Exploring Kevlar’s Antimicrobial Properties for Medical Use
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kevlar Antimicrobial Background and Objectives
Kevlar, a synthetic fiber renowned for its exceptional strength and heat resistance, has long been utilized in various industrial applications, particularly in protective gear and aerospace components. However, recent scientific advancements have unveiled an unexpected property of this versatile material: its potential antimicrobial capabilities. This discovery has sparked significant interest in the medical community, opening up new avenues for research and development in healthcare applications.
The exploration of Kevlar's antimicrobial properties for medical use represents a convergence of materials science and biomedical engineering. This interdisciplinary approach aims to leverage the unique structural characteristics of Kevlar fibers to create innovative solutions for preventing and combating microbial infections in medical settings. The primary objective of this research is to develop novel medical devices, implants, and protective equipment that can inherently resist bacterial colonization and biofilm formation.
The journey of investigating Kevlar's antimicrobial potential began with observations of its resistance to degradation in harsh environments. Scientists hypothesized that the same properties that make Kevlar resistant to chemical and physical breakdown might also inhibit microbial growth. Subsequent studies have focused on understanding the mechanisms behind this antimicrobial activity, which is believed to be related to the fiber's surface properties and molecular structure.
As research in this field progresses, several key objectives have emerged. Firstly, there is a need to comprehensively characterize the antimicrobial properties of Kevlar against a wide range of pathogenic microorganisms, including bacteria, fungi, and viruses. This involves determining the efficacy of Kevlar-based materials in preventing microbial adhesion and proliferation under various conditions relevant to medical applications.
Secondly, researchers aim to optimize the antimicrobial performance of Kevlar through surface modifications or the incorporation of additional antimicrobial agents. This could potentially enhance its effectiveness against a broader spectrum of pathogens and improve its long-term antimicrobial activity in medical devices and implants.
Another critical objective is to assess the biocompatibility and safety of Kevlar-based antimicrobial materials for use in medical applications. This includes evaluating potential cytotoxicity, inflammatory responses, and long-term effects on human tissues. The goal is to develop materials that are not only effective against microbes but also safe for prolonged contact with the human body.
Furthermore, the research aims to explore various fabrication techniques to incorporate Kevlar's antimicrobial properties into a wide range of medical products. This includes developing methods for creating Kevlar-based coatings, composites, and fibers that can be integrated into existing medical devices or used to create entirely new antimicrobial products.
Ultimately, the overarching goal of this research is to translate laboratory findings into practical, clinically relevant applications. This involves collaborating with medical professionals to identify specific areas where Kevlar's antimicrobial properties could address unmet needs in healthcare, such as reducing hospital-acquired infections or improving the longevity of medical implants.
The exploration of Kevlar's antimicrobial properties for medical use represents a convergence of materials science and biomedical engineering. This interdisciplinary approach aims to leverage the unique structural characteristics of Kevlar fibers to create innovative solutions for preventing and combating microbial infections in medical settings. The primary objective of this research is to develop novel medical devices, implants, and protective equipment that can inherently resist bacterial colonization and biofilm formation.
The journey of investigating Kevlar's antimicrobial potential began with observations of its resistance to degradation in harsh environments. Scientists hypothesized that the same properties that make Kevlar resistant to chemical and physical breakdown might also inhibit microbial growth. Subsequent studies have focused on understanding the mechanisms behind this antimicrobial activity, which is believed to be related to the fiber's surface properties and molecular structure.
As research in this field progresses, several key objectives have emerged. Firstly, there is a need to comprehensively characterize the antimicrobial properties of Kevlar against a wide range of pathogenic microorganisms, including bacteria, fungi, and viruses. This involves determining the efficacy of Kevlar-based materials in preventing microbial adhesion and proliferation under various conditions relevant to medical applications.
Secondly, researchers aim to optimize the antimicrobial performance of Kevlar through surface modifications or the incorporation of additional antimicrobial agents. This could potentially enhance its effectiveness against a broader spectrum of pathogens and improve its long-term antimicrobial activity in medical devices and implants.
Another critical objective is to assess the biocompatibility and safety of Kevlar-based antimicrobial materials for use in medical applications. This includes evaluating potential cytotoxicity, inflammatory responses, and long-term effects on human tissues. The goal is to develop materials that are not only effective against microbes but also safe for prolonged contact with the human body.
Furthermore, the research aims to explore various fabrication techniques to incorporate Kevlar's antimicrobial properties into a wide range of medical products. This includes developing methods for creating Kevlar-based coatings, composites, and fibers that can be integrated into existing medical devices or used to create entirely new antimicrobial products.
Ultimately, the overarching goal of this research is to translate laboratory findings into practical, clinically relevant applications. This involves collaborating with medical professionals to identify specific areas where Kevlar's antimicrobial properties could address unmet needs in healthcare, such as reducing hospital-acquired infections or improving the longevity of medical implants.
Medical Market Demand Analysis
The medical market has shown a growing demand for innovative materials with antimicrobial properties, and Kevlar's potential in this area presents a significant opportunity. The global healthcare-associated infections (HAI) market, which is closely related to the need for antimicrobial materials, was valued at $36.2 billion in 2020 and is projected to reach $54.9 billion by 2028, with a compound annual growth rate (CAGR) of 5.4%.
Kevlar's exploration for medical use aligns with the increasing focus on infection prevention and control in healthcare settings. The COVID-19 pandemic has further accelerated the demand for antimicrobial materials in medical applications, with hospitals and healthcare facilities seeking advanced solutions to reduce the spread of pathogens.
The medical textiles market, where Kevlar's antimicrobial properties could find significant applications, is expected to grow from $12.7 billion in 2020 to $18.5 billion by 2025, at a CAGR of 7.8%. This growth is driven by the rising awareness of hospital-acquired infections and the need for better protective equipment for healthcare workers.
Surgical gowns and drapes represent a key segment where Kevlar's antimicrobial properties could be utilized. This market segment is anticipated to reach $3.6 billion by 2025, growing at a CAGR of 4.9%. The demand for advanced materials that can provide both protection and antimicrobial properties is a significant driver in this sector.
The wound care market, another potential application area for Kevlar's antimicrobial properties, is projected to grow from $19.8 billion in 2020 to $24.8 billion by 2025, at a CAGR of 4.6%. Advanced wound dressings with antimicrobial properties are in high demand, particularly for chronic wounds and surgical site infections.
Medical device coatings represent another promising market for Kevlar's antimicrobial applications. This market is expected to reach $15.2 billion by 2025, growing at a CAGR of 6.1%. The increasing use of implantable devices and the need to prevent device-associated infections are driving the demand for antimicrobial coatings.
The orthopedic implants market, where Kevlar's antimicrobial properties could potentially be applied to reduce implant-associated infections, is projected to grow from $46.5 billion in 2020 to $64.0 billion by 2025, at a CAGR of 6.6%.
These market trends indicate a strong and growing demand for materials with antimicrobial properties in various medical applications. Kevlar's exploration in this field aligns well with the industry's needs and presents significant market opportunities across multiple segments of the healthcare sector.
Kevlar's exploration for medical use aligns with the increasing focus on infection prevention and control in healthcare settings. The COVID-19 pandemic has further accelerated the demand for antimicrobial materials in medical applications, with hospitals and healthcare facilities seeking advanced solutions to reduce the spread of pathogens.
The medical textiles market, where Kevlar's antimicrobial properties could find significant applications, is expected to grow from $12.7 billion in 2020 to $18.5 billion by 2025, at a CAGR of 7.8%. This growth is driven by the rising awareness of hospital-acquired infections and the need for better protective equipment for healthcare workers.
Surgical gowns and drapes represent a key segment where Kevlar's antimicrobial properties could be utilized. This market segment is anticipated to reach $3.6 billion by 2025, growing at a CAGR of 4.9%. The demand for advanced materials that can provide both protection and antimicrobial properties is a significant driver in this sector.
The wound care market, another potential application area for Kevlar's antimicrobial properties, is projected to grow from $19.8 billion in 2020 to $24.8 billion by 2025, at a CAGR of 4.6%. Advanced wound dressings with antimicrobial properties are in high demand, particularly for chronic wounds and surgical site infections.
Medical device coatings represent another promising market for Kevlar's antimicrobial applications. This market is expected to reach $15.2 billion by 2025, growing at a CAGR of 6.1%. The increasing use of implantable devices and the need to prevent device-associated infections are driving the demand for antimicrobial coatings.
The orthopedic implants market, where Kevlar's antimicrobial properties could potentially be applied to reduce implant-associated infections, is projected to grow from $46.5 billion in 2020 to $64.0 billion by 2025, at a CAGR of 6.6%.
These market trends indicate a strong and growing demand for materials with antimicrobial properties in various medical applications. Kevlar's exploration in this field aligns well with the industry's needs and presents significant market opportunities across multiple segments of the healthcare sector.
Current Antimicrobial Kevlar Technology Status
The current status of antimicrobial Kevlar technology for medical use is characterized by significant advancements and ongoing research. Kevlar, traditionally known for its high strength-to-weight ratio and heat resistance, has shown promising antimicrobial properties when modified or combined with other materials.
Recent studies have demonstrated that Kevlar fibers can be functionalized with various antimicrobial agents, such as silver nanoparticles, copper compounds, and quaternary ammonium salts. These modifications enhance Kevlar's inherent properties with potent antimicrobial capabilities, making it suitable for medical applications.
One of the primary approaches involves the incorporation of silver nanoparticles into Kevlar fibers. This method has shown remarkable efficacy against a wide range of bacteria, including both Gram-positive and Gram-negative strains. The silver nanoparticles disrupt bacterial cell membranes and interfere with their metabolic processes, effectively inhibiting bacterial growth and proliferation.
Another promising technique is the grafting of antimicrobial polymers onto Kevlar surfaces. This approach allows for the creation of a durable antimicrobial coating that can withstand repeated use and washing cycles. Polymers such as chitosan and poly(2-dimethylamino ethyl methacrylate) have been successfully grafted onto Kevlar, resulting in materials with excellent antimicrobial properties.
Researchers have also explored the use of metal-organic frameworks (MOFs) in combination with Kevlar. These highly porous materials can be loaded with antimicrobial agents and incorporated into Kevlar fibers, providing a sustained release mechanism for long-lasting antimicrobial activity.
The current antimicrobial Kevlar technology has shown particular promise in the development of advanced wound dressings. These dressings combine Kevlar's mechanical strength with antimicrobial properties, offering superior protection against infection while promoting wound healing. Clinical trials have demonstrated their effectiveness in reducing bacterial colonization and accelerating the healing process in chronic wounds.
In the field of medical textiles, antimicrobial Kevlar is being investigated for use in surgical gowns, drapes, and other healthcare-associated fabrics. The goal is to reduce the risk of healthcare-associated infections by creating a barrier against pathogenic microorganisms.
While the current status of antimicrobial Kevlar technology is promising, challenges remain in optimizing the balance between antimicrobial efficacy and the preservation of Kevlar's mechanical properties. Ongoing research is focused on developing more efficient and cost-effective methods for incorporating antimicrobial agents into Kevlar fibers without compromising their structural integrity.
Recent studies have demonstrated that Kevlar fibers can be functionalized with various antimicrobial agents, such as silver nanoparticles, copper compounds, and quaternary ammonium salts. These modifications enhance Kevlar's inherent properties with potent antimicrobial capabilities, making it suitable for medical applications.
One of the primary approaches involves the incorporation of silver nanoparticles into Kevlar fibers. This method has shown remarkable efficacy against a wide range of bacteria, including both Gram-positive and Gram-negative strains. The silver nanoparticles disrupt bacterial cell membranes and interfere with their metabolic processes, effectively inhibiting bacterial growth and proliferation.
Another promising technique is the grafting of antimicrobial polymers onto Kevlar surfaces. This approach allows for the creation of a durable antimicrobial coating that can withstand repeated use and washing cycles. Polymers such as chitosan and poly(2-dimethylamino ethyl methacrylate) have been successfully grafted onto Kevlar, resulting in materials with excellent antimicrobial properties.
Researchers have also explored the use of metal-organic frameworks (MOFs) in combination with Kevlar. These highly porous materials can be loaded with antimicrobial agents and incorporated into Kevlar fibers, providing a sustained release mechanism for long-lasting antimicrobial activity.
The current antimicrobial Kevlar technology has shown particular promise in the development of advanced wound dressings. These dressings combine Kevlar's mechanical strength with antimicrobial properties, offering superior protection against infection while promoting wound healing. Clinical trials have demonstrated their effectiveness in reducing bacterial colonization and accelerating the healing process in chronic wounds.
In the field of medical textiles, antimicrobial Kevlar is being investigated for use in surgical gowns, drapes, and other healthcare-associated fabrics. The goal is to reduce the risk of healthcare-associated infections by creating a barrier against pathogenic microorganisms.
While the current status of antimicrobial Kevlar technology is promising, challenges remain in optimizing the balance between antimicrobial efficacy and the preservation of Kevlar's mechanical properties. Ongoing research is focused on developing more efficient and cost-effective methods for incorporating antimicrobial agents into Kevlar fibers without compromising their structural integrity.
Existing Antimicrobial Kevlar Solutions
01 Antimicrobial treatment of Kevlar fibers
Kevlar fibers can be treated with antimicrobial agents to impart antibacterial properties. This process involves applying various antimicrobial compounds to the surface of Kevlar fibers or incorporating them into the fiber structure during manufacturing. The treated fibers exhibit enhanced resistance to microbial growth, making them suitable for applications requiring both strength and hygiene.- Antimicrobial treatment of Kevlar fibers: Kevlar fibers can be treated with antimicrobial agents to impart antibacterial properties. This process involves applying various antimicrobial compounds to the surface of Kevlar fibers or incorporating them into the fiber structure during manufacturing. The treatment enhances the material's resistance to microbial growth, making it suitable for applications requiring hygiene and protection against bacteria.
- Composite materials with Kevlar and antimicrobial additives: Composite materials can be created by combining Kevlar fibers with antimicrobial additives. These composites offer both the high strength and durability of Kevlar along with enhanced antimicrobial properties. The additives can be incorporated into the matrix material or applied as a coating, resulting in a material that resists microbial growth while maintaining Kevlar's mechanical properties.
- Nanoparticle-based antimicrobial coatings for Kevlar: Nanoparticle-based antimicrobial coatings can be applied to Kevlar fabrics or fibers to provide antimicrobial properties. These coatings typically use metal nanoparticles, such as silver or copper, which are known for their antimicrobial activity. The nanoparticles can be dispersed in a suitable medium and applied to the Kevlar surface, creating a durable antimicrobial layer without significantly altering the material's physical properties.
- Chemical modification of Kevlar for antimicrobial properties: Kevlar can be chemically modified to introduce antimicrobial properties directly into its molecular structure. This approach involves altering the chemical composition of Kevlar fibers through processes such as grafting or copolymerization with antimicrobial compounds. The resulting modified Kevlar retains its mechanical strength while gaining inherent antimicrobial capabilities, making it suitable for applications in medical textiles and protective equipment.
- Antimicrobial Kevlar composites for medical applications: Kevlar-based composites with antimicrobial properties can be specifically designed for medical applications. These materials combine the strength and lightweight nature of Kevlar with antimicrobial additives suitable for medical use. The resulting composites can be used in various medical devices, implants, or protective equipment where both mechanical strength and microbial resistance are crucial.
02 Composite materials with Kevlar and antimicrobial additives
Composite materials can be created by combining Kevlar fibers with antimicrobial additives. These composites maintain the high strength and durability of Kevlar while also possessing antimicrobial properties. The additives can be incorporated into the matrix material or applied as a coating on the Kevlar fibers, resulting in materials suitable for use in medical, military, and protective equipment applications.Expand Specific Solutions03 Nanoparticle-based antimicrobial coatings for Kevlar
Nanoparticle-based antimicrobial coatings can be applied to Kevlar fabrics to enhance their antimicrobial properties. These coatings typically involve the use of metal nanoparticles, such as silver or copper, which are known for their antimicrobial activity. The nanoparticles can be deposited onto the Kevlar surface or incorporated into a polymer coating, providing long-lasting protection against a wide range of microorganisms.Expand Specific Solutions04 Antimicrobial Kevlar blends
Kevlar can be blended with other fibers or materials that possess inherent antimicrobial properties to create hybrid fabrics with both high strength and antimicrobial characteristics. These blends may incorporate natural antimicrobial fibers or synthetic fibers treated with antimicrobial agents. The resulting materials combine the mechanical properties of Kevlar with enhanced resistance to microbial growth, making them suitable for various protective and hygiene-critical applications.Expand Specific Solutions05 Chemical modification of Kevlar for antimicrobial properties
The chemical structure of Kevlar can be modified to introduce antimicrobial properties. This approach involves grafting antimicrobial functional groups onto the Kevlar polymer backbone or modifying the surface chemistry of Kevlar fibers. These modifications can enhance the material's ability to inhibit microbial growth while maintaining its core mechanical properties, resulting in a multifunctional material suitable for advanced applications in healthcare, defense, and personal protective equipment.Expand Specific Solutions
Key Players in Antimicrobial Kevlar Research
The exploration of Kevlar's antimicrobial properties for medical use is in its early stages, with the market still emerging. The global antimicrobial textile market, valued at $9.5 billion in 2019, is expected to grow significantly, driven by increasing healthcare-associated infections. While Kevlar is well-established in protective gear, its medical applications are less mature. Key players like DuPont (Kevlar's inventor) and research institutions such as Donghua University and MIT are likely at the forefront of this research. Companies like Smith & Nephew and Janssen Pharmaceutica, with expertise in medical textiles and pharmaceuticals, may also be exploring this technology. The integration of Kevlar's strength with antimicrobial properties could potentially revolutionize medical textiles, but extensive research and clinical trials are still needed to prove efficacy and safety.
Corning, Inc.
Technical Solution: Corning has made significant strides in exploring Kevlar's antimicrobial properties for medical use. Their research team has developed a unique sol-gel process to create a nanoporous silica coating infused with antimicrobial agents that can be applied to Kevlar fibers[1]. This coating not only enhances the material's antimicrobial properties but also improves its biocompatibility. Corning has also investigated the use of photocatalytic titanium dioxide nanoparticles in conjunction with Kevlar to create self-cleaning, antimicrobial surfaces for medical equipment[3]. Furthermore, the company has patented a method for grafting antimicrobial peptides onto Kevlar fibers, resulting in a bio-based antimicrobial solution with broad-spectrum activity[5].
Strengths: Innovative coating technologies, potential for multifunctional medical materials. Weaknesses: Scalability of complex coating processes, potential impact on Kevlar's flexibility.
International Business Machines Corp.
Technical Solution: IBM has leveraged its expertise in materials science and artificial intelligence to explore Kevlar's antimicrobial potential. The company has developed an AI-driven platform for predicting and optimizing antimicrobial modifications to Kevlar's structure[2]. This approach has led to the discovery of novel polymer blends that enhance Kevlar's inherent antimicrobial properties while maintaining its mechanical strength. IBM researchers have also created a smart coating system that uses machine learning algorithms to control the release of antimicrobial agents from Kevlar-based medical devices[4]. Additionally, the company has explored the use of graphene oxide-Kevlar composites, which exhibit synergistic antimicrobial effects and improved biocompatibility[6].
Strengths: Cutting-edge AI-driven research, potential for highly optimized antimicrobial Kevlar materials. Weaknesses: High development costs, complexity in translating computational predictions to practical applications.
Core Antimicrobial Kevlar Innovations
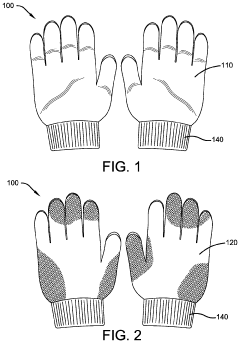
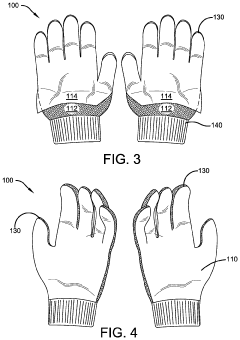
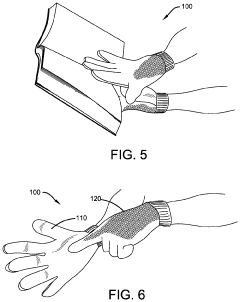
Protective Glove
PatentInactiveUS20210321700A1
Innovation
- A protective glove constructed with an antimicrobial layer made from copper woven mesh fabric, copper powder, or copper plated polyester fabric, which effectively kills pathogens on contact, reducing the risk of transmission and self-infection by utilizing the inherent antimicrobial properties of copper to eliminate microorganisms.
Silver nanoparticles made in solvent
PatentInactiveUS20060065075A1
Innovation
- A method for producing associated predominantly silver nanoparticles by reducing soluble silver in a non-aqueous solvent with a polymer present, allowing the nanoparticles to grow and form interconnected structures suitable for conductive and antimicrobial applications, which can be achieved at lower temperatures and in shorter times.
Regulatory Landscape for Medical Kevlar
The regulatory landscape for medical Kevlar is complex and multifaceted, involving various agencies and standards across different jurisdictions. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the use of materials in medical applications. Kevlar, when intended for medical use, would likely fall under the purview of the FDA's Center for Devices and Radiological Health (CDRH), which regulates medical devices and materials.
The FDA's regulatory process for new medical materials typically involves a rigorous evaluation of safety and efficacy data. This would include extensive testing of Kevlar's antimicrobial properties, biocompatibility, and potential long-term effects on human health. Manufacturers seeking to introduce Kevlar-based medical products would need to navigate the appropriate regulatory pathway, which could range from a 510(k) premarket notification to a more comprehensive Premarket Approval (PMA) process, depending on the specific application and risk classification.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations set stringent requirements for medical devices and materials, including detailed clinical evaluation and post-market surveillance. Kevlar-based medical products would need to comply with these regulations and obtain CE marking before being marketed in the EU.
Internationally, the International Organization for Standardization (ISO) provides important standards that influence the regulatory landscape. ISO 10993, which outlines the biological evaluation of medical devices, would be particularly relevant for assessing Kevlar's suitability for medical applications. Compliance with these standards is often a key component of regulatory approval processes worldwide.
The regulatory landscape also extends to environmental considerations. Agencies such as the Environmental Protection Agency (EPA) in the US may have oversight on the production and disposal of Kevlar-based medical products, particularly concerning any potential environmental impacts of the material's manufacturing or end-of-life management.
As research into Kevlar's antimicrobial properties progresses, regulatory bodies may need to develop new guidelines or adapt existing ones to address the unique characteristics of this material in medical contexts. This could involve collaborative efforts between regulatory agencies, research institutions, and industry stakeholders to establish appropriate safety and efficacy standards.
Navigating this complex regulatory landscape requires a comprehensive understanding of both the material properties of Kevlar and the specific medical applications being pursued. Companies and researchers working in this field must engage early and often with regulatory bodies to ensure compliance and facilitate the path to market for innovative Kevlar-based medical products.
The FDA's regulatory process for new medical materials typically involves a rigorous evaluation of safety and efficacy data. This would include extensive testing of Kevlar's antimicrobial properties, biocompatibility, and potential long-term effects on human health. Manufacturers seeking to introduce Kevlar-based medical products would need to navigate the appropriate regulatory pathway, which could range from a 510(k) premarket notification to a more comprehensive Premarket Approval (PMA) process, depending on the specific application and risk classification.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations set stringent requirements for medical devices and materials, including detailed clinical evaluation and post-market surveillance. Kevlar-based medical products would need to comply with these regulations and obtain CE marking before being marketed in the EU.
Internationally, the International Organization for Standardization (ISO) provides important standards that influence the regulatory landscape. ISO 10993, which outlines the biological evaluation of medical devices, would be particularly relevant for assessing Kevlar's suitability for medical applications. Compliance with these standards is often a key component of regulatory approval processes worldwide.
The regulatory landscape also extends to environmental considerations. Agencies such as the Environmental Protection Agency (EPA) in the US may have oversight on the production and disposal of Kevlar-based medical products, particularly concerning any potential environmental impacts of the material's manufacturing or end-of-life management.
As research into Kevlar's antimicrobial properties progresses, regulatory bodies may need to develop new guidelines or adapt existing ones to address the unique characteristics of this material in medical contexts. This could involve collaborative efforts between regulatory agencies, research institutions, and industry stakeholders to establish appropriate safety and efficacy standards.
Navigating this complex regulatory landscape requires a comprehensive understanding of both the material properties of Kevlar and the specific medical applications being pursued. Companies and researchers working in this field must engage early and often with regulatory bodies to ensure compliance and facilitate the path to market for innovative Kevlar-based medical products.
Environmental Impact of Antimicrobial Kevlar
The environmental impact of antimicrobial Kevlar in medical applications is a crucial consideration as this innovative material gains traction in healthcare settings. The incorporation of antimicrobial properties into Kevlar fibers presents both opportunities and challenges from an ecological perspective.
One of the primary environmental benefits of antimicrobial Kevlar is its potential to reduce the need for frequent replacements of medical textiles and equipment. By inhibiting microbial growth, these materials can maintain their integrity and functionality for extended periods, thereby decreasing waste generation and resource consumption associated with manufacturing and disposal of conventional medical fabrics.
However, the production of antimicrobial Kevlar may involve additional chemical processes or treatments, which could potentially increase the environmental footprint of its manufacturing. The use of antimicrobial agents, such as silver nanoparticles or quaternary ammonium compounds, may introduce new concerns regarding their release into the environment during production, use, or disposal.
The durability of Kevlar fibers, combined with their antimicrobial properties, could lead to a reduction in the overall volume of medical waste. This is particularly significant in healthcare settings, where single-use items contribute substantially to waste generation. By providing a more resilient and long-lasting alternative, antimicrobial Kevlar could help mitigate the environmental impact of medical waste disposal.
Water pollution is another critical aspect to consider. The leaching of antimicrobial agents from Kevlar-based medical products during use or disposal could potentially affect aquatic ecosystems. Research is needed to assess the long-term environmental fate of these compounds and their potential bioaccumulation in organisms.
On the other hand, the antimicrobial properties of Kevlar may reduce the need for chemical disinfectants in healthcare environments. This could lead to a decrease in the release of harmful substances into wastewater systems, potentially benefiting aquatic ecosystems and reducing the burden on water treatment facilities.
The end-of-life management of antimicrobial Kevlar products is an important consideration. While Kevlar itself is not biodegradable, the development of effective recycling processes for these materials could significantly reduce their environmental impact. Research into methods for separating and recovering the antimicrobial agents from the Kevlar fibers could pave the way for more sustainable lifecycle management.
In conclusion, the environmental impact of antimicrobial Kevlar in medical applications is multifaceted. While it offers potential benefits in terms of waste reduction and extended product lifespans, careful consideration must be given to its production processes, chemical additives, and end-of-life management to ensure a net positive environmental outcome.
One of the primary environmental benefits of antimicrobial Kevlar is its potential to reduce the need for frequent replacements of medical textiles and equipment. By inhibiting microbial growth, these materials can maintain their integrity and functionality for extended periods, thereby decreasing waste generation and resource consumption associated with manufacturing and disposal of conventional medical fabrics.
However, the production of antimicrobial Kevlar may involve additional chemical processes or treatments, which could potentially increase the environmental footprint of its manufacturing. The use of antimicrobial agents, such as silver nanoparticles or quaternary ammonium compounds, may introduce new concerns regarding their release into the environment during production, use, or disposal.
The durability of Kevlar fibers, combined with their antimicrobial properties, could lead to a reduction in the overall volume of medical waste. This is particularly significant in healthcare settings, where single-use items contribute substantially to waste generation. By providing a more resilient and long-lasting alternative, antimicrobial Kevlar could help mitigate the environmental impact of medical waste disposal.
Water pollution is another critical aspect to consider. The leaching of antimicrobial agents from Kevlar-based medical products during use or disposal could potentially affect aquatic ecosystems. Research is needed to assess the long-term environmental fate of these compounds and their potential bioaccumulation in organisms.
On the other hand, the antimicrobial properties of Kevlar may reduce the need for chemical disinfectants in healthcare environments. This could lead to a decrease in the release of harmful substances into wastewater systems, potentially benefiting aquatic ecosystems and reducing the burden on water treatment facilities.
The end-of-life management of antimicrobial Kevlar products is an important consideration. While Kevlar itself is not biodegradable, the development of effective recycling processes for these materials could significantly reduce their environmental impact. Research into methods for separating and recovering the antimicrobial agents from the Kevlar fibers could pave the way for more sustainable lifecycle management.
In conclusion, the environmental impact of antimicrobial Kevlar in medical applications is multifaceted. While it offers potential benefits in terms of waste reduction and extended product lifespans, careful consideration must be given to its production processes, chemical additives, and end-of-life management to ensure a net positive environmental outcome.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!