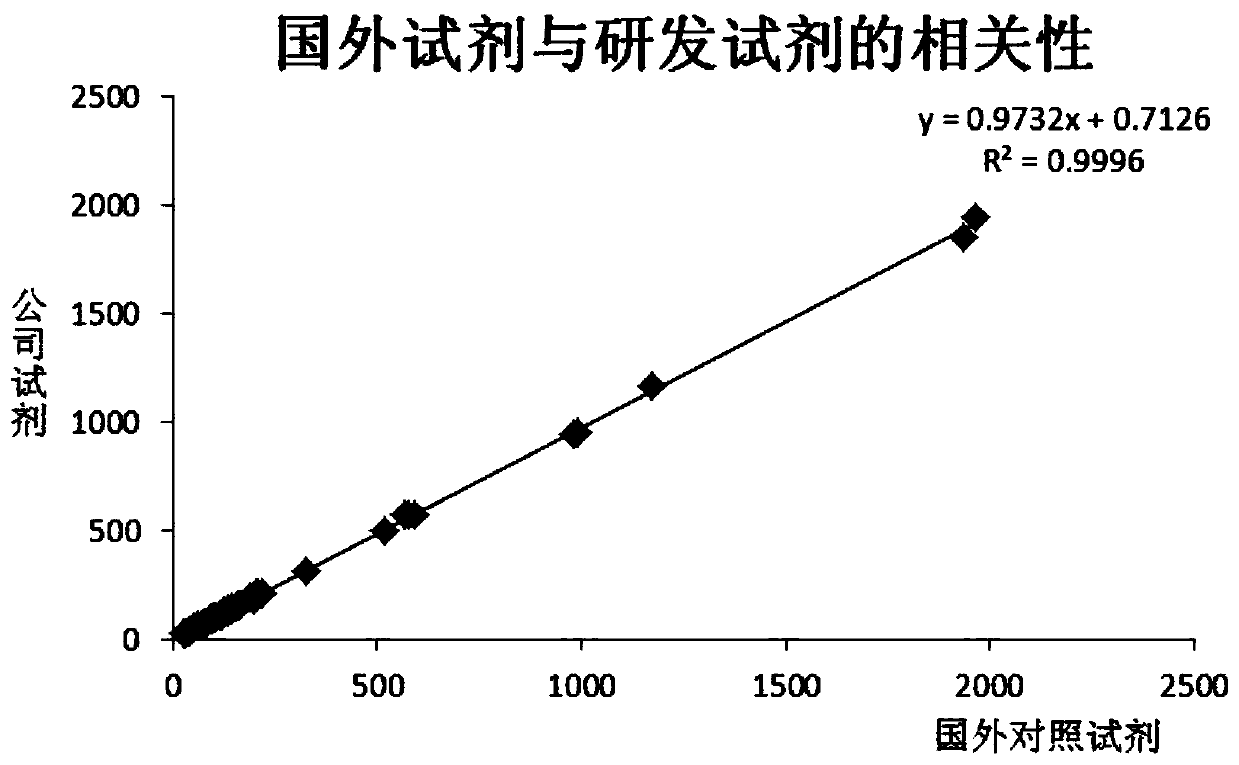
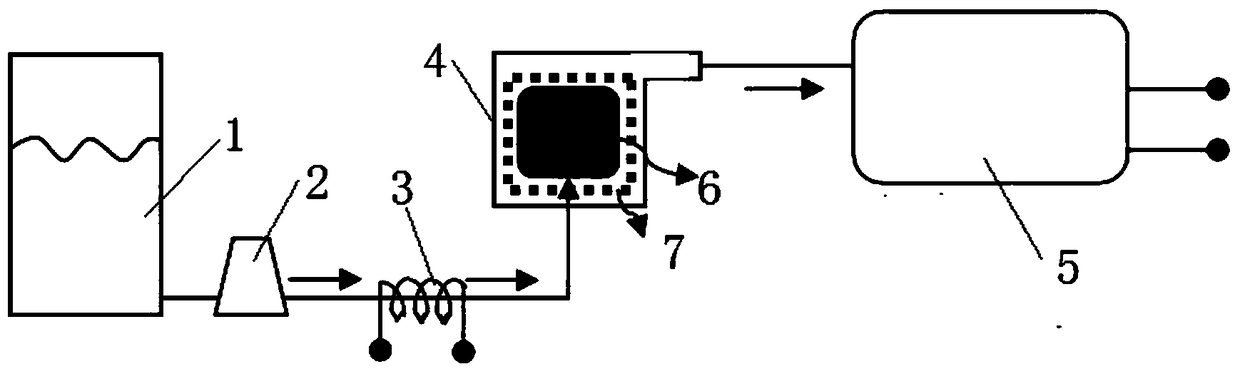
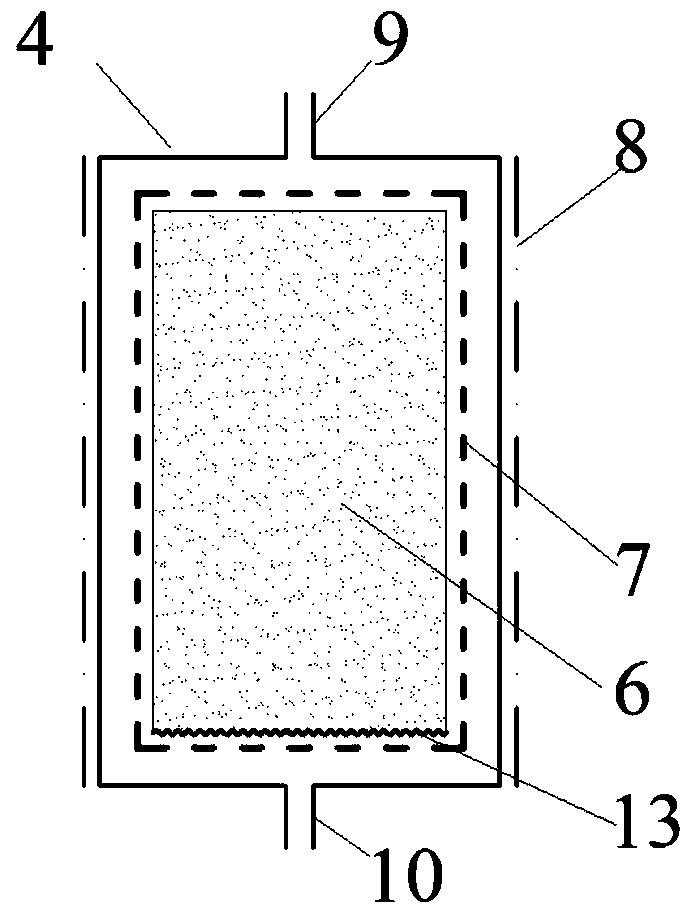
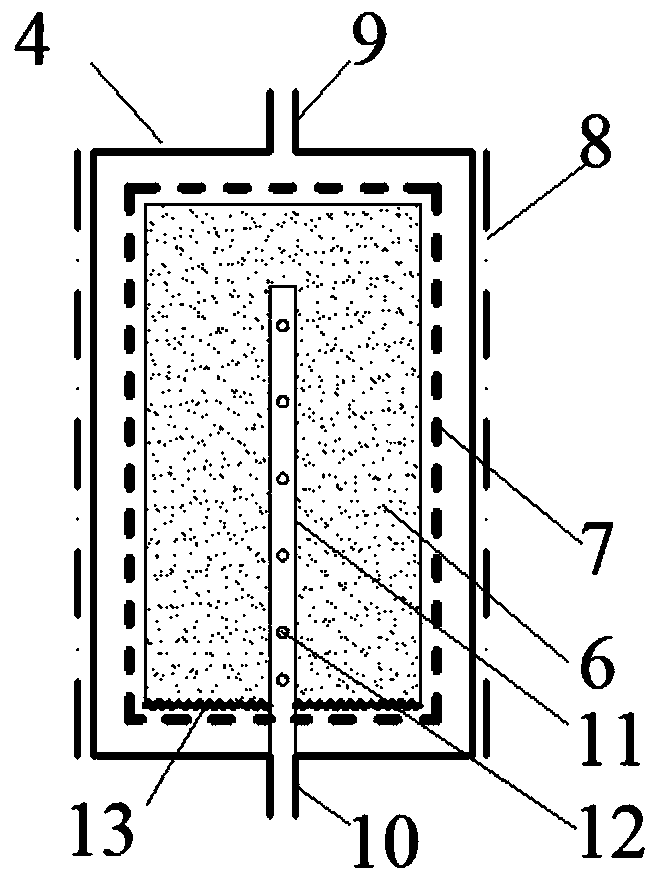
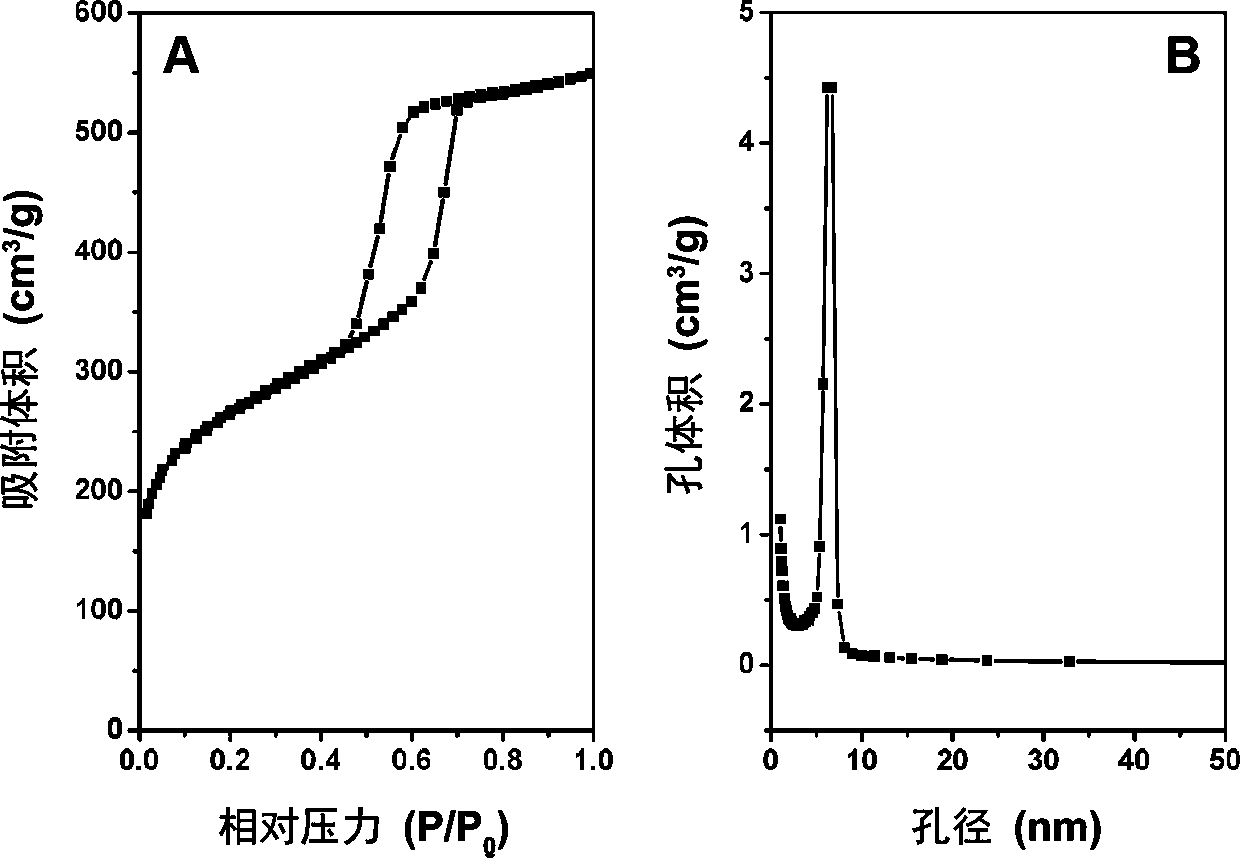
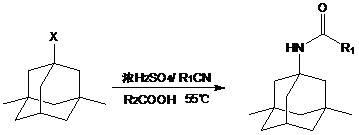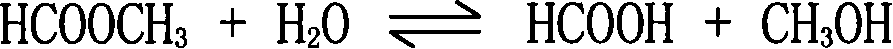Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
132results about How to "Promote hydrolysis reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
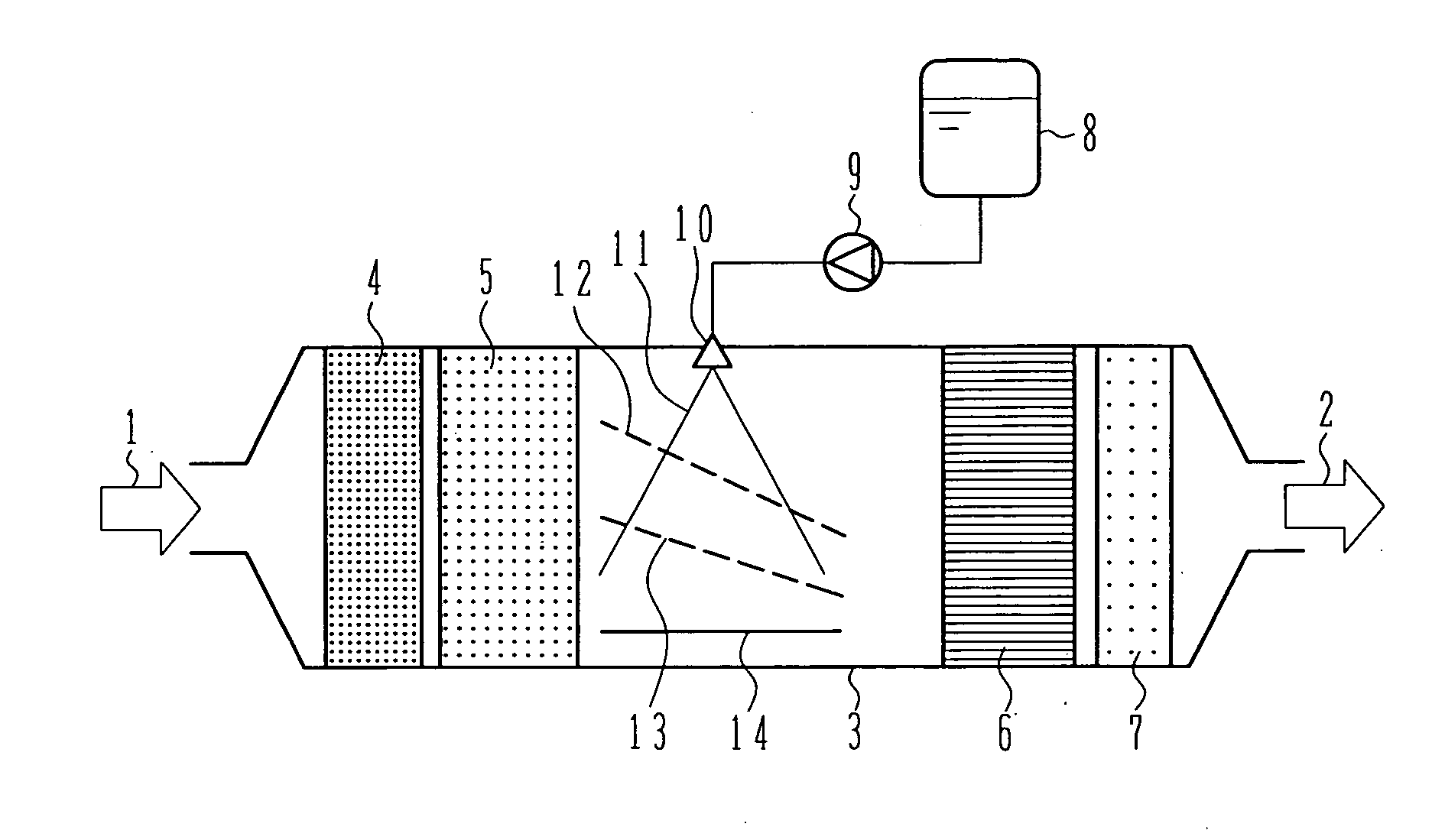
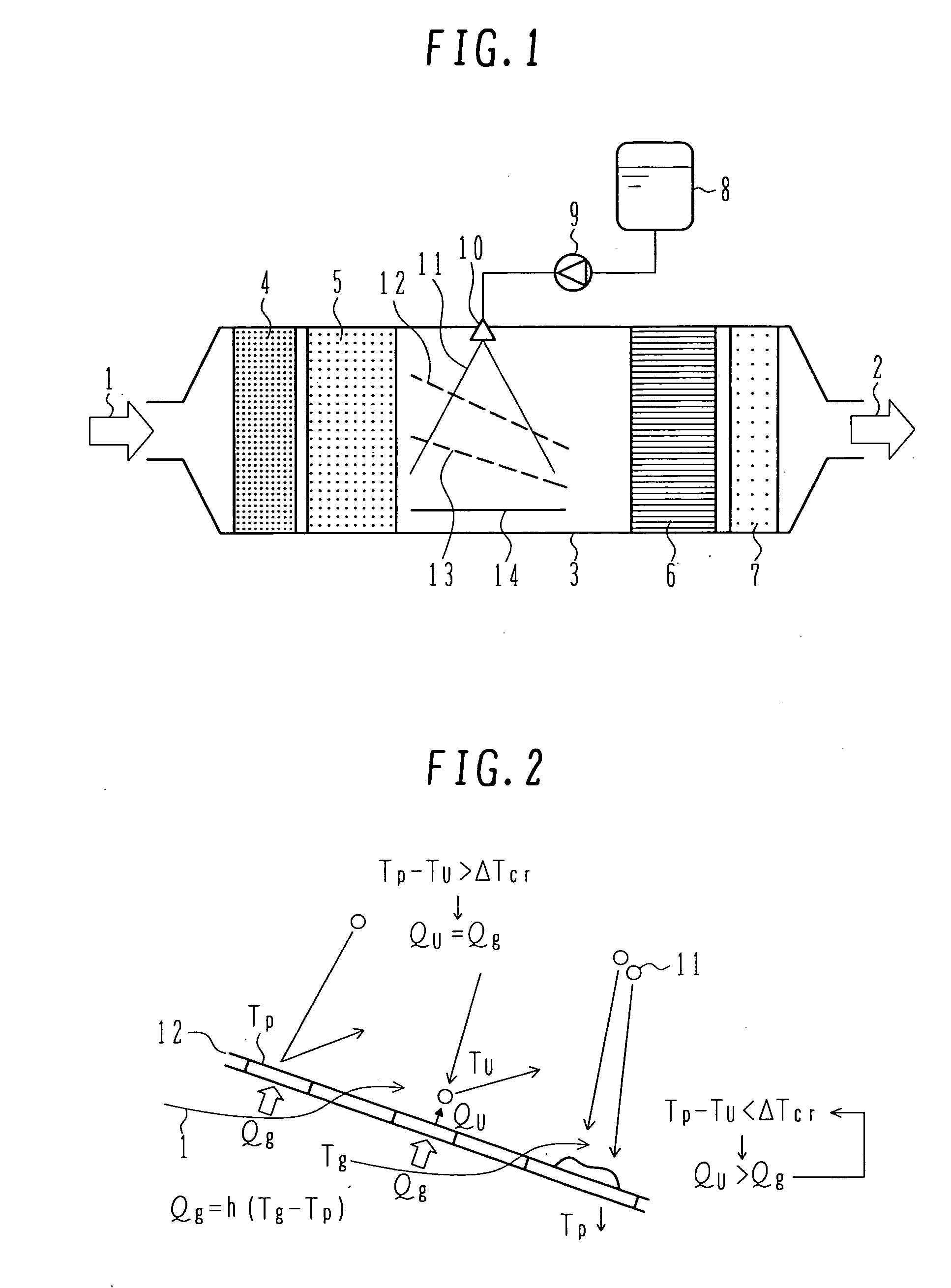
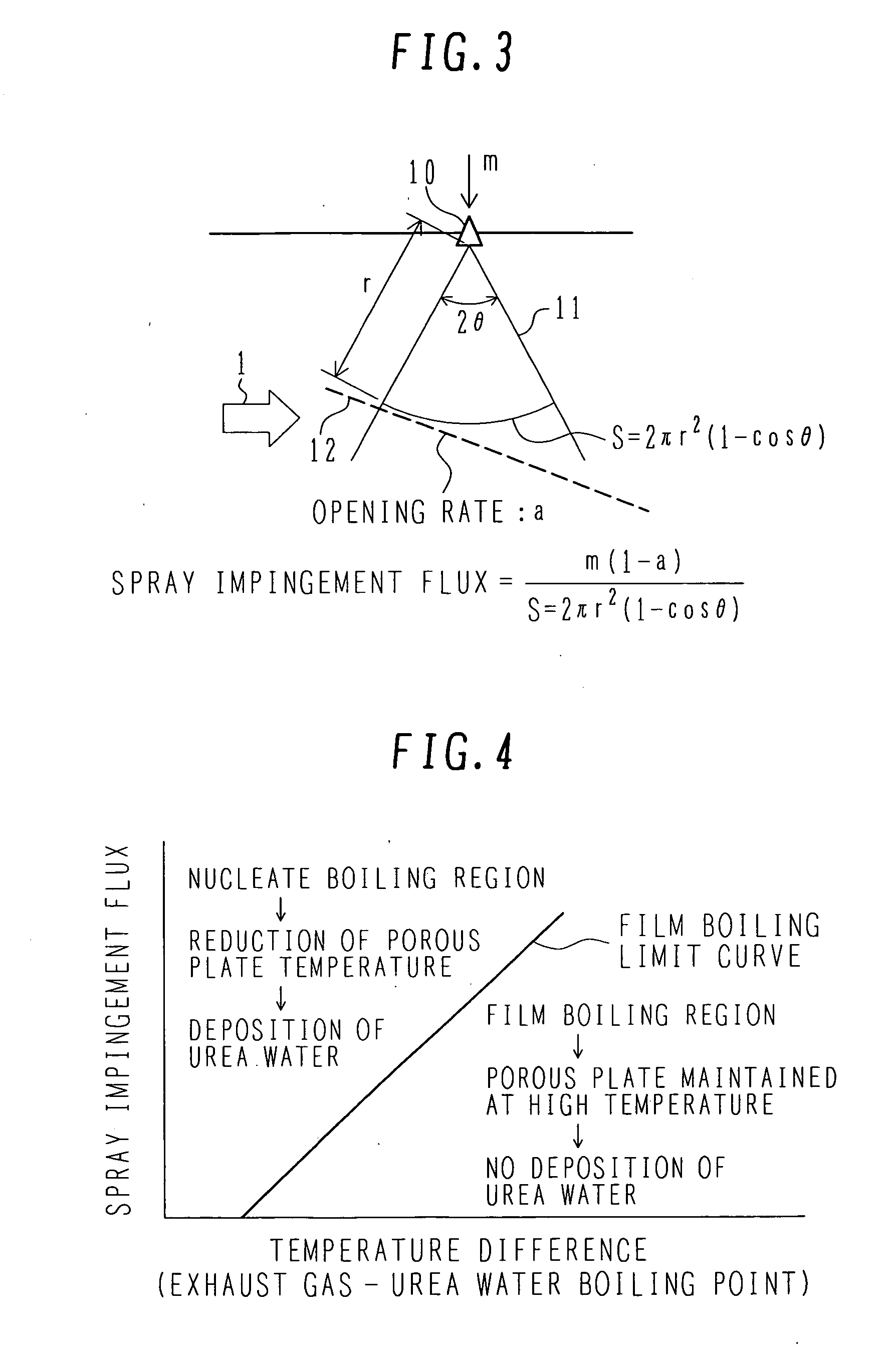
Exhaust aftertreatment system using urea water
InactiveUS20070036694A1Avoid depositionReduced responseCombination devicesInternal combustion piston enginesHandling systemMultiple stages
An exhaust aftertreatment system comprises an injector for injecting urea water into an exhaust duct, and a denitration catalyst disposed downstream of the injector with respect to a flow of exhaust gas. The exhaust aftertreatment system reduces nitrogen oxides in the exhaust gas by the denitration catalyst while using ammonia produced from the urea water injected from the injector. The urea water is injected along a direction of the flow of the exhaust gas within the exhaust duct, and a porous plate is disposed in multiple stages in a space of the exhaust duct such that droplets of the injected urea water impinge against the porous plate before reaching a wall surface of the exhaust duct. A surface of the porous plate subjected to the impingement of the droplets is arranged to face downstream with respect to the flow of the exhaust gas. Deposition of the urea water is prevented by causing film boiling when the droplets impinge against the porous plate, and the urea water reflected by the porous plate is uniformly dispersed into the exhaust gas. Thus, the urea water is uniformly dispersed into the exhaust gas without increasing a pressure loss of the exhaust gas. The urea water is prevented from depositing on the wall surface and producing a precipitate in the form of a solid.
Owner:HITACHI HIGH-TECH CORP +2
Process for producing composite semipermeable membrane
InactiveUS6521130B1Increase volumePromote hydrolysis reactionReverse osmosisWater/sewage treatment bu osmosis/dialysisPolymer sciencePolyamide
When forming a separating functional layer containing crosslinked polyamide, by carrying this out in the presence of 1) carboxylic acid ester with a total of 8 or more carbons, or 2) carboxylic acid, it is possible to provide a composite semipermeable membrane which, while still maintaining a high rejection rate, is more outstanding in its water permeability than hitherto.
Owner:TORAY IND INC
Efficient cellulose nanocrystalline preparing method
ActiveCN105777913AHigh yieldImproved particle size distributionPulp properties modificationWashing/displacing pulp-treating liquorsInorganic saltsCellulose
The invention provides an efficient and quick cellulose nanocrystalline preparing method.According to the method, a cellulose raw material is pretreated with aqueous alkali and then subjected to acidolysis, and inorganic salt containing metal ions is added during acidolysis to serve as a promoter to promote acidolysis reaction.Reaction condition is mild and easy to control, raw materials needed by reaction are simple and easy to obtain, and the method can be widely applied to preparation of cellulose nanocrystalline from various cellulose raw materials.
Owner:QINGDAO UNIV OF SCI & TECH
Method for preparing acellular matrix
InactiveCN101274106AStrong decellularization specificityStrong specificityTissue regenerationProsthesisDiseasePhospholipase
The invention discloses a method of preparing acellular matrixes by using phospholipase. The method of the invention is characterized in that stand-by organ tissue is first pre-treated and then added into solution containing the phospholipase to prepare the acellular matrixes under a controlled condition; the prepared acellular matrixes are then washed. By adopting the preparation method of the invention, the obtained acellular matrix can have good physical property and biological function. Therefore, the preparation method in the invention is not only a great breakthrough in the tissue engineering, but also opens a new way for clinical treatment of diseases. The preparation method of the invention has the advantages of reliable theory, simple and flexible process technique, good product reproducibility and is very easy to be industrialized.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
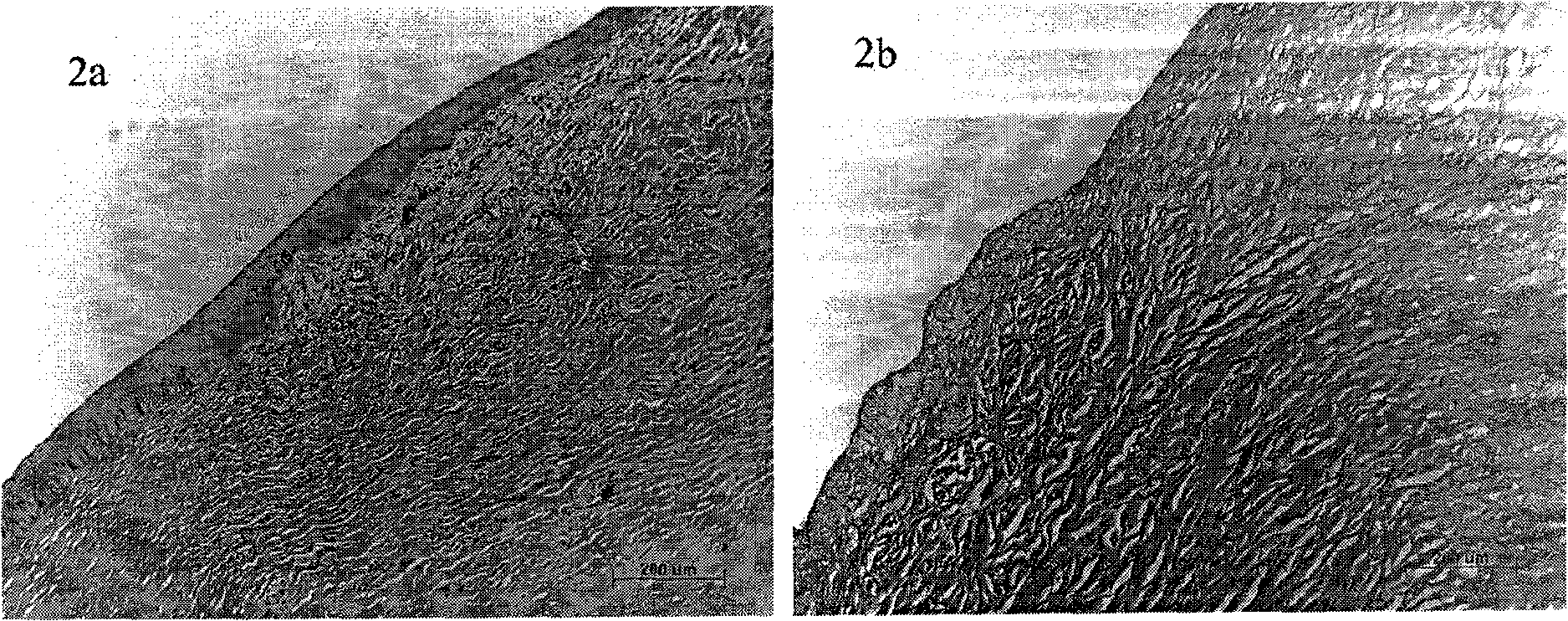
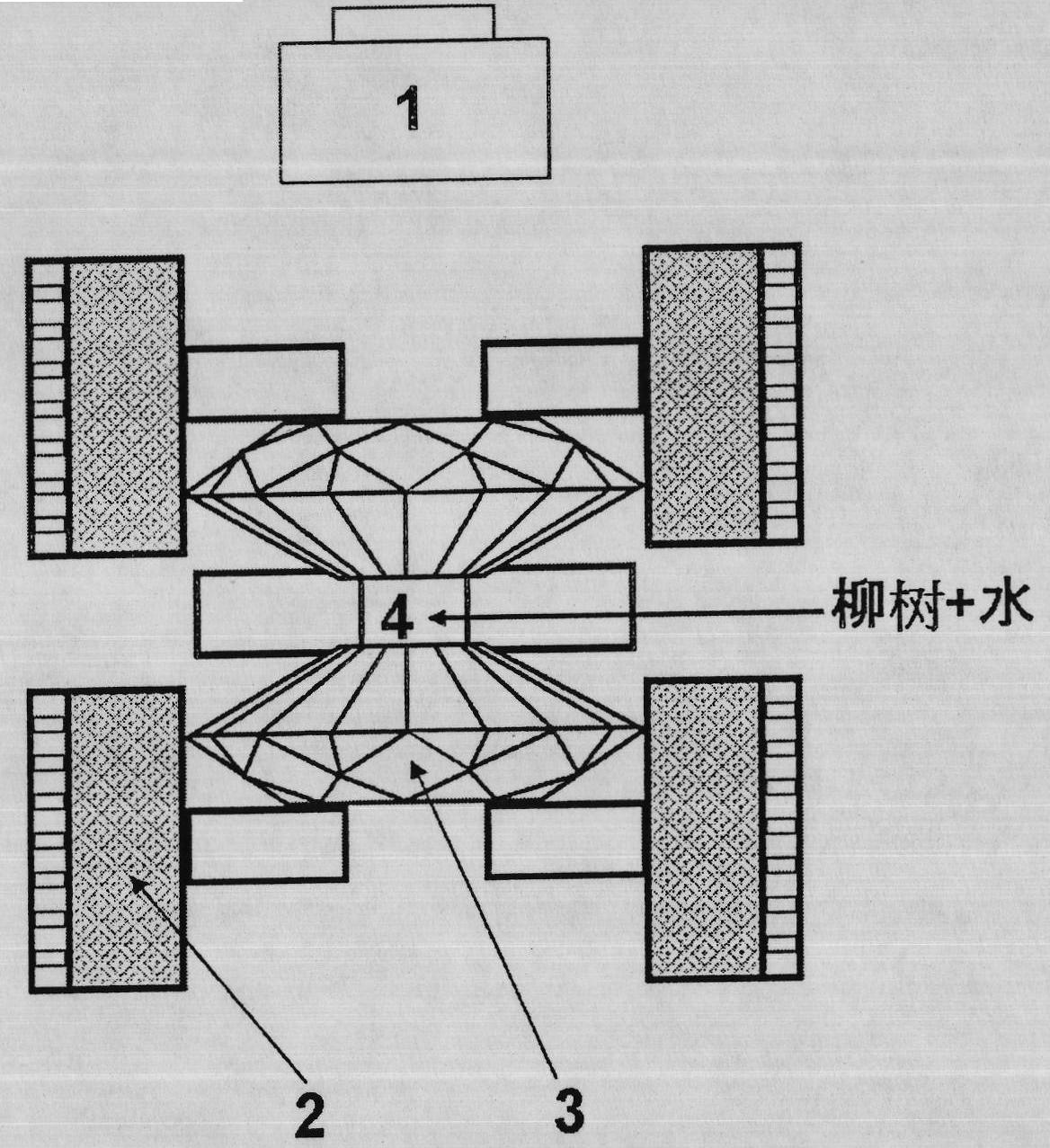
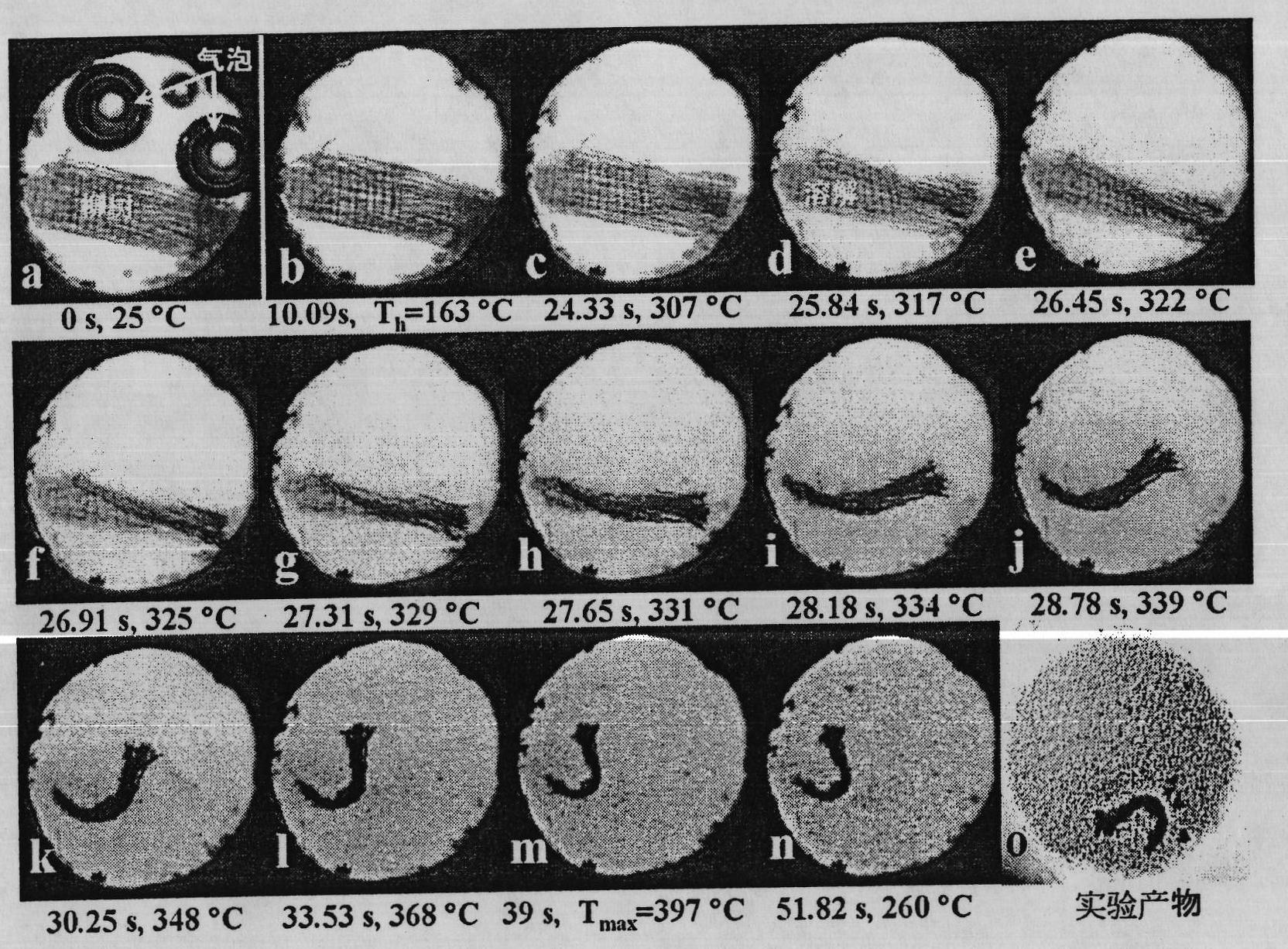
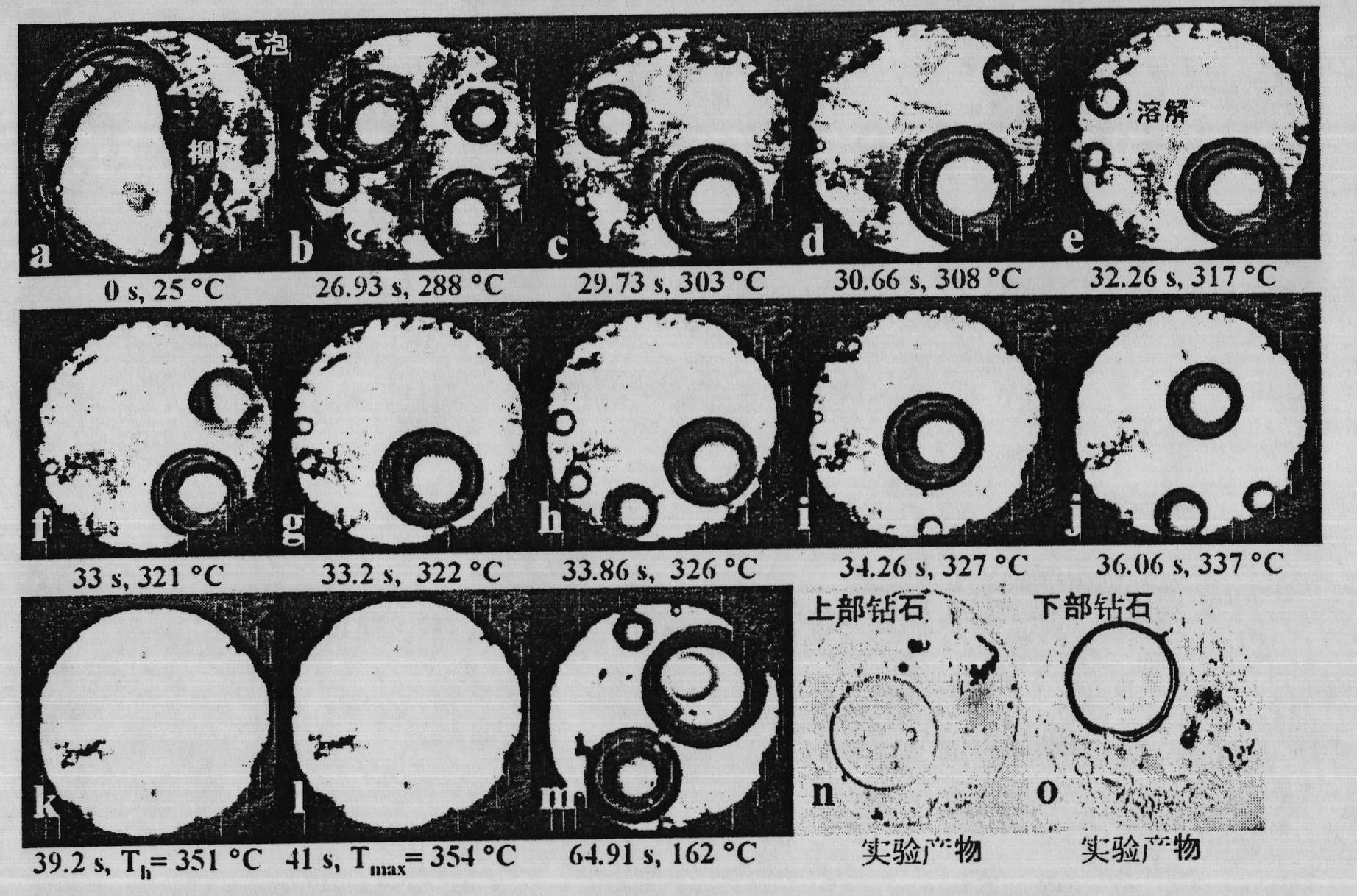
Method for dissolving and quickly hydrolyzing lignocellulose biomass as well as device and application thereof
ActiveCN101974161AReduce pollutionQuick mixOther chemical processesOrganic compound preparationCellulosePtru catalyst
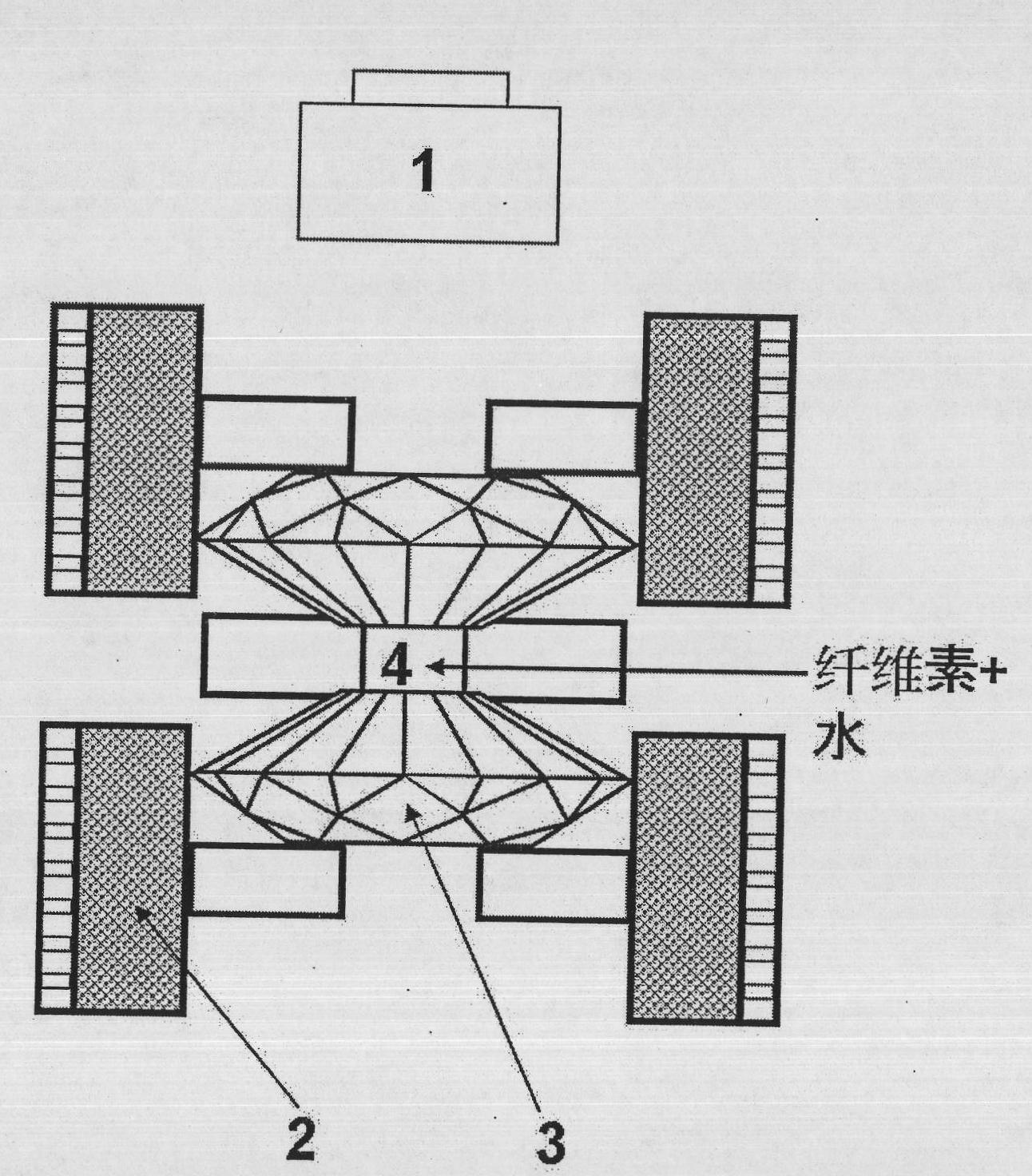
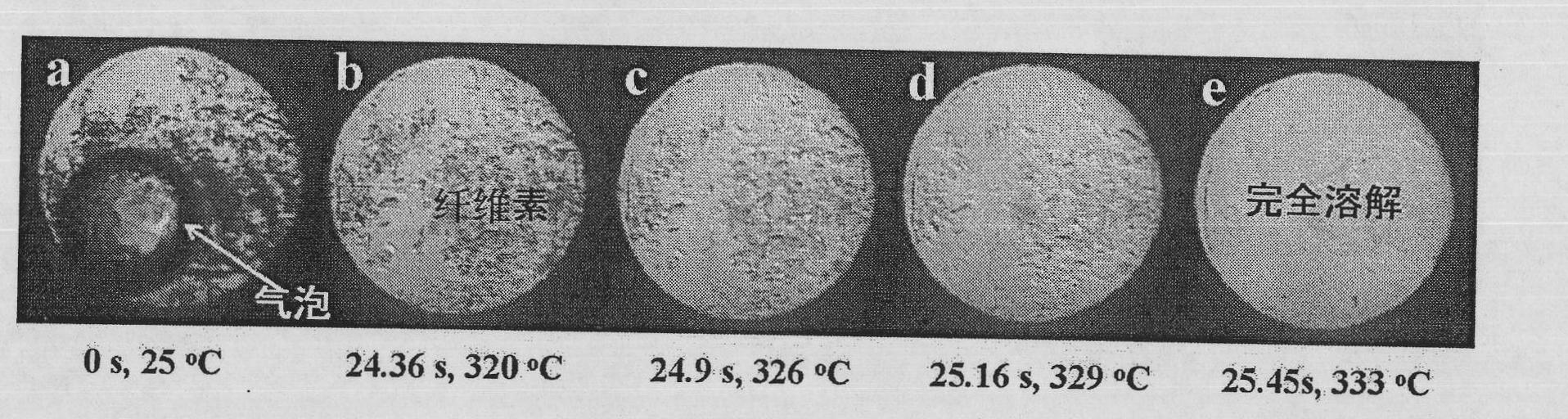
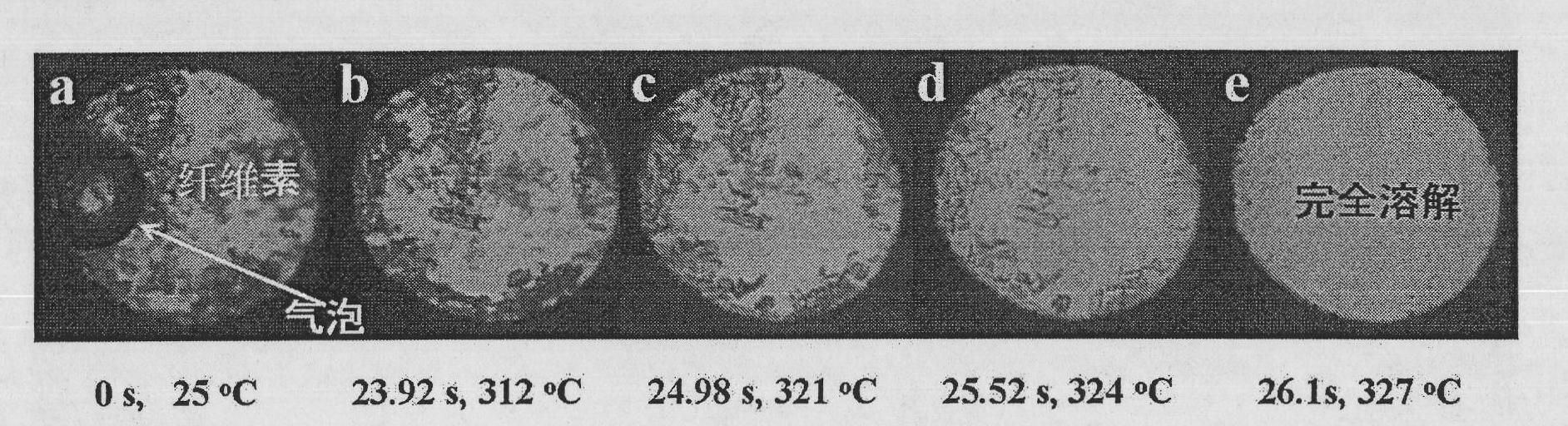
The invention discloses a method and device for dissolving and quickly hydrolyzing lignocellulose biomass. The method comprises the following steps of: placing lignocellulose biomass in pure water, and quickly heating to 330-403 DEG C within 3.38-21.79 s. 89-99% of lignocellulose biomass is dissolved and quickly hydrolyzed into saccharides. The dissolution of the lignocellulose biomass enables the subsequent hydrolysis reaction to be carried out in a homogeneous phase condition. Meanwhile, the solvated biomass can be conveniently applied to a high-pressure flow reactor, and the biomass is continuously pretreated and hydrolyzed into saccharides and other kinds of biofuel and products. The invention has the advantages of no use of any catalyst, no environmental pollution, simple and convenient process and good market application prospects, and belongs to green sustainable industries encouraged by China.
Owner:XISHUANGBANNA TROPICAL BOTANICAL GARDEN CHINESE ACAD OF SCI
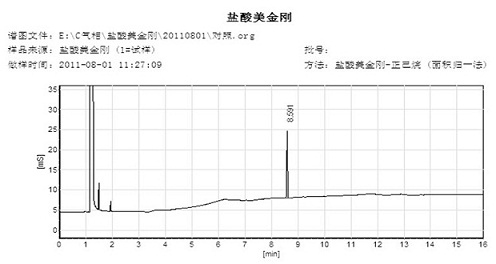
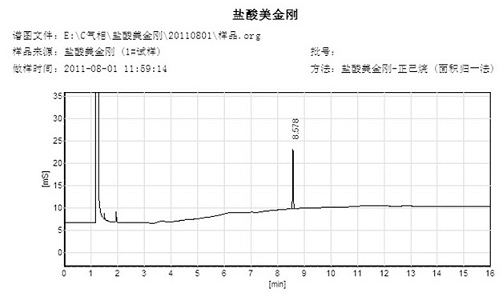
Synthetic method of memantine hydrochloride
InactiveCN102432473AShort timeSolve the difficulty of stirringOrganic compound preparationAmino compound preparationMemantine HydrochlorideMethylene Dichloride
The invention discloses a synthetic method of memantine hydrochloride serving as a medicament for treating dementia. The method comprises the following steps of: reacting halogenated dimethyladamantane with nitrile, concentrated sulfuric acid and organic acid to obtain 1-acetamido-3,5-dimethyladamantane crystal serving as an intermediate for synthesizing memantine hydrochloride; hydrolyzing 1-acetamido-3,5-dimethyladamantane with alcohol and alkali to generate raw memantine; and extracting and salting to obtain memantine hydrochloride. In the method, the organic acid is taken as a solvent, so that the problems of low yield, difficulty in stirring and a series of problems caused by difficulty in stirring are solved; compared with an original process, the method has the advantages that: five steps for distilling the solvent under reduced pressure, adding water, extracting methylene dichloride, recovering the solvent under reduced pressure and recrystallizing are reduced, the reaction post-treatment operation is simplified greatly, and reaction post-treatment is easy and convenient; operation is easier and more convenient, so that the time consumption of a memantine hydrochloride synthesizing process is lowered, and the efficiency is increased; compared with an original process, the method has the advantages that: the step for extracting methylene dichloride serving as an organic solvent is eliminated, so that higher economic efficiency and environmental friendliness are achieved; and more importantly, the acetamide reaction yield is increased from lower than 70 percent to over 90 percent.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Stable alpha-amylase detection kit
The invention provides a stable alpha-amylase detection kit. The stable alpha-amylase detection kit is composed of a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from the following components: 0.5-100mM buffer solution, 20-200mM NaCl, 10-100mM CaCl2, 1-10g / L sodium dodecyl benzene sulfonate, 0.1-10KU / L alpha-glucosidase, 0.1-10g / L stabilizer and 0.1-10g / L preservative; the reagent R2 is prepared from the following components: 0.5-100mM buffer solution, 0.5-10g / L 4,6-ethylidene-nitrophenylcarbimide-alpha-D-maltoheptaoside, 1-10g / L sodium dodecyl benzene sulfonate, 0.1-10g / L stabilizer and 0.1-10g / L preservative. The sodium dodecyl benzene sulfonate is added, so that the performances of the kit are improved, the kit has the good repetitiveness, high sensitivity and wide linear range, and reagents are stable.
Owner:上海睿康生物科技有限公司
Hydrogen production method and device with magnesium hydride as hydrogen storage material
The invention relates to the technical field of hydrogen production, and in particular, relates to a hydrogen production device with magnesium hydride as a hydrogen storage material, wherein the hydrogen production device includes a reaction chamber; a massive magnesium hydride filler layer is arranged in the reaction chamber. Provided is a hydrogen production method with magnesium hydride as thehydrogen storage material; massive magnesium hydride is prepared by mixing and pressing MgH2 and other chemical substances (M), the MgH2 / M ratio is 10 wt.%-80 wt.%, and the massive magnesium hydride is shaped as particles or cakes, wherein the particles are spheres or irregular spheres with the diameter of 1-50 mm, and the cakes are round cakes or cakes with other shapes with the bottom diameter or side length of 1-100 mm and the height of 1-50 mm. The massive magnesium hydride is stacked and filled in the reaction chamber, the void between the particles becomes larger and the energy density of the filler increases so as to be conducive to full contact reaction of water vapor to all parts of the filler to produce hydrogen with high efficiency. At the same time, hydrogen gas escapes from the filler more smoothly to prevent the sudden increase of the pressure in the reaction chamber. The start-up time of reaction is shortened by adding a reaction promoter, and the quality of hydrogen gasis improved by adding a gas purifier.
Owner:武汉市能智达科技有限公司
Preparation method of micro-arc oxidation-composite chemical nickel plating coating layer on surface of magnesium alloy
InactiveCN104141138APromote hydrolysis reactionAvoid PolymerizationAnodisationLiquid/solution decomposition chemical coatingSurface brightnessMicro arc oxidation
The invention discloses a preparation method of a micro-arc oxidation-composite chemical nickel plating coating layer on the surface of a magnesium alloy, and belongs to the technical field of metal surface treatment. The preparation method comprises the following steps: firstly, the micro-arc oxidation is performed for the surface of a magnesium alloy matrix to form a porous ceramic coating layer on the surface; then, the hole sealing treatment is performed for the matrix with the porous ceramic coating layer by adopting nanometer self-assembly penetrant containing nickel salt; the nickel preplating treatment is performed for the matrix, after the hole sealing treatment, by adopting alcohol solution of sodium borohydride; and finally, the composite chemical nickel plating treatment is performed for the matrix, after the nickel preplating treatment, to form the micro-arc oxidation-composite chemical nickel plating coating layer on the surface of the magnesium alloy. The micro composite coating layer, prepared by the method, has better protective thickness, good binding force with the matrix, good corrosion resistance, high hardness, good surface brightness and full luster, effectively solves the protective requirements of high wear resistance and corrosion resistance of the magnesium alloy, and provides powerful guarantee to the further application of the magnesium alloy.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Method for preparing catalyst carrier Al2O3 powder by activating and hydrolyzing metallic aluminium under ultrasound-electric field coupling
InactiveCN101829607APromote activationThe principle is simpleCatalyst carriersCatalyst activation/preparationSpecific volumeAluminium
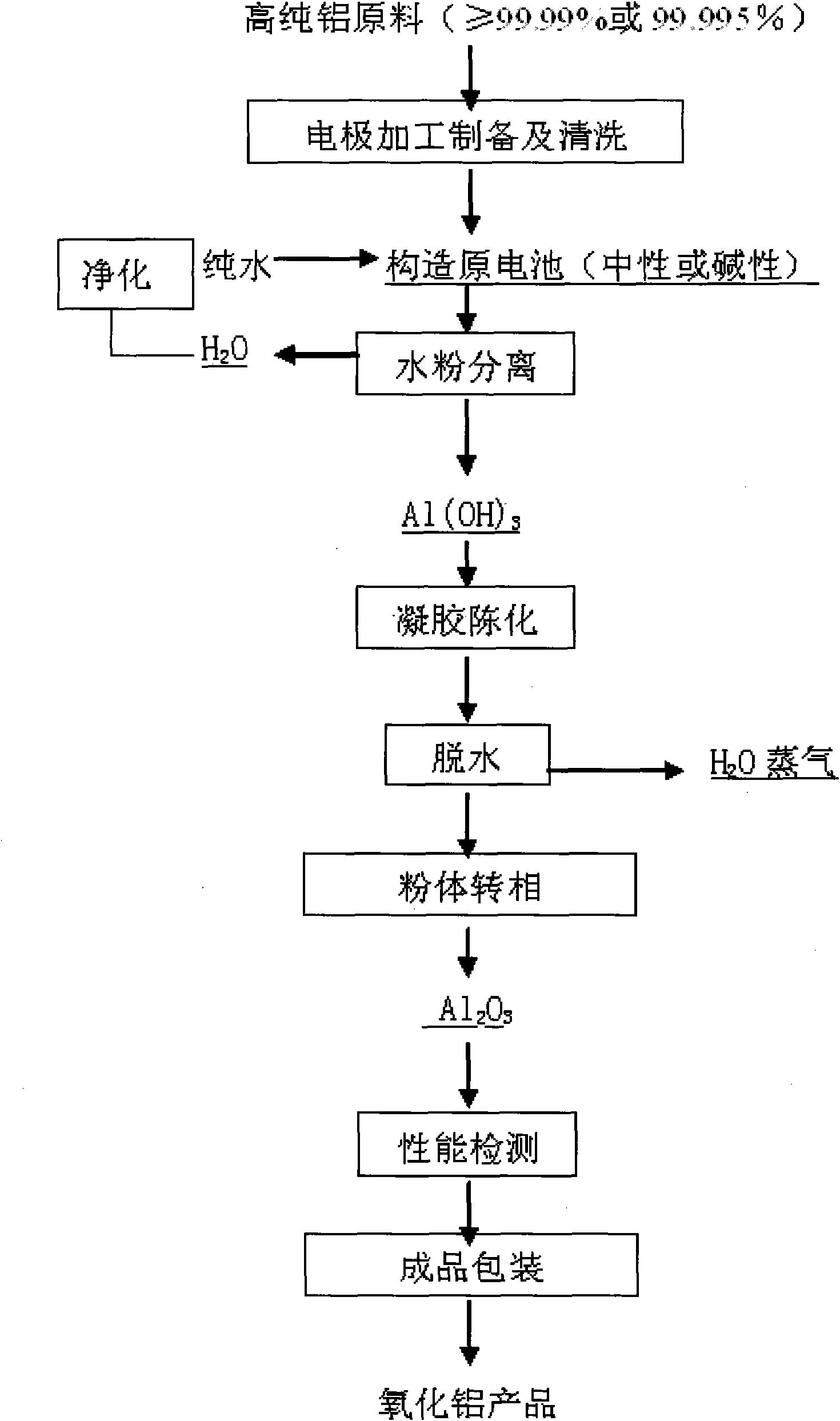
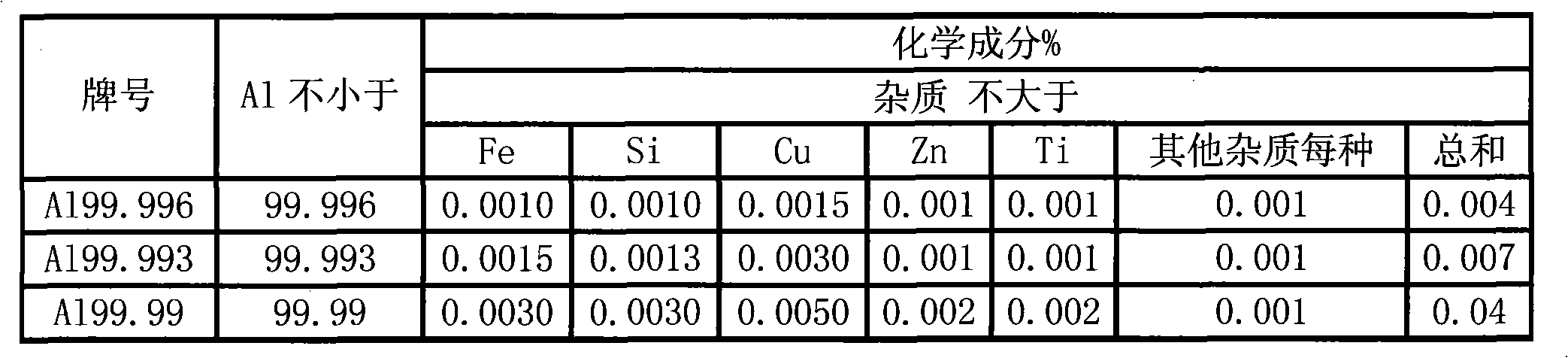
The invention discloses a new process for preparing high purity aluminum oxide powder. The processed is carried out based on the fundamental principle of ultrasound electrochemistry, wherein electrode reactions in primary cells between metallic aluminium and air is formed under ultrasound action in a pure water system, and in-situ activation is carried out on the surface of the high purity metallic aluminium by the cavitation of an ultrasonic field, which can promote the activation of the surface of the metallic aluminium and the primary cell reaction between the metallic aluminium and the air as well as the hydrolysis reaction of the metallic aluminium. The method has simple principle, concise process, short technological procedures, low specific volume and energy consumption, environmental protection and no pollution. As under the entire ultrasound-electric field coupling condition, the reaction is moderate and controllable, and the obtained high purity Al (OH) 3 gel has small primary particle size, uniform particle size distribution, high activity, good flowability, particle appearance approximate to sphere and easy purity control. The obtained Al (OH) 3 gel is processed then by thermal treatment, and the high purity aluminum oxide powder with different forms can be obtained at different thermal treatment temperatures. The product has high purity, excellent particle characteristics and incomparable advantages over the products prepared by other production methods in the aspects of purity and particle shape.
Owner:昆明珀玺金属材料有限公司
Method for completely dissolving and rapidly hydrolyzing cellulose and application thereof
InactiveCN101824156ARapid hydrolysisReduce manufacturing costBiofuelsFermentationCelluloseAlkaline water
The invention discloses a method for completely dissolving and rapidly hydrolyzing cellulose and application thereof. Cellulose is put into an acid solution with the concentration of 10<-7> to 1M [H<+>] or in an alkaline solution with the concentration of 10<-7> to 1M [OH<->], and the volume ratio of solid to liquid is (0.003 to 1.05) to 1; the acid solution of 10-7 to 1M [H<+>] or the alkaline solution of 10<-7> to 1M [OH<->] is heated to be at the temperature of 261 to 352 DEG C; the products of the step 1 and the step 2 are mixed and put in a reactor; the concentration of the cellulose is 0.1 % to 35%; the concentration of the mixed material solution is regulated to be acid 10<-7> to 1M [H<+>] or alkaline 10<-7> to 1M [OH<->]; the water density is 587 to 997 kg / m<3>, the pressure is set to be 6 to 584MPa, heating rate is 7.8 to 14.8 DEG C / s, the mixture is rapidly heated to be at the temperature of 261 to 352 DEG C, and the cellulose can be completely dissolved in 0.8 to 2 seconds. The method first realizes the complete dissolution and rapid hydrolysis of cellulose at lower temperature, thereby greatly lowering production cost, improving production safety, prolonging service life of equipment, and thus having favorable application prospect.
Owner:XISHUANGBANNA TROPICAL BOTANICAL GARDEN CHINESE ACAD OF SCI
Master batch for improving durability of polyglycolide (PGA) and preparation method of master batch
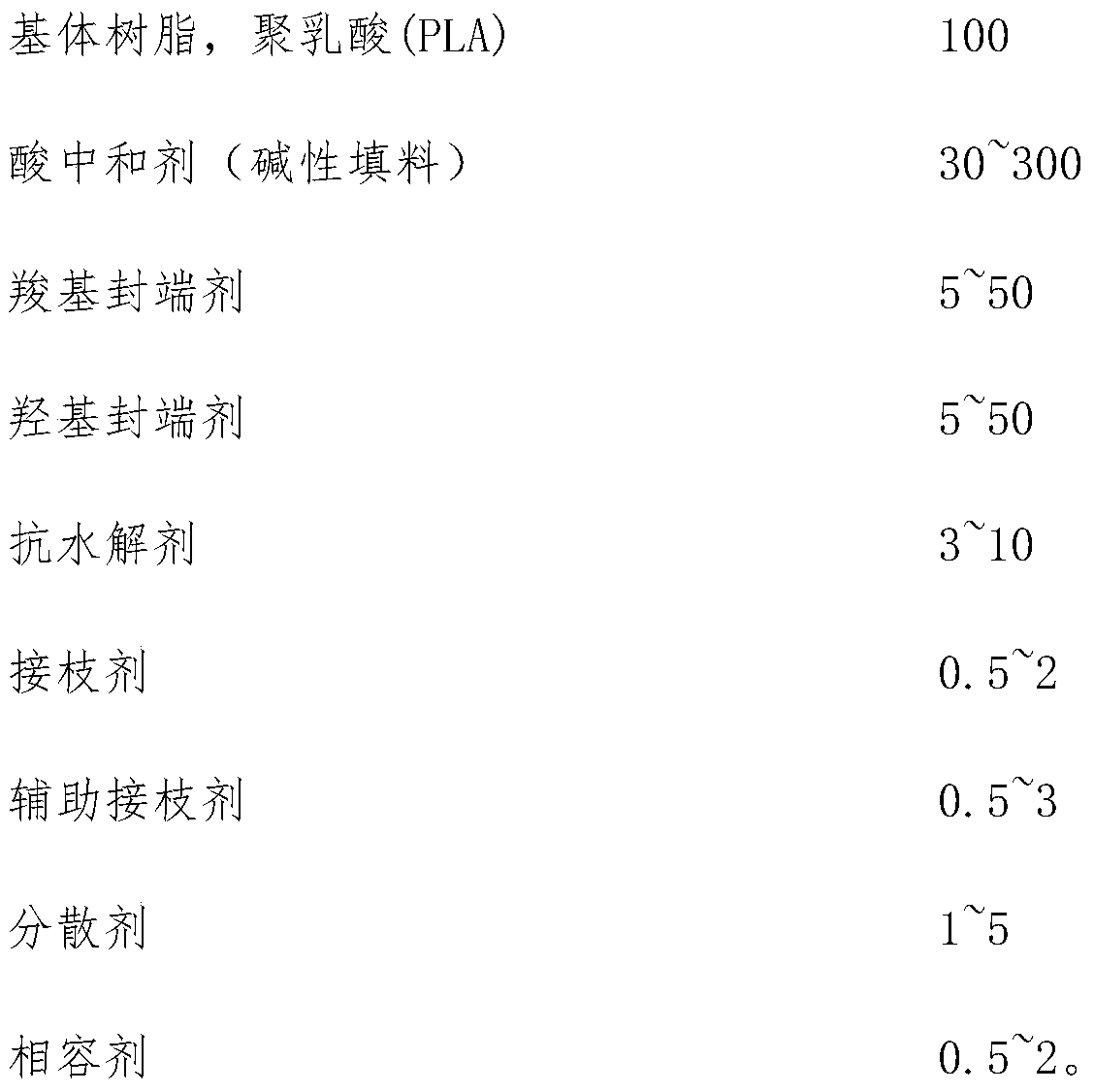
The invention relates to a master batch for improving durability of polyglycolide (PGA). The master batch is prepared from the following components in parts by mass: 100 parts of matrix resin, 30-300parts of an acid neutralizer, 5-50 parts of a carboxyl end-capping reagent, 5-50 parts of a hydroxyl end-capping reagent, 3-10 parts of an anti-hydrolysis agent, 0.5-2 parts of a grafting agent, 0.5-3parts of an auxiliary grafting agent, 1-5 parts of a dispersing agent and 0.5-2 parts of a compatilizer. The master batch for improving the durability of polyglycollide has the characteristics of convenience in processing and use and small addition amount, can be mixed with polyglycollide particles for injection molding or extrusion processing, and has an excellent effect in improving the durability and shelf life of a polyglycollide product in a room-temperature environment.
Owner:JIANGSU JINJU ALLOY MATERIAL
Production method of silicon dioxide aerogel functional material
InactiveCN109851380AHigh compressive strengthImprove physical crosslink strengthCeramicwareLayered structureIon
The invention relates to a production method of a silicon dioxide aerogel functional material, and belongs to the technical field of aerogel. According to the production method of the silicon dioxideaerogel functional material, through carbonization treatment, the silicon dioxide aerogel functional material is produced, not only can an original nano-porous network structure be maintained, but also the silicon dioxide aerogel functional material has a plurality of excellent property such as a high specific surface area, low mass density and nanoscale continuous pores, and the mechanical performance is good; and montmorillonoid is a two-dimensional molecular-sieve-like porous substance, due to the fact that the montmorillonoid has performance of a large specific surface area, large adsorption capability and the like, the montmorillonoid is applied to a wide variety of aspects of adsorption, hydrogen storage, catalysis, bioseparation, electron devices, chromosorb supports and the like, through carbonization treatment, the swelling capability of a layered structure and exchangeable performance of positive ions of the montmorillonoid are used for inserting the inorganic positive ions into layers of the montmorillonoid, and the layers of the montmorillonoid are distracted to form two-dimensional pore channels with openings, so that the adsorption capability and the mechanical performance of the silicon dioxide aerogel functional material are improved.
Owner:谢吉萍
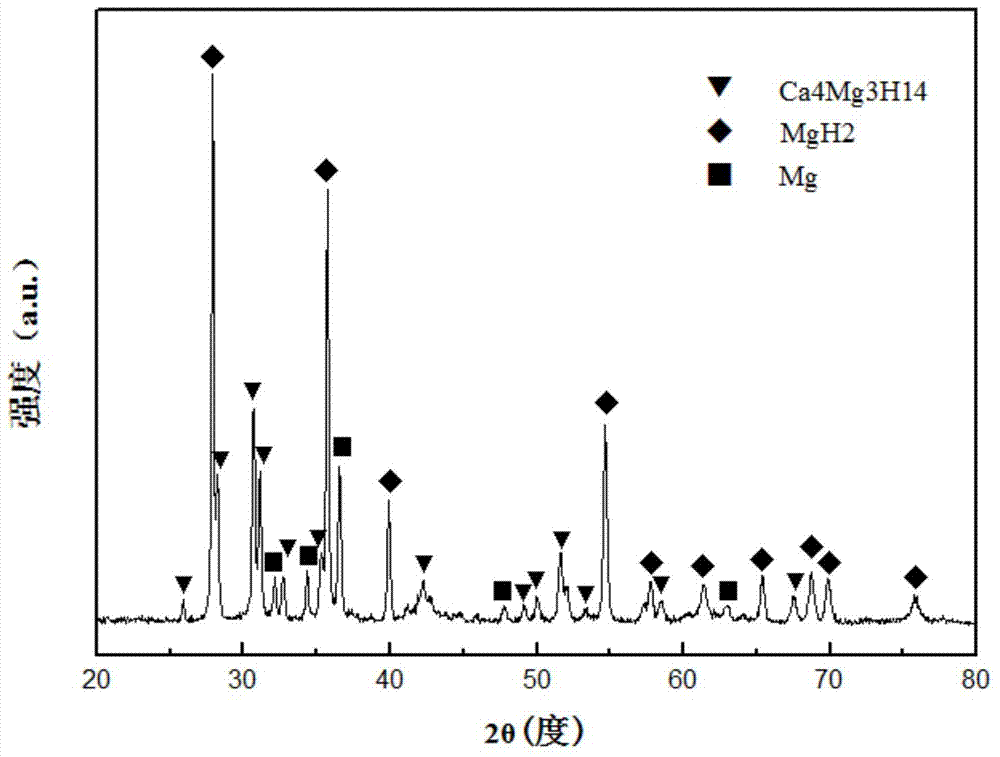
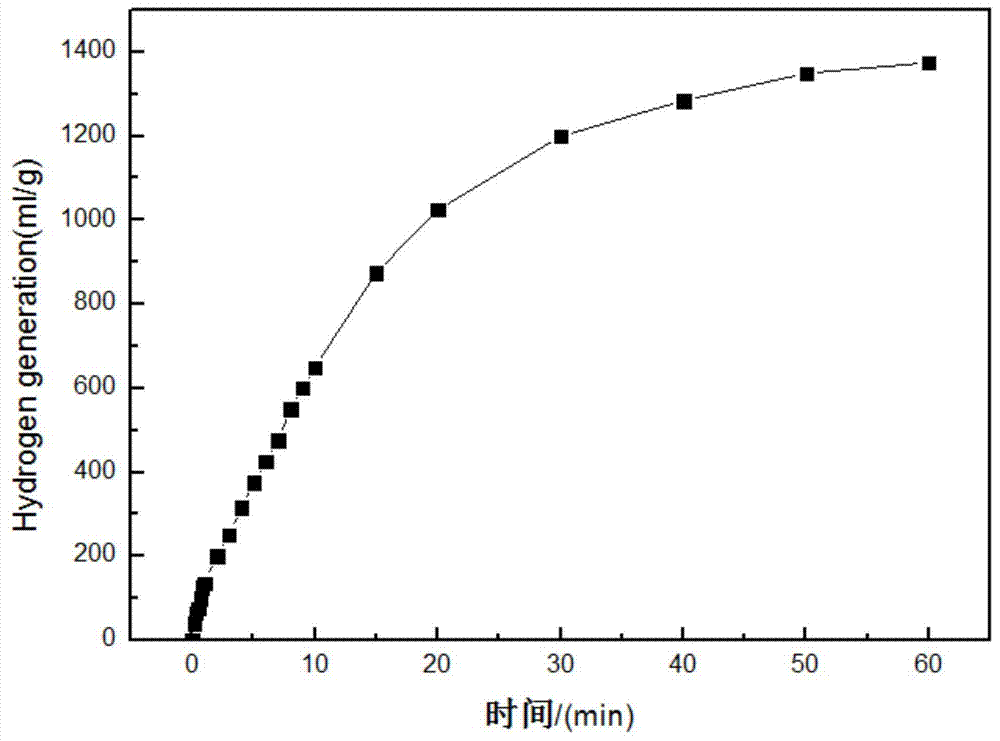
Magnesium-calcium-based hydride powder for wide-temperature zone hydrolysis hydrogen generation and preparation method for magnesium-calcium-based hydride powder
ActiveCN103787273AAvoid formingQuick releaseAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen productionGeneration rateRoom temperature
The invention discloses magnesium-calcium-based hydride powder for hydrolysis hydrogen generation. The magnesium-calcium-based hydride powder comprises the following components in percentage by mass: 14.2 to 50.2 percent of Ca4Mg3H14, 34.8 to 85.7 percent of MgH2 and 0.1 to 15 percent of Mg. In order to increase the hydrolysis hydrogen generation rate, other hydrides and / or salts can also be compounded, and the mass content of the other hydrides is 0 to 5 percent based on the total mass of the components Ca4Mg3H14, MgH2 and Mg; the mass content of the salts is 0 to 11 percent based on the total mass of the components Ca4Mg3H14, MgH2 and Mg; the content of the other hydrides and the content of the salts is not 0 simultaneously. According to the preparation method for the magnesium-calcium-based hydride powder, a magnesium-calcium alloy is activated for hydrogen absorption after being crushed and ball-milled. According to the magnesium-calcium-based hydride powder for the hydrolysis hydrogen generation, the hydrogen yield can be increased on the premise of ensuring the reaction safety and controllability, and particularly the hydrogen yield at room temperature and low temperature is increased.
Owner:SICHUAN UNIV
Method for preparing composite nickel coating on surface of magnesium alloy micro-arc oxidation film
InactiveCN103695905AInhibition of polymerizationImprove overall protection performance and bonding performanceSuperimposed coating processMicro arc oxidationNickel coating
The invention discloses a method for preparing a composite nickel coating on the surface of a magnesium alloy micro-arc oxidation film, and belongs to the technical field of metal surface treatment. The method comprises the following steps: putting a magnesium alloy matrix in a silicate series electrolyte to prepare a micro-arc oxidation ceramic layer, carrying out surface hole sealing and pre-nickeling treatment of the matrix ceramic layer by adopting a nickel salt-containing self-assembled nano-penetrant and an alcoholic solution of sodium borohydride, and carrying out composite chemical nickeling treatment to form the composite nickel coating on the surface of the film. The method is an improvement on tradition chemical nickeling technologies, and adopts a palladium salt-free activation process at room temperature, so the composite chemical nickeling operation is simplified, and the pollution of the process to the environment is reduced. The prepared composite coating has the advantages of good protection thickness, good bonding force with the substrate, good corrosion resistance, high hardness, good surface brightness, shininess, effective solving of the protection requirements on the high wear resistance and the corrosion resistance of a magnesium alloy, and provision of a powerful guarantee for the further application of the magnesium alloy.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Method for performing catalytic stereoselective separation on 2,3-diphenylpropionic acid enantiomer by adopting bio-enzyme
The invention discloses a new chiral separation method of a 2,3-diphenylpropionic acid single enantiomer, i.e., a method for synthesizing the 2,3-diphenylpropionic acid single enantiomer by performing enzyme-catalyzed enantioselective hydrolysis on a 2,3-diphenylpropionic acid enantiomer. 2,3-diphenylpropionate enantiomer is hydrolyzed by utilizing high selectivity and high catalysis efficiency of Candida antarctica lipase A and a solubilization effect of cyclodextrin on a 2,3-diphenylpropionate enantiomer is utilized, so that hydrolysis reaction of the 2,3-diphenylpropionate enantiomer in a phosphate buffering solution is enhanced; the conversion rate of a substrate and the optical purity of a product are respectively up to 44.79 percent and 98.24 percent, and the stereoselectivity E is greater than 276. According to the method disclosed by the invention, the problems of low optical purity, low yield, environment pollution and the like in a general separation technique are overcome; by adopting the method, the high conversion rate and the high selectivity required by hydrolysis of the 2,3-diphenylpropionate enantiomer can be realized, so that the aims of no-toxicity and no-harmlessness of a separation chiral compound, mild reaction conditions, simpleness of equipment, convenience in operation, low cost and the like are fulfilled.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing formic acid
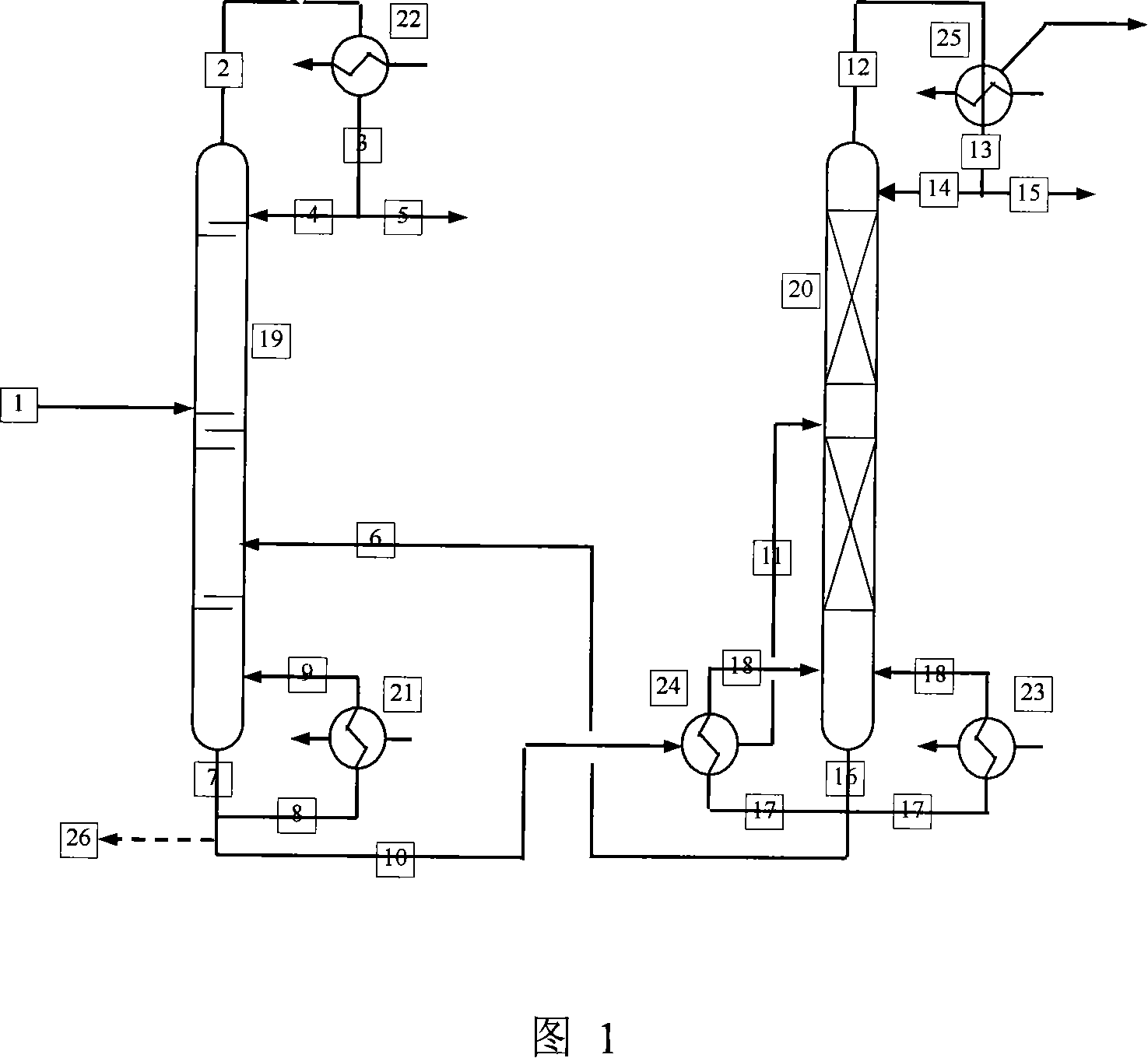
ActiveCN101125795AControl incidenceFull recoveryOxygen-containing compound preparationOrganic compound preparationDecreased energyFormate Esters
The invention discloses a preparation method of methanoic acid, main steps of which are that: (1) compound containing water, methanoic acid, methanol and methyl formate is produced by hydrolyzing methyl formate; (2) methanol, water and methyl formate are separated from the compound containing water, methanoic acid, methanol and methyl formate by pressurizing and distilling, and then methanoic acid containing water is taken out of tower bottom. The method for preparing methanoic acid can effectively prevent reversing esterification, increase hydrolyzing reaction, reduce recycle ratio of materiel, improve manufacturing ability of hydrolyzing system equipment and greatly decrease energy consumption.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Preparation method of magnesium alloy surface micro-arc oxidized nano self-assembling composite protective coating
InactiveCN103668393AMeet the protection requirementsWide variety of sourcesAnodisationPretreated surfacesWater basedMicro arc oxidation
The invention discloses a preparation method of a magnesium alloy surface micro-arc oxidized nano self-assembling composite protective coating, and belongs to the technical field of magnesium alloy surface treatment. The method comprises the following steps: firstly performing micro-arc oxidation on a magnesium alloy matrix surface to form a porous ceramic layer, spraying modified water-based paint on the porous ceramic layer surface to form a water-based coating so as to form a composite protective coating composed of the porous ceramic layer and the water-based coating on the magnesium alloy matrix surface. The multilayer composite protective coating formed on the magnesium alloy surface has the features of good binding force, good compactness, high hardness, high corrosion resistance, high wear resistance and the like; the preparation method is simple, convenient and environment-friendly; the composite protective coating is an expected composite protective coating of the magnesium alloy application, and the protective requirement on the magnesium alloy product in severe environment is satisfied.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Ordered mesoporous silica material with pore wall being rich in micropore structures and preparation thereof
The invention relates to an ordered mesoporous silica material with a pore wall being rich in micropore structures. The material is formed by sequentially dissolving a triblock copolymer nonionic surfactant, organic carboxylic acid and a silicon source in anhydrous ethanol containing a small amount of deionized water, and then sequentially performing solvent thermal treatment, solvent removal andcalcination treatment. The ordered mesoporous silica material has a highly orderly two-dimensional hexagonal mesoporous structure, high porosity, large specific surface area and uniform and adjustablemesoporous aperture, contains a large quantity of micropores, with adjustable contents, in the mesoporous wall, and shows a broader application prospect compared with the traditional microporous materials and mesoporous material in the fields of adsorption, separation and catalysis.
Owner:TAIYUAN UNIV OF TECH
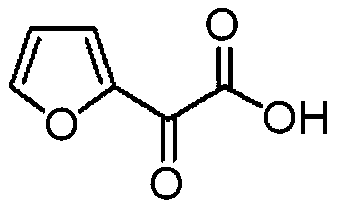
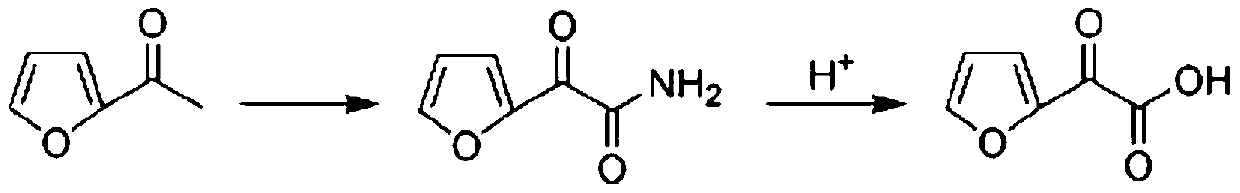
Preparation method of furanone acid
ActiveCN110003151APromote hydrolysis reactionEnhanced leaving activityOrganic chemistryChemical industryFuran
The invention belongs to the technical field of medical chemical industry, and particularly relates to a preparation method of furanone acid. The method comprises the following steps of: adding dropwisely a sodium nitrite aqueous solution into a concentrated sulfuric acid and long-chain alcohol aqueous solution, separating an ester layer to obtain nitrite after the dropwise addition is finished; dissolving 2-acetyl furan in a dilute hydrochloric acid solution, adding concentrated sulfuric acid and a catalyst, controlling temperature, adding dropwisely the nitrite, continuously stirring to react after the dropwise addition is finished, adjusting pH after the reaction is finished, filtering to recover the catalyst, and adding an extracting agent into filtrate to extract long-chain alcohol generated by the reaction and unreacted acetyl furan, wherein a water phase is a furanone acid aqueous solution. By the adoption of the preparation method of the furanone acid, an adopted oxidant only needs to slightly exceed the amount of a substrate, so that generation of toxic gas nitrogen oxide is reduced compared with oxidation of sodium nitrite; and the reaction condition is mild, the use of the catalyst accelerates a hydrolysis process, the side reaction is reduced, the yield is improved and more than 82.5%, and a conversion rate of the 2-acetyl furan reaches more than 95%.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
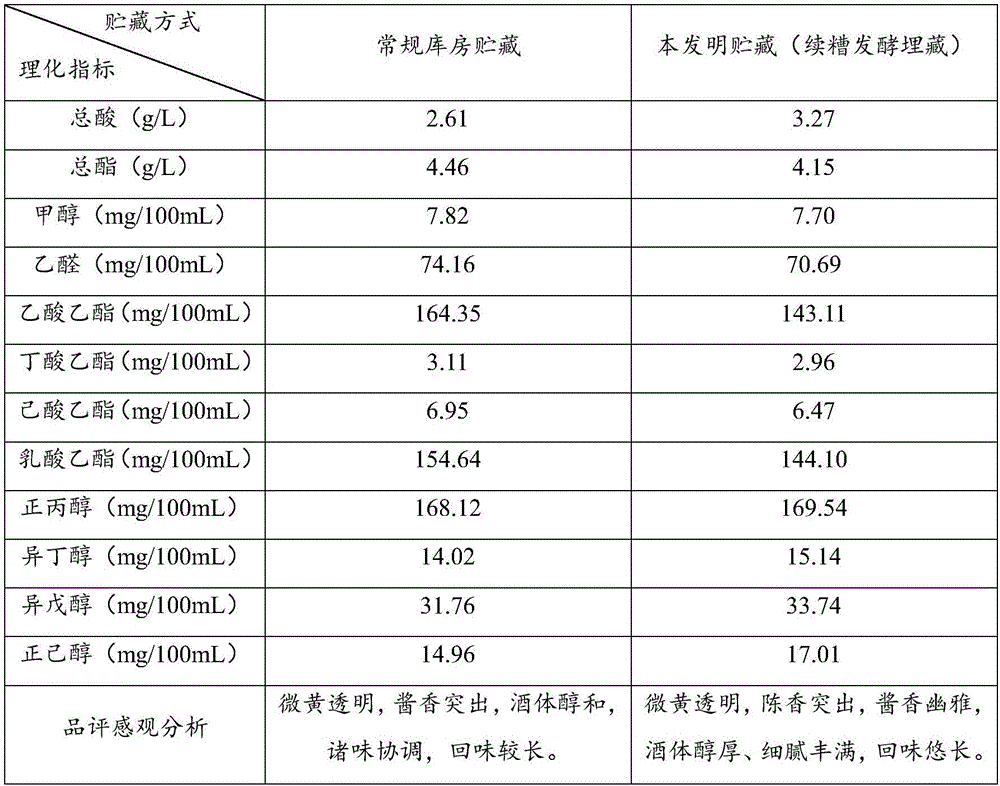
Maotai-flavor baijiu continuous mixed fermentation burying method
InactiveCN105695235ASimple operation processGood cellaring effectAlcoholic beverage preparationAdditive ingredientPurplish red
The invention belongs to the technical field of brewing and particularly relates to a Maotai-flavor baijiu continuous mixed fermentation burying method. The method includes steps: performing combinational primary blending of fresh baijiu; naturally ageing in a baijiu storehouse, performing secondary combination, blending and flavoring; proportionally mixing, and carrying out stacking fermentation for baijiu burying in pits; transferring blended finished baijiu into jars in the pits, covering the jars with lids, hermetically tying with kraft paper and waterproof cloth, and filling each jar with fermented grains subjected to stacking fermentation to a neck position; completely sealing the jars and the pits with unique purplish red mud in Maotai Town, and burying for two years; taking original baijiu from fermented grains in the pits as flavoring liquor, and adding into long-time stored baijiu according to different proportions, so that finished baijiu products are obtained. The defects of large ageing quality difference, long storage time and slow capital turnover of existing baijiu storage methods are overcome, aroma, flavor and medicinal ingredients of baijiu are increased, and baijiu quality is improved while healthcare value of baijiu is increased.
Owner:MOUTAI INST +2
Quantum nanocrystalline complex and preparation method thereof
InactiveCN108841387AGood coating effectImprove stabilityMaterial nanotechnologyNanoopticsChemistryCompound s
The invention provides a quantum nanocrystalline complex and a preparation method thereof. The method comprises the following steps: (1) preparing a quantum nanocrystalline solution; (2) adding a porous material into the quantum nanocrystalline solution in the step (1), and drying to obtain composite powder; (3) adding an oxide precursor solution into the composite powder obtained in the step (2),carrying out a hydrolysis reaction, and drying, thereby obtaining the quantum nanocrystalline complex. The preparation method provided by the invention realizes dual protection of the porous materialsubstrate and the oxide protective layer on the quantum nanocrystalline, and has high stability; moreover, the preparation method provided by the invention is simple in process, and no catalyst is added, that is, an excellent hydrolysis reaction can be carried out. The operation is convenient, and the prepared quantum nanocrystalline complex has excellent application prospects in the fields of illumination, display and the like.
Owner:SHENZHEN PLANCK INNOVATION TECH CO LTD +1
Aluminum alloy material capable of being used for online hydrogen supply
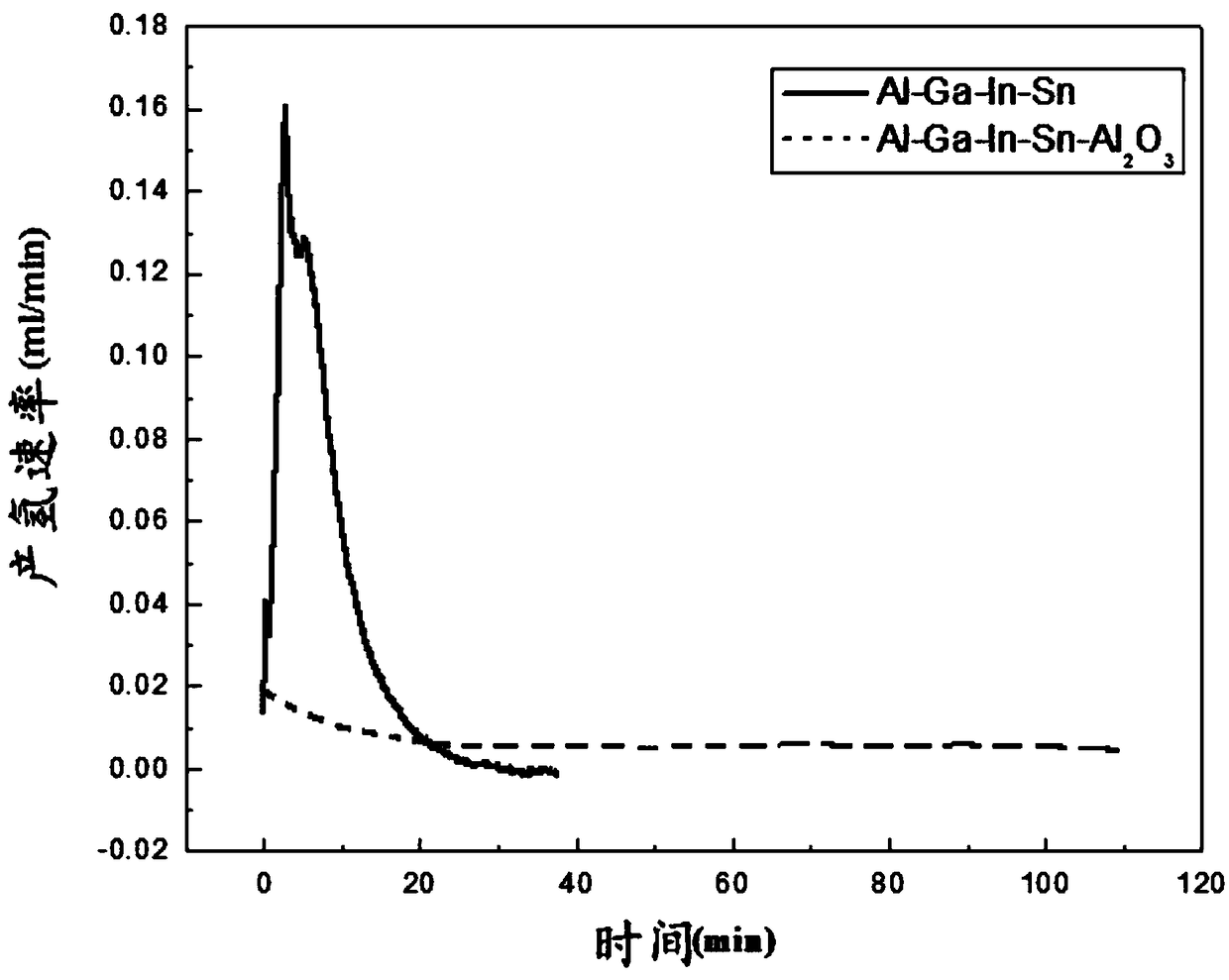
The invention discloses an aluminum alloy material capable of being used for online hydrogen supply. According to the aluminum alloy material, Al2O3 powder is introduced into an Al-Ga-In-Sn alloy capable of achieving hydrogen generation through hydrolysis to obtain an Al-Ga-In-Sn-Al2O3 alloy, wherein the content of the Al2O3 is not more than 8% by weight; and a fusion casting method is adopted forpreparation. Researches show that an Al2O3 doped aluminum alloy has the good hydrogen production performance, hydrogen can be generated immediately after the alloy is in contact with water, and a reaction does not have delaying time. The Al-Ga-In-Sn-Al2O3 alloy has the stable hydrogen production rate and can be used for online hydrogen supply through hydrolysis, and a stable hydrogen source is provided for proton exchange membrane fuel cells.
Owner:JILIN UNIV
Method for resourceful full-mass utilization of rape stalks
ActiveCN109136293AReduce dosagePromote hydrolysis reactionSugar derivativesBiofuelsWastewaterHydrolysis
The invention belongs to the field of xylose extracted by explosion and ethanol prepared by fermentation, and particularly relates to a method for resourceful full-mass utilization of rape stalks. Themethod comprises the following steps: pretreating raw materials at first; then preparing a compound fertilizer by screw extruded explosion, xylose extraction, impurity removal by hydrolysis, enzymolysis fermentation process and fermentation residues; and treating and reusing waste water. Resourceful full-mass utilization of the rape stalks is realized, the production cost for fermenting ethanol is reduced, and a production process is environmentally friendly.
Owner:SICHUAN GOLDEN ELEPHANT SINCERITY CHEM CO LTD
Method for preparing ACE (Angiotensin Converting Enzyme) inhibitory peptides through compound enzymatic hydrolysis of feta protein
InactiveCN103215331APromote generationStrong inhibitory activityFermentationNeutral proteaseSide effect
The invention provides a method for preparing ACE (Angiotensin Converting Enzyme) inhibitory peptides through compound enzymatic hydrolysis of feta protein. The method comprises the following steps of: centrifuging and degreasing recovered goat milk taken as a raw material, preparing casein by an isoelectric precipitation method, carrying out compound hydrolysis on the casein through neutral protease and trypsase under the conditions of specific temperature and pH, optimizing the hydrolysis condition, treating the hydrolytic process with ultrasound waves to obtain small-molecule ACE inhibitory peptides and determining the activity of the small-molecule ACE inhibitory peptides. The inhibitory proportion of an ACE inhibitory peptide product prepared by the method can reach above 80%; the ACE inhibitory peptide product has small molecular mass, can be absorbed by a human body in the form of complete peptide fragments and cannot cause the immunological rejection of the human body; and the ACE inhibitory peptide product has an obvious antihypertensive effect on hypertensive, has no effect on people with normal blood pressure and has the characteristics of little dosage, no toxic or side effect, mild property and strong physiological function.
Owner:SHAANXI UNIV OF SCI & TECH
Method for synthesizing mesoporous gamma-Al2O3 by anion/cation dual-hydrolysis
InactiveCN104229847AReduce usageGood hydrothermal stabilityAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationInorganic saltsFiltration
The invention relates to a method for synthesizing mesoporous gamma-Al2O3 by anion / cation dual-hydrolysis, which is characterized in that cheap and accessible inorganic salts Al(NO3)3 and NaAlO2 are used as aluminum sources to synthesize the mesoporous gamma-Al2O3 material by using Pluronic P123 as a structural guide agent and using dual-hydrolysis as the guiding ideology. The method comprises the following steps: dissolving P123 in distilled water; after the solution becomes clear and transparent, adding Al(NO3)3.9H2O solid powder, and dissolving by stirring; meanwhile, weighing a certain amount of NaAlO2, and dissolving in distilled water; while intensely stirring, dropwisely adding the NaAlO2 solution into the Al(NO3)3 solution; after finishing the addition, continuing the intense stirring for 4 hours; transferring the reaction gel into a stainless steel reaction kettle, and crystallizing at 60-120 DEG C for 1-4 days; and taking out, carrying out vacuum filtration, washing, drying to obtain a surfactant-alumina organic-inorganic composite, and roasting the composite in a muffle furnace for 2 hours to obtain the mesoporous alumina.
Owner:PETROCHINA CO LTD +1
Method used for preparing nano-microcrystalline cellulose via graphite oxide assistant-catalysis acid-hydrolysis
The invention discloses a method used for preparing nano-microcrystalline cellulose via graphite oxide assistant-catalysis acid-hydrolysis. The method comprises following steps: a sulfuric acid graphite oxide mixed solution with a certain concentration is prepared; microcrystalline cellulose powder is added slowly with stirring; after reaction, distilled water is added to stop reaction; centrifugation extraction is carried out; collected nano-microcrystalline cellulose colloid is subjected to dialysis until pH value is changed to be 7; and at last nano-microcrystalline cellulose powder is obtained via freeze drying. The main innovative points are that: nano-microcrystalline cellulose is obtained via graphite oxide assistant-catalysis acid-hydrolysis of microcrystalline cellulose; hydrolysis of neighboring carboxy groups with phenolic hydroxyl groups is promoted by the flexible and soft layered structures of graphite oxide and abundant carbonyl groups; yield of nano-microcrystalline cellulose is increased; and the method possesses important theoretical significance and is high in practical value.
Owner:SHANGHAI TONNOR MATERIAL SCI
Method of preparing nano-zirconia-doped tungsten oxide
ActiveCN106315679ASolve the problem of coarse grainEvenly distributedMaterial nanotechnologyTungsten oxides/hydroxidesFiltrationAmmonium metatungstate
The invention relates to a method of preparing nano-zirconia-doped tungsten oxide. The method includes the following steps that a distilled water solution of ammonium metatungstate is prepared, a concentrated nitric acid solution is added dropwise into the distilled water solution of ammonium metatungstate under the condition of continuous stirring, ammonium metatungstate, zirconium nitrate and urea are subjected to hydrothermal reaction according to the molar ratio of (0.25-2.8):(0.05-0.5):1, supernate prepared through the reaction is poured out, sediment is washed with distilled water until the pH value of the washing solutions of the two times is 6-8, the washing solutions of the two times are mixed and stirred for 2 h through a stirrer after being mixed uniformly, suction filtration, drying and calcining are carried out on the prepared mixed solution, and the product is prepared after air cooling to room temperature. The method is simple in process, and effectively solves the problem of large tungsten alloy grains.
Owner:HENAN UNIV OF SCI & TECH
Device and method for producing hydrogen from magnesium hydride
The invention discloses a device for producing hydrogen from magnesium hydride. The device comprises: a hydrolysis reaction tank; a feeding unit for conveying magnesium hydride into the hydrolysis reaction tank; and a reaction promoting solution conveying unit for conveying a reaction promoting solution into the hydrolysis reaction tank, wherein the reaction promoting solution is a mixed solutionof a MgCl 2 solution and a MgSO 4 solution, and the reaction promoting solution is fed into the hydrolysis reaction tank prior to the magnesium hydride. The invention also discloses a method for producing hydrogen from magnesium hydride. The method comprises the following steps: feeding the mixed solution of the MgCl2 solution and the MgSO4 solution into the hydrolysis reaction tank as the reaction promoting solution; feeding magnesium hydride into the hydrolysis reaction tank, and reacting the magnesium hydride with water in the hydrolysis reaction tank to form hydrogen; and continuously injecting the reaction promoting solution into the hydrolysis reaction tank during the reaction of the magnesium hydride raw material and water. The device and the method, which adopt the mixed solution of the MgCl2 solution and the MgSO4 solution as the reaction promoting solution to promote the progress of the hydrolysis reaction, greatly accelerate the hydrogen production by the hydrolysis reaction.
Owner:中国科学院嘉兴轻合金技术工程中心
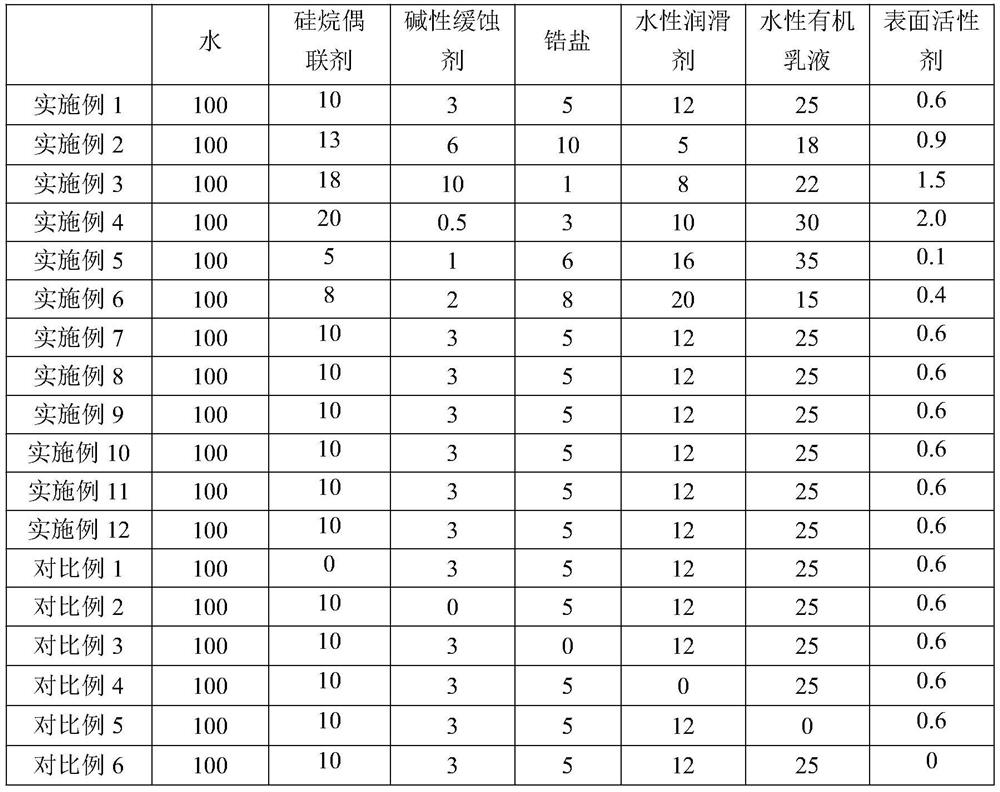
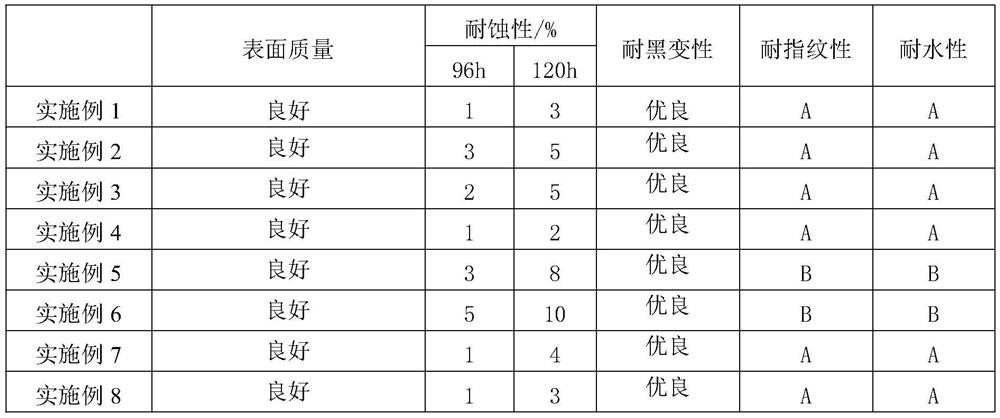
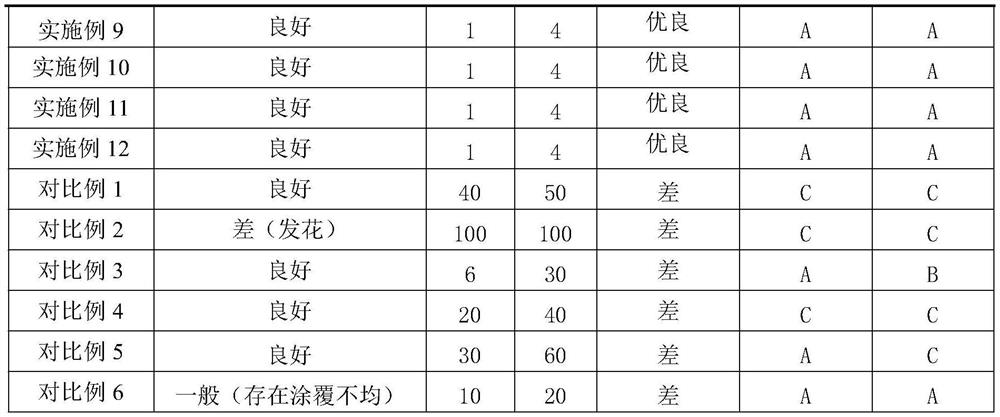
Surface treatment liquid for zinc-aluminum-magnesium alloy steel plate as well as preparation method and use method of surface treatment liquid
PendingCN114133848AGood self-lubricating performanceImprove compactnessHot-dipping/immersion processesAnti-corrosive paintsSteel platesActive agent
The invention belongs to the technical field of zinc-aluminum-magnesium alloy coated steel plates, and particularly relates to a zinc-aluminum-magnesium alloy coated steel plate surface treatment liquid and a preparation method and a use method thereof. The invention firstly provides a zinc-aluminum-magnesium alloy coated steel plate surface treatment liquid which comprises the following components in parts by weight based on 100 parts by weight of water: 5-20 parts by weight of silane coupling agent, 0.5-10 parts by weight of alkaline corrosion inhibitor, 1-10 parts by weight of zircon salt, 5-20 parts by weight of water-based lubricant, 15-35 parts by weight of water-based organic emulsion and 0.1-2.0 parts by weight of surfactant. The steel plate surface treatment liquid is suitable for a zinc-aluminum-magnesium steel plate with coating components of 0.5-6% of Al and 0.2-3.0% of Mg, an environment-friendly coating on the zinc-aluminum-magnesium surface can be compact and uniform, the coating is prevented from making contact with water vapor and oxygen in the environment, the corrosion resistance, blackening resistance, water resistance, fingerprint resistance, coatability and self-lubricating performance of the surface coating are remarkably improved, and the service life of the surface coating is prolonged. And the use requirements of stamping and coating of users are met.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com