Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
826 results about "Nickel titanium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
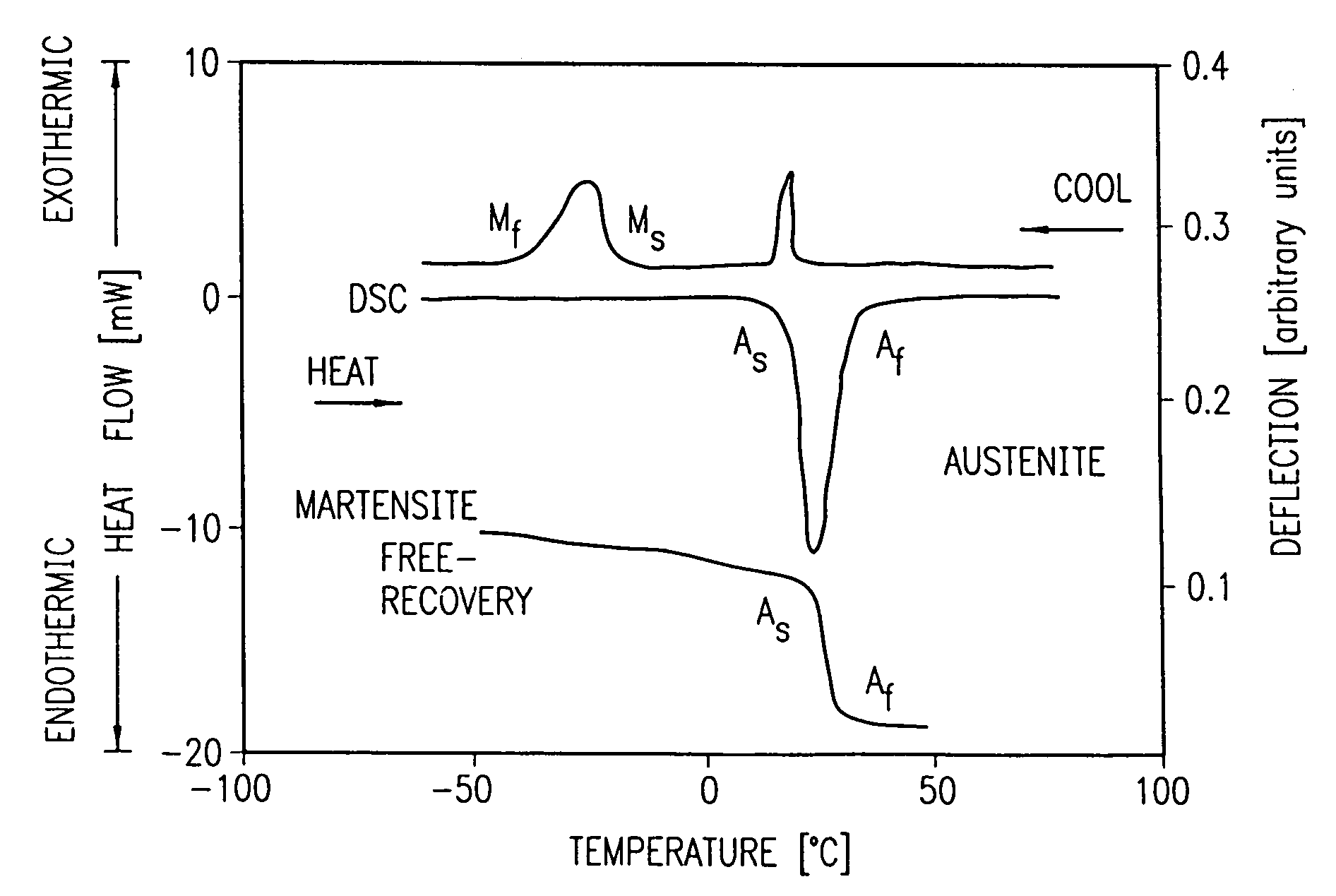
Nickel titanium, also known as Nitinol (part of shape memory alloy), is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages e.g. Nitinol 55, Nitinol 60.
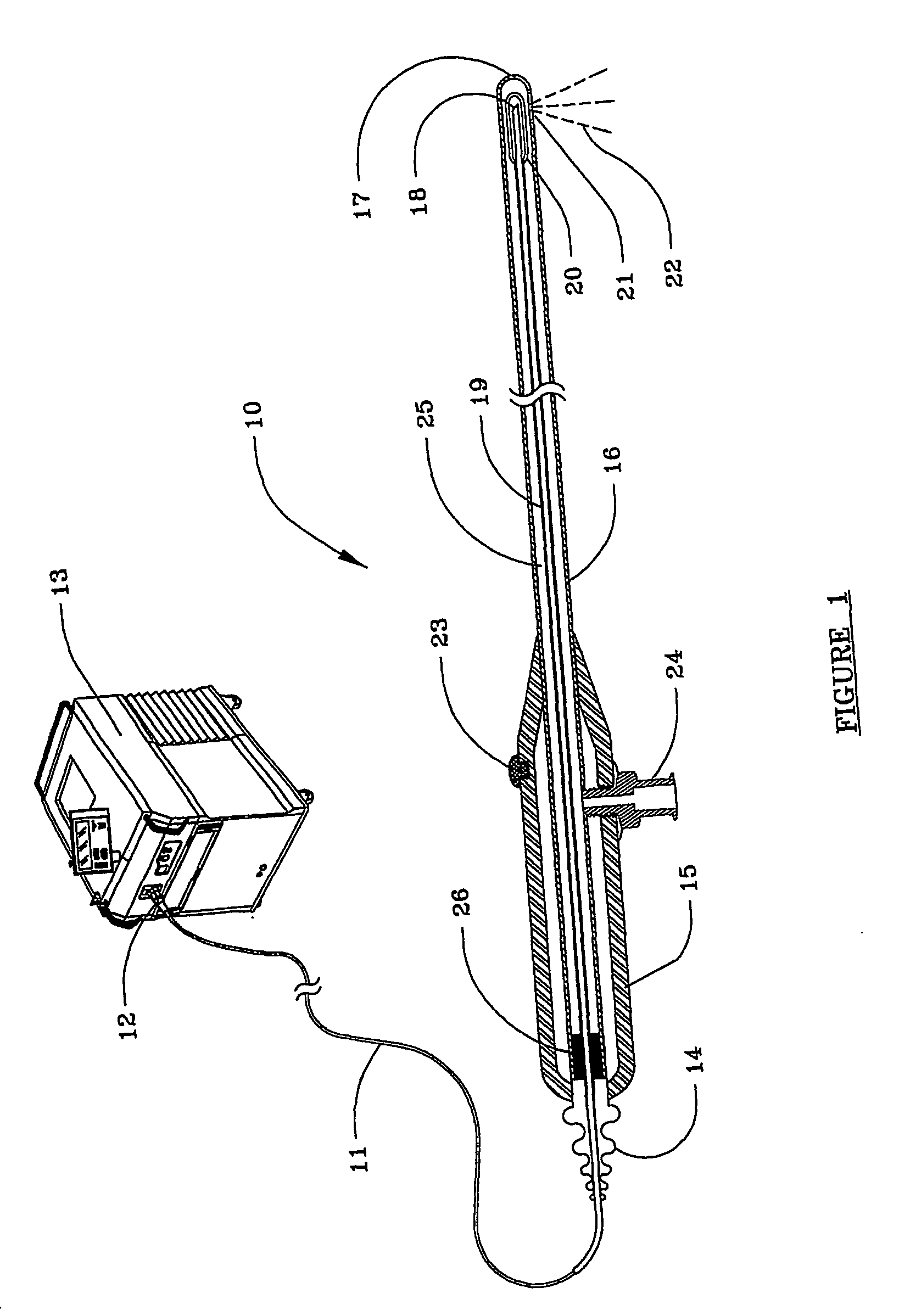
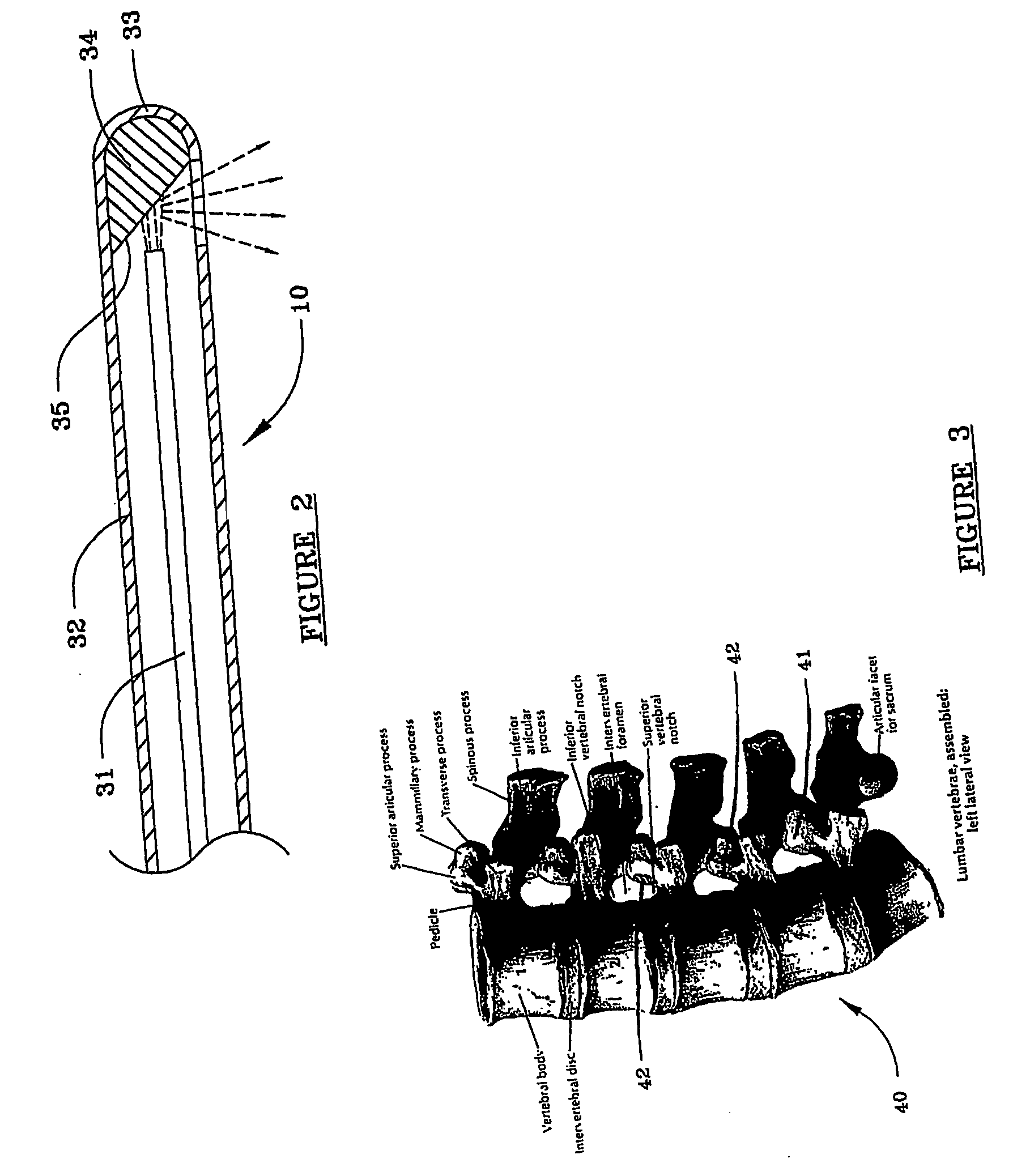
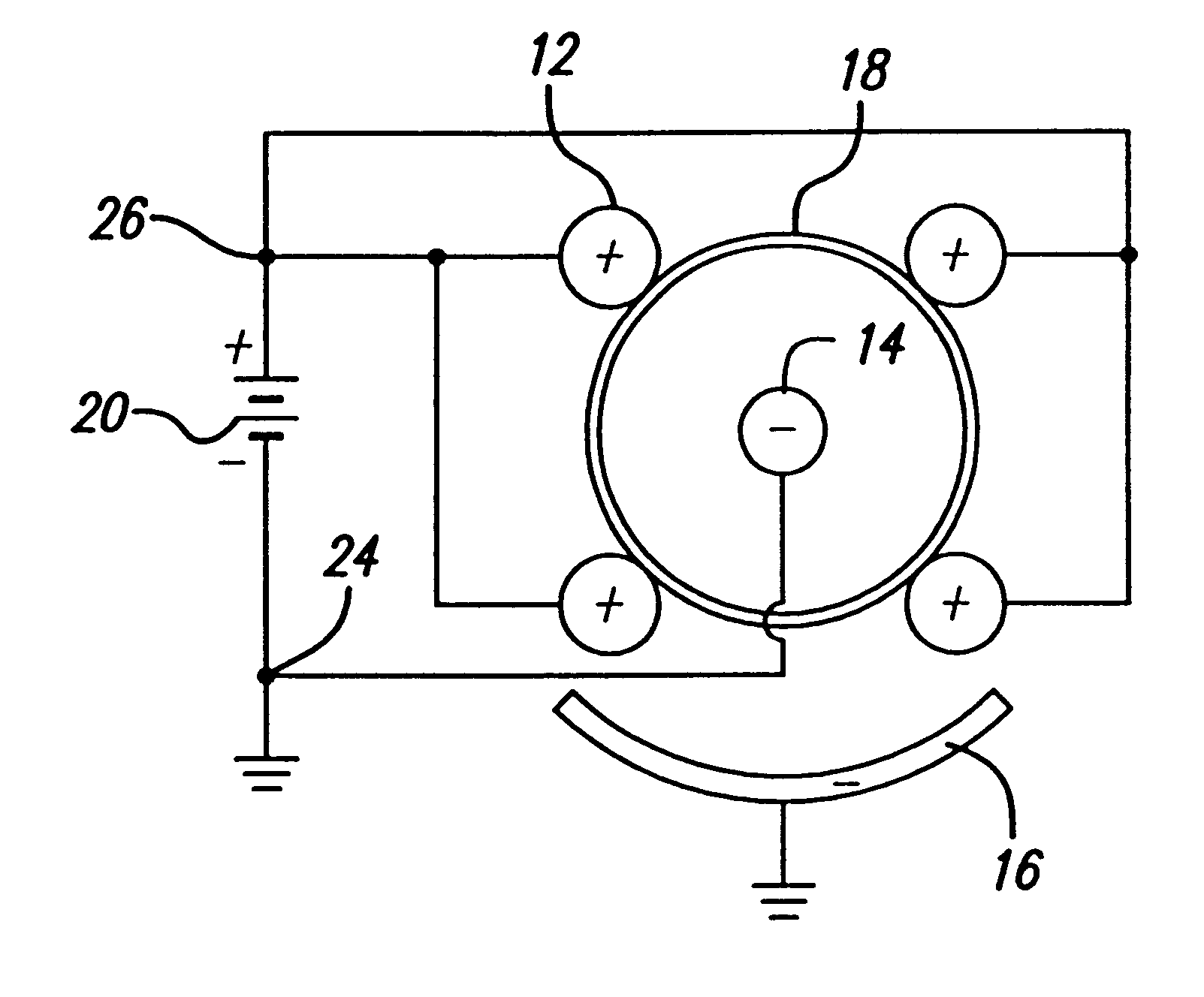
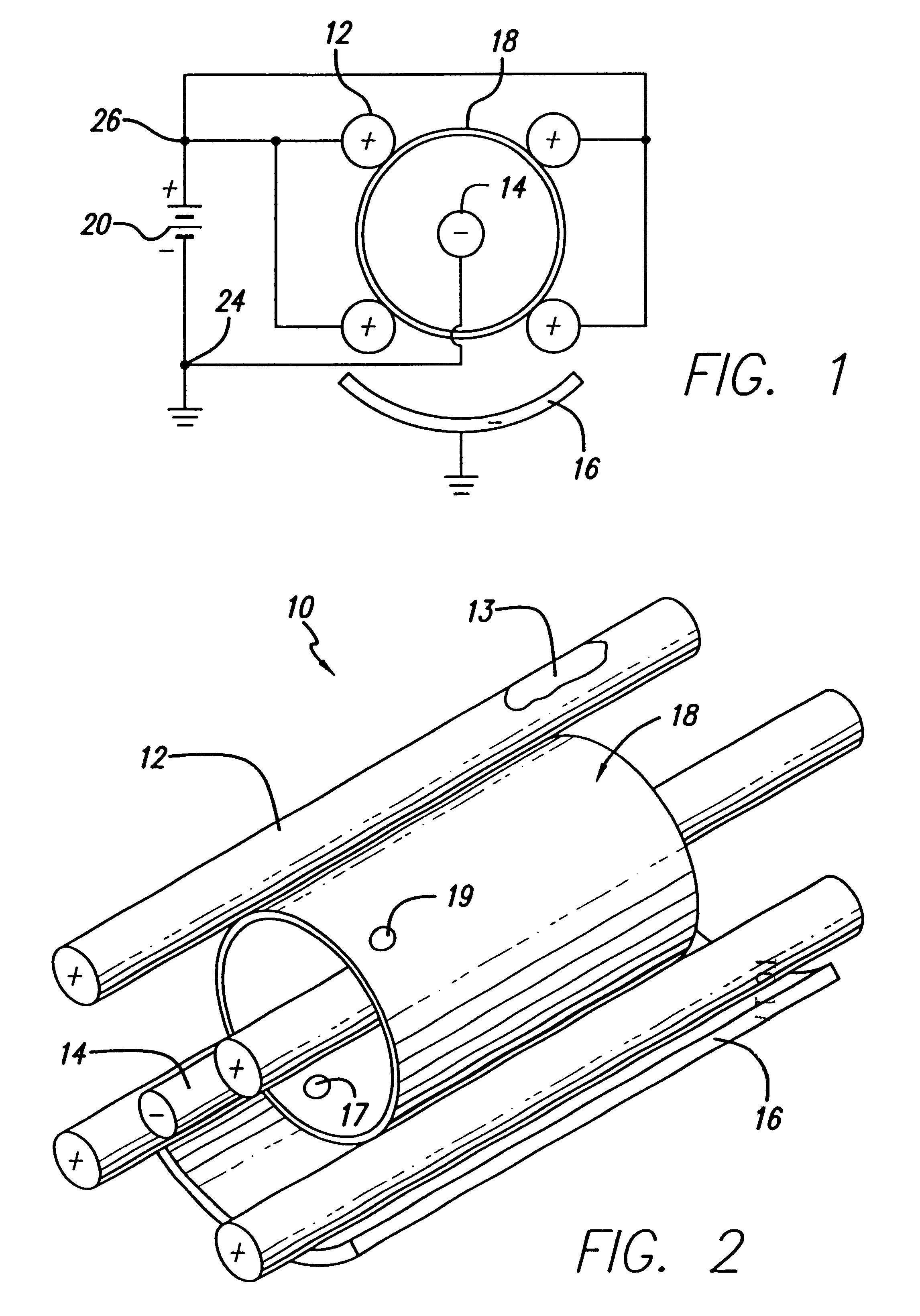
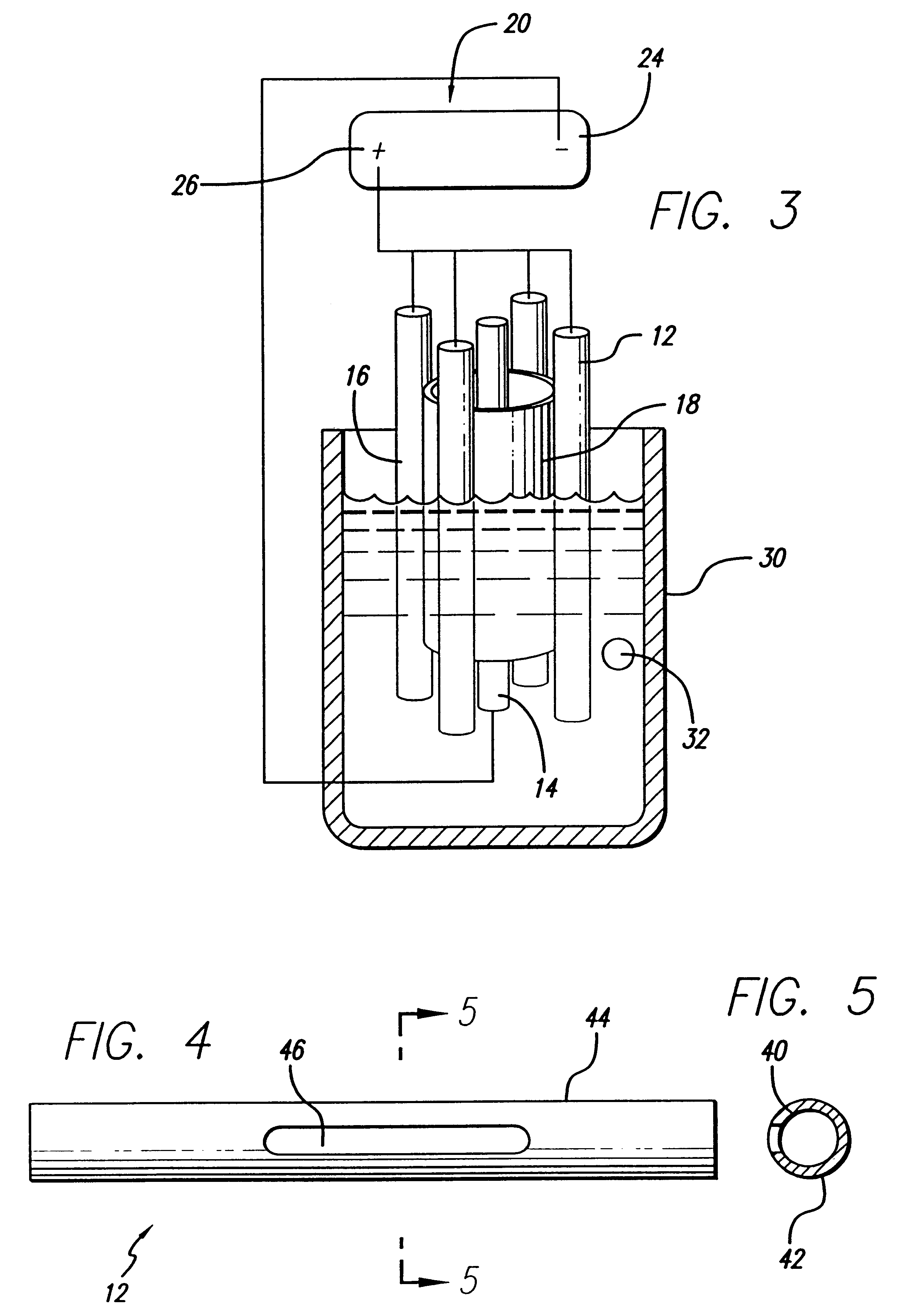
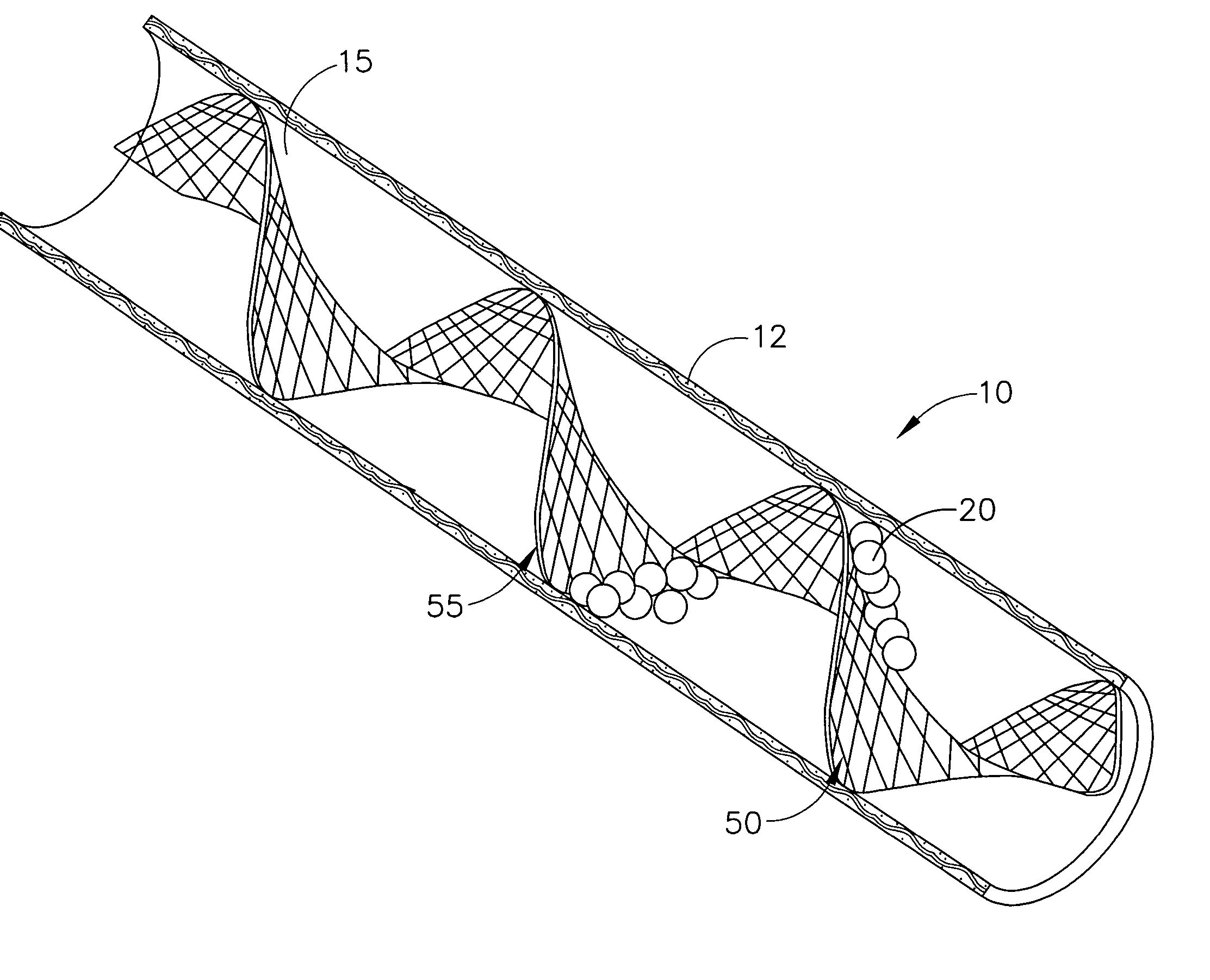
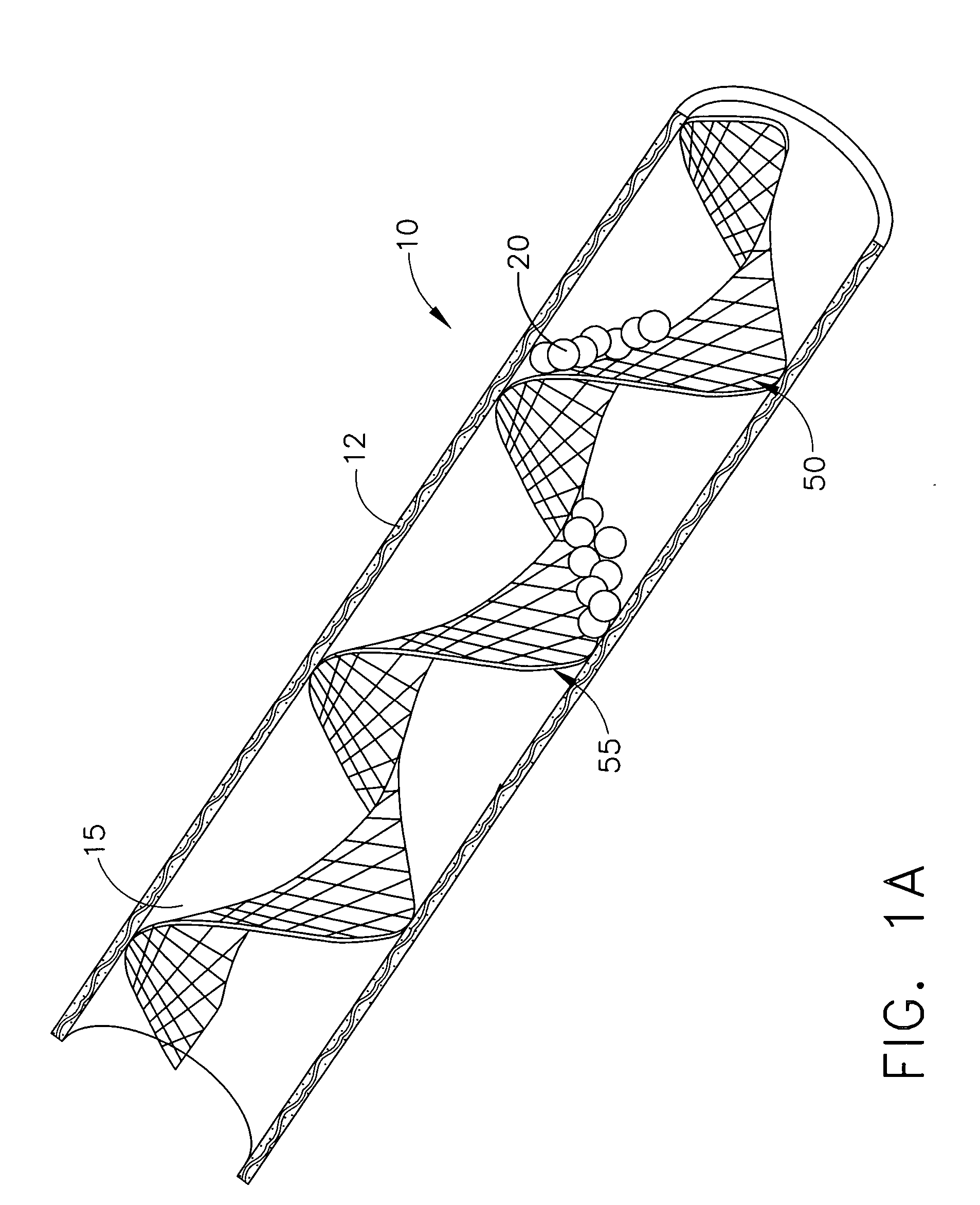
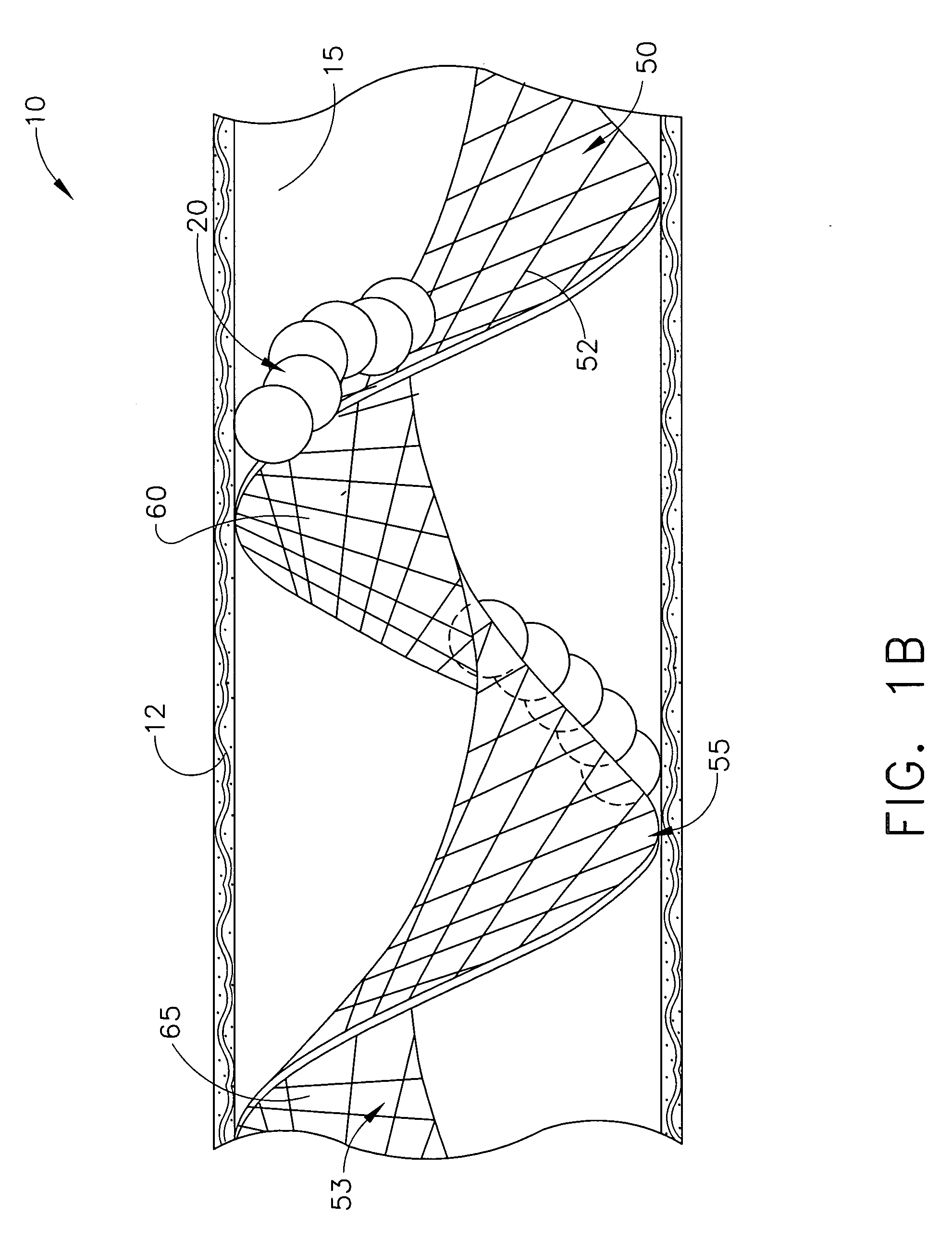
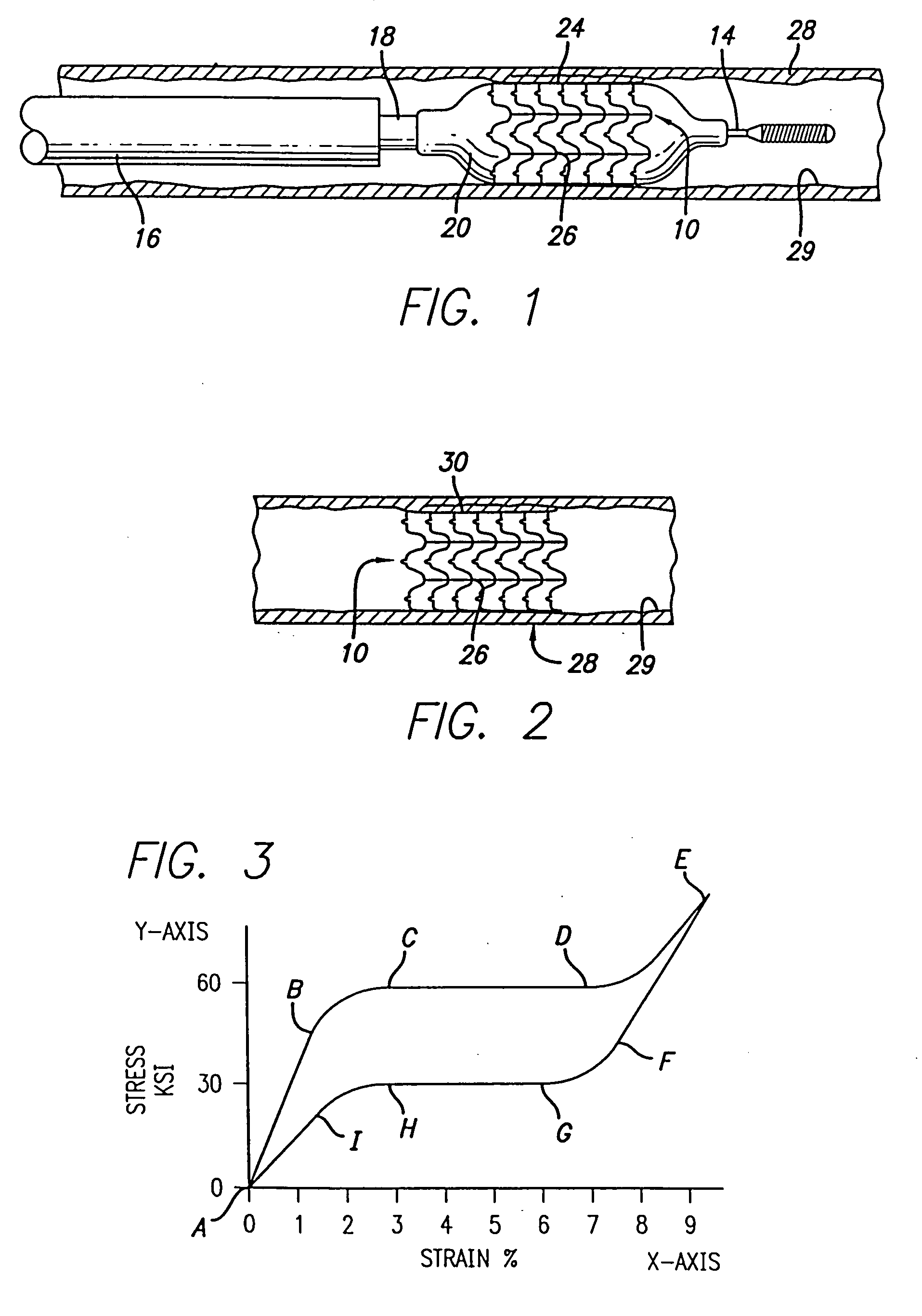
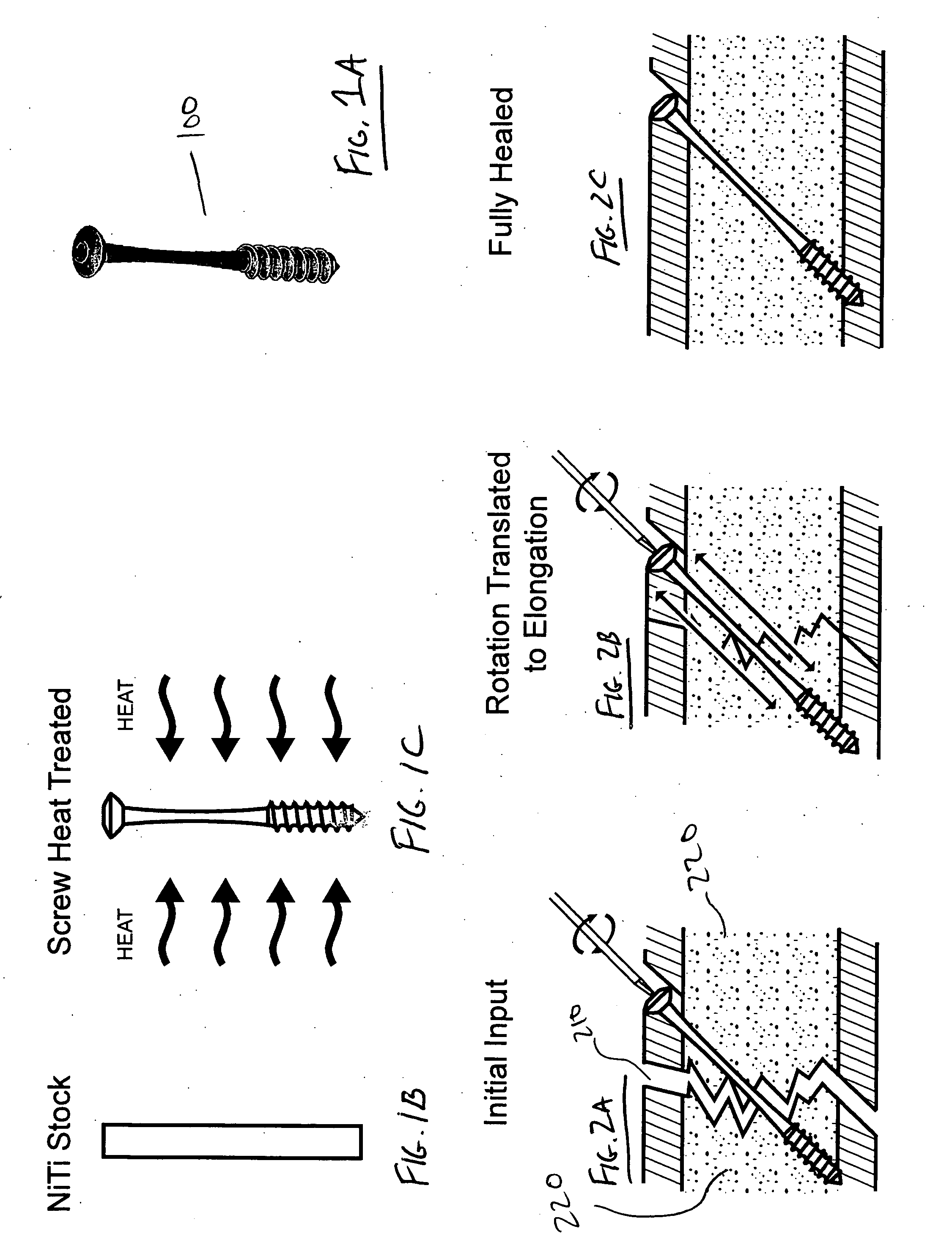
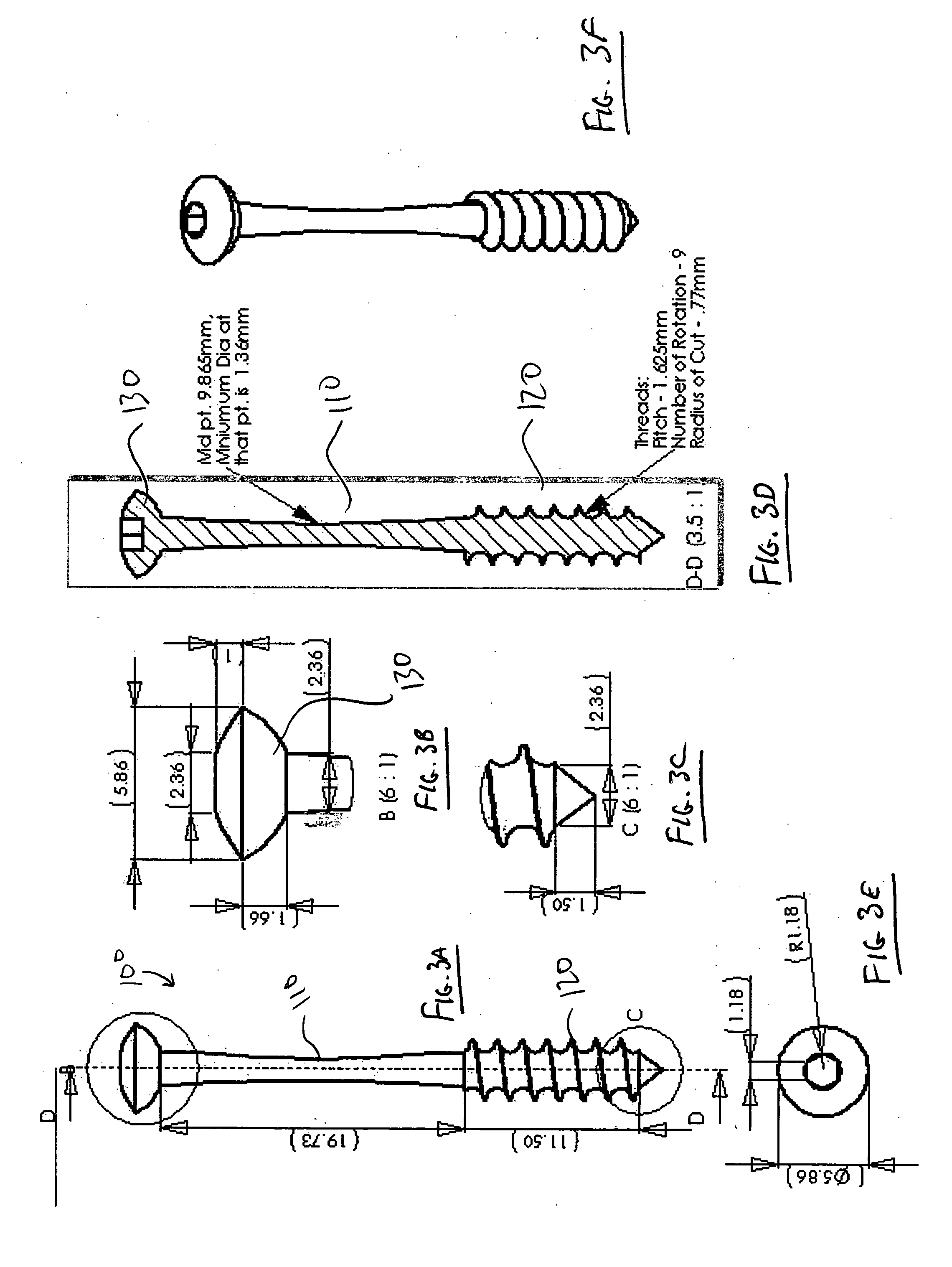
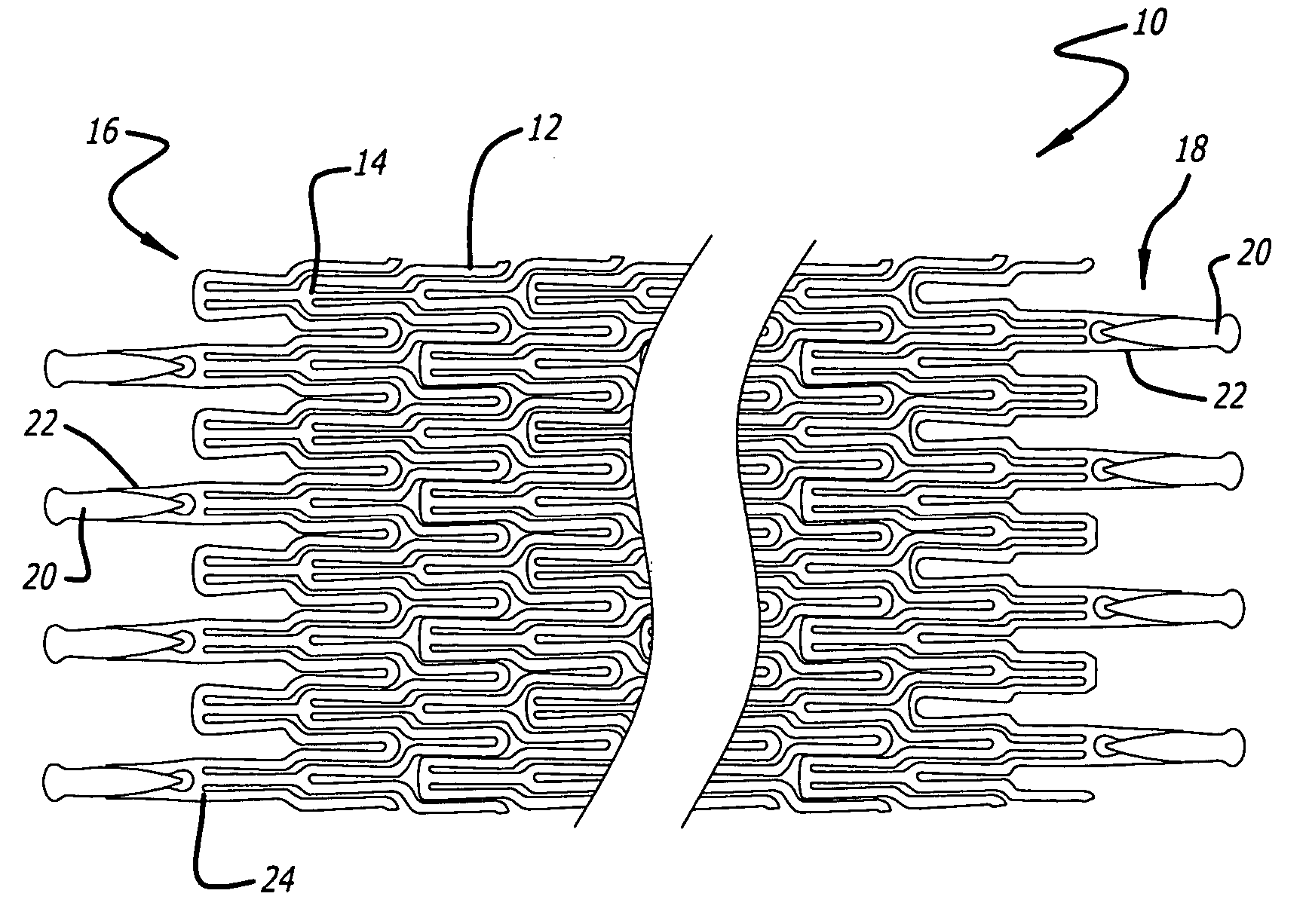
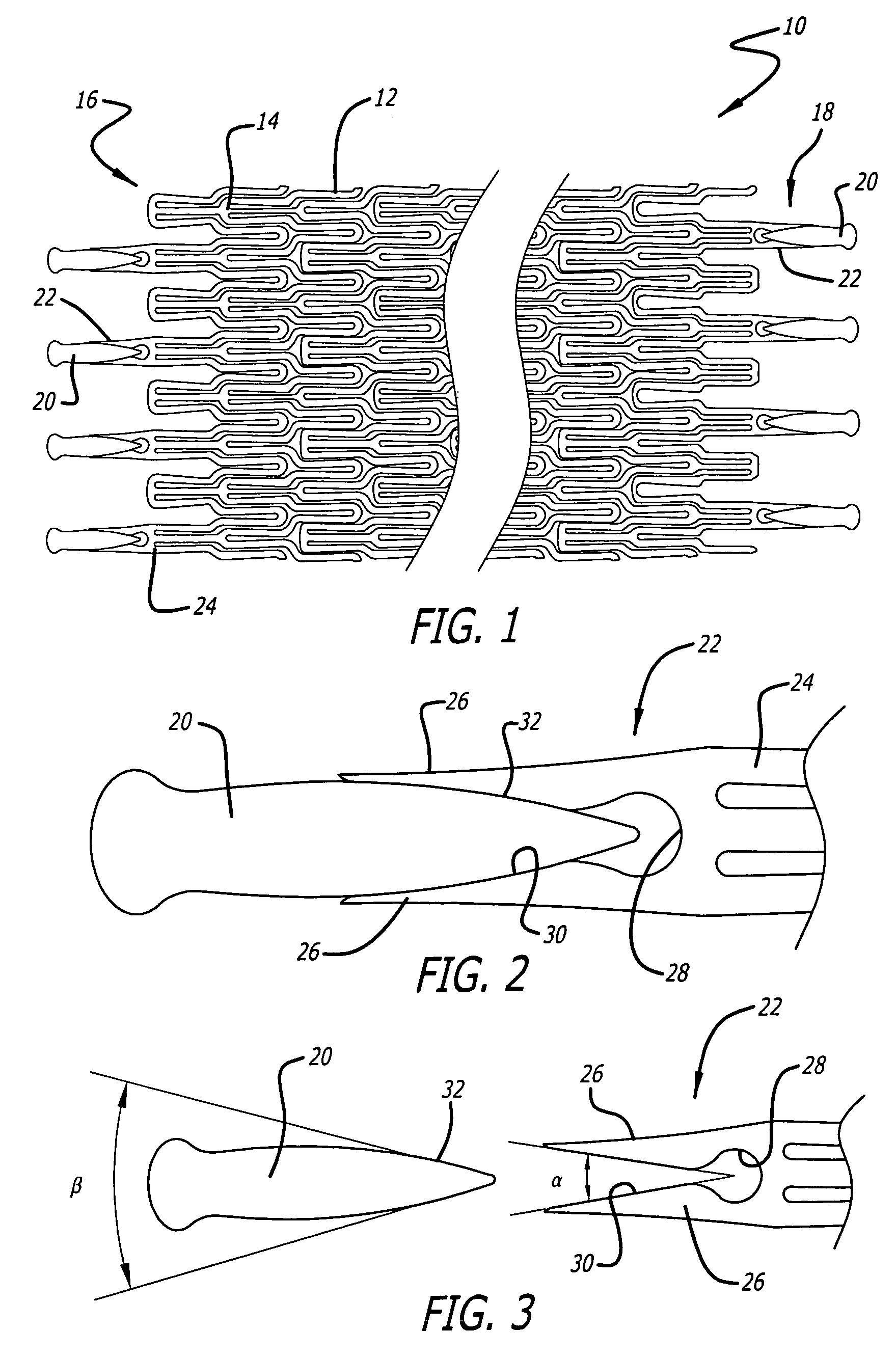
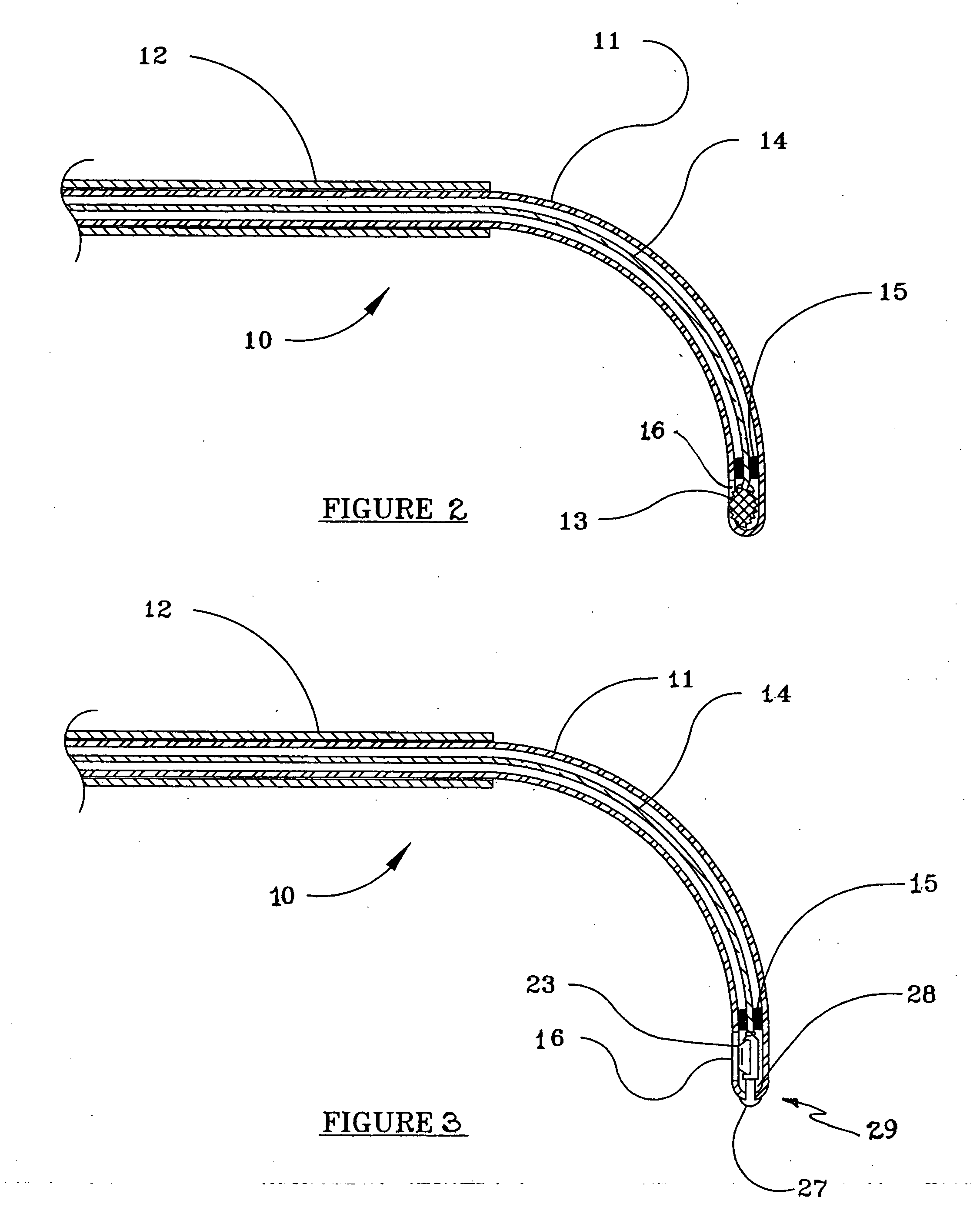
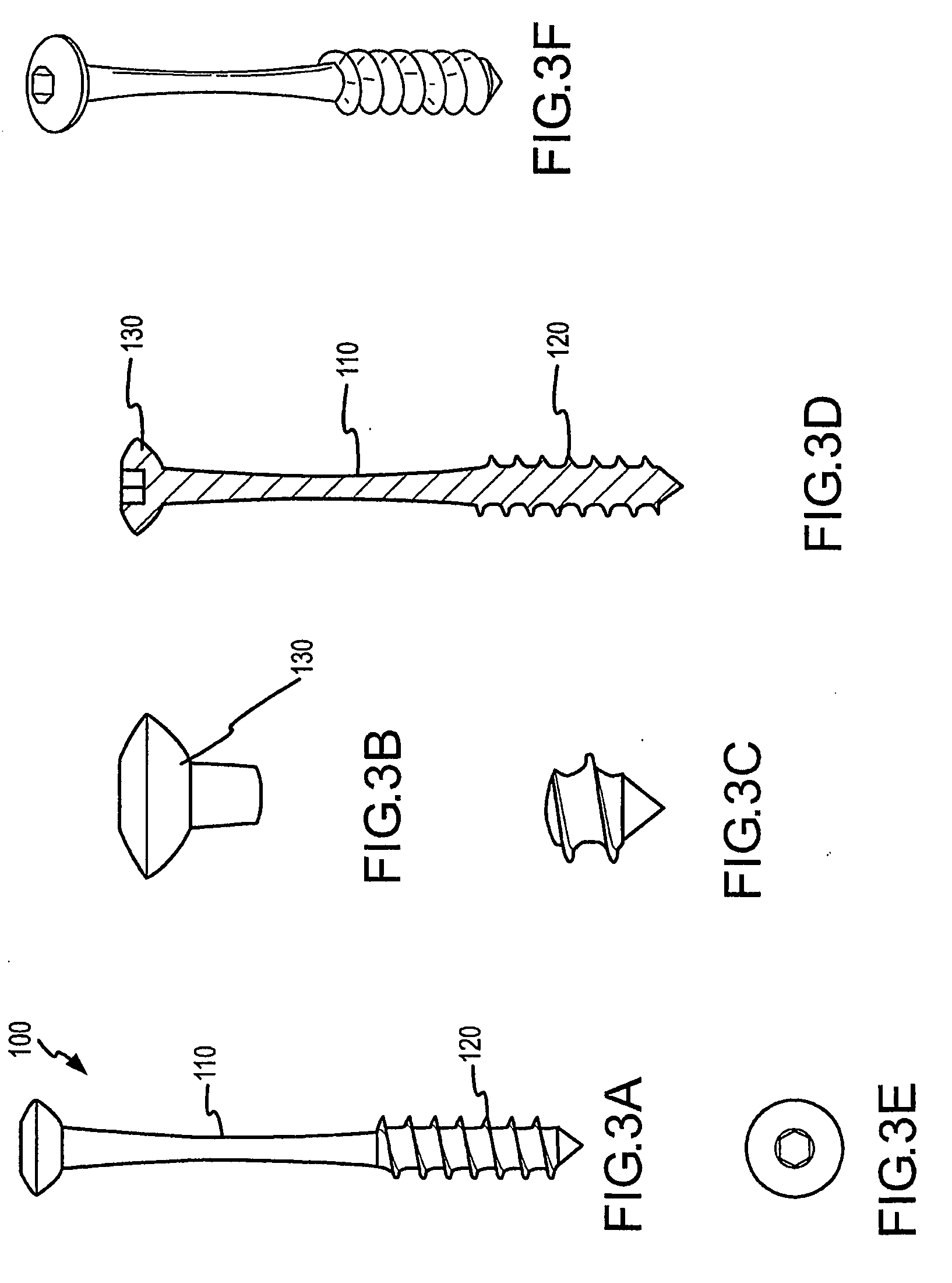
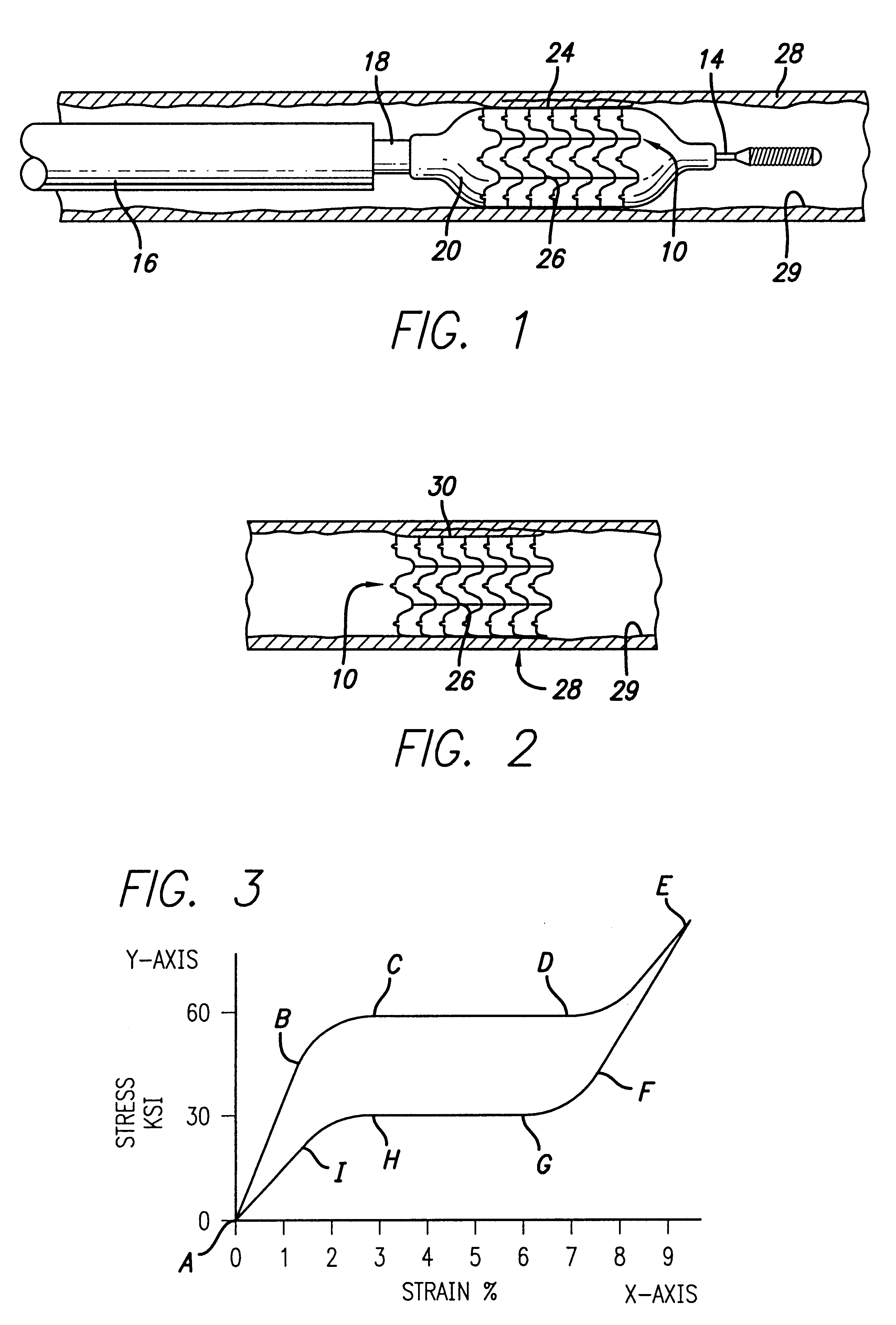
Devices and methods for minimally invasive treatment of degenerated spinal discs
InactiveUS20050222681A1Accurate spacingBone debris is eliminatedBone implantJoint implantsExpandable cageRadio frequency
Spinal stabilization devices and their methods of insertion and use to treat degenerated lumbar, thoracic or cervical spinal discs in minimally invasive, outpatient procedures are described. In one embodiment, the spinal stabilization device is an expandable cage made of a coil or perforated cylindrical tube with a bulbous or bullet-shaped distal end and a flat or rounded proximal end. In a preferred embodiment, the spinal stabilization device is mechanically expanded to a larger diameter or is made of a superelastic nickel-titanium alloy which is thermally programmed to expand to a relatively larger diameter when a pre-determined transition temperature below body temperature is reached. To treat a degenerated disc, a guide wire is inserted into the disc and an endoscope is inserted through a posterolateral puncture in the back and advanced up to the facet of the spine. Mechanical tools or laser energy, under endoscopic visualization, are used to remove or vaporize a portion of the facet bone, creating an opening into the foraminal space in the spine for insertion of an endoscope, which enables the disc, vertebra and nerves to be seen. The passageway is expanded, mechanical tools or laser of RF energy are used to make a tunnel into the disc, and a delivery cannula is inserted up to the opening of the tunnel. An insertion tool is used to insert one or more spinal stabilization devices into the tunnel in the disc, preserving the mobility of the spine, while maintaining the proper space between the vertebra. Laser or radio frequency (RF) energy is used to coagulate bleeding, vaporize or remove debris and shrink the annulus of the disc to close, at least partially, the tunnel made in the disc.
Owner:TRIMEDYNE
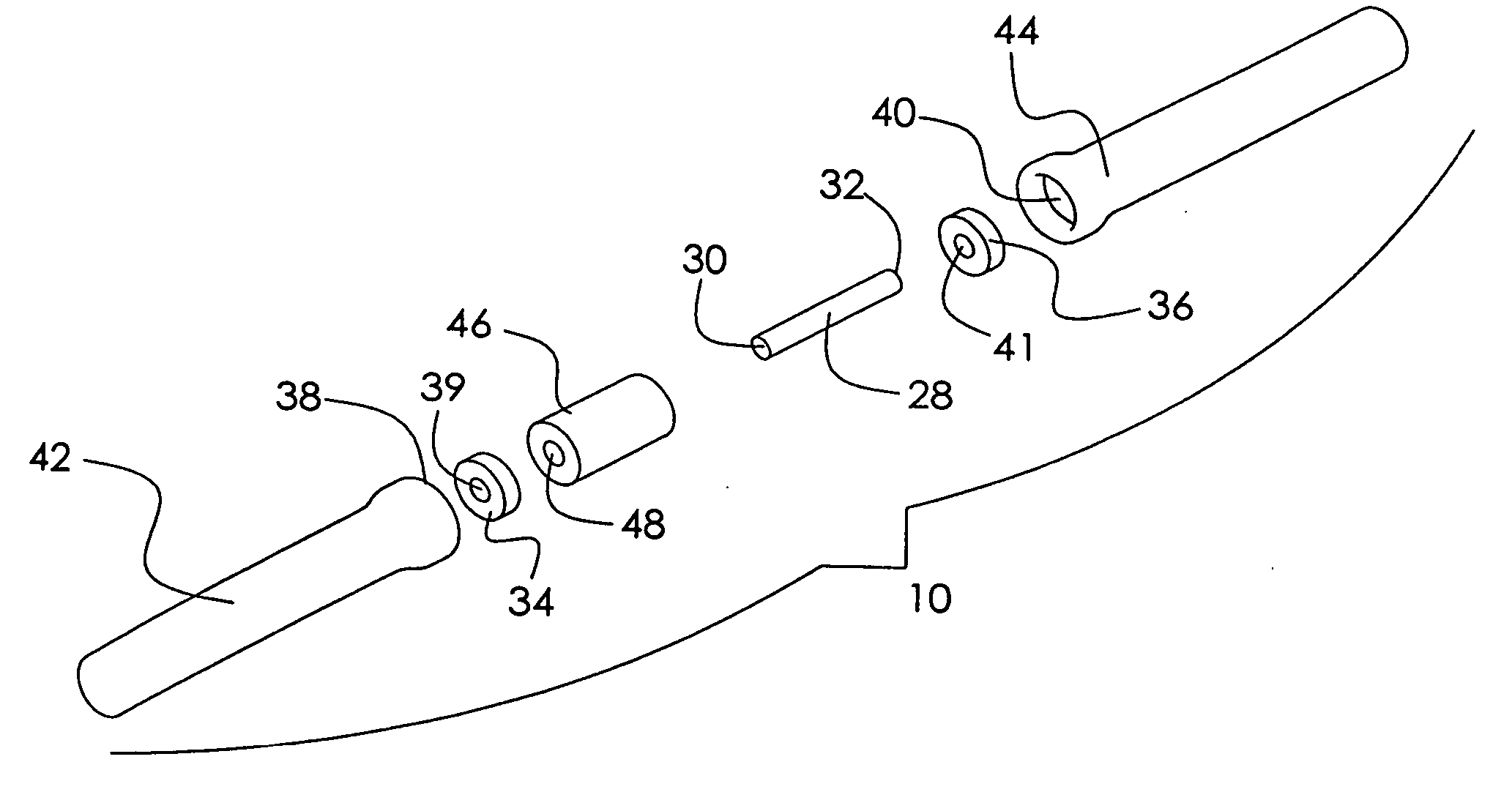
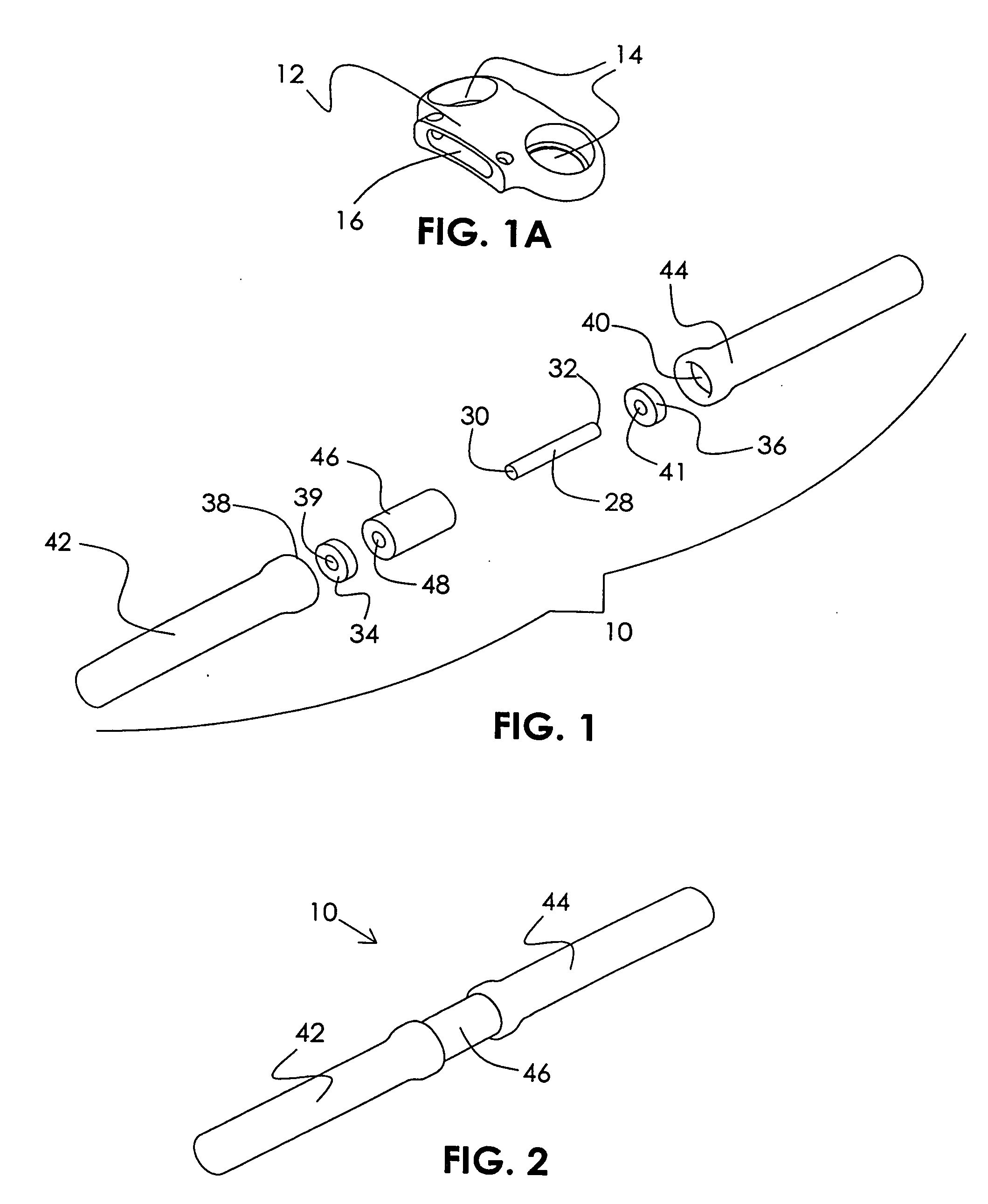
Mobile spine stabilization device
InactiveUS20060264937A1Limit range of motionConstraining bending forceInternal osteosythesisJoint implantsSpinal columnMedicine
An orthopedic device is described for stabilizing the spinal column between first and second vertebral bodies. The device has first and second screws adapted for fixation to the first and second vertebral bodies, respectively. The device further includes an elongated ligament with a first end connected to the first screw and the second end operatively connected with the second screw. The ligament is made preferably of a nickel titanium alloy selected to have ductile inelastic properties at body temperature and is capable of continuous plastic deformation to allow relative constrained motion between the vertebral bodies. In a preferred embodiment the second pedicle screw includes a bearing for receiving the ligament in a slideably engageable relationship. The device further includes optional first and second dampening members surrounding the ligament for restraining the spinal column during flexion and extension. Other preferred devices and kits containing such devices are also described.
Owner:WHITE PATRICK
Orthopedic stabilization device
InactiveUS20060264935A1Limit range of motionRestore range of motionInternal osteosythesisJoint implantsSpinal columnLigament structure
An orthopedic device is described for stabilizing the spinal column between first and second vertebral bodies. The device has first and second anchors adapted for fixation to the first and second vertebral bodies, respectively. The device further includes an elongated bridge with first and second ends operatively connected to the first and second anchors, respectively. The bridge contains an implantable ligament made preferably of a nickel titanium alloy selected to have inelastic properties at body temperature, wherein the bridge is capable of continuous plastic deformation to allow relative constrained motion between the vertebral bodies. In a preferred embodiment the device further includes a dampening member surrounding the ligament, which may be a wire, tube or band. In another preferred embodiment the device includes rigid rod members each correspondingly retained with either end of the ligament, and independently attached to the vertebral bodies with anchors. The rigid rod members are correspondingly connected to either end of the ligament. In yet another preferred embodiment, the device includes plate segments correspondingly retained with either end of the ligament, and independently attached to the vertebral bodies with a plurality of anchors. Other preferred devices and kits containing such devices are also described.
Owner:WHITE PATRICK
Electro-polishing fixture and electrolyte solution for polishing stents and method
An electro-polishing fixture for polishing stents which incorporates multiple anodes in contact with the stent and a center cathode disposed coaxially within the interior of the stent and a curved exterior cathode disposed about the perimeter of the stent. The invention further includes an electrolyte solution adapted for polishing stents composed of nickel-titanium alloy and a method of using the electrolyte in combination with the electro-polishing fixture.
Owner:ABBOTT CARDIOVASCULAR
Device for filtering blood in a vessel with helical elements
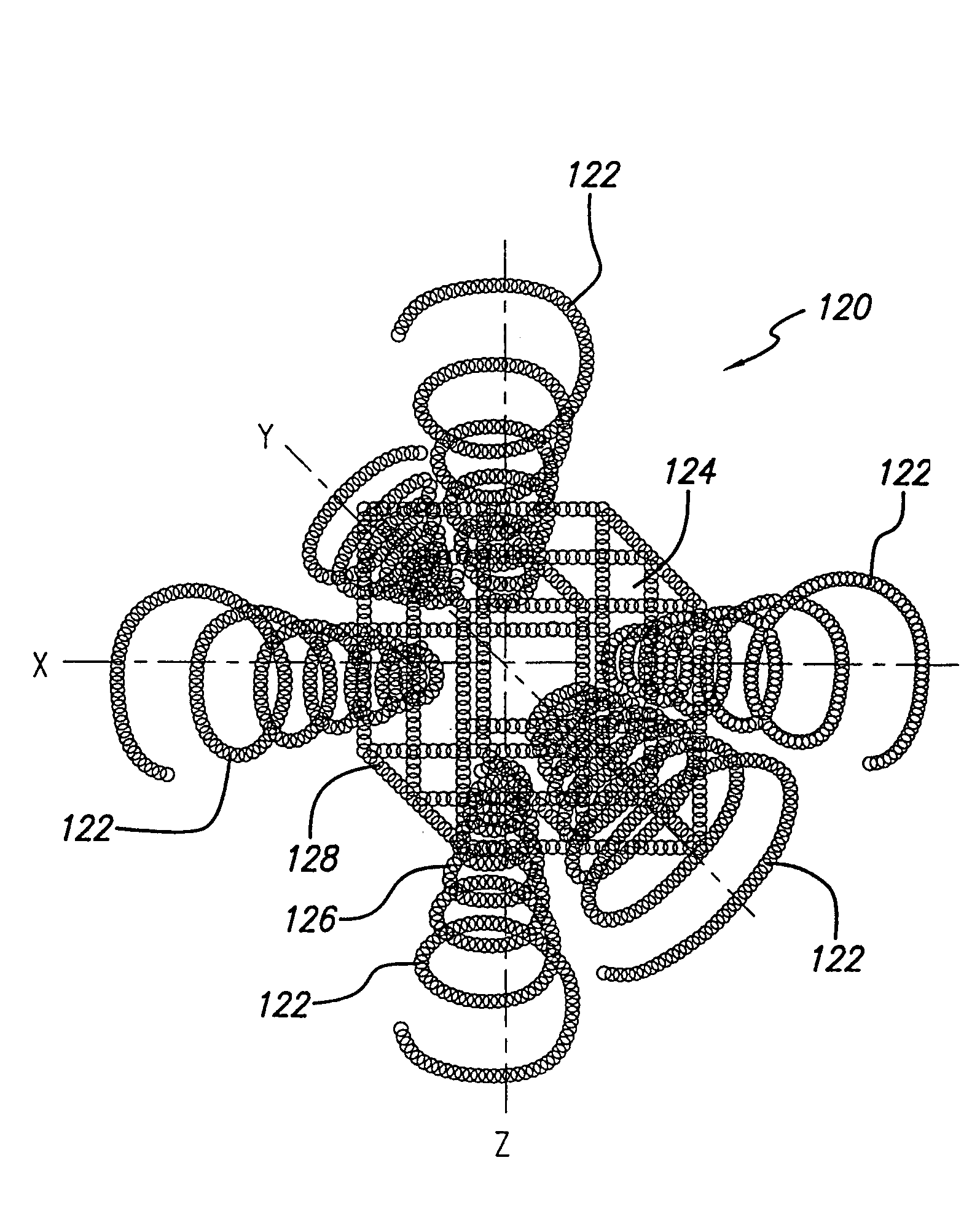
InactiveUS20060020286A1Efficient capturePreventing pulmonary embolismHaemofiltrationSurgeryMedicineEmbolus
A device for capturing an embolus within a vessel of a patient's body has at least one helix made of a mesh material. The at least one helix has a plurality of turns helically arranged around a longitudinal axis. The mesh material has a plurality of pores therein and the pores have a size ≧120 μm. The at least one helix includes a plurality of panels wherein the panels are movable from a collapsed state to an expanded state when placed within a vessel. The mesh material is made of a self-expanding material such as nickel titanium in one embodiment. In another embodiment, the device has a double helix arrangement.
Owner:CORDIS CORP
Radiopaque nitinol alloys for medical devices
Owner:ABBOTT CARDIOVASCULAR
Methods for manufacturing a clip and clip
The present invention relates to a method of making clips which can be used to engage body tissue for the purpose of closing wounds. Such clips are generally annular in shape and have radially inwardly extending tines. It is often desirable for such clips to have a small lateral dimension, but manufacturing difficulty has been encountered in making small clips because of the difficulty in cutting materials accurately when attempting to produce a clip with closely packed elements. The present invention avoids these difficulties by first forming a precursor which, in one embodiment, has the tines extending radially outwardly from the annular body and then forms the clip by inverting the precursor such that the tines extend radially inwardly. In an alternate embodiment, the precursor is formed with an over-sized lateral dimension and then compressed inwardly to bring the tines closer together and to reduce the lateral dimension of the precursor. It is preferred to manufacture such clips from a superelastic alloy such as nickel-titanium, in which case the inverted or compressed precursor must be heated and quenched to heat set the clip in its final shape.
Owner:INTEGRATED VASCULAR SYST
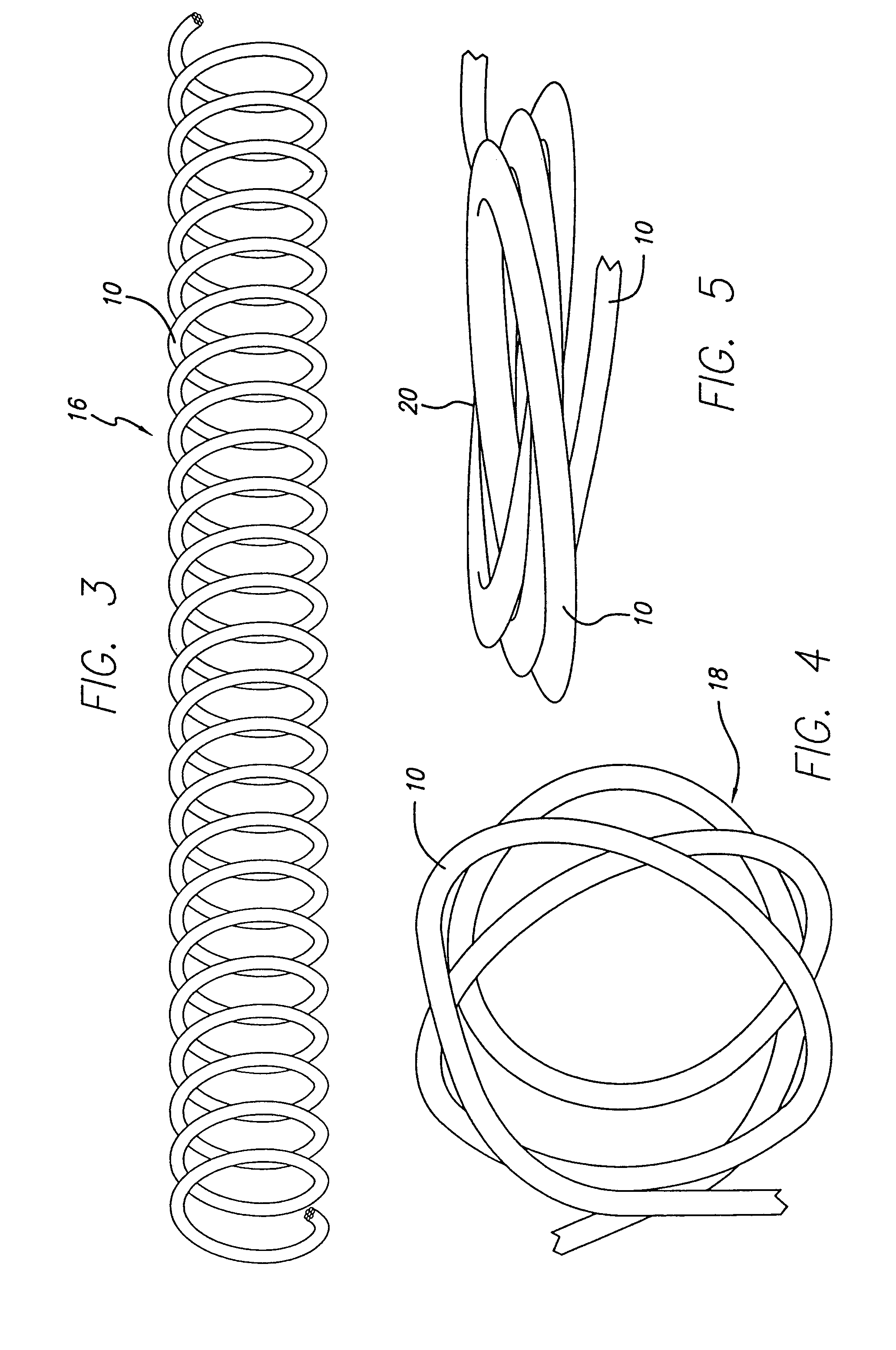
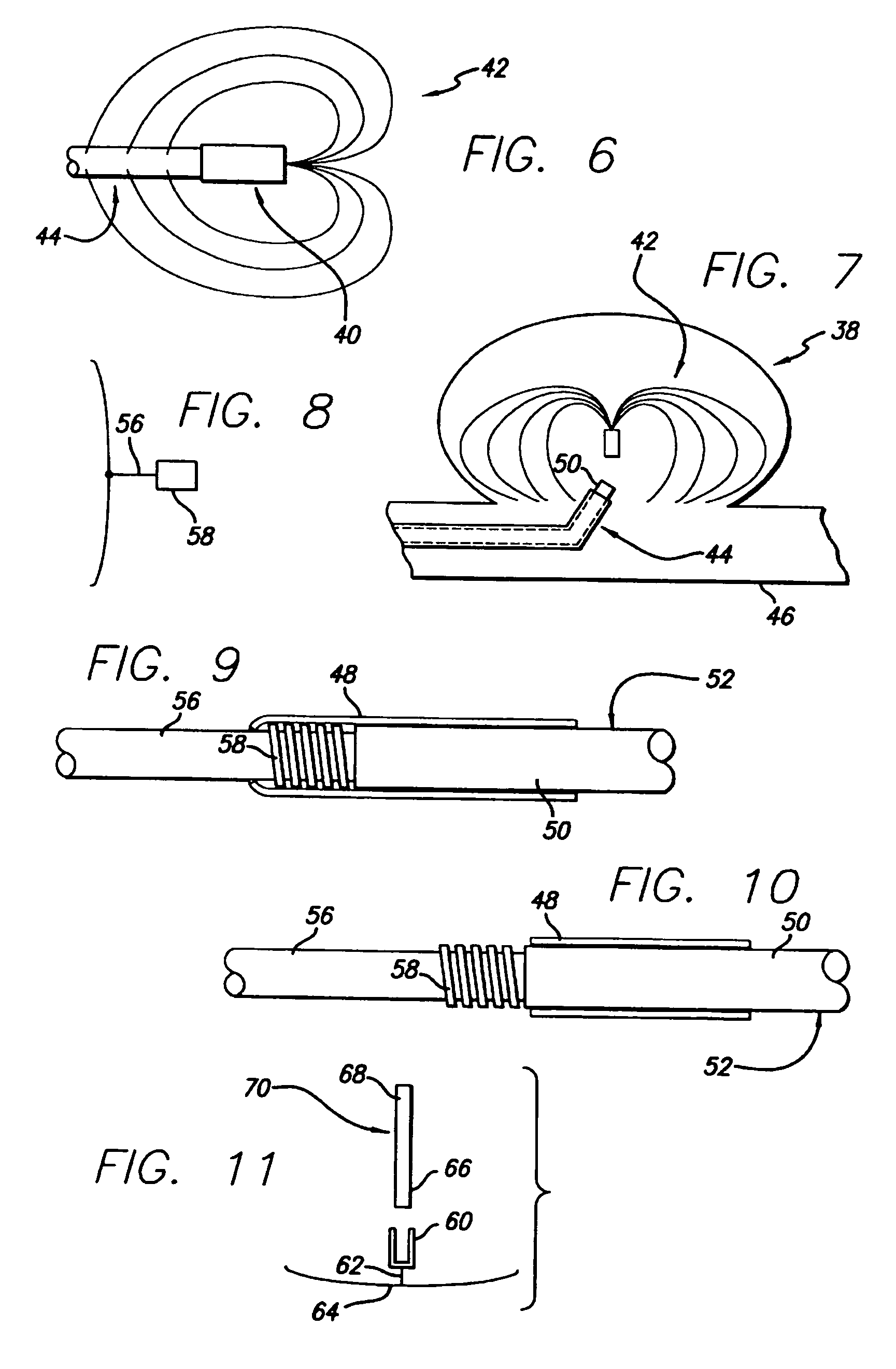
Occlusive coil manufacture and delivery
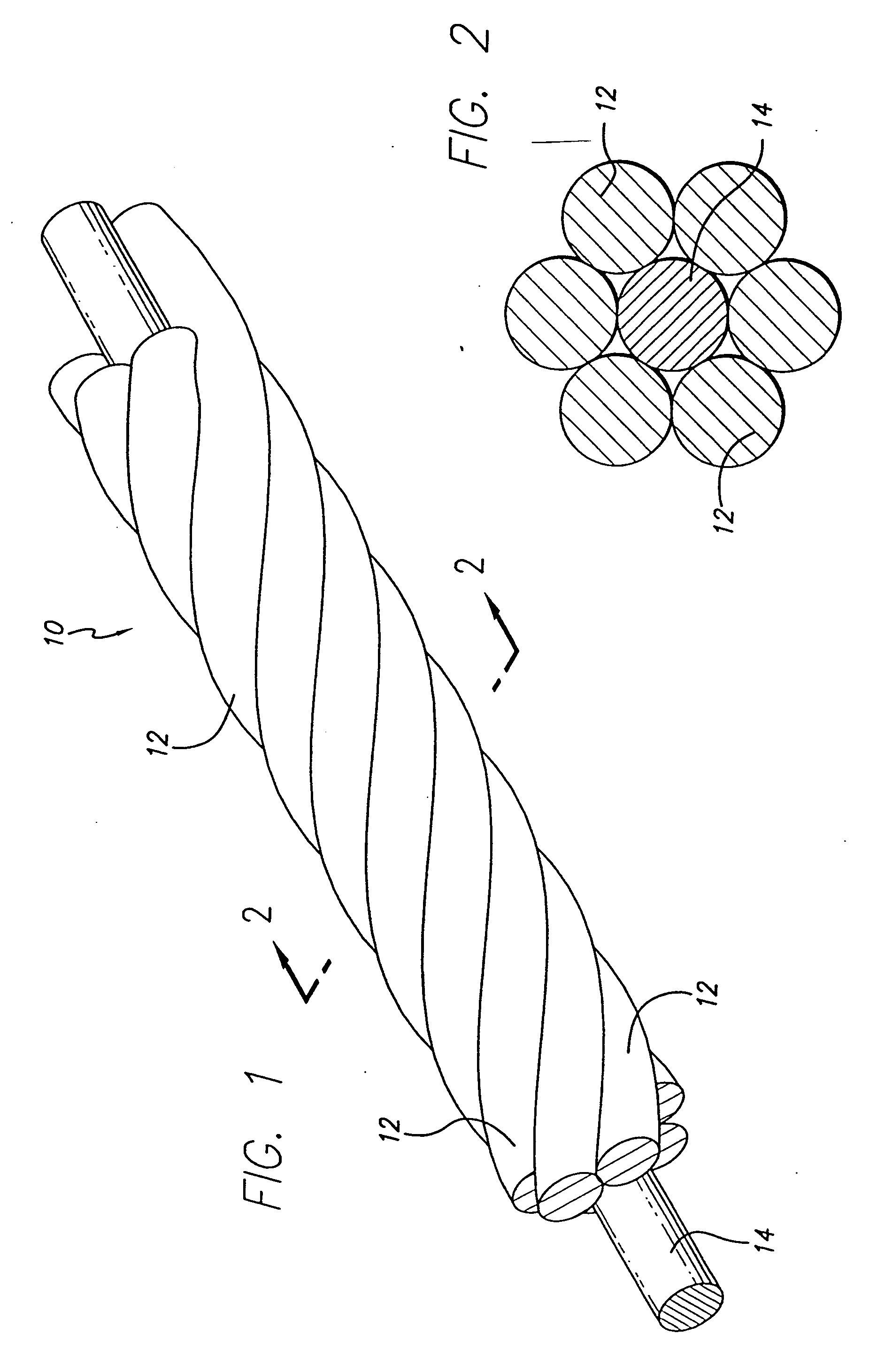
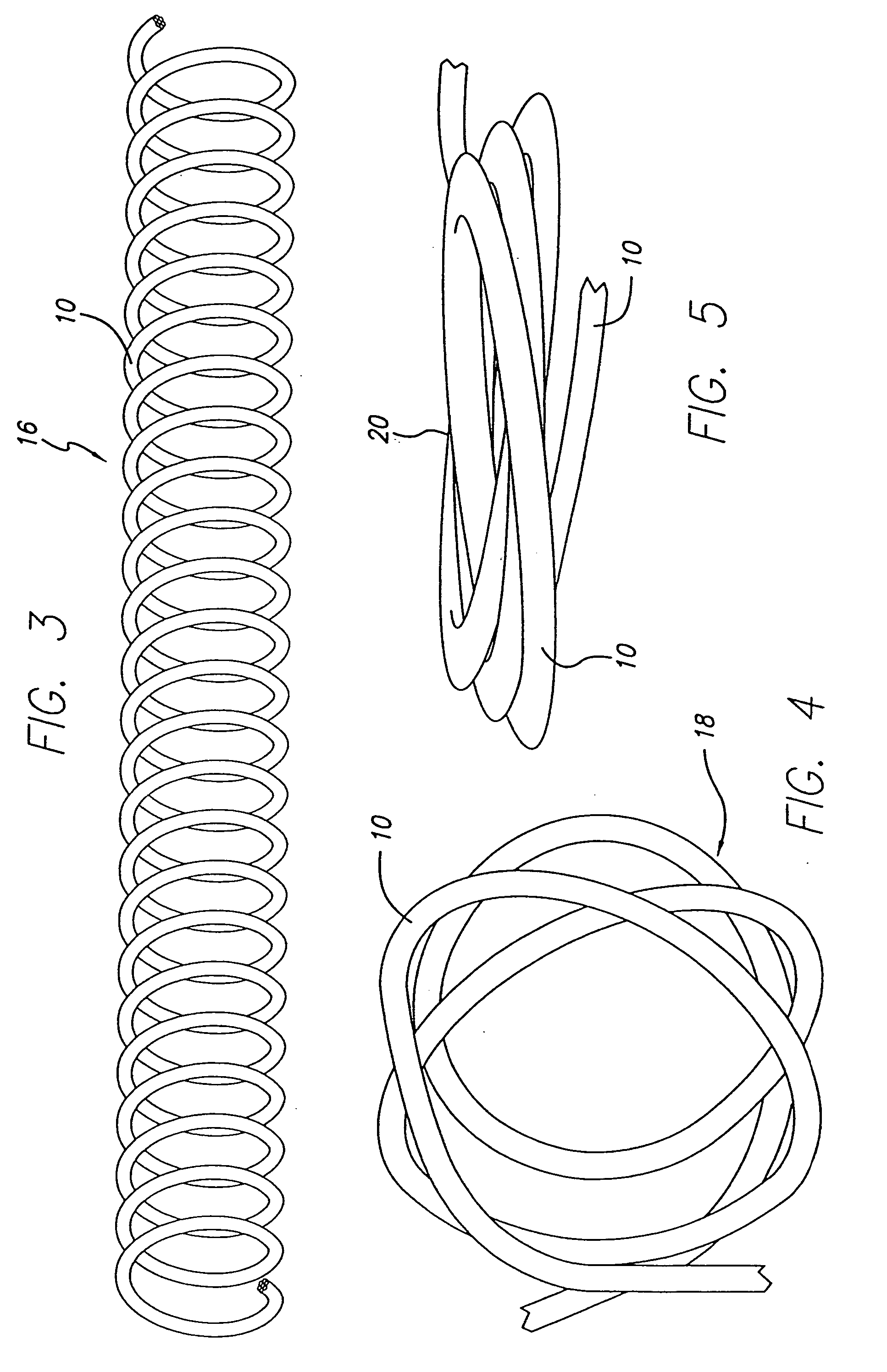
The present invention includes a coiled wire formed of a shape memory material for implantation into an anatomical defect. After implantation of one or more of the coiled wires according to the present invention, the defect is occluded and thereby corrected or treated. Prior to implantation, the coiled wire is generally elongated and thereafter it reverts to a predetermined shape that is suitable for occluding the defect. At least one clip having at least two prongs may be provided on the wire for attachment to body tissue. Preferably the wire is made of nickel-titanium. In an alternative embodiment, the coil includes a plurality of layers. At least one of these layers is formed of a shape memory material.
Owner:JAYARAMAN SWAMINATHAN
Osteosynthetic implants and methods of use and manufacture
ActiveUS20050240190A1Promote repairSmall sectionSuture equipmentsInternal osteosythesisBone fixation devicesBiomedical engineering
The present invention provides bone fracture fixation devices, systems and methods of use and manufacture. One such bone fixation device includes an elongate element having a responsive zone. The element is adapted to be coupled to the bone so that the responsive zone is positioned adjacent a fracture site in the bone. The responsive zone is adapted to apply a desired pressure to the bone when coupled thereto. In some embodiments, the responsive zone comprises a shape memory material, which may be nickel titanium or Nitinol, to apply compressive pressure across the fracture site for longer periods of time than standard bone screws.
Owner:MEDSHAPE
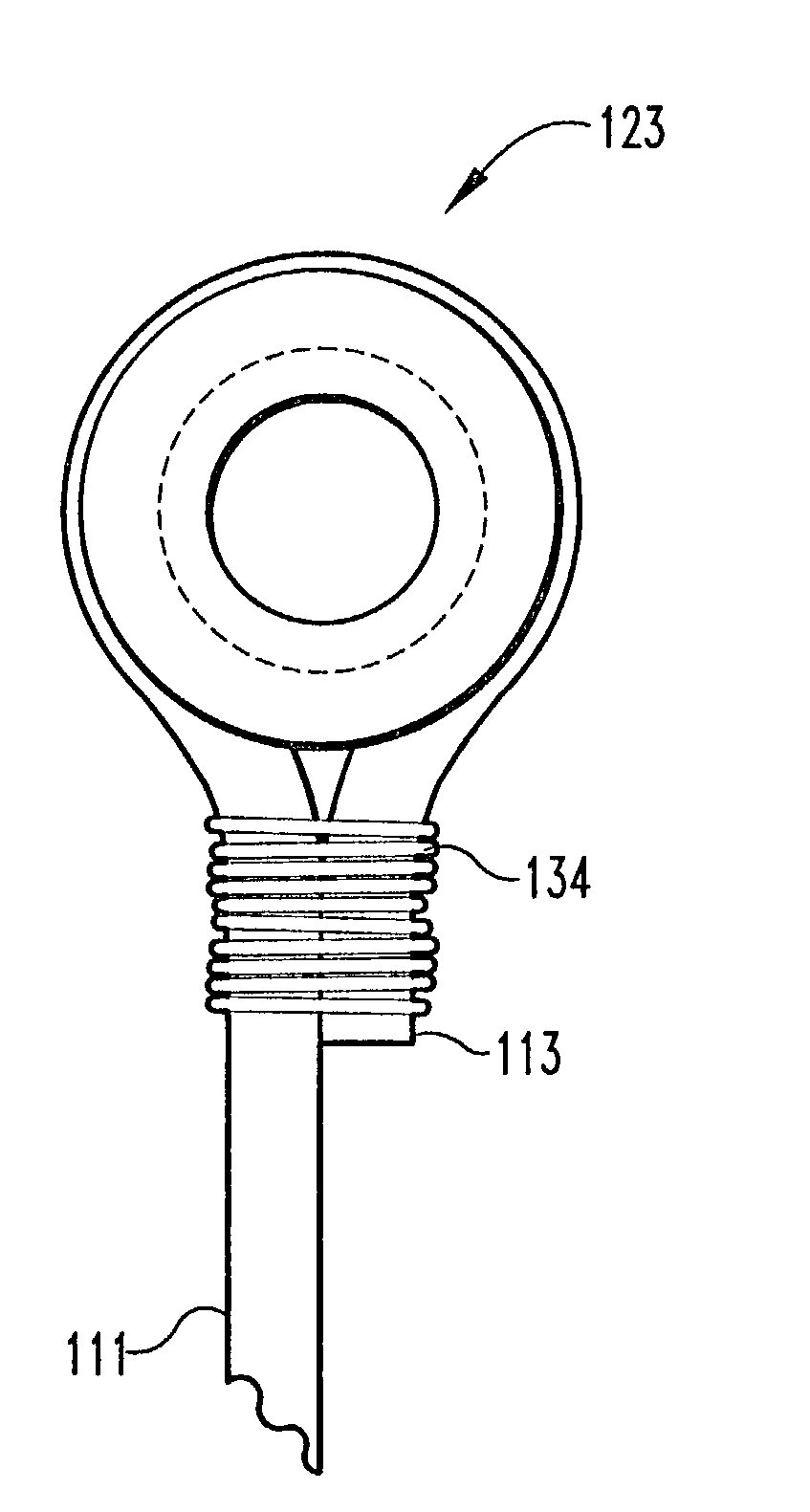
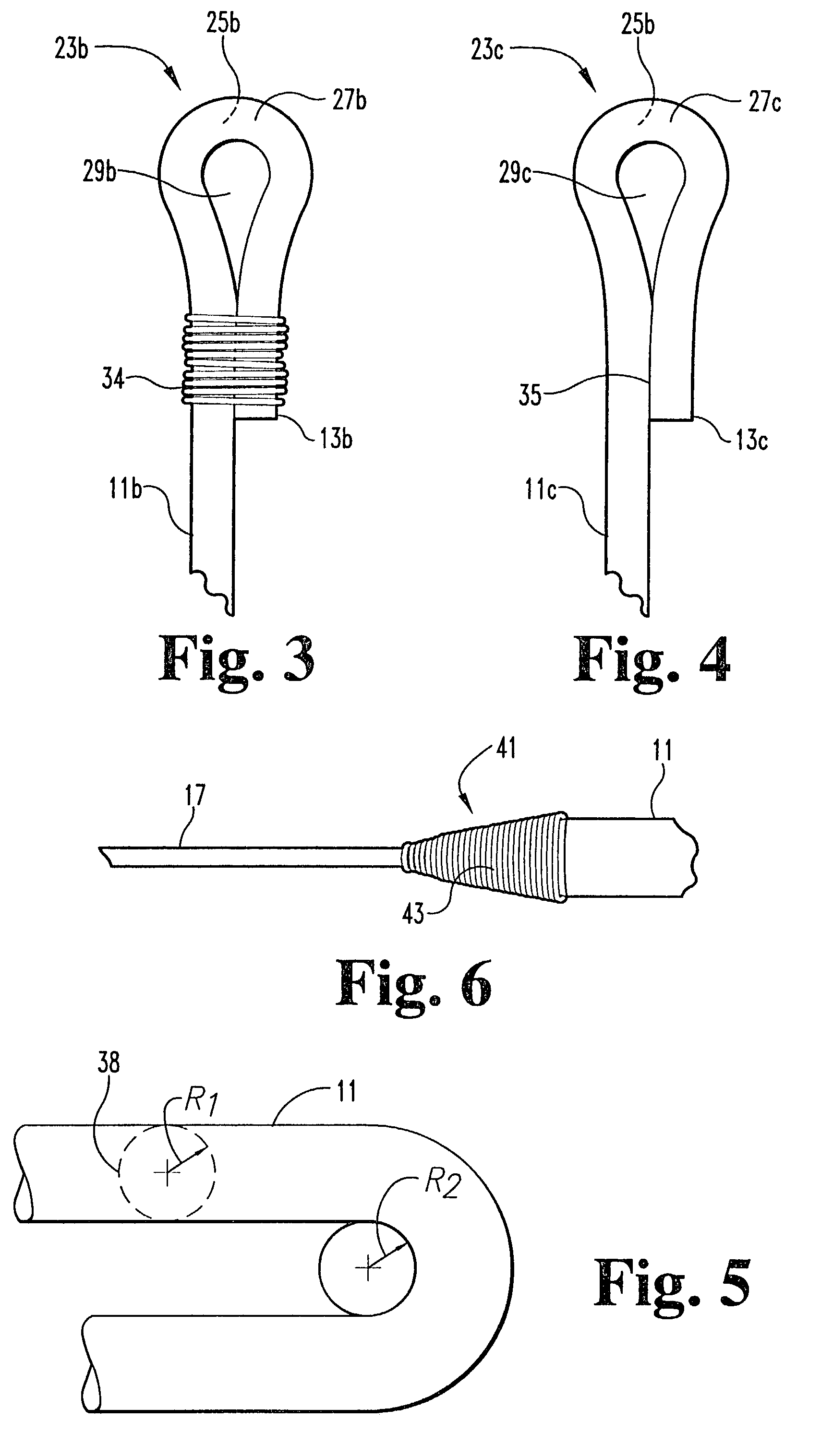
Adjustable spinal tether
An improved apparatus is provided to allow for an adjustable length tether for use in the spine and other parts of the body. The tether comprises an artificial strand with an eyelet formed in one end, the other end being looped through the eyelet. The other end is then secured with respect to the eyelet by a crimp, the excess length being cut off after the length of the tether has been given an appropriate tension. Alternatively, the eyelet end may be formed around a grommet. The crimp may be separate from the grommet or a part of the grommet. The mechanism by which the length is adjusted in some cases will take advantage of the shape memory properties of alloys such as nickel-titanium.
Owner:WARSAW ORTHOPEDIC INC
Radiopaque markers for medical devices
InactiveUS20050060025A1High level of radiopacitySufficient radiopacityStentsBlood vesselsIridiumRhenium
An implantable medical device includes a structural body made from a superelastic material and includes one or more marker holders integrally formed on the structural body. Each marker holder is designed to hold a radiopaque marker which has a level of radiopacity greater than the superelastic material. The radiopaque marker can be made from a nickel-titanium alloy which includes a ternary element. The ternary element can be selected from the group of elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, and hafnium. In one form, the marker holder includes a pair of projecting fingers connected together at a notched region to cooperatively create a particular-shaped opening. This opening, in turn, is adapted to receive a similarly shaped portion formed on the radiopaque marker. In one form, the radiopaque marker includes an inner core which is partially, or completely, encased by an outer layer. This inner core can be made from a highly radiopaque material while the outer layer is formed from a material that is easier to weld to the marker.
Owner:ABBOTT VASCULAR SOLUTIONS
Device for replacing aortic valve membrane or pulmonary valve membrane percutaneously
The invention relates to a novel percutaneous aortic valve and pulmonary valve exchanger, which is a self-expand support with biological valve, formed by the support in special shape and made from nickel titanium alloy skeleton and the three-blade one-way opening valve formed by pig heart, wherein, the support has fixing and supporting functions, and the pig heart forms three valves fixed in the support. The invention has little hurt, high safety and reduced complication.
Owner:孔祥清
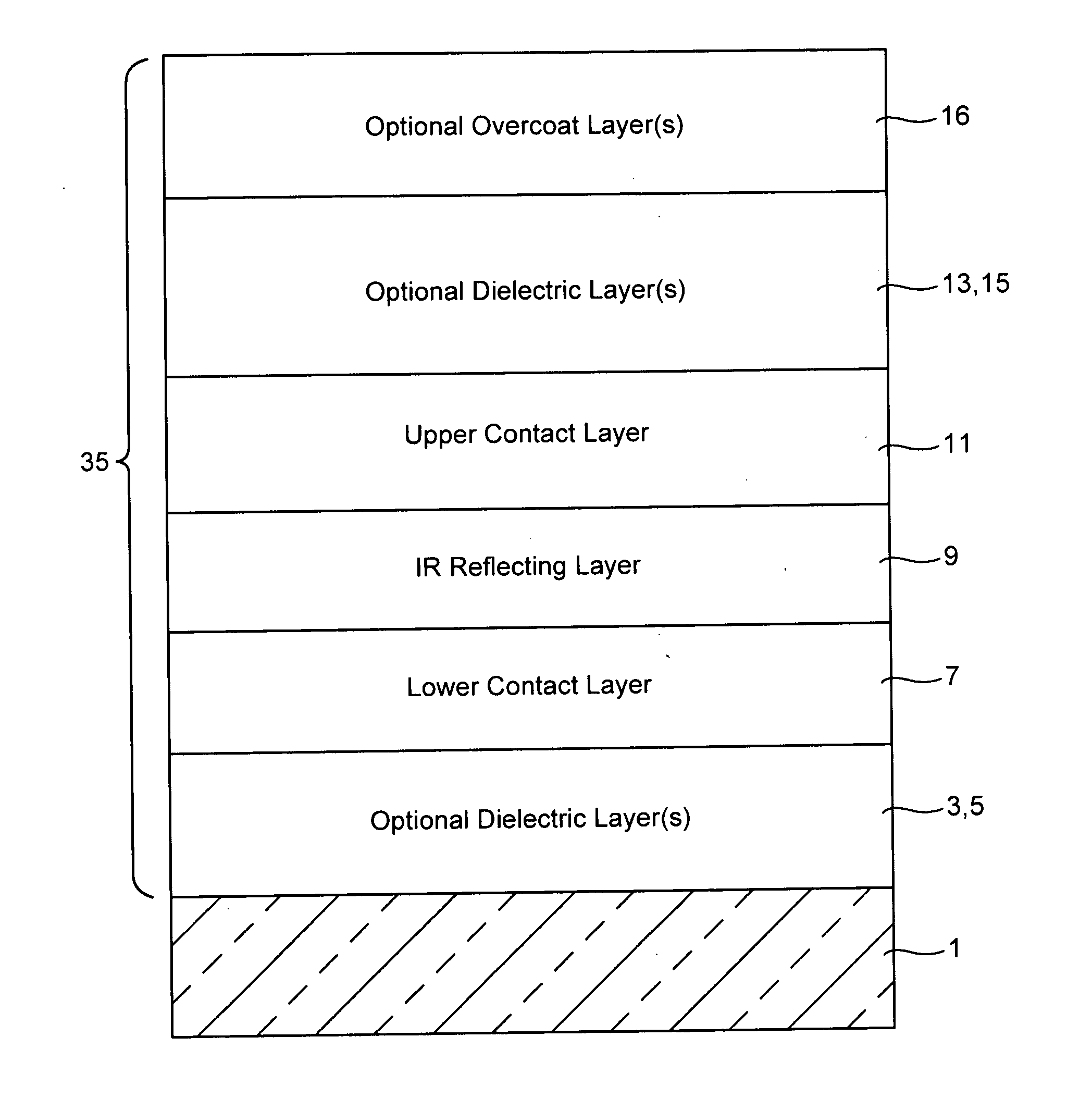
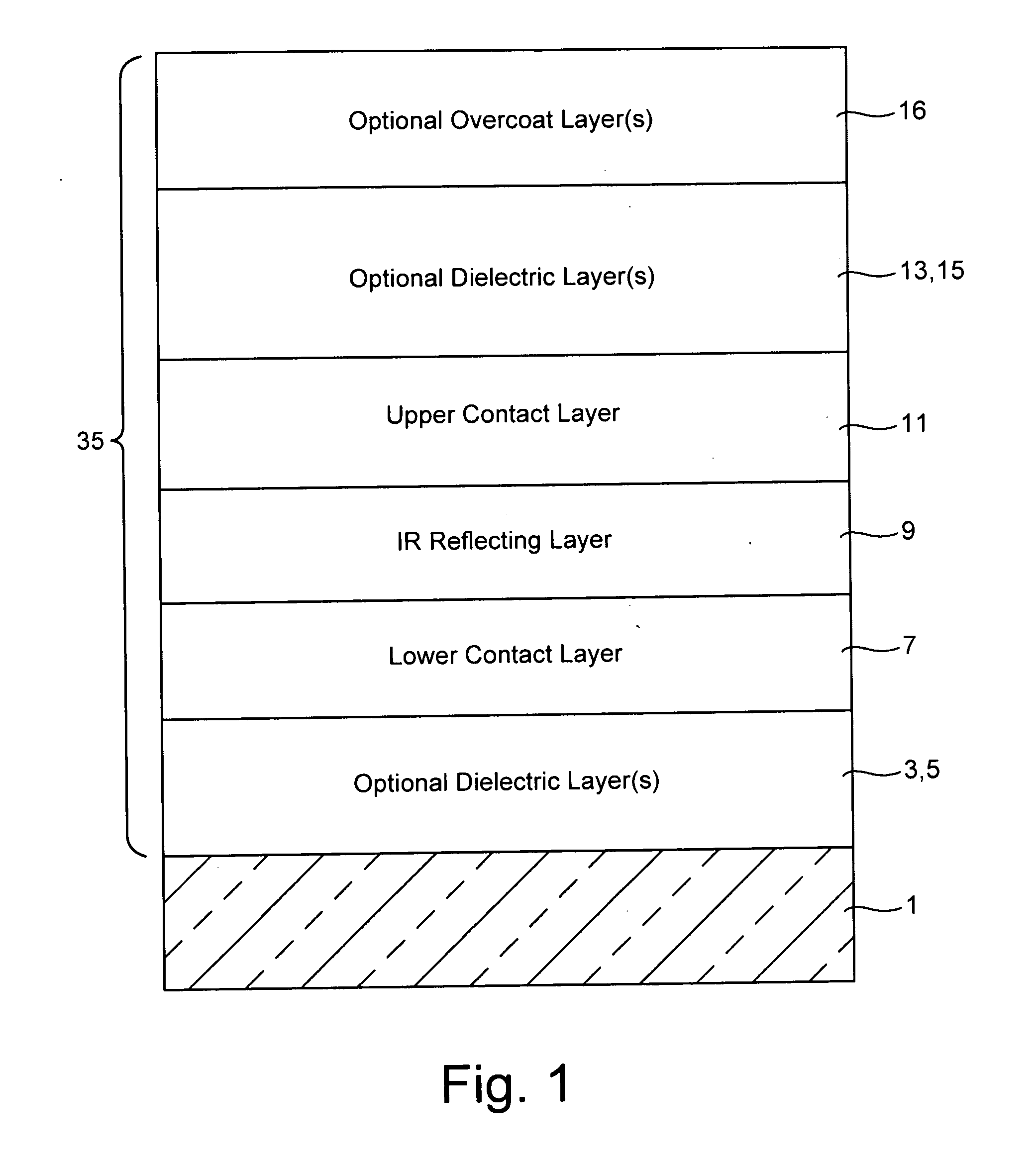
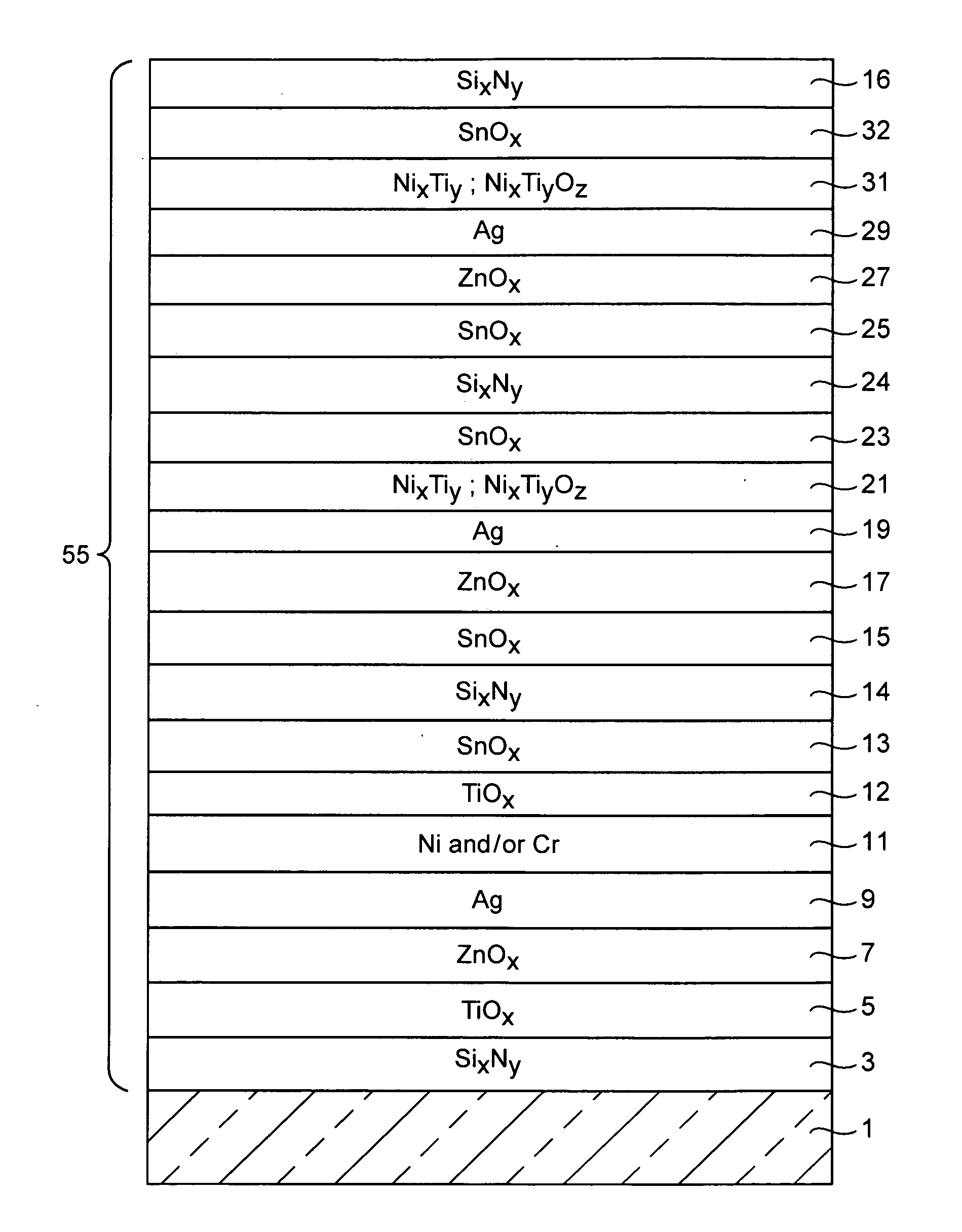
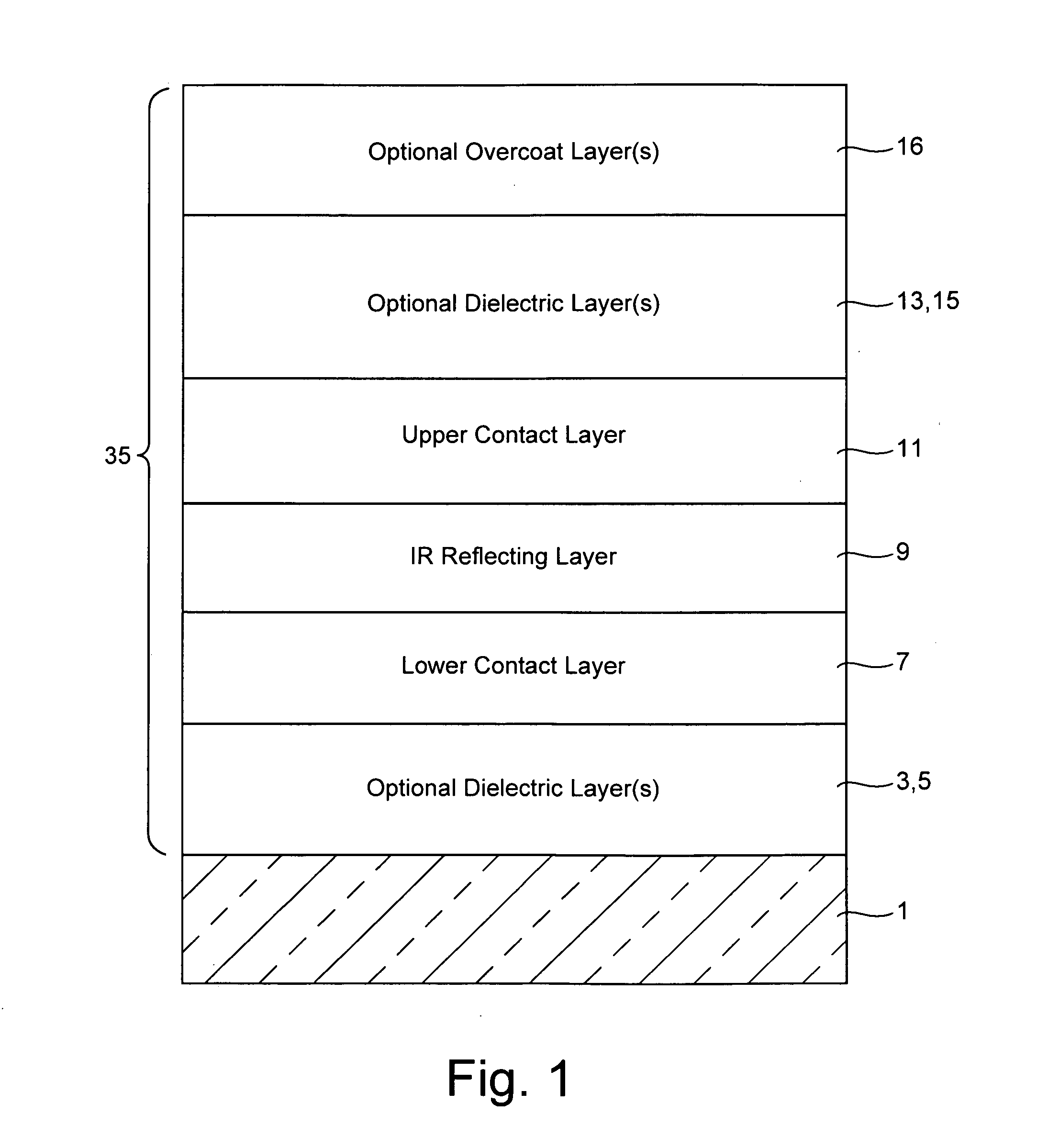
Coated article including low-emissivity coating, insulating glass unit including coated article, and/or methods of making the same
InactiveUS20120219821A1Reduce sheet resistanceImprove transmittancePretreated surfacesLaminationInsulated glazingLow emissivity
Certain example embodiments relate to a coated article including at least one infrared (IR) reflecting layer of a material such as silver or the like in a low-E coating, and methods of making the same. In certain cases, at least one layer of the coating is of or includes nickel and / or titanium (e.g., NixTiyOz). The provision of a layer including nickel titanium and / or an oxide thereof may permit a layer to be used that has good adhesion to the IR reflecting layer, and reduced absorption of visible light (resulting in a coated article with a higher visible transmission). When a layer including nickel titanium oxide is provided directly over and / or under the IR reflecting layer (e.g., as a barrier layer), this may result in improved chemical and mechanical durability. Thus, visible transmission may be improved if desired, without compromising durability; or, durability may simply be increased.
Owner:GUARDIAN EURO S A R L +1
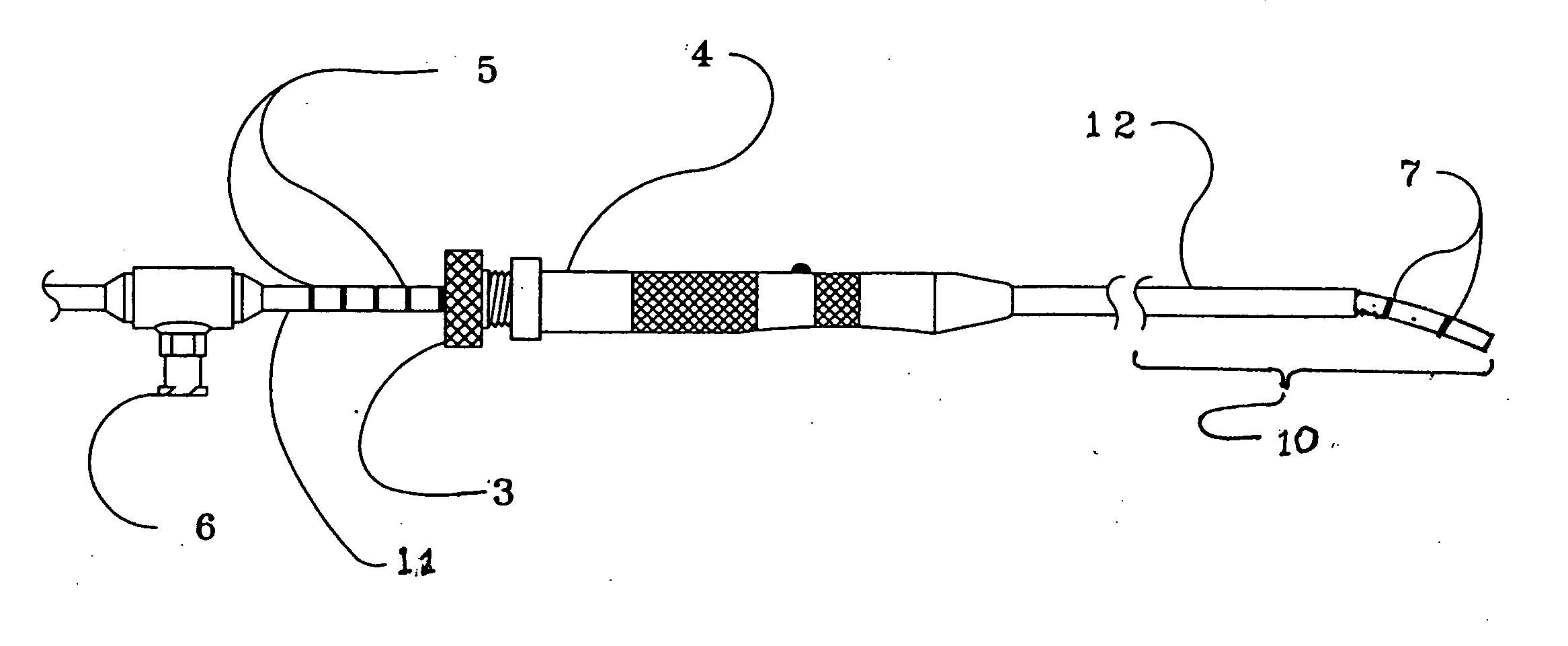
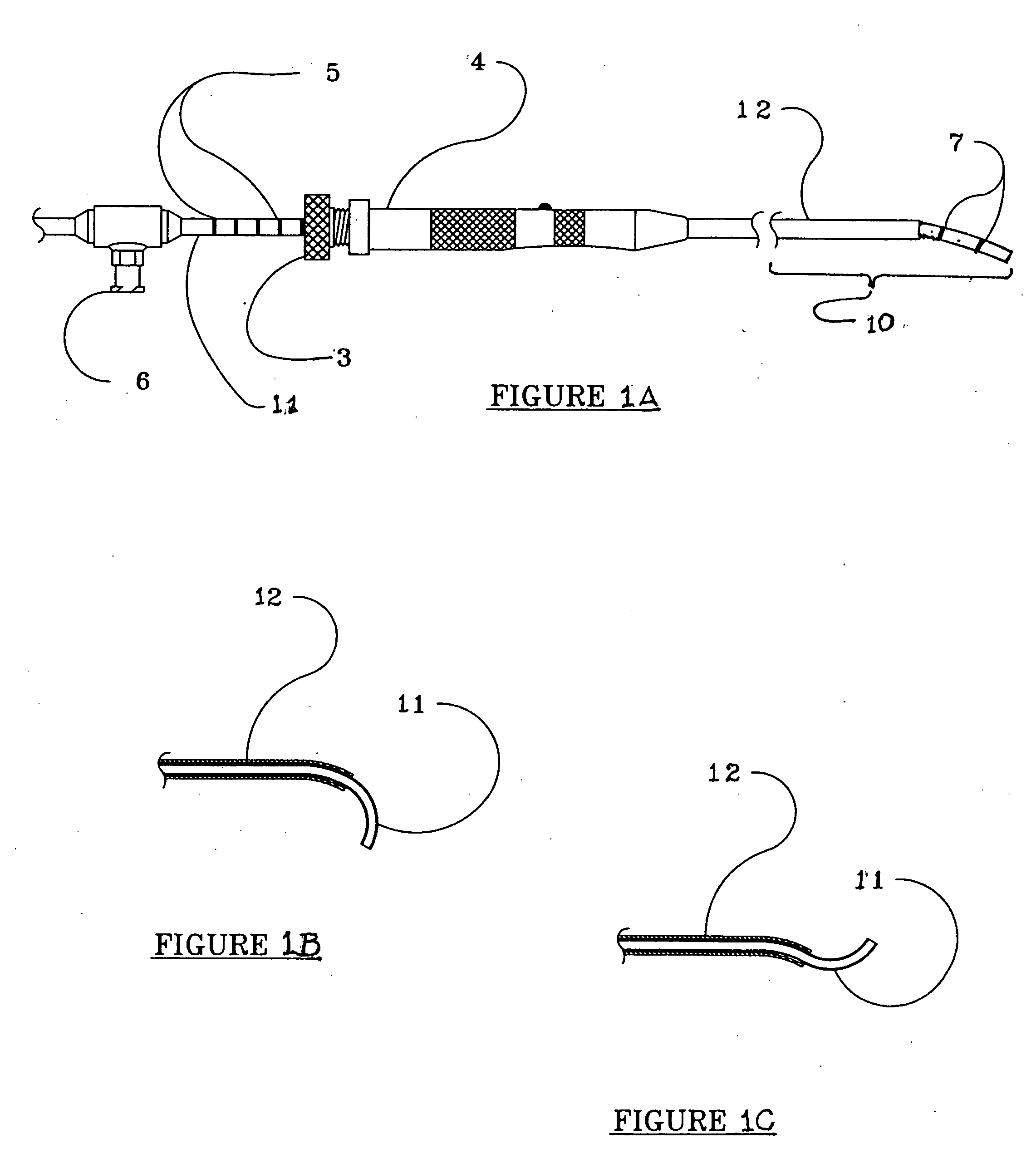
Angular deflection apparatus for use in confined spaces and method of use
InactiveUS20050187537A1Avoiding excessive coagulation and edemaElectrotherapyCatheterCurve shapeMetal alloy
A tube having a distal end portion made of a curved, flexible, shape retentive material such as superelastic, nickel-titanium memory metal alloy which has been heat-treated to retain a desired curved shape. The tube translates within and is constrained by a rigid sleeve, which may alternately be an instrument channel of an endoscope. When the distal end portion of the tube is extended from the sleeve, it returns to its original curved shape. Markings about the proximal and, or distal end portions of the tube enable the operator to know to what extent the distal end portion of the tube has been extended from the sleeve even when the distal end portion of the tube is not visible. The tube may be used for supporting and activating a cutting, abrading, coagulating, shrinking, or vaporizing device that is brought near or into contact with a tissue surface.
Owner:TRIMEDYNE
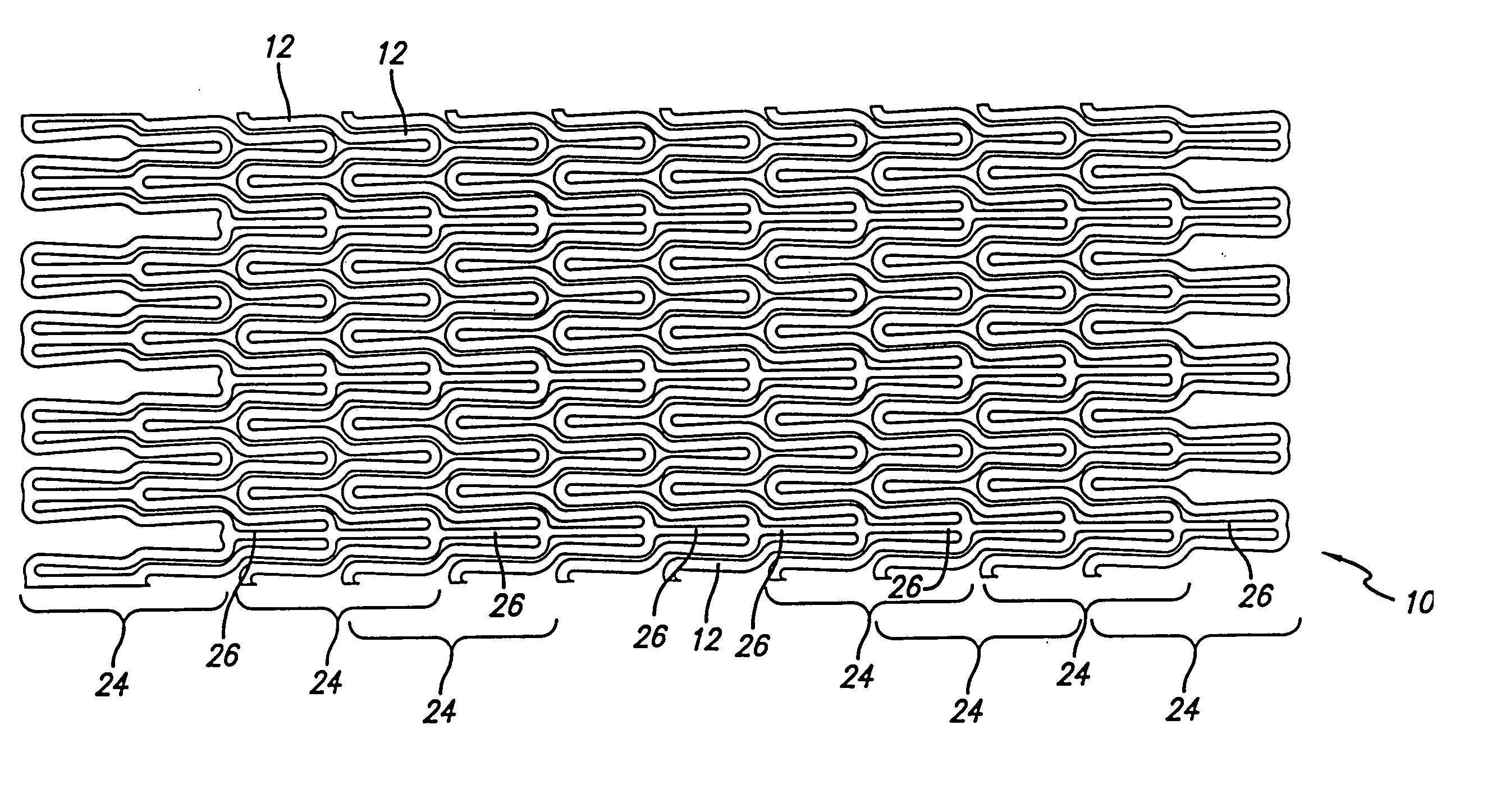
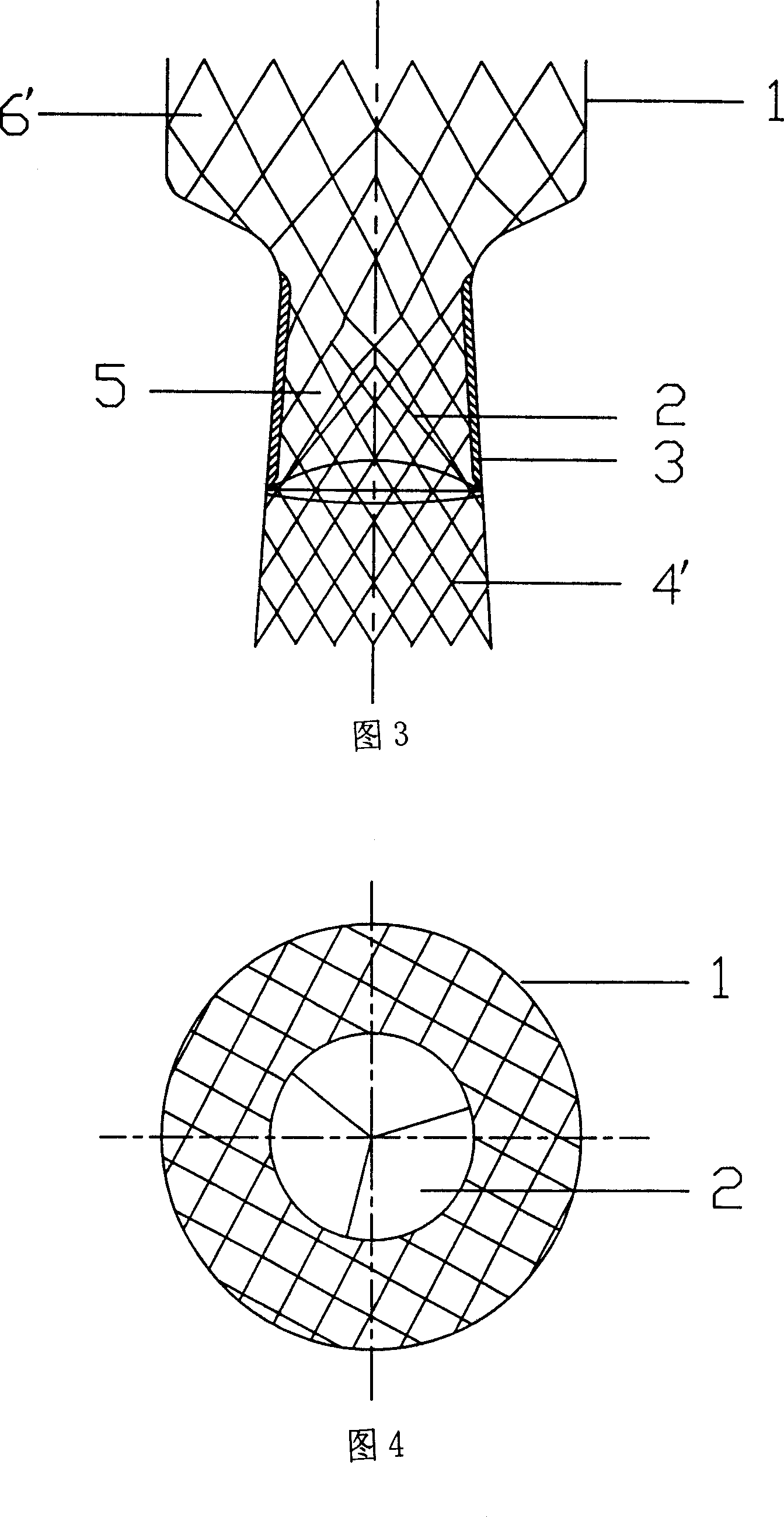
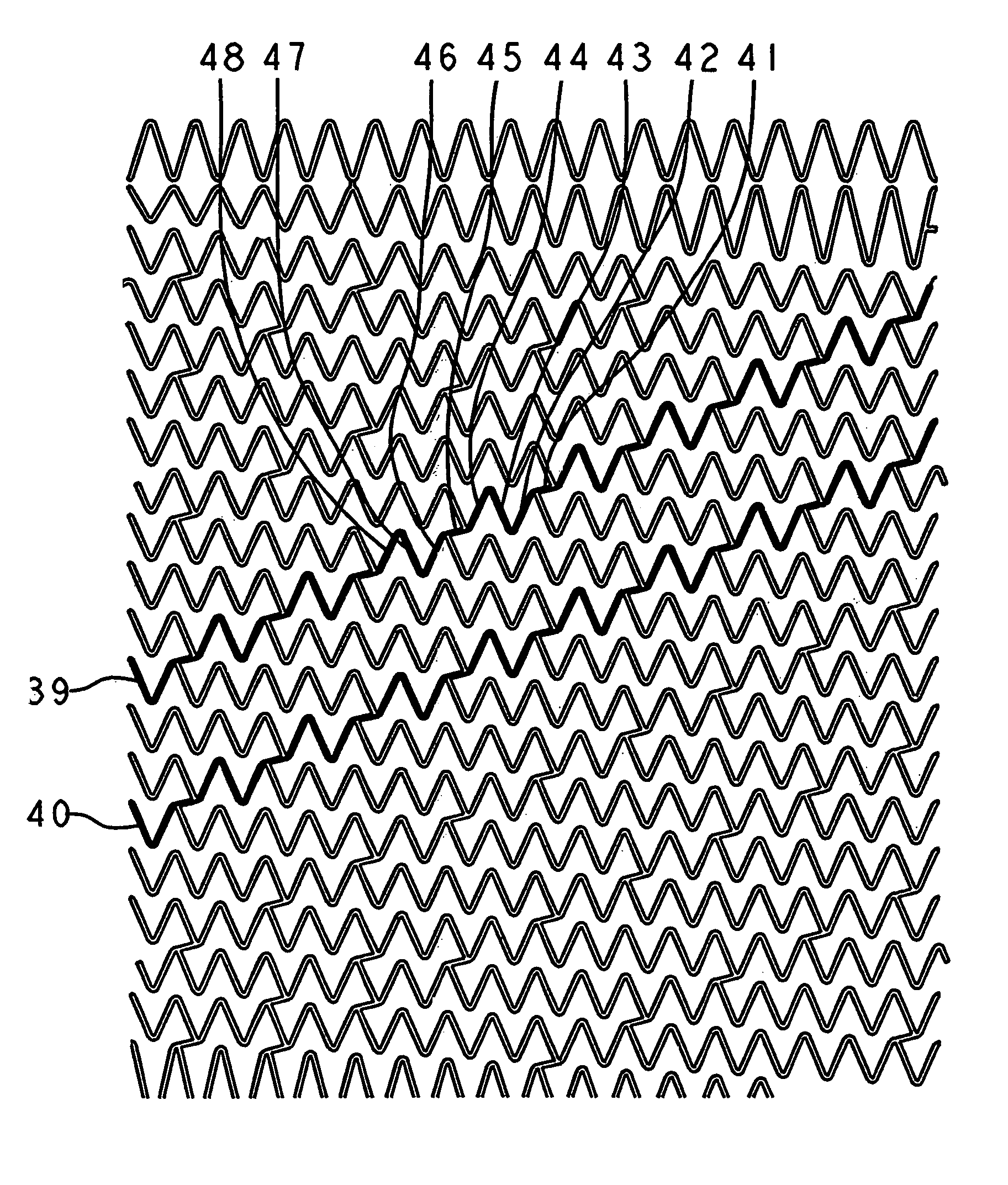
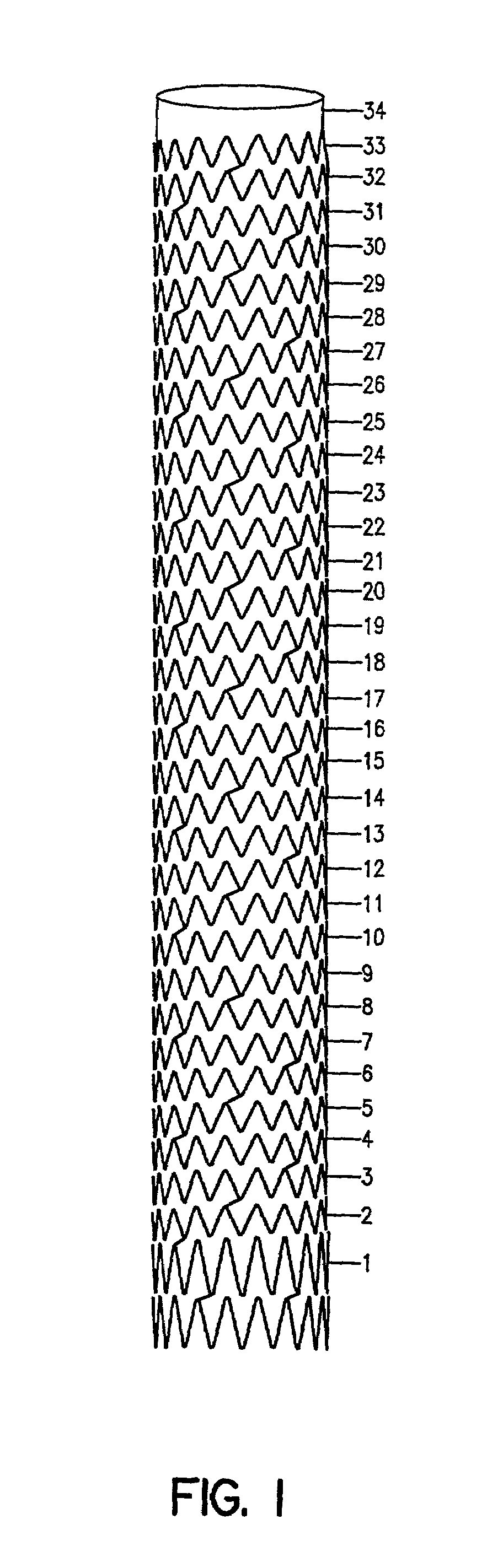
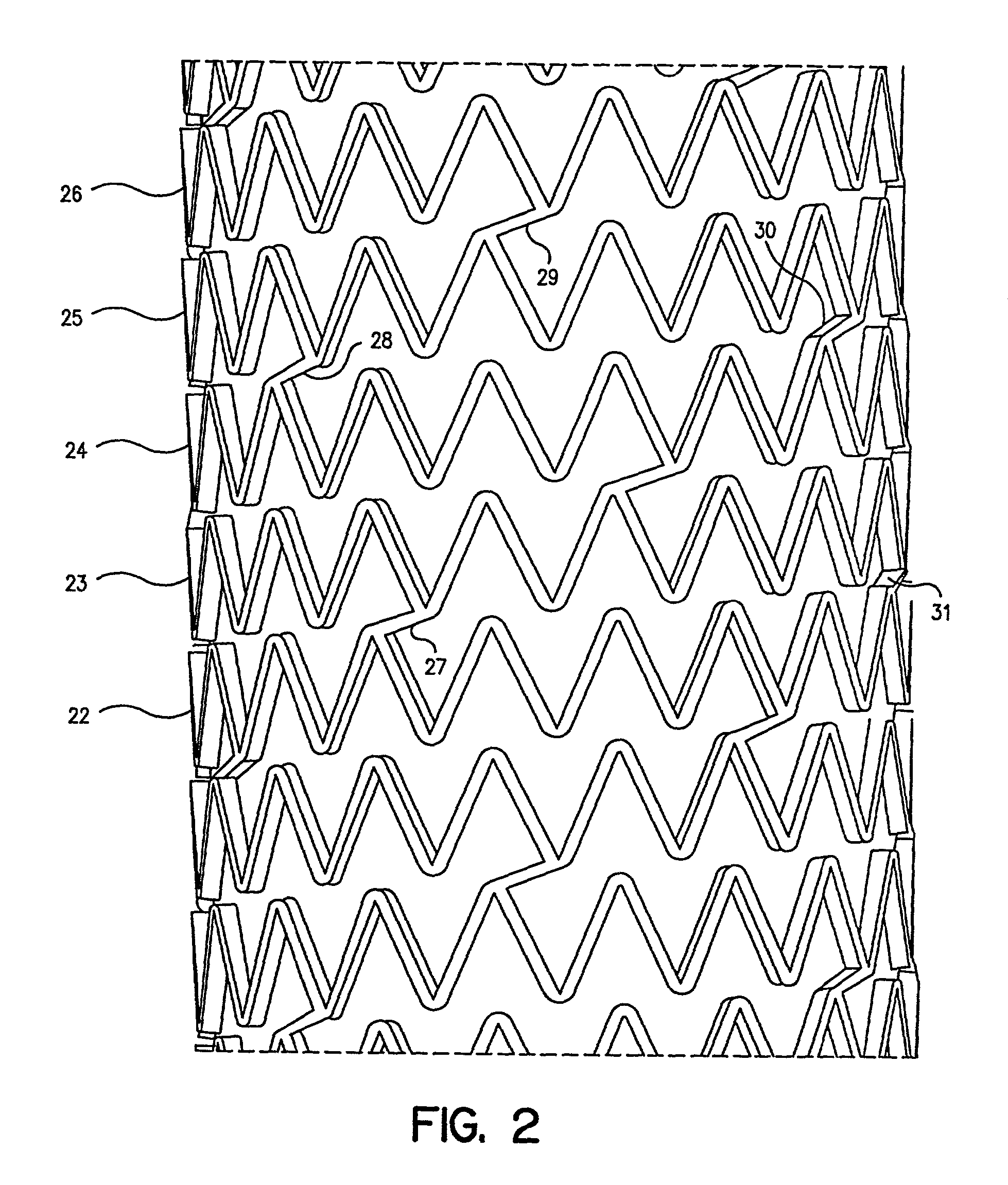
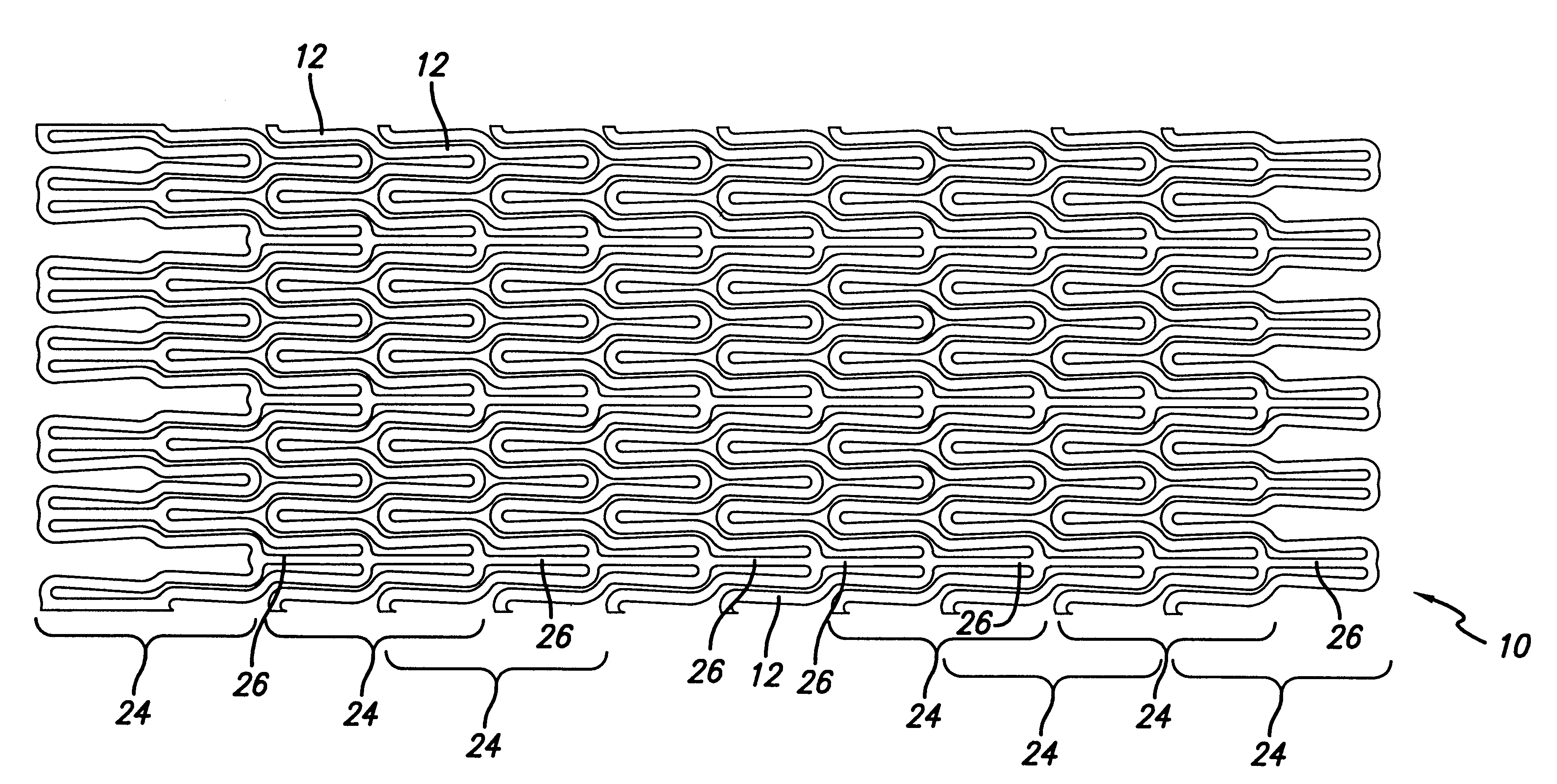
Self-expanding stent
The stent of this invention is a self-expanding stent created by a scaffolding lattice. The stent may be made from a nickel-titanium alloy. The lattice is formed from two different types of helices that proceed circumferentially in opposite directions along the longitudinal axis of the stent. The helices have no free ends. The first type of helix is formed by a series of undulations and the second type of helix is formed from a series of connection elements. The undulations may be in a zigzag or sinusoidal pattern. The connection elements connect the junction points lying on adjacent turns of the first type of helix. The junction points are formed by the ascending and descending arms of the undulations or zigzags. The ends of the stent may be formed by a closed circumferential element which is linked by connection elements to a transition zone. The transition zone is formed by a closed loop that connects directly to the first helix. The amplitude of the undulations or zigzags forming the transition zone increases from the closed loop to the point connecting the transition zone with the first type of helix. The closed circumferential element may be made from a radiopaque material. The scaffolding lattice design of the stent provides a stent having a high degree of flexibility as well as radial strength.
Owner:ORBUSNEICH MEDICAL PTE LTD
Vasoocclusive device for treatment of aneurysms
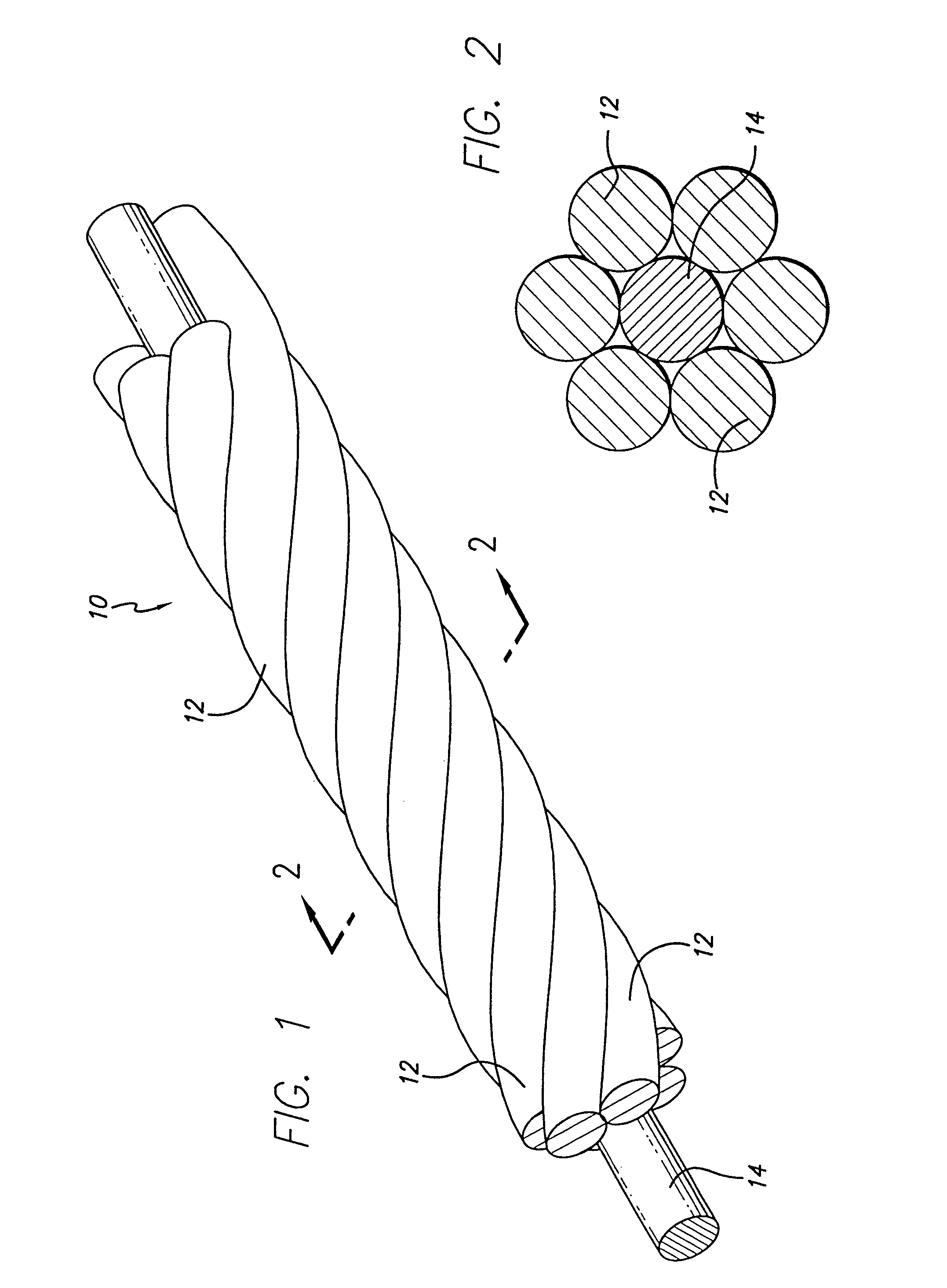
The device is formed from a multi-stranded micro-cable having a plurality of flexible strands of a shape memory material and at least one radiopaque strand. The strands can be made of a shape memory nickel titanium alloy, that is highly flexible at a temperature appropriate for introduction into the body via a catheter, and that after placement will take on the therapeutic shape. The radiopaque strand can have a core strand with a plurality of intermittently spaced apart enlarged radiopaque portions that may be a plurality of beads of radiopaque material spaced apart and mounted on the core strand, or a plurality of coils intermittently wound about and spaced apart on the core strand. A polyhedral occlusive device is also provided, adapted to be inserted into a portion of a vasculature for occluding a portion of the vasculature, for use in interventional therapy and vascular surgery.
Owner:MICRUS ENDOVASCULAR CORP
Osteosynthetic Implants and Methods of Use and Manufacture
ActiveUS20080269808A1Promote repairSmall sectionSuture equipmentsInternal osteosythesisBone fixation devicesBiomedical engineering
Owner:MEDSHAPE
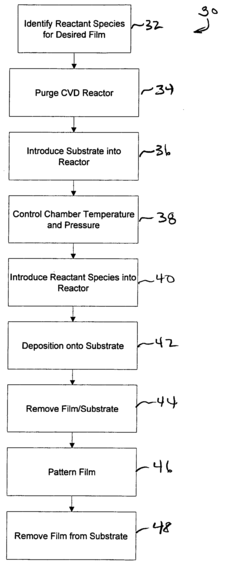
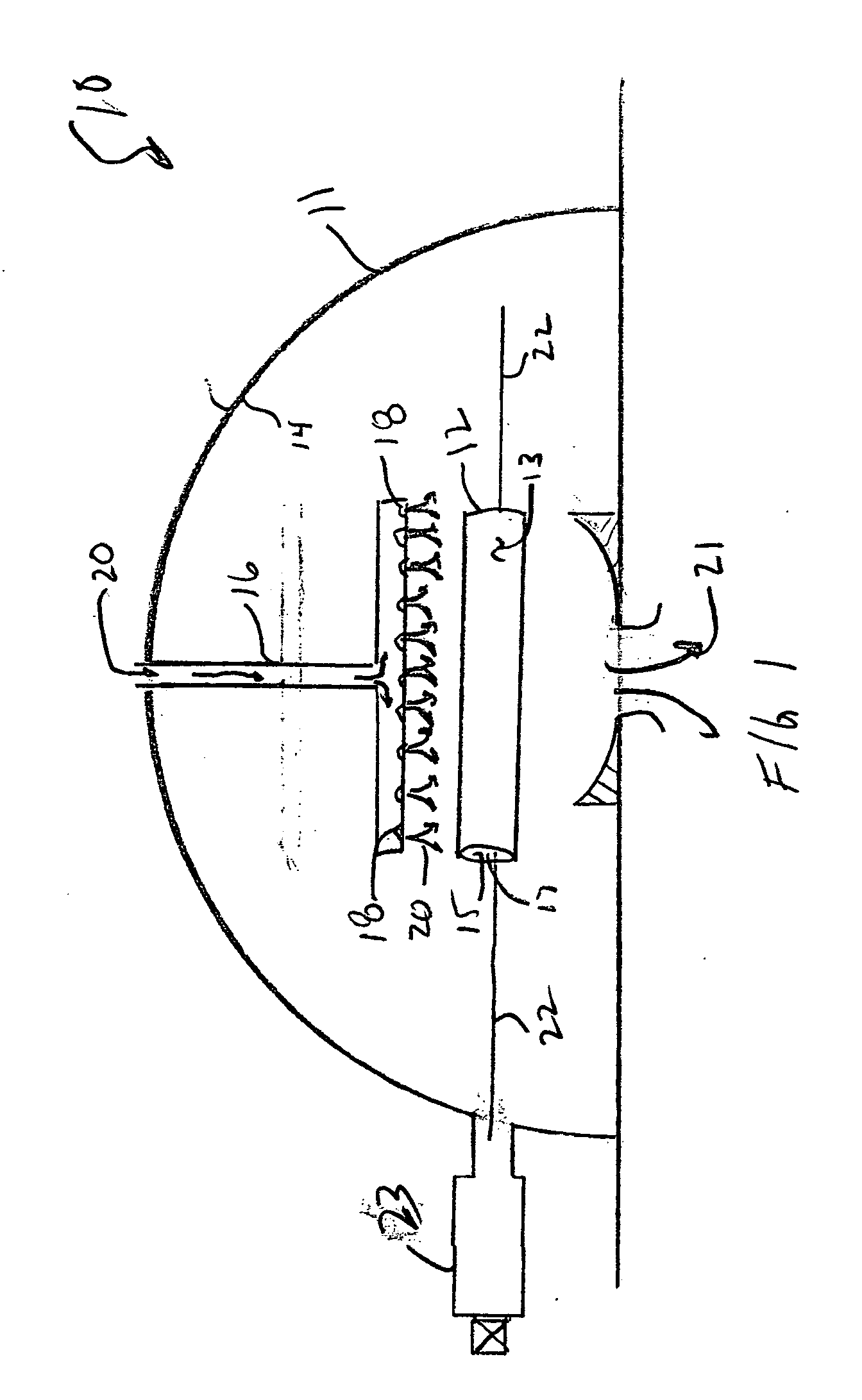
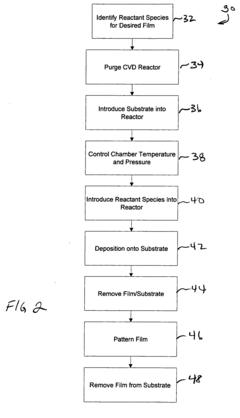
Methods of making shape memory films by chemical vapor deposition and shape memory devices made thereby
A method of depositing shape memory or superelastic thin films by chemical vapor deposition (CVD) and medical devices made thereby, including stents, grafts, stent-grafts, stent covers, occlusive and filter membranes and drug-delivery devices. The method entails a thin film is deposited on a substrate surface using a CVD reaction in the production of a film of nickel-titanium shape memory or superelastic alloy. Such nickel-titanium-based shape memory or superelastic alloys may be binary nickel-titanium alloys or may include additional compounds to form ternary, quaternary, or higher level alloys.
Owner:VACTRONIX SCI LLC
Avoiding stress-induced martensitic transformation in nickel titanium alloys used in medical devices
A process for assembling a medical device made from a nickel-titanium alloy for use in a mammalian body while avoiding the formation of stress-induced martensite and a medical device used in combination with a delivery system for deployment into the mammalian body are disclosed. By heating the nickel-titanium alloy of the medical device to a temperature above Md, and deforming and installing the device into a delivery system or holding capsule, it is possible to avoid the formation of stress-induced martensite in the stent, which stays in the austenitic phase throughout.
Owner:ABBOTT LAB INC
Vasoocclusive device for treatment of aneurysms
The three-dimensional device is adapted to be inserted into a portion of a vasculature for occluding the portion of the vasculature for use in interventional therapy and vascular surgery. The device is formed from a multi-stranded micro-cable having a plurality of flexible strands of a shape memory material and at least one radiopaque strand. The flexible strands in a multi-stranded micro-cable of the device can be helically wound, or can be configured as parallel, longitudinal strands, and can also be formed to have a secondary, three-dimensional therapeutic configuration, such as helical, conical, spherical, or other geometric shapes. The strands can be made of a shape memory nickel titanium alloy, that is highly flexible at a temperature appropriate for introduction into the body via a catheter, and that after placement will take on the therapeutic shape. The device can also include a therapeutic agent, and can be bundled by an outer cover to constrain the strands of the micro-cable about a longitudinal axis to produce a composite banded cable. The radiopaque strand can have a core strand with a plurality of intermittently spaced apart enlarged radiopaque portions that may be a plurality of beads of radiopaque material spaced apart and mounted on the core strand, or a plurality of coils intermittently wound about and spaced apart on the core strand. A polyhedral occlusive device is also provided, adapted to be inserted into a portion of a vasculature for occluding a portion of the vasculature, for use in interventional therapy and vascular surgery. One or more therapeutic fibers can also be woven into the coil to enhance treatment of the side after placement of the device.
Owner:DEPUY SYNTHES PROD INC
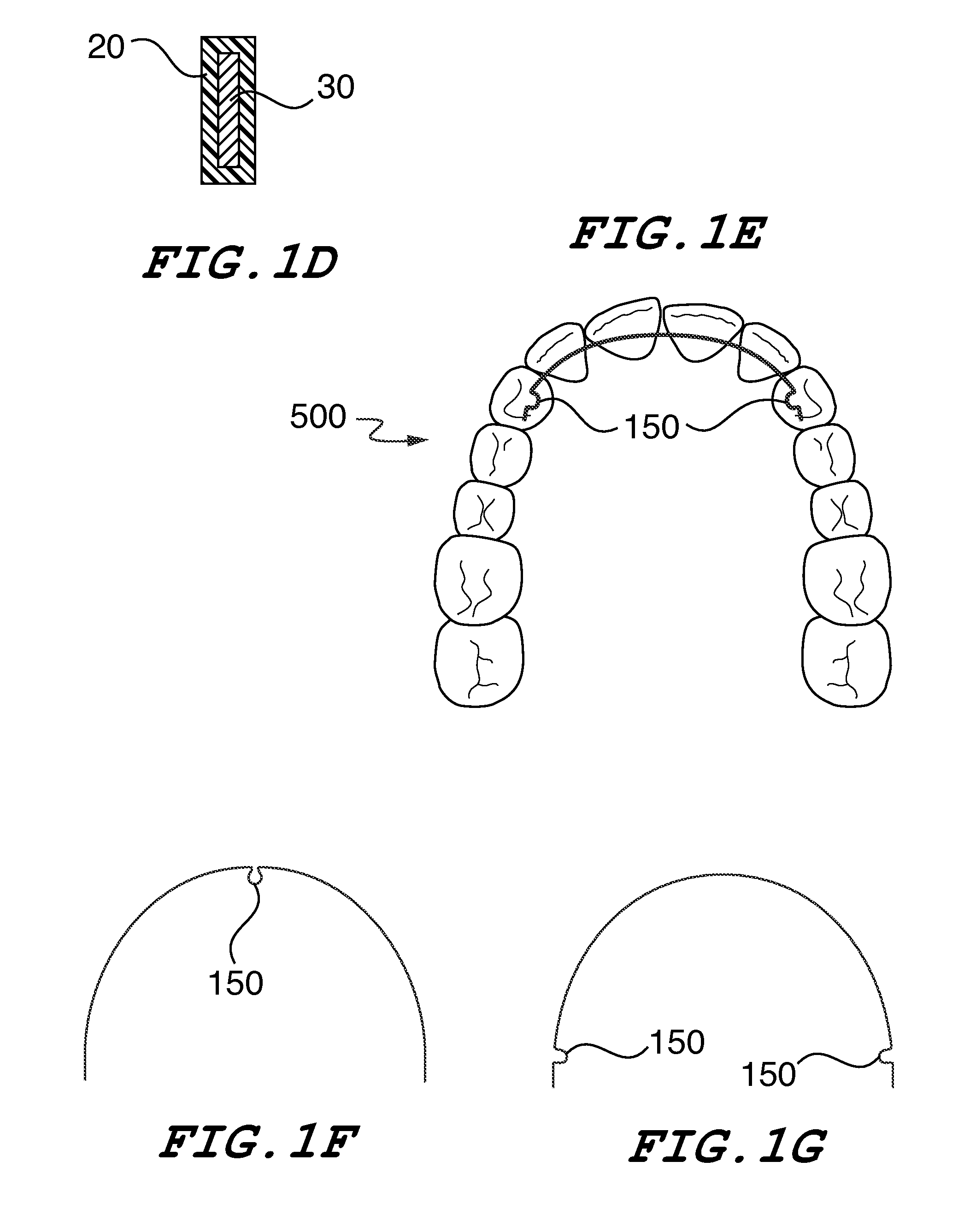
Orthodontic Wire Alignment System and Method
ActiveUS20140302448A1Minimize contaminationPrecise positioningArch wiresBracketsLingual surfaceResin-Based Composite
Disclosed is a system and method for treating mal-alignment of teeth using super-elastic nickel titanium, heat activated nickel titanium coated or uncoated orthodontic wires with composite resins in order to effectuate desired tooth alignment. The composite resin is formed into beads that hold the wire in place preferably on the lingual surface of the teeth. Alternate embodiments using composite brackets are disclosed. The overall purpose of this invention is to provide a close contact, low profile orthodontic system, in particular, a lingual orthodontic system that is significantly more comfortable than existing orthodontic systems, completely concealed and effective.
Owner:ALTA SMILES LLC
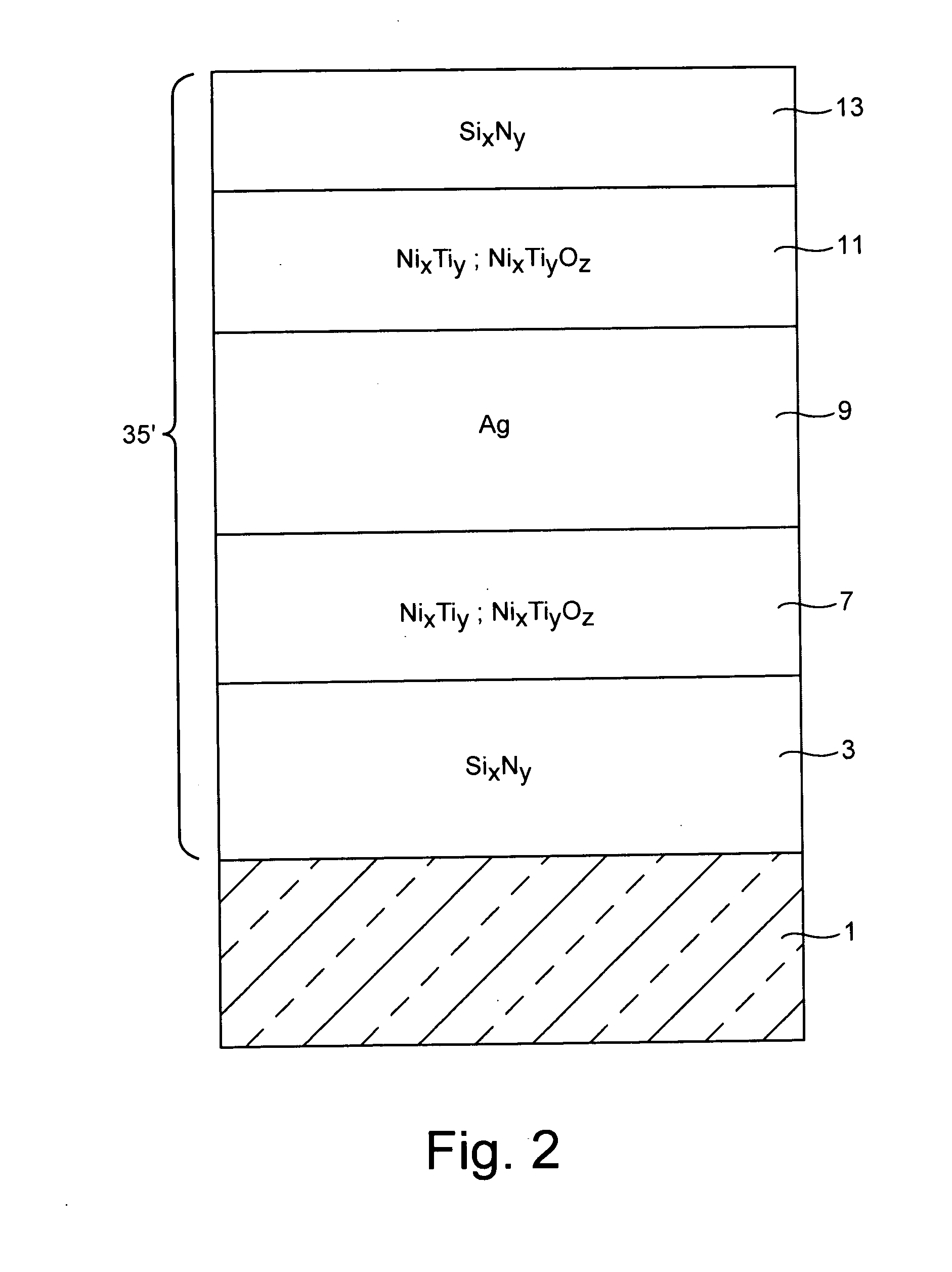
Barrier layers comprising Ni and/or Ti, coated articles including barrier layers, and methods of making the same
InactiveUS20120225224A1Reduce sheet resistanceImprove transmittanceVacuum evaporation coatingSputtering coatingTitaniumReflective layer
Certain example embodiments relate to a coated article including at least one infrared (IR) reflecting layer of a material such as silver or the like in a low-E coating, and methods of making the same. In certain cases, at least one layer of the coating is of or includes nickel and / or titanium (e.g., NixTiyOz). The provision of a layer including nickel titanium and / or an oxide thereof may permit a layer to be used that has good adhesion to the IR reflecting layer, and reduced absorption of visible light (resulting in a coated article with a higher visible transmission). When a layer including nickel titanium oxide is provided directly over and / or under the IR reflecting layer (e.g., as a barrier layer), this may result in improved chemical and mechanical durability. Thus, visible transmission may be improved if desired, without compromising durability; or, durability may simply be increased.
Owner:GUARDIAN GLASS LLC
Radiopaque nitinol alloys for medical devices
InactiveUS6855161B2Enhance alloy 's formabilityEnhance thermomechanical propertyStentsSurgeryIridiumRhenium
A radiopaque nitinol medical device such as a stent for use with or implantation in a body lumen is disclosed. The stent is made from a superelastic alloy such as nickel-titanium or nitinol, and includes a ternary element selected from the group of chemical elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, or hafnium. The added ternary element improves the radiopacity of the nitinol stent comparable to that of a stainless steel stent of the same size and strut pattern coated with a thin layer of gold. The nitinol stent has improved radiopacity yet retains its superelastic and shape memory behavior and further maintains a thin strut / wall thickness for high flexibility.
Owner:ABBOTT CARDIOVASCULAR
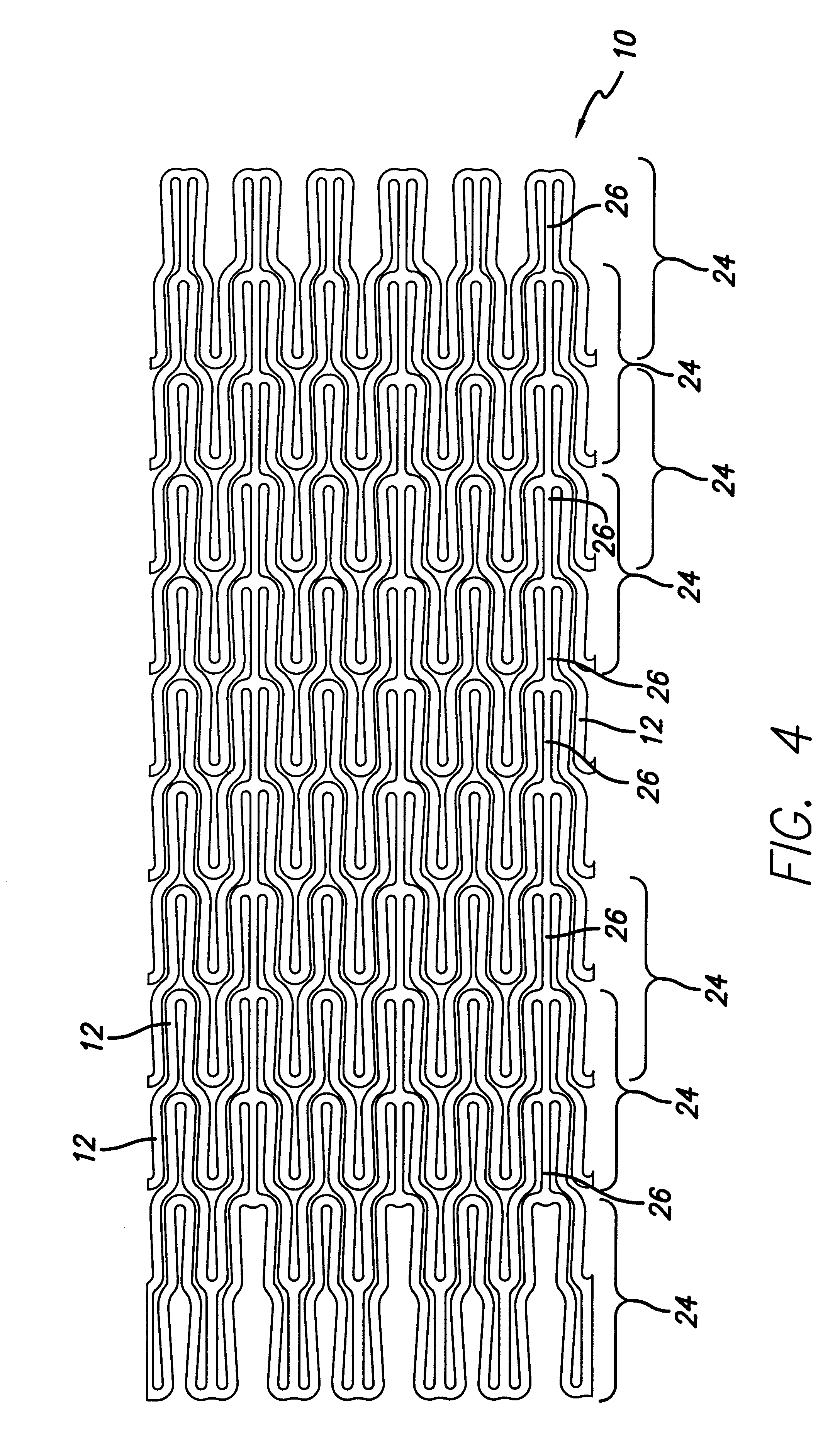
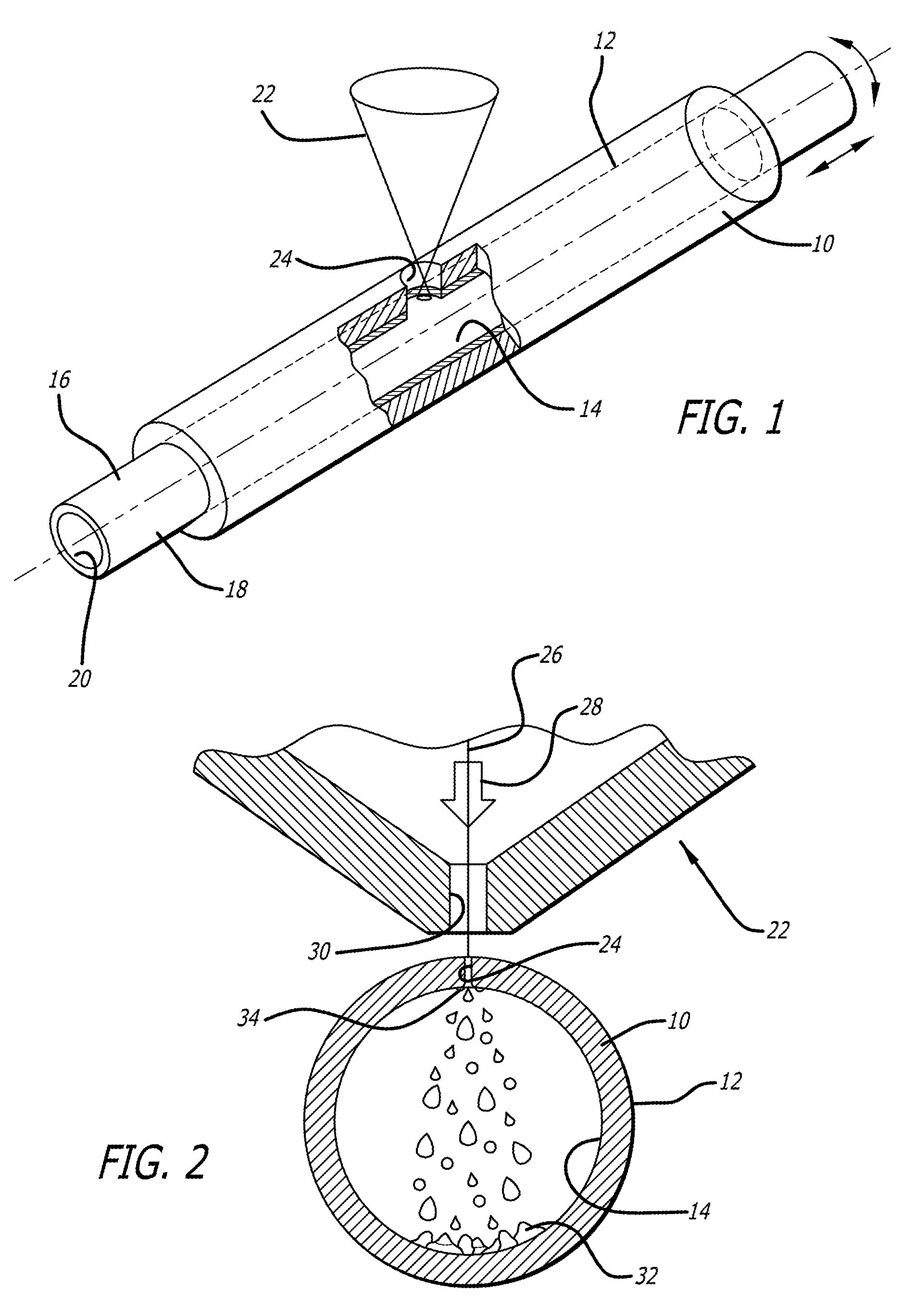
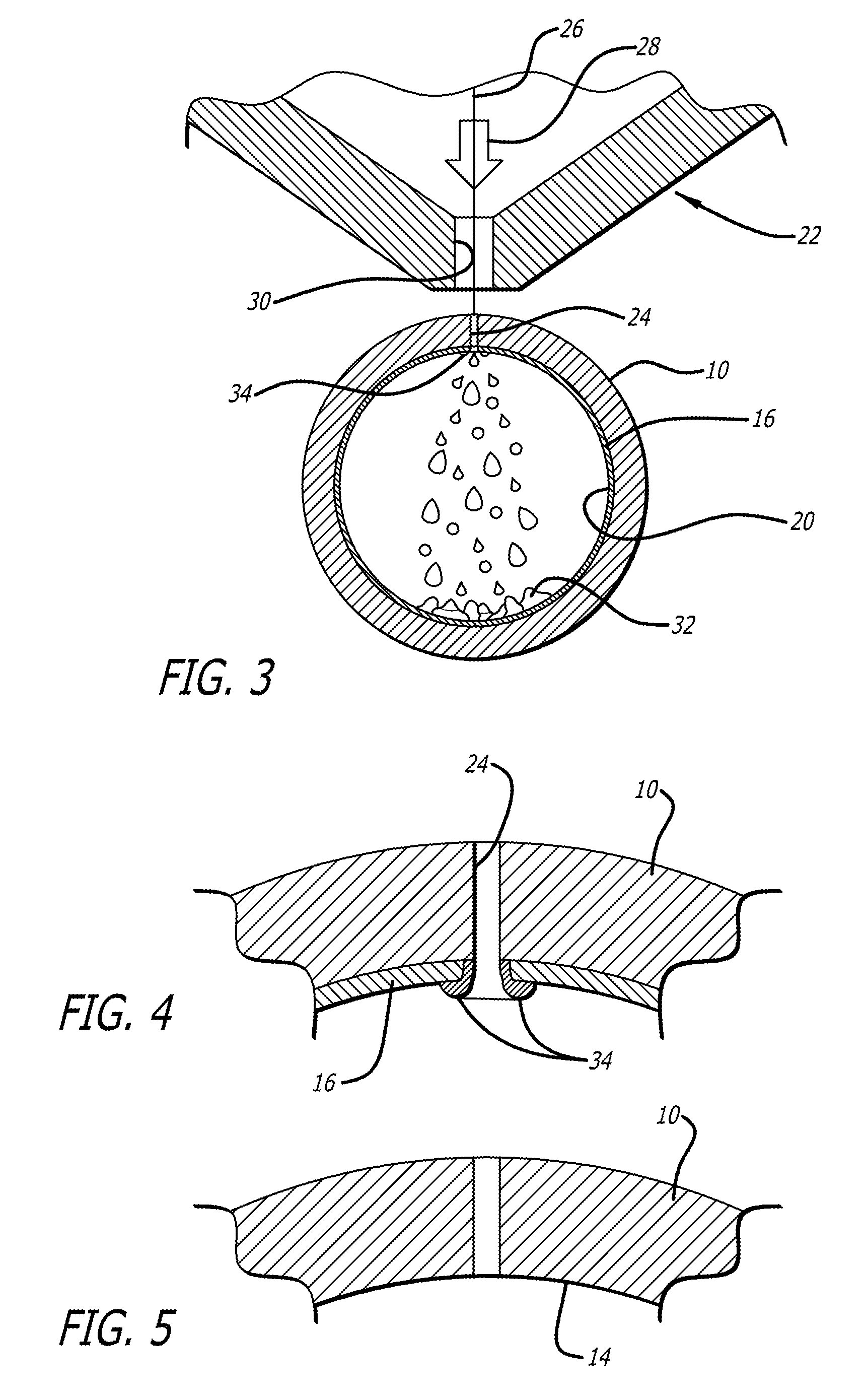
Methods and systems for laser cutting and processing tubing to make medical devices
Methods for making devices include providing a tubular member to be formed into a device, placing a removable sacrificial block material in the lumen of the tubular member and laser cutting the tubular member. A doping material can be added to the melted portion of the tubular member to promote the formation of brittle slag. A fixture can be used to hold a cut workpiece in order to ream sacrificial material from the surface of the workpiece. Pressurized gas can be supplied to the inner lumen of the tubular member to cause slag to form on the outside surface, rather than the inner surface, of the tubular member. A tubular member made from nickel-titanium alloy can be tightly adhered to a sacrificial sleeve utilizing the phase changes associated with nickel-titanium. A rotating mandrel can be placed within the lumen of the tubular member during laser cutting. A mandrel which includes an enlarged diameter section causes the workpiece to expand slightly within its elastic deformation range to dislodge islands from the workpiece. Such a mandrel could be formed from a tubular member which has a central lumen that can be used to deliver a pressurized medium to “blast” islands from the workpiece.
Owner:ABBOTT CARDIOVASCULAR
Implantable antenna
InactiveUS20090149918A1Enhanced tissue ingrowthIncrease signal strengthSpinal electrodesImplantable neurostimulatorsImplanted deviceEngineering
An antenna implantable through minimally invasive techniques, preferably comprising a coil with conductive probes is provided. The antenna is preferably superelastic nickel-titanium having an insulative coating. The antenna may conduct a signal originating from a device external to the body of the implantee, or from another implanted device connected to the antenna depending on whether the antenna is employed for sending, receiving, or transceiving signals. Signals may contain data, operational commands, and may be used to transfer power. The implantable antenna may be connected to another implanted device, such as a blood pressure monitor, or may be implanted as a stand-alone device for purposes such as stimulating tissue.
Owner:CORDIS CORP
Expandable curvilinear strut arrangement for deployment with a catheter to repair an aneurysm
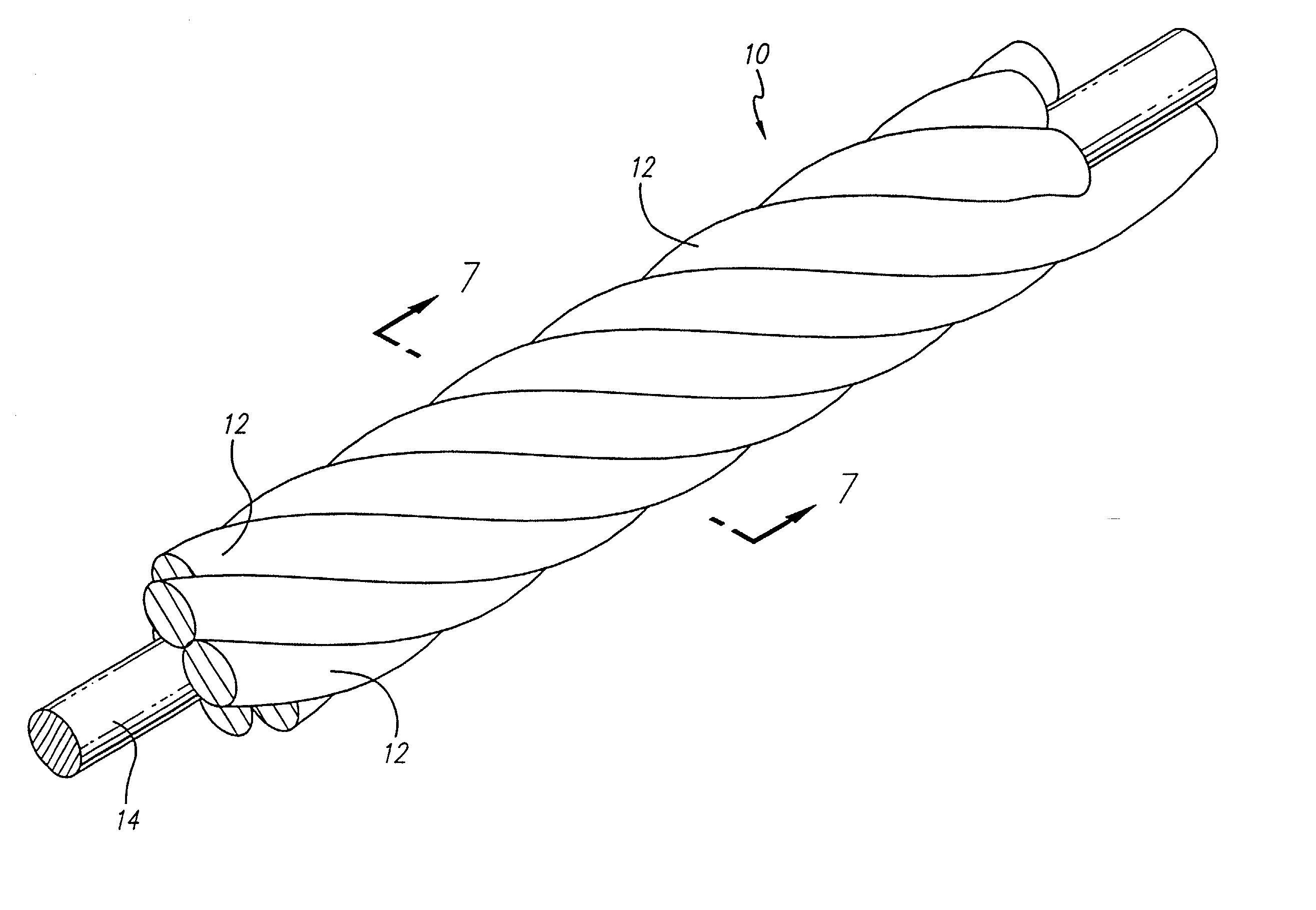
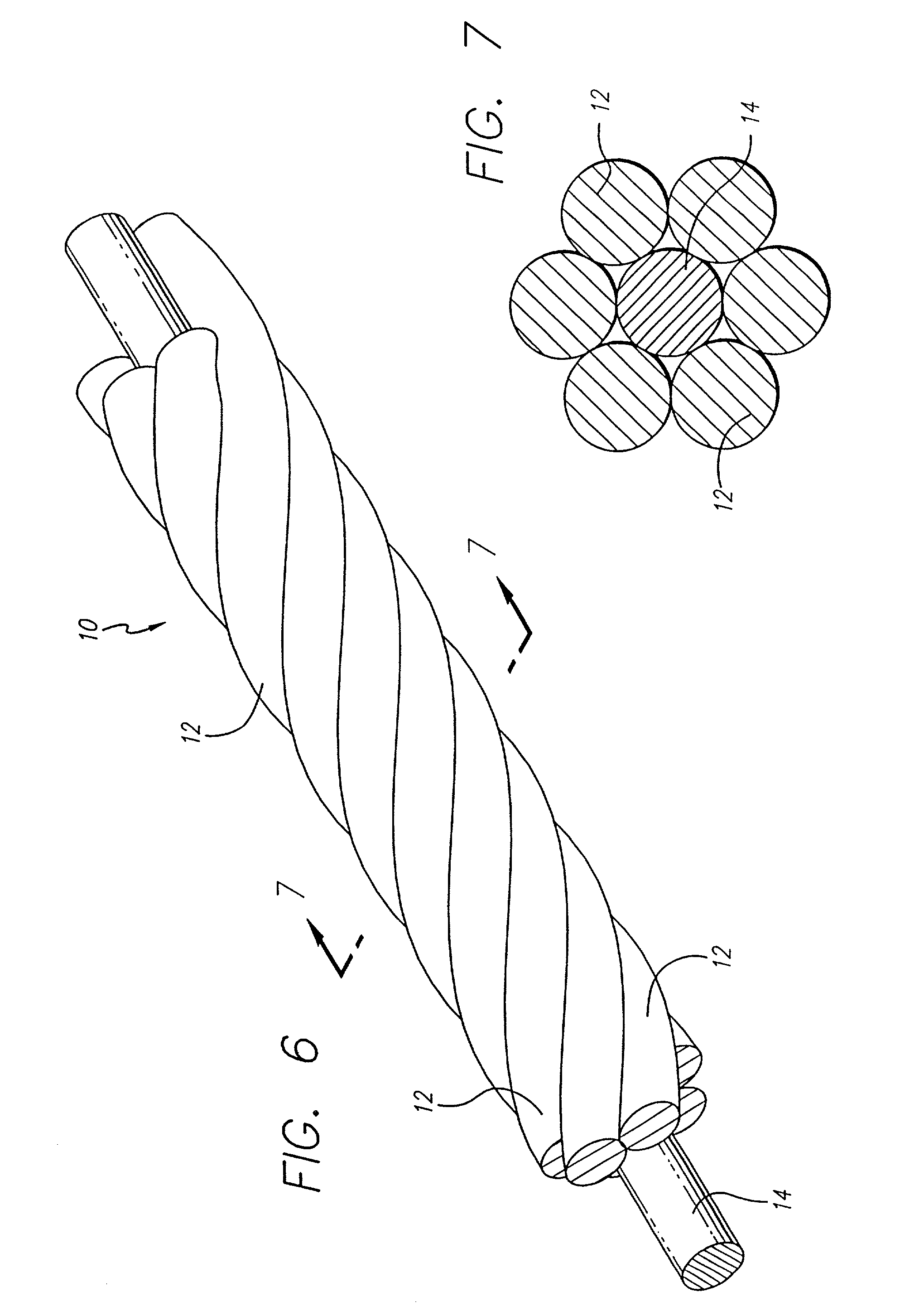
The vasoocclusive apparatus can be used in a method of interventional therapy and vascular surgery by inserting the apparatus into a portion of a vasculature, for treatment of a body vessel such as an aneurysm in conjunction with a secondary vasoocclusive device to be placed within the vessel. The vasoocclusiveVasooclusive apparatus includes a plurality of strut members connected together at a central hub that extend from a collapsed position to an expanded configuration to cross the neck of the aneurysm, dividing the neck into smaller openings, allowing the deployment of the secondary vasoocclusive device within the aneurysm but preventing migration of the secondary vasoocclusive device from the aneurysm. The strut members can be made from a twisted cable of strands of a superelastic material, such as a shape memory nickel titanium alloy, with at least one radiopaque strand. A shape memory collar is provided for detachably mounting the vasoocclusive apparatus to a pusher member and for detaching the vasoocclusive apparatus for deployment when a desired placement within an aneurysm to be treated and out of a parent vessel is achieved.
Owner:MICRUS CORP
Thin film vascular stent and biocompatible surface treatment
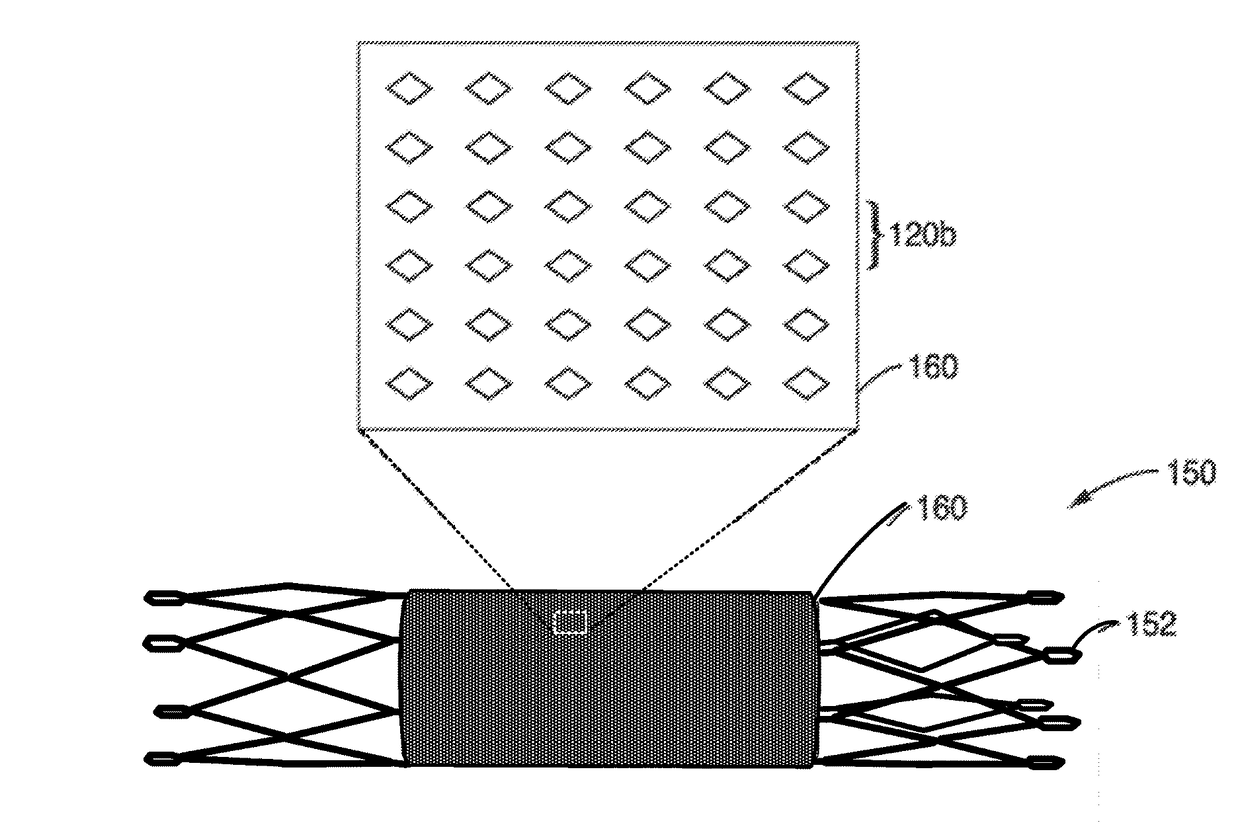
A vascular implant, comprising a sheet comprising thin film nickel titanium (NiTi), wherein the sheet has at least one super-hydrophilic surface having a water contact angle of less than approximately 5 degrees. The sheet is configured to have a compacted form having a first internal diameter and a deployed form having a second internal diameter larger than the first internal diameter. The sheet may be delivered into a blood vessel in the compacted form and expanded to its deployed form at a treatment location within the blood vessel, wherein the stent is configured to expand onto an internal surface of the blood vessel and exert a radial force on said internal surface.
Owner:RGT UNIV OF CALIFORNIA
Vasoocclusive coil
InactiveUS7070608B2Provide bending stiffnessDesired bendingDilatorsDiagnostic markersEngineeringAlloy
The vasoocclusive coil has a primary coil configuration with a helical loop at at least one end. The terminal helical loop can have a J-shaped configuration, preferably with a loop diameter of about 2 mm. The coil is preferably provided with helical loops at both ends, with helical loop at the proximal and distal ends of the coil acting as an anchor to prevent the coil from coming free from the location being treated and escaping into the vasculature. In a presently preferred embodiment both ends of the coil have a J-shape. In another presently preferred aspect, the vasoocclusive coil includes one or more loops intermediate the ends of the coil. The device is formed from a multi-stranded micro-cable having a plurality of flexible strands of a shape memory material and at least one radiopaque strand. The strands can be made of a shape memory nickel titanium alloy, that is highly flexible at a temperature appropriate for introduction into the body via a catheter, and that after placement will take on the therapeutic shape.
Owner:MICRUS CORP
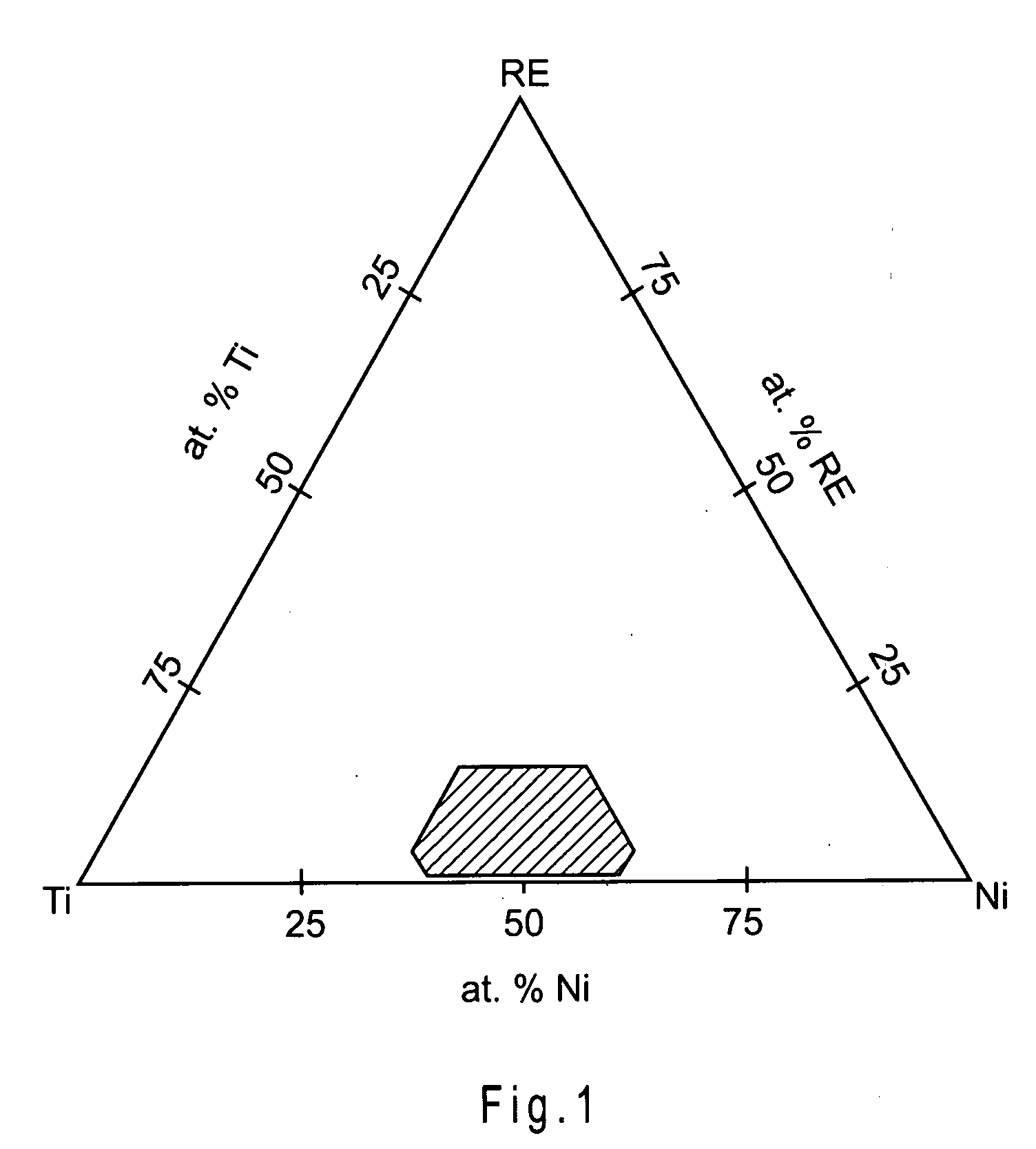
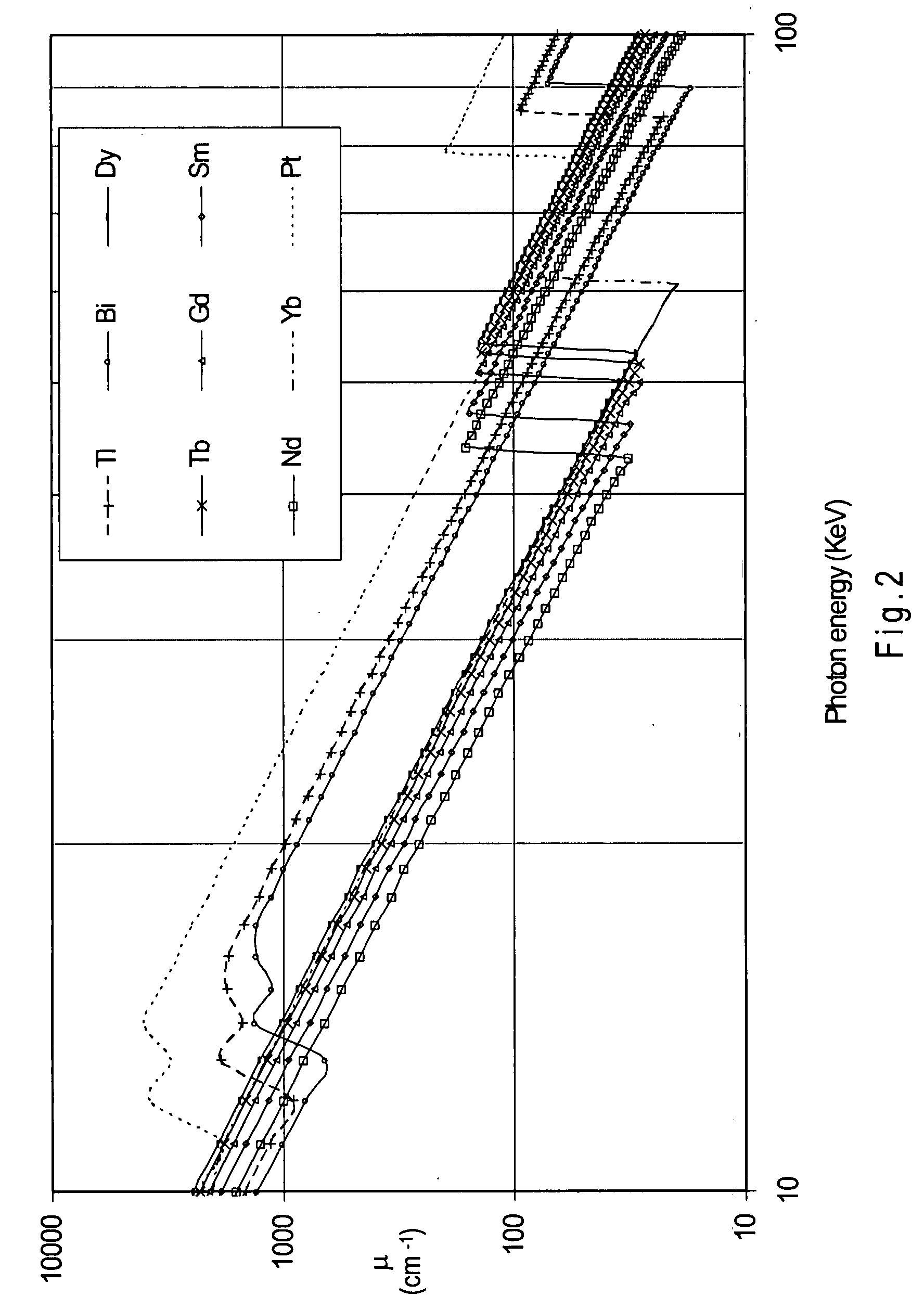
Nickel-titanium alloy including a rare earth element
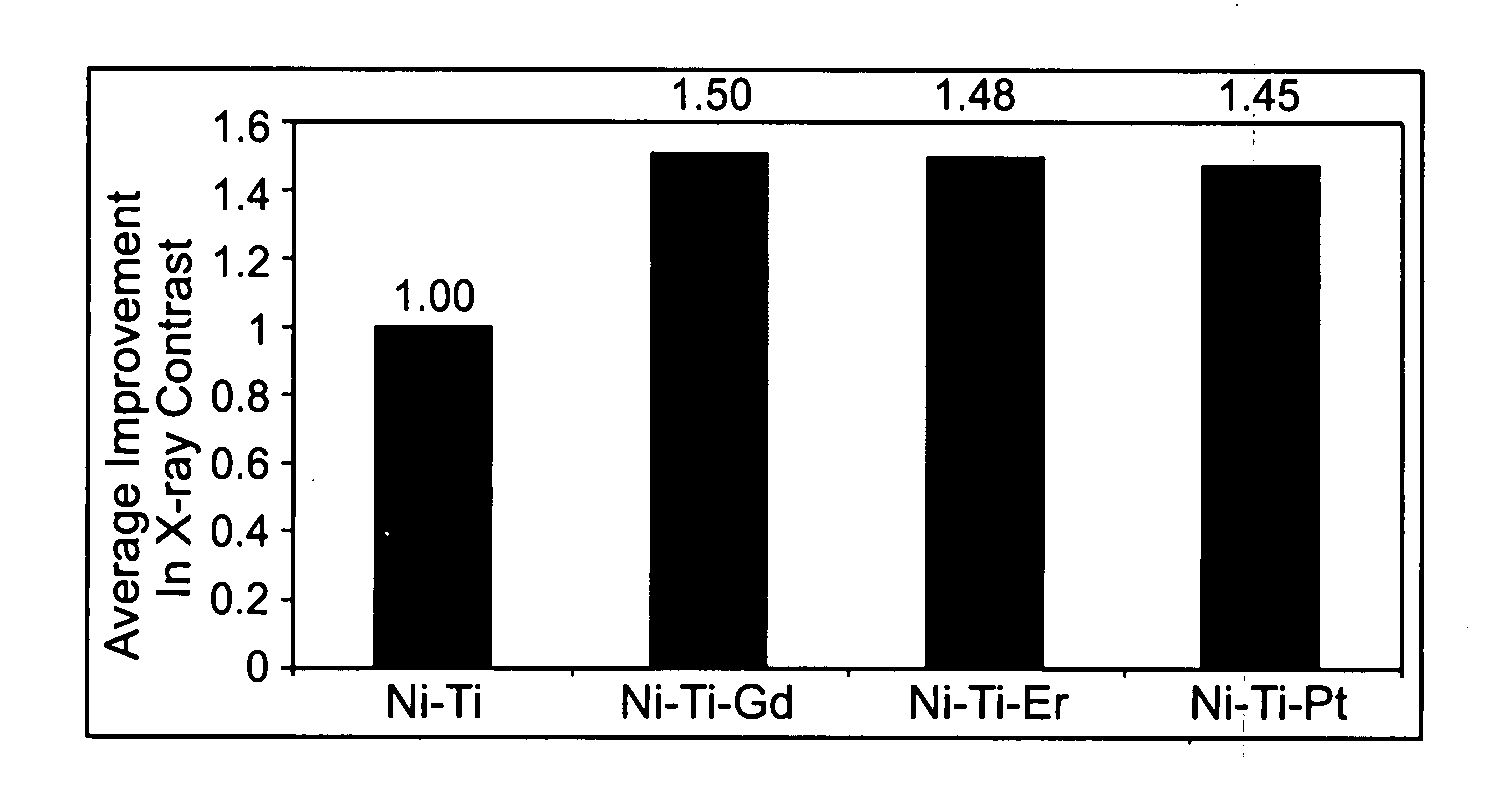
Disclosed herein is a nickel-titanium alloy comprising nickel, titanium, and at least one rare earth element. The nickel-titanium alloy comprises from about 34 at. % to about 60 at. % nickel, from about 34 at. % to about 60 at. % titanium, and from about 0.1 at. % to about 15 at. % at least one rare earth element. The nickel-titanium alloy may further include one or more additional alloying elements. In addition to radiopacity, the nickel-titanium alloy preferably exhibits superelastic or shape memory behavior. Medical devices comprising the nickel-titanium alloy and a method of making them are also disclosed.
Owner:COOK MEDICAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com