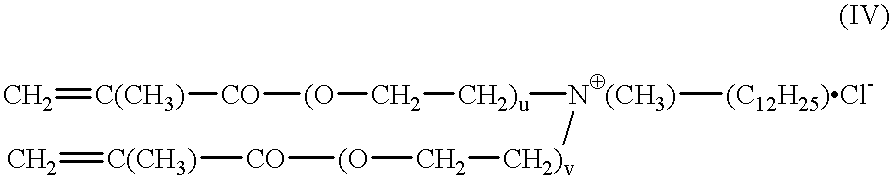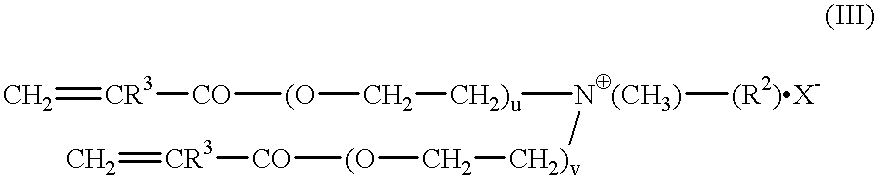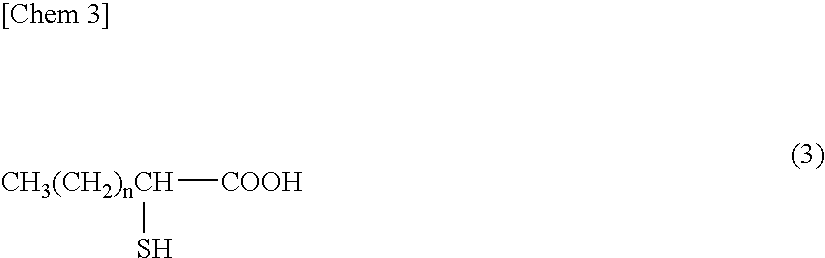Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4339 results about "Sulphate salt" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
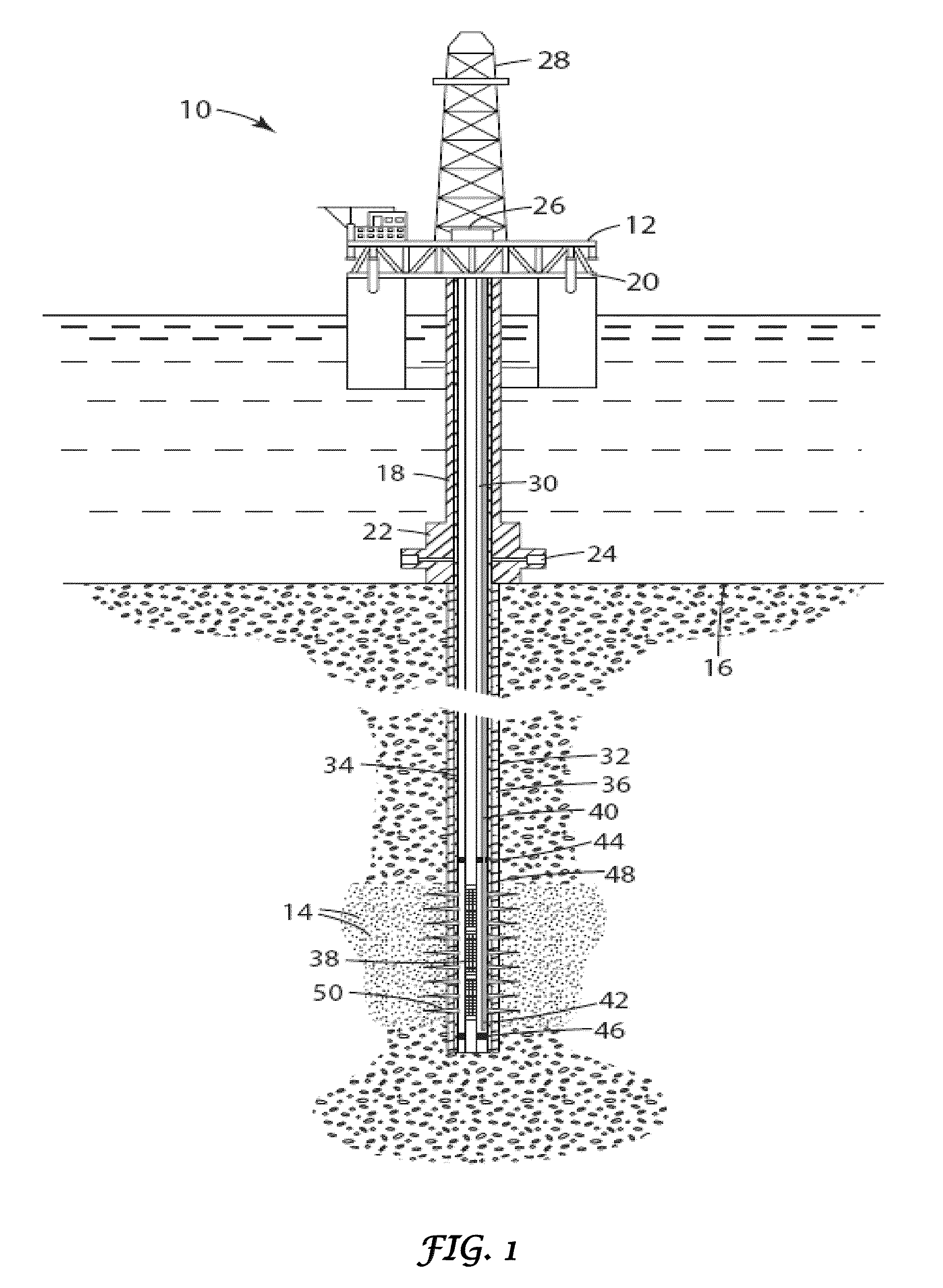
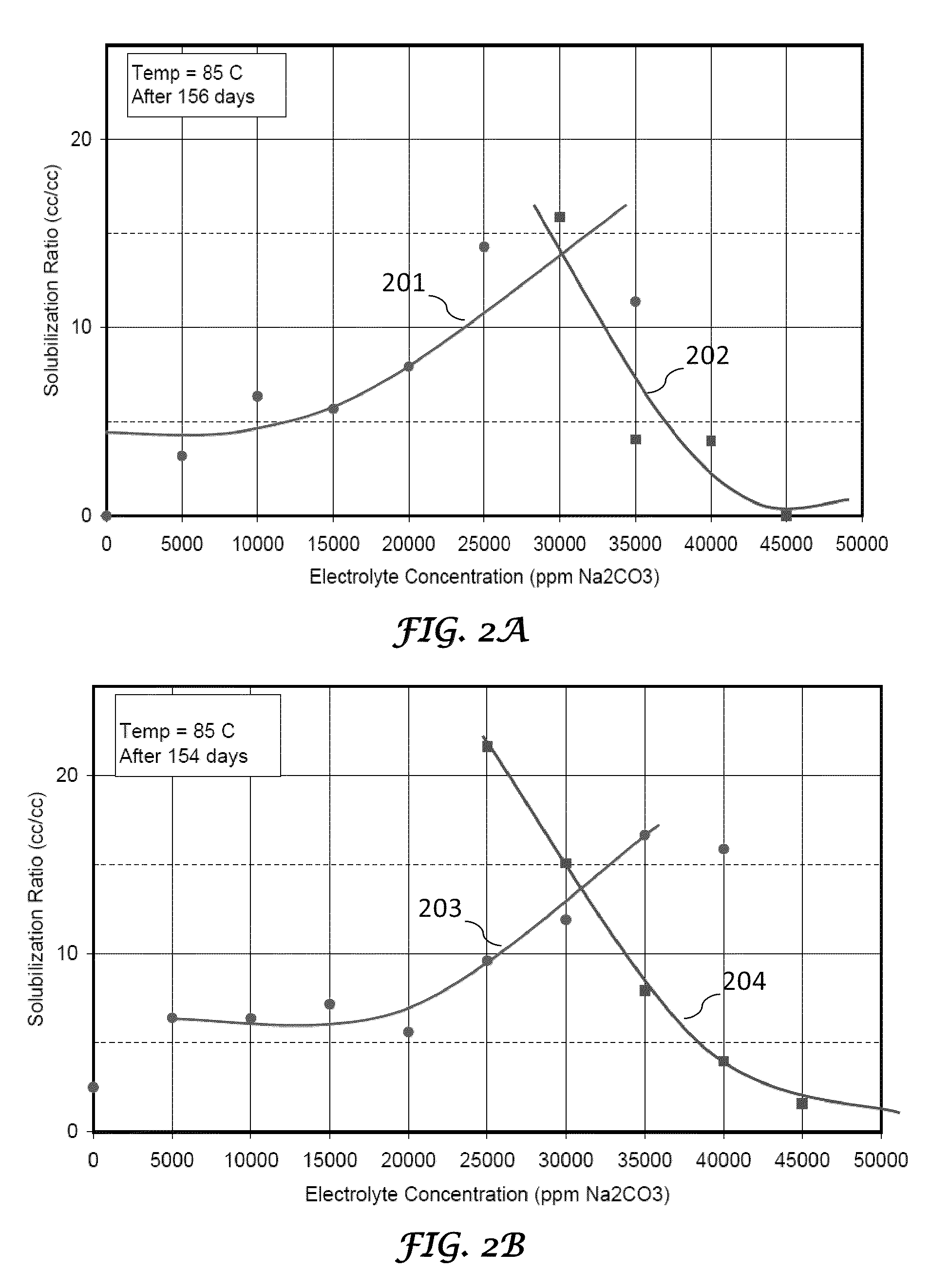
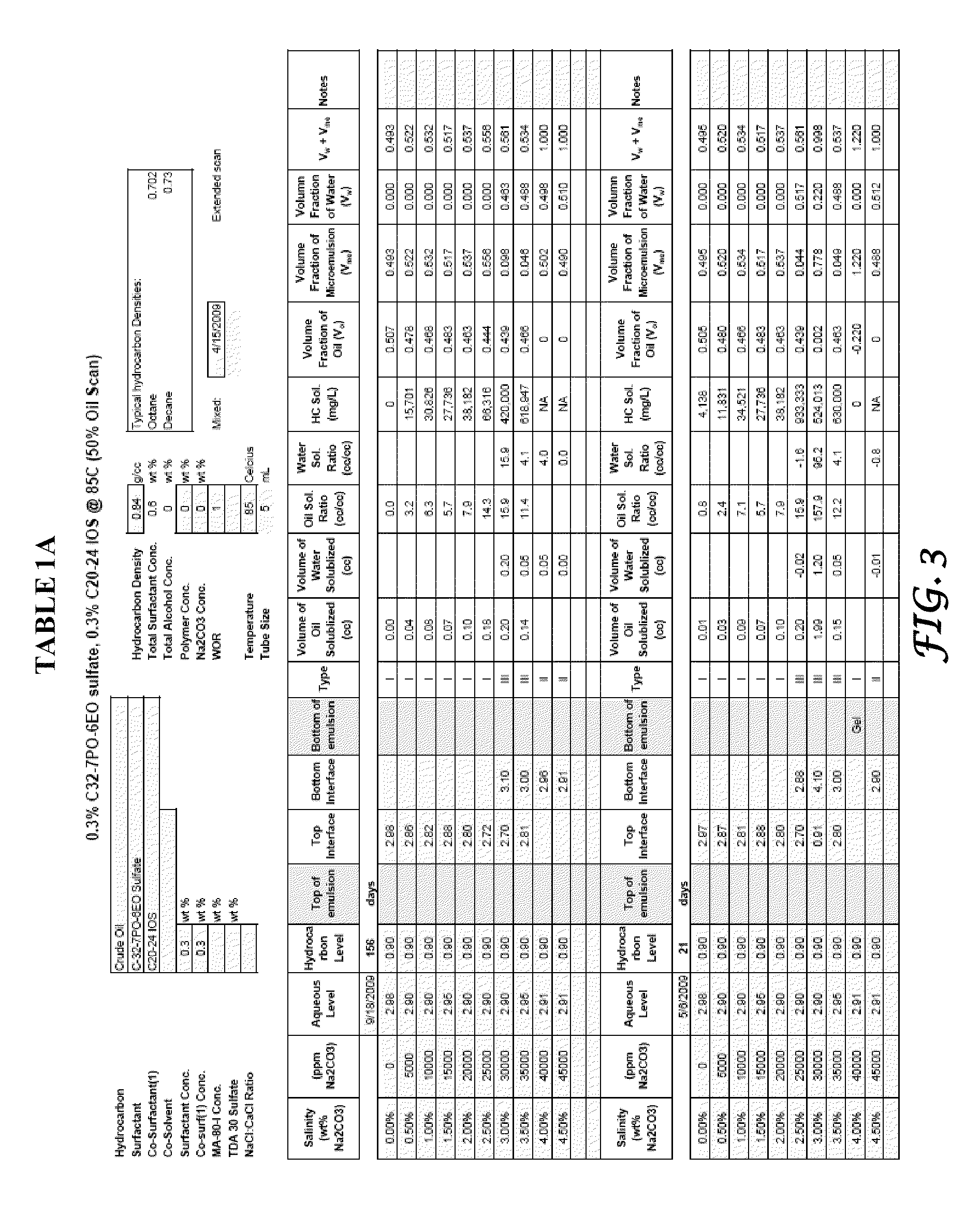
Method of manufacture and use of large hydrophobe ether sulfate surfactants in enhanced oil recovery (EOR) applications
ActiveUS8211837B2Improve usabilityLow costOrganic chemistryOrganic compound preparationSulfationAlcohol
The present invention describes the method of making anionic ether sulfate surfactants by alkoxylation of a GA using PO and / or EO followed by a sulfation reaction. The GA of the present invention is made by a facile and inexpensive method that involves high temperature base catalyzed dimerization of a linear alcohol. The ether sulfate surfactants of the present invention find uses in EOR applications where it is used for solubilization and mobilization of oil and for environmental cleanup.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Polymers with anti-microbial properties
The invention relates to polymers with antimicrobial properties, consisting of: a) 99-40 wt % non functional vinylically polymerizable monomers and b) 1-60 wt % functional vinylically polymerizable monomers of general formula (I) wherein V=vinyl, (meth)acroyl, allyl or styryl, A=a possibly available linking unit, which can be alkyl, aryl, arylalkyl or hydroxy alkyl, which can also be interrupted by hetero atoms, e.g. by hetero atoms in urethane, carbonate, ester, amide or ether groups, wherein y=0 or is 1, Hsp=a hydrophilic spacer of general formula (i) -(O-CH2-CH2)r- and / or (ii) -(O-CH2-CH(CH3))s with r=0-40, s=0-40 and r+s=2-40, also m=1.2 or 3 and R1=CH3, ethyl or benzyl, R2=an alkyl radical with 8-20 C-atoms, wherein t=1, 2 or 3 and X-=Cl-, Br-, I- or alkyl sulphate.
Owner:EVONIK ROEHM GMBH
Hydrogen peroxide disinfectant with increased activity
InactiveUS6346279B1High activityReduced activityBiocideInorganic phosphorous active ingredientsDisinfectantPhosphoric acid
An acidic aqueous hydrogen peroxid solution is provided, with improved disinfectant activity. Concentrated solutions preferably contain up to about 8% and as-used concentrations contain about 0.5% peroxide. The solution also contains from 0.1 to 5.0% of at least one acid compound, e.g. phosphoric and / or a phosphonate with from 1 to 5 phosphonic acid groups, and from 0.02 to 5% of at least one anionic surfactant. The surfactant is selected from C8 to C16-alkyl aryl sulphonic acids, sulphonated C12 to C22 carboxylic acids, C8 to C22-alkyl diphenyl oxide sulphonic acids, naphthalene sulphonic acids, C8 to C22 alkyl sulphonic acids, and alkali metal and ammonium salts thereof, and alkali metal C8 to C18 alkyl sulphates, and mixtures thereof. Most preferably the solution has an emulsifier, e.g. a salt of an alkylated diphenyl oxide. The solution may also contain corrosion inhibitors and / or lower alcohols.
Owner:VIROX TECH
Aqueous suspension of agrochemical
InactiveUS6306414B1Low viscosityEffective dispersionOrganic active ingredientsBiocideSuspended particlesPhenyl Ethers
The present invention relates to an aqueous suspension comprising (i) a compound of the formula:or a salt thereof, (ii) a condensate of formaldehyde with an aromatic sulfonic acid or a salt thereof or a polyoxyalkylene allyl phenyl ether sulfate, and (iii) an absorptive water-soluble polymer. The aqueous suspension of the invention can be used with advantage as a stable aqueous suspension of low viscosity providing for excellent delivery from a container, with excellent dispersibility in diluent water and excellent long-term fluidity free from caking due to precipitation of the suspended particles.
Owner:SUMITOMO CHEM CO LTD
Water-soluble film
ActiveUS20090291282A1Good water solubilitySynthetic resin layered productsInksPolymer sciencePolyvinyl alcohol
The present invention relates to a water-soluble film, which is a polyvinyl alcohol film comprising a polyvinyl alcohol resin (A), wherein the water-soluble film has a time for dissolution in water at 20° C. of not more than 60 seconds in terms of a film thickness of 76 μm, a b-value of the film is not more than 0.5, and a b-value of the film after leaving at 80° C. for 72 hr is not more than 1.0, preferably a water-soluble film comprising: a polyvinyl alcohol resin (A); at least two kinds of plasticizers (B); and a sulfite salt (C), wherein the content of the plasticizer (B) based on 100 parts by weight of the polyvinyl alcohol resin (A) is 5 to 50 parts by weight and the content ratio of the sulfite salt (C) to the plasticizer (B) (C / B: weight ratio) is more than 0.02 and not more than 0.35. According to the invention, there is provided a water-soluble film comprising a PVA resin as a main component, wherein the film is less likely to cause coloration at the time of film formation and coloration with time even upon contact with a chemical.
Owner:MITSUBISHI CHEM CORP
Reactive compositions for fluid treatment
InactiveUS20030196966A1Perfluorocarbons/hydrofluorocarbons captureLoose filtering material filtersFiltrationPhosphate
A method and device for the chemical conversion, filtration and / or purification of fluids water or other solutions containing microbiological and chemical contaminants, such as fluids containing arsenic, chlorine, bacteria, viruses, and cysts, where the fluid is passed through a purification material composed of fluid treatment carbon, metal phosphates, metal oxides, reduced metals, metal silicates, metal sulfates, metal carbonates, metal hydroxides, or combinations thereof. The material may be included in a fixed binder matrix.
Owner:WATERVISIONS INT
Non-thrombogenic and anti-thrombogenic polymers
InactiveUS6096798AEasy to attachReducing the thrombin-antithrombin complex concentrationCosmetic preparationsImpression capsPolymer scienceThrombogenicity
PCT No. PCT / GB97 / 01173 Sec. 371 Date Apr. 30, 1999 Sec. 102(e) Date Apr. 30, 1999 PCT Filed Apr. 30, 1997 PCT Pub. No. WO97 / 41164 PCT Pub. Date Nov. 6, 1997Polymers having non-thrombogenic properties can be prepared by copolymerizing monomers of at least three classes selected from (a) monomers having sulphate groups, (b) monomers having sulphonate groups, (c) monomers having sulphamate groups, (d) monomers having polyoxyalkylene ether groups, and (e) monomers having zwitterionic groups. The polymers can additionally be provided with anti-thrombogenic properties by including an additional comonomer having a pendant heparin (or hirudin, warfarin or hyaluronic acid) group. The polymers can be used as coating materials for medical devices, such as tubing or connectors, in order to provide them with non-thrombogenic, and optionally anti-thrombogenic, properties.
Owner:BIOINTERACTIONS
Recovery of common salt and marine chemicals from brine
InactiveUS6776972B2High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Prolonged release bioadhesive therapeutic systems
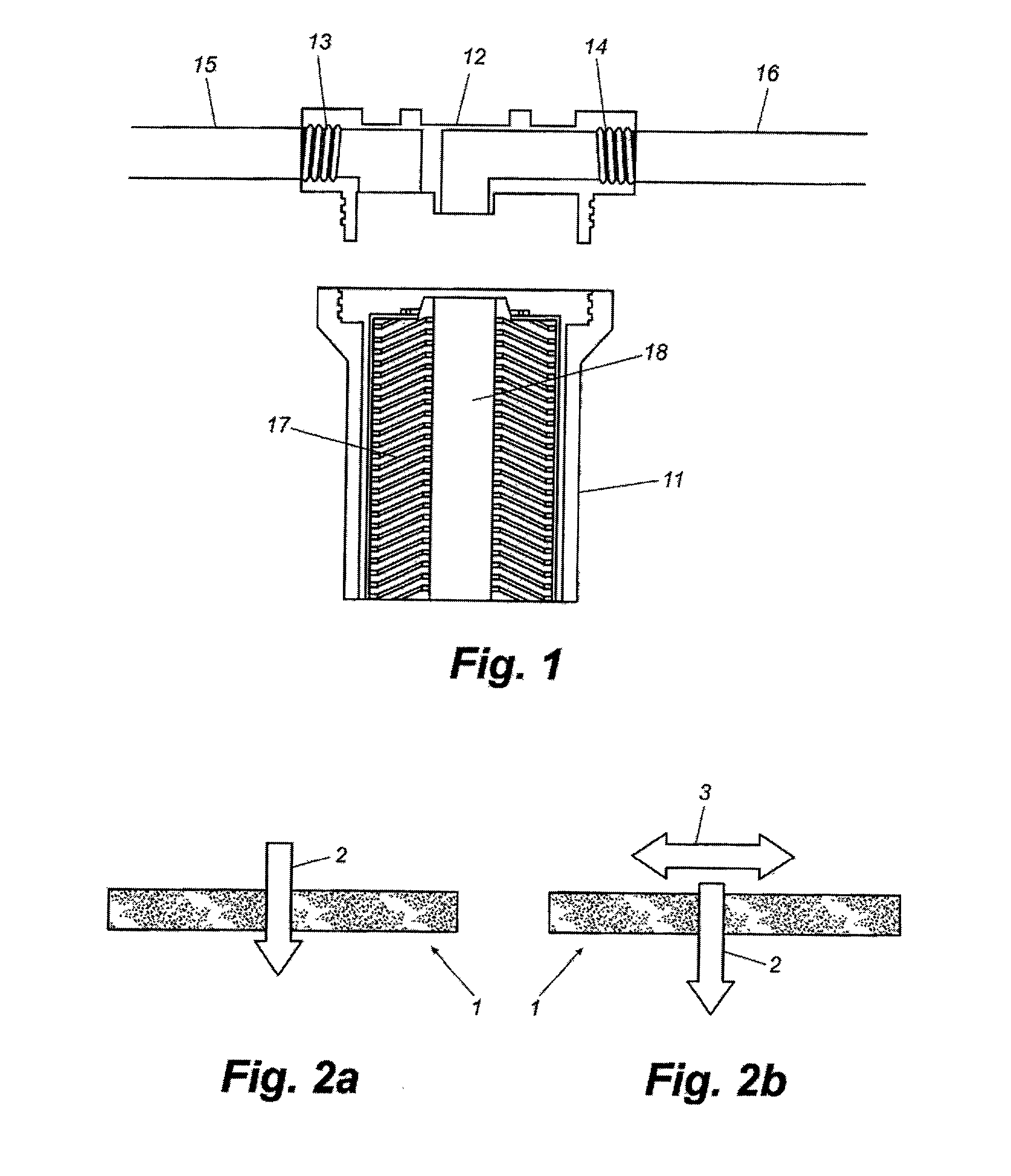
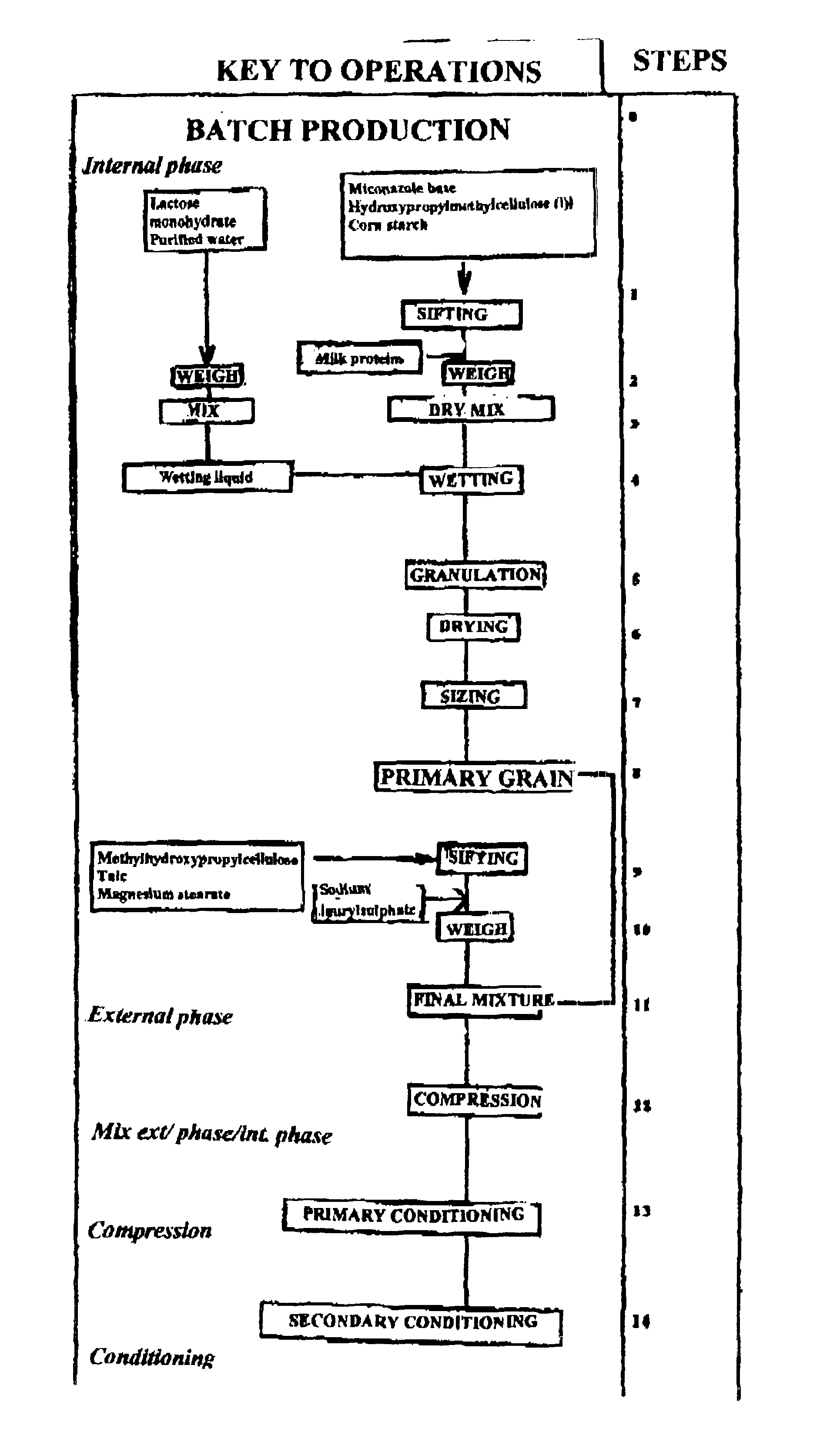
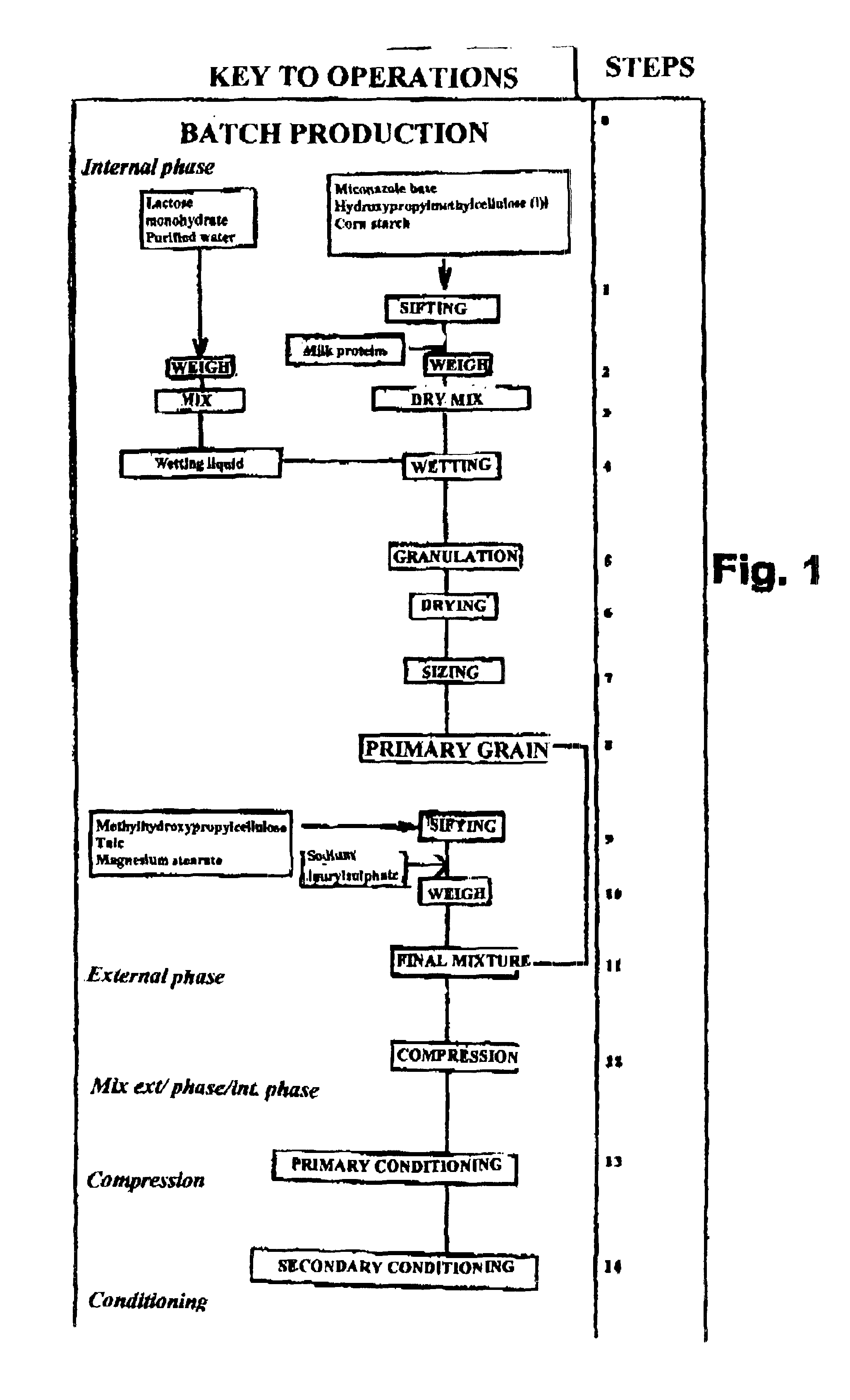
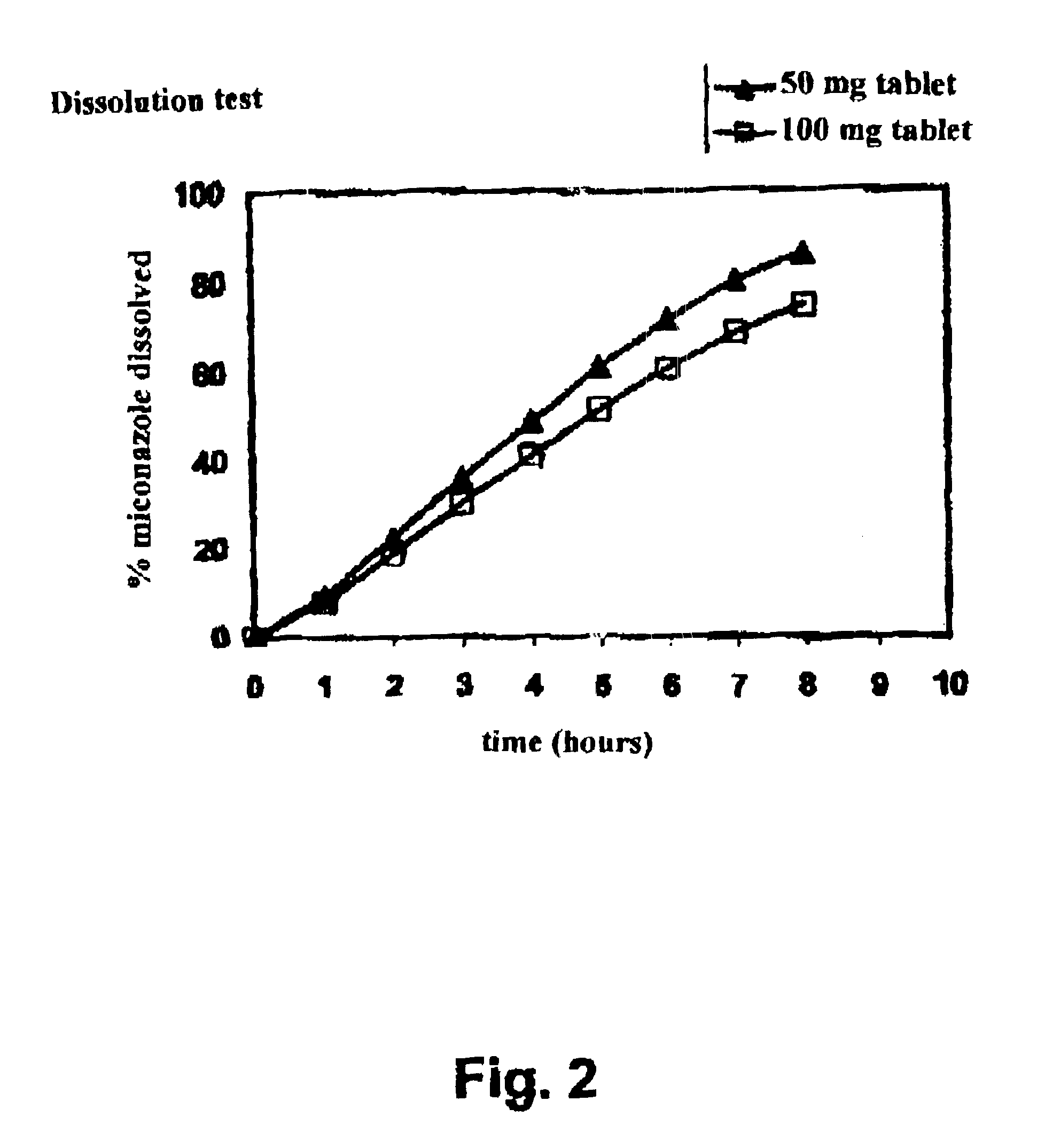
The present invention concerns a prolonged release bioadhesive mucosal therapeutic system containing at least one active principle, with an active dissolution test of more than 70% over 8 hours and to a method for its preparation. The bioadhesive therapeutic system may be in tablet form and may contain quantities of natural proteins representing at least 50% by weight of active principle and at least 20% by weight of the tablet, between 10% and 20% of a hydrophilic polymer, and compression excipients, and may contain between 4% and 10% of an alkali metal alkylsulphate to reinforce the local availability of active principle and between 0.1% and 1% of a monohydrate sugar.
Owner:VECTANS PHARMA
Dispersant and foaming agent combination
InactiveUS20040028956A1Low densityReduce weightConstruction materialOther chemical processesAlkaline earth metalAir entrainment
The present invention provides a dispersant and foaming agent combination that is useful in the production of gypsum wallboard and other aqueous cementitious products, a method of forming a gypsum wallboard and a gypsum wallboard. The dispersant in the combination according to the invention is a naphthalene sulfonate-aldehyde condensate alkali salt polymer having a weight average molecular weight of from about 17,000 to about 47,000. The alkali is preferably an alkali metal and / or an alkaline earth metal. The aldehyde is preferably formaldehyde. The foaming agent used in the combination according to the invention is a soap, preferably an alkali salt of an alkyl ether sulfate and / or an alkyl sulfate. The combination of a high molecular weight dispersant and a foaming agent produces a gypsum wallboard core effect that more efficiently entrains air (i.e., creates void space), thereby lowering overall board weight without detrimentally affecting strength. A gypsum wallboard formed using the dispersant and foaming agent combination according to the invention exhibits a higher nail pull value than gypsum wallboard formed using a conventional dispersant and a foaming agent at the same solids loading ratio.
Owner:GEO SPECIALTY CHEM
Oligomeric alkyl glyceryl sulfonate and/or sulfate surfactant mixture and a detergent composition comprising the same
Owner:THE PROCTER & GAMBLE COMPANY
Sludge curing agent and application thereof
ActiveCN101081718AGood boardIncreased durabilitySludge treatment by de-watering/drying/thickeningSolid waste managementSludgeSlag
The present invention is sludge curing agent and its application, and belongs to the field of soil treating chemicals technology. The sludge curing agent includes powdered components and liquid components, the powdered components include cement clinker 30-60 weight portions, slag 30-60 weight portions, lime 3-8 weight portions, gypsum 1-7 weight portions and other sulfates 1-7 weight portions; and the liquid components include polyacrylamide 5-30 weight portions, polyaluminum chloride 0-20 weight portions, mannitol 0-30 weight portions, lignosulfonate 20-80 weight portions, lignosulfonate-iron or chromium ion complex 0-30 weight portions, alkylphenol ethoxylate 0.2-2 weight portions, tannin 0-10 weight portions, humate 0-10 weight portions, and alpa-olefin sulfonate 0.2-2.5 weight portions. The sludge curing agent has low cost, small consumption, high cumulate strength and high cumulate water tolerance, and may be applied widely.
Owner:BEIJING ZHONGYONGJI FIRMING AGENT TECH DEV
Salt solution for colon cleansing
The field of colonic diagnostic and surgical procedures is hampered by the lack of optimal means available to cleanse the colon. A compromise between convenient, distasteful, solid or low volume, hyperosmotic solutions which cause considerable fluid and electrolyte imbalances in patients and large volume, difficult to consume, iso-osmotic solutions has had to be made heretofore. This invention describes a low volume, hyper-osmotic solution consisting of sulfate salts with and with out polyethylene glycol. Unlike prior art, this composition is useful for the cleansing of the bowel and, in lower volumes, as a laxative, without producing clinically significant changes in bodily function.
Owner:BRAINTREE LAB
Method to decrease iron sulfide deposits in pipe lines
InactiveUS6866048B2Inorganic/elemental detergent compounding agentsAnionic surface-active compoundsPhosphoniumSulfate
This invention provides a method of treating a dry or processed fluid pipe line susceptible to the build-up of iron sulfide deposits by complexing the iron sulfide in the pipe lines. The method of the present invention introduces the composition on a continuous or a batch basis to a gas pipe line. The composition is made of a solution of 1) water, 2) [tetrakis(hydroxymethyl)phosphonium]sulfate or chloride, and 3) a soluble ammonium salt, such as ammonium chloride or the like.
Owner:COASTAL CHEM CO L L C
Mixed anionic surfactant composition for oil recovery
InactiveUS20070191633A1Enhanced overall recoveryMinimum adsorptionOrganic chemistryDrilling compositionSulfateSURFACTANT BLEND
A composition for recovering oil from subterranean formation by injecting an aqueous fluid containing from about 0.05 to about 2.0% by weight of a bi-functional surfactant or a mixture of surfactants containing one or more of the following structures;Optionally the aqueous fluid may contain mixtures of individual surfactants having carboxylic, and sulfonate or sulfate functionalities. The remainder of the composition includes water or brine, a cosolvent and optionally a viscosity control agent, and optionally an alkali.
Owner:BERGER CHRISTIE HUIMIN
Low-foaming hydrogen peroxide cleaning solution for organic soils
InactiveUS6686324B2Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsParticulatesAlkane
A low-foaming cleaning solution and dry particulate formulation which can be diluted with water, deionized water, or mixtures thereof, to form the cleaning solution. The cleaning solution has an alkaline pH, which is preferably from about 8 to about 11.5 and consists essentially of at least one low foaming surfactant in a concentration of from about 0.005% to about 40% w / w of the total solution, at least one active oxygen releasing compound in an amount effective to produce a hydrogen peroxide concentration of from about 0.005% to about 50% w / w of the total solution, at least one builder in a concentration of from about 0.001% to about 50% w / w of the total solution, and at least one diluent selected from the group consisting of water, deionized water, and mixtures thereof. The at least one surfactant is selected from the group consisting of C3-C8 alkane sulfonates, C3-C8 alkyl sulfates, C1-C7 alkyl naphthalene sulfonates, polyoxyethylene / polyoxypropylene block copolymers having a polyoxypropylene molecular weight of from about 1500 to about 8500, of which less than about 30% of the total molecular weight is due to the polyoxyethylene portion, and mixtures thereof. The at least one active oxygen releasing compound is selected from the group consisting of hydrogen peroxide, at least one source of hydrogen peroxide, and mixtures thereof.
Owner:JOHNSONDIVERSEY INC
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
Process for Producing Self-Assembling Peptide Derivatives
ActiveUS20150175663A1Economical and efficientMass productionPeptide/protein ingredientsImmunoglobulinsTrifluoroacetic acidDisulfuric acid
An object of the present invention is to provide a process capable of producing a self-assembling peptide derivative that is useful in the fields of regenerative medicine and surgery in large quantities and in an economical and efficient manner. In particular, provided is a production process employing a combination of (i) a step of convergently constructing a sequence with use of a common repeating unit consisting of a specific amino acid sequence and (ii) a step of first isolating the peptide derivative as a disulfuric acid salt, a tetramethanesulfonic acid salt or a tetra(trifluoroacetic acid (TFA) salt), and then subjecting the peptide salt to a salt exchange reaction to yield a tetrahydrochloric acid salt.
Owner:MENICON CO LTD
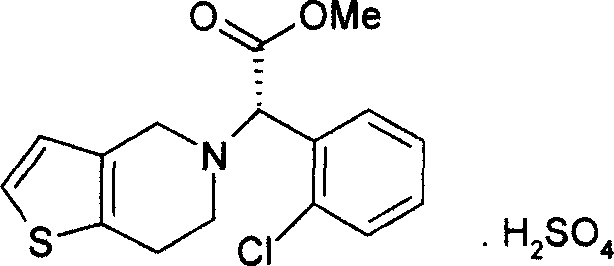
Clopidogrel sulfate solid preparation, and its preparing method
ActiveCN1935119AOrganic active ingredientsPharmaceutical delivery mechanismClopidogrel HydrochlorideSilica gel
The present invention relates to a solid preparation of clopidogrel hydrochloride and its preparation method. It is characterized by that in said sold preparation the palmitoleostearin and micropowder silica gel are added, and its preparation method adopts a grinding equivalent progressively-increasing method so as to raise the stability and safety of said solid preparation.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
FeS and Fe0 composite and preparation method and application thereof
InactiveCN105174414AEfficient degradationHigh reactivityIron sulfidesWater/sewage treatment by reductionWastewaterSurface water
The invention belongs to the field of chemical materials, and particularly relates to a FeS and Fe0 composite and a preparation method and application thereof. The FeS and Fe0 composite is formed by compositing nano FeS and nano Fe0, the surface of the nano Fe0 is coated with the nano FeS, and the molar ratio of the nano FeS to the nano Fe0 is 2:1 to 15:1. According to the FeS and Fe0 composite and the preparation method and application thereof, the preparation process is simple, convenient, environmentally friendly and feasible, the prepared FeS and Fe0 composite is high in reaction activity, capable of efficiently and rapidly reducing and adsorbing heavy metal ions in waste water within a wider temperature range, a pH value range and a dissolved oxygen content range and further capable of rapidly and efficiently activating hydrogen peroxide and persulfate to make a system generate hydroxyl free radicals and sulfate free radicals to degrade and mineralize organic pollutants, and therefore the FeS and Fe0 composite can be widely applied to degradation of the organic pollutants in surface water and underground water.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Non-linear associating water-soluble quadripolymer, and preparation and use thereof
InactiveCN101463116AImprove rigidityHigh temperature resistanceTransportation and packagingMixingIonChemistry
The invention discloses a non-linear associated water soluble quadripolymer as well as a preparation method and use thereof, comprising: adding 20 parts of acrylamide, 1-20 parts of nionic monomer or / and cationic monomer, 0.1-15 parts of macromonomer, 0.05-10 parts of hydrophobic monomer, 0.1-50 parts of surfactant and 60-1000 parts of deionized water to a three-necked reaction flask, adjusting the pH to be 3-9, adding 0.002-1 part of initiator (persulphate) at 30-75 DEG C after introducing N2 for 30min, reacting for 8-36h to obtain the quadripolymer PACH, diluting with water, and obtaining concentrated PACH solution. The macromonomer with a long chain and the hydrophobic monomer with a molecular association function are simultaneously introduced in a copolymer PACH, which can obtain the best synergistic viscosifying between rigid conformation of a molecular chain and molecular association and salt resistance. The non-linear associated water soluble quadripolymer is obtained. The copolymer is prepared into aqueous solution with the mass concentration of 0.2-3g / l and the surfactant concentration of 0.01-2mmol / l, added to a mixing vessel with a stirring device and evenly stirred at the room temperature to obtain a viscosified, salt-resistant and shear-resistant polymer oil displacement agent. The PACH has dual functions of a viscosifier and a high molecular surfactant; minute amount of a low molecular surfactant is added to the PACH solution, thus improving the apparent viscosity of the solution, reducing the surface tension of solution and the water-oil interfacial tension, and being beneficial to improving the crude oil recovery ratio. A copolymer PABE is prepared into the aqueous solution with the mass concentration of 0.05-7% to obtain high molecular surfactant with excellent surface activity, and the high molecular surfactant is used as an emulsifier, a demulsifier, a solubilizer and a wetting agent.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Method of preventing hydrogen sulfide odor generation in an aqueous medium
ActiveUS20060006120A1Water treatment compoundsSpecific water treatment objectivesBiotechnologyChemical treatment
The present invention relates to a fast acting chemical treatment for preventing the generation of hydrogen sulfide odor by the microbial metabolic activities of sulfate reducing bacteria. Specifically, the invention relates to a method for preventing hydrogen sulfide odor generation in a sulfur species-containing aqueous medium, which includes adding to the aqueous medium an effective amount for the purpose of a sulfide scavenger treatment selected from the group consisting of glyoxal, triazine, n-chlorosuccinimide, and mixtures thereof.
Owner:BL TECH INC
Synthesizing microcapsules of storing energy through phase change by using method of emulsion polymerization
A process for synthesizing the phase-change energy-accumulating microcapsules by emulsion polymerizing method features that the liposoluble organic phase-change material, vinyl or bivinyl monomers, water, non-ionic or anionic surfactant and the persulfate, percarbonate, perborate, hydrogen peroxide, or their redox pair take part is core-shell polymerizing reaction in emulsion to obtain said microcapsules.
Owner:DONGHUA UNIV
Grinding aid for slag cement
The invention provides a grinding aid for slag cement. The grinding aid provided by the invention comprises the following components by weight percent: 5-20wt% of alcohol amine compound, 0.1-5wt% of hydroxypropyl methyl cellulose, 5-30wt% of polyhydric alcohol compound, 0.5-10wt% of sodium hexametaphosphate, 1-10wt% of soluble sulfate, 1-8wt% of sodium dodecyl benzene sulfonate and the balance of water. In the grinding agent provided by the invention, the hydroxypropyl methyl cellulose is beneficial for stimulating the activity of the slag, forming stable hydrous products in the reaction process, improving the property of the cement, improving the strength of the cement and improving the stability of the liquid grinding aid at the same time. Experimental result shows that the grinding aid is capable of increasing the breaking strength of the cement to 9.5MPa and increasing the compressive strength to about 55MPa.
Owner:SHANDONG HONGYI TECH +1
L-sulforamidate type chiral ionic liquid and its prepn
InactiveCN1383920AEasy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSide productIon
The present invention relates to a new type chemical material and its preparation. L-sulforamidate-type chiral ionic liquid is prepared through the full reaction of aqueous L-amino acid solution with98% sulfuric acid. The produced L-sulforamidate-type chiral ionic liquid has the features of both ionic liquid and chiral matter. The reaction has high product purity, low cost, no exhausted pollutant and no side product, and is suitable for application in large scale industrial production. The new material is capable of becoming environmentally friendly important chemical material.
Owner:EAST CHINA NORMAL UNIVERSITY
Sulfate-free or substantially sulfate-free personal care cleansing compositions
Disclosed are personal care cleansing compositions including:a) water;b) up to about 10 wt %, based on the total weight of the personal care cleansing composition, of a surfactant selected from the group consisting of an anionic surfactant, an amphoteric surfactant, a nonionic / anionic surfactant mixture, and combinations thereof;c) a rheology modifying polymer;d) a cationic-substituted guar; ande) a copolymer of acrylamidopropyltrimonium chloride and acrylamide;wherein the personal care cleansing composition is sulfate-free or substantially sulfate-free; and their use in personal care, such as hair care, is also disclosed.
Owner:HERCULES LLC
Biological carbon prepared form grapefruit skin, preparation method and application thereof
ActiveCN106000303ALarge specific surface areaStrong adsorption capacityOther chemical processesWater contaminantsSodium polyacrylateAcrylic acid
The invention discloses a preparation method of biological carbon by using grapefruit skin. The method is as below: drying and pulverizing grapefruit skin, and mixing the grapefruit skin with an aqueous solution of catalyst, and conducting a hydrothermal carbonization reaction; after the hydrothermal carbonization reaction, washing and drying to obtain the biological carbon. The temperature of the hydrothermal carbonization reaction is 160 DEG to 260 DEG C; the catalyst is at least one selected from phosphoric acid, sulfuric acid, hydrochloric acid, acetic acid, citric acid, acrylic acid, sodium polyacrylate, sodium polystyrene sulfonate, zinc chloride, and stannic chloride. In addition, the invention also comprises the biological carbon which is prepared by the method and the application of the biological carbon in the adsorption of heavy metals. The biological carbon material obtained by the invention has the advantages of microsphere particle shape, uniform particle size and high adsorption capacity, and can be applied to the field of adsorption and remediation of heavy metal pollutants or organic pollutants.
Owner:HUNAN AGRICULTURAL UNIV
Clean production process of plateau sulfate type boron-lithium salt lake brine
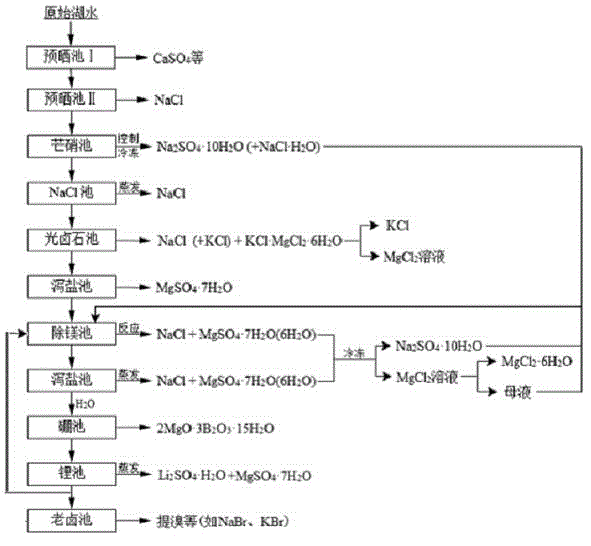
InactiveCN102910652AHigh purityReduce the ratio of magnesium to lithiumChemical industryAlkali metal halide purificationHydration reactionSylvinite
The invention relates to a clean production process of plateau sulfate type boron-lithium salt lake brine. The process comprises the following steps of: (1) arranging a pre-airing pond, a mirabilite pond, a NaCl pond, a carnallite pond, an epsom salt pond I, a magnesium removing pond, an epsom salt pond II, a boron pond, a lithium pond and an old brine pond; (2) controlling the sodium ion concentration in plateau sulfate type boron-lithium salt lake brine, precipitating mirabilite out in winter to obtain brine A, naturally evaporating the brine A, and salting out to obtain brine B; (3) naturally evaporating the brine B, and precipitating sylvine and carnallite out in sequence to obtain brine C; (4) naturally evaporating the brine C, precipitating an epsom salt out, and performing solid-liquid separation to obtain brine D and a solid A; (5) blending the brine D with mirabilite, removing magnesium to obtain brine E, and naturally evaporating brine E to obtain brine F and a solid B; (6) performing a hydration reaction on brine F, naturally evaporating, and precipitating reservoir water / inderite and brine G out; and (7) evaporating brine G or refrigerating for precipitating lithium sulfate, and processing the lithium sulfate into a corresponding product. The process has the advantages of comprehensive utilization of natural energy, saving in energy and environment friendliness.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com