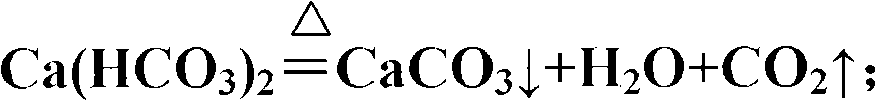Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
479results about "Ammonium halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
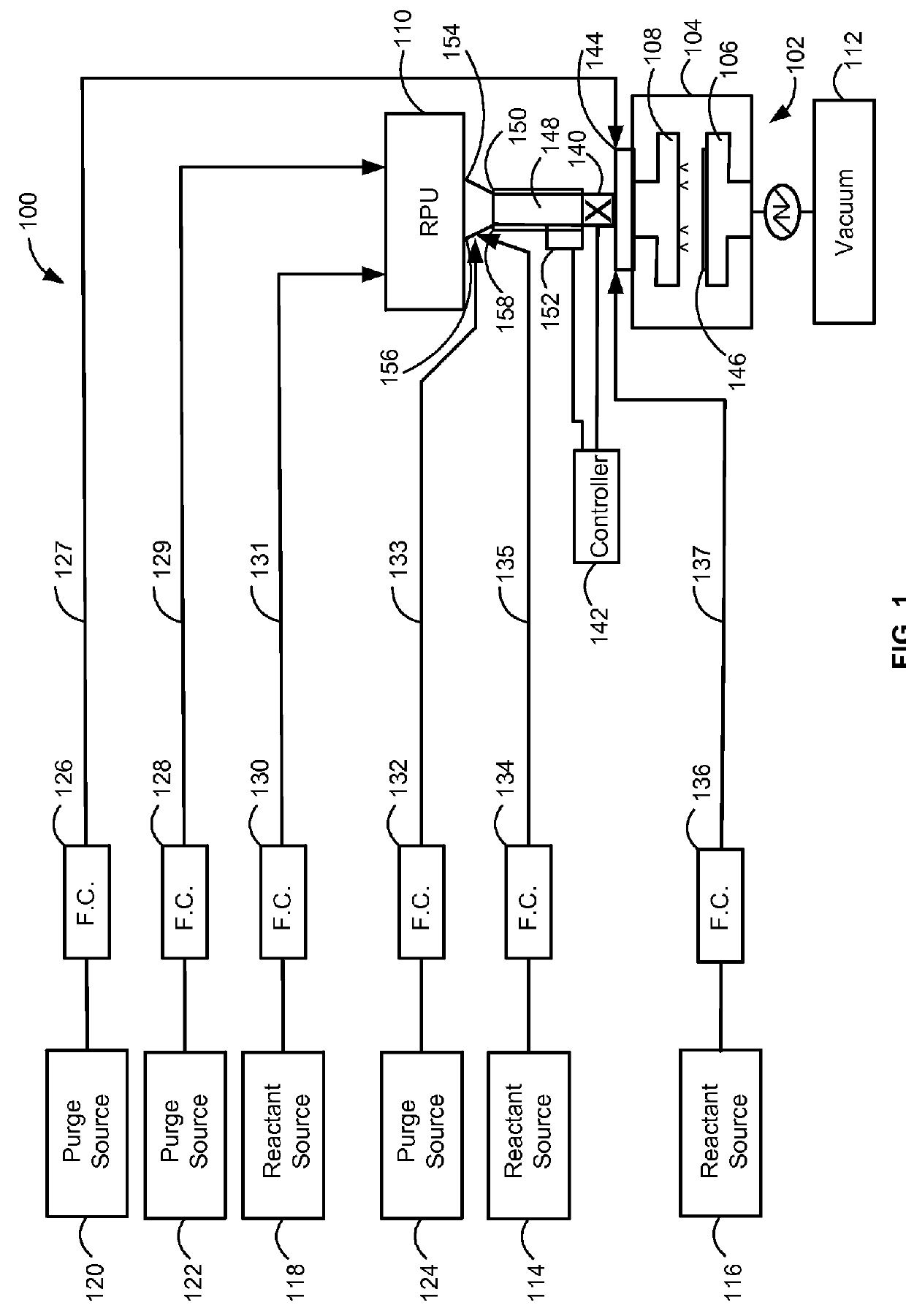
Method and system for in situ formation of gas-phase compounds
ActiveUS20160051964A1Process can be usedProcess control/regulationCell electrodesCompound aGas phase
A system and method for providing intermediate reactive species to a reaction chamber are disclosed. The system includes an intermediate reactive species formation chamber fluidly coupled to the reaction chamber to provide intermediate reactive species to the reaction chamber. A pressure control device can be used to control an operating pressure of the intermediate reactive species formation chamber, and a heater can be used to heat the intermediate reactive species formation chamber to a desired temperature.
Owner:ASM IP HLDG BV
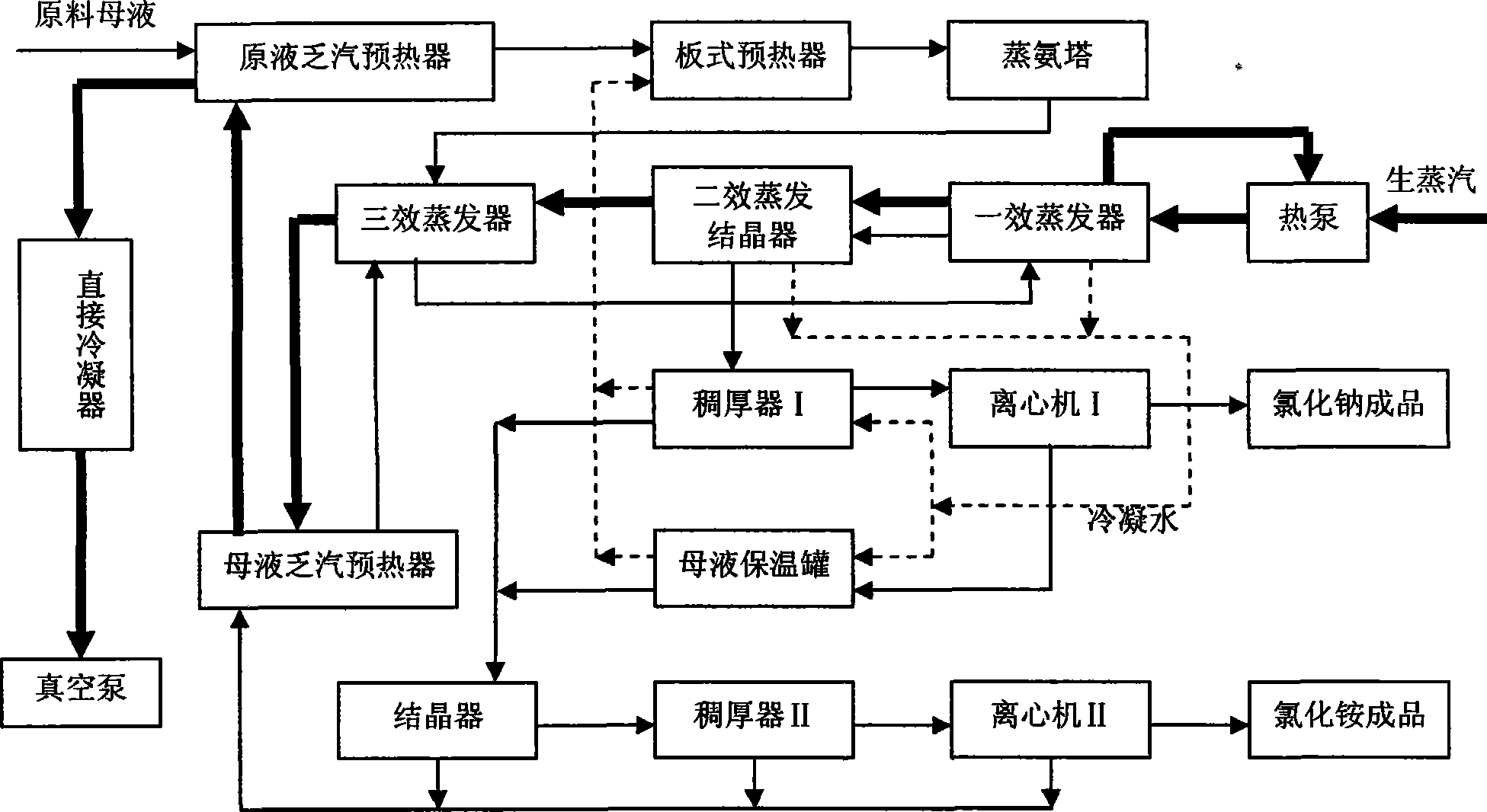
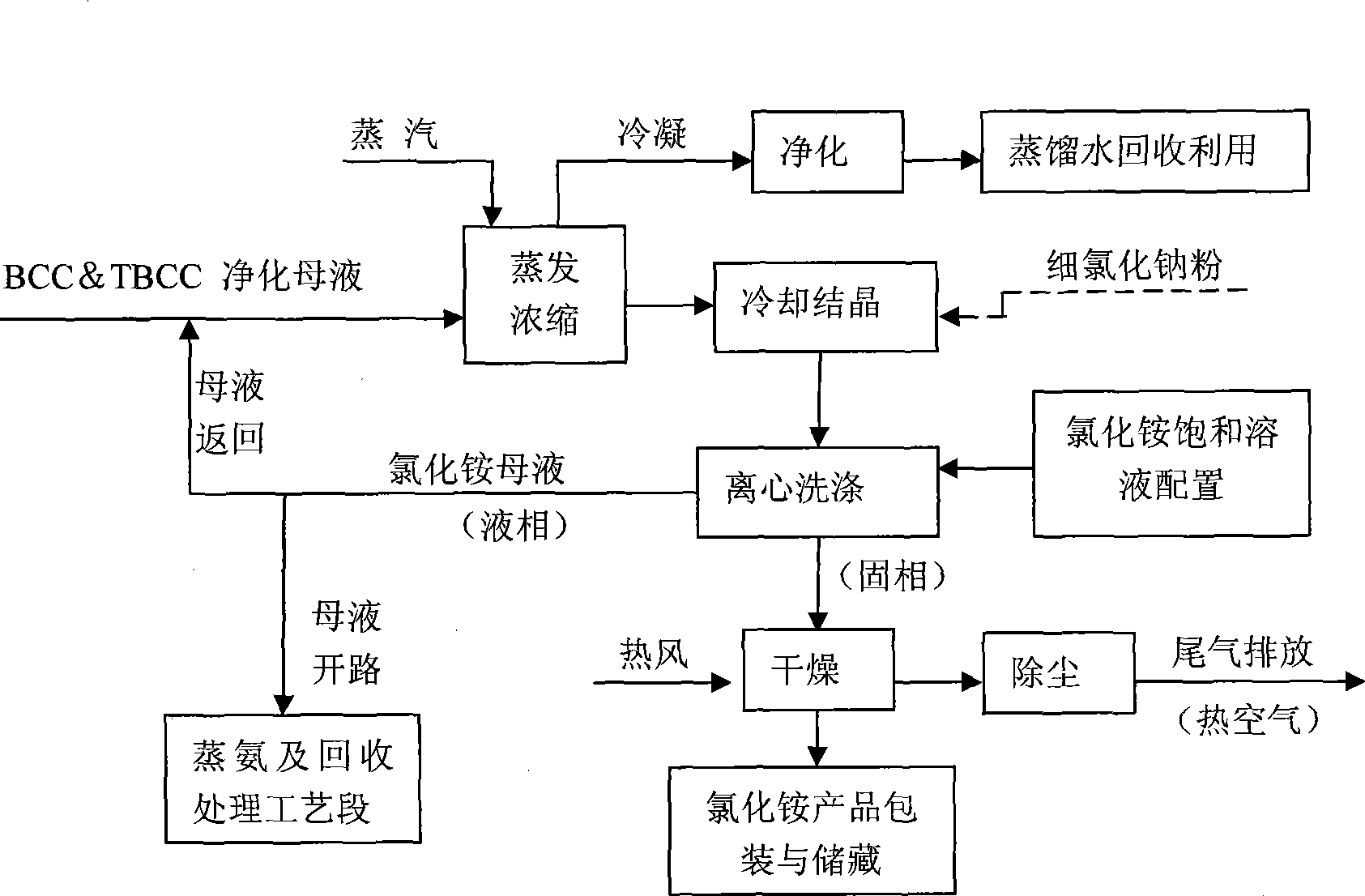
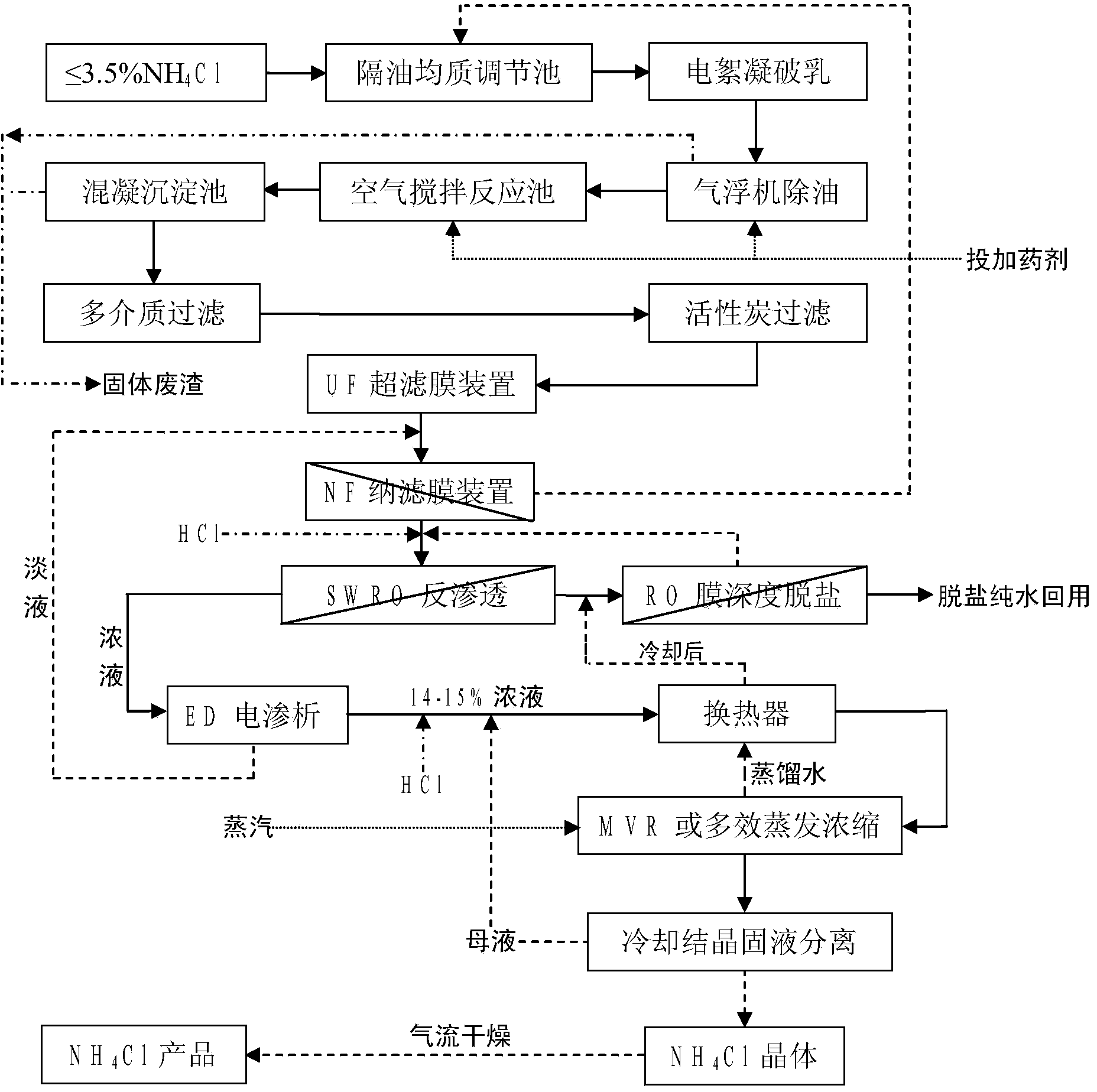
Process method for recovering ammonium chloride and sodium chloride from waste water containing ammonium chloride and sodium chloride
InactiveCN101544437AFully recycleReduce energy consumptionEnergy inputSolution crystallizationSodium bicarbonateDecomposition
The invention relates to a process method for recovering ammonium chloride and sodium chloride from waste water containing the ammonium chloride and the sodium chloride, which produces the ammonium chloride and the sodium chloride by using mother solution which is generated in a process for producing sodium bicarbonate by natural bittern double decomposition reaction and contains the ammonium chloride and the sodium chloride as raw materials. The method adopts ammonium still, evaporation, crystallization and separation process to treat, wherein the evaporation adopts multiple-effect, a heat pump and a vacuum evaporation process, and selects a falling film evaporator and a forced circulation type evaporator to perform triple-effect mixed-flow procedure, so that sodium chloride is crystallized and separated in the evaporation; and the ammonium chloride is crystallized and separated by cooling after the evaporation. The method effectively reduces the operation temperature of the equipment, can repeatedly use secondary steam and condensed water, reduces erosion of the ammonium chloride solution to the equipment, saves the energy, reduces the cost, improves the production efficiency, and reduces environmental pollution.
Owner:HEBEI UNIV OF TECH +1
Cleaning treatment method of dying industrial acidic wastewater
ActiveCN102826673AAchieve recyclingAvoid it happening againAmmonium sulfatesMultistage water/sewage treatmentSulfateWastewater
The invention provides a cleaning treatment method of dying industrial acidic wastewater. After purified by measurements of neutralization, decolouring, oxidation, condensation, separation and the like, wastewater is reused as synthetic bottom water and dye filter cake washing water for dye production, thus avoiding discharge of a lot of colored wastewater with high COD value during a traditional dye production process. By a method of using a by-product ammonium sulfate or ammonium chloride, generation of calcium sulfate is avoided during neutralization of wastewater by the use of lime. Therefore, the dangerous solid waste calcium sulfate slug treatment problem is solved, and the cleaning production purposes of water saving, emission reduction and synergy are reflected.
Owner:ZHEJIANG LONGSHENG GROUP +1
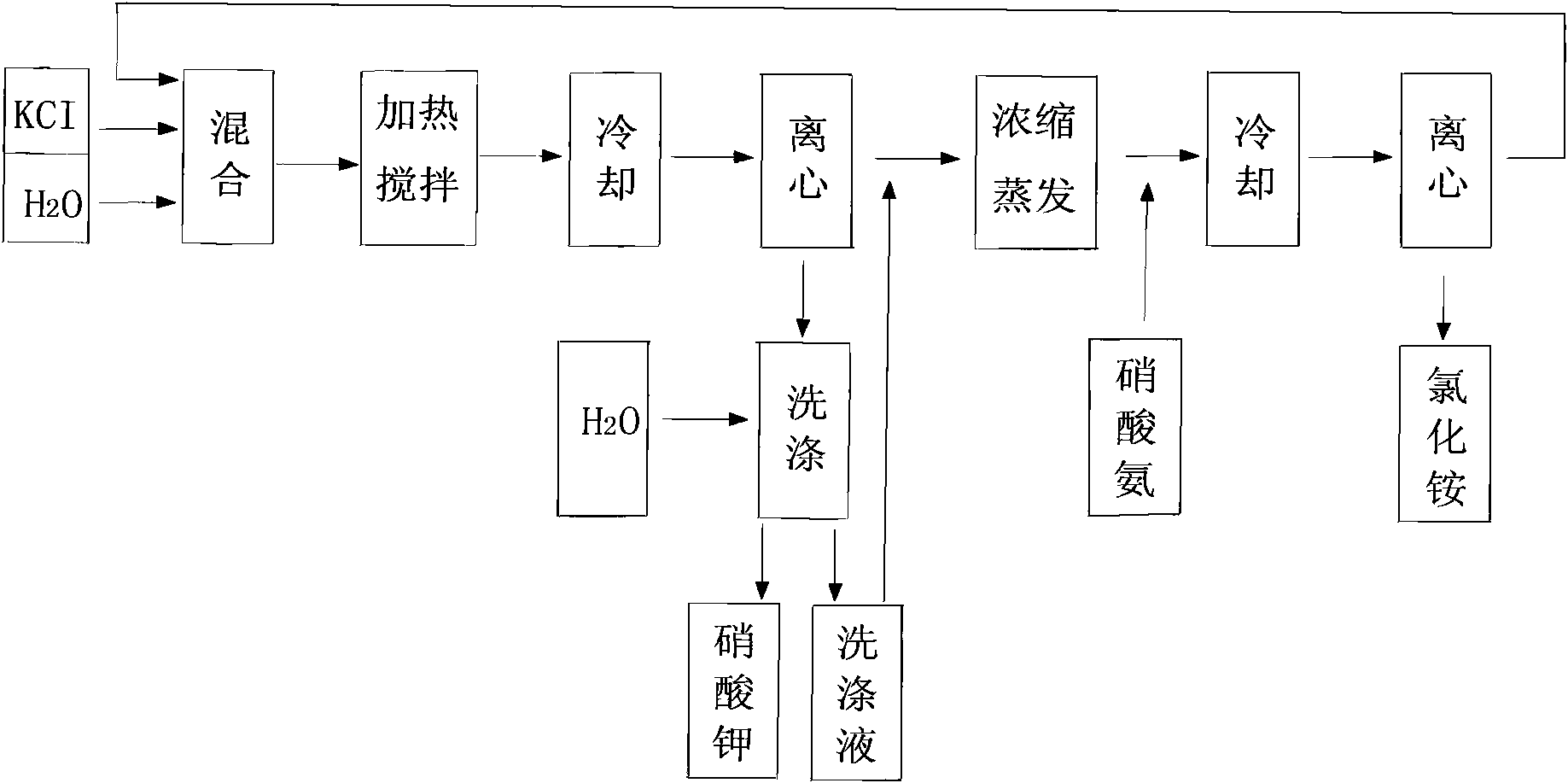
Method for preparing potassium nitrate and ammonium chloride employing double decomposition reaction
InactiveCN101628723ACreate pollutionRealize the concentrated evaporation processAlkali metal nitrate preparationAmmonium halidesDecompositionIon exchange
A method for preparing potassium nitrate and ammonium chloride employing double decomposition reaction comprises the following steps: dissolving ammonium nitrate and potassium chloride in water according to a defined ratio at 110 DEG C, continuously adding potassium chloride and water, heating while stirring to ensure that potassium nitrate is in supersaturation state, after stopping heating, cooling the solution in a vacuum cooling crystallizer to 36-40 DEG C to separate potassium nitrate crystal, placing the potassium nitrate crystal in a centrifugal machine with a filter cloth lining to obtain coarse potassium nitrate, then washing the potassium nitrate with cold water, drying to obtain the finished potassium nitrate; in addition, adding ammonium nitrate in mother solution I and cleaning solution to adjust solution concentration so that ammonium chloride can reach supersaturation state, using a vacuum concentration device to perform negative pressure evaporation, separating and precipitating ammonium chloride by centrifuging and obtaining a solid ammonium chloride product, wherein, when dissolving ammonium nitrate and potassium chloride, the ratio of ammonium ion to chlorine ion is 1:2 and when using the centrifugal machine to obtain the coarse potassium nitrate, the separated mother solution is another mother solution I sharing the same saturation point of potassium nitrate and ammonium chloride. The solution of feed liquid circular reaction overcomes the defects of the prior art that the price of potassium nitrate used in reaction is high, the resource of potassium nitrate is in short supply and the cost of devices used in ion-exchange method is high, thus being applicable to the production of potassium nitrate.
Owner:湖南丹化农资有限公司
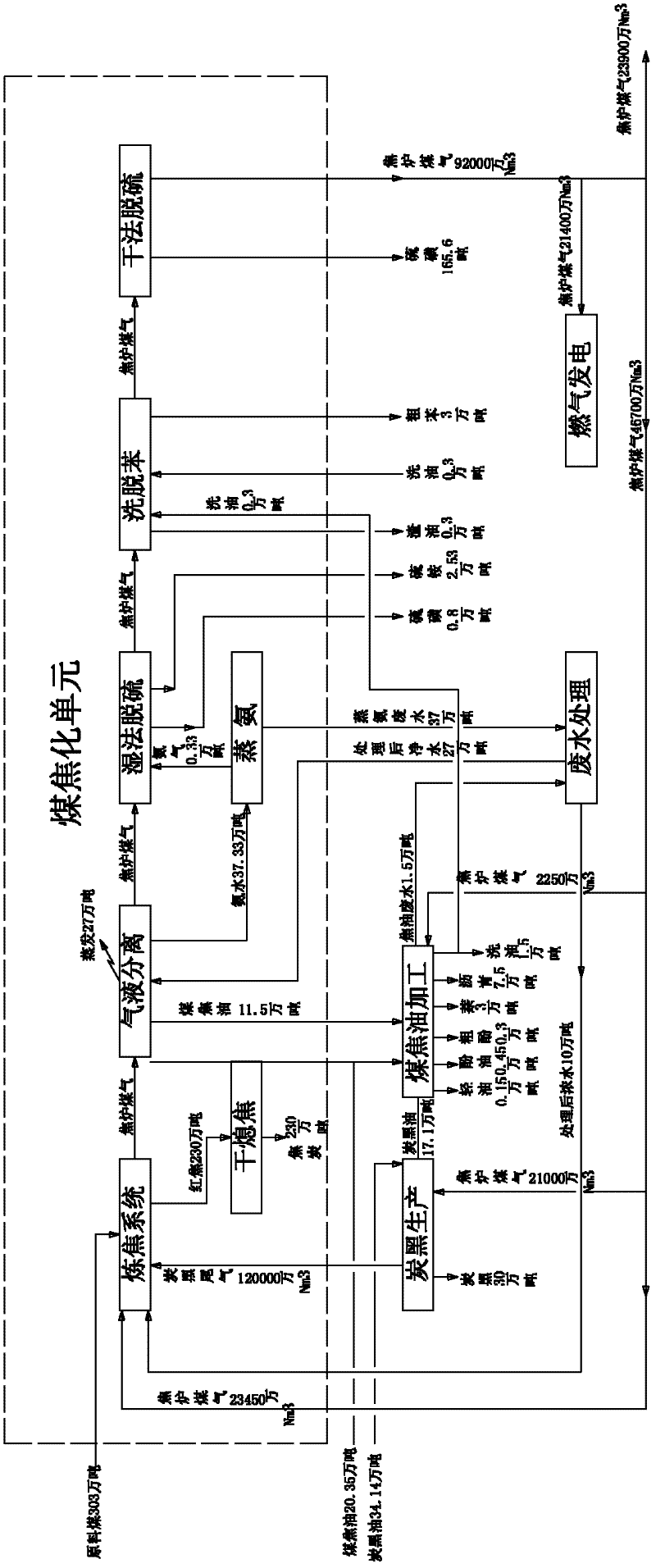
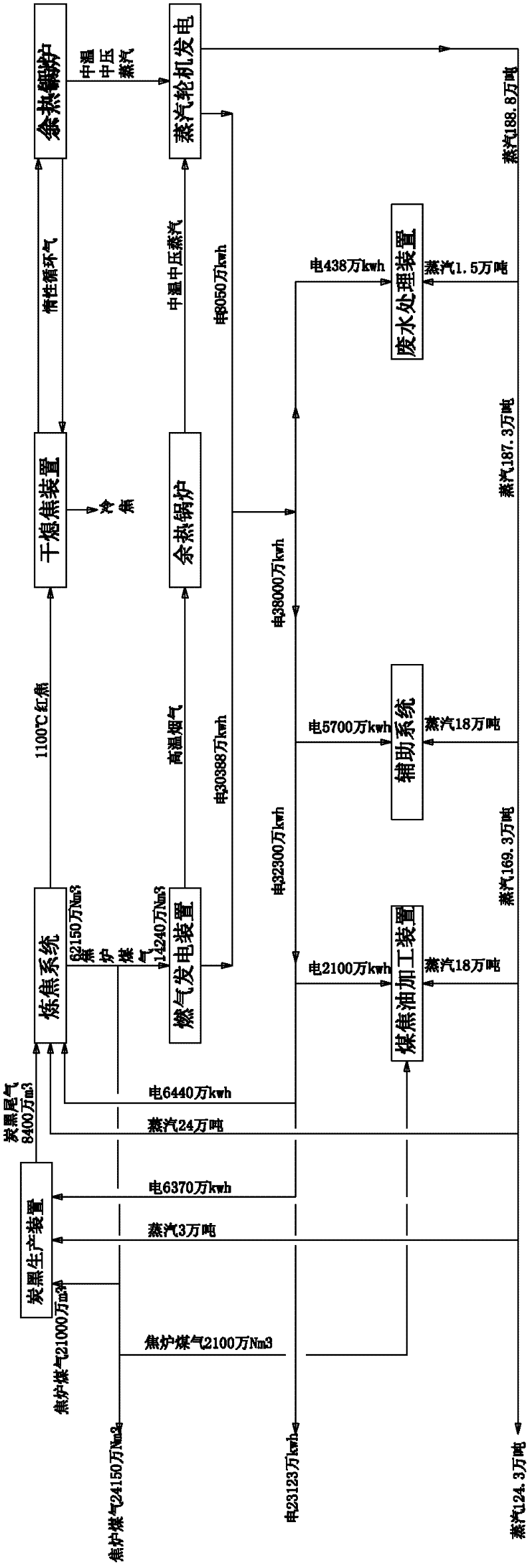
Green circular economy technology with coal coking as the main part
ActiveCN102229806AHigh aromatic contentQuality improvementPigmenting treatmentCoke quenchingCogenerationEngineering
The invention provides a green circular economy technology with coal coking as the main part. The technical scheme of the technology is as follows: a coal coking part, a coal tar processing part, a carbon black part and a part of combined power and thermoelectricity generation by coke oven gas are organically combined to form the relationship of industrial metabolism, symbiosis and coupling; suchmeasures as differentially utilizing tail gas of carbon black, recovering energy of coke drying quenching, reducing, recycling and changing the three wastes into resources, etc., enable resources to be saved, production cost to be reduced and pollution to be lessened; therefore, economic benefits of coal coking enterprises are maximized and the green circular economy technology is realized indeed. The invention also provides an apparatus for realizing the green circular economy technology with coal coking as the main part.
Owner:JINNENG SCI & TECH
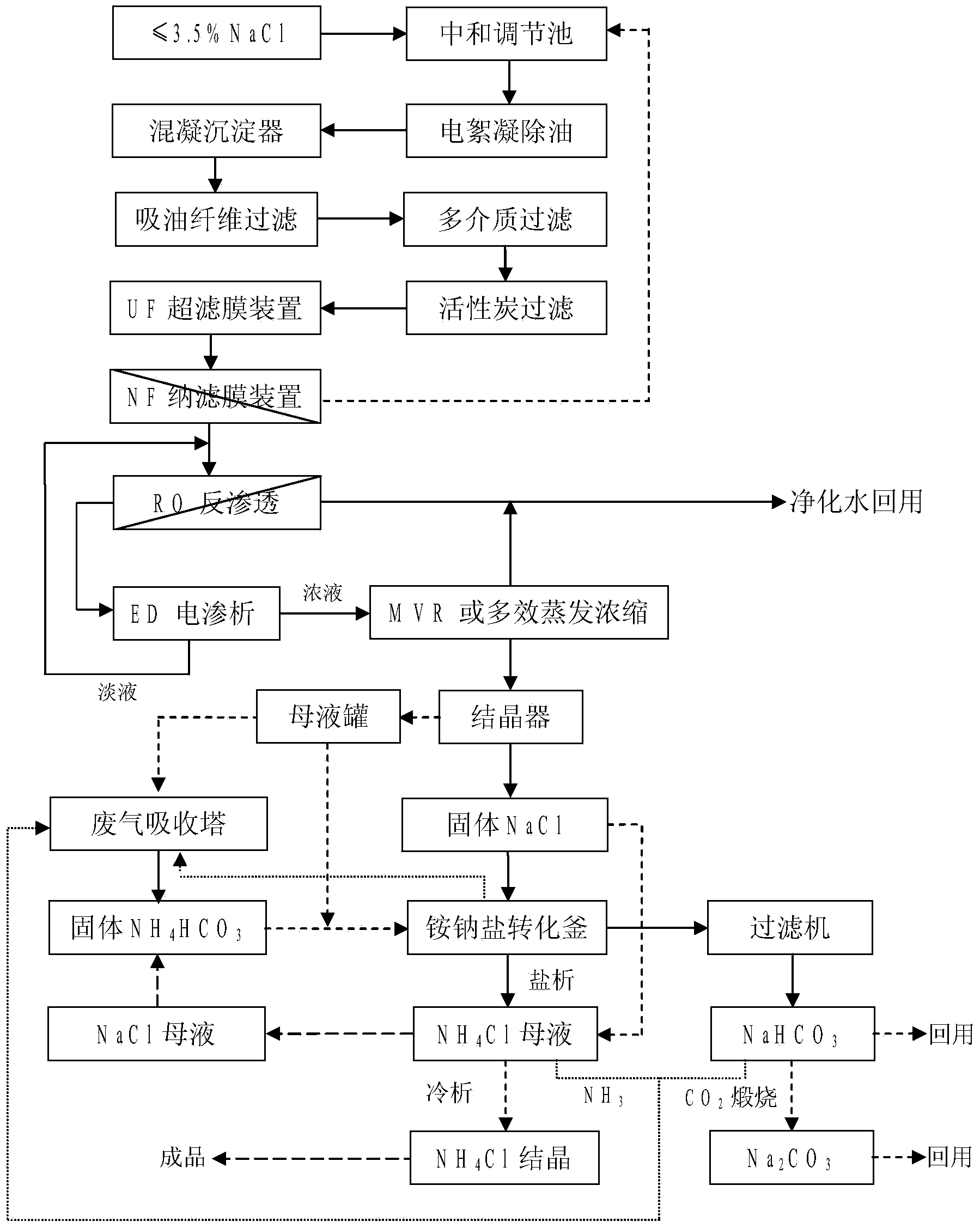
Combined treatment method for sodium chloride-containing wastewater generated in dressing and smelting of rare earth
InactiveCN103449653AImprove environmental protection measuresAvoid volatile lossGeneral water supply conservationMultistage water/sewage treatmentSodium bicarbonateUltrafiltration
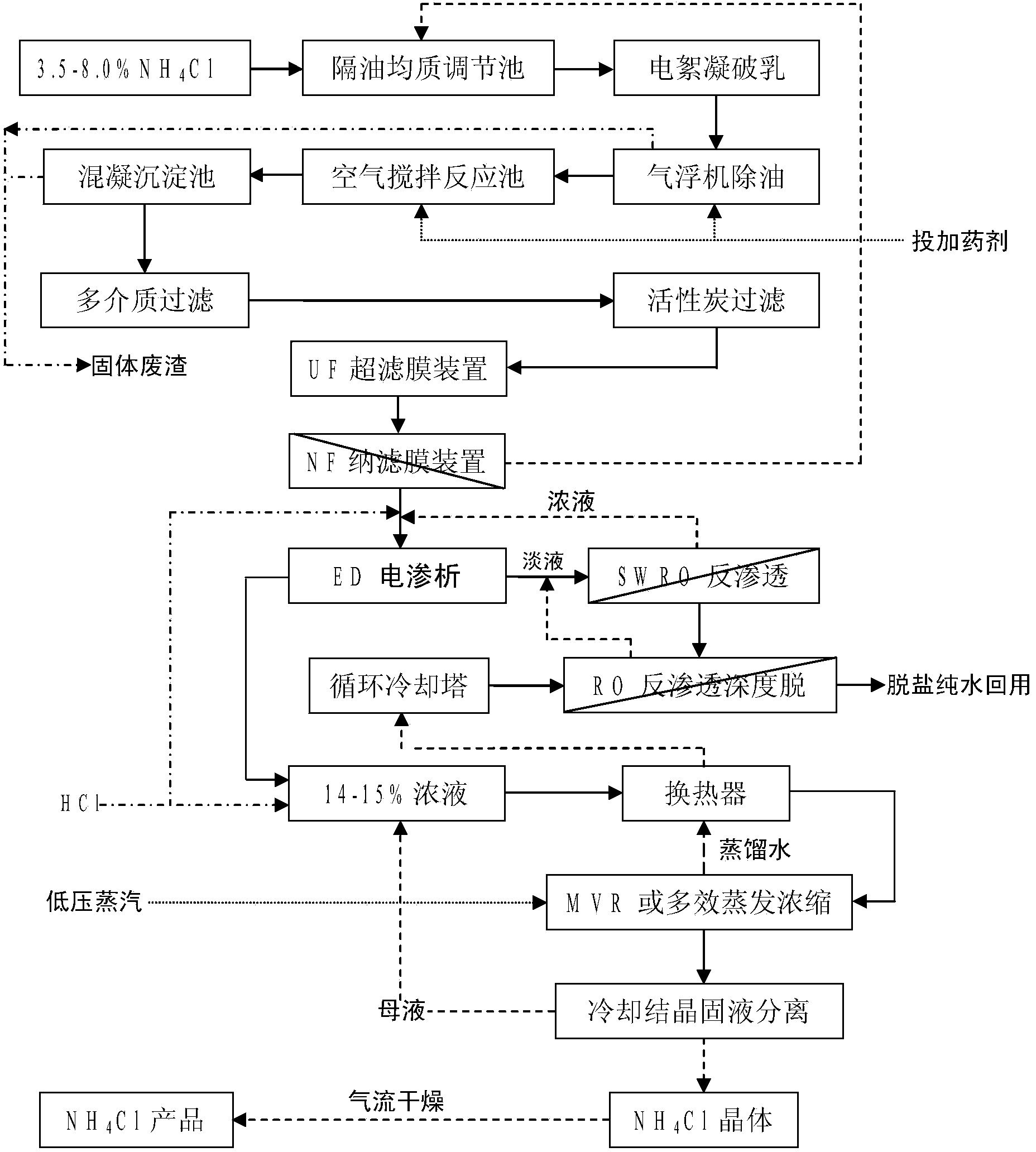
The invention relates to a combined treatment method for sodium chloride-containing wastewater generated in dressing and smelting of rare earth. According to the method, a series of desalination and concentration treatments such as deoiling, neutralizing, homogenizing, aeration, flocculation, ultrafiltration, nanofiltration, reverse osmosis, electroosmosis and MVR (mitral valve replacement) concentration are carried out on the wastewater, so that recyclable purified water is obtained; ammonium bicarbonate is added into the sodium chloride extracted from the wastewater, so that the sodium salt can be converted and regenerated into sodium bicarbonate, sodium carbonate and ammonium chloride products for dressing and smelting of the rare earth. According to the method, the recovery rate of the wastewater is high, the effluent quality is stable, the conversion rate of the sodium salt is high, and no three wastes are discharged; production equipment is compact, simple to operate, and easy to realize automation control; the problem that the sodium chloride-containing wastewater and other similar industrial wastewater generated in dressing and smelting of rare earth are difficult to recycle is solved.
Owner:YANSHAN UNIV
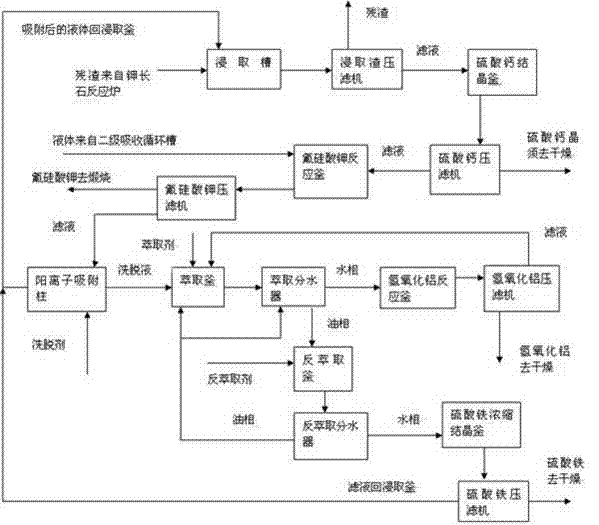
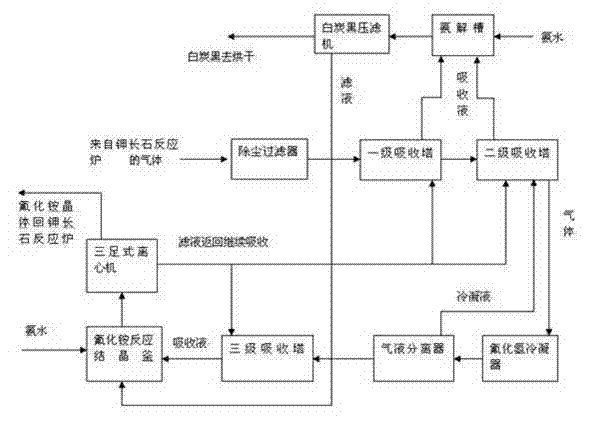
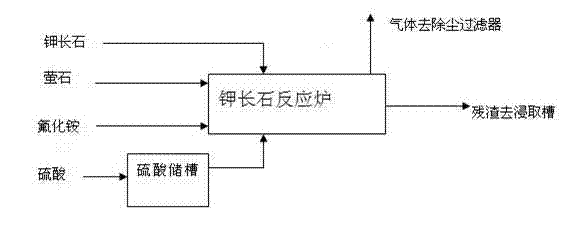
Process for decomposing potassium feldspar by adopting low-temperature semidry method for comprehensive utilization
ActiveCN103172074AAvoid wastingLower requirementSilicon halogen compoundsSilicaAluminium hydroxideDecomposition
The invention relates to potassium feldspar decomposition and comprehensive utilization technology and in particular relates to a process for decomposing potassium feldspar by adopting a low-temperature semidry method for comprehensive utilization. The process comprises the steps of fully mixing potassium feldspar, fluorite and sulfuric acid, then adding the mixture to a converter reactor to react at 180-250 DEG C, separating SiF4 and HF generated through a reaction from a system under the condition of negative pressure, carrying out absorption with ethanol and water solution to prepare white carbon black, recovering fluorine resources in the process from the solution in the forms of ammonium fluoride and other aids by adopting the method of adding ammonia water and applying the recovered fluorine sources to decomposition of potassium feldspar and carrying out a series of processes such as extraction on the solids after a reaction to obtain calcium sulfate whisker, potassium fluosilicate, aluminium hydroxide and ferric sulfate products. Compared with the method for decomposing potassium feldspar by a high temperature method, the process has the advantages that the reaction conditions are mild and the requirements for equipment are lower; the white carbon black is directly prepared through gas hydrolysis, thus avoiding the tedious course from solid phase separation; and the fluorine resources introduced to the decomposition course are recycled by adopting the mode of ammonium fluoride, thus avoiding waste of the fluorine resources.
Owner:LUOYANG FLUORIDE & POTASSIUM TECH +1
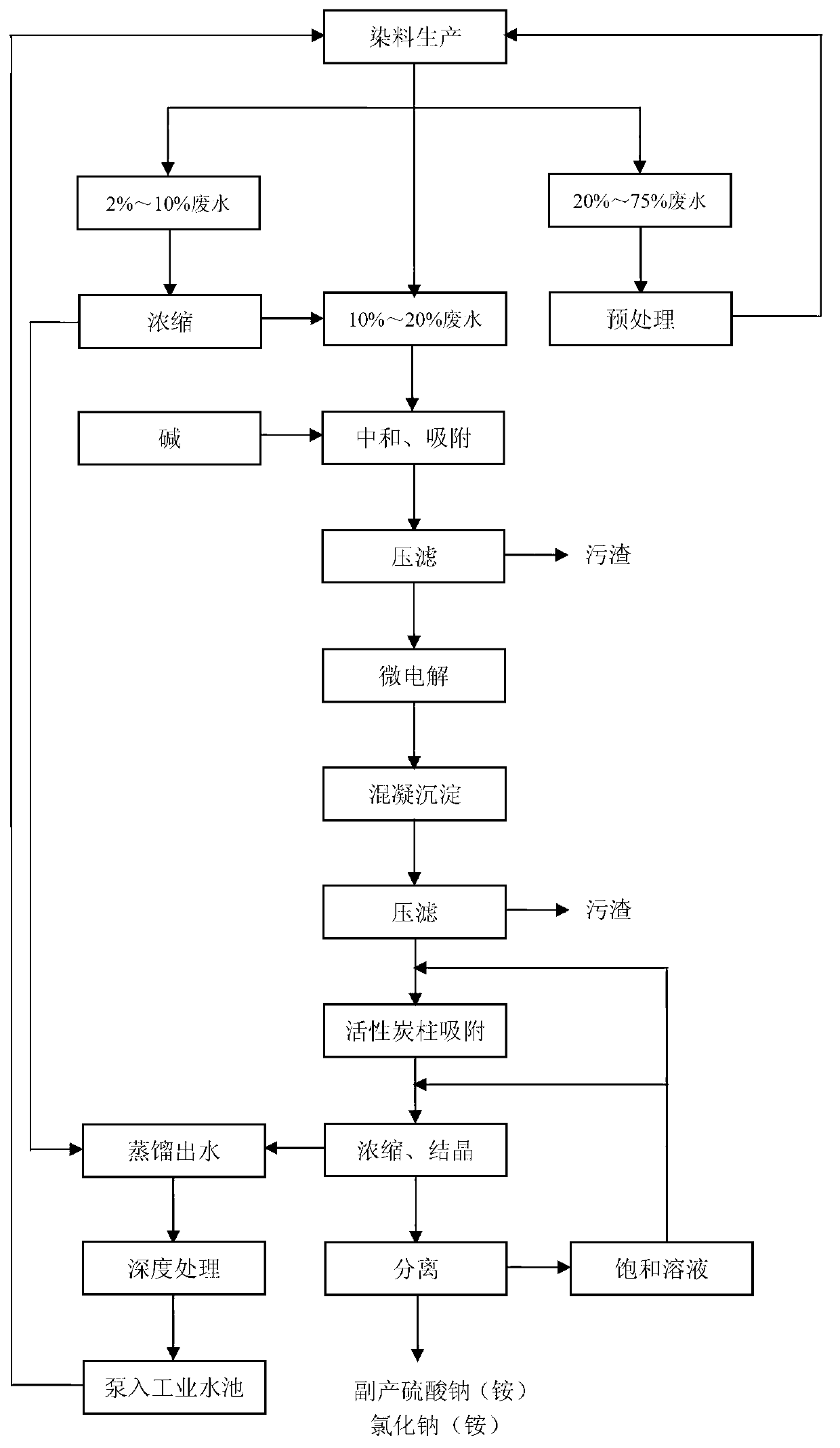
Dye acidic waste water treatment method and device
ActiveCN103130370AWide pH rangeLow costAmmonium sulfatesMultistage water/sewage treatmentAutomatic controlWater discharge
The invention relates to a dye acidic waste water treatment method and device. The method comprises steps as follows: separately collected dye waste acid is subjected to distilling concentration, neutralization, adsorption, micro-electrolysis, coagulative precipitation, activated carbon column adsorption, concentrating crystallization and separation through an automatic control system to prepare sodium (ammonium) sulfate or sodium (ammonium) chloride; and the distilled water is subjected to advanced treatment to reach the industrial reuse water standard, enters an industrial water storage tank, and is circulated to the dye production procedure. The method and device are especially suitable for industries generating abundant waste sulfuric acid and waste hydrochloric acid, such as production of dyes, titanium white and the like. The abundant waste sulfuric acid and waste hydrochloric acid generated in the production process can be utilized to prepare sodium (ammonium) sulfate or sodium (ammonium) chloride, thereby recycling acidic waste water, and changing wastes into valuable substances; and meanwhile, the distilled water can be circulated into industrial production after advanced treatment, thereby reducing the waste water discharge.
Owner:ZHEJIANG DIBANG CHEM
Energy-saving evaporation treatment process of high-salinity wastewater
ActiveCN102964019AStrong adsorption and removal abilityHigh purityCalcium/strontium/barium chloridesMultistage water/sewage treatmentInorganic saltsIndustrial effluent
The invention provides an energy-saving evaporation treatment process of high-salinity wastewater. The energy-saving evaporation treatment process comprises an evaporating system, a concentrating system, a drying system and a heat circulating system. Grading preheating, heating evaporation, adsorption purification, concentration, adsorption purification and high-temperature drying are successively carried out on the high-salinity wastewater treated by a wastewater treatment system to obtain the finished product of inorganic salt, heat exhausted in the evaporation, concentration and drying processes is recycled, and wastewater is discharged. By adopting the technical scheme, wastewater discharged finally meets the national industrial wastewater discharge standard, the purity of the obtained inorganic salt is as high as 99.3%, and the energy-saving effect is remarkable.
Owner:山东大明消毒科技有限公司
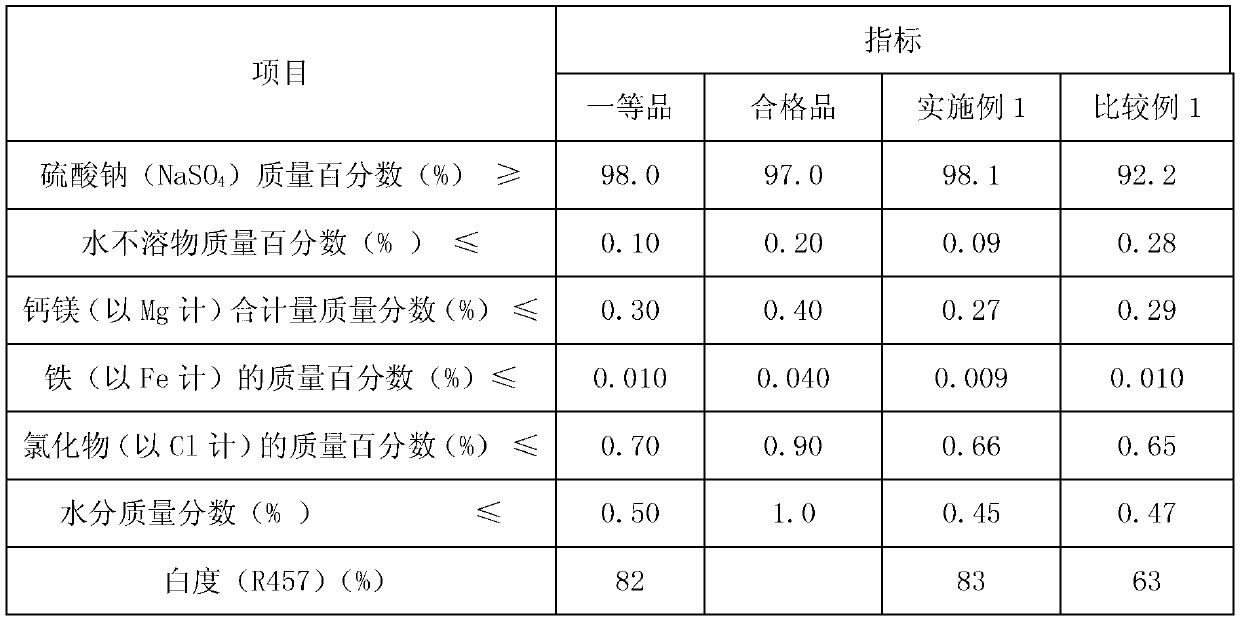
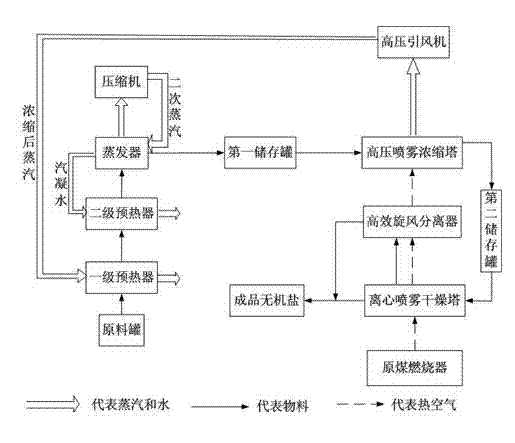
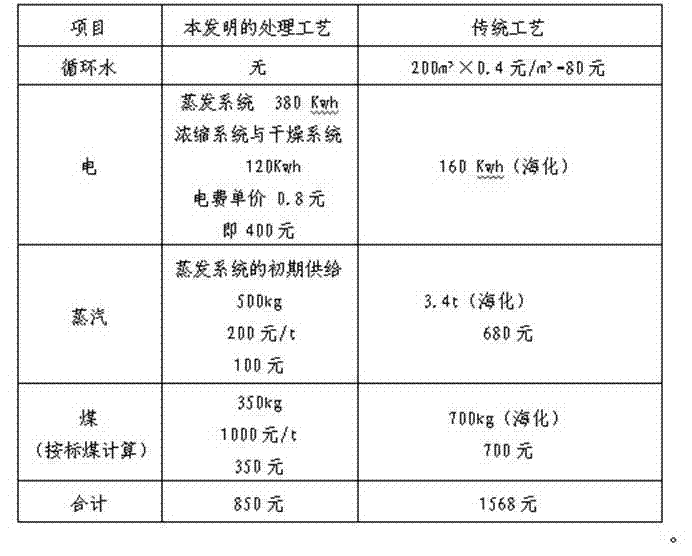
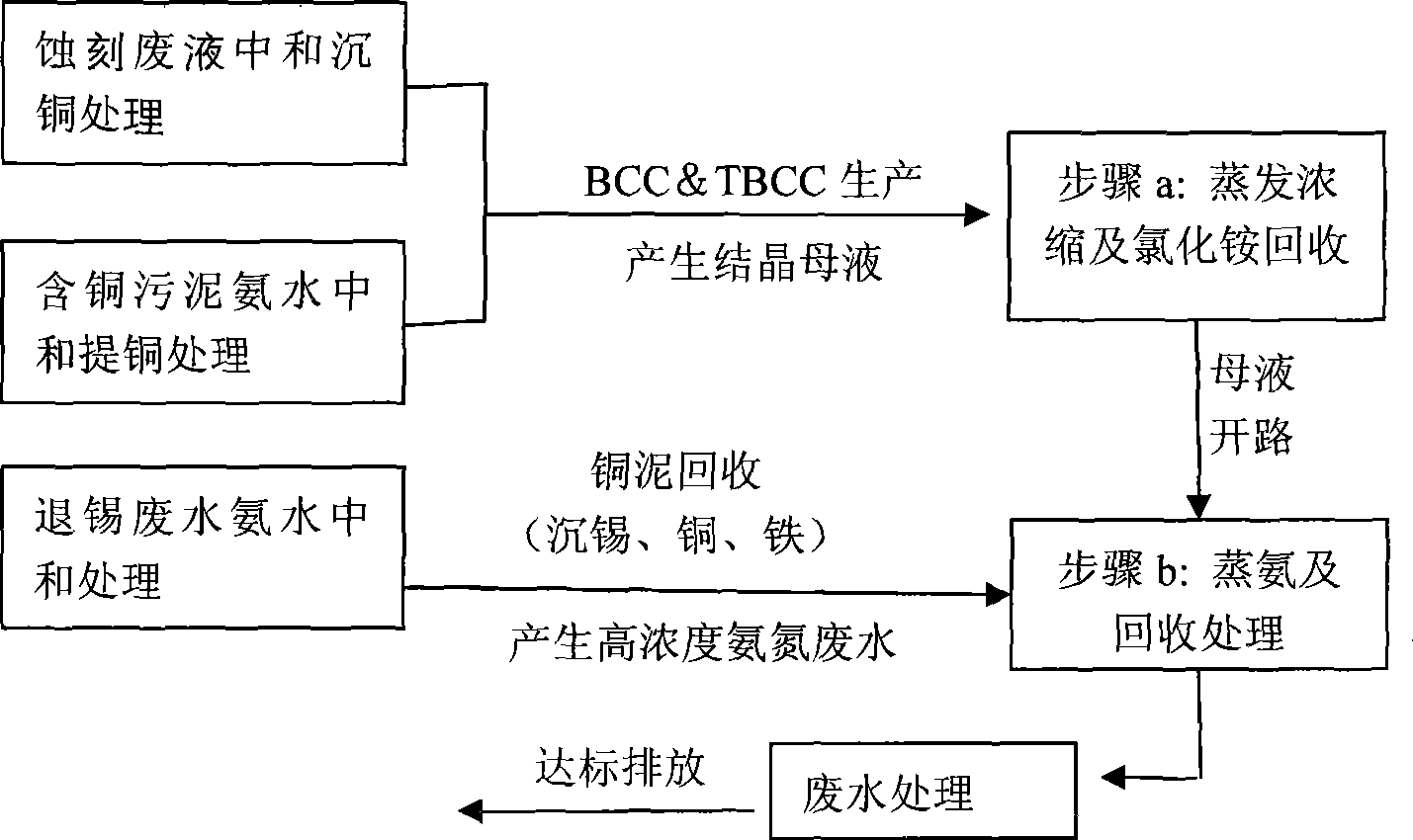
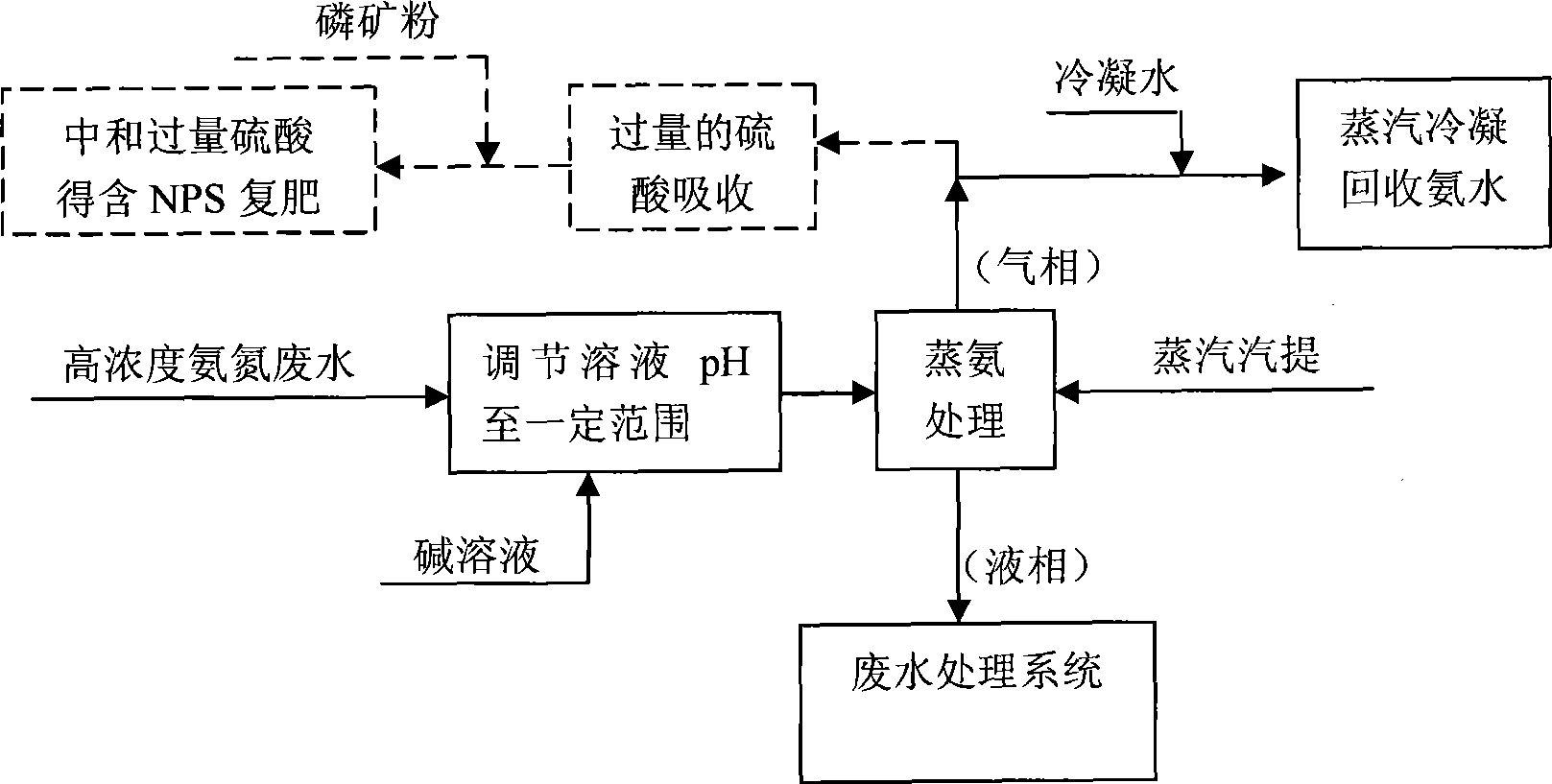
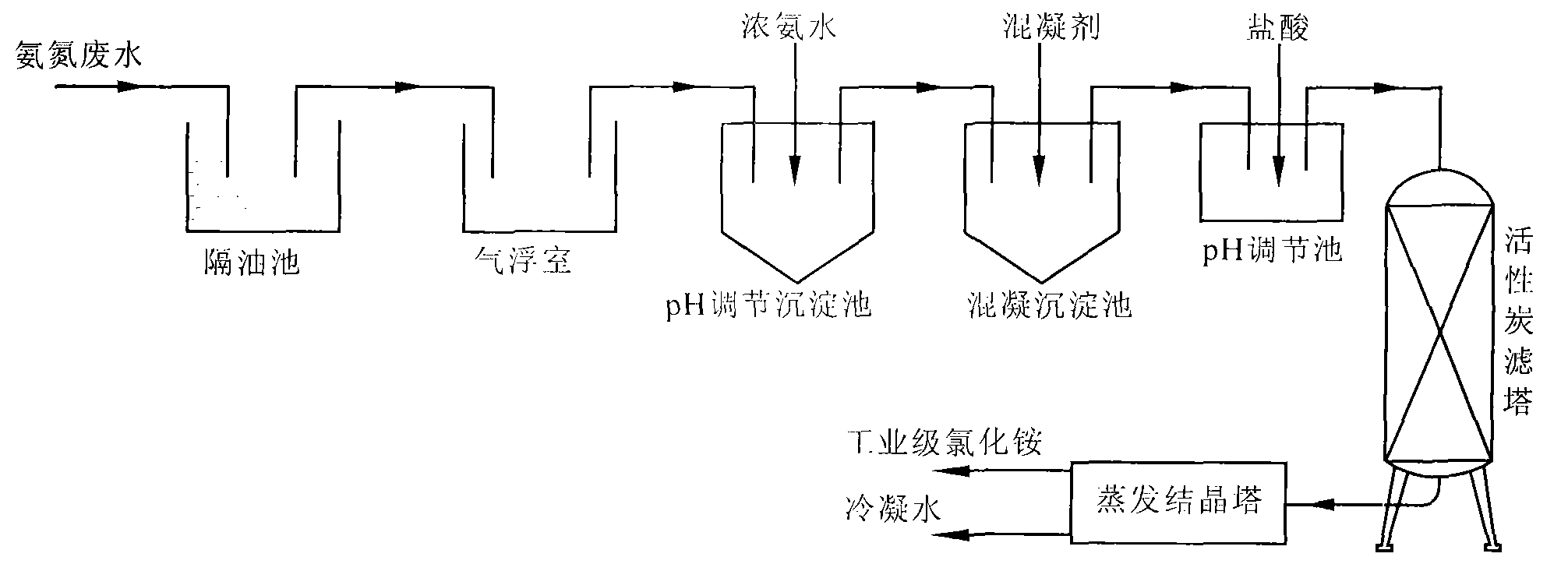
Method for recovery processing of ammonia nitrogen from printed circuit board waste liquid
ActiveCN101391799ARealize resource utilizationEasy to handleClimate change adaptationSewage/sludge fertilisersHigh concentrationLiquid waste
The invention relates to a method for recycling ammonian in ammonian waste water with high concentration, in particular to a method for recycling ammonian in printed wiring board waste water; crystallization mother liquid containing ammonium chloride with high concentration which is generated from processes such as producing alkaline copper chloride, Alpha crystallization type alkaline copper, blue vitriod and the like by using wiring board etching waster water, firstly is evaporated, concentrated and crystallized by one or two combination in multiple effect evaporation technique of mechanical compression, evaporating and compression with heat so as to recycle most ammonium chloride; secondly, Ph is adjusted and ammonian is evaporated so as to recycle ammonia or ammonium sulphate; ammonian waste water generated from removing tin water from ammonia and recycling tin mud are treated by evaporating ammoniac after directly adjusting Ph; and the residual sewage is further treated in the sewage treatment system. By using the method, both of the removal rates of ammonian waste water for wiring board and the recovery rate of ammonia are more than 99.8 percent; the method has good treatment effect, high efficiency, little amount of steam usage, low running cost, and good popularization and application prospect.
Owner:深圳市宝安东江环保技术有限公司
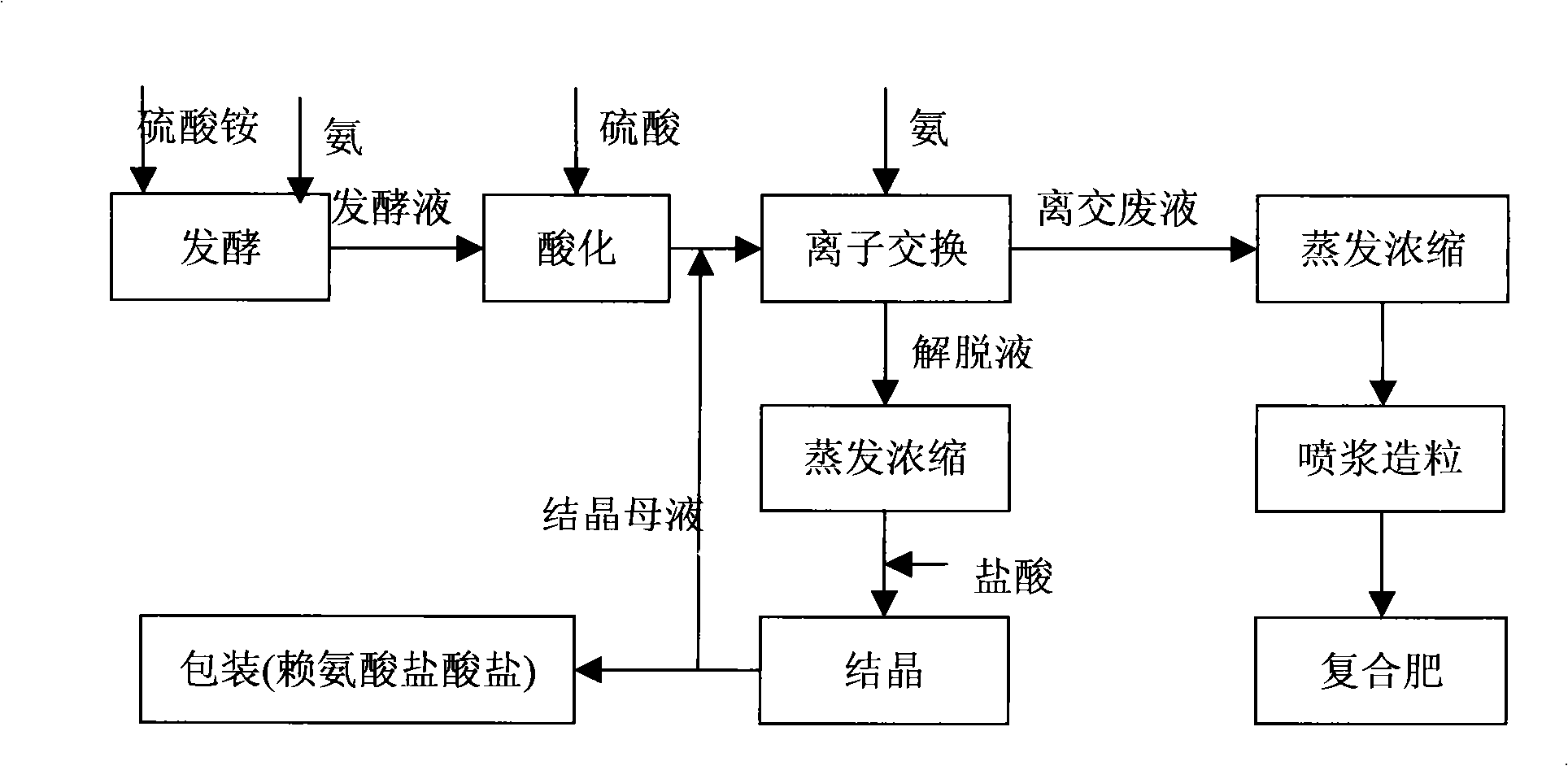
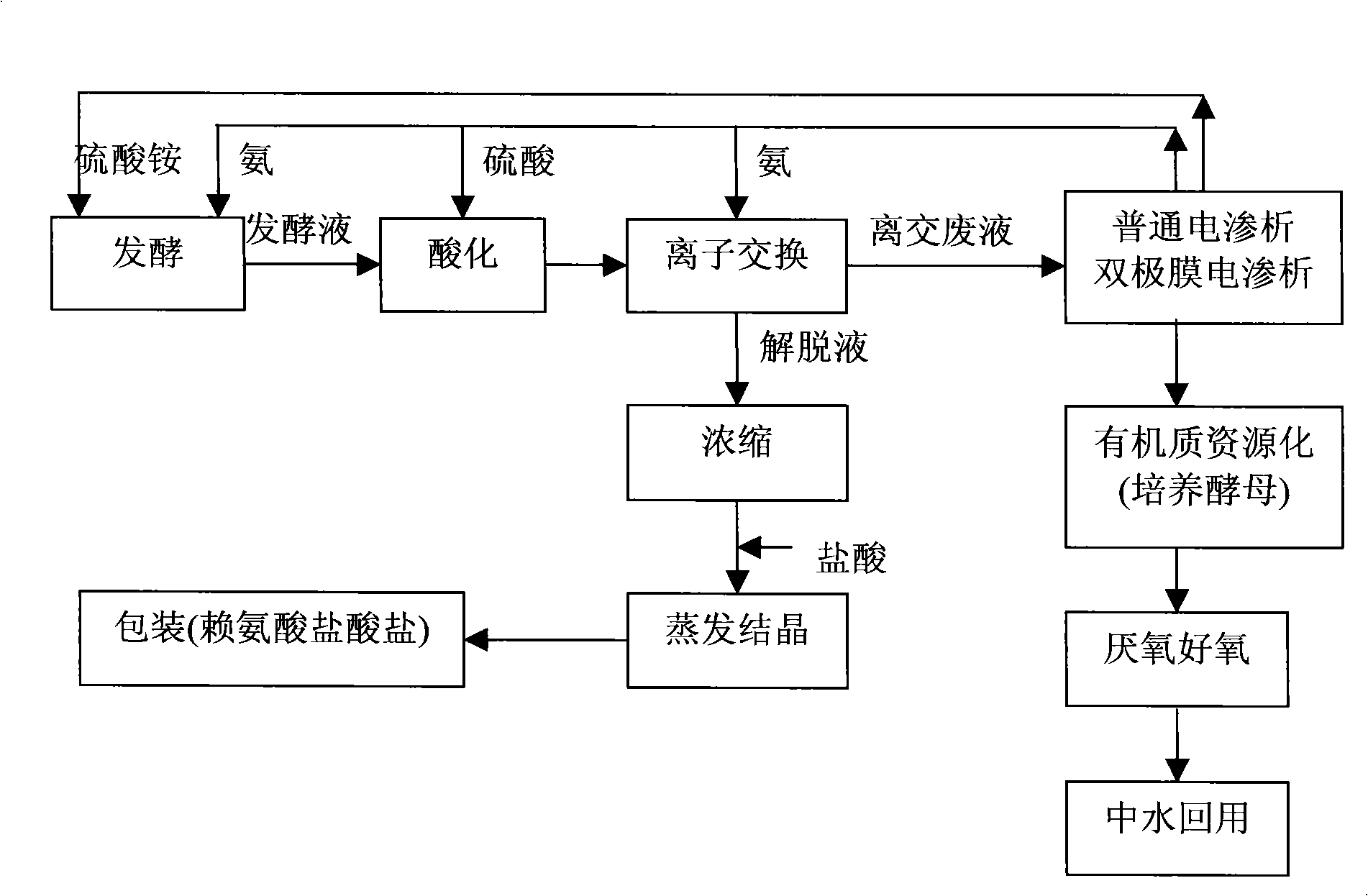
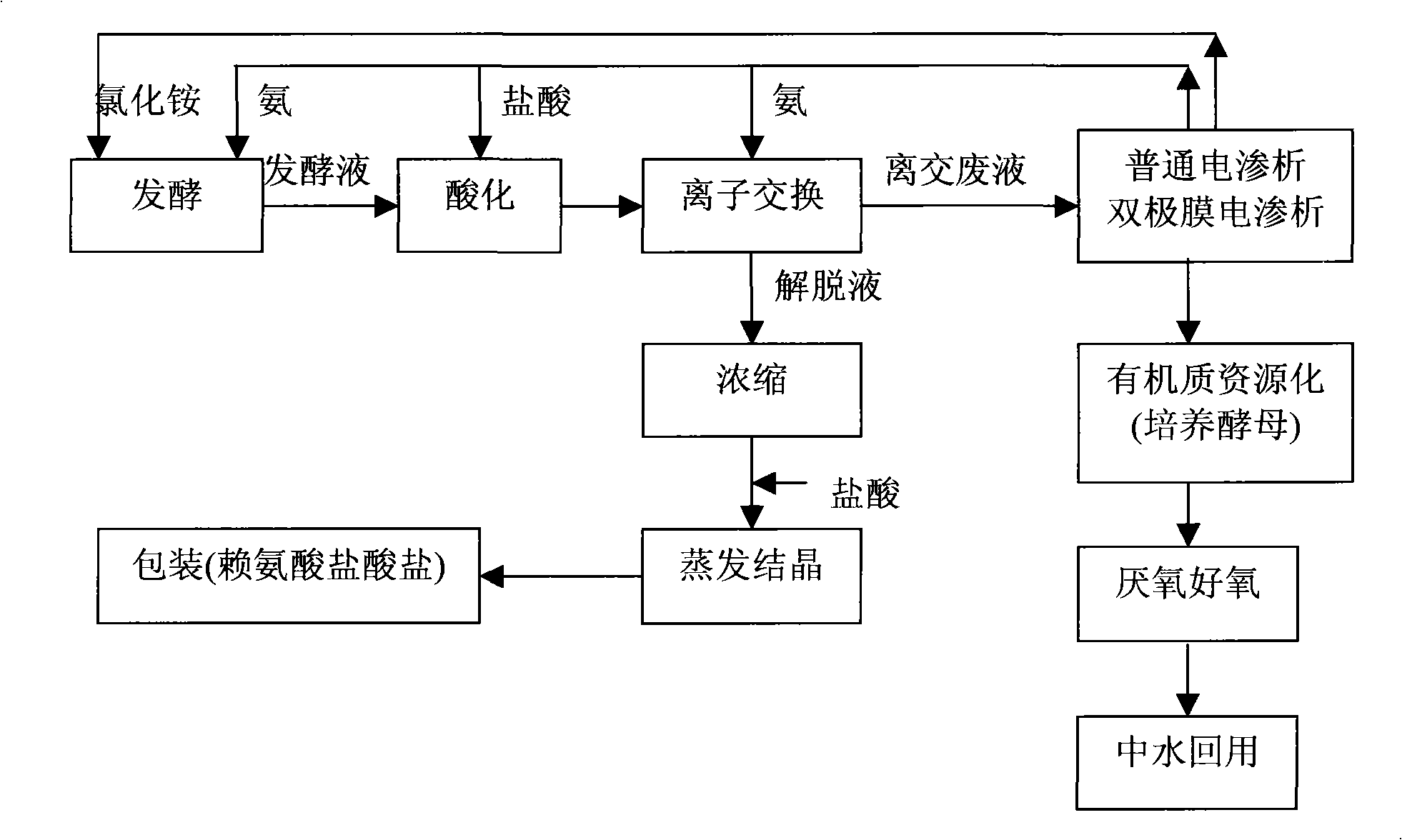
Method for processing ion exchange waste liquor of lysine production by fermentation method
InactiveCN101407350AFast growthLarge biomassAmmonium nitratesDispersed particle separationIon exchangeLysine fermentation
The invention belongs to the ferment industry, in particular relates to a treatment method of ion exchange waste liquor in lysine production by a fermentation method. The invention adopts a common electrodialysis technology to recycle ammonium sulfate, ammonium chloride or ammonium nitrate in the lysine ion exchange waste liquor produced by extracting the lysine from lysine fermentation production to water solution of ammonium sulfate, ammonium chloride or ammonium nitrate, or adopts a bipolar membrane electrodialysis technology to regenerate sulfuric acid, hydrochloric acid or nitric acid and NH3 by ammonium sulfate, ammonium chloride or ammonium nitrate in the lysine ion exchange waste liquor produced by extracting the lysine from the lysine fermentation production, or adopts the combination of the common electrodialysis technology and the bipolar membrane electrodialysis technology to recycle ammonium sulfate, ammonium chloride or ammonium nitrate in the lysine ion exchange waste liquor produced by extracting the lysine from the lysine fermentation production and regenerate sulfuric acid, hydrochloric acid or nitric acid and NH3. The invention realizes the resource recycling of the ion exchange waste liquor and clean production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Struvite circulating crystallization method for treating synthetic ammonia wastewater
InactiveCN102336504AReduce processing costsGuaranteed removal rateBio-organic fraction processingMultistage water/sewage treatmentMagnesium saltRaw material
The invention relates to a struvite circulating crystallization method for treating synthetic ammonia wastewater. The method comprises the following steps of: A, adding soluble phosphate and magnesium salt into the synthetic ammonia wastewater at the ammonium nitrogen concentration of 1,000-2,065mg / L; B, adding alkali into the acquired struvite solid, and performing pyrolysis at the temperature of between 80 and 100DEG C for 1 to 3 hours; C, treating the synthetic ammonia wastewater by using a pyrolysis solid product, and adding a small amount of magnesium salt, wherein ammonia generated in the pyrolysis process is absorbed by a dilute acid solution, and the obtained ammonium salt is used as a raw material for producing a fertilizer; and D, using struvite which cannot be recycled as a sustained-release fertilizer. For the synthetic ammonia wastewater at the ammonium nitrogen concentration of 1,000-2,065mg / L, the struvite can be recycled for 3 to 6 times, the ammonia nitrogen removal rate is higher than 87 percent, the ammonium nitrogen concentration of effluent is lower than 200mg / L, agents are saved through recycle, and nitrogen resources are recycled. The pretreated wastewater meets the requirement of an A / O biochemical treatment process on nitrogen and phosphorus, and can be subjected to biochemical treatment further.
Owner:TONGJI UNIV
Method for processing production waste water of propylene oxide
InactiveCN101337745ARealize environmental protection and decontaminationCalcium/strontium/barium carbonatesWater/sewage treatment bu osmosis/dialysisEpoxyCalcium bicarbonate
A method for processing industrial wastewater of epoxy propane relates to a processing method of wastewater produced by epoxy propane, in particular to the treatment and the re-utilization of calcium chloride in wastewater. The invention provides a method for processing the industrial wastewater of the epoxy propane, which is used for processing and better utilizing the calcium oxide in the industrial waste water of the epoxy propane. The method comprises the following steps: firstly, generating calcium bicarbonate and calcium chloride by utilizing the reaction of ammonium bicarbonate and ammonium chloride; secondly, generating calcium carbonate precipitate, water and carbon dioxide through thermally decomposing the calcium bicarbonate; and thirdly, generating the calcium carbonate precipitate and the water by utilizing the reaction of calcium hydroxide in the wastewater and CO2 generated by thermally decomposing the calcium bicarbonate.
Owner:LIAONING DAZE ENVIRONMENT ENG
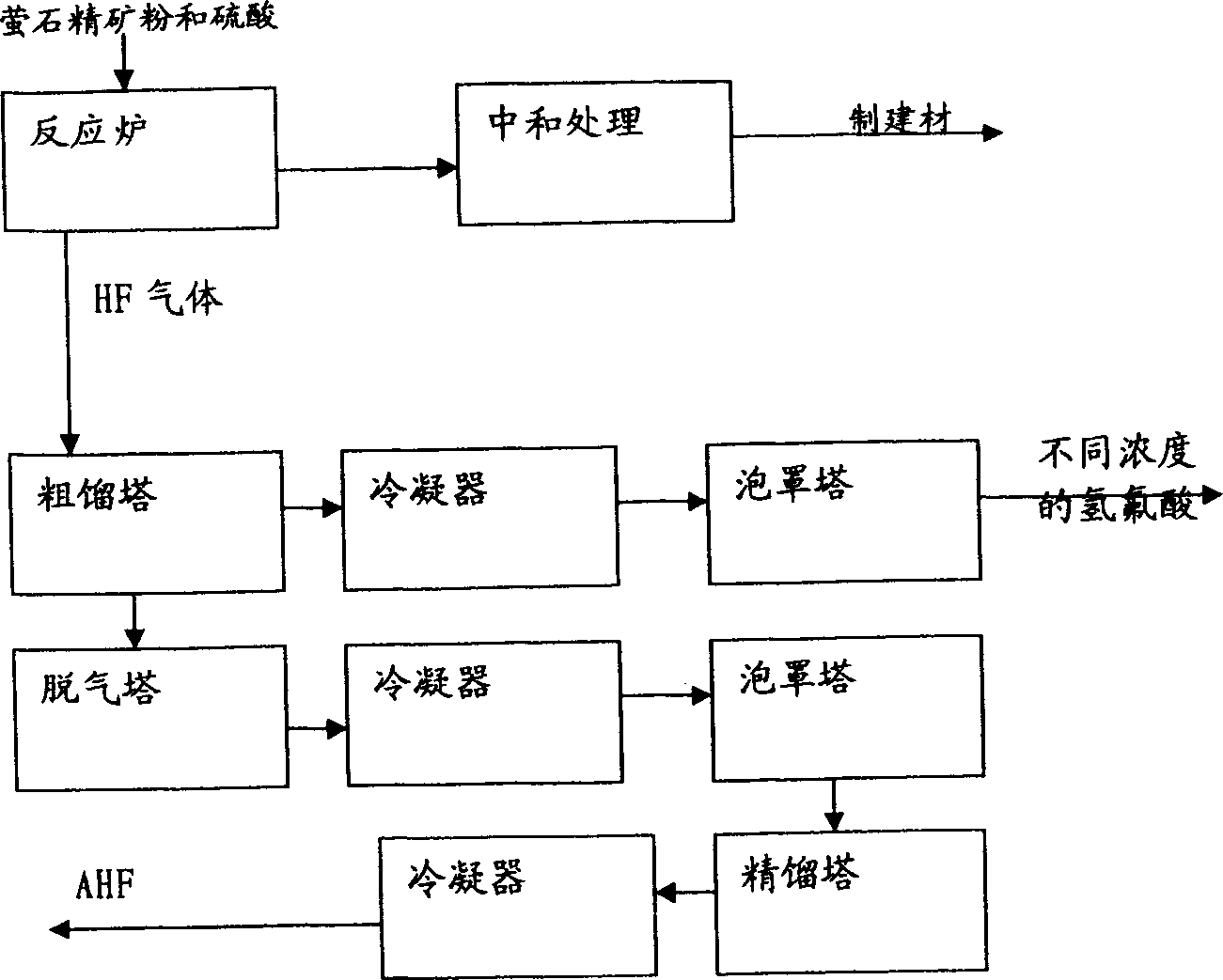
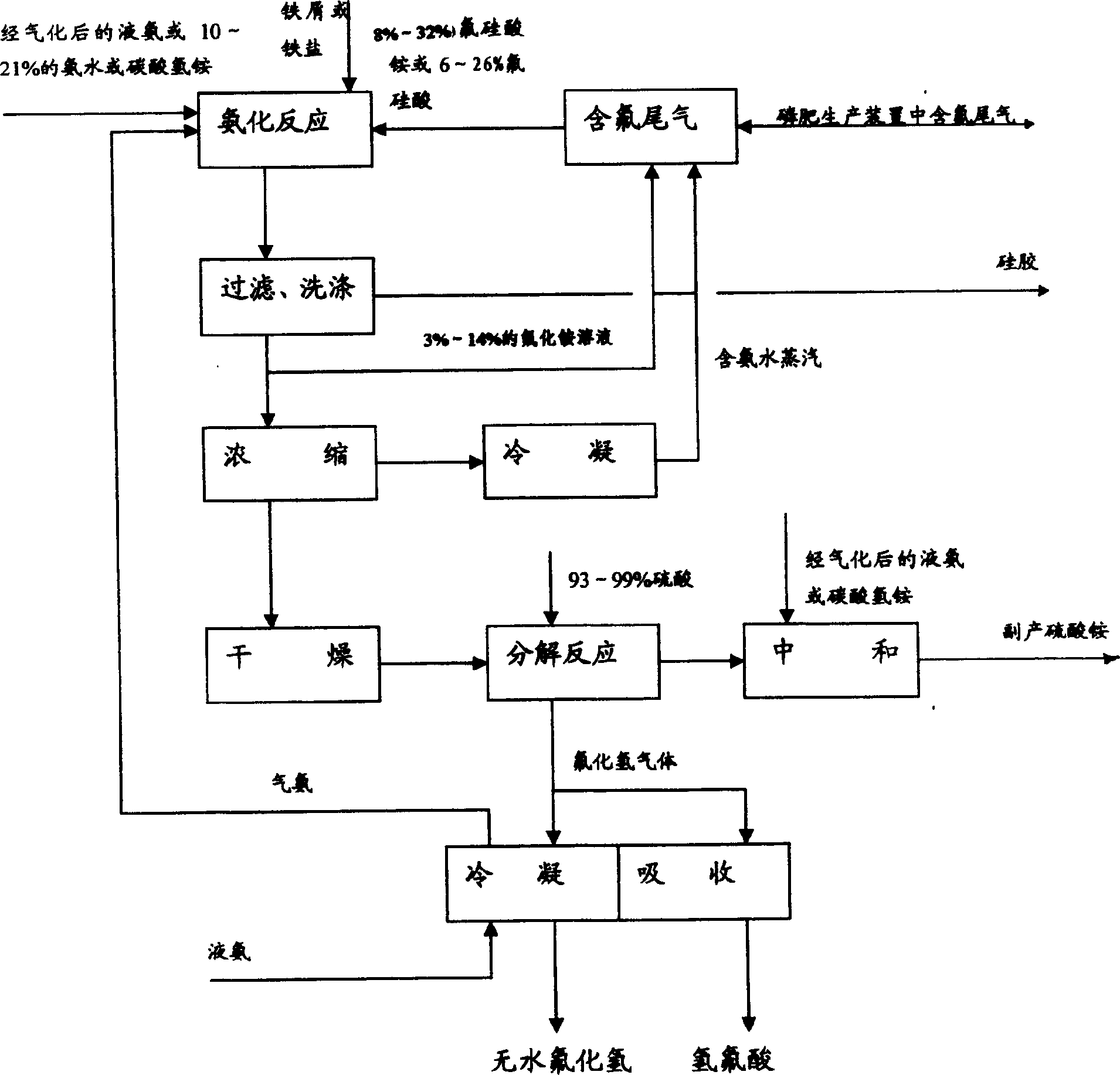
Method for comprehensively utilizing phosphate fertilizer by-product
InactiveCN1554570AReasonable designReduce material requirementsSolid waste disposalFluorine/hydrogen-fluorideIron saltsAmmonium fluorosilicate
The method of comprehensively utilizing phosphate fertilizer by-product includes utilizing ammonium fluoride solution of 3-14% concentration to absorb side product fluorine containing tail gas ammonium fluorosilicate solution; adding iron scrap or iron salt to fluorosilicic acid as one other by-product to eliminate phosphorus; adding liquid ammonia, ammonia water or ammonium bicarbonate; filtering, washing, and eliminating silica precipitate; returning ammonium fluoride solution to phosphate fertilizer absorbing system, concentration and drying to obtain ammonium hydrogen fluoride; reacting solid ammonium hydrogen fluoride and sulfuric acid at high temperature to obtain HF gas, absorbing HF gas to obtain hydrofluoric acid, purification and condensation to obtain anhydrous HF; and mixing the mixture of ammonium sulfate and ammonium bisulfate with ammonium bicarbonate or ammonia in a mixer, curing and crushing to ammonium sulfate fertilizer.
Owner:云南三环化工有限公司 +1
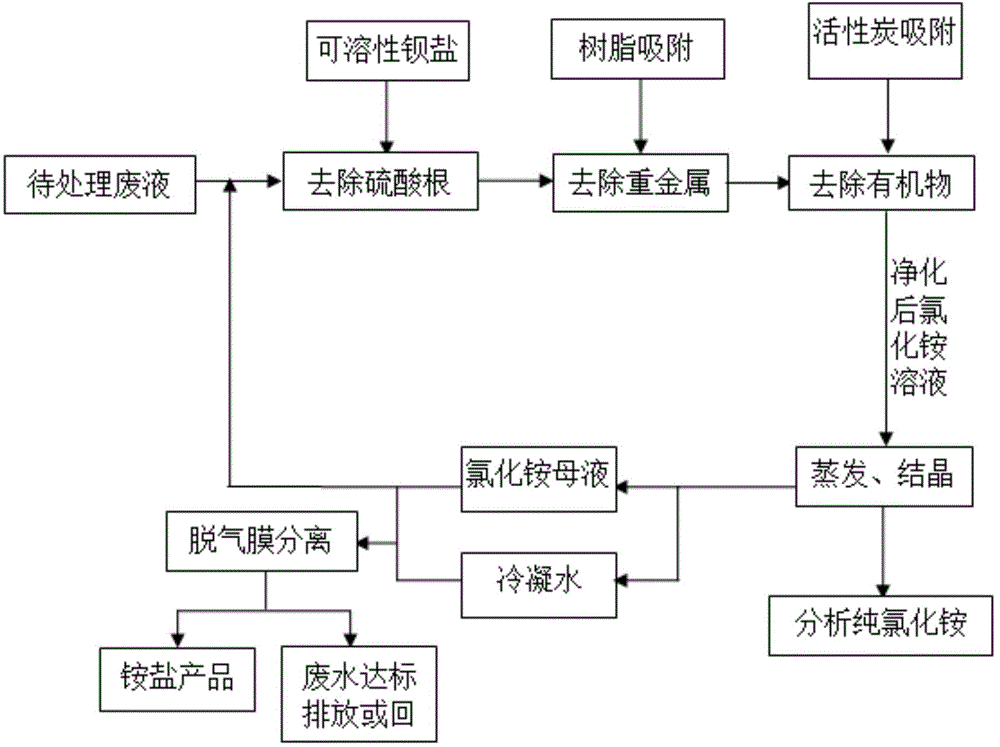
Method for recycling and processing ammonia-nitrogen-containing wastewater in circuit board etching waste liquor recycling industry
ActiveCN104591465AImprove hydrophobicityProlong hydrophilization timeMultistage water/sewage treatmentAmmonium halidesLiquid wasteHigh concentration
The invention discloses a method for recycling and processing ammonia-nitrogen-containing wastewater in circuit board etching waste liquor recycling industry, relates to a regenerating method for ammonia-nitrogen-containing wastewater in circuit board etching waste liquor recycling industry, and aims to provide an ammonia-nitrogen-containing wastewater recycling method which is low in operation cost, high in ammonia-nitrogen pollutant eliminating rate and high in utilization rate. By adopting a combined mode of an impurity removal workshop section, MVR (melt volume flow rate) and a super-hydrophobic degassing membrane separation technology, heavy-metal-recycled ammonia-nitrogen-containing wastewater of the circuit board etching waste liquor is processed to obtain analytically pure ammonium chloride, and the ammonia-nitrogen content of the processed outlet water is not higher than 8mg / L. By recycling and performing up-to-standard processing on the ammonia-nitrogen-containing wastewater, the method can save the energy resources, can safely and effectively process high-concentration ammonia-nitrogen-containing hazardous wastes and can obtain a high-quality ammonium salt product. The method has the remarkable characteristics of recycling resources, being low in energy consumption, simple to operate, low in operation cost, convenient to manage, free of secondary pollution, high in ammonia-nitrogen pollutant reduction rate, high in utilization rate and the like.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
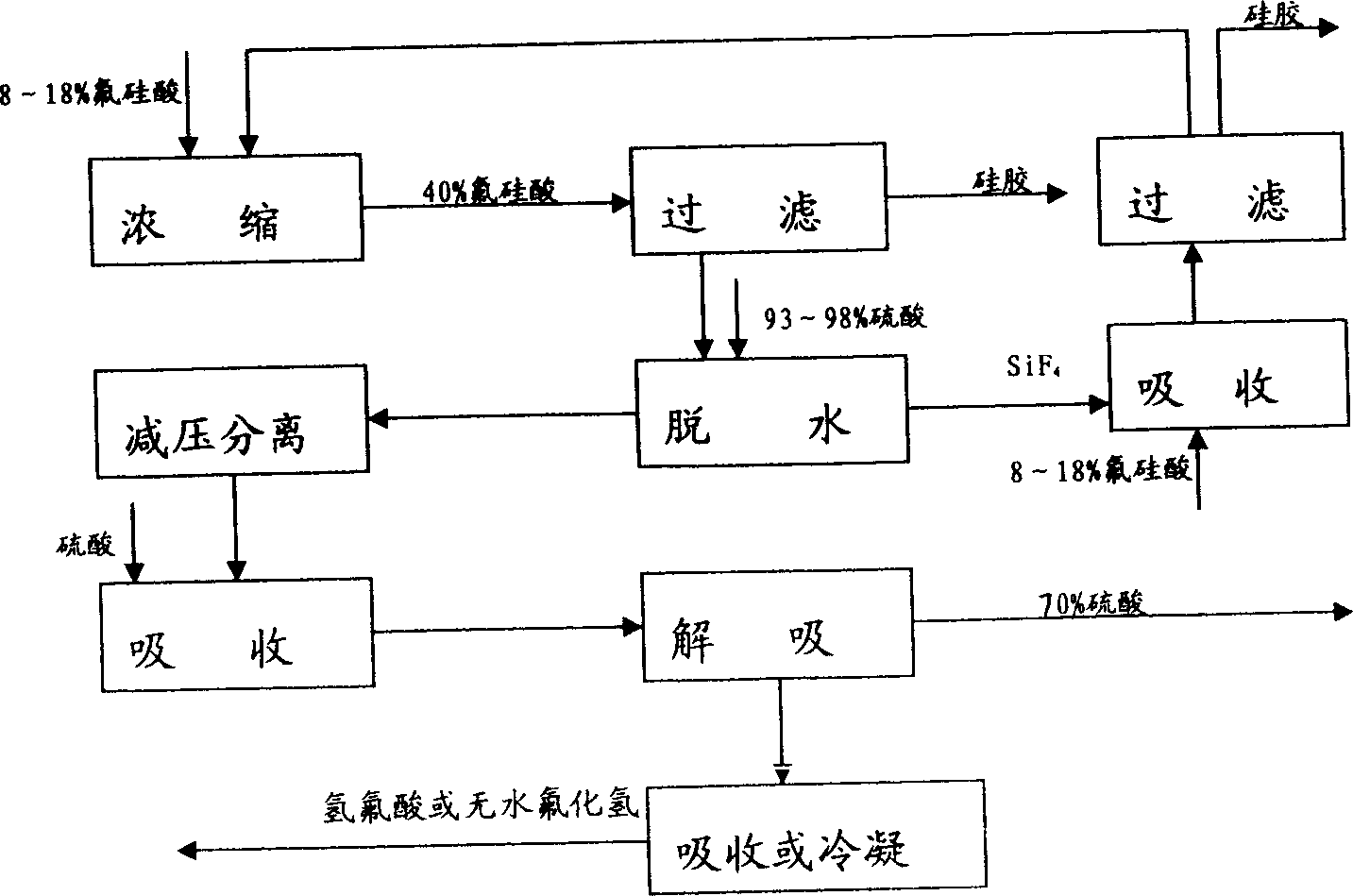
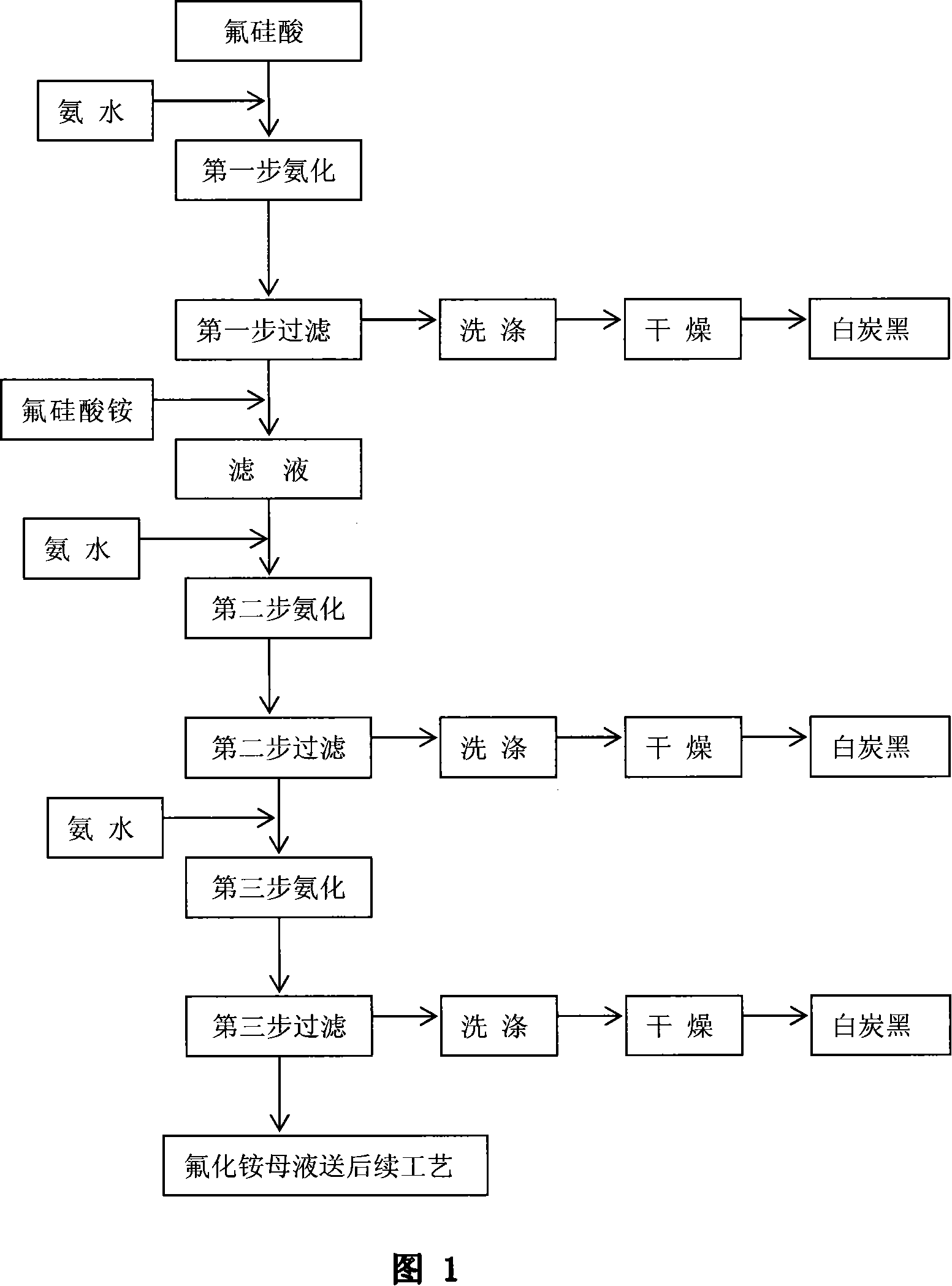
Method for preparing big-compensating forcing white carbon black and high concentration ammonium fluoride through aminating of fluosilicic acid
ActiveCN101139094AFacilitate subsequent processingReduce energy consumptionSilicon oxidesAmmonium halidesHigh concentrationSal ammoniac
A preparation method of highly reinforced white carbon black and high concentration ammonium fluoride through fluosilicic acid ammoniation relates to a preparation method of chemical product, in particular relating to a preparation method of inorganic power material. The invention carries through ammoniation reaction by steps with the fluosilicic acid and ammonia. The white carbon black is acquired after filtrating, washing and drying. The invention is characterized in that the ammonia is added in by steps and the filter is carried through by steps. The steps are that a, 30 to 70 percent of the total ammonia is added in the fluosilicic acid to carry through ammoniation reaction, the reaction liquid is aged, filtrated to acquire a filter cake and a filter liquid; the ammonium fluorosilicate is added in the filter liquid acquired in step a and then the ammonia is added to carry through ammoniation reaction, the reaction liquid is aged, filtrated to acquire the filter cake and the filter liquid; c, the left ammonia is added in the filter liquid acquired in step b and then the reaction liquid is aged and the aged liquid is filtrated to acquire the filter cake and high concentration ammonium fluoride mother liquid; d, the filter cakes acquired by filtrating are respectively washed and dried to prepare the white carbon black.
Owner:YUNNAN YUNTIANHUA
Compound coagulant used for treating rare-earth highly concentrated ammonian wastewater to recover industry ammonium chloride and treatment method
InactiveCN101555053ASimple processing methodLow costFatty/oily/floating substances removal devicesMultistage water/sewage treatmentRare earthPolyacrylamide
The invention provides a compound coagulant used for treating rare-earth highly concentrated ammonian wastewater to recover industry ammonium chloride and a treatment method, belonging to the technical field of chemical environment protection and resource recovery. The compound coagulant is a mixed liquor of ammonium oxalate, ammonium sulphate, heavy metal precipitator DTCR and polyacrylamide (PAM). The treatment method for treating wastewater by using the compound coagulant comprises the following steps: firstly, oil substances in the wastewater are removed through a separation tank and an air floating chamber; secondly, the wastewater enters a pH adjusting precipitation tank to adjust the pH value to be 7.5-8.5; thirdly, the wastewater enters a coagulative precipitation tank, the compound coagulant is added, the wastewater after being coagulated and precipitated enters the pH adjusting precipitation tank to adjust the pH value to be 2.5-4, the wastewater after being adjusted the pH value enters an active carbon filter tower to be absorbed and filtered and then is evaporated and concentrated under the condition of negative pressure. The compound coagulant has simple treatment method, low cost and good effect. The treatment method has short process and simple and easy treatment, not only solves the problem of the pollution of the highly concentrated ammonian wastewater, but also achieves the purpose of recovering valuable materials.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Treatment for wastewater in para-nitraniline production and method for resource recovery and use
InactiveCN101244878AImprove adsorption capacityAchieve recyclingOrganic chemistryMultistage water/sewage treatmentAbsorption capacityPhosphate
The invention discloses a method for nitroaniline wastewater treatment and recycling, comprising a plurality of steps: cool p-nitroaniline mother liquor wastewater and crystals are allowed to be separated out; filter the wastewater after crystal precipitation and remove the free ammonia in the filtrate; filter the effluent ever treated at the procedure B and change PH value to acidity or weak alkalinity; use the viscose-based active carbon fiber made by phosphate-impregnated high-temperature steam activation to absorb the p-nitroaniline wherein; evaporate and concentrate the absorbed water; precipitate crystal and filter out ammonium chloride crystal. The active carbon fiber has large absorption capacity and fast absorption speed for p-nitroaniline and can be utilize repeatedly, which makes the recovery rate of p-nitroaniline near 100%. The method has the advantages that the method can fully recover p-nitroaniline and free ammonia in the p-nitroaniline wastewater and the byproduct of ammonium chloride, realizing the unification of wastewater treatment and resources recovery; the method is of great economic and practical value in the treatment of p-nitroaniline wastewater.
Owner:NANJING UNIV
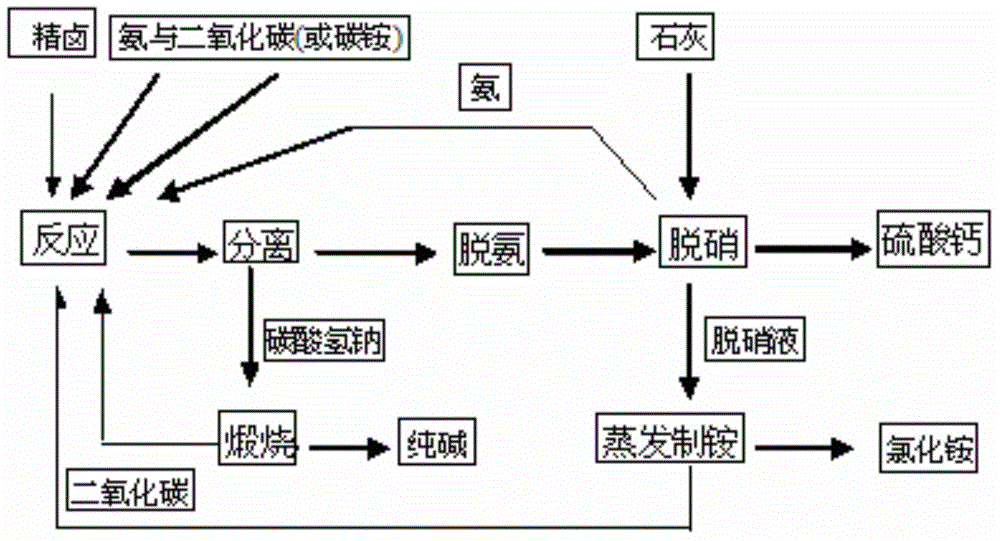
Technology for combined production of sodium carbonate and ammonium chloride through sodium sulfate type brine thermal cycle method
InactiveCN105000579AAdaptableThe main product is of high qualityAmmonium halidesCarbonate preparationSodium bicarbonateSodium sulfate
The invention discloses a technology for combined production of sodium carbonate and ammonium chloride through a sodium sulfate type brine thermal cycle method. The technology comprises the following steps: (1) carrying out a metathesis reaction on sodium sulfate type brine, ammonia and carbon dioxide as raw materials, separating to obtain sodium bicarbonate and an alkali production mother liquor containing ammonium chloride, sodium sulfate, sodium chloride, ammonium bicarbonate and ammonium carbonate, and calcining the obtained sodium bicarbonate to obtain a sodium carbonate product; (2) preheating the alkali production mother liquor to carry out high temperature removal of ammonium bicarbonate and ammonium carbonate in order to obtain a deaminized mother liquor containing ammonium chloride, sodium sulfate and sodium chloride; (3) adding lime with the amount equal to that of sodium sulfate to the deaminized mother liquor, and reacting to remove sodium sulfate in order to obtain calcium sulfate, ammonia and a denitrified mother liquor containing ammonium chloride and sodium chloride; and (4) evaporating the denitrified mother liquor, and separating to obtain ammonium chloride and an ammonium production mother liquor. The technology has the characteristics of high quality of main products, strong adaptability of the raw materials, low cost, low energy consumption, closed loop, and no discharge of three wastes.
Owner:CHINA LIGHT IND INT ENG CO LTD
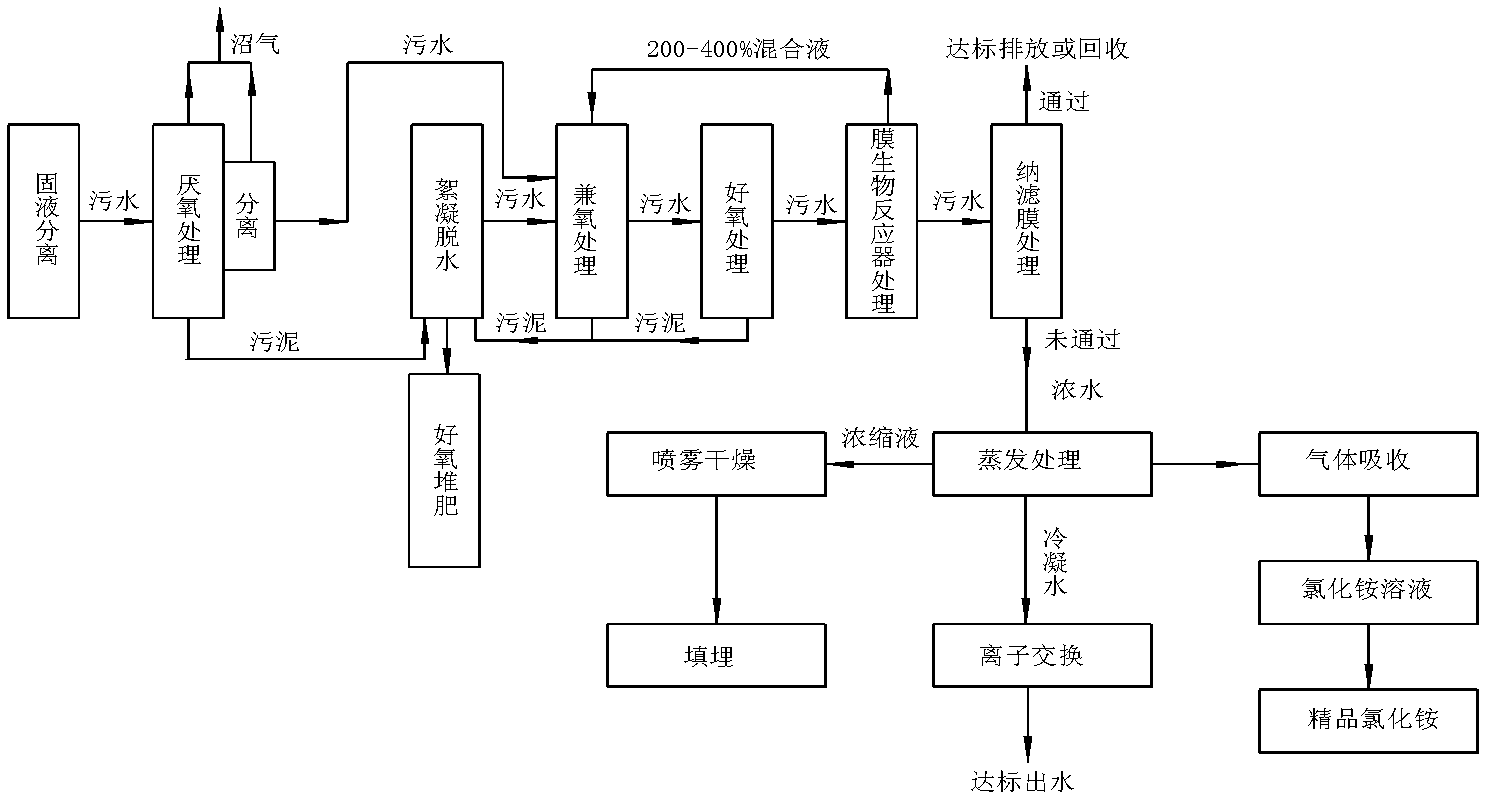
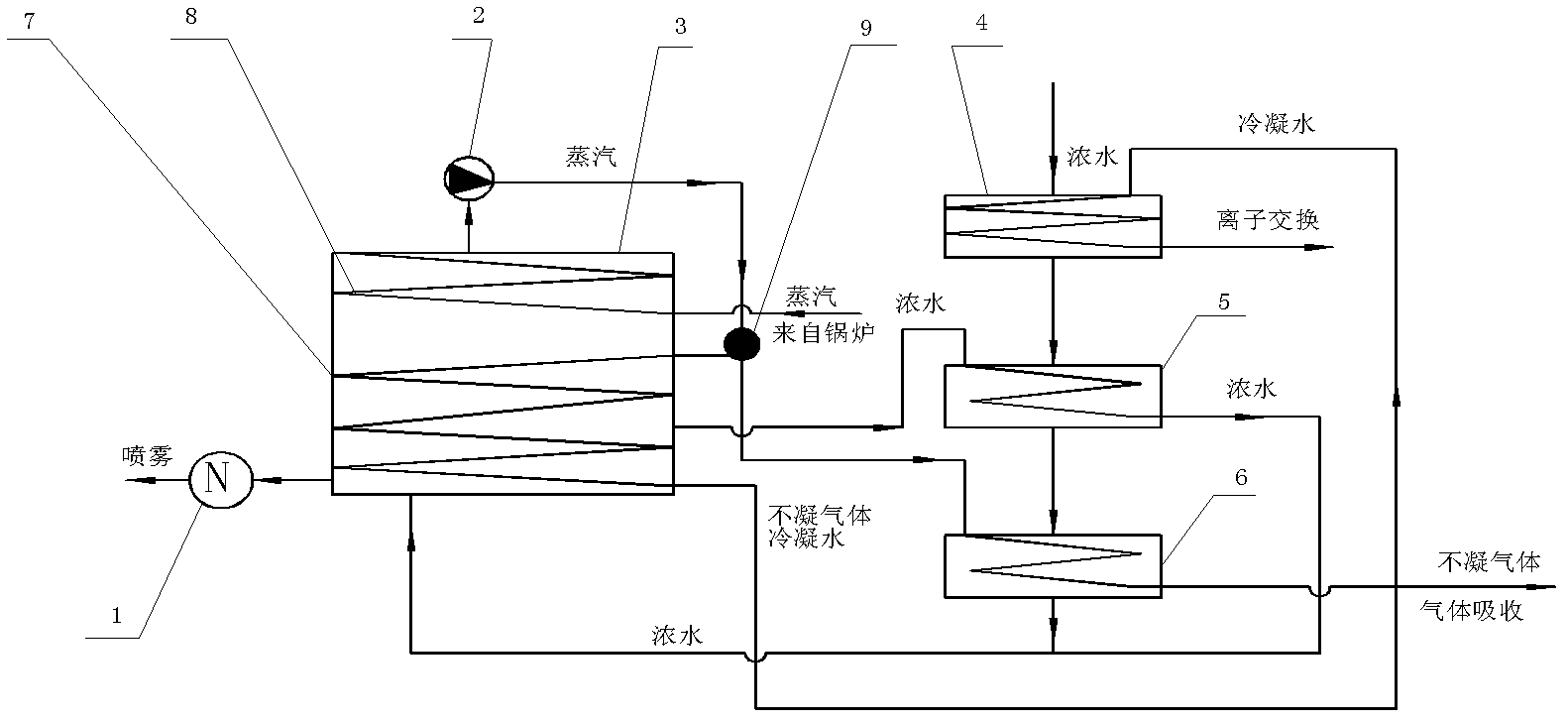
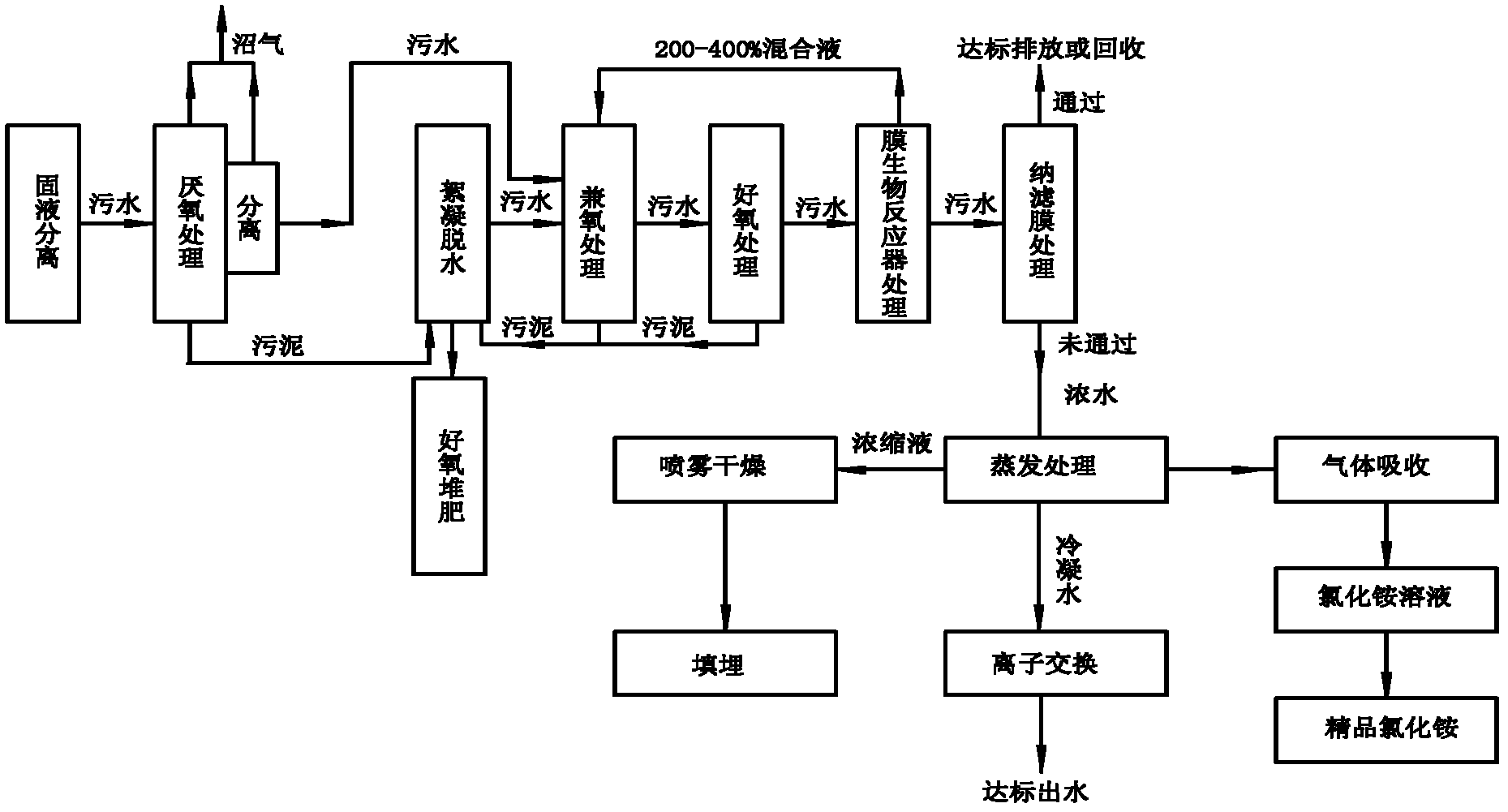
Treatment method of landfill leachate
ActiveCN102557339AHarmlessImplement resourcesSludge treatment by de-watering/drying/thickeningBio-organic fraction processingEvaporationIon exchange
The invention relates to a treatment method of a landfill leachate, which comprises the step of treating the landfill leachate by adopting a multi-stage processing method of solid-liquid separation, anaerobic treatment, aerobic treatment, membrane bioreactor treatment, nanofiltration membrane, evaporation, ion exchange and spray drying. Due to the adoption of the treatment method, the problem that the future trouble of difficult treatment is finally generated due to higher and higher ammonia nitrogen and chemical oxygen demand COD in the landfill leachate caused by recharging is solved.
Owner:北京昊业怡生科技有限公司
Method for preparing potassium nitrate by means of double decomposition
ActiveCN101659431AReduce churnHigh product contentAlkali metal nitrate preparationAmmonium halidesFiltrationDecomposition
The invention discloses a method for preparing potassium nitrate by means of double decomposition. The method comprises the following steps: taking potassium chloride, ammonium nitrate and water solution as raw materials, and controlling the concentrations of Cl<-> and NH4<+> in the reaction solution to be 14-19% and 6-9.6% respectively, wherein the temperature of the reaction solution is 85-95 DEG C; lowering the temperature of a solution obtained from the reaction till a temperature of 0-20 DEG C is realized, crystallizing and precipitating KNO3 semi-finished product, and obtaining a filtrate mother liquor I by means of filtration; flushing KNO3 crystals with water till the Cl<-> concentration is 1-2% and the NH4<+> concentration is 1-3%; and recovering the flushing solution; positioningthe KNO3 crystals in a double decomposition device so that the KNO3 crystals can be fully dissolved, and controlling the solution concentration to be 44-52 Be; lowering the temperature for purpose ofprecipitating the KNO3 crystal, drying the KNO3 crystal till the water content of the KNO3 is less than or equal to 0.1%, and then obtaining the KNO3 finished product. In the method, the double decomposition device is used during the double decomposition reaction process and no stirring device is needed, therefore, the contact reaction of the reaction solution is more complete, and balance, continuous cyclic industrial production, productivity improvement and production cost reduction are realized in the precondition of ensuring 'two highs'.
Owner:江西金利达钾业有限责任公司
Method for treating rare earth ammonium chloride wastewater
InactiveCN102531025ASimple processHigh recovery rateMultistage water/sewage treatmentRare earth metal compoundsCarbon sinkRare earth
The invention discloses a method for treating rare earth ammonium chloride wastewater, belonging to the field of wastewater treatment. The method for treating the rare earth ammonium chloride wastewater comprises the steps of: dissolving ammonium chloride rare earth, carrying out organic phase saponification, carrying out extraction separation, recycling wastewater, stripping, carbon-sinking, washing, evaporating, cooling and crystallizing. According to the method, ammonium chloride raffinate and carbon sink mother liquor which are generated by extraction separation on the rare earth are used as the solvents of the ammonium chloride rare earth for recycling so that the ammonium chloride in the wastewater is increased, the ammonium chloride is favorably evaporated and condensed, and energy resource consumption is reduced. Meanwhile, washing liquor of rare earth carbonate precipitates is recycled for preparing a precipitating agent, thus water resources are saved. The mass percent of the ammonium chloride in the recycled finished product is 91.7-96.5 percent in the terms of dry basis; and the economic benefit is remarkable, the wastewater is recycled, and zero emission of the rare earth wastewater is recycled.
Owner:江西德盛精细化学品有限公司
Method for continuously preparing white carbon black and ammonium fluoride by carrying out fluosilicic acid ammoniation
ActiveCN101863482AContinuous processProcess stabilitySilicaAmmonium halidesSal ammoniacHexafluorosilicic acid
The invention discloses a method for continuously preparing white carbon black and ammonium fluoride by carrying out fluosilicic acid ammoniation, which relates to a chemical production method, in particular to a method for preparing the white carbon black and the ammonium fluoride. The method comprises the following steps: (a) at a temperature between minus 5 and 30 DEG C, starting a stirrer and an air compressor, blowing compressed air into an outlet pipe of a reactor by the air compressor and adding fluosilicic acid and ammonia into the reactor simultaneously; (b) when the fluosilicic acid and the ammonia are added to the position of an overflow pipe in the reactor, opening an emptying valve to continuously feed and discharge materials and controlling a PH value of the reaction, wherein the period of the continuous reaction is 24 hours; (c) in the reaction process, filtering the materials discharged by the step (b) to obtain a white carbon black filter cake and filtrate of ammonium fluoride, and washing and drying the white carbon black filter cake to obtain a white carbon black finished product, wherein the filtrate is ammonium fluoride solution; and (d) after performing a reaction for 24 hours in the reactor, emptying the materials, cleaning the reactor to be used next time and producing the white carbon black finished product by using the materials according to the step (c), wherein the ammoniation yield of the process can reach 99.99 percent. The method of the invention effectively overcomes the defects of the existing intermittent technology and has the advantages of continuous, stable and simple process and long operation period.
Owner:YUNNAN YUNTIANHUA +1
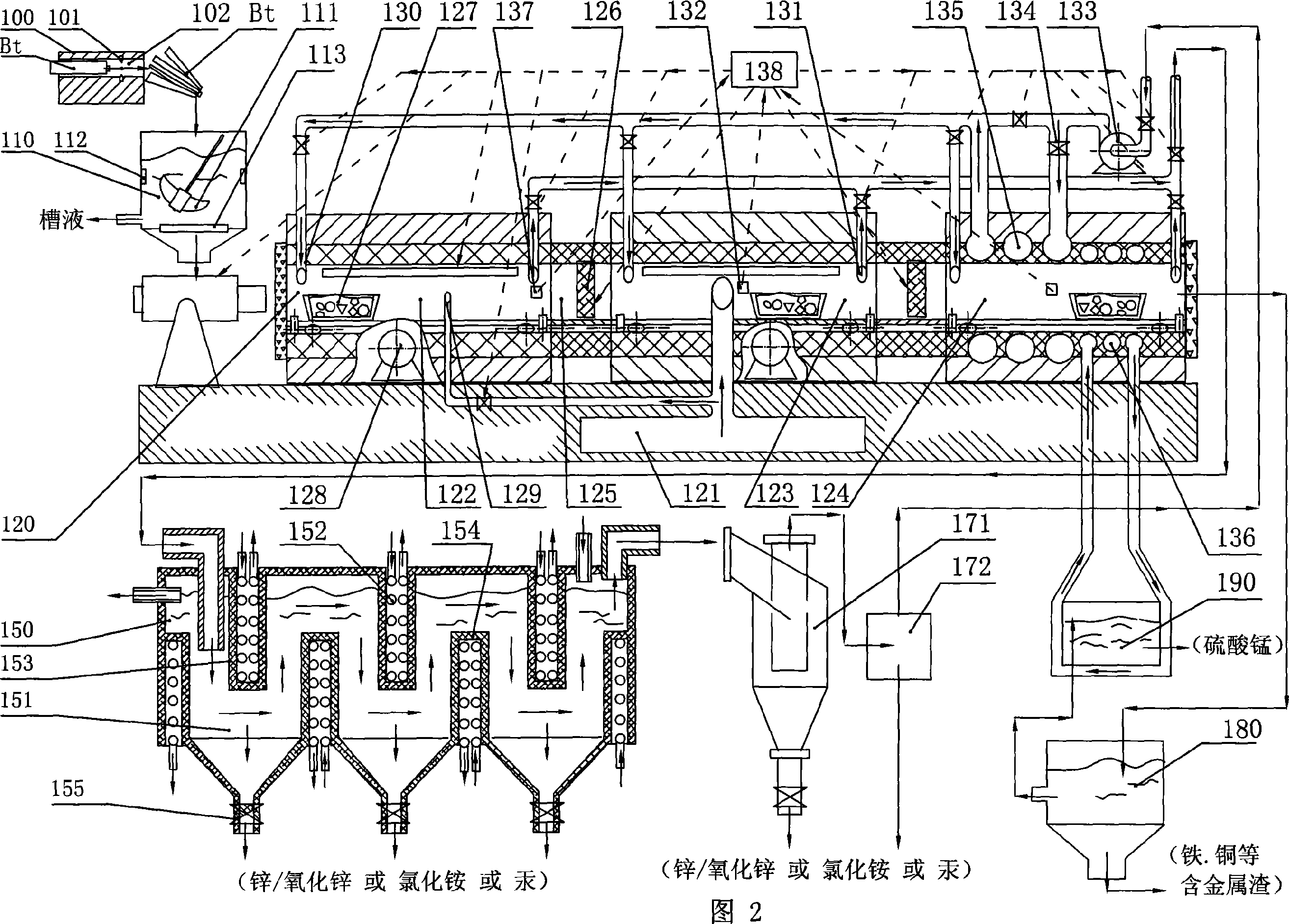
Selectivity volatilization reclaiming technique for waste zinc-manganese battery and reclaiming system thereof
ActiveCN101054187AGood for acid soluble recoveryFull recoveryZinc oxides/hydroxidesReclaiming serviceable partsSurface layerSulfate
The present invention relates to a selective volatilization recovery technology for abandoned zinc-manganese battery, which comprises the steps of that: the surface layer of the battery is broken and scrubbed, thereby the tank liquor, washed up surface layer matter and contained matter are obtained; then the remaining solid containing manganese / ferric oxide and high pure gas are obtained by high temperature carbonization in a properly closed roaster, wherein the temperature inside of the roaster is controlled; the multiple scrubbed tank liquor is heated and concentrated to obtain concentrate which is further heated and cooled to obtain ammonium chloride with a high purity; the distilled off hot gas is guided into a condensation and recovery container and is cooled to be separated and collected thereof, thereby the powder containing zinc and / or zinc oxide is obtained; the remaining solid is subjected to acid soluble to obtain the manganese sulfate primordial solution which is then subjected to oxidation, alkali-settling and separation so as to obtain the purified manganese sulfate solution. In the present invention, the bulk inclusion in the zinc-manganese battery can be completely recovered, the recovered product has a higher purity, the recovery operation and the equipment thereof is simple, and secondary pollution will not occur basically.
Owner:GEM CO LTD
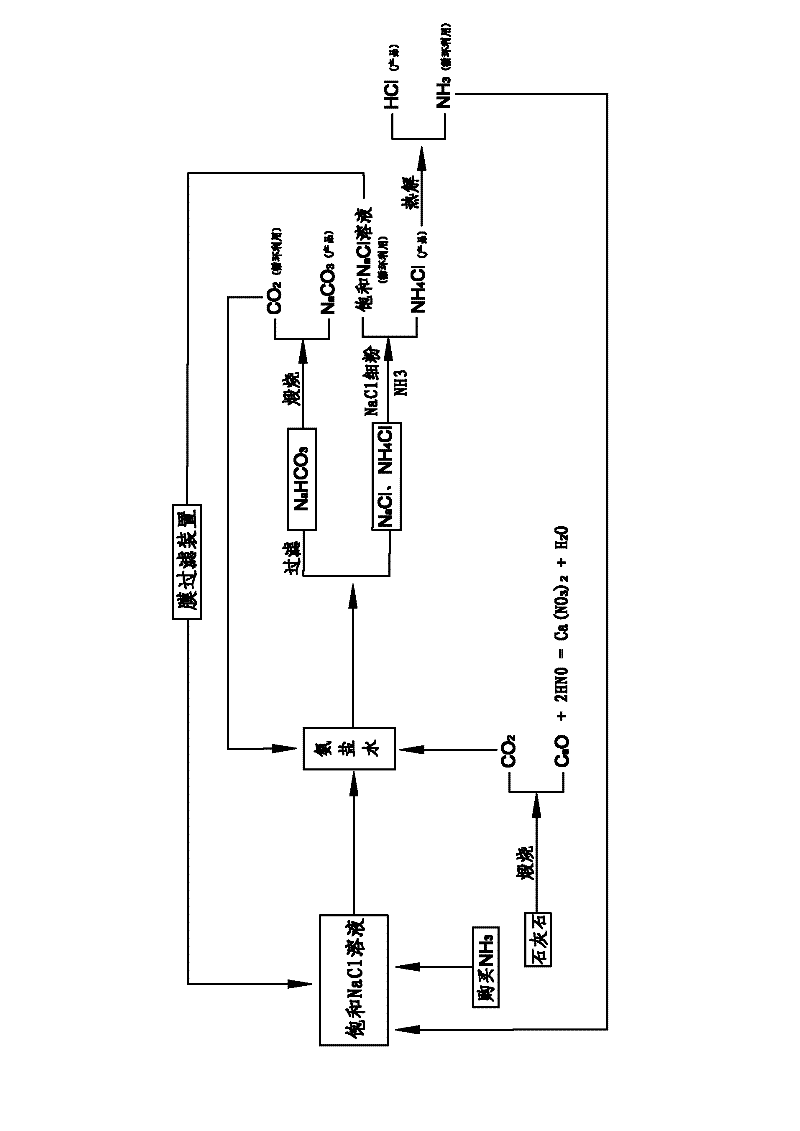
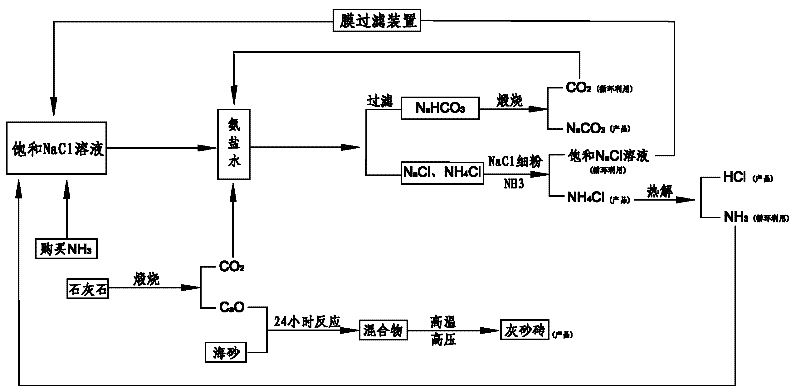
Comprehensive soda ash producing process and product application thereof
ActiveCN102531001AIncrease profitReduce manufacturing costCalcium/strontium/barium nitratesAmmonium halidesSodium bicarbonateProcess engineering
The invention relates to the technical field of soda ash production, and especially relates to a comprehensive soda ash producing process and product applications thereof; the process comprises the following process steps: introducing ammonia gas into a saturated sodium chloride solution to prepare ammoniacal brine; introducing carbon dioxide generated in limestone calcination into the ammoniacal brine to generate a mixture of sodium bicarbonate, sodium chloride and ammonium chloride; filtering the mixture to obtain sodium bicarbonate, sodium chloride and ammonium chloride; calcining the sodium bicarbonate to generate soda ash and carbon dioxide, recycling the carbon dioxide; adding sodium chloride fine powder and ammonia gas into the mixed filtrate of ammonium chloride and sodium chloride to generate ammonium chloride and sodium chloride solutions; separating impurities from the sodium chloride solution by a membrane filter, recycling the sodium chloride solution. The process has the advantages of low production cost, high soda ash whiteness, high sodium chloride utilization rate, no water waste or solid waste generation, environment protection, safety, simple processes, full use of geographic advantages in our country, low raw material purchase cost, waste change into things of value, high economic benefits, and good social benefits.
Owner:GUANGDONG DAZHONG AGRI SCI CO LTD
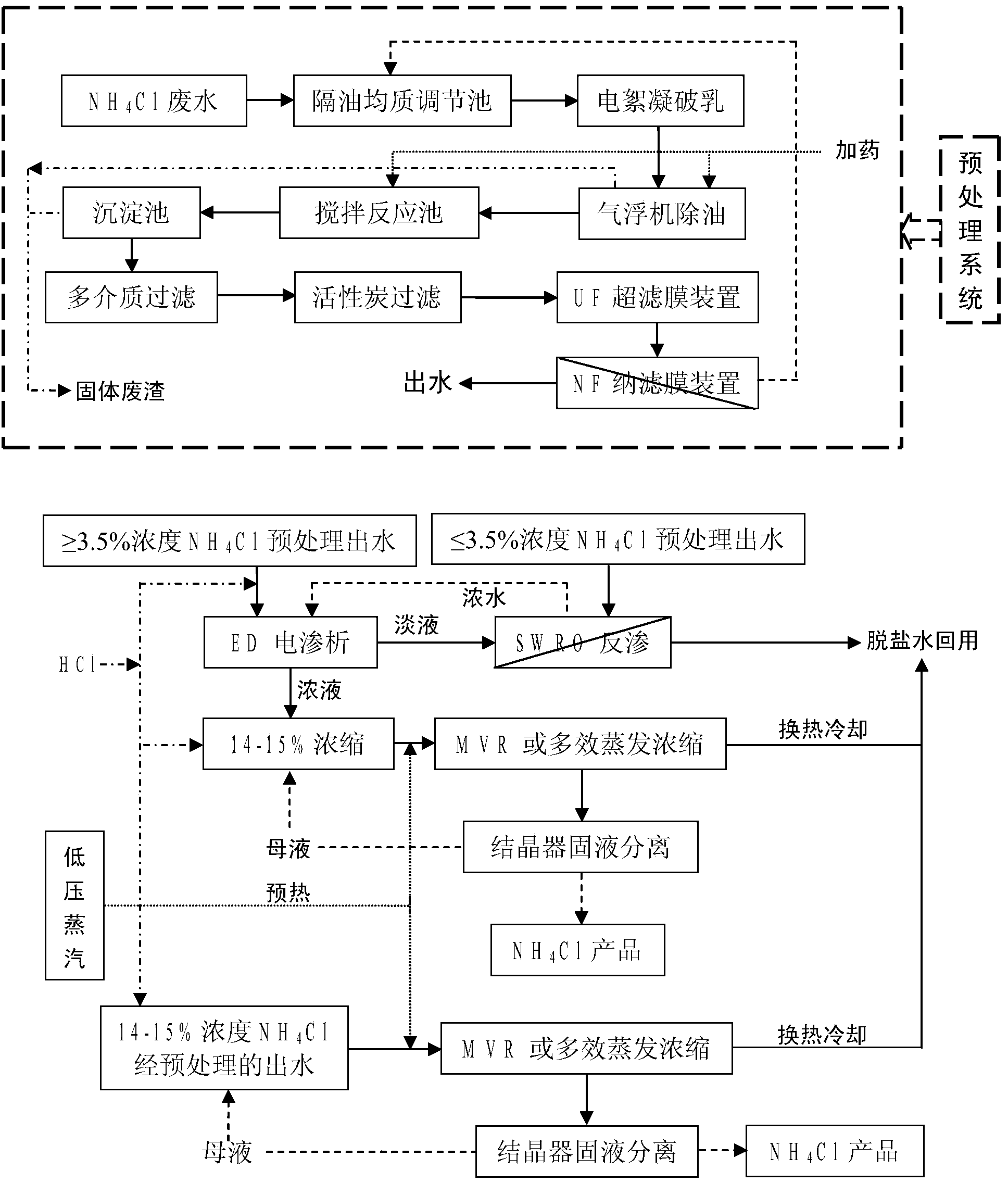
Combined treatment method for ammonia chloride waste water through rare earth extraction separation
InactiveCN104140174AReduce processing costsProduction unit is compactGeneral water supply conservationMultistage water/sewage treatmentResource utilizationReverse osmosis
The invention provides a combined treatment method for ammonia chloride waste water through rare earth extraction separation. The method mainly comprises the steps that the waste water is subjected to a series of desalting and concentration treatment through oil separation, homogenizing, aerating, electric flocculation demulsification, degreasing through an air floating machine, precipitation of calcium, magnesium and heavy metal ions through chemical feeding, microfiltration, ultrafiltration, nanofiltration, reverse osmosis, electrodialysis, evaporation, concentration and the other processes, and then desalted and purified water capable of being utilized cyclically and ammonia chloride products conforming to agricultural standards are obtained. The waste water treatment process is mature, stable and low in treatment cost; production equipment is complete and easy to operate, and automatic industrial control can be easily achieved; zero emission and resource utilization in the treatment process of the ammonia chloride waste water, with different concentrations, generated through rare earth extraction separation are well achieved.
Owner:YANSHAN UNIV
Method for producing ammonium bifluoride by recovering fluorine resource from fluorine-containing silicon slag
ActiveCN102491370AReduce dosageIncrease concentrationSolid waste disposalAmmonium halidesHydrogen fluoridePhysical chemistry
The invention discloses a method for producing ammonium bifluoride by recovering a fluorine resource from fluorine-containing silicon slag. The method comprises the following steps of: dissolving fluorine-containing silicon slag and an ammonium fluoride solution, cooling an obtained ammonium fluosilicate solution, introducing gas ammonia, and precipitating white carbon black out; filtering and washing to obtain a white carbon black solid and an ammonium fluoride solution; introducing anhydrous hydrogen fluoride into the ammonium fluoride solution, uniformly mixing the anhydrous hydrogen fluoride with the ammonium fluoride solution, and fully reacting till the pH of the solution is 2-4 to obtain an ammonium hydrogen fluoride of which the mass concentration is 21-37 percent; mixing with a crystallized ammonium hydrogen fluoride mother solution, and performing triple-effect evaporation and concentration till the mass concentration of the ammonium hydrogen fluoride is 60-80 percent; feeding into a crystallizer, and cooling and crystallizing for 3-7 hours to obtain an ammonium hydrogen fluoride solid-liquid mixture; and separating to obtain an ammonium bifluoride product and an ammonium bifluoride mother solution. The method has the advantages of low using amount of fluorine hydride, high concentration of ammonium bifluoride, low energy consumption in a concentrating process, low production cost, easiness and convenience for operating, and easiness for realizing industrialization.
Owner:WENGFU (GRP) CO LTD +1
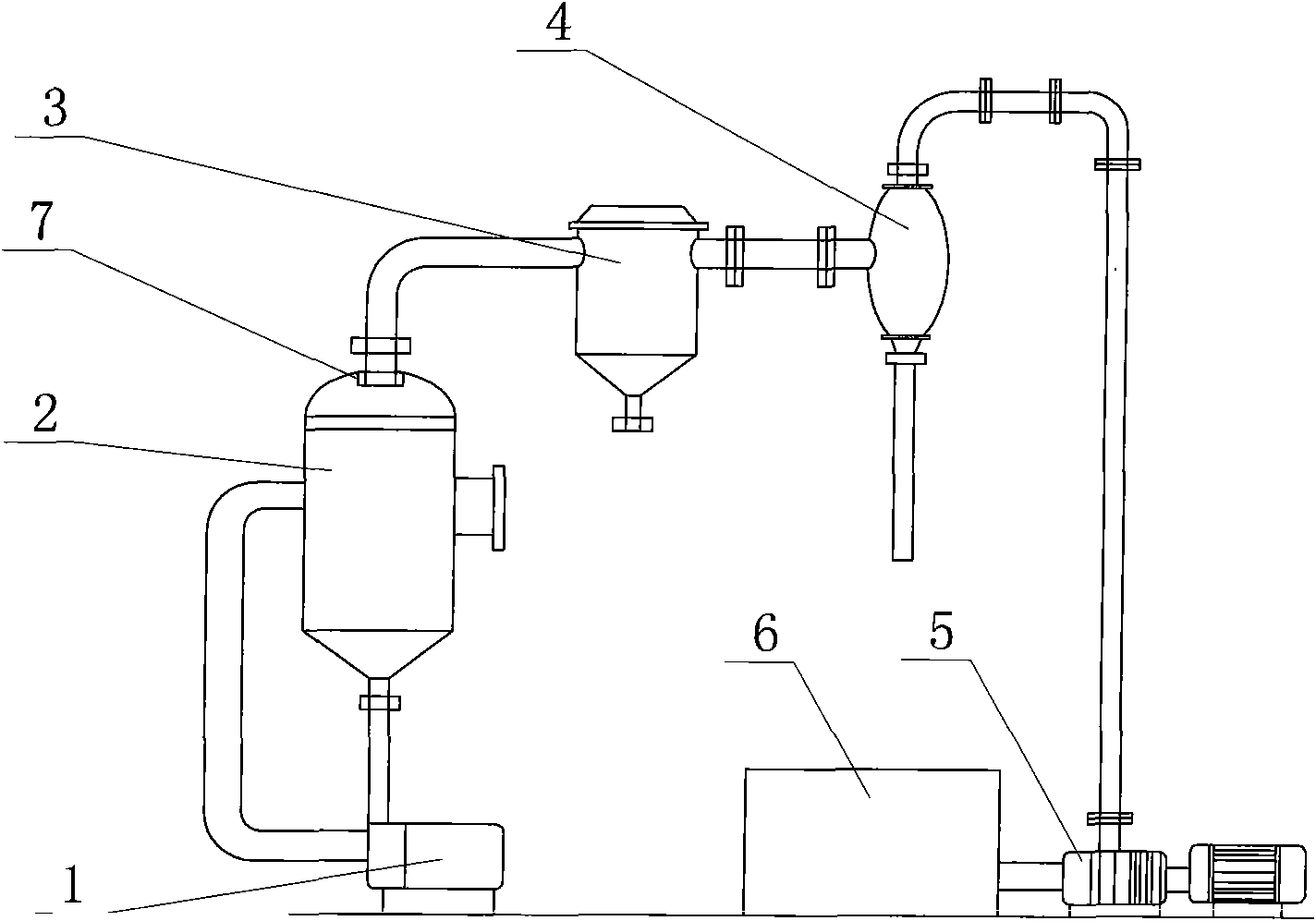
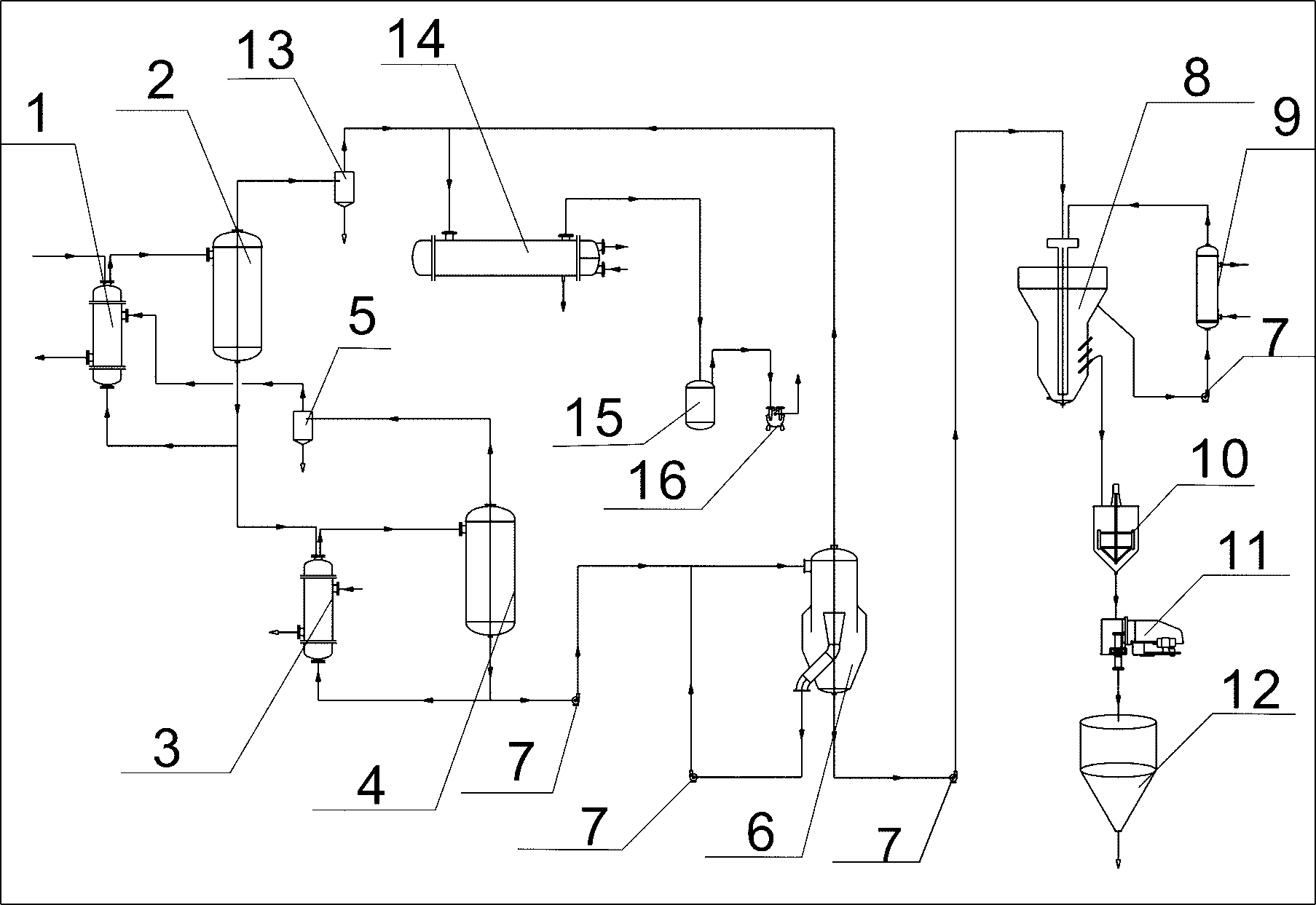
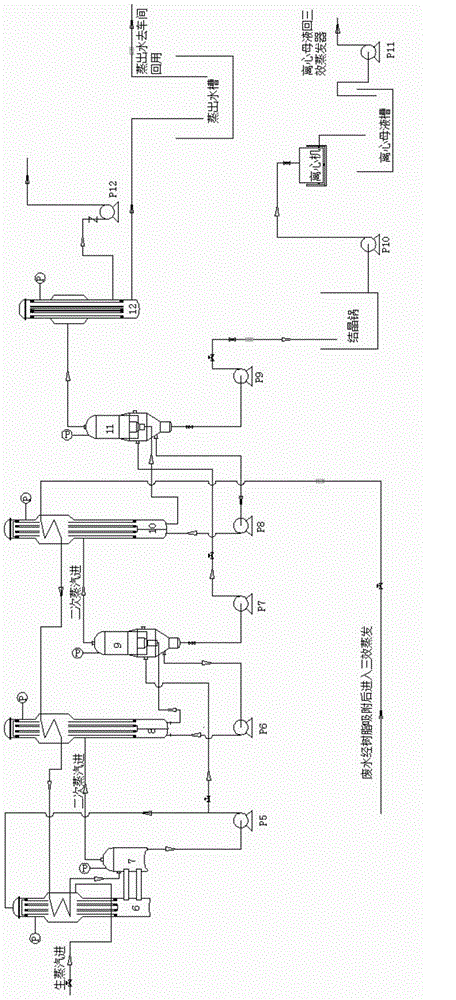
Method and device for recycling ammonium chloride from glycine mother liquor
The invention relates to a method and a device for recycling ammonium chloride from glycine mother liquor. The method is specially implemented as follows: after carrying out 2nd effect evaporation and concentration on glycine mother liquor containing ammonium chloride, carrying out continuous separation by crystallization on the obtained object so as to obtain ammonium chloride. The method comprises the following specific steps of: carrying out preliminary vacuum evaporation and concentration on glycine mother liquor by using an I-effect evaporator; feeding the obtained object into the I-effect evaporator to carrying out further evaporation and concentration on the obtain object; feeding the obtained object into a flash vessel, and carrying out flash vaporization and concentration on the obtained object in a vacuum state, so that the concentration of the obtained flash vaporization and concentration liquor reaches 45 + / -3%; feeding the flash vaporization and concentration liquor into a continuous crystallizer and carrying out circulating heat exchange and cooling crystallization; carrying out thickening and centrifugal separation on the obtained product so as to obtain an ammonium chloride product. In the whole process, the steam application amount is small, and the evaporation speed is rapid, so that the method and device disclosed by the invention have the characteristics of high efficiency and energy conservation, and are suitable for being widely used in workshops.
Owner:HUBEI XINGFA CHEM GRP CO LTD
Method for producing high-purity ammonium bifluoride
The invention discloses a method for producing high-purity ammonium bifluoride, which comprises the following steps of: (1) under the stirring condition, introducing anhydrous hydrogen fluoride into saturated solution of ammonium fluoride until the pH value of the reaction liquid ranges from 1 to 4, and stopping introducing the anhydrous hydrogen fluoride, wherein the temperature is controlled between 25 and 50 DEG C in the reaction process; (2) cooling the reaction liquid to the temperature of between 5 and 20 DEG C, and keeping the temperature for 2 to 4 hours until crystals separate out; and (3) filtering, drying a filter cake to obtain the product of high-purity ammonium bifluoride, and recycling the filter liquor. The method for producing the high-purity ammonium bifluoride has the advantages of small equipment investment, simple process, easy operation, and low moisture content, high purity, high dissolving speed, convenient use, hard agglomeration, and long-term storage of the product ammonium bifluoride. The mother solution is circulated in a closed loop, and the whole technical process has no liquid waste discharge. The raw material ammonium fluoride is prepared by ammonolysis of a byproduct fluosilicic acid in the phosphate fertilizer industry, and the method has the advantages of easily obtained raw materials, low cost, and low production cost. The method provides a new fluoride source, and solves the problem of environmental pollution in the phosphate fertilizer industry.
Owner:焦作市增氟科技有限公司
Processing method of aniline intermediate production wastewater
ActiveCN104803529AUninterrupted adsorptionImprove connectivityMultistage water/sewage treatmentAmmonium halidesDevice formFiltration
A processing method of aniline intermediate production wastewater comprises the following steps: (1) mother liquor wastewater containing sodium chloride or ammonium chloride during the production process of an aniline intermediate firstly undergoes physical filtration to remove impurities; (2) after filtration, the mother liquor wastewater enters an N-staged series resin adsorption device, wherein N is a natural number being greater than or equal to 2; the N-staged series resin adsorption device is an N+1 towers N-staged series resin adsorption device formed by series connection of N+1 adsorption towers filled with resin columns; one of the adsorption towers is a standby tower; the whole resin adsorption device ceaselessly carries out wastewater adsorption and resin desorption regeneration; (3) after the mother liquor wastewater undergoes adsorption by the resin adsorption device, physical filtration is carried out to remove impurities; (4) the filtered mother liquor wastewater enters a multiple-effect evaporation device to undergo condensation crystallization, an ammonium chloride or sodium chloride solid is finally separated, and the mother liquor returns to the multiple-effect evaporation device to continuously undergo condensation crystallization; and (5) a desorption solution obtained from the step (2) is reused. The processing method provided by the invention is a genuine continuous clear production technology.
Owner:ZHEJIANG LONGSHENG GROUP +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com