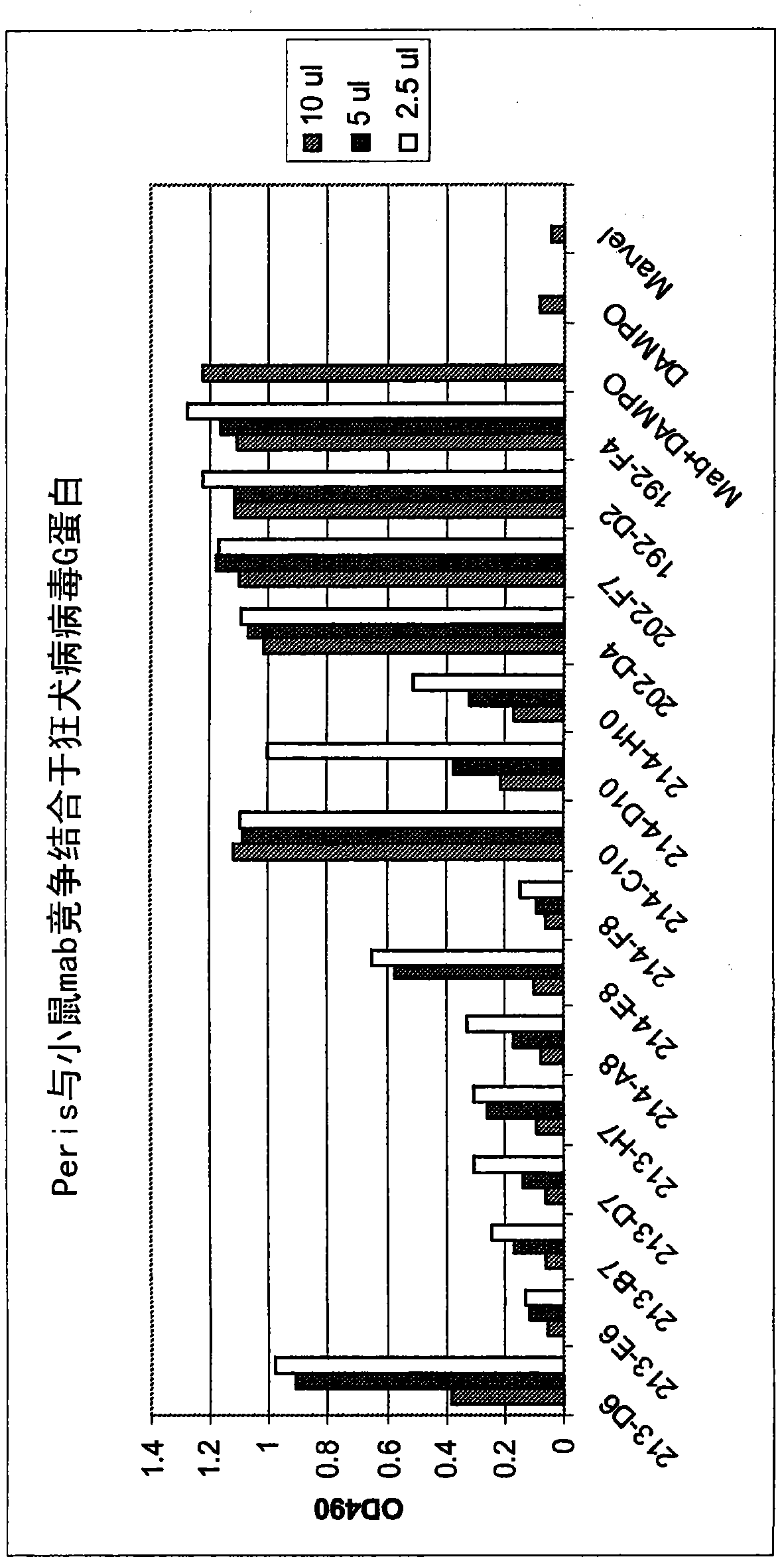
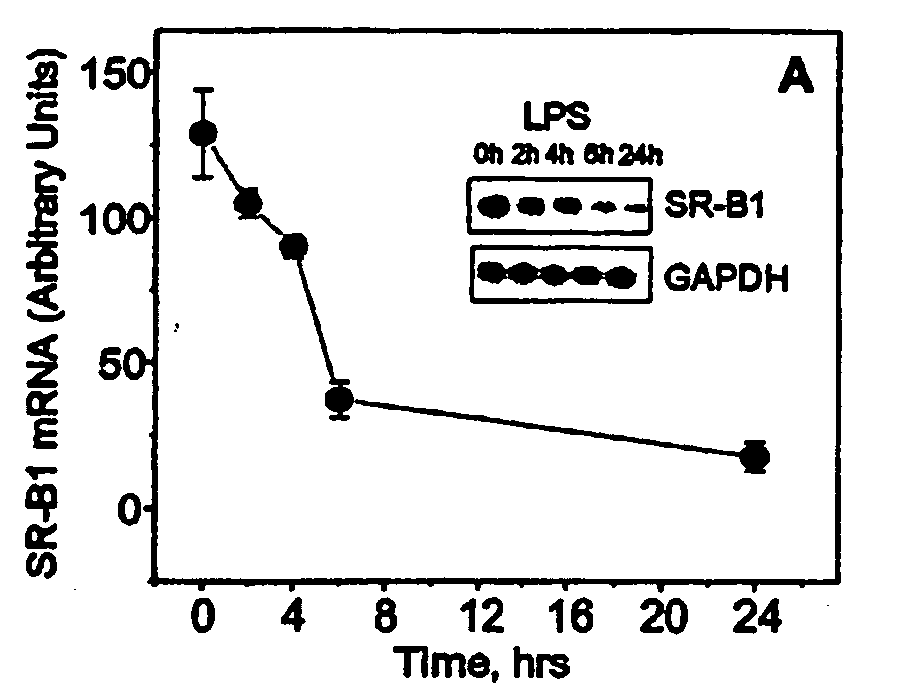
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
206 results about "Virus Envelope Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
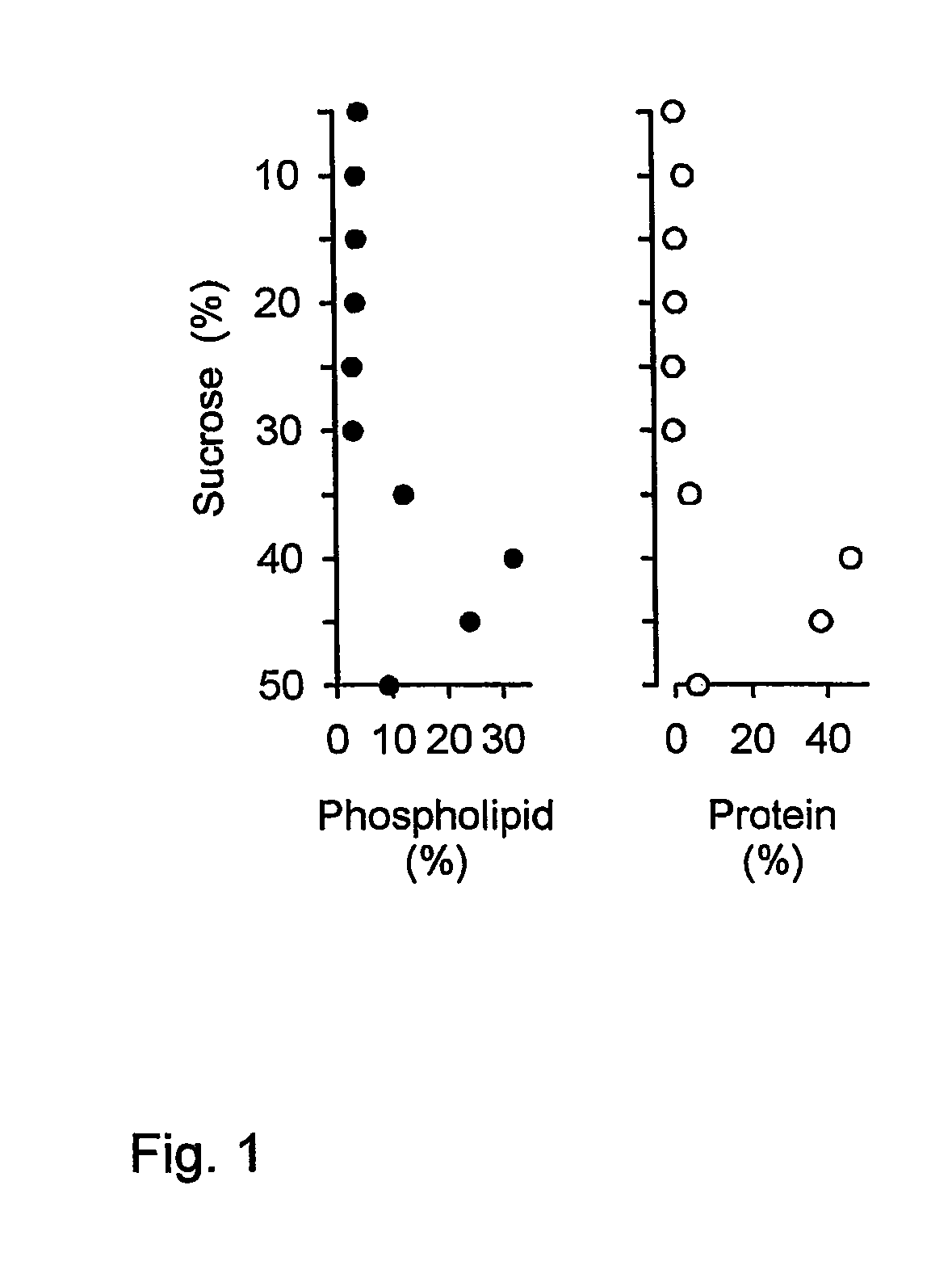
Some viruses (e.g. HIV and many animal viruses) have viral envelopes covering their protective protein capsids. The envelopes are typically derived from portions of the host cell membranes (phospholipids and proteins), but include some viral glycoproteins. They may help viruses avoid the host immune system.
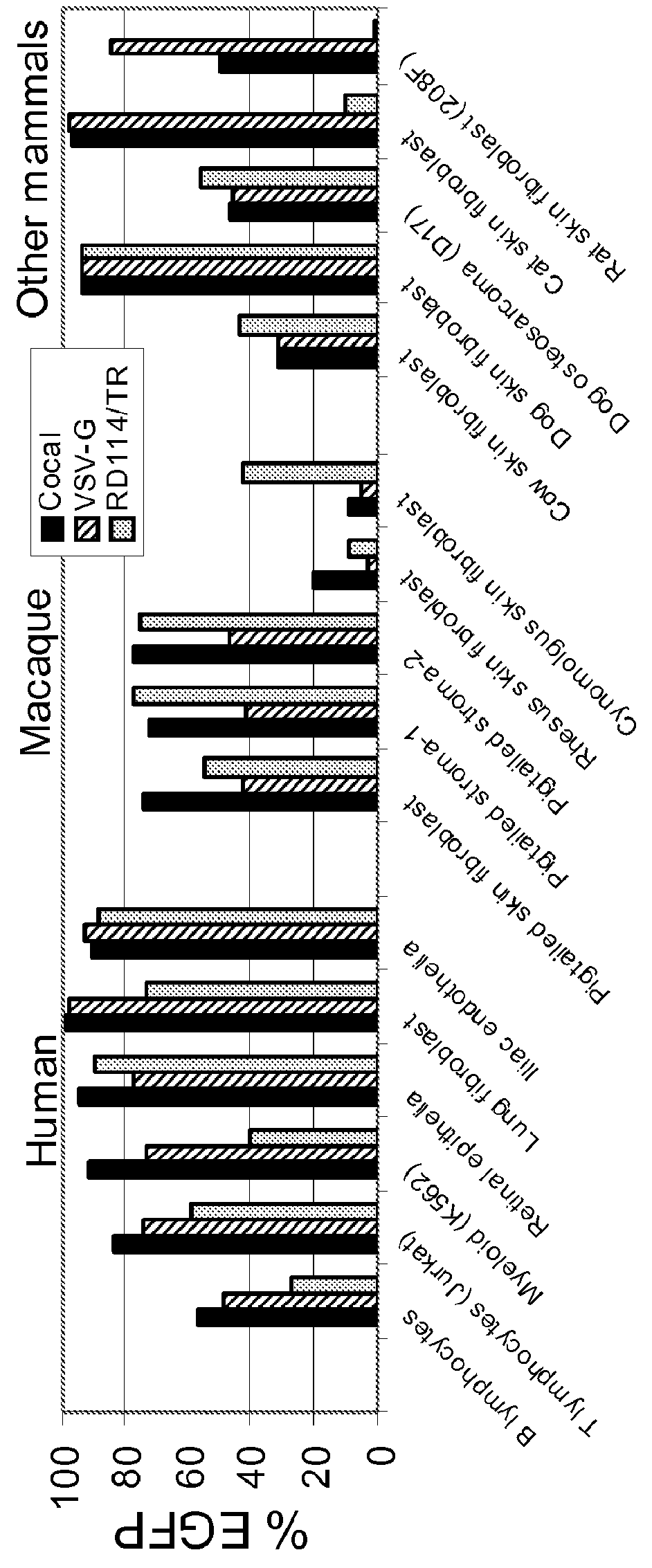
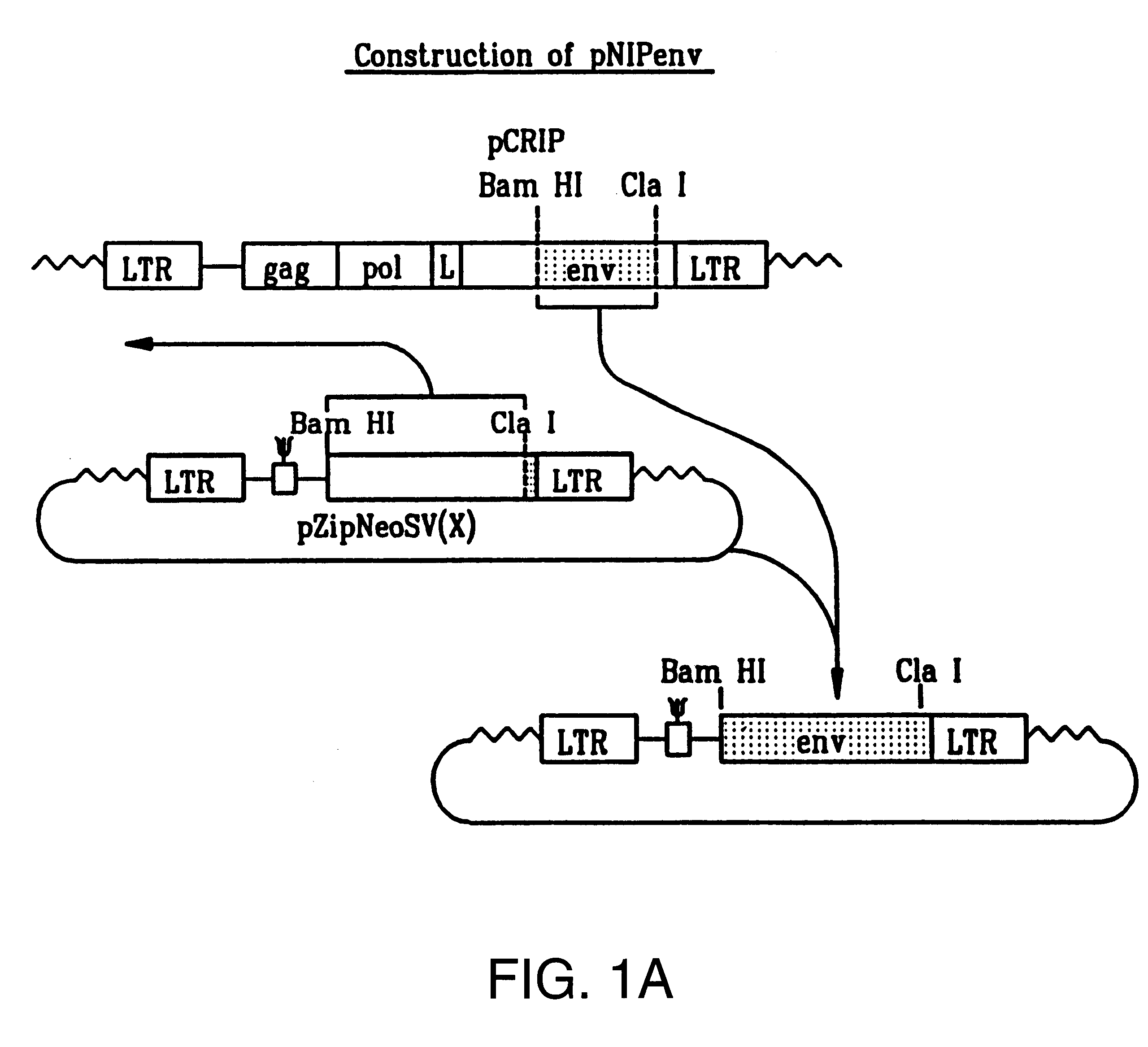
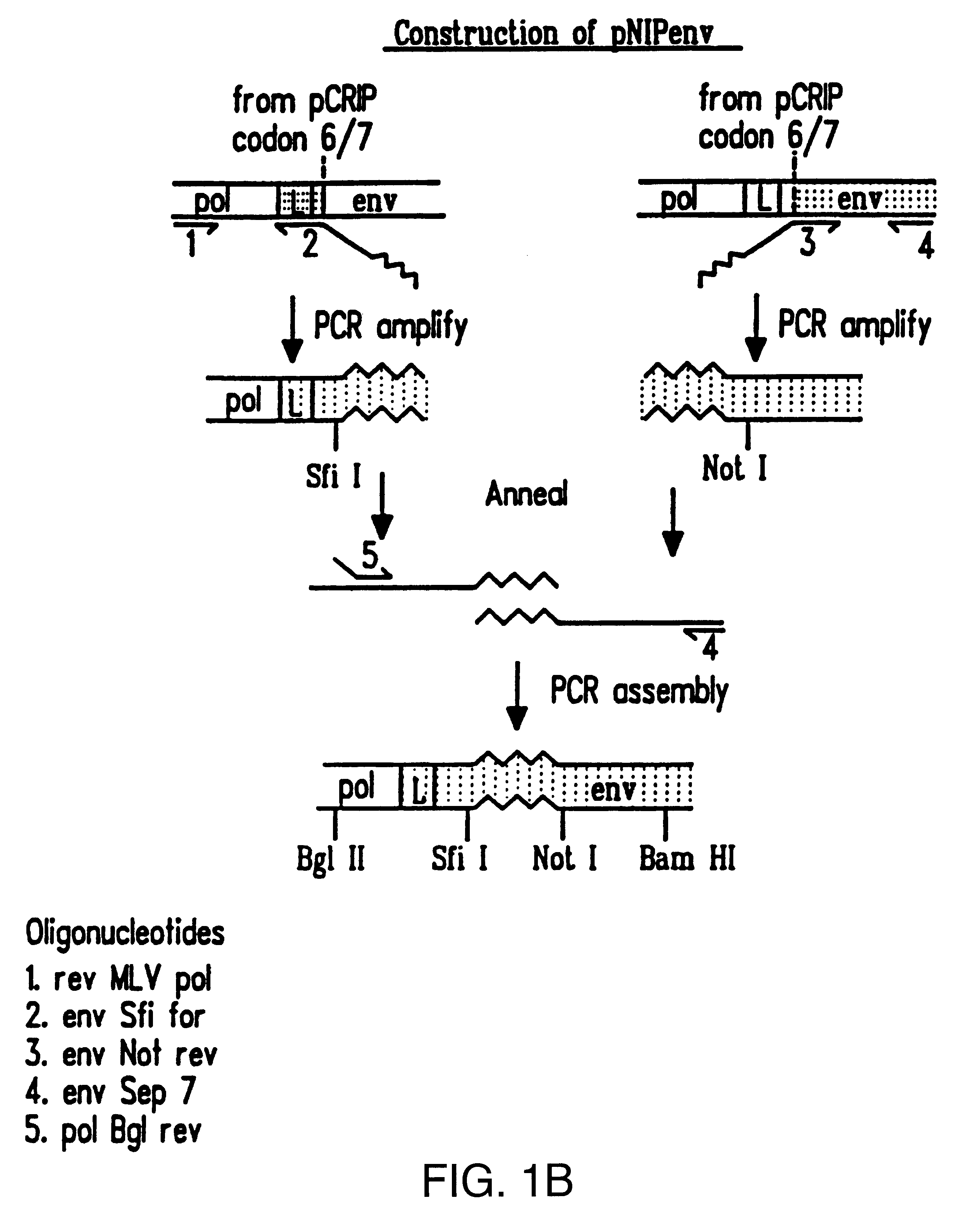
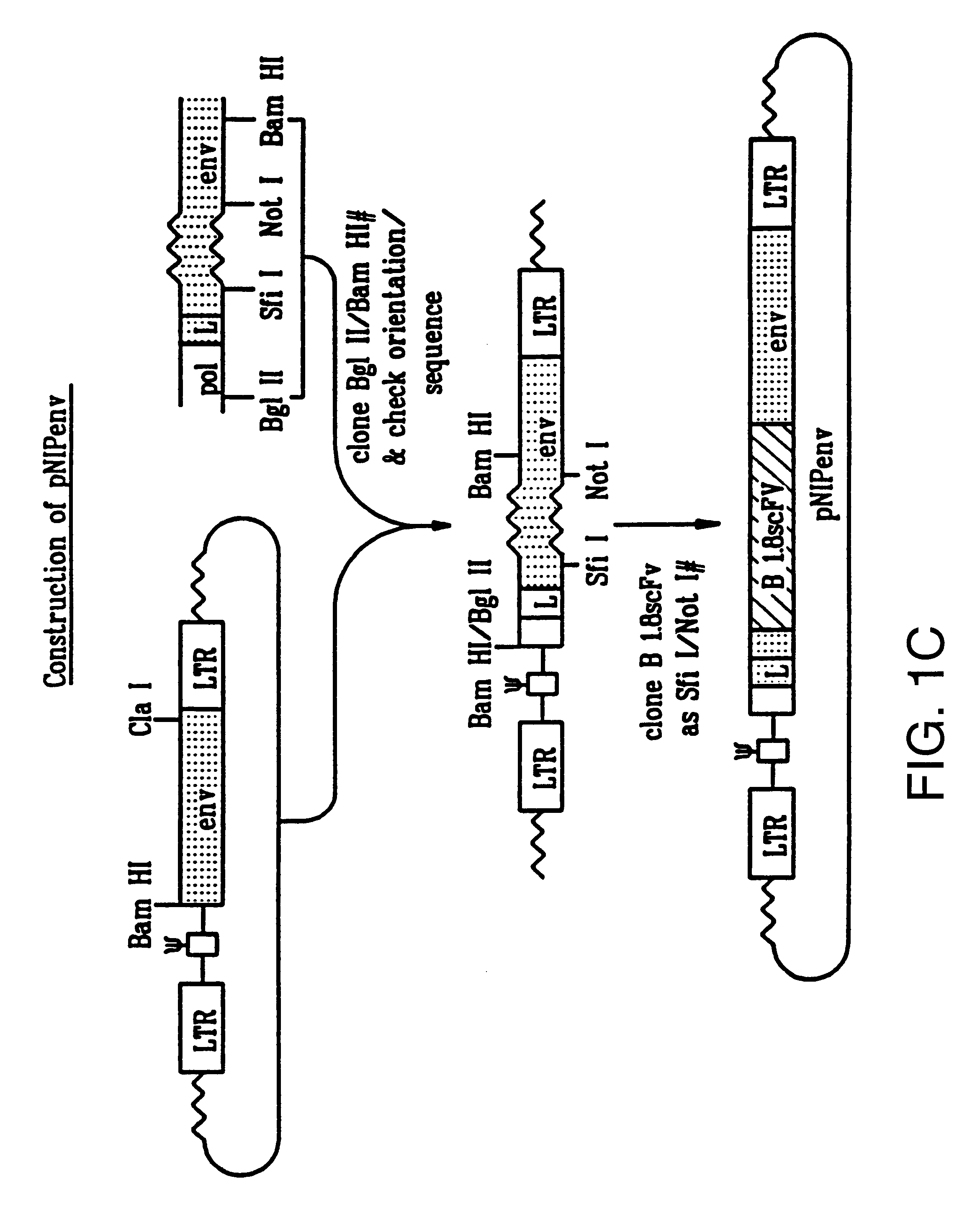
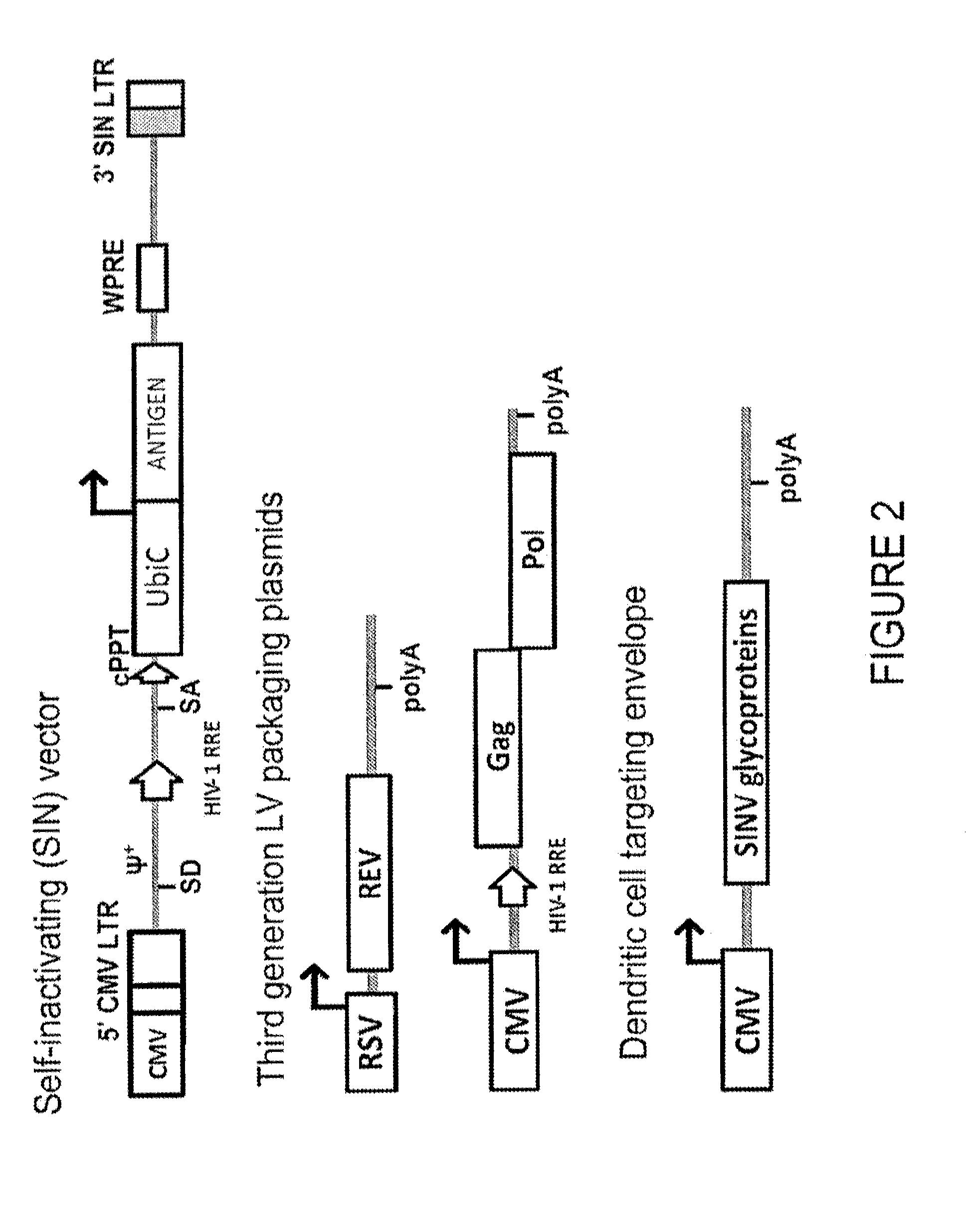
Cocal vesiculovirus envelope pseudotyped retroviral vectors
InactiveUS20120164118A1Increase serum stabilitySsRNA viruses negative-senseBiocideCocal vesiculovirusVirus-Retrovirus
Provided herein are Cocal vesiculovirus envelope pseudotyped retroviral vectors that exhibit high titers, broad species and cell-type tropism, and improved serum stability. Disclosed Cocal vesiculovirus envelope pseudotyped retroviral vectors may be suitably employed for gene therapy applications and, in particular, for the ex vivo and in vivo delivery of a gene of interest to a wide variety of target cells.
Owner:FRED HUTCHINSON CANCER RES CENT
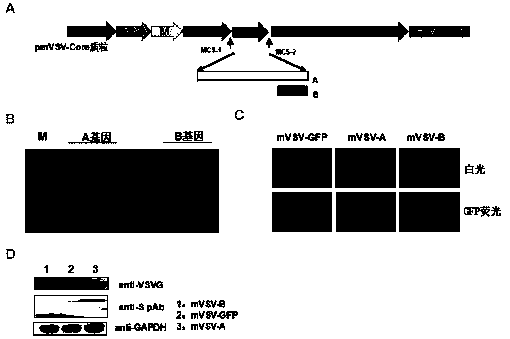
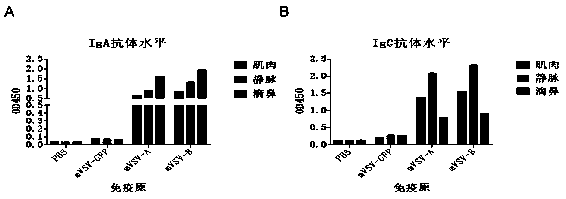
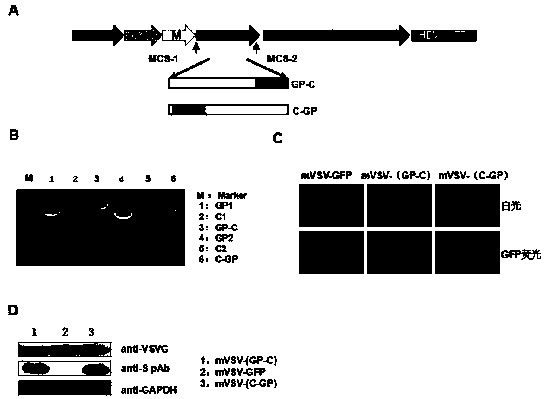
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Targeted gene delivery for dendritic cell vaccination
Methods and compositions are provided for delivery of a polynucleotide encoding a gene of interest, typically an antigen, to a dendritic cell (DC). The virus envelope comprises a DC-SIGN specific targeting molecule. The methods and related compositions can be used to treat patients suffering from a wide range of conditions, including infection, such as HIV / AIDS, and various types of cancers.
Owner:CALIFORNIA INST OF TECH
Method of targeted gene delivery using viral vectors
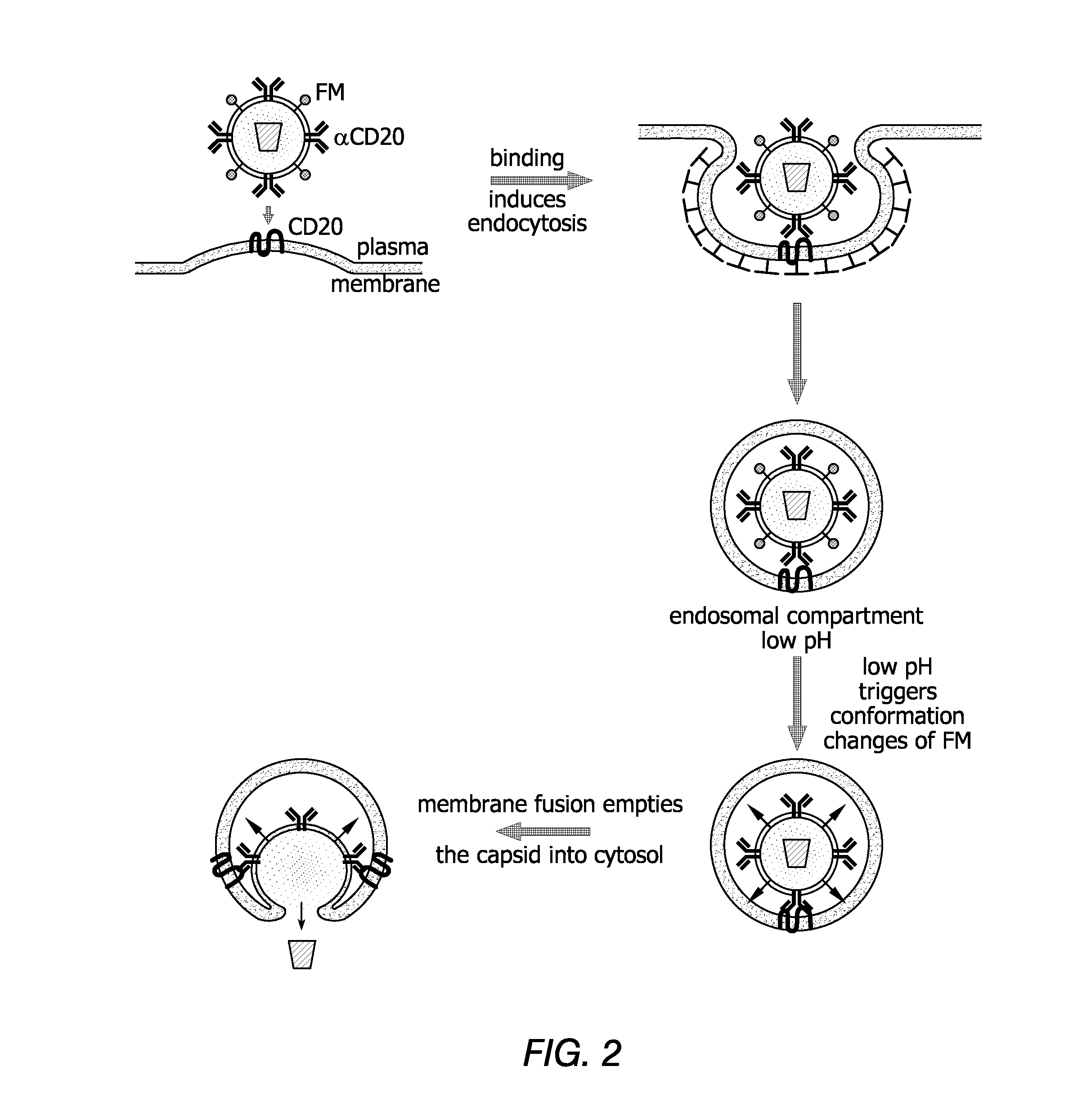
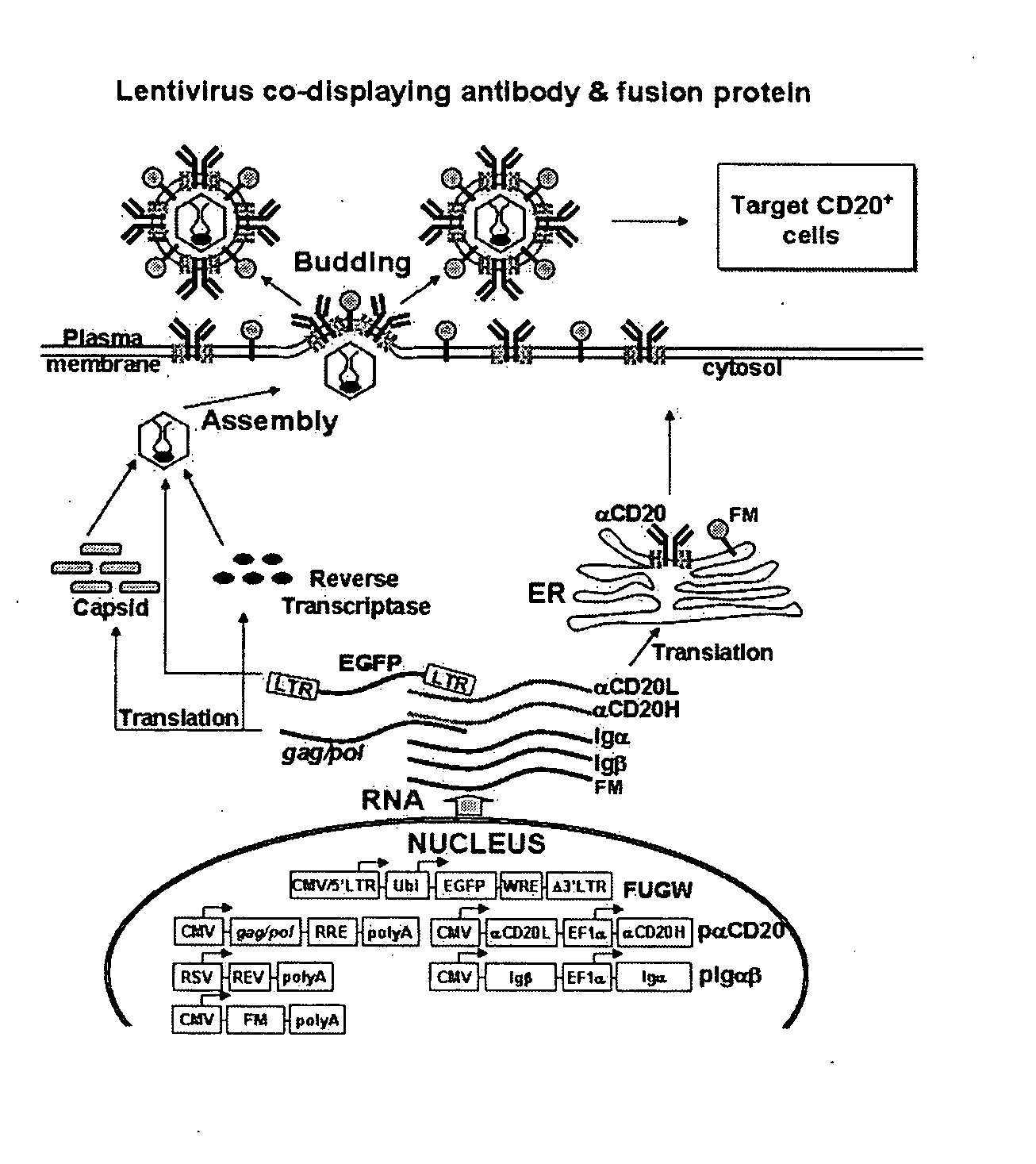
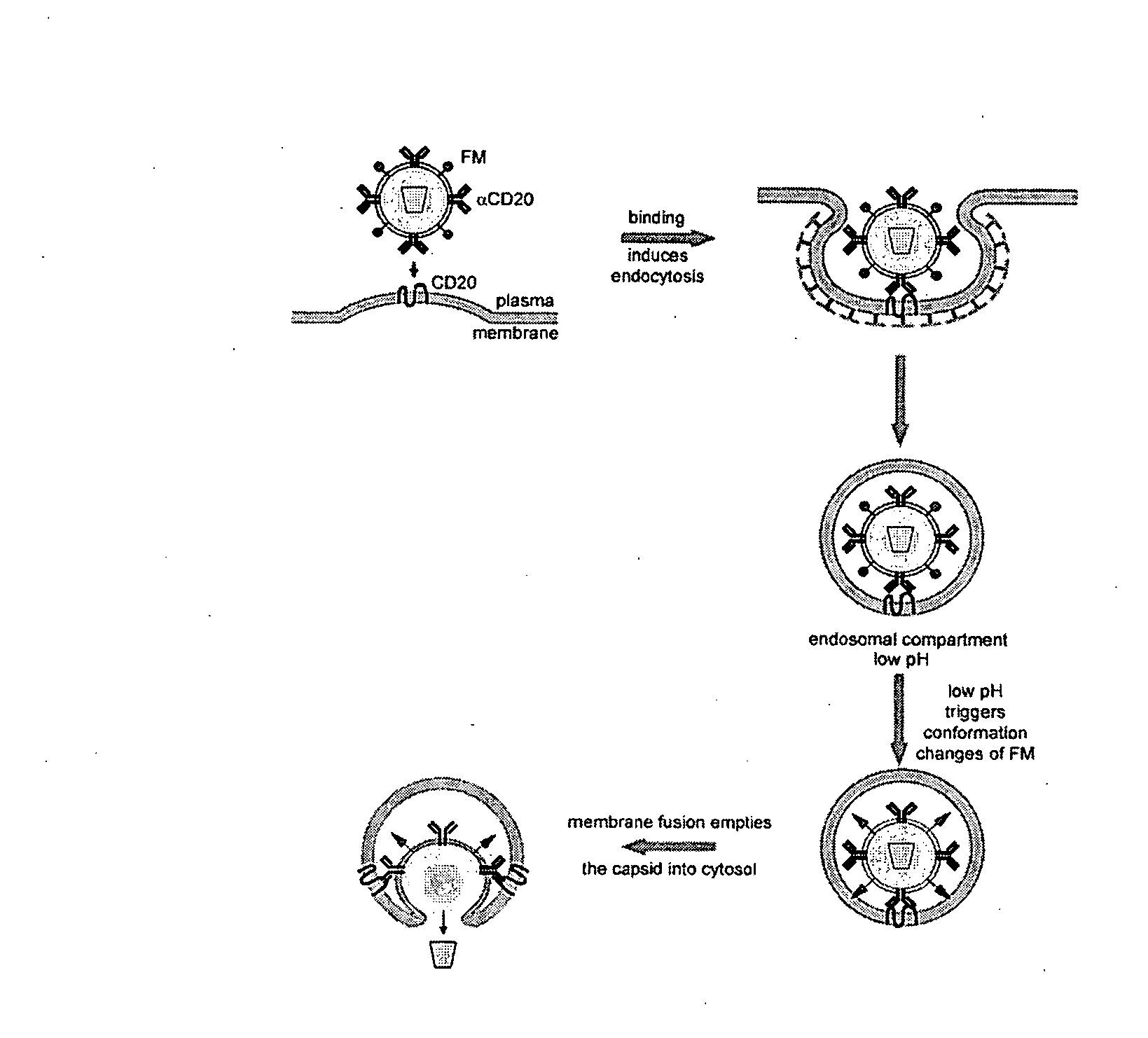
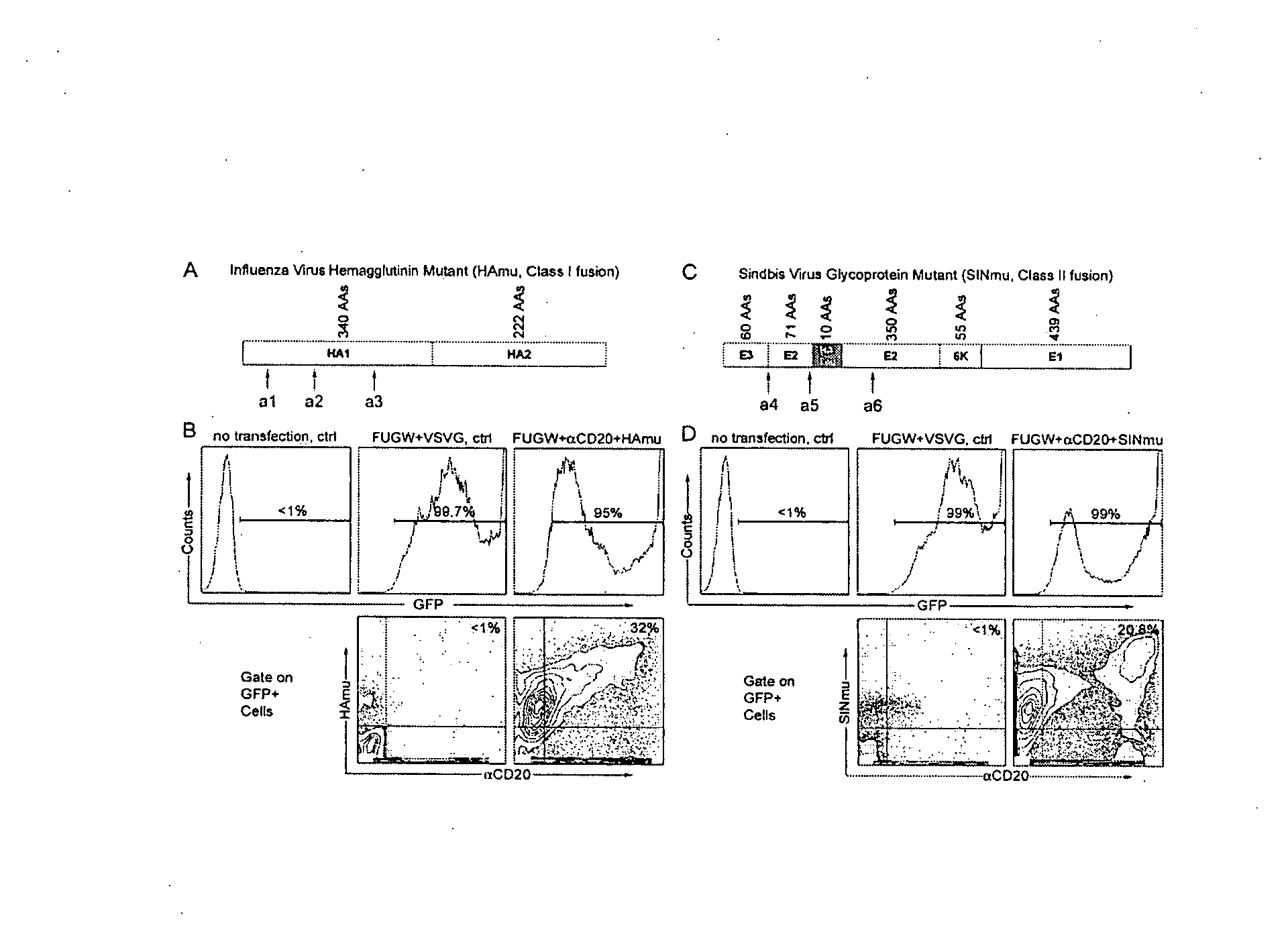
Methods and compositions are provided for delivering a polynucleotide encoding a gene of interest to a target cell using a virus. The virus envelope comprises a cell-specific binding determinant that recognizes and binds to a component on the target cell surface, leading to endocytosis of the virus. A separate fusogenic molecule is also present on the envelope and facilitates delivery of the polynucleotide across the membrane and into the cytosol of the target cell. The methods and related compositions can be used for treating patients having suffering from a wide range of conditions, including infection, such as HIV; cancers, such as non-Hodgkin's lymphoma and breast cancer; and hematological disorders, such as severe combined immunodeficiency.
Owner:CALIFORNIA INST OF TECH
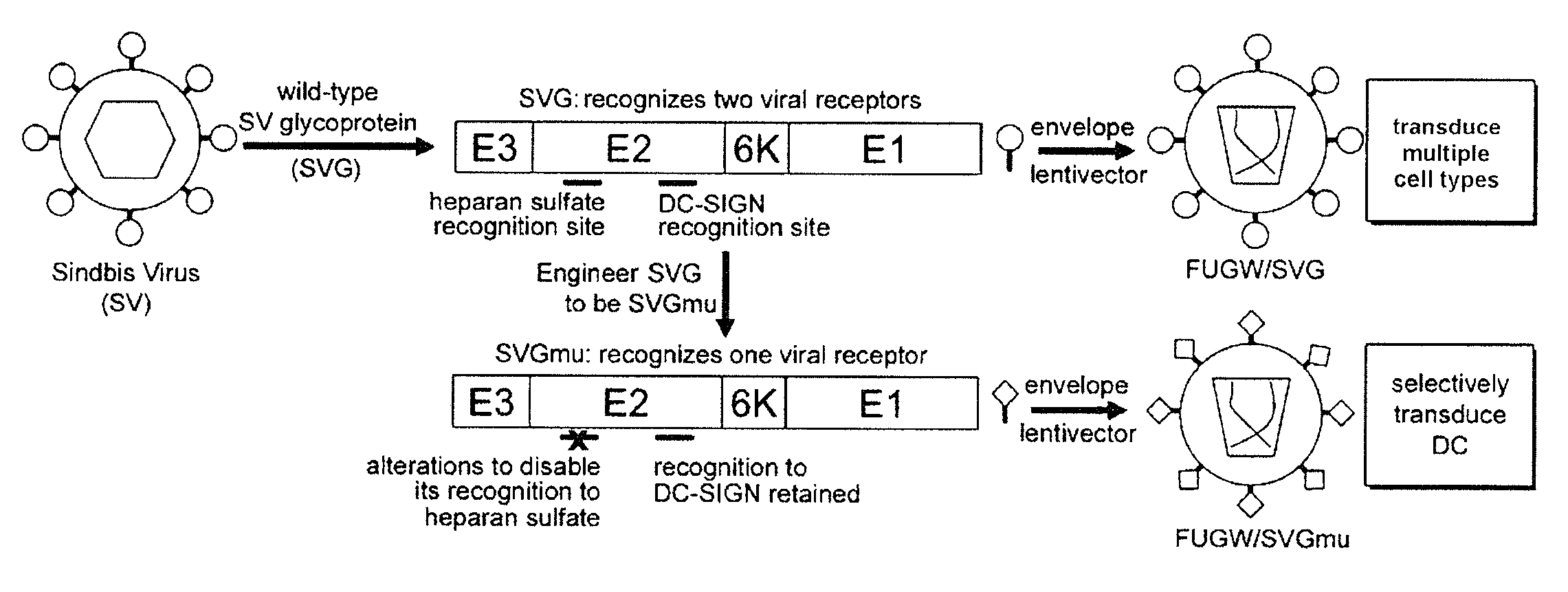
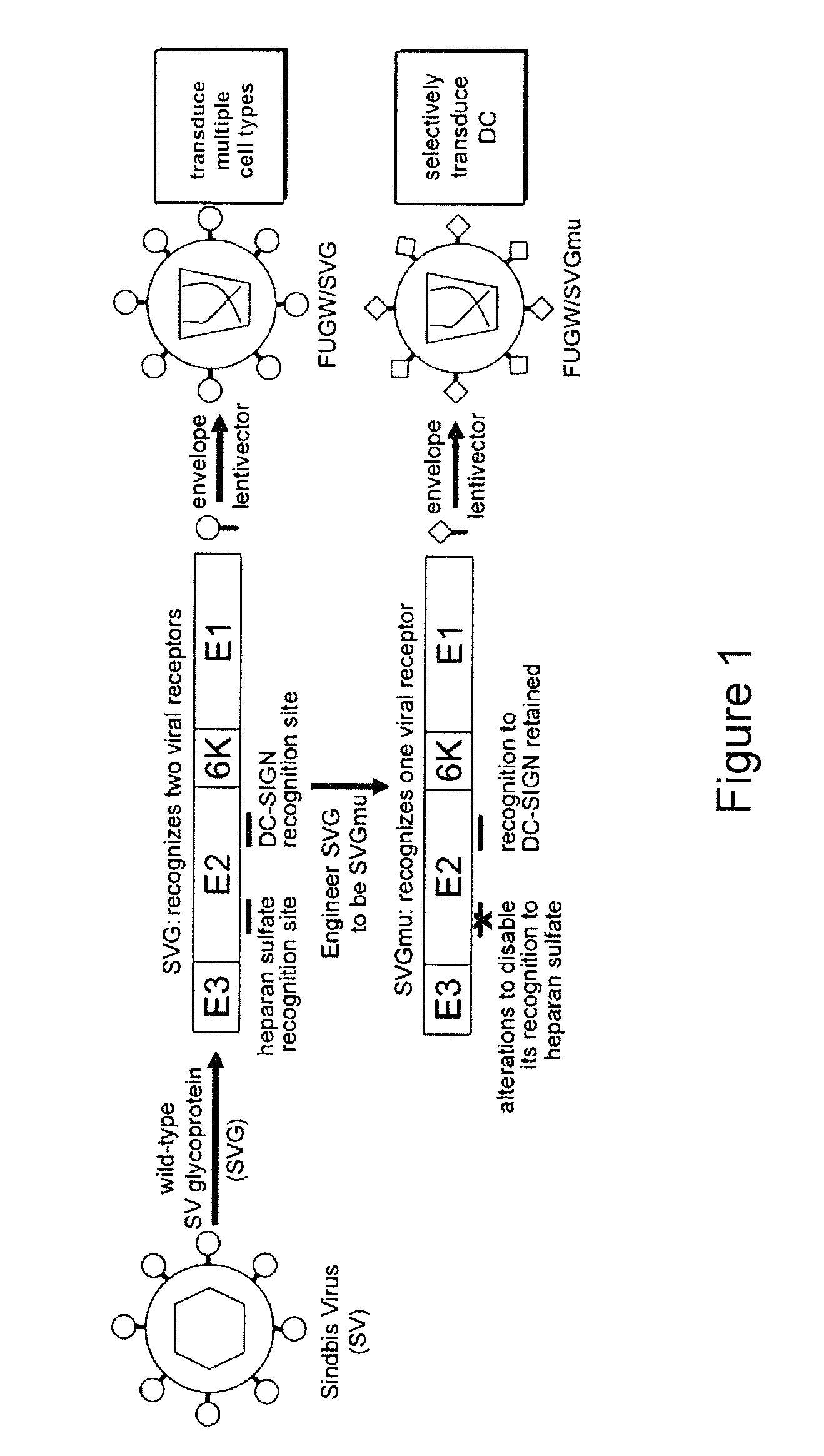
Lentiviral vectors pseudotyped with a sindbis virus envelope glycoprotein
InactiveUS20110064763A1Promote infectionSsRNA viruses positive-senseAntiviralsDendritic cellSindbis virus
Lentiviral vector particles comprising a Sindbis virus E2 glycoprotein variant and a lentiviral vector genome comprising a sequence of interest are provided. A lentiviral vector particle comprising: (a) an envelope comprising a Sindbis virus E2 glycoprotein variant; and (b) a lentiviral vector genome comprising a sequence of interest; wherein the E2 glycoprotein variant facilitates infection of dendritic cells by the lentiviral vector particle, and wherein the E2 glycoprotein variant has reduced binding to heparan sulfate compared to a reference sequence (HR strain).
Owner:IMMUNE DESIGN CORP
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
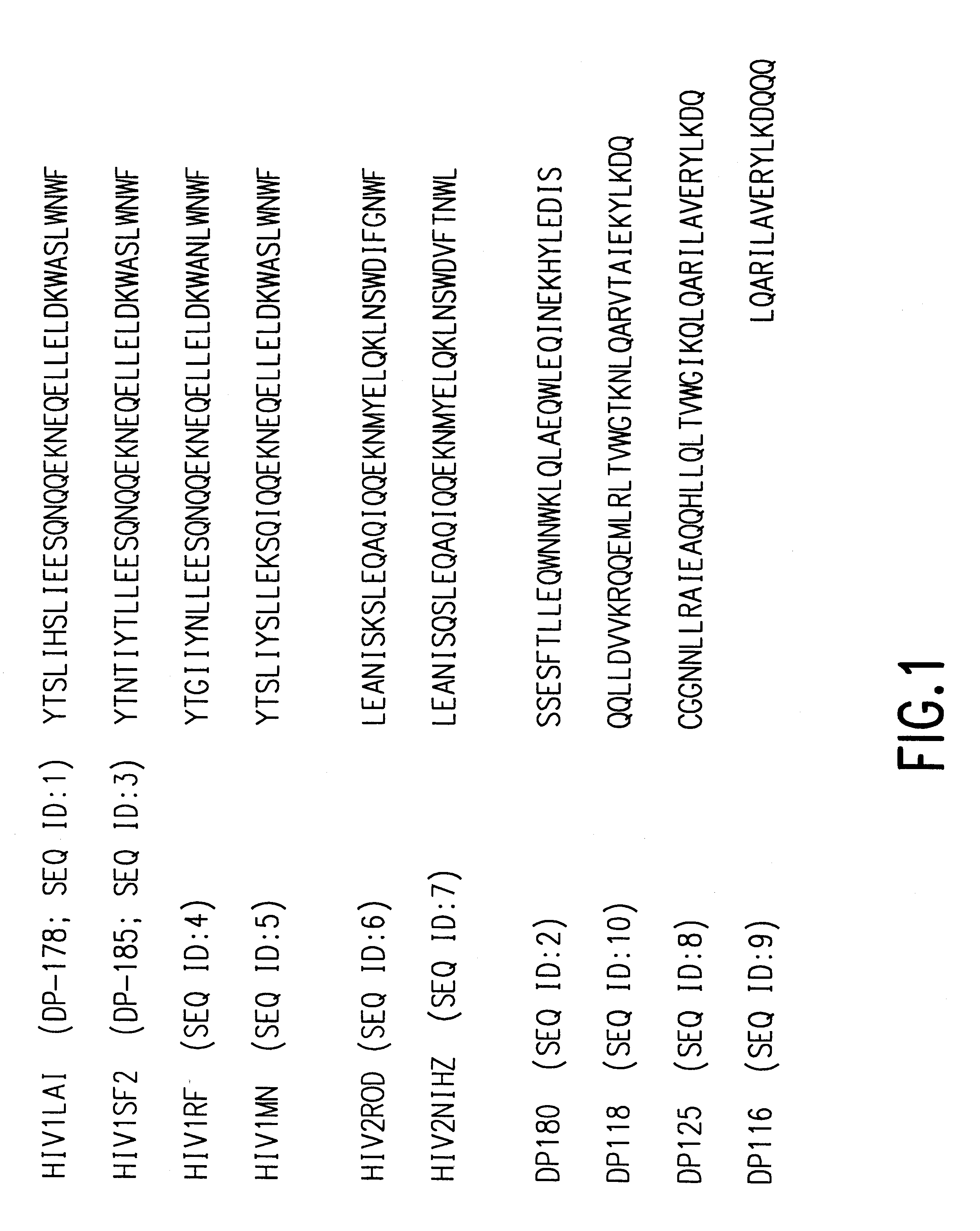
Methods for the inhibition of epstein-barr virus transmission employing anti-viral peptides capable of abrogating viral fusion and transmission
InactiveUS6518013B1SsRNA viruses negative-sensePeptide/protein ingredientsCell membraneViral life cycle
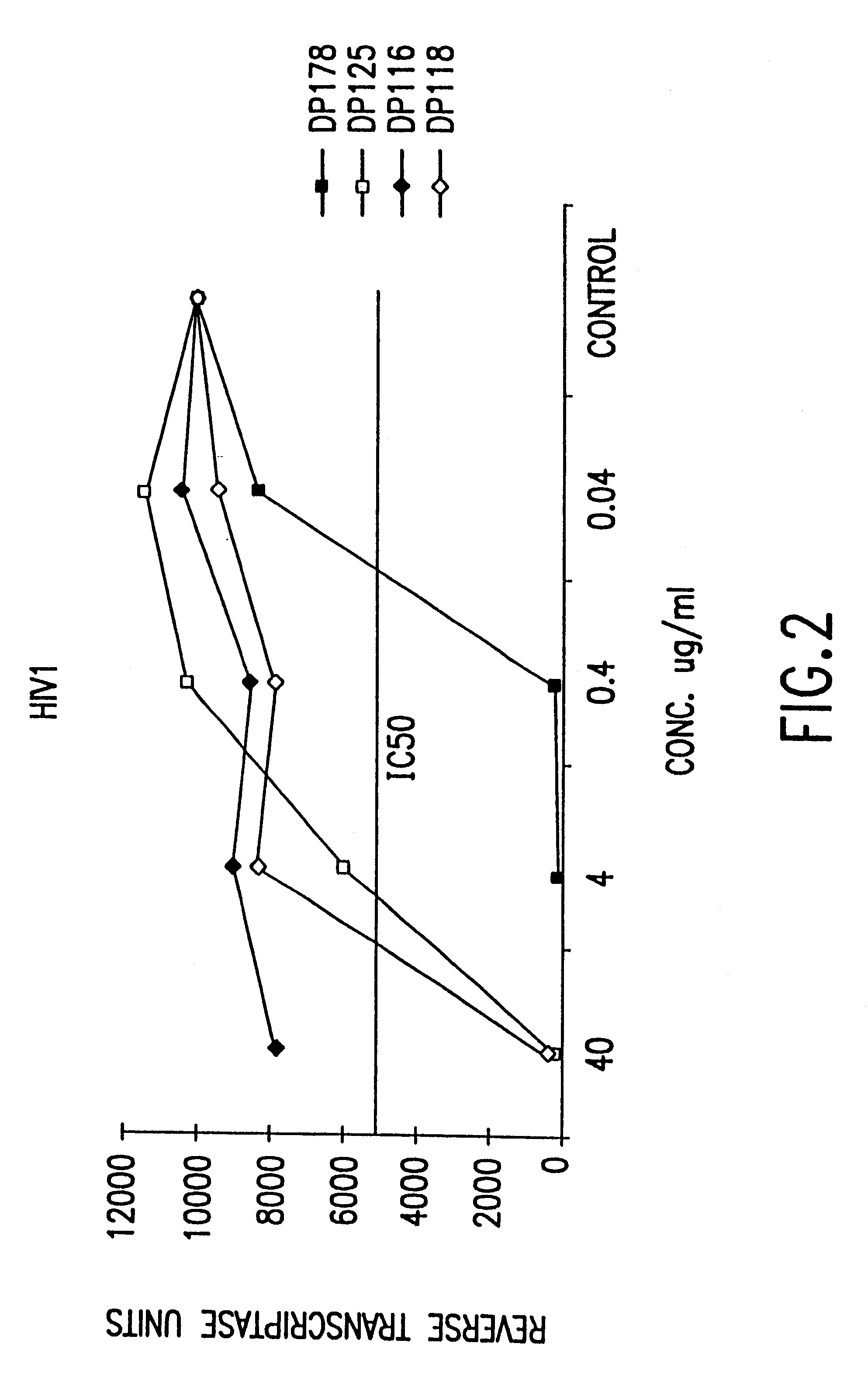
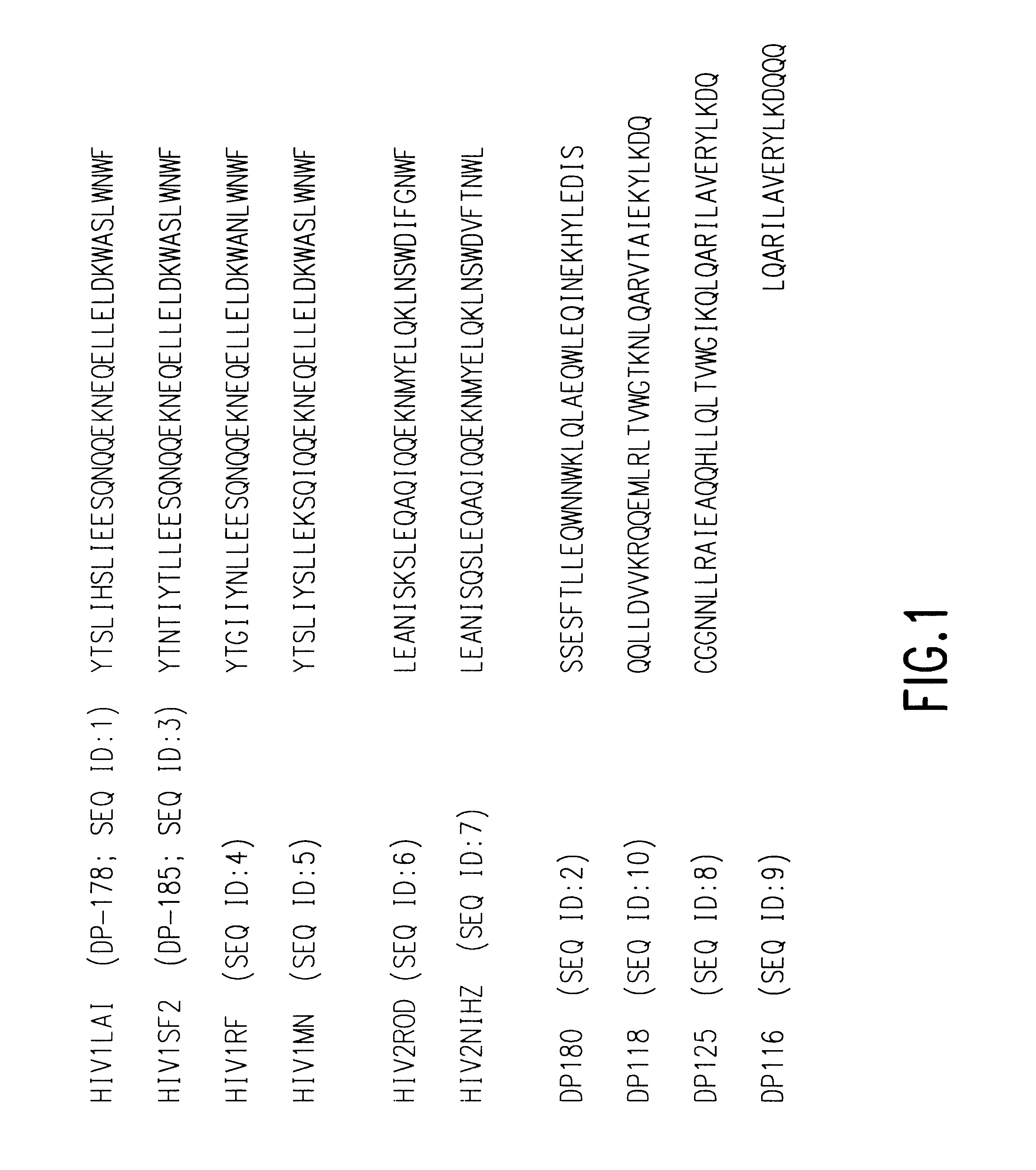
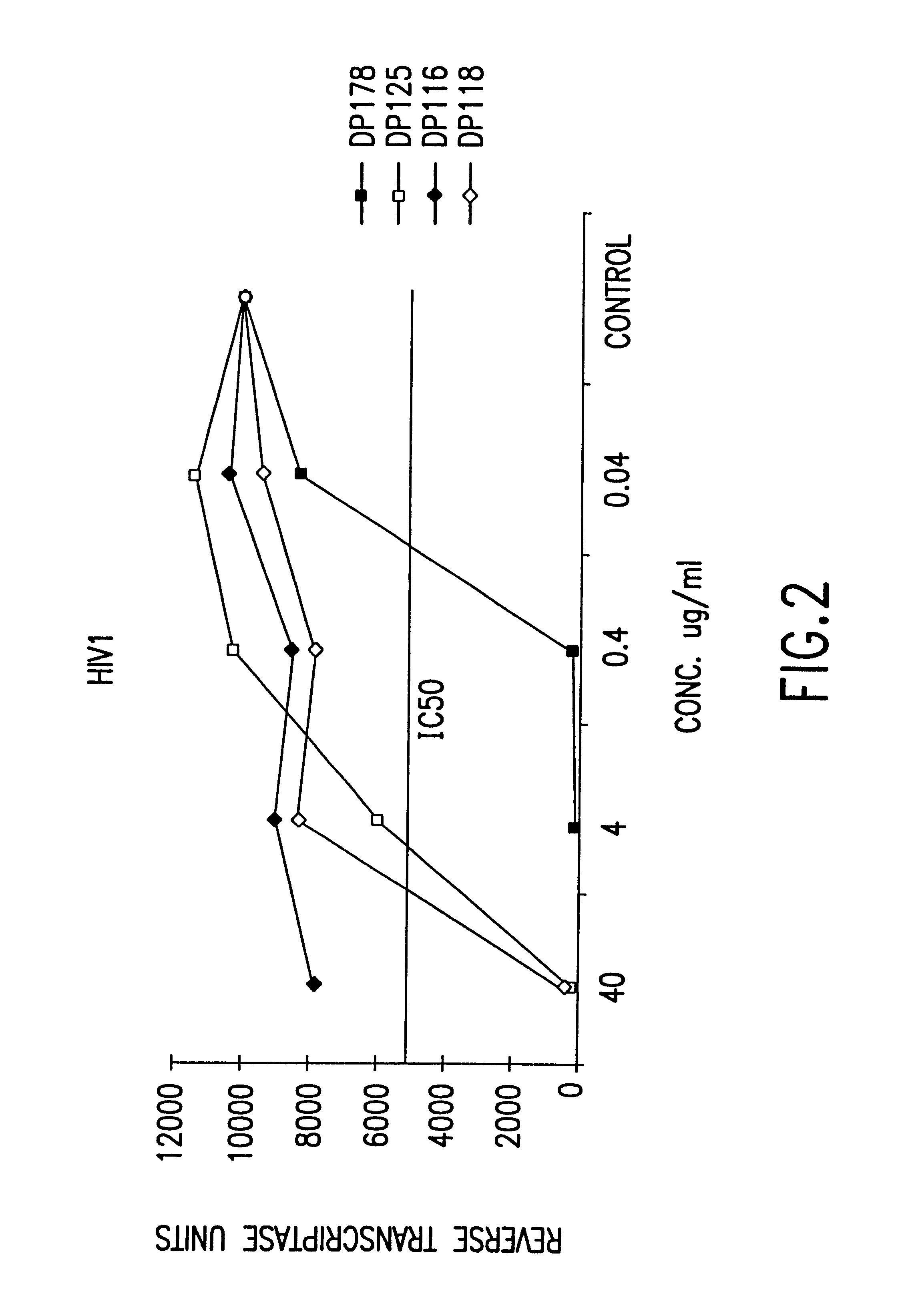
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1(HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI 5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the Epstein-Barr virus (EBV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the EBV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of EBV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of EBV infections.
Owner:TRIMERIS
Method of targeted gene delivery using viral vectors
Methods and compositions are provided for delivering a polynucleotide encoding a gene of interest to a target cell using a virus. The virus envelope comprises a cell-specific binding determinant that recognizes and binds to a component on the target cell surface, leading to endocytosis of the virus. A separate fusogenic molecule is also present on the envelope and facilitates delivery of the polynucleotide across the membrane and into the cytosol of the target cell. The methods and related compositions can be used for treating patients having suffering from a wide range of conditions, including infection, such as HIV; cancers, such as non-Hodgkin's lymphoma and breast cancer; and hematological disorders, such as severe combined immunodeficiency.
Owner:CALIFORNIA INST OF TECH
Glycosylated modified primate lentivirus envelope polypeptides
InactiveUS6908617B1Improving immunogenicityPeptide/protein ingredientsViral antigen ingredientsAdjuvantBinding site
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has at least two of the glycosylation sites proximal to the CD4 binding site or chemokine receptor site altered so that the alteration prevents glycosylation at that site or where glycosylation sites distal to these sites have been derivatized with a molecular adjuvant, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has both changes. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp 120.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method of preventing virus: cell fusion by inhibiting the function of the fusion initiation region in rna viruses having class i membrane fusogenic envelope proteins
ActiveUS20060280754A1Prevent and inhibit infectionSsRNA viruses negative-senseSsRNA viruses positive-senseCell membraneDisease cause
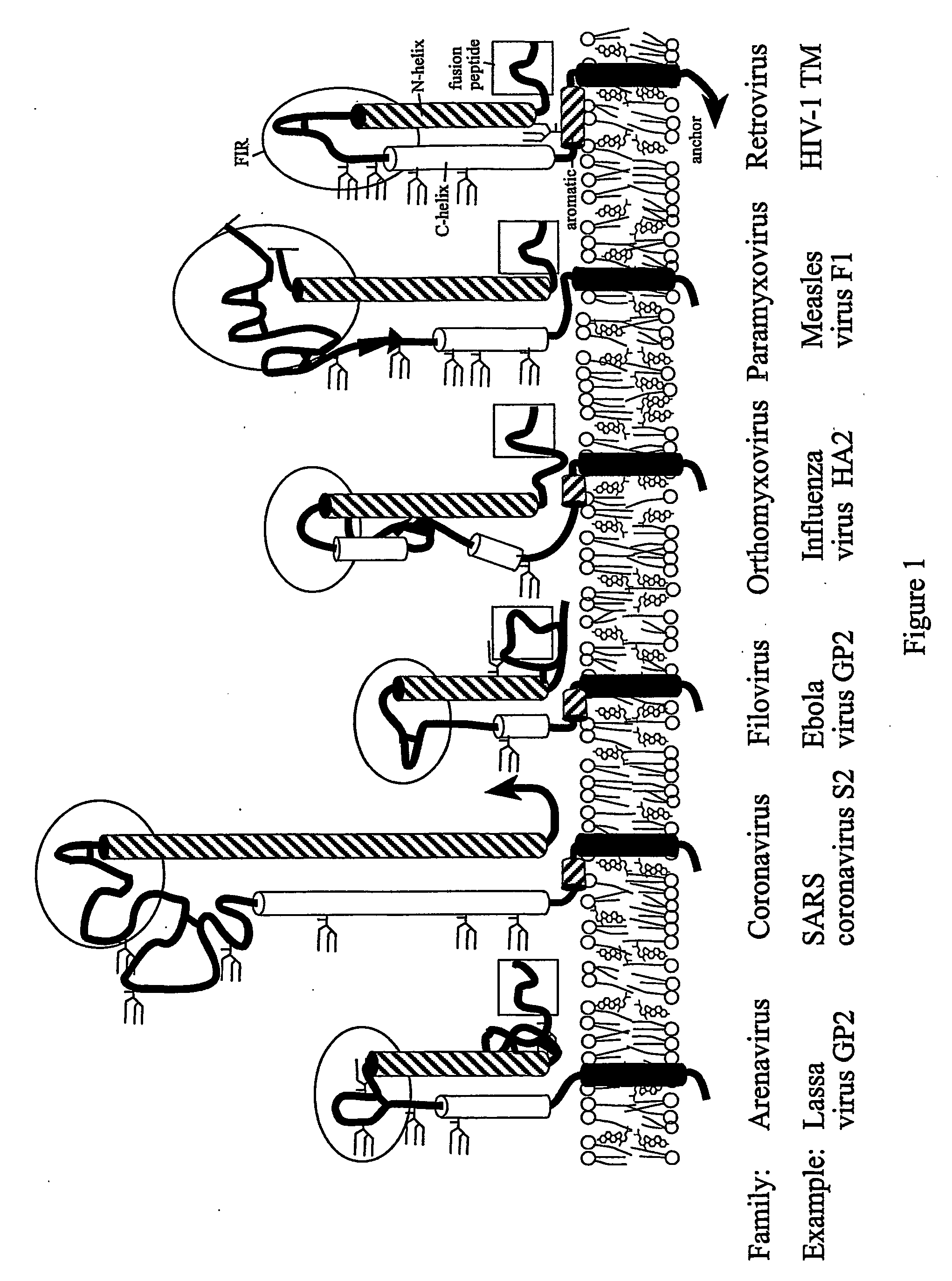
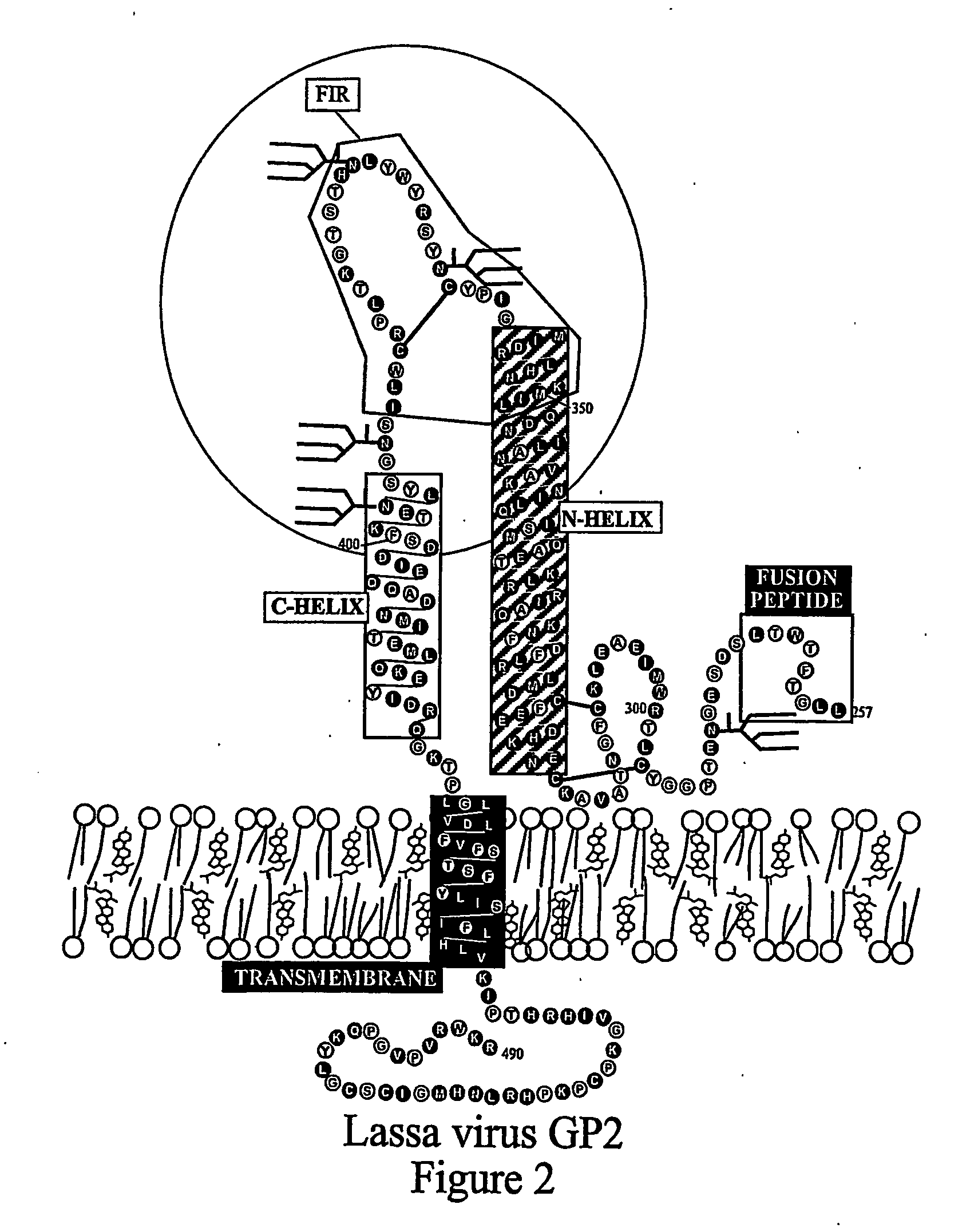
The present invention relates to a method of preventing or inhibiting viral infection of a cell and / or fusion between the envelope of a virus and the membranes of a cell targeted by the virus (thereby preventing delivery of the viral genome into the cell cytoplasm, a step required for. viral infection). The present invention particularly relates to the families of RNA viruses, including the arenaviruses, coronaviruses, filoviruses, orthomyxoviruses, paramyxoviruses, and retroviruses, having Class I membrane fusion proteins as the fusion proteins that mediate this fusion process. The present invention provides for a method of identifying a conserved motif or domain called the fusion initiation region (FIR) in these viruses. The present invention further provides for methods of preventing infection by such viruses, by interfering with their FIR. The present invention further provides for methods of treatment and prophylaxis of diseases induced by such viruses.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS OF THE
Stabilized viral envelope proteins and uses thereof
This invention provides an isolated nucleic acid which comprises a nucleotide segment having a sequence encoding a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention also provides a viral envelope protein comprising a viral surface protein and a corresponding viral transmembrane protein wherein the viral envelope protein contains one or more mutations in amino acid sequence that enhance the stability of the complex formed between the viral surface protein and transmembrane protein. This invention further provides methods of treating HIV-1 infection.
Owner:PROGENICS PHARMA INC
Stabilized primate lentivirus envelope glycoproteins
InactiveUS7048929B1Improve stabilityImproving immunogenicityAntibody mimetics/scaffoldsVirus peptidesEnv GlycoproteinsDisulfide bond
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has cysteine amino acid residues introduced to create disulfide bonds, a cavity is filled with hydrophobic amino acids, a Proresidue is introduced at a defined turn structure of the protein, or the hydrophobicity is increased across the interface between different domains, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has more than one of those characteristics. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp120.
Owner:DANA FARBER CANCER INST INC +1
Methods for producing high titre vectors and compositions used in such methods
InactiveUS6969598B2Reduce chanceQuantity minimizationGenetic material ingredientsVirus peptidesNucleotideViral envelope
A method for producing viral vectors is described using packaging and producer cell lines is described. The producer cell comprises: (i) a first nucleotide sequence (NS) encoding a toxic viral envelope protein operably linked to a promoter; wherein the promoter is operably linked to at least one copy of a TRE; (ii) a second NS wherein the second NS comprises a sequence encoding a tetracycline modulator; (iii) a third NS encoding a retrovirus nucleocapsid protein; and (iv) a fourth NS comprising a retroviral sequence capable of being encapsidated in the nucleocapsid protein such that the retroviral vector particle titre obtainable from the producer cell is regulatable by tetracycline and an initial stimulus with sodium butyrate or functional analogues thereof.
Owner:OXFORD BIOMEDICA (UK) LTD +1
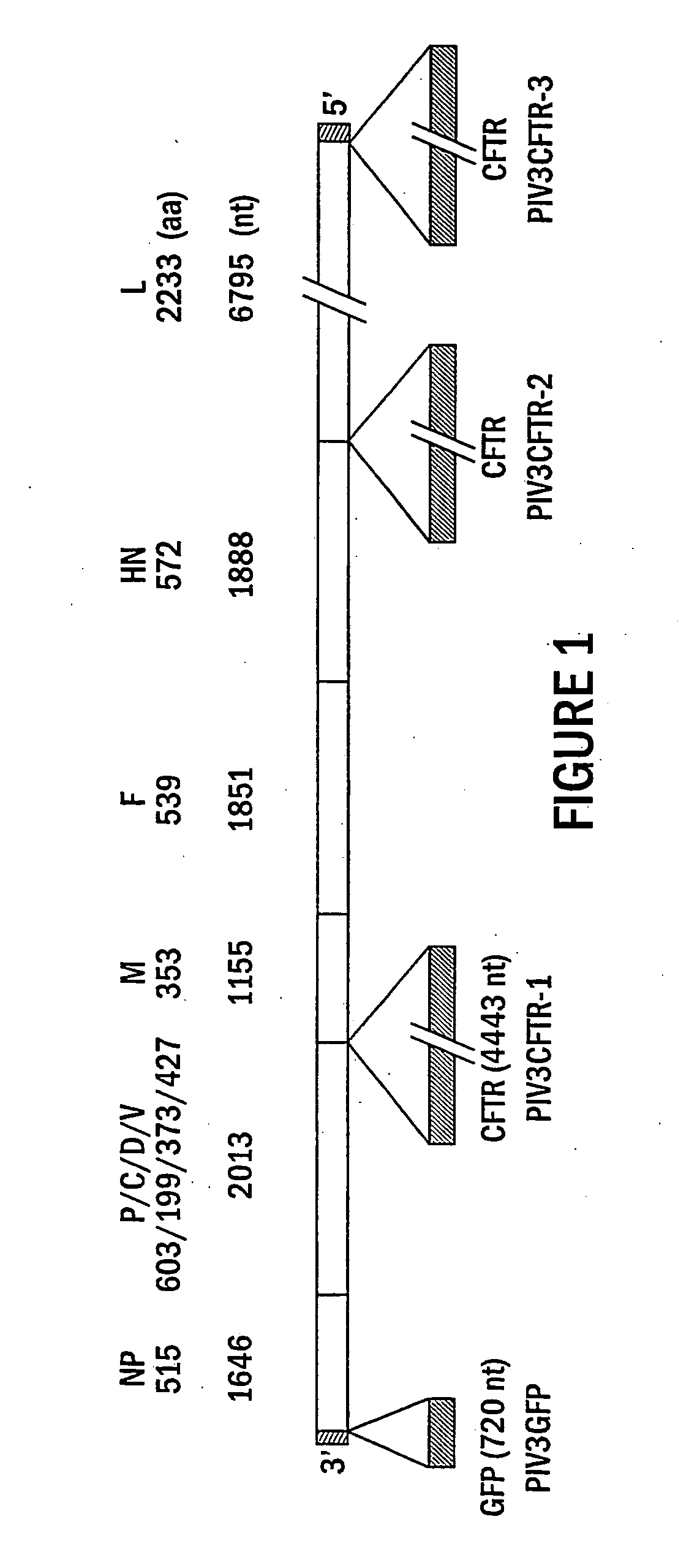
Paramyxoviruses as gene transfer vectors to lung cells
The present invention provides infectious recombinant viral vectors (e.g., parainfluenza virus (PIV) and a respiratory syncytial virus (RSV) vectors) comprising a viral genome comprising a heterologous nucleic acid of interest. Also provided are pseudotyped recombinant viral vectors comprising (i) a viral envelope and (ii) a viral genome comprising heterologous nucleic acids of interest. The viral envelope comprises a structural protein selected from the group consisting of envelope proteins from PIV and / or RSV. Further provided are methods of delivering heterologous nucleic acids of interest into airway epithelial cells comprising introducing viral vectors of the present invention comprising nucleic acids of interest into airway epithelial cells so that the nucleic acids of interest are expressed therein.
Owner:RUSH PRESBYTERIAN ST LUKES MEDICAL CENT +2
Production of virus and purification of viral envelope proteins for vaccine use
Immunogenic envelope glycoproteins are produced from enveloped virus, such as of the paramyxoviridae family, particularly PIV-3 and RSV, by culturing the virus in the substantial absence of exogenous serum proteins, isolating the virus from the tissue culture, solubilizing the envelope glycoproteins and isolating the solubilized envelope glycoproteins by chromatography.
Owner:CONNAUGHT LAB
Methods for the inhibition of respiratory syncytial virus transmission
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1 (HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the respiratory syncytial virus (RSV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the RSV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of RSV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of RSV infections.
Owner:TRIMERIS
Virus envelope vector for gene transfer
InactiveUS6913923B2Safe and highly efficientLow cytotoxicitySsRNA viruses negative-senseBiocideGene transferVirus Envelope Proteins
A gene transfer vector is prepared by introducing an exogenous gene into an inactivated virus envelope, through a freezing and thawing treatment or mixing with a detergent. There are also provided a pharmaceutical composition for gene therapy containing this gene transfer vector, a kit containing this gene transfer vector, and a gene transfer method employing this gene transfer vector.
Owner:GENOMIDEA INC
Composition Having Antitumor Effect
ActiveUS20080226674A1Improve immunityImproving tumor antigen-presenting capabilitySsRNA viruses negative-senseHeavy metal active ingredientsAntitumor immunityAdjuvanticity
The present invention is intended to provide a pharmaceutical composition for delivering a chemotherapeutic, preferably an anticancer drug, into cells or into a living organism, using a viral envelope vector, and provides a pharmaceutical composition comprising a chemotherapeutic encapsulated in, or used in combination with, a viral envelope vector having an adjuvanticity as an active ingredient. Thereby it is possible to introduce an anticancer drug encapsulated in a viral envelope vector directly into a tumor, with coadministration of another anticancer drug so as to induce tumor cell-specific antitumor immunity also thanks to the adjuvant action of HVJ-E, and hence to regress the tumor. The present invention also provides a pharmaceutical composition comprising a viral envelope vector and a chemotherapeutic as active ingredients.
Owner:IMMUNOMEDICINE INC
Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases
InactiveCN102112155AAntibody mimetics/scaffoldsImmunoglobulins against virusesViral diseaseBioinformatics
The present invention relates in part to amino acid sequences that are directed against and / or that can specifically bind to an envelope protein of a virus, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such amino acid sequences.
Owner:ABLYNX NV
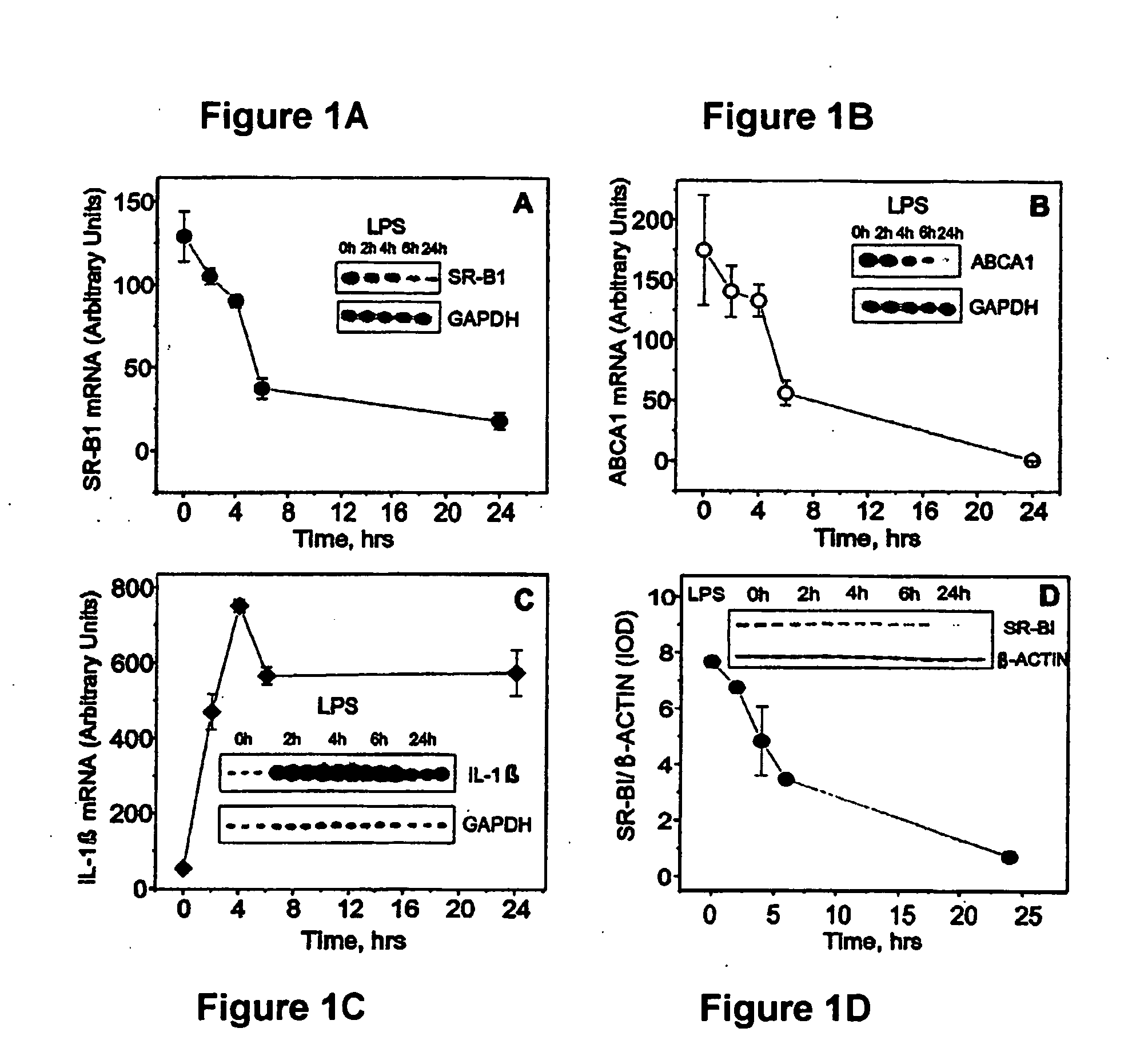
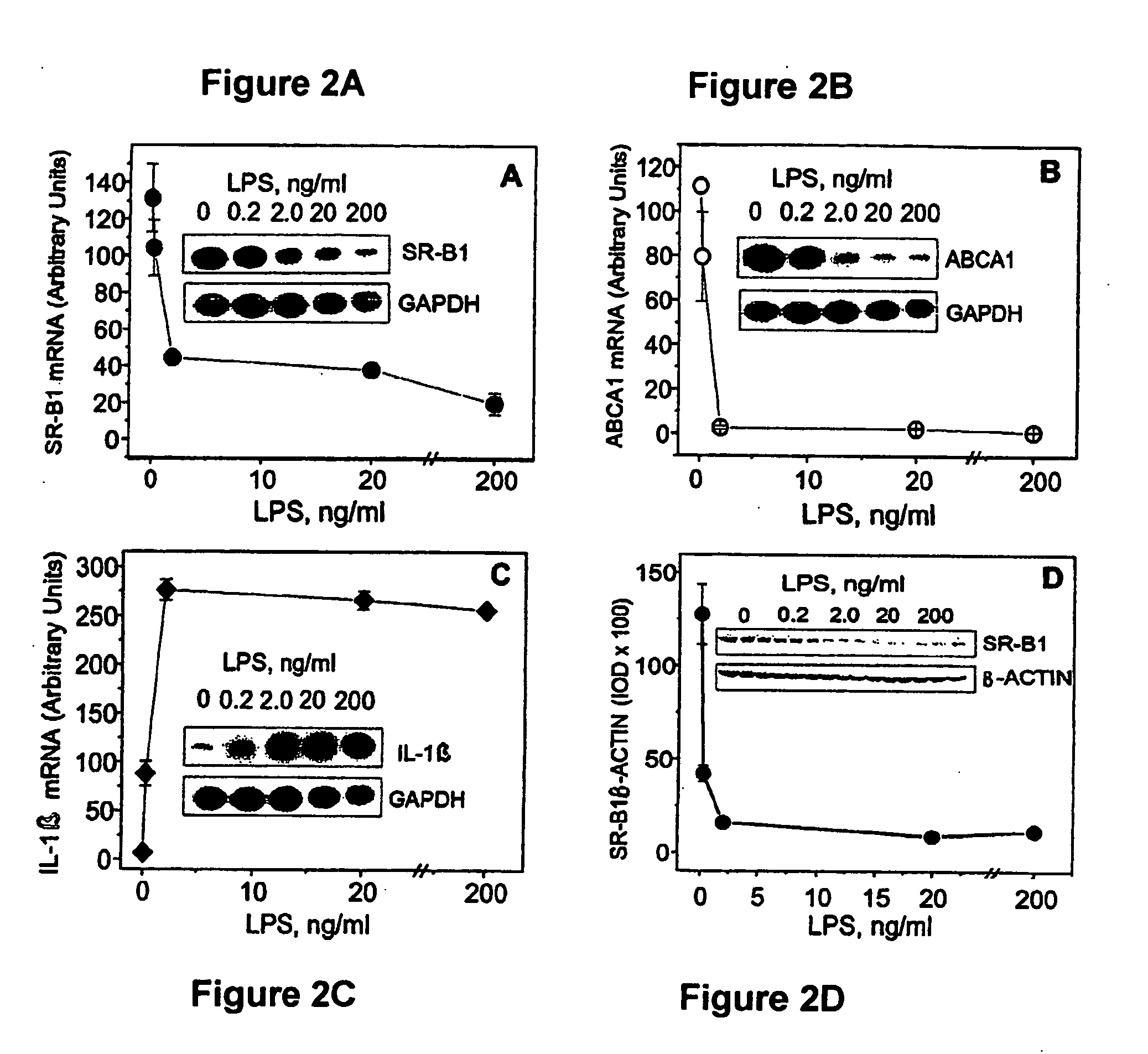
Scavenger Receptor B1 (Cla-1) Targeting for the Treatment of Infection, Sepsis and Inflammation
This invention relates to methods and compositions for the treatment of sepsis, inflammation or infection. In particular, the invention concerns the use of molecule(s) that target SR-BI, which is also referred to as CLA-1 (SR-BI / CLA-1), to treat sepsis, bacterial and viral infections, and inflammatory diseases. SRB I / CLA-1 ligands contributing to the pathogenesis of disease include LPS, LTA, viral envelope proteins, beta-amyloid, serum Amyloid A and / or heat shock proteins.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NIH
Virus-like particles (VLPs) comprising heterologous multiple membrane spanning proteins
Enveloped virus vectors are described which comprise a cellular virus receptor protein and which are capable of fusing with a cell which comprises a viral envelope protein to which the cellular virus receptor protein is cognate. Enveloped virus vectors comprising a plurality of cellular virus receptor proteins are also described. Methods for making the enveloped virus vectors are described, as are methods of using the enveloped virus vectors. The invention further relates to a lipoparticle comprising a membrane spanning protein, and the lipoparticle can be attached to a sensor surface. The invention relates to methods of producing and using the lipoparticle to, inter alia, assess protein binding interactions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Recombinant subunit dengue virus vaccine
ActiveUS20130216575A1Prevention and treatmentAcceptable safety profileSsRNA viruses positive-senseViral antigen ingredientsAdjuvantImmune senescence
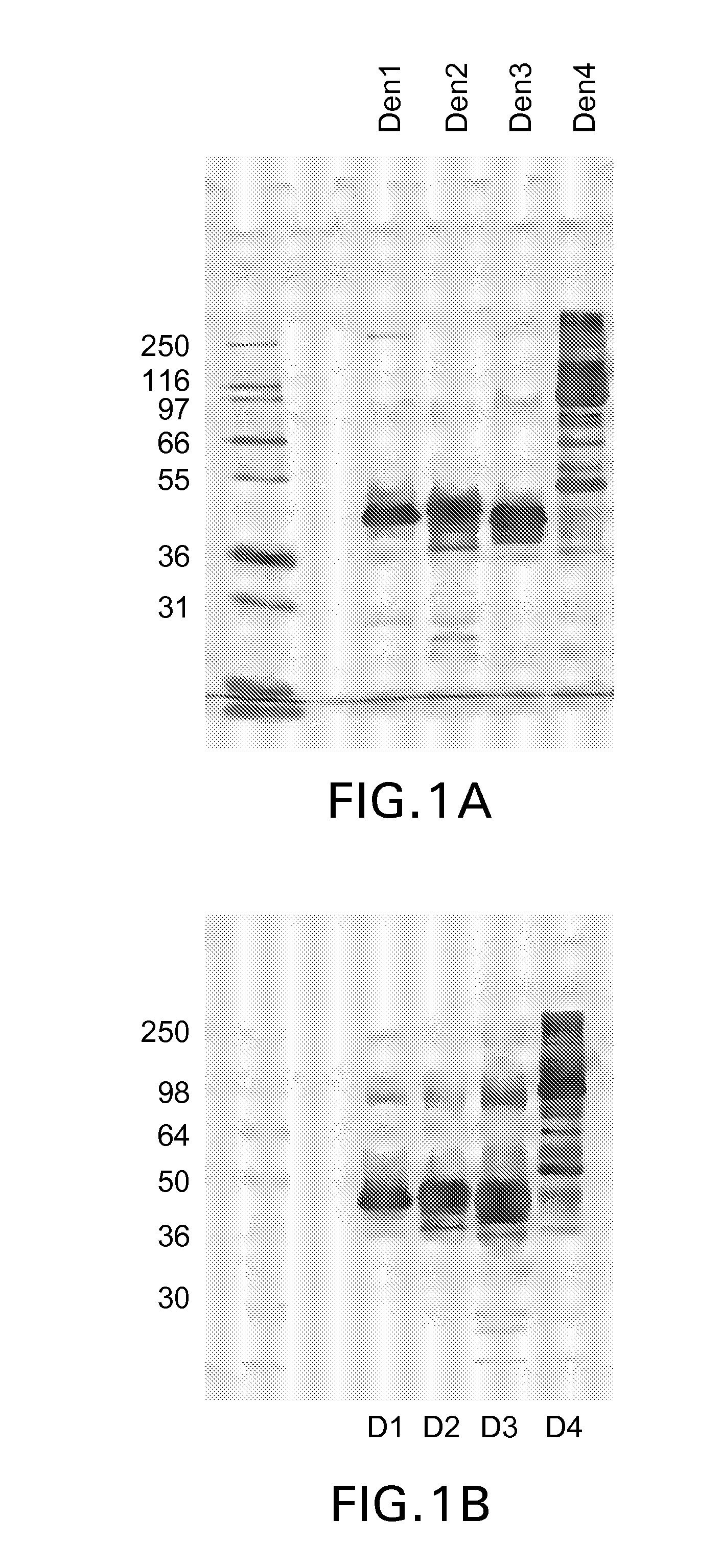
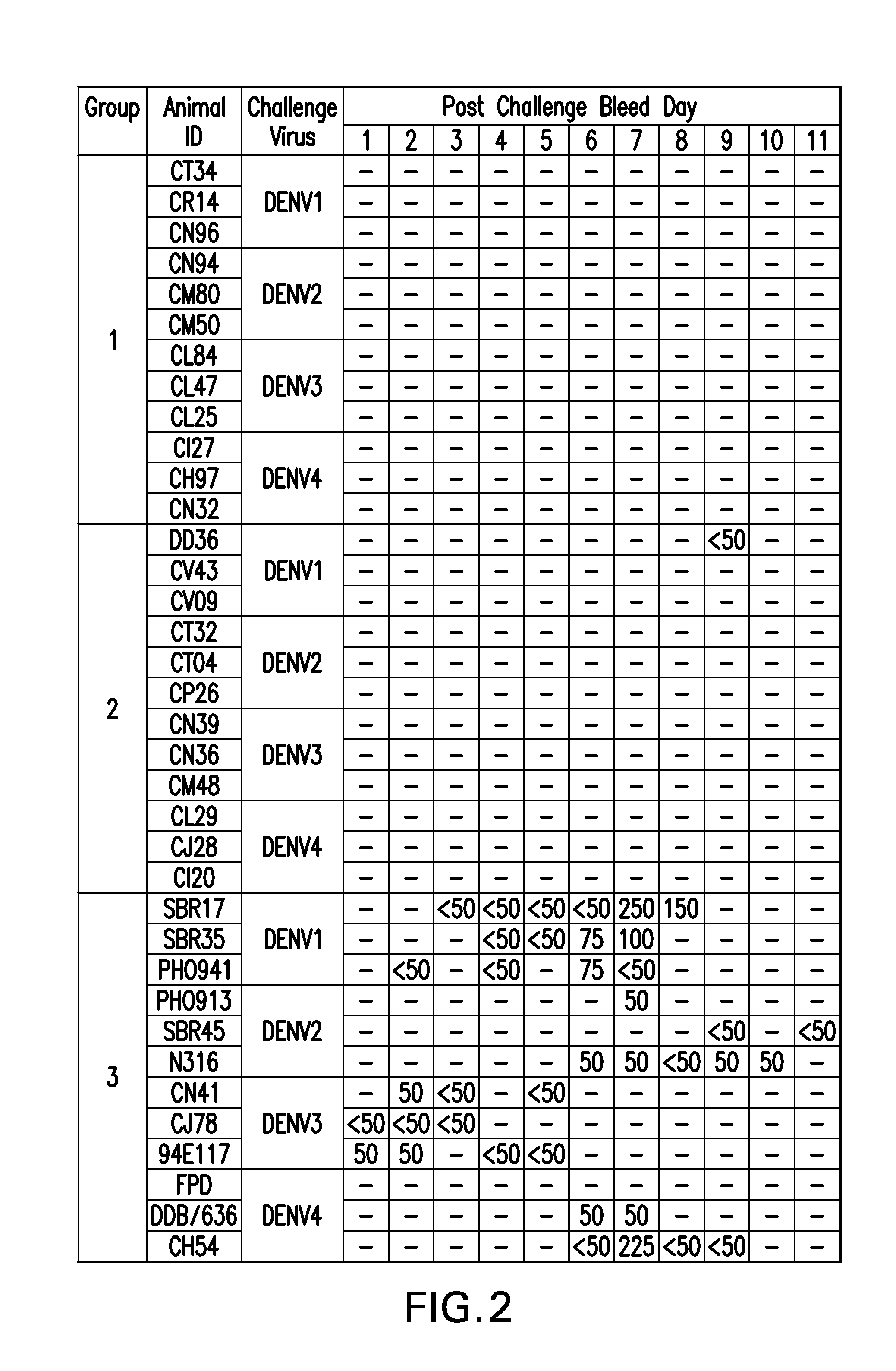
The present invention provides dengue virus vaccines and immunogenic compositions for administration to human subjects. The vaccine compositions of the present invention comprise recombinantly produced monomeric and / or dimeric forms of truncated dengue virus envelope glycoprotein that, when formulated together with an adjuvant and a pharmaceutically acceptable carrier, induce balanced tetravalent immune responses. In preferred embodiments of the compositions described herein, the DEN4 protein component is a dimeric form of DEN4. The compositions are designed to be acceptable for use in the general population, including immunosuppressed, immunocompromised, and immunosenescent individuals. Also provided herein are methods of inducing a protective immune response in a human patient population by administering the compositions described herein to the patients.
Owner:MERCK SHARP & DOHME LLC
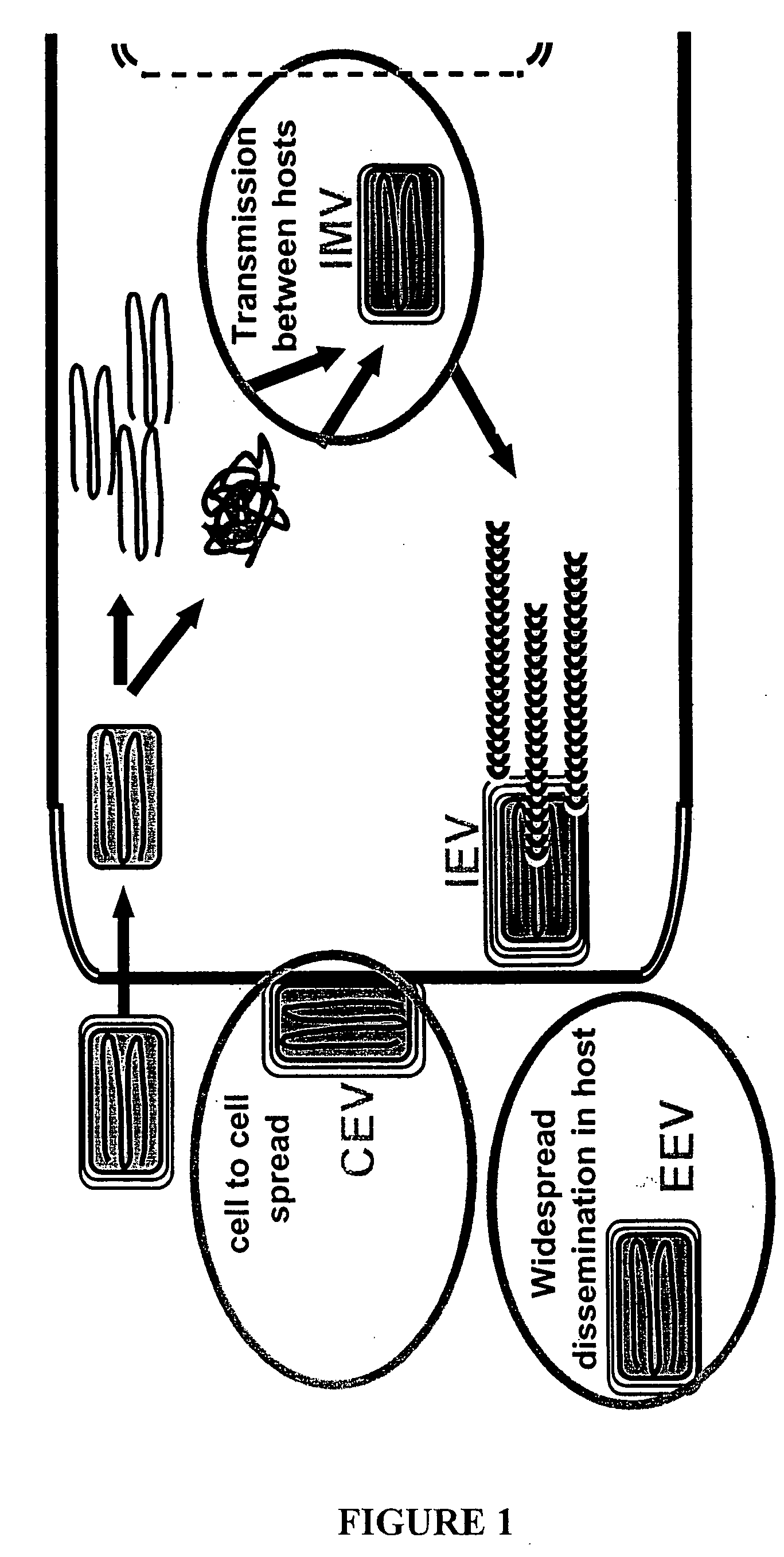
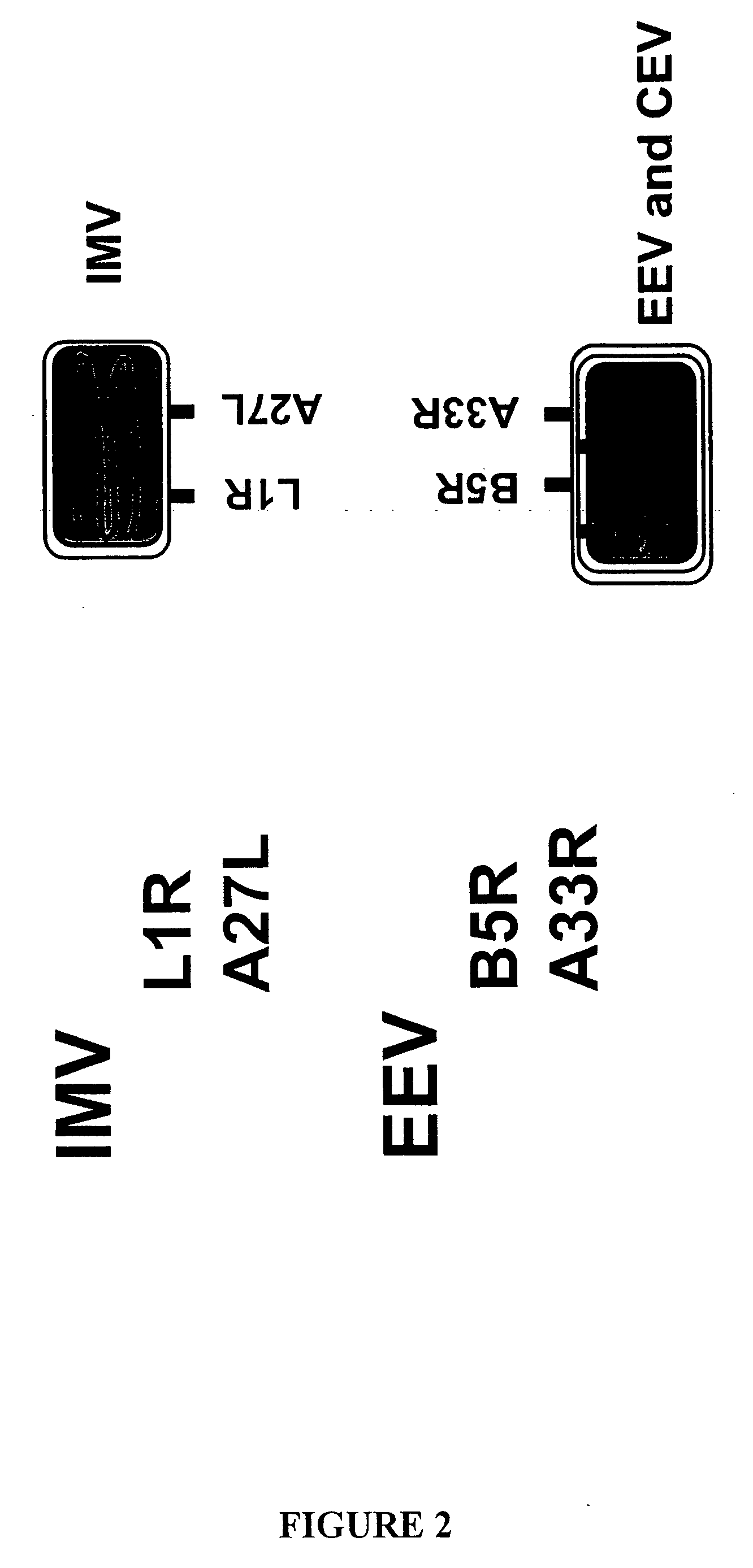
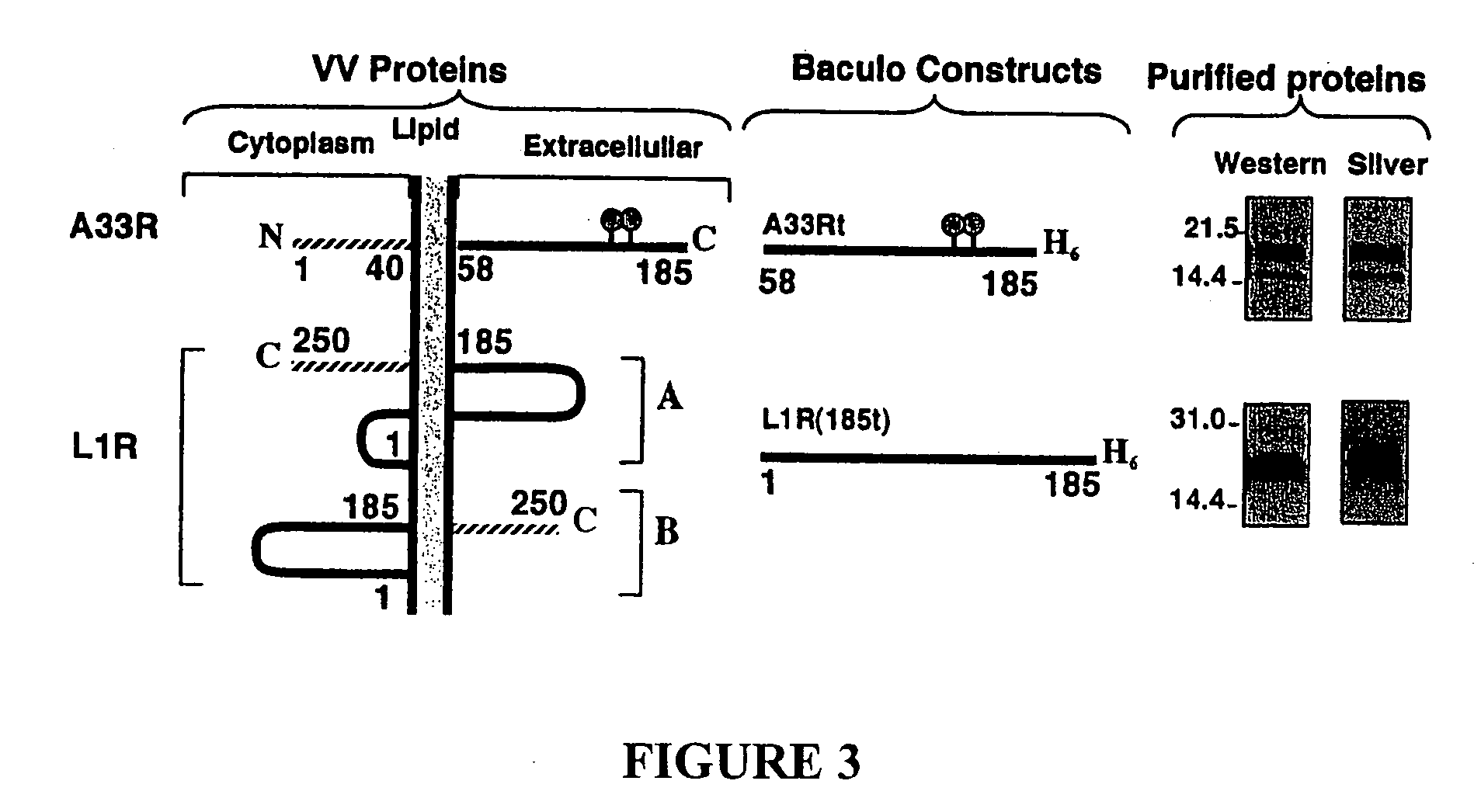
Compositions, methods and kits relating to poxvirus subunit vaccines
ActiveUS20060062800A1Organic active ingredientsGenetic material ingredientsPoxvirus InfectionsAntibody
The invention is directed to a poxvirus vaccine comprising a soluble truncated poxvirus envelope protein. The invention is also directed to a vaccine comprising a nucleic acid encoding such proteins. Also included is an antibody which specifically binds to the proteins and nucleic acid encoding the same, as well as methods of preventing and treating a poxvirus infection using the afore-mentioned vaccine, antibody, protein, and nucleic acid encoding them.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH +2
Materials and methods relating to the transfer of nucleic acid into quiescent cells
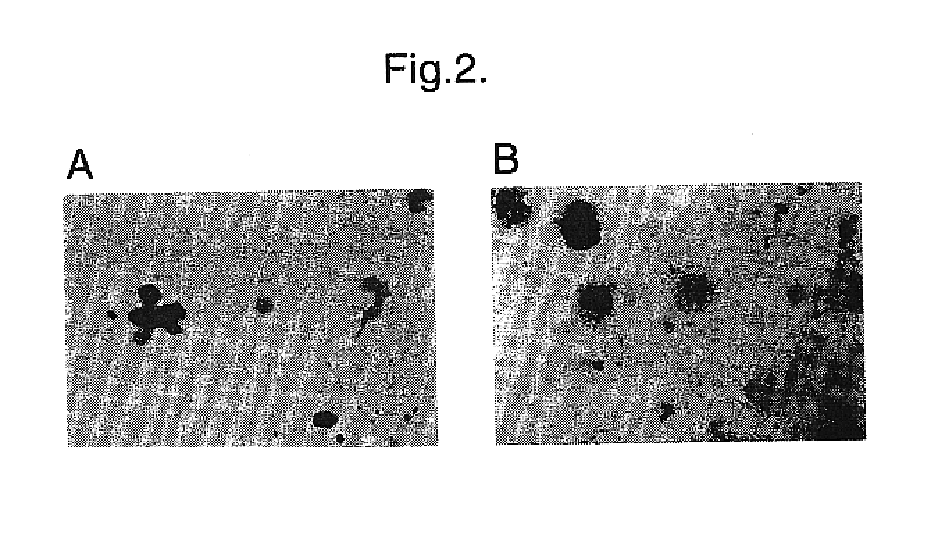
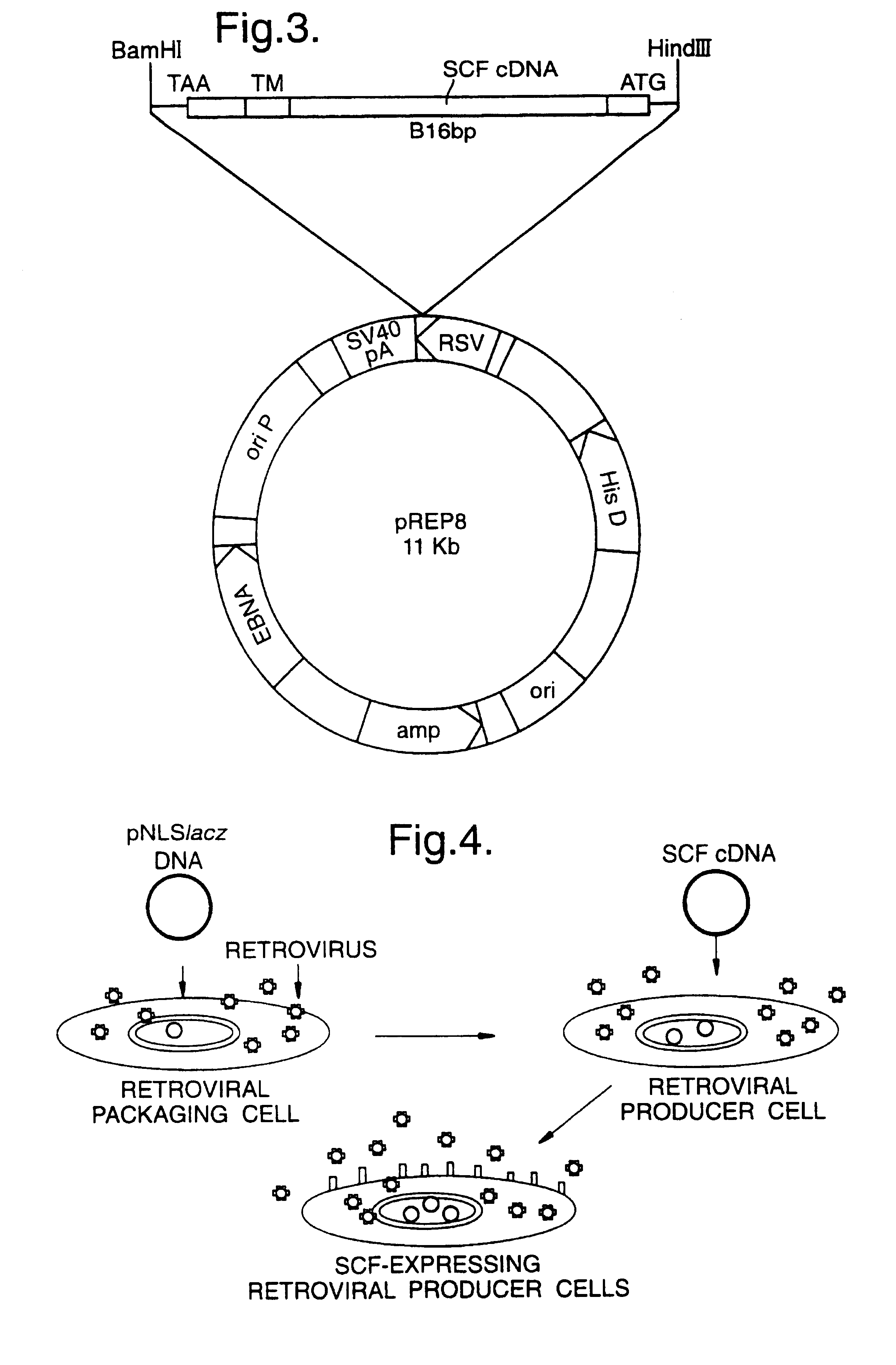
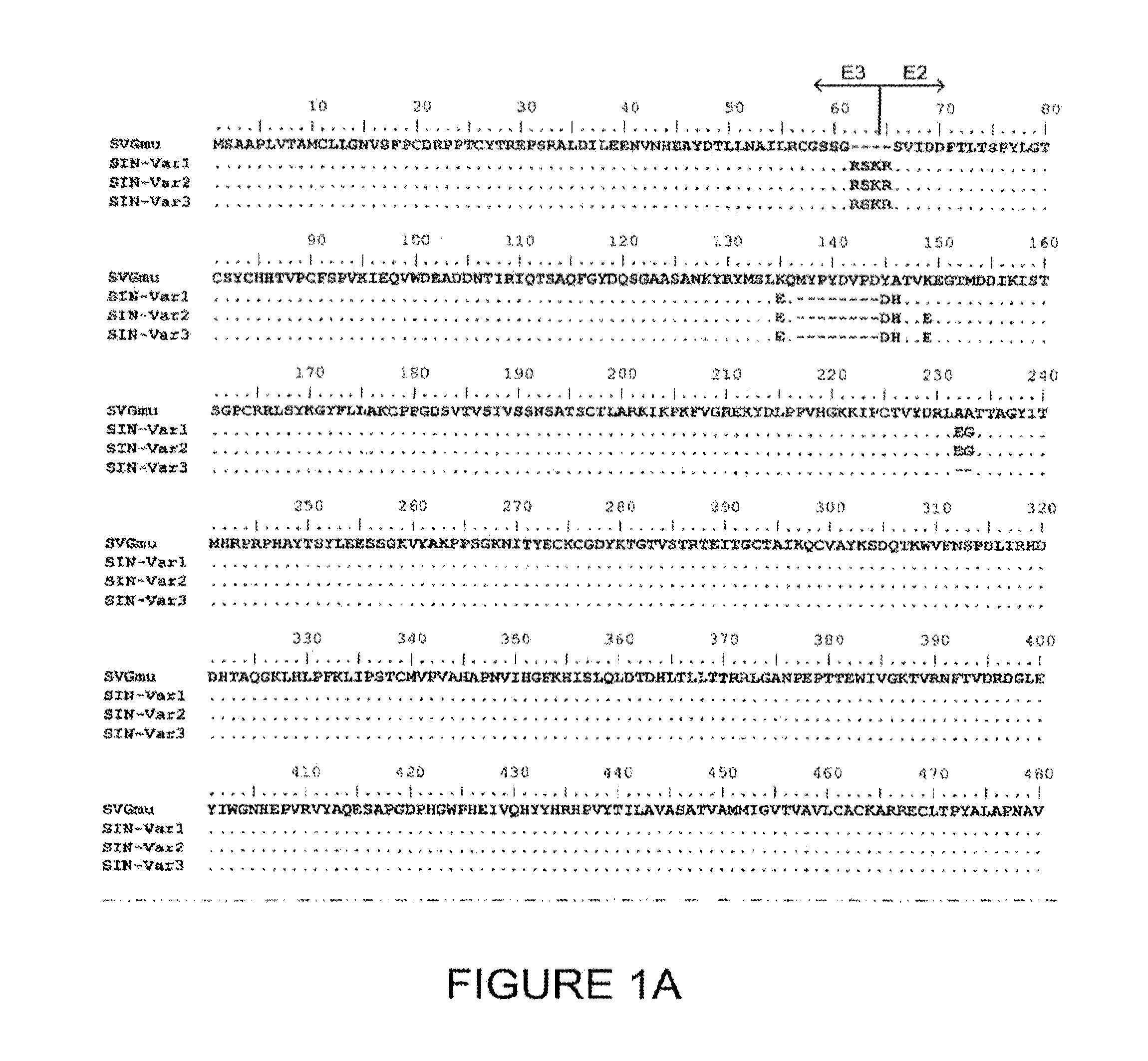
Materials and methods for transferring nucleic acid encoding a polypeptide for treating a disease or disorder into populations of quiescent cells such as haematopoietic stem cells (HSCs), using retroviral packaging cell lines and retroviral particles expressing and display a growth factor such as stem cell factor (SCF) on the cell surface or as a fusion with a viral envelope protein. The present invention also relates to compositions comprising the retroviral packaging cell lines and retroviral particles, and their use in methods of medical treatment, in vivo and ex vivo.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Lentiviral Vectors Pseudotyped with a Sindbis Virus Envelope Glycoprotein
ActiveUS20120039932A1Promote infectionSsRNA viruses positive-senseViral antigen ingredientsDendritic cellSindbis virus
Lentiviral vector particles comprising a Sindbis virus E2 glycoprotein variant and a lentiviral vector genome comprising a sequence of interest are provided. A lentiviral vector particle comprising: (a) an envelope comprising a Sindbis virus E2 glycoprotein variant; and (b) a lentiviral vector genome comprising a sequence of interest; wherein the E2 glycoprotein variant facilitates infection of dendritic cells by the lentiviral vector particle, and wherein the E2 glycoprotein variant has reduced binding to heparan sulfate compared to a reference sequence (HR strain).
Owner:IMMUNE DESIGN CORP
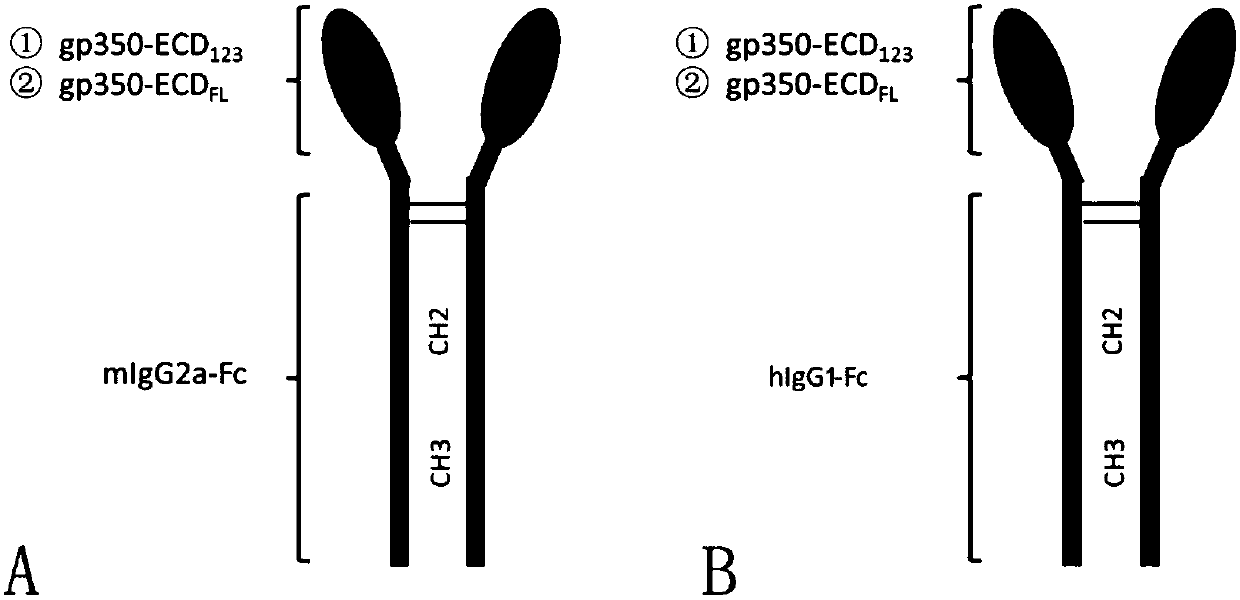
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
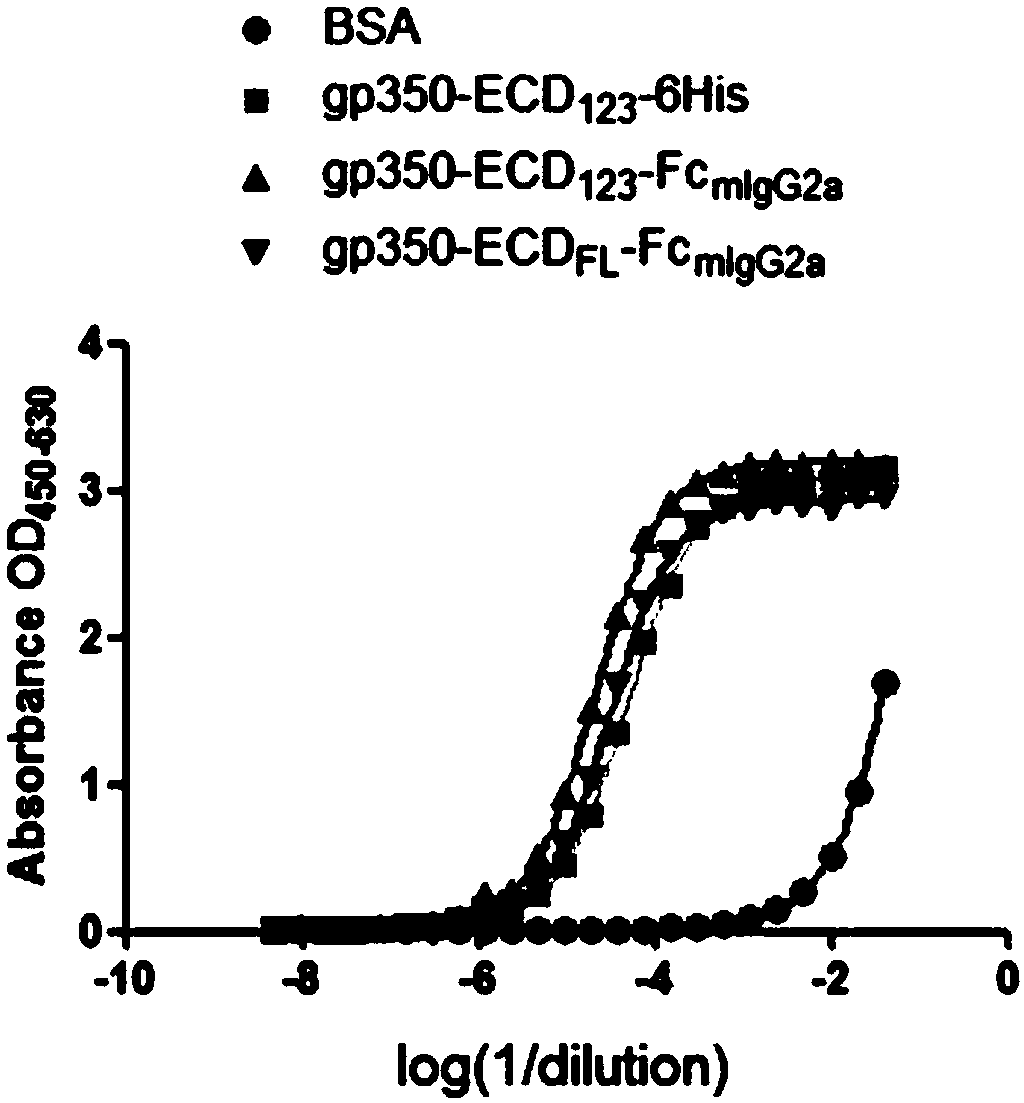
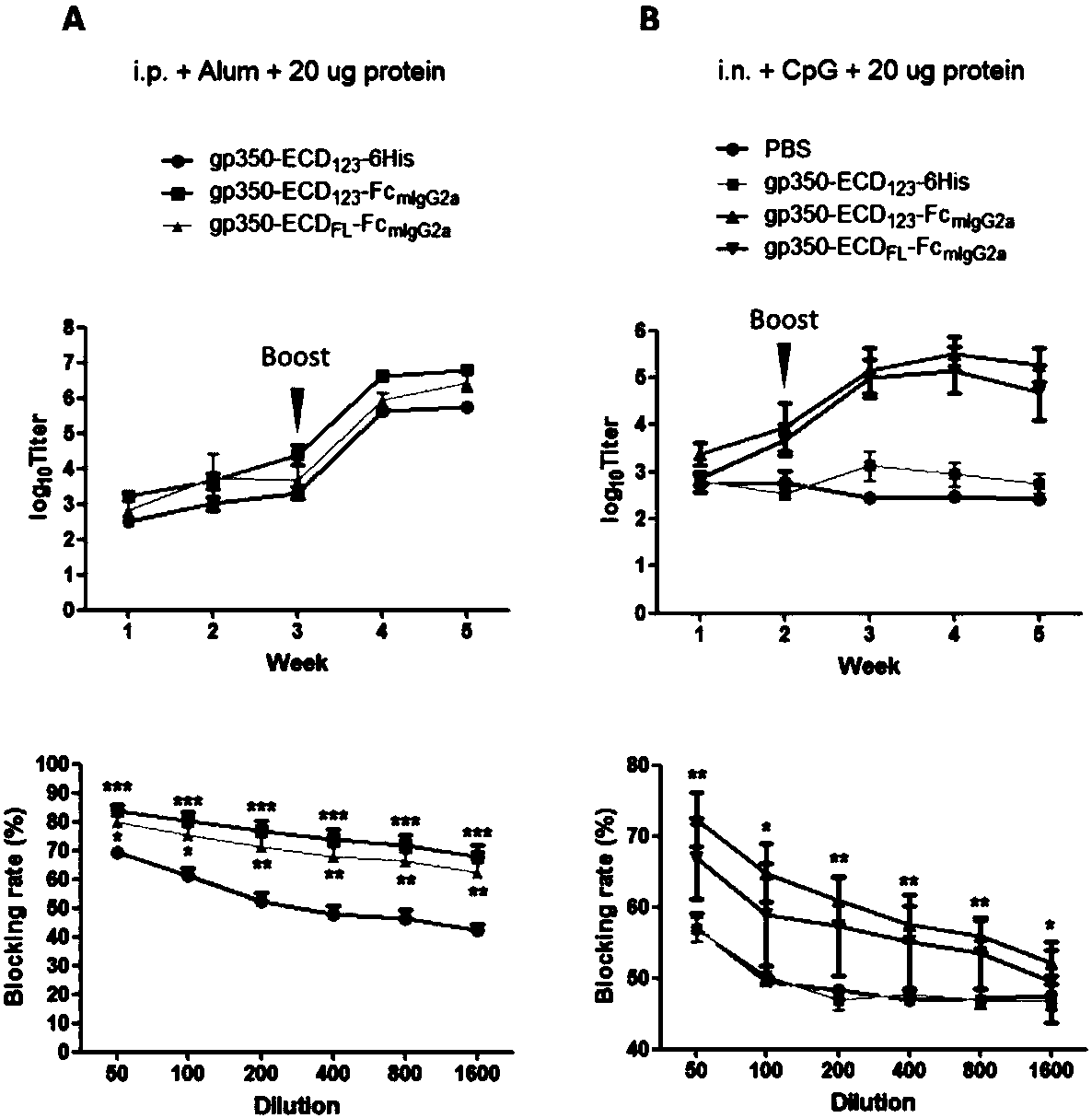
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
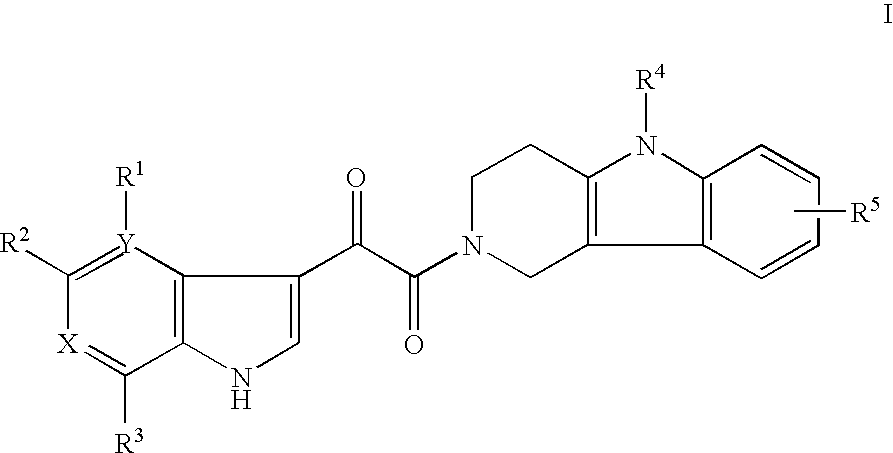
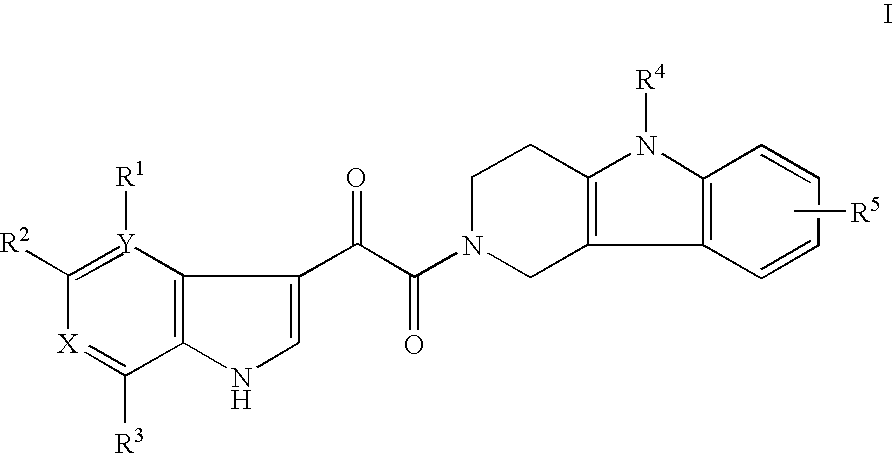
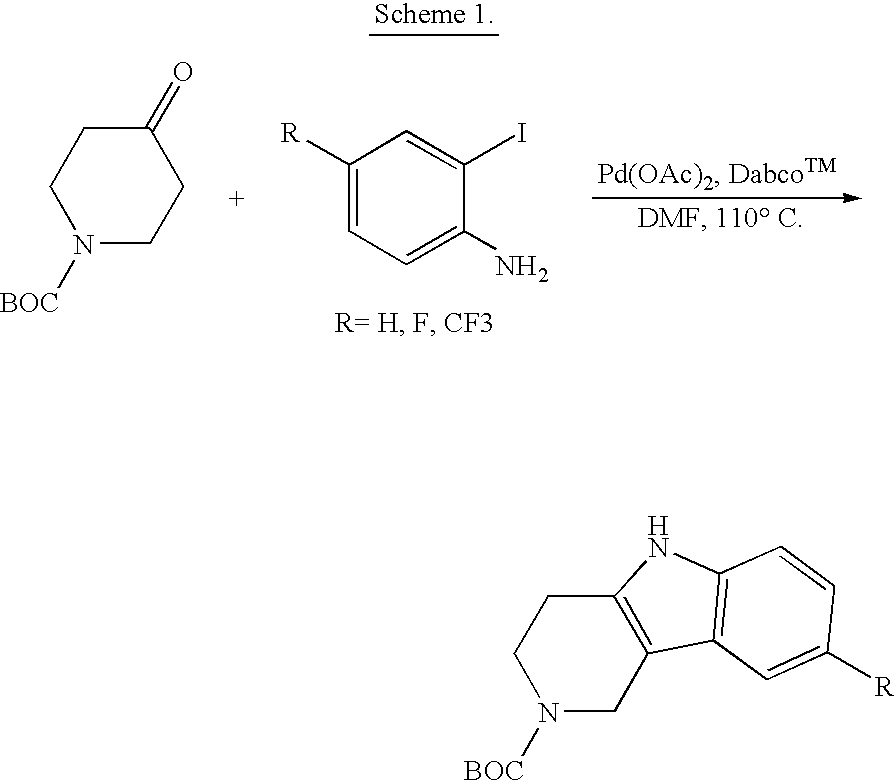
Tetrahydrocarboline antiviral agents
The invention encompasses a series of tetrahydrocarboline compounds of Formula I which inhibit HIV entry by attaching to the exterior viral envelop protein gp120 and interrupting the viral entry process, possibly by interfering with the cellular receptor CD4. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Virosome-like-particles
ActiveUS7901920B2Effective dissolutionAntibacterial agentsSsRNA viruses negative-senseVirosomePhospholipid
The invention relates to the production of virosome-like-particles. The invention provides a method for producing a virosome-like-particle comprising contacting an enveloped virus with a solution containing a short-chain phospholipid allowing solubilisation of the viral envelope of said virus further comprising removing short-chain phospholipid from said solution allowing formation of a functionally reconstituted viral envelope.
Owner:BESTEWIL HOLDING BV
Recombinant phages influenza vaccine
ActiveCN101015691AImproving immunogenicityEasy accessAntiviralsViruses/bacteriophagesMicrosphereBacteriophage
This invention relates to the field of molecular biology and vaccinology, a phage display broad spectrum influenza vaccine, and further relates to preparation method of phage display broad spectrum influenza vaccine. Present influenza vaccine can not provide protect aim at different influenza virus owing to high Variation of influenza virus. The invention constructs a universal recombinant phage influenza vaccine by inserting extracellular region M2 coding gene of highly conserved Type An influenza virus envelope protein M and part coding gene of B influenza virus highly conserved envelope protein BM 2 to bacteriophage T7, and prepares to recombinant phage influenza microsphere vaccine. After protective inoculation, the vaccine can provide protective action against different hypotype of type Ainfluenza virus and type B influenza virus.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
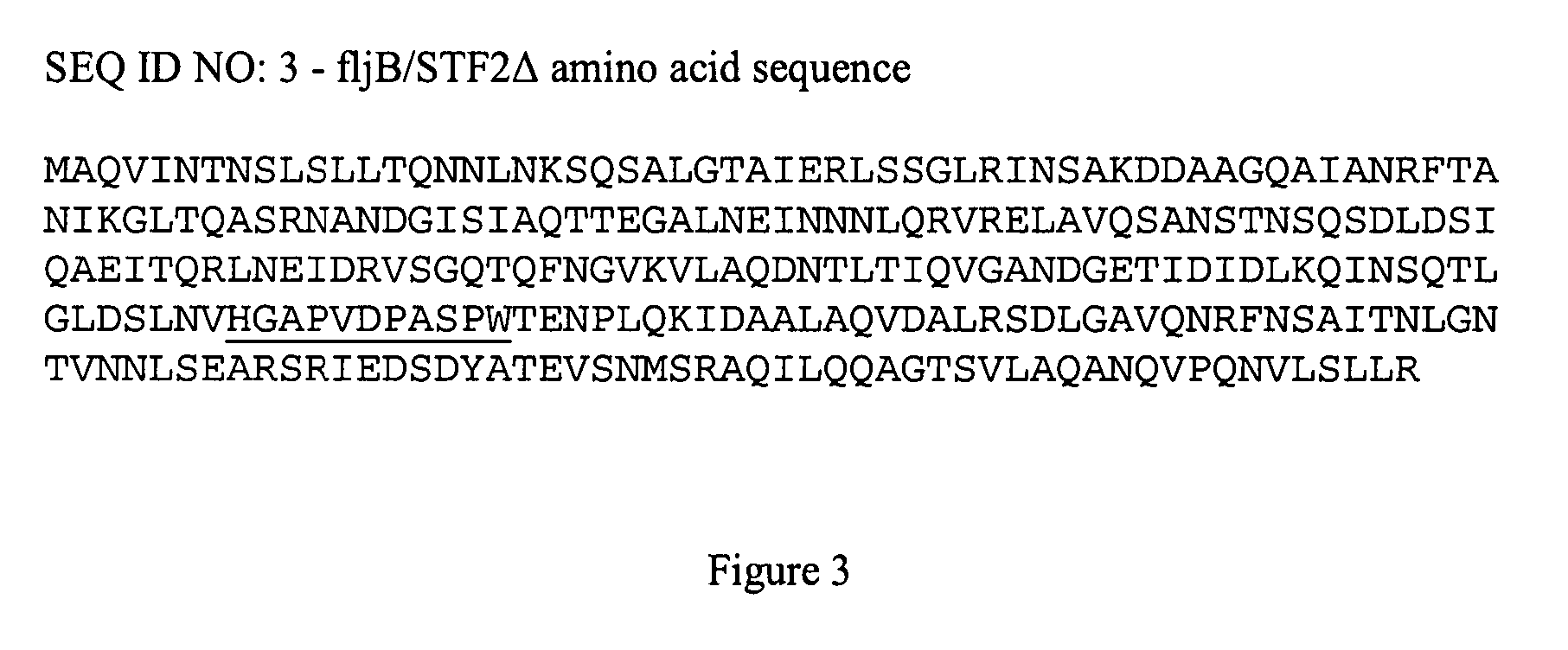
Fusion proteins comprising flagellin and dengue viral envelope proteins
InactiveUS8574588B2Effective immunogencityEliminate side effectsSsRNA viruses positive-sensePeptide/protein ingredientsViral envelopeProtective immunity
Fusion proteins comprise at least a portion of at least one flagellin and at least a portion of at least one Dengue viral envelope protein. The fusion proteins are used to stimulate an immune response and protective immunity in a subject.
Owner:VAXINNATE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com