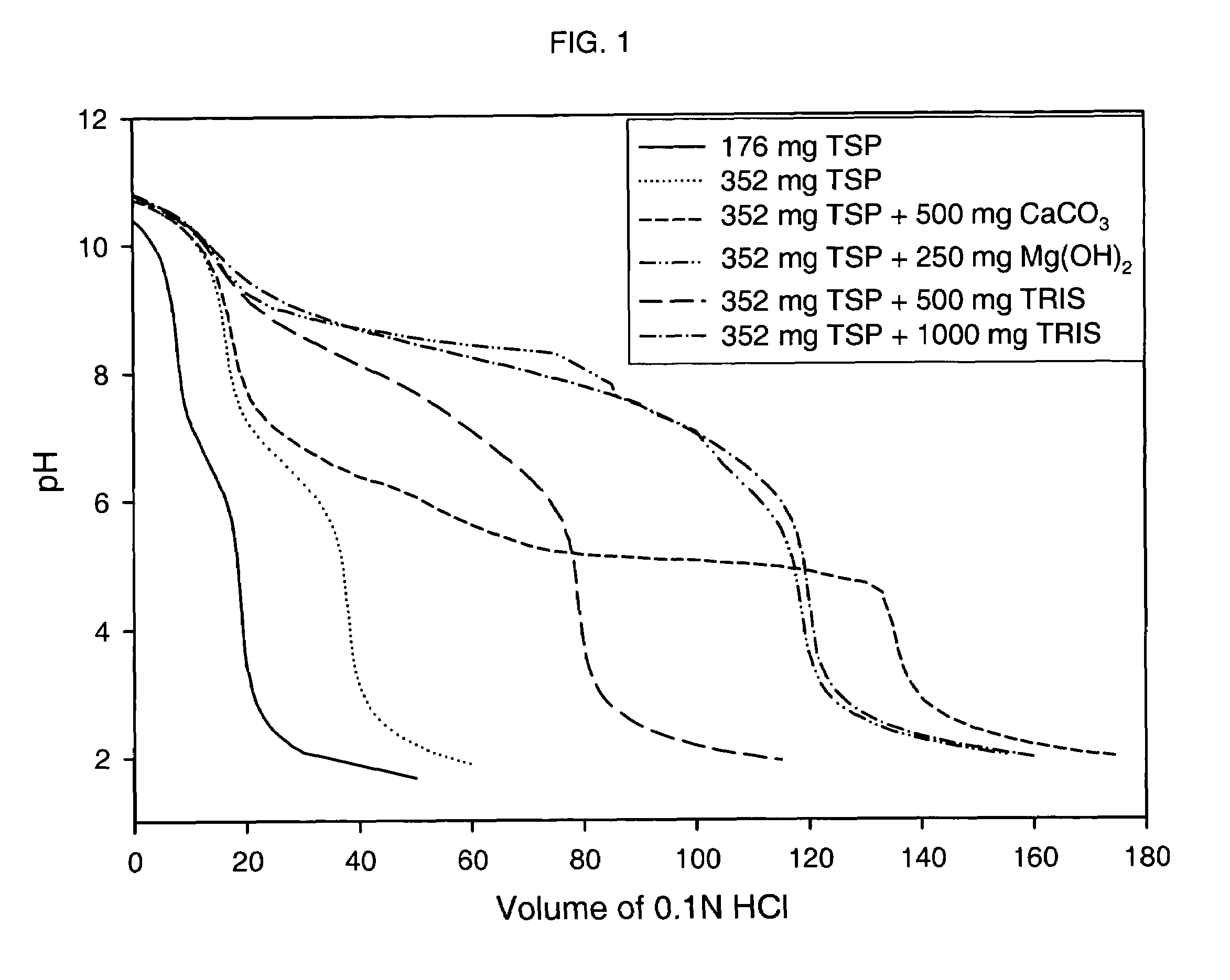
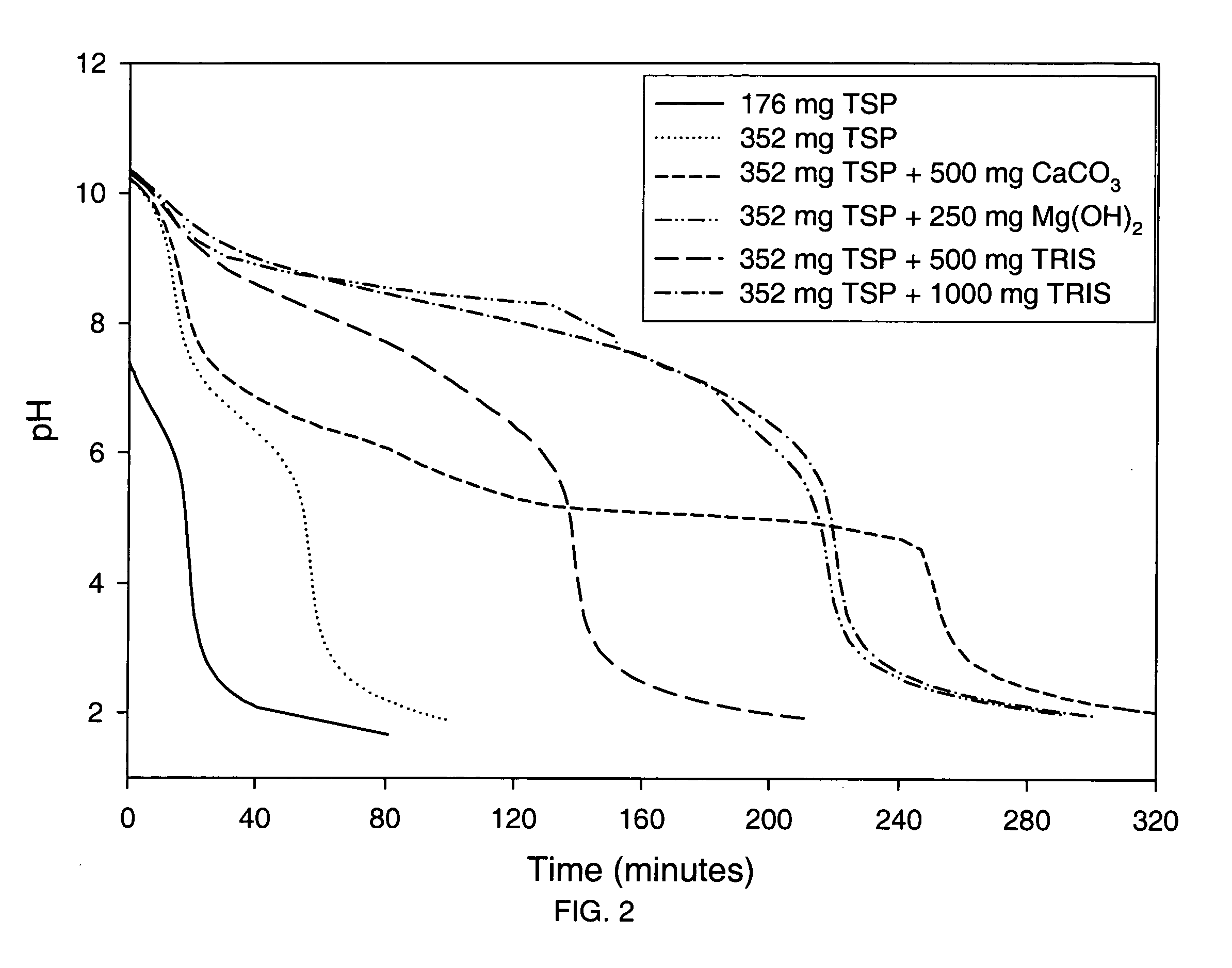
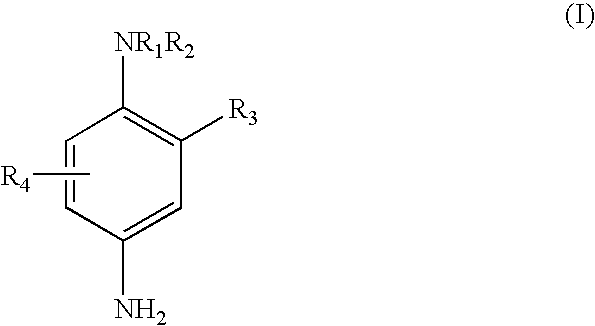
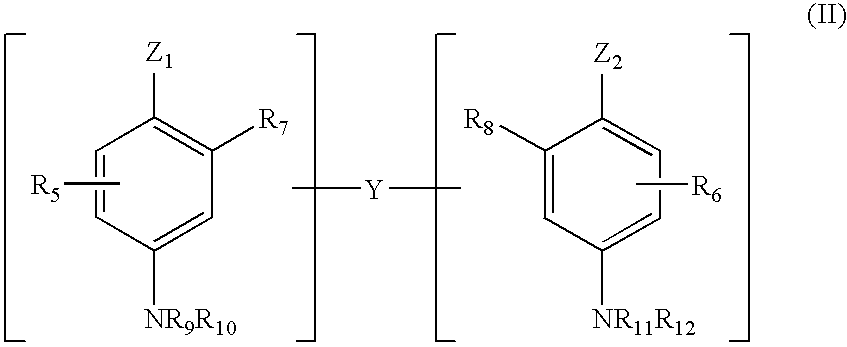
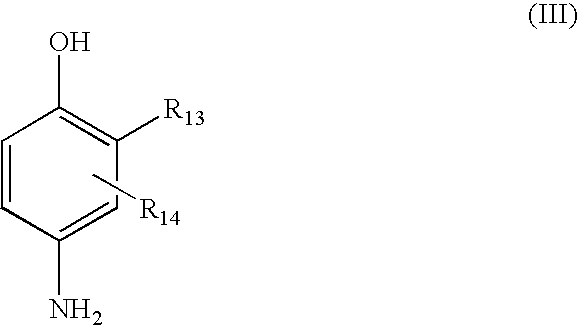
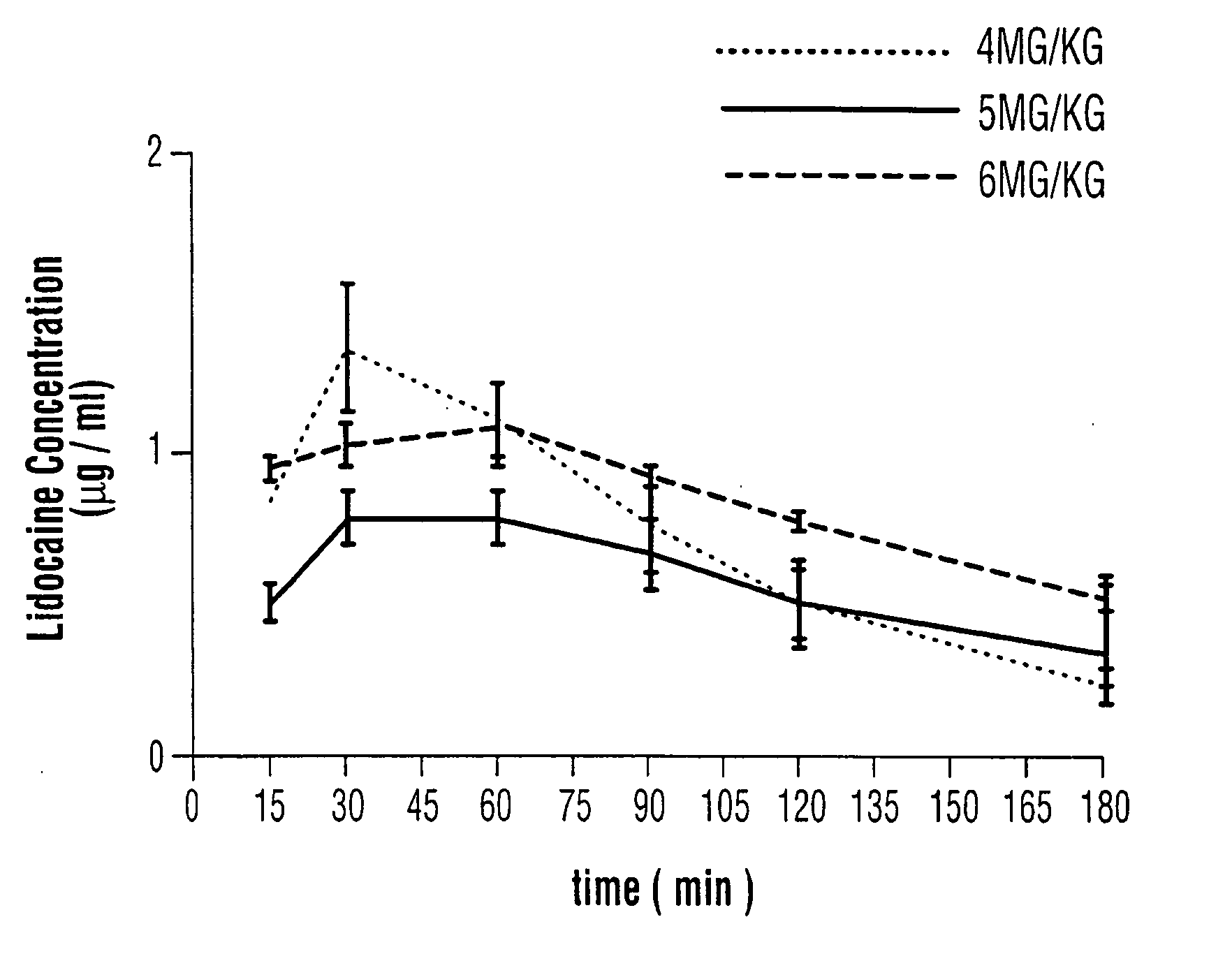
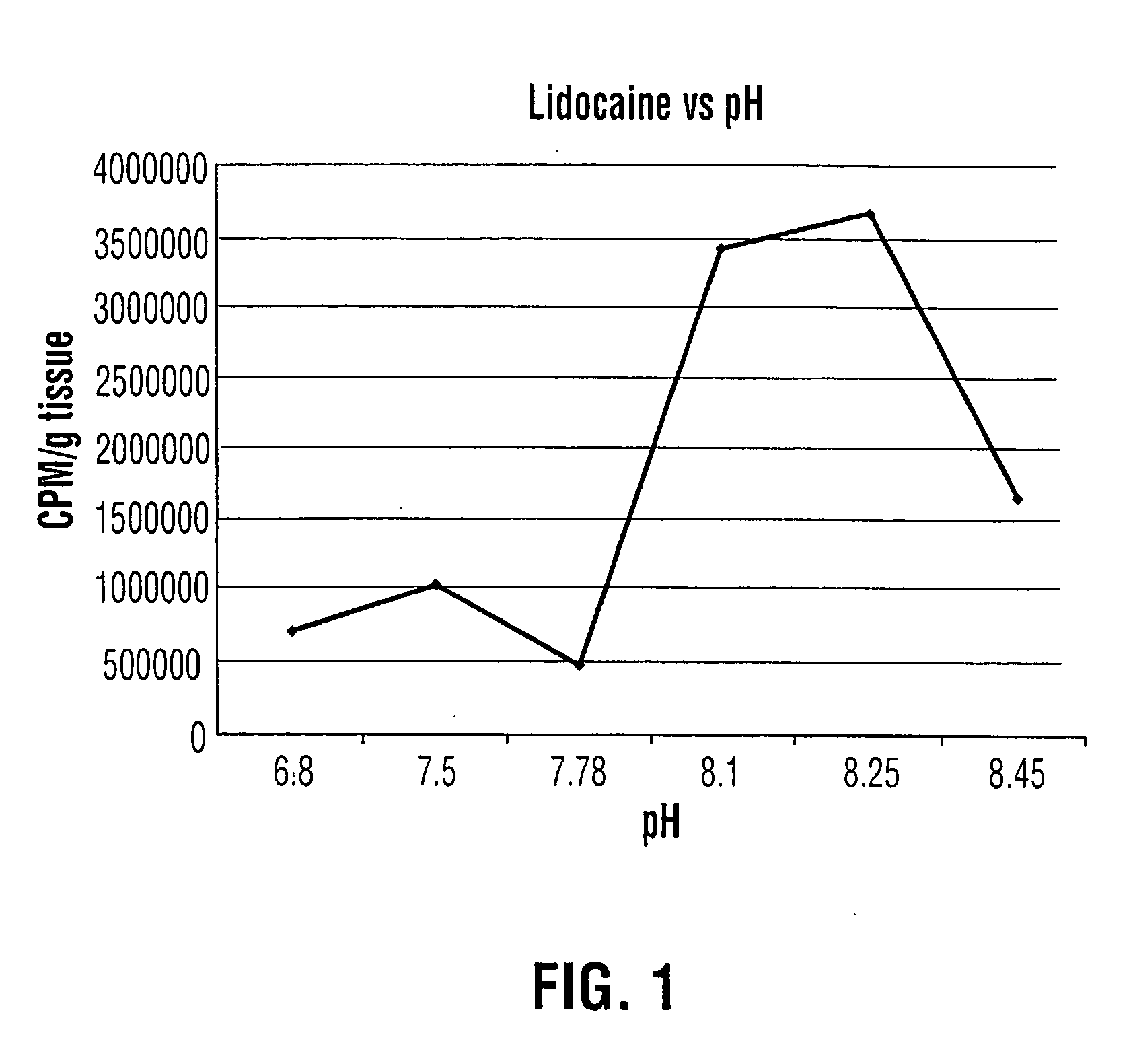
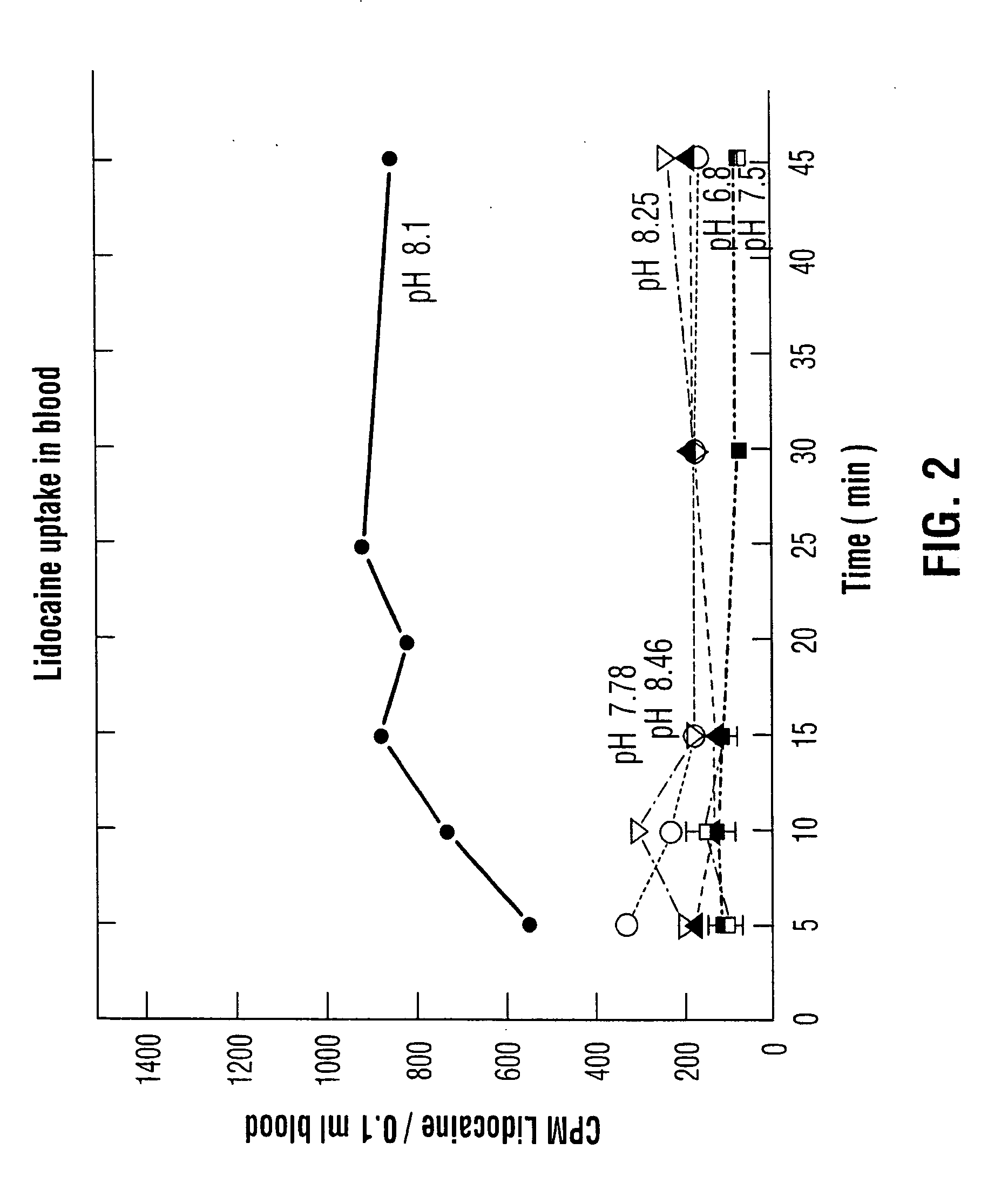
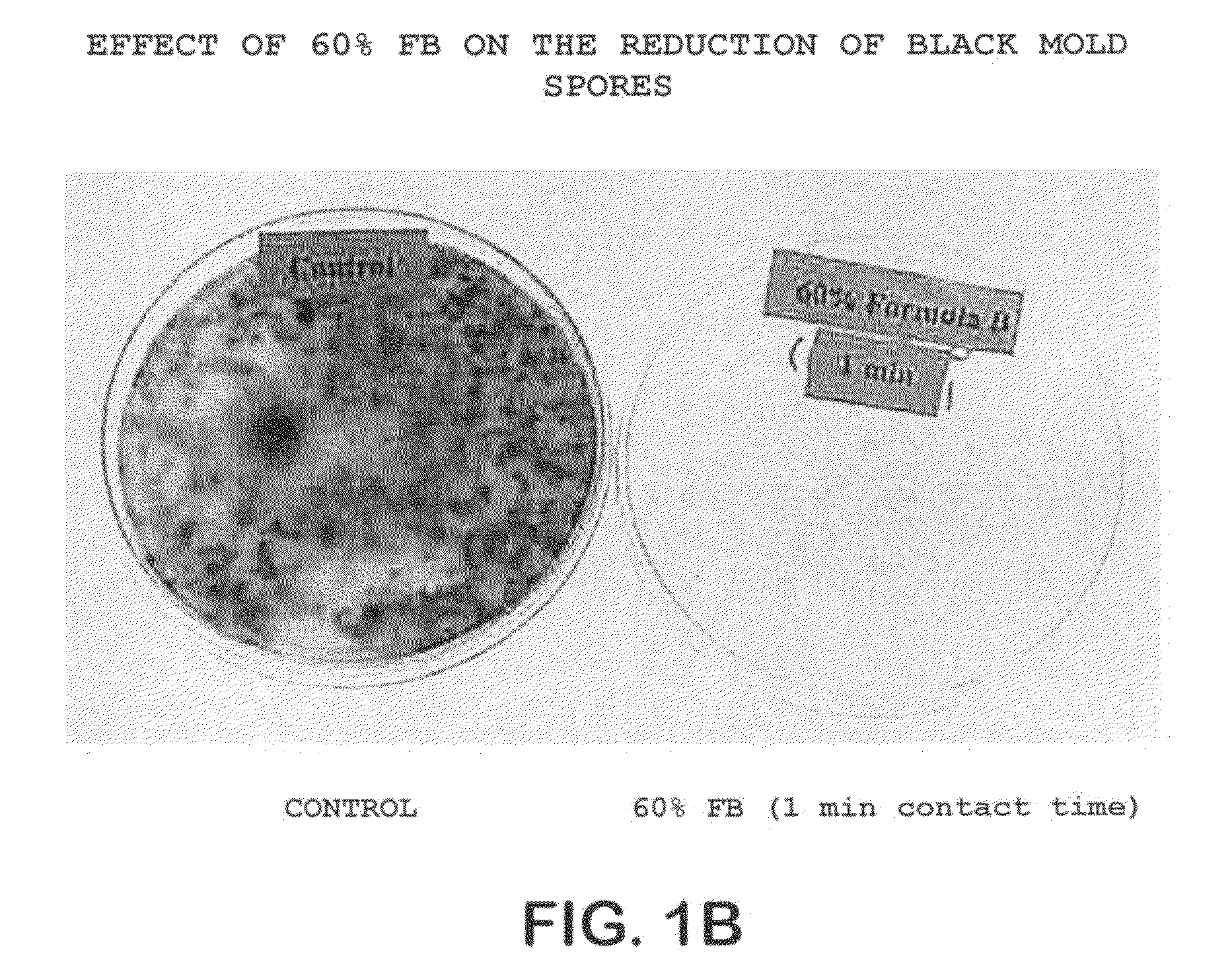
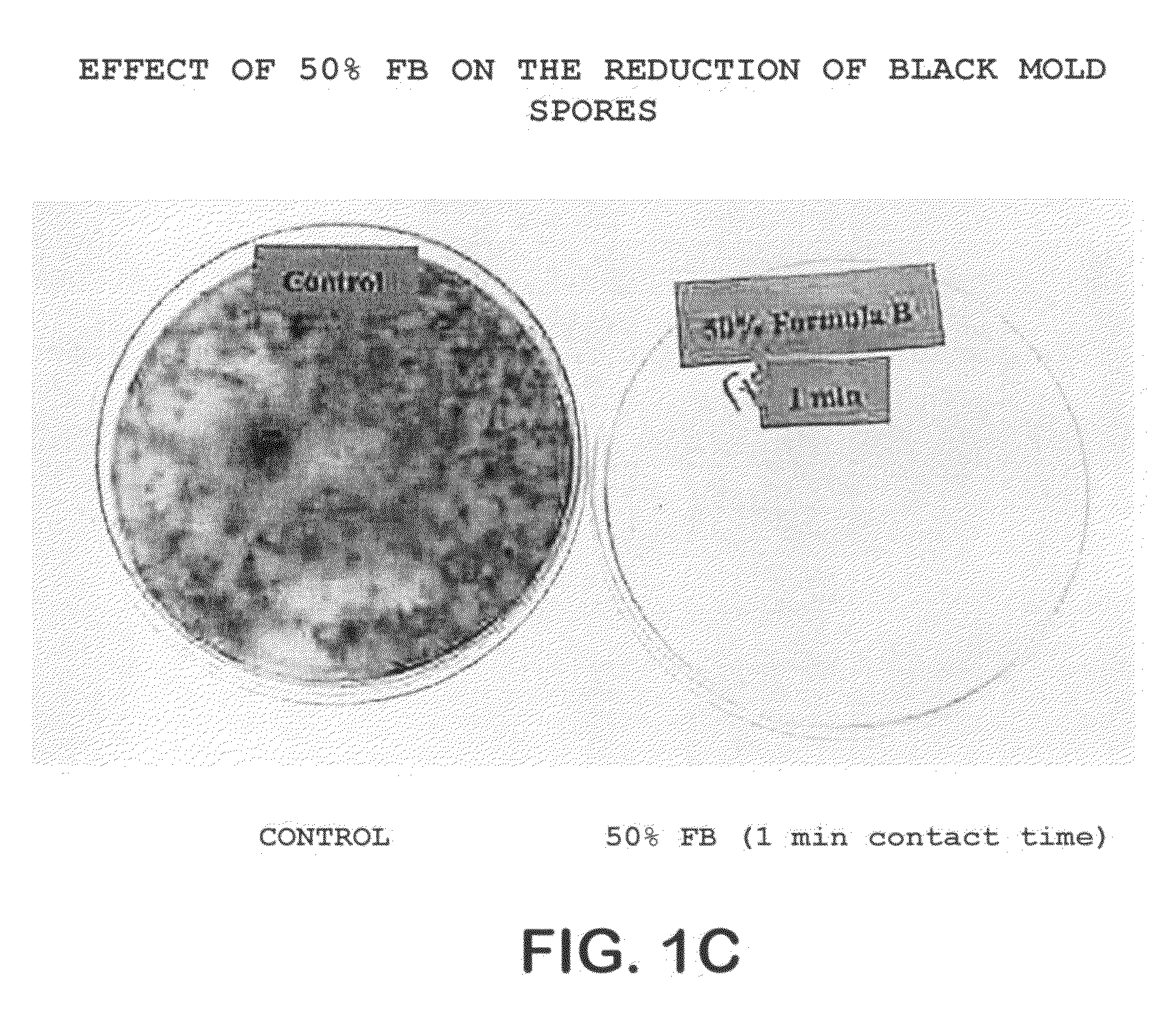
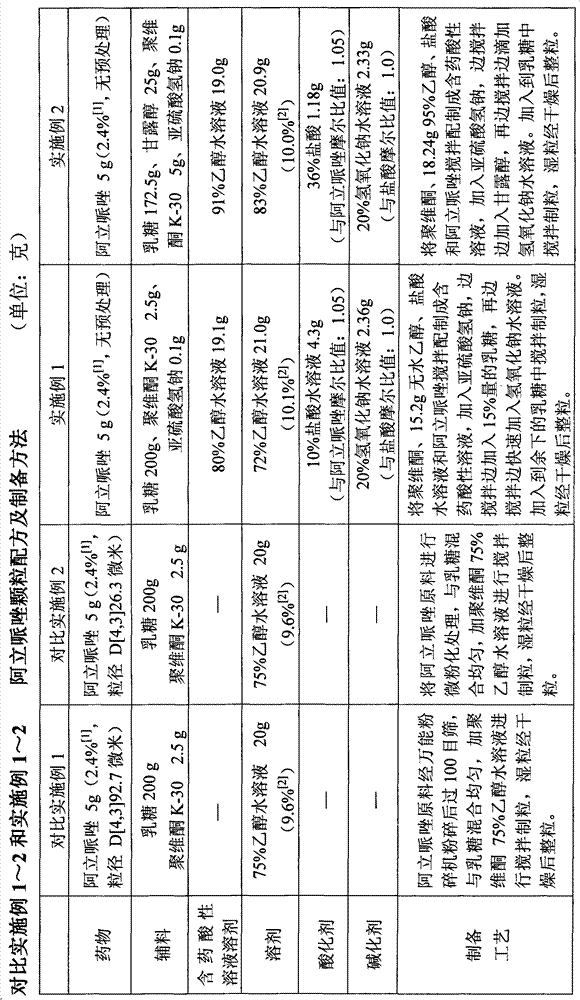
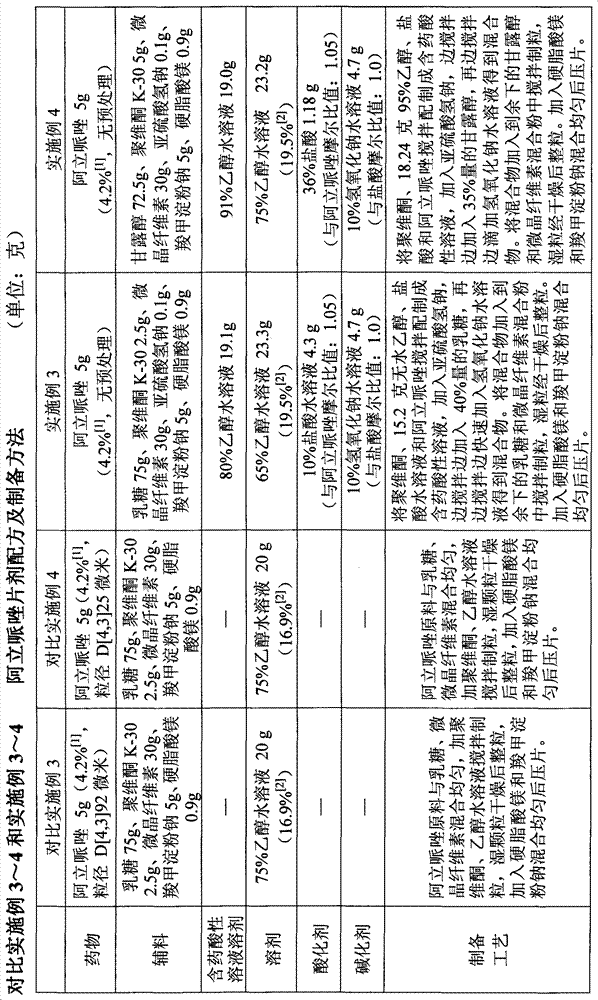
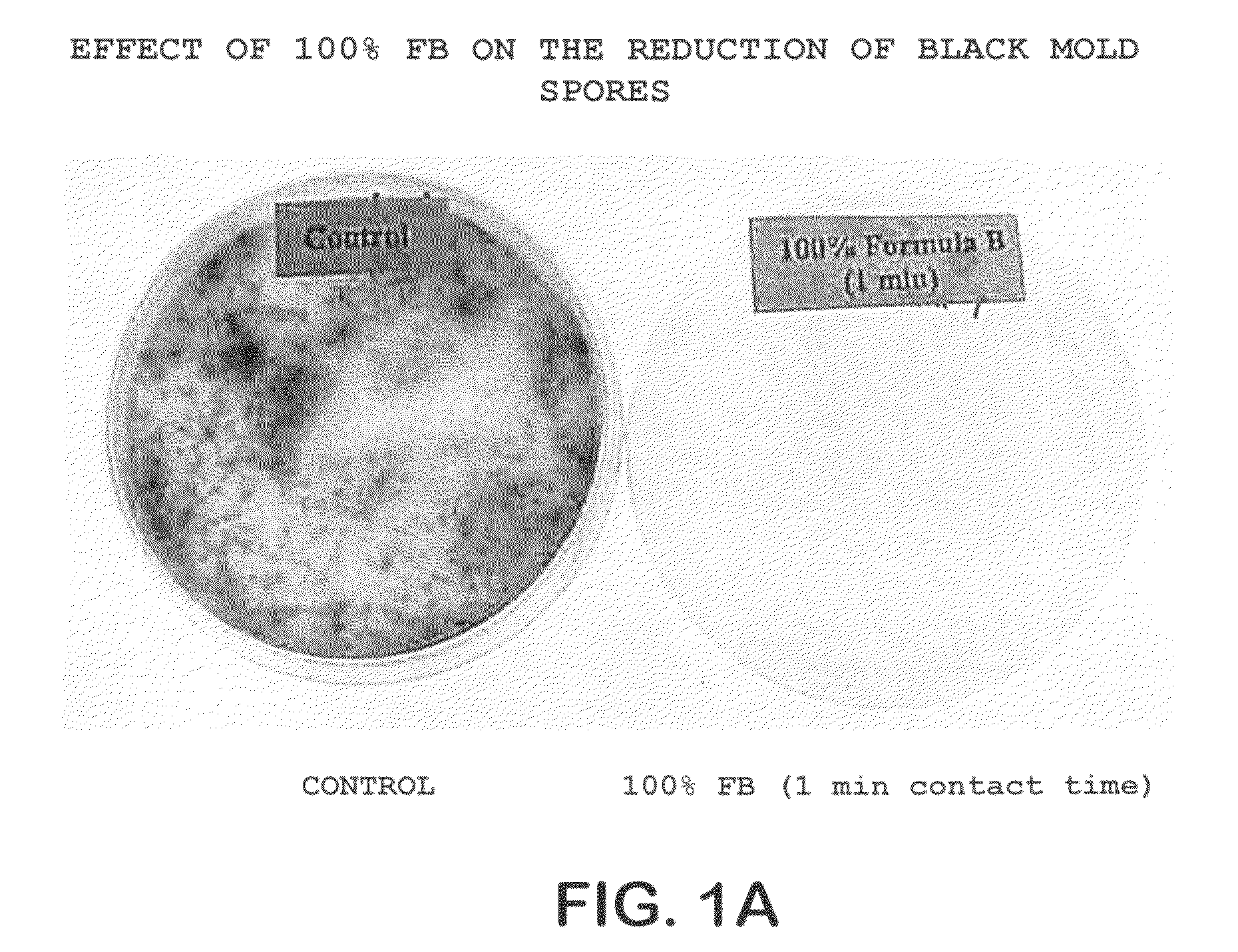
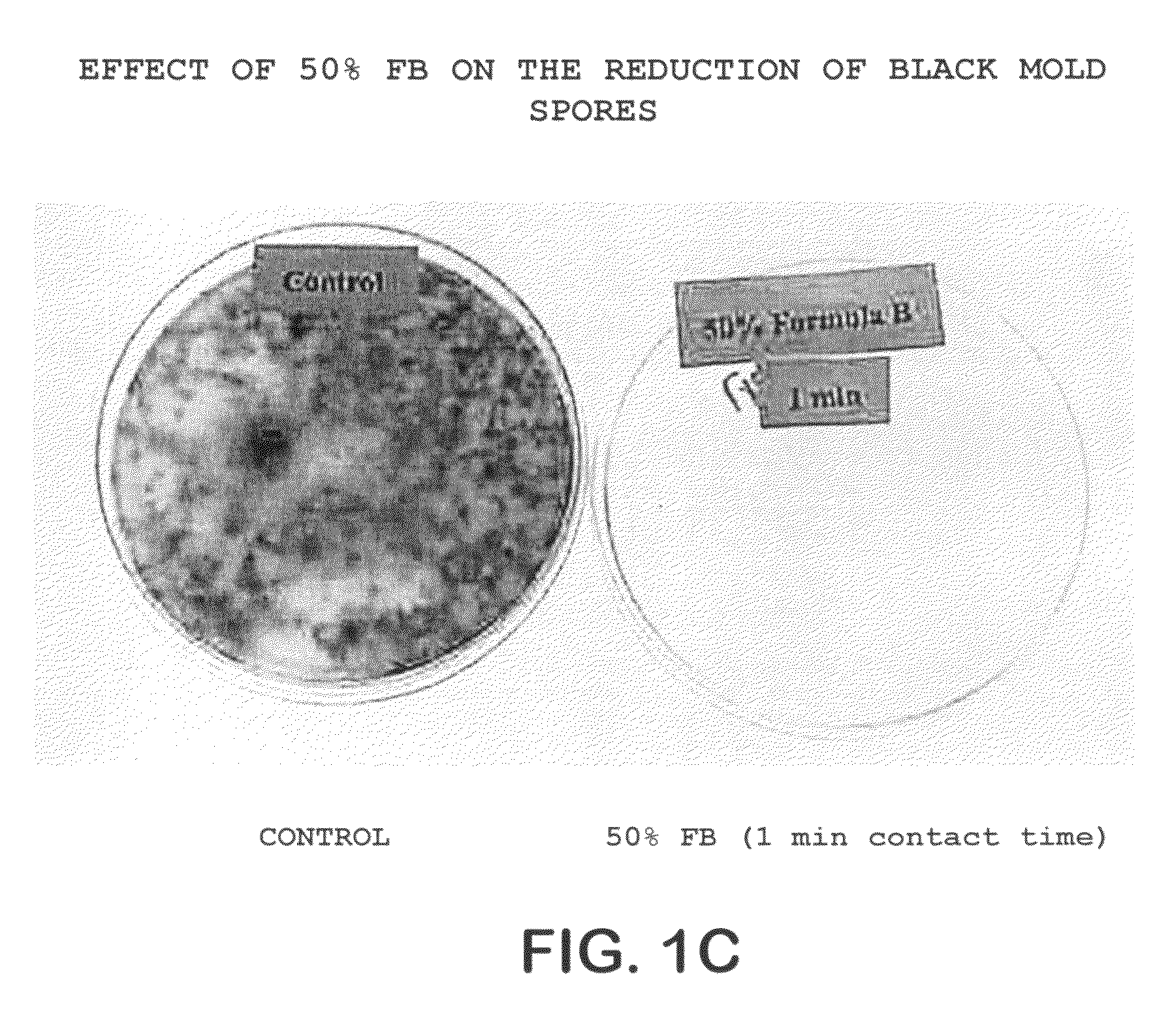
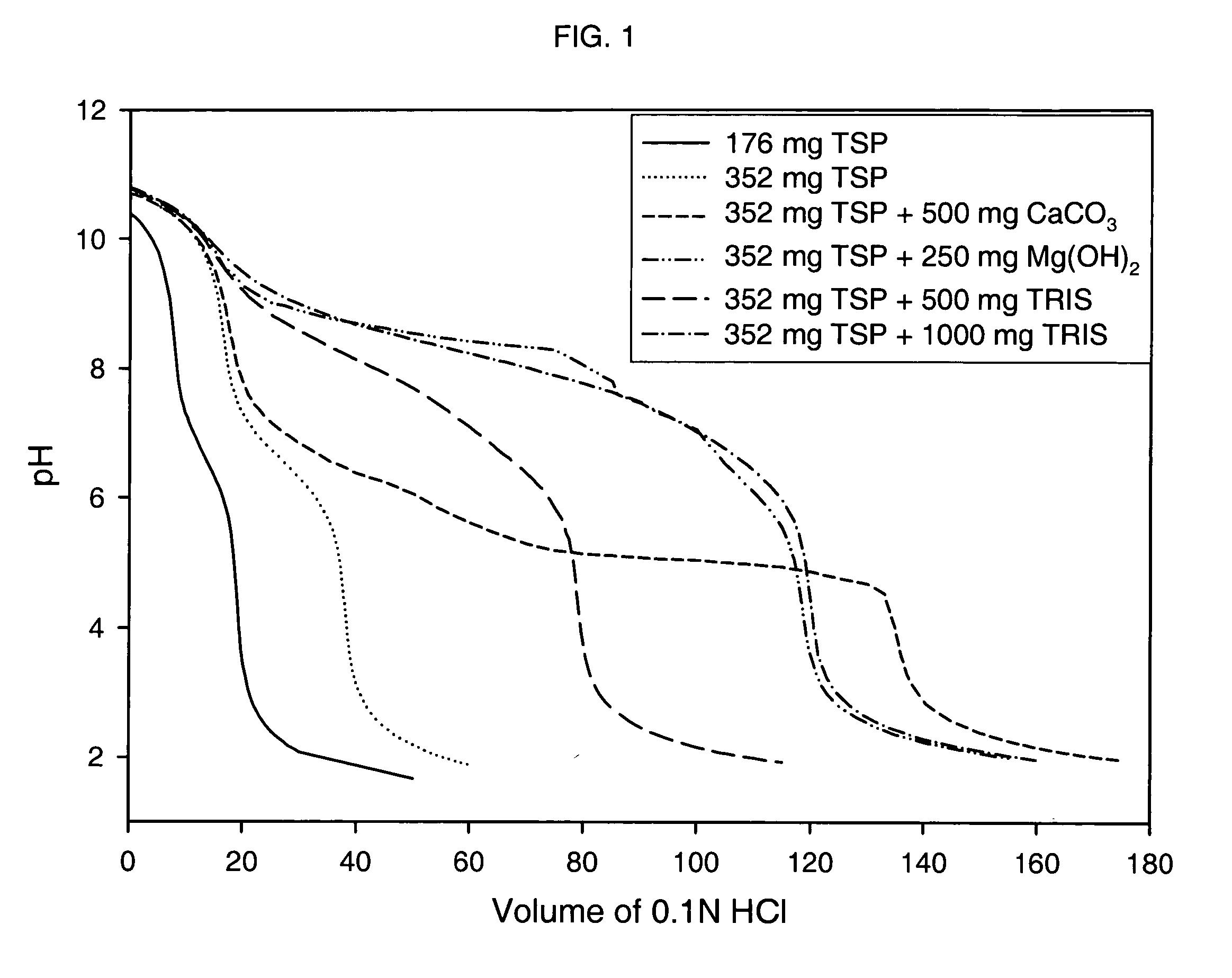
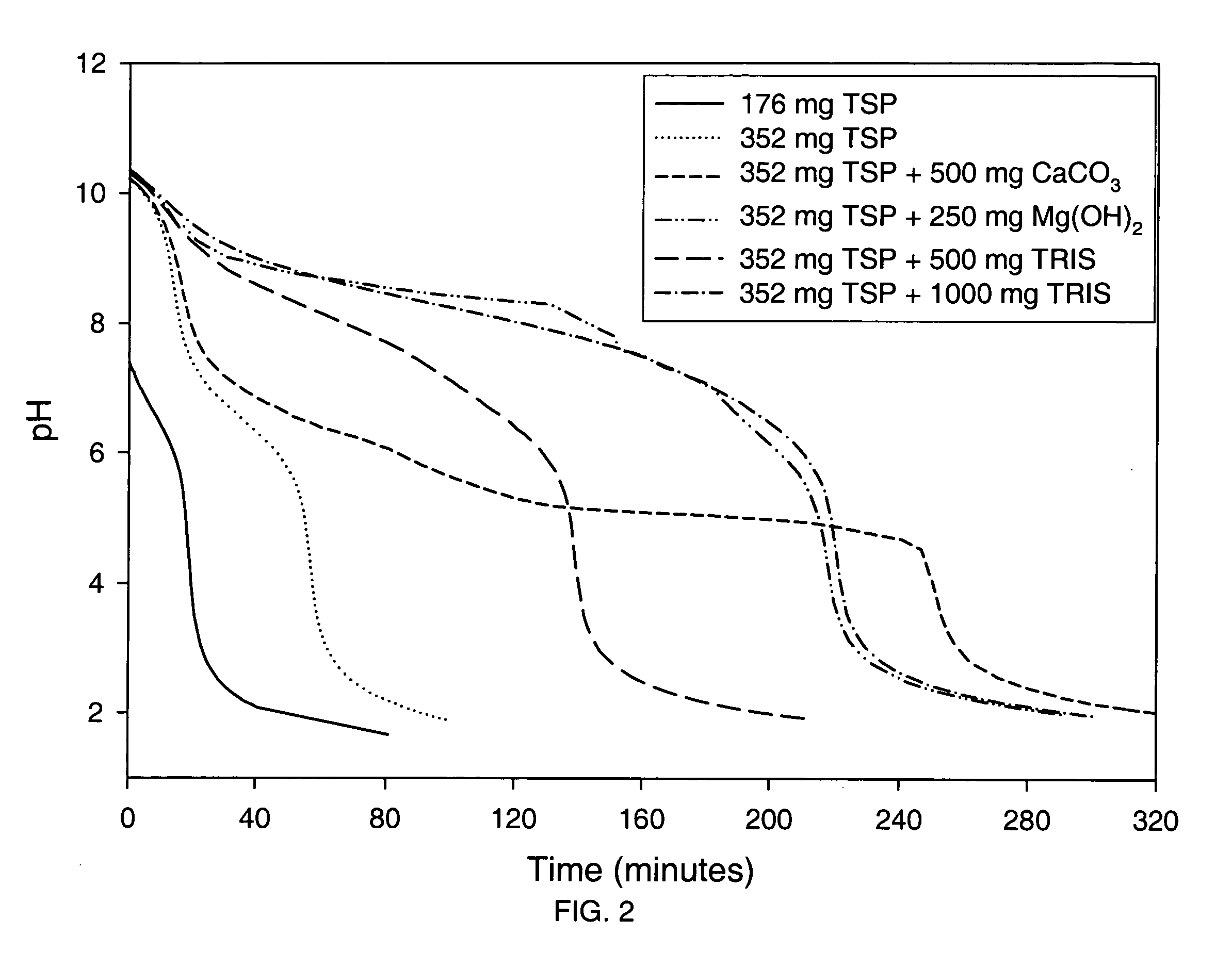
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Alkalinizing agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
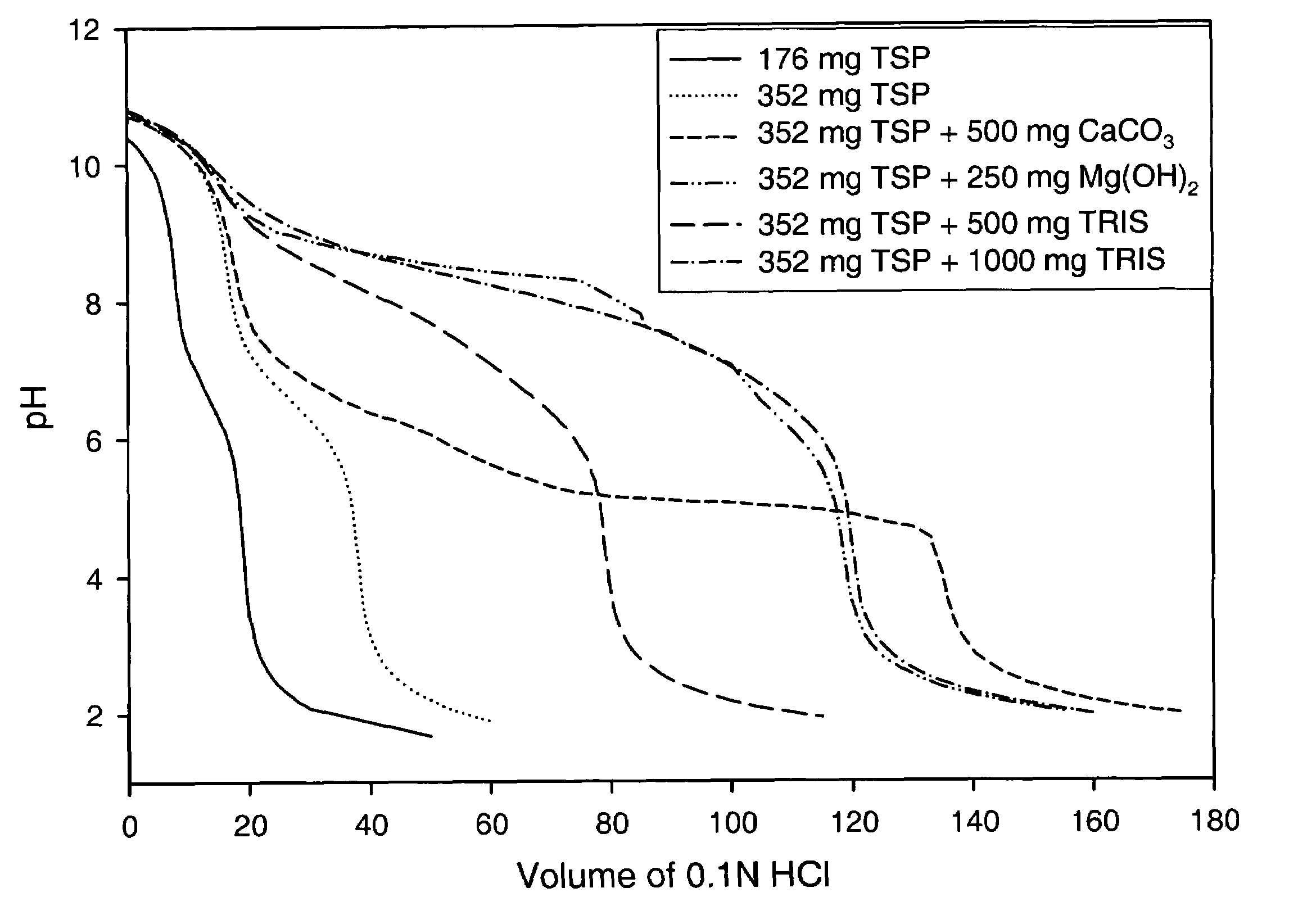
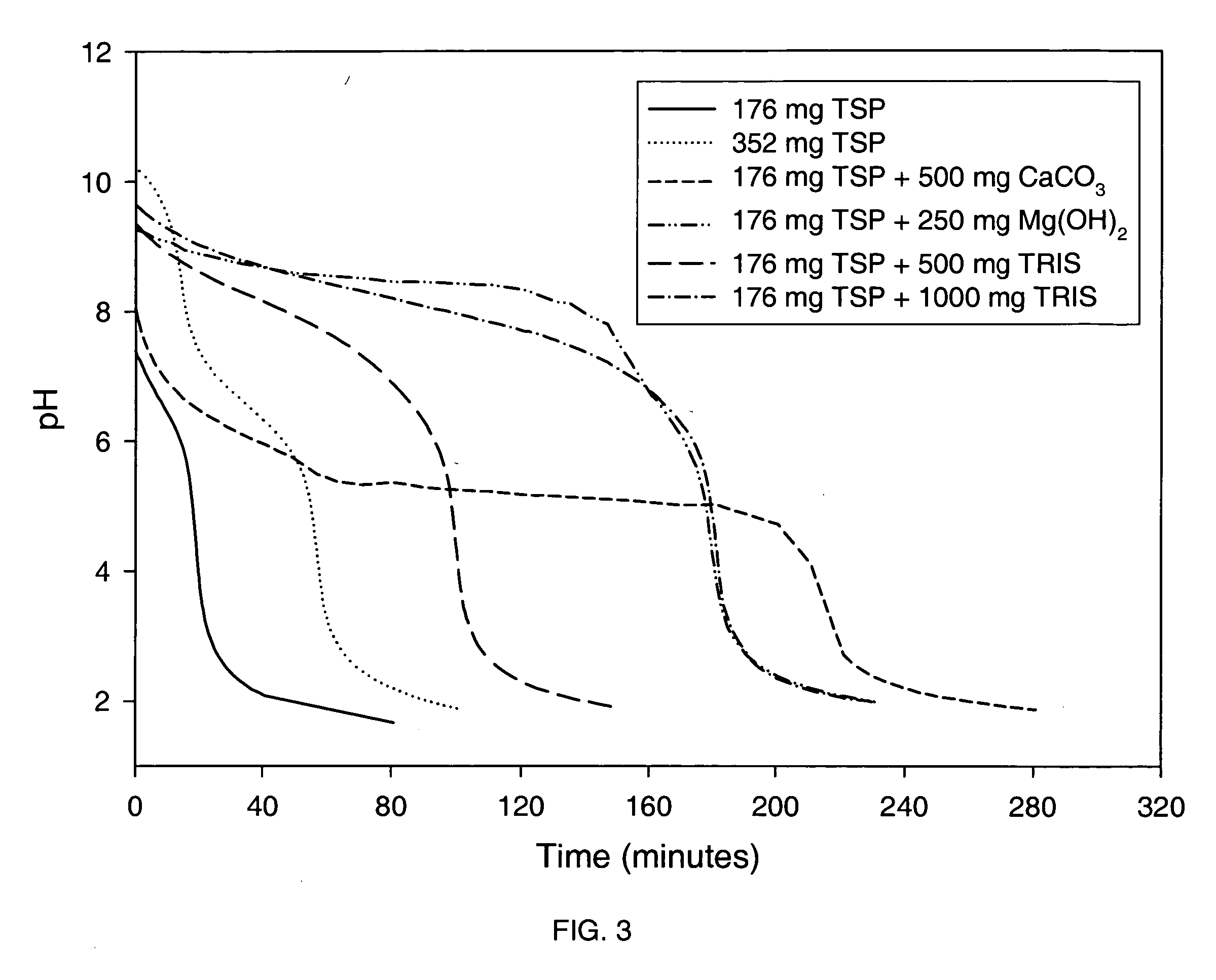
Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to renal failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizing agent. Others include potassium citrate, calcium carbonate, sodium lactate and calcium acetate.
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
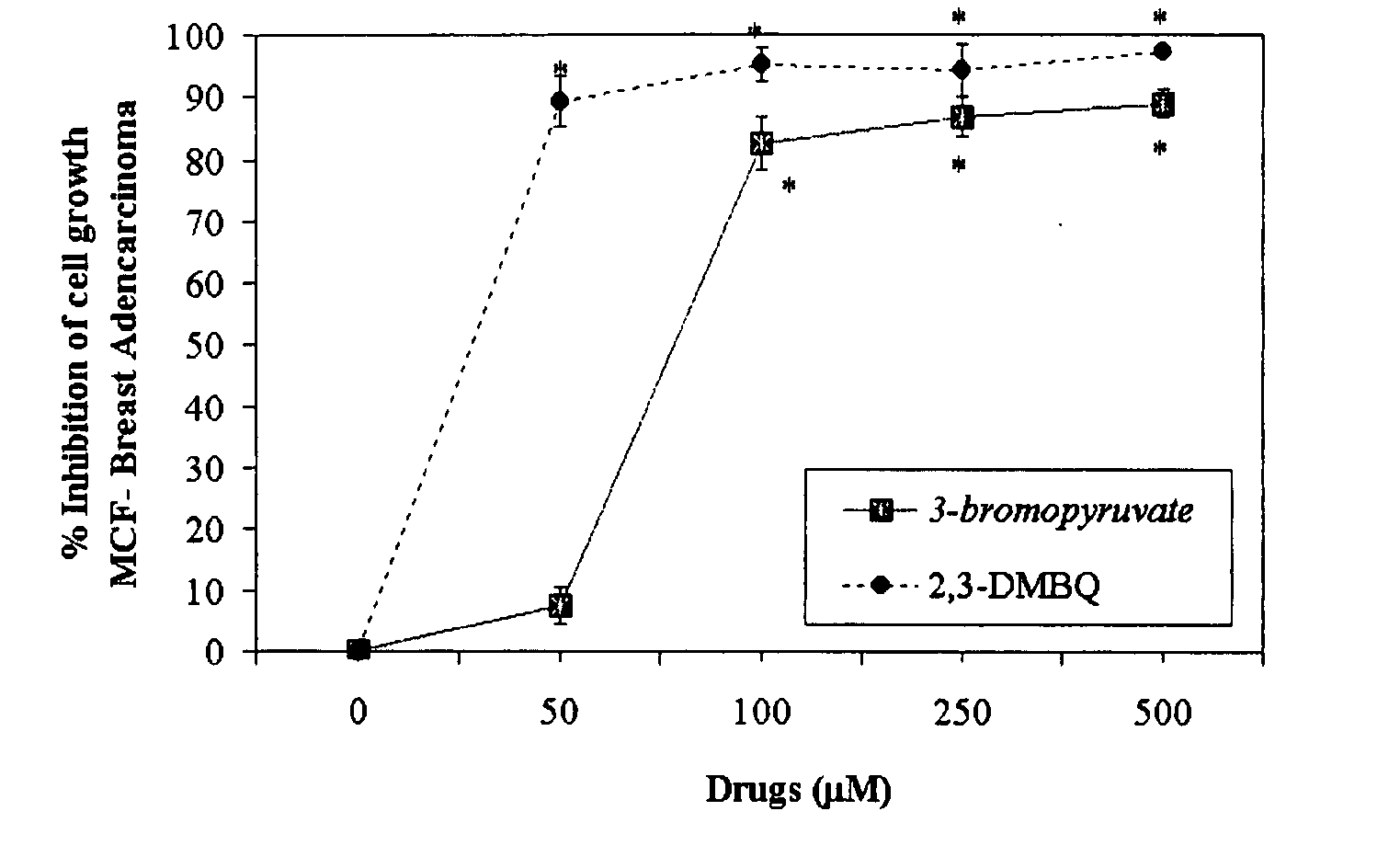
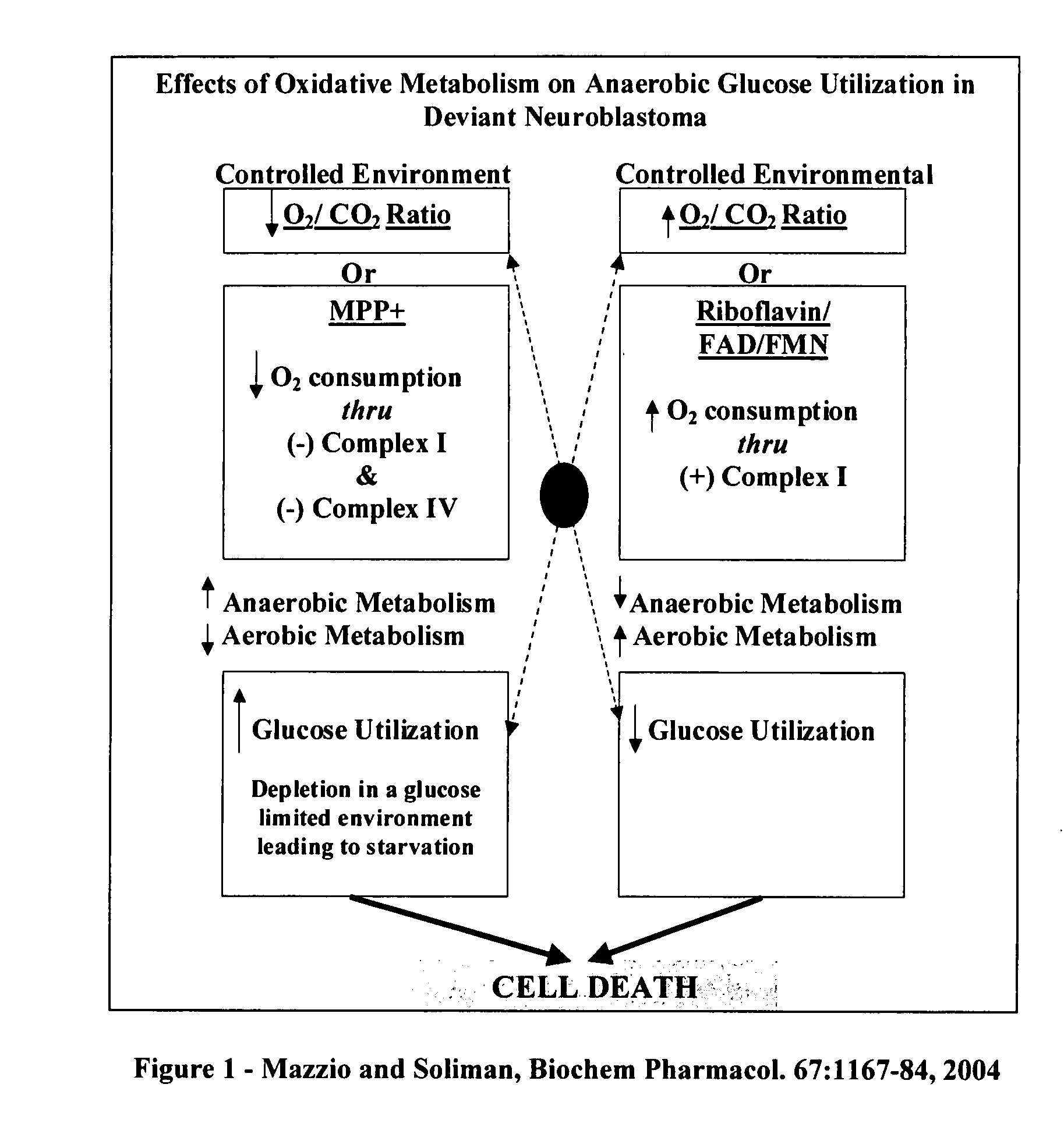
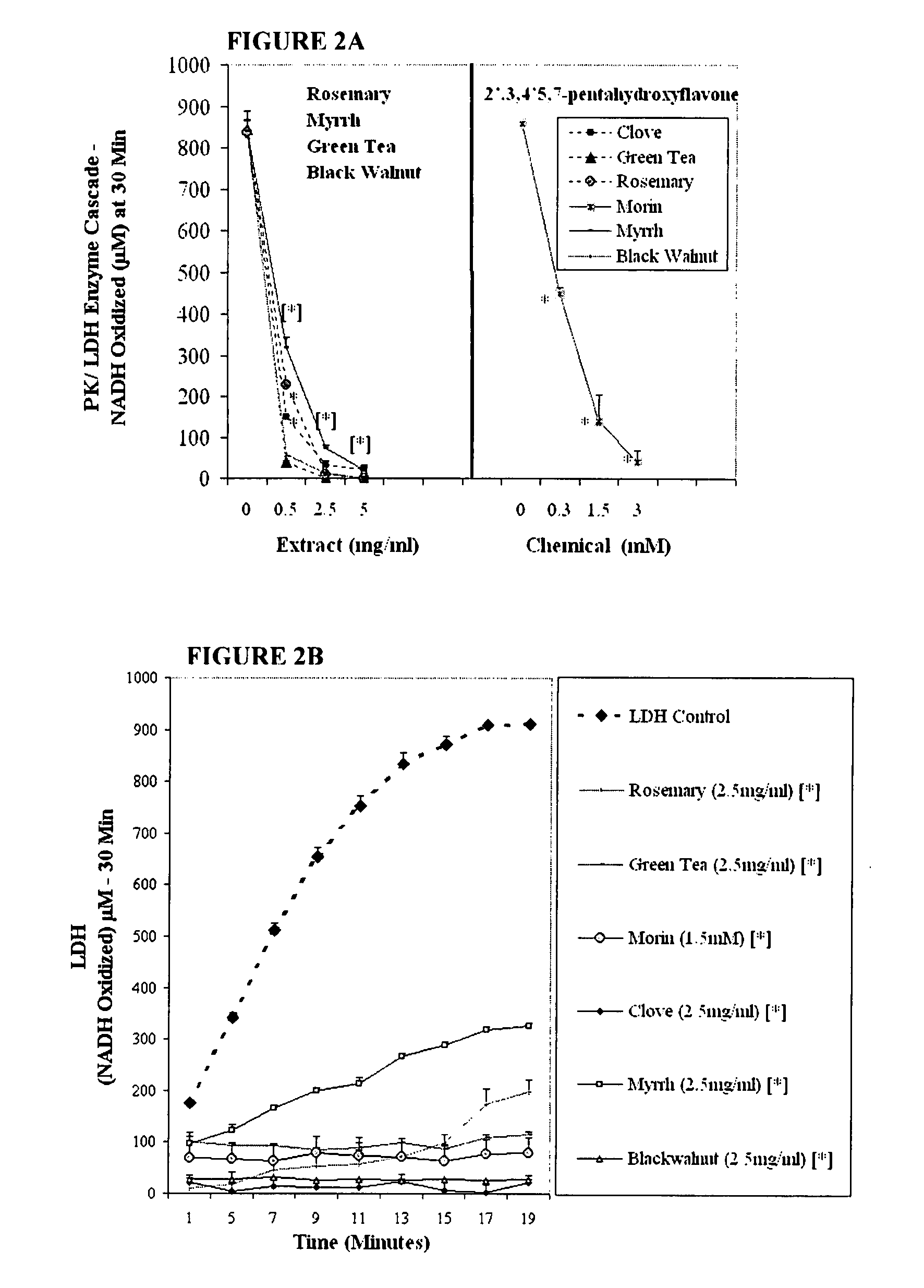
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Composition useful for the oxidation dyeing of human keratinous fibres
A composition useful for the oxidation dyeing of human keratinous fibers and in particular hair containing, in a cosmetically acceptable medium based on water and at a basic pH, at least one oxidation dye and an alkalinizing agent containing at least one alkali metal, alkaline-earth metal or ammonium metasilicate and at least one alkanolamine, and the dyeing method using this composition.
Owner:LOREAL SA
Topical anesthesia of the urinary bladder
InactiveUS20050238733A1Reduce concentrationReduce absorptionBiocideInorganic active ingredientsSodium bicarbonateCystoscopy
An aqueous solution of local anesthetic is instilled into the urinary bladder in sufficient concentration with the addition of an alkalinizing agent such as sodium bicarbonate to elevate the intra-vesical pH to approximately 8.0. The combination is left in situ in the bladder for at least fifteen minutes to allow time for absorption of the base form of the local anesthetic. This method provides safe and effective topical anesthesia to allow pain-free cystoscopic biopsy and cautery of bladder lesions such as bladder cancer, and provides a means to treat inflammatory conditions of the bladder such as chronic interstitial cystitis and acute bacterial cystitis.
Owner:HENRY RICHARD
System for Highlighting Hair
The present invention relates to a system to highlight the hair. The system comprises a device (10) and a composition (50). Device (10) comprises a first portion movably joined to a second portion. Composition (50) comprises a percentage of hydrogen peroxide and a percentage of an alkalizer by weight of said composition (50). The weight percentages of hydrogen peroxide and of an alkalizer are defined by equation (I): X+1.5Y≧15 (I) wherein X is the percentage by weight of hydrogen peroxide by weight of the composition (50) and wherein Y is the percentage by weight of an alkalizer by weight of the composition (50). Said composition (50) comprises at least 2% of hydrogen peroxide by weight of said composition (50) and said alkalizer is an inorganic salt selected from the group consisting of sodium silicate, sodium metasilicate, potassium hydrogen carbonate, ammonium carbonate, ammonium hydrogen carbonate and sodium hydrogen carbonate and mixtures thereof. Said composition (50) allows to perform highlighting when applied with the device (10) as described herein.
Owner:WELLA OPERATIONS US LLC
Methods and composition for treating a material
ActiveUS20090324514A1Unprecedented speedUnprecedented economyBiocideCosmetic preparationsMicroorganismCompound (substance)
A composition and method are described for sanitizing or otherwise treating a material such as a non-living surface, living tissue, soil or atmosphere which may be contaminated by a toxin, chemical warfare agent, insect, prion, microorganism or other infectious agent. The composition generally includes an aqueous composition mixture that includes: (a) lower alkanol; (b) an alkalinating agent, and (c) a fatty acid salt and / or ester. The components are present in an effective amount to sanitize (or treat) a material (or modifying a chemical contained thereon) to which the composition is applied. Also described are methods of making the composition.
Owner:URTHTECH
Preparing method of calcium stearate lubricating agent for making paper
The invention relates to a calcium stearate lubricant for papermaking and a preparation method thereof. The preparation method is characterized in that the method comprises the following steps of: heating water to 50 to 95 DEG C, adding an emulsifying agent A, a dispersant and a defoaming agent, stirring, adding molten stearic acid, an alkalinizing agent and an emulsifying agent B, reacting, adding antibacterial agent, and stirring to obtain the final product. The calcium stearate lubricant has a viscosity range of 70 to 360 mPa.S, particle diameter range of 400 to 800 nm and solid content weight of 40 to 79%. The lubricant is prepared by one-step synthesis to solve the disadvantages of secondary processing of the lubricant such as large cost, large particle diameter, larger viscosity and low efficiency. The lubricant prepared by the method has the advantages of small particle diameter, high solid content, low viscosity, good mechanical and chemical stability, no yellowing, no special smell, high use efficiency, etc.
Owner:SHANGHAI DONGSHENG NEW MATERIALS
Aripiprazole pharmaceutic preparation and preparation method thereof
ActiveCN102846543ANo adverse effectsDrug effectOrganic active ingredientsNervous disorderPharmaceutical drugPharmaceutical Aids
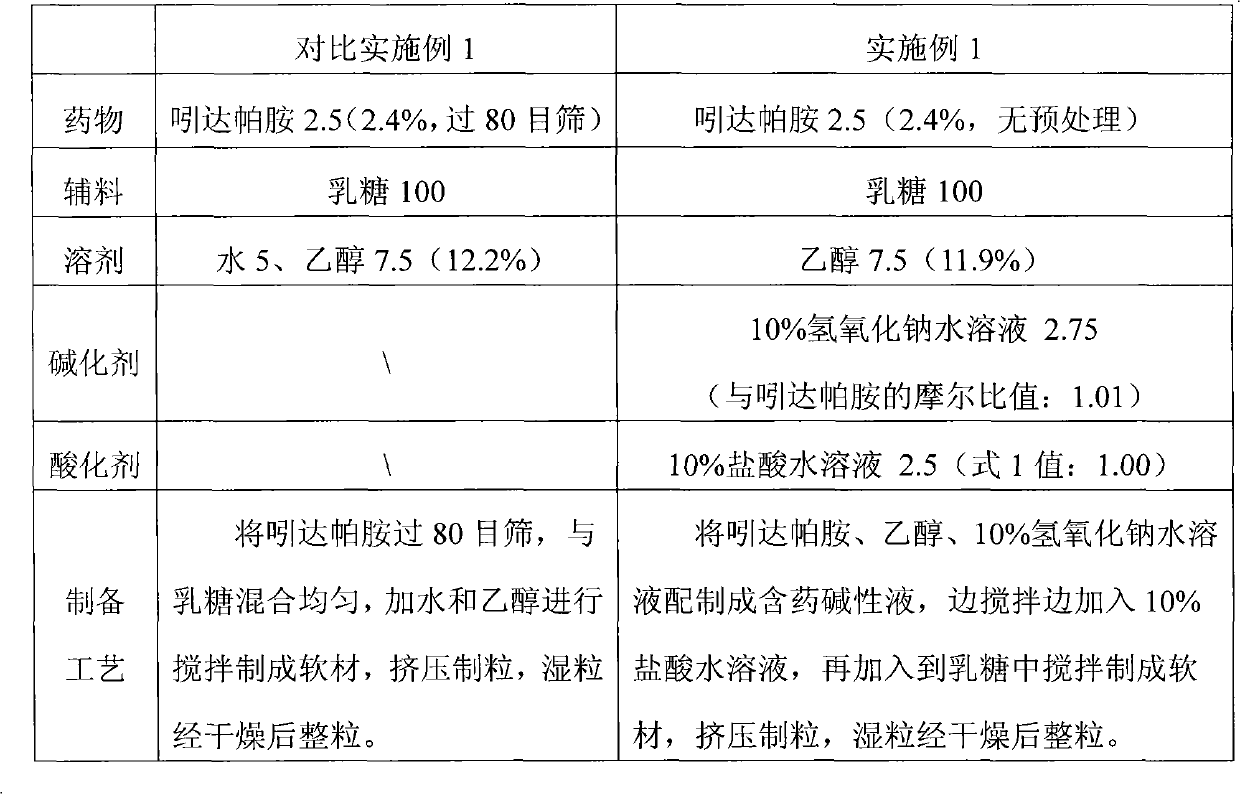
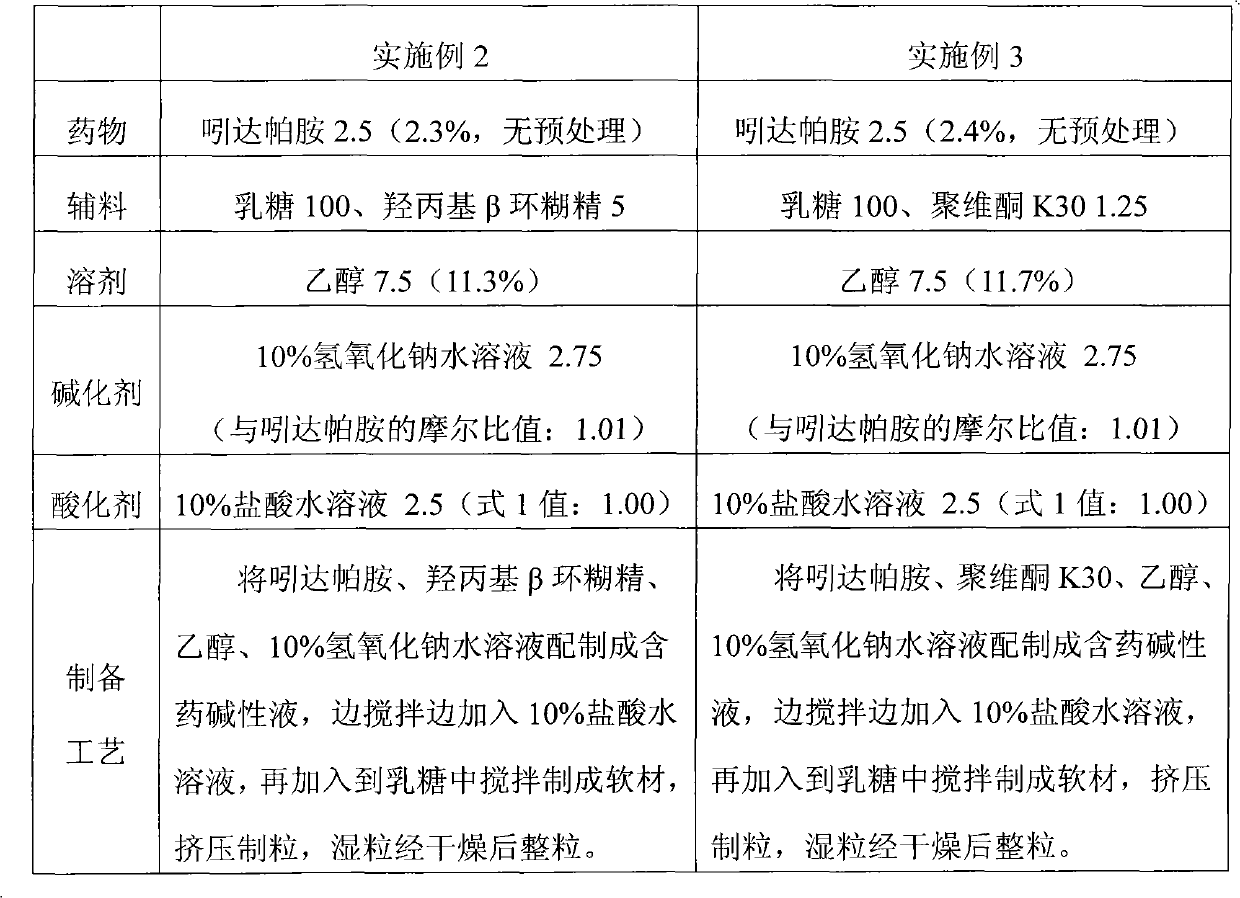
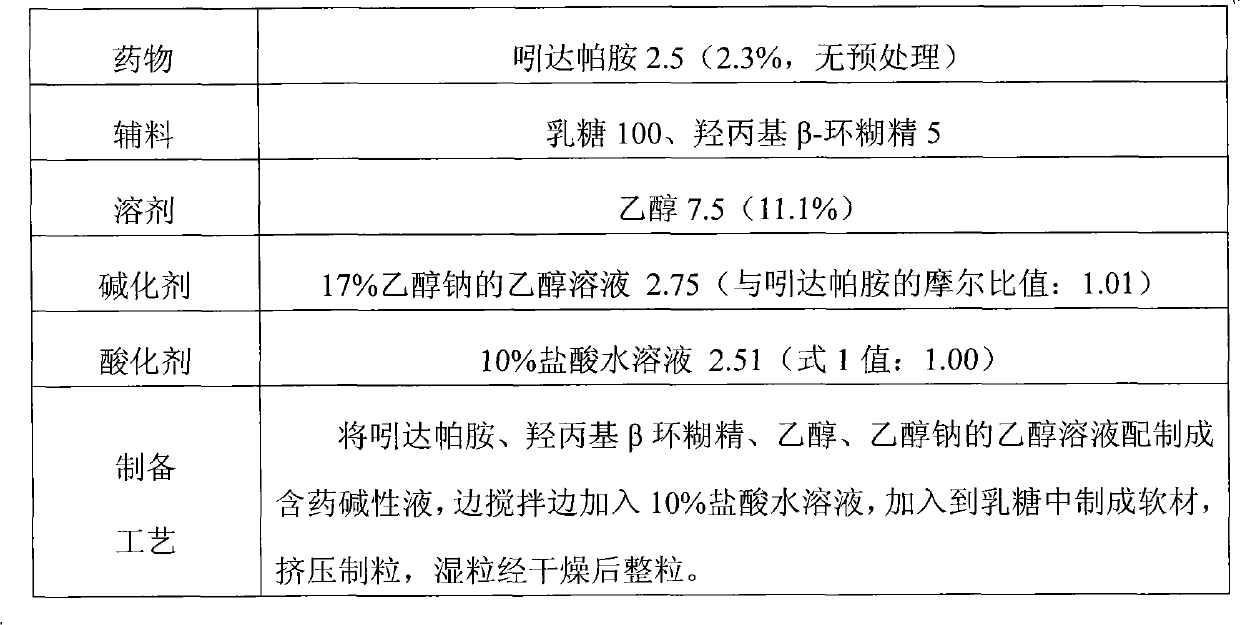
The invention provides a preparation method of an aripiprazole pharmaceutic preparation, which comprises the following steps: dissolving aripiprazole in an acidic solution containing an acidifying agent to prepare a drug-containing acidic solution; then performing wet granulation or preparing a suspension by the drug-containing acidic solution, an alkalizer and an auxiliary material to obtain the aripiprazole pharmaceutic preparation; the auxiliary material comprises an anti-oxidant. The invention also provides an aripiprazole pharmaceutic preparation prepared by the method. The aripiprazole pharmaceutic preparation obtained by the preparation method of the invention has significantly reduced amounts of related materials, good dissolution property and stability, high bioavailability, less individual difference, and improved wetability and content uniformity of drugs which are difficult to dissolve. The method of the invention is simple in operation, and low in cost, requires no special equipment, and is easily applicable to industrial production; especially, the method eliminates the effect of raw material forms on preparation quality, and avoids defects of severe pollution, great loss, and severe potential safety hazard caused by aripiprazole pretreatment.
Owner:SHANGHAI ZHONGXI PHARMACEUTICAL CO LTD
Composition for the oxidation dyeing of keratin fibers, comprising particular fatty alcohols, a liquid fatty substance and a cationic polymer
The present invention relates to a composition for dyeing keratin fibres, comprising: one or more oxidation dyes; one or more basifying agents; one or more non-oxyalkylenated fatty substances that are liquid at room temperature in a content of less than or equal to 20% by weight relative to the total weight of the composition; one or more oxidizing agents; one or more oxyethylenated fatty alcohols with a number of oxyethylene units of greater than or equal to 10; one or more oxyethylenated fatty alcohols with a number of oxyethylene units of less than 10; one or more non-oxyethylenated fatty alcohols that are solid at room temperature; and one or more cationic polymers. The present invention also relates to a process for dyeing keratin fibres using such a composition, and also to a kit for preparing the said composition.
Owner:LOREAL SA
Methods and composition for treating a material
ActiveUS8143309B2Improving germicidalReduce transmissionCosmetic preparationsBiocideMicroorganismEnvironmental engineering
A composition and method are described for sanitizing or otherwise treating a material such as a non-living surface, living tissue, soil or atmosphere which may be contaminated by a toxin, chemical warfare agent, insect, prion, microorganism or other infectious agent. The composition generally includes an aqueous composition mixture that includes: (a) lower alkanol; (b) an alkalinating agent, and (c) a fatty acid salt and / or ester. The components are present in an effective amount to sanitize (or treat) a material (or modifying a chemical contained thereon) to which the composition is applied. Also described are methods of making the composition.
Owner:URTHTECH
Method for raising thermal denaturation temperature of hide and hide powder through THP salt-nano clay combination tannage
InactiveCN101696456ATanning treatmentPre-tanning chemical treatmentThermal denaturationAlkalinizing agent
The invention discloses a method for raising thermal denaturation temperature of hide and hide powder through THP salt-nano clay combination tannage. The method comprises: adding softened hide or hide powder and water in a mass ratio of (0.5-1):1 to a reactor; adding a certain amount of salt and formic acid; adding THP salt and stirring for 3 to 5 hours; adding nano clay and stirring for 3 to 5 hours; extracting alkali till the pH of bath is between 5.0 and 6.0, continuing to stir for 2 hours and then standing the obtained product overnight; and stirring the obtained product for 30 minutes next day and then performing washing. The method can effectively raise the thermal denaturation temperature of hide collagen up to over 115 DEG C through the THP salt-nano clay combination tannage. Due to the specific structure of the nano clay added in the method, during alkali extraction in the later stage of tanning, tanning liquor is automatically alkalized and mitigated in effect, thereby helping to stabilize product quality, and the consumption of alkalization agent can be reduced. Meanwhile, the THP salt and the nano clay in the method containing no heavy metal salts are environment-friendly materials, and the tanning process is a clean leather-making technique.
Owner:SICHUAN UNIV
Azithromycin dosage forms with reduced side effects
ActiveUS20050123627A1Reduce gastrointestinal side effectsAntibacterial agentsBiocideOral suspensionsGLYCERYL MONOBEHENATE
An oral dosage form comprising azithromycin and an effective amount of an alkalizing agent. Preferably, said oral dosage form comprises an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule. Additionally disclosed is an oral suspension comprising azithromycin, an effective amount of an alkalizing agent and a vehicle. Preferably, the azithromycin is in multiparticulate form wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Also disclosed is a method for reducing gastrointestinal side effects, associated with administering azithromycin to a mammal, comprising contiguously administering azithromycin and an effective amount of alkalizing agent to said mammal wherein the frequency of gastrointestinal side effects is lower than that experienced by administering an equal dose of azithromycin without said alkalizing agent. Further disclosed is a method of treating a bacterial or protozoal infection in a mammal in need thereof comprising contiguously administering to said mammal a single dose of an oral dosage form wherein said oral dosage form comprises azithromycin and an effective amount of an alkalizing agent. Additionally disclosed are azithromycin multiparticulates comprising azithromycin, a surfactant; and a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Preparation method of medicament solid preparation and obtained medicament solid preparation
ActiveCN102552161ASmall particle sizeSmall individual differencesSulfonylurea active ingredientsPill deliveryMedicineBULK ACTIVE INGREDIENT
Owner:SHANGHAI ZHONGXI PHARMA
System for highlighting hair
The present invention relates to a system to highlight the hair. The system comprises a device (10) and a composition (50). Device (10) comprises a first portion movably joined to a second portion. Composition (50) comprises a percentage of hydrogen peroxide and a percentage of an alkalizer by weight of said composition (50). The weight percentages of hydrogen peroxide and of an alkalizer are defined by equation (I): X+1.5Y≧15 (I) wherein X is the percentage by weight of hydrogen peroxide by weight of the composition (50) and wherein Y is the percentage by weight of an alkalizer by weight of the composition (50). Said composition (50) comprises at least 2% of hydrogen peroxide by weight of said composition (50) and said alkalizer is an inorganic salt selected from the group consisting of sodium silicate, sodium metasilicate, potassium hydrogen carbonate, ammonium carbonate, ammonium hydrogen carbonate and sodium hydrogen carbonate and mixtures thereof. Said composition (50) allows to perform highlighting when applied with the device (10) as described herein.
Owner:WELLA OPERATIONS US LLC
Amine-free voc-free metal working fluid
The present application concerns an aqueous metalworking composition comprising in a concentrate or after dilution of a concentrate with water in a diluent: 0.002 to 40 % by weight of component (a) which is a lubricity agent comprising at least one water-insoluble compound (a) having at least one hydrophobic aliphatic chain and at least one polar group and having a water solubility at 20 DEG C of less than 0.1 g / liter; 0.002 to 40 % by weight of component (b)comprising at least one water-soluble corrosion inhibiting compound(b) having a water-solubility at 20 DEG C of more than 0.1g / liter; 0.002 to 45 % by weight of at least one emulsifying and dispersing agent(c)which contains at least one emulsifying and / or dispersing compound (c) which is water-soluble, water-miscible or water-dispersable and which is selected from the group consisting of non-ionic, anionic and zwitterionic surfactants; 0.002to 30 % by weight of an alkalinity agent (d) containing at least one alkaline water-soluble compound (d) selected from the group consisting of hydroxides and carbonates; and 0.004 to 99 % by weight of a transport component(e)containing predominantly water. Further on, the present application concerns a method of use of such aqueous metalworking composition as a coolant, as a lubricant, etc., and concerns further on a method to prepare an aqueous metalworking composition and a metalworking process.
Owner:CHEMETALLGMBH
Method for controllably synthesizing nano zinc oxide based on solvent heat
InactiveCN102515245AEvenly distributedEasy to operateZinc oxides/hydroxidesNanotechnologyPyrrolidinonesEngineering
The invention belongs to the field of nano material synthesis and application, and in particular relates to a method for synthesizing nano zinc oxide based on solvent heat. According to the invention, zinc acetate dehydrate is used as a raw material, sodium hydroxide is used as an alkalinizing agent, and polyvinylpyrrolidone is used as an additive. The method comprises the following synthesis steps of: firstly, adding ethanol in a hydrothermal kettle until the filling degree is 0.6; adding the additive polyvinylpyrrolidone, the raw material zinc acetate dehydrate and the alkalinizing agent sodium hydroxide in the solution, and stirring for 20-40 minutes; sealing the hydrothermal kettle and then placing the sealed hydrothermal kettle in an oven, heating to 80-160 DEG C and maintaining the temperature to be constant for 0.5-3 hours; and finally, naturally cooling, centrifuging the obtained product, washing the product with deionized water and ethanol, and drying to obtain the nano zinc oxide products with different morphologies.
Owner:SHENYANG POLYTECHNIC UNIV
Preparation method of salt-resistant carboxymethyl starch
The invention relates to a preparation method of salt-resistant carboxymethyl starch. A mixed solvent of ethanol and isopropyl alcohol serves as a reaction medium, and multi-step modification of cassava starch is performed in slurry taking the mixed solvent as a medium. The method comprises the following specific steps: swelling the starch to prepare starch milk; adding a surfactant and performing modification such as alkalization and etherification by an 'acid pretreatment process'; and adding a crosslinking agent for a micro-crosslinking reaction. In the method provided by the invention, the surfactant is added as a penetrant, the reagent molecules are promoted to penetrate and spread into the starch particles, and the alkalization efficiency and etherification efficiency are improved; the generation of side reactions can be reduced by adding an etherifying agent before an alkalizer, thus the alkalization and etherification are carried out at the same time, and more thorough etherification reaction is guaranteed; finally, the starch is subjected to micro-crosslinking so as to improve the structure viscosity and enhance the salt resistance; the product viscosity 4% exceeds 40,000, the substitution degree is over 1.0, the salt-viscosity ratio goes beyond 0.5, and the product can be applied to multiple technical fields instead of CMC.
Owner:HANGZHOU HONGBO NEW MATERIALS
Polymeric aluminum chloride flocculant preparation method using phthalocyanine green waste water
InactiveCN1544348AAchieve the purpose of useReduce pollutionWater/sewage treatment by ion-exchangeIron halidesAluminium chlorideLiquid product
A process for preparing polymerized aluminium chloride flocculating agent from phthalocyanine green waste water comprising, placing the type 335 weak alkaline anion exchange resin into adsorbing column, flowing dilute hydrochloric acid through the adsorbing column, rinsing the resin column with water or deionized water, processing the resin column by weak alkaline liquor, finally rinsing the adsorbing column with water or deionized water until water exhibits weak alkalinity, adjusting the pH of phthalocyanine green waste water, making it pass through the pretreated and transformed type 335 weak alkaline anion exchange resin column from normal temperature to 50 deg. C, and passing through another ion exchange resin adsorbing column, thus obtaining the phthalocyanine green waste water whose copper ion concentration meets second level standard. By concentrating the phthalocyanine green waste water treated by the resin under the condition of stirring and heating, adding in alkalinizing agents simultaneously for reaction, adjusting waste water till it exhibits weak acidity, continuously stirring and conducting heating reaction, the liquid product or solid end product can be obtained.
Owner:NANJING UNIV
Composition and method of treatment for urogenital conditions
The present invention is directed to a composition and method for treating uro-genital conditions. One embodiment disclosed is a pharmaceutical composition for use in the treatment of uro-genital conditions wherein said composition comprises a KPV dimer, a first preservative agent, a solvent, an alkalizer, an acrylic acid-based polymer, a second preservative agent and a gelatinizing agent. Another embodiment of the invention is disclosed wherein the composition comprises CKPV (SEQ ID NO: 5) dimer, API, Carbopol®, NF, propylparaben, NF; methylparaben, NF; propylene glycol, USP; edetic acid (EDTA), USP; 2 M sodium hydroxide solution (NaOH); and sterile water for injection, USP. Also disclosed are methods and indications for use of the disclosed composition.
Owner:MSH PHARMA INC
Aripiprazole medicament formulation and preparation method therefor
ActiveUS20140135343A1No adverse effectsOrganic active ingredientsNervous disorderAntioxidantMedicine
The preparation method includes the following steps: dissolving aripiprazole in an acidic solution having an acidifier so as to obtain a medicament having acidic solution; then, performing a wet granulation on or preparing a suspension with the obtained medicament having acidic solution, an alkalizer, and an excipient so as to obtain the aripiprazole medicament formulation; the excipient comprising an antioxidant. The aripiprazole medicament formulation obtained through the preparation method has a significantly reduced amount of related substances, great solubility, great stability, high bioavailability, reduced individual differences, and enhanced wettability and content uniformity of insoluble medicaments.
Owner:SHANGHAI ZHONGXI PHARMA
Sedative hypnotic pharmaceutical preparation and its preparation method
ActiveCN102846538AChange surface propertiesImprove wettabilityOrganic active ingredientsNervous disorderOrganic acidSubstance content
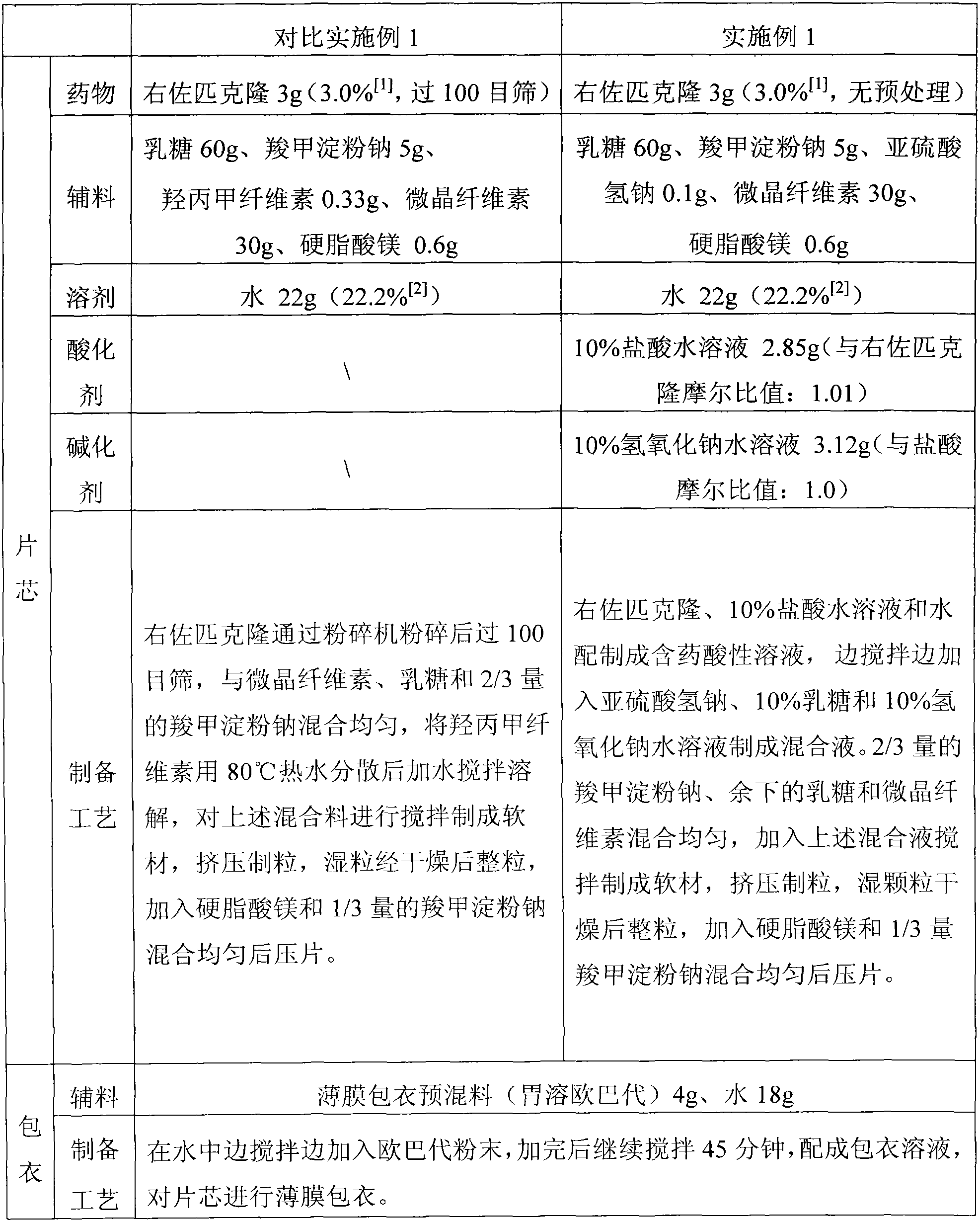
The invention discloses a preparation method of a sedative hypnotic pharmaceutical preparation. The preparation method includes the steps of dissolving active ingredient dexzopiclone or zopiclone in acidifier-containing acidic solution to give drug-containing acidic solution, and then performing wet granulation with the obtained drug-containing acidic solution, basifier and adjuvant. The acidifier is hydrochloric acid, and the basifier is sodium hydroxide. One or more of organic weak acid, acid salt and conjugate base of organic weak acid are added before or during the addition of the basifier. The invention also discloses the sedative hypnotic pharmaceutical preparation prepared by the method. The inventive preparation method has no potential safety hazard, simple and convenient operation, low pollution and loss, low cost, and good process controllability. The obtained pharmaceutical preparation has excellent dissolution and stability, and lower related substance content.
Owner:SHANGHAI ZHONGXI PHARMA
Bubble-formulation hair dye and preparation method thereof
The invention relates to a bubble-formulation hair dye and a preparation method thereof. The hair dye consists of two components, namely a component A and a component B. The component A consists of a dye mixture, a surfactant, a film forming agent, an alkalizer, water-soluble lanoline, water-soluble silicone oil, a humectant, an antioxidant, a heavy metal chelating agent and an essence and is prepared by the following steps of: adding water with the temperature between 45 and 55 DEG C to the film forming agent, and stirring for dissolution; adding the alkalizer, the water-soluble lanoline, the water-soluble silicone oil, the humectant, the antioxidant, the heavy metal chelating agent and the dye mixture, and stirring for dissolution; and finally adding the surfactant and the essence, stirring for dissolution, and filtering the mixture. The component B consists of hydrogen dioxide and phosphoric acid and is prepared by the following steps of: adding the hydrogen dioxide into water, and stirring for dissolution; adjusting the pH value to between 2 and 4 by using the phosphoric acid; and filtering the mixture to discharge the material. The hair dye is characterized in that: the production process is simple; the hair dye has the bubble formulation, is easy to spread uniformly and has good hair dyeing effect; and a user can optionally change the colour of the hair at home.
Owner:GUANGZHOU YOUNGRACE COSMETIC LTD
Enteric film coating composition containing enteric polymer micronized with detackifier
Dry, enteric, film-coating compositions and aqueous dispersions containing the same are disclosed. When applied to orally-ingestible substrates such as oral solid dosage forms, the film coatings are capable of preventing the substrates from disintegrating in media with pH values from about 1 to about 4.5 or higher values. One preferred film-coating composition contains a micronized intermediate comprised of an acrylic resin and talc. Advantageously and surprisingly, the preferred film-coating composition does not contain an alkalizing agent.
Owner:BPSI HLDG LLC
Method for removing cloud point of sea water drasticlly and producing acid waste-water neutralizer
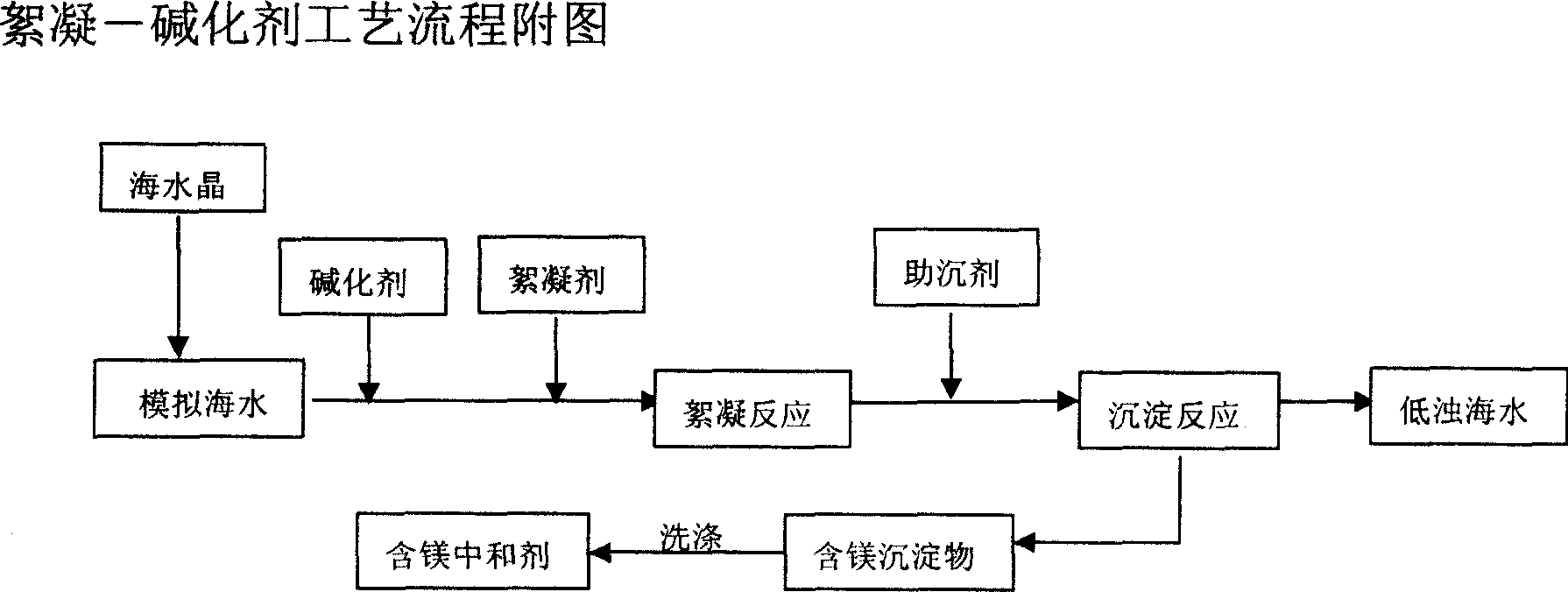
InactiveCN1982230AReduce turbidityQuick filterSeawater treatmentWater/sewage treatment by neutralisationWater desalinationMagnesium salt
Alkali treatment for sea water with sodium hydrate as basifier is carried out by regulating sea-water pH value by basifier addition, reacting magnesium salt with alkali to generate magnesium hydrate with strong adsorptive function, and adsorbing rapidly electric colloid, suspended substance, microbe, bacterial and algae. It has faster flocculation depositing speed, gentle reactive condition, simple process and less investment. It can be used as acid waster-water neutralizer and magnesium hydrate slurry.
Owner:TIANJIN UNIV OF SCI & TECH
Composition for colouring keratin fibres
ActiveUS20190117541A1Stable in timeNot damage hairCosmetic preparationsHair cosmeticsAlkalizing agentHair dyes
The present invention refers to a hair dye comprising a) an alkalinising agent consisting of at least one amino acid having a pKa of greater than 10.00 and at least one ester of a fatty acid and glycerol polyethoxylate; and b) at least one oxidation colourant. In particular, the composition of the invention does not comprise any further alkalinizing agent.Kits and methods for colouring hair based on this composition also fall within the scope of this invention.
Owner:BEAUTY & BUSINESS SPA
Method for extracting high-purity calcium oxide from carbide slag
InactiveCN102092756AHigh extraction rateShorten the timeCalcium/strontium/barium oxides/hydroxidesEmulsionHydrogen
The invention relates to a method for extracting high-purity calcium oxide from carbide slag. The method is characterized by comprising the following steps: drying the carbide slag; carrying out high temperature roasting pretreatment; preparing carbide slag emulsion; carrying out centrifugal separation to produce a calcium chloride leaching liquid; acidifying the leaching liquid; adding a precipitator ammonium oxalate in the acidified solution; dropwise adding a basifier in the solution to neutralize hydrogen ions in the solution, and obtaining the precipitated ammonium oxalate by centrifugal separation; re-drying the separated ammonium oxalate particles; and roasting the dried ammonium oxalate particles at a high temperature to obtain the calcium oxide product with the purity of 98-99%. The invention provides the method for extracting the calcium oxide from the carbide slag by using the precipitator ammonium oxalate. The method is scientific and reasonable, and can meet industrial production requirement so that the extraction efficiency of the calcium oxide product is high, the extraction time is short, the cost is low, and the purity is up to 98-99%.
Owner:NORTHEAST DIANLI UNIVERSITY
Method of Synthesizing Ferrate
ActiveUS20160304356A1Process is safe and fastReduce the amount requiredHeavy metal active ingredientsIron compoundsIron saltsFree cooling
A method of synthesizing ferrate, which includes the steps of: (a) weighing and obtaining iron salts, activating agents, alkalinizing agents and oxidizing agents solution; (b) mixing uniformly the iron salts, the activating agents and the alkalinizing agents, heating to 30˜398° C. and maintaining for 1 min˜60 min to obtain a mixture; (c) adding the oxidizing agents solution to the mixture with an adding time of less than 10 minutes, then obtaining a precursor; and (d) natural cooling the precursor, then mixing the precursor with water and stirring evenly to obtain a final product of ferrate, wherein a volume ratio of the precursor and the water is 1:1˜5. The method involves low power consumption, low temperature, low explosion risk, non-complicated steps and procedures, short synthetic time and high ferrate conversion efficiency. The method produces ferrate of high yield and good stability, and is suitable for producing ferrate composite pharmaceuticals in industrialized mass production.
Owner:HARBIN INST OF TECH
Choline-silicic acid complex with osmolytes and divalent trace elements
ActiveUS20120015047A1Improve bioavailabilityRaise the pHBiocideHeavy metal active ingredientsOligomerSilicic acid
The invention relates to a biological preparation comprising orthosilicic and silicic acid, a primary (“constant / first”) osmolyte choline and a weak alkalinizing agent without free hydroxyl groups and to a method for preparing the preparation, comprising: i) hydrolyzing a silicon comprising choline solution thereby forming a choline stabilized orthosilicic acid and oligomers solution; ii) alkalizing the choline orthosilicic acid and oligomers solution by adding a weak alkalizing agent without hydroxyl groups; and iii) optionally adding a divalent trace element and / or secondary osmolyte, to biological preparation obtainable and its uses.
Owner:BIO MINERALS
Process for modification of biopolymers
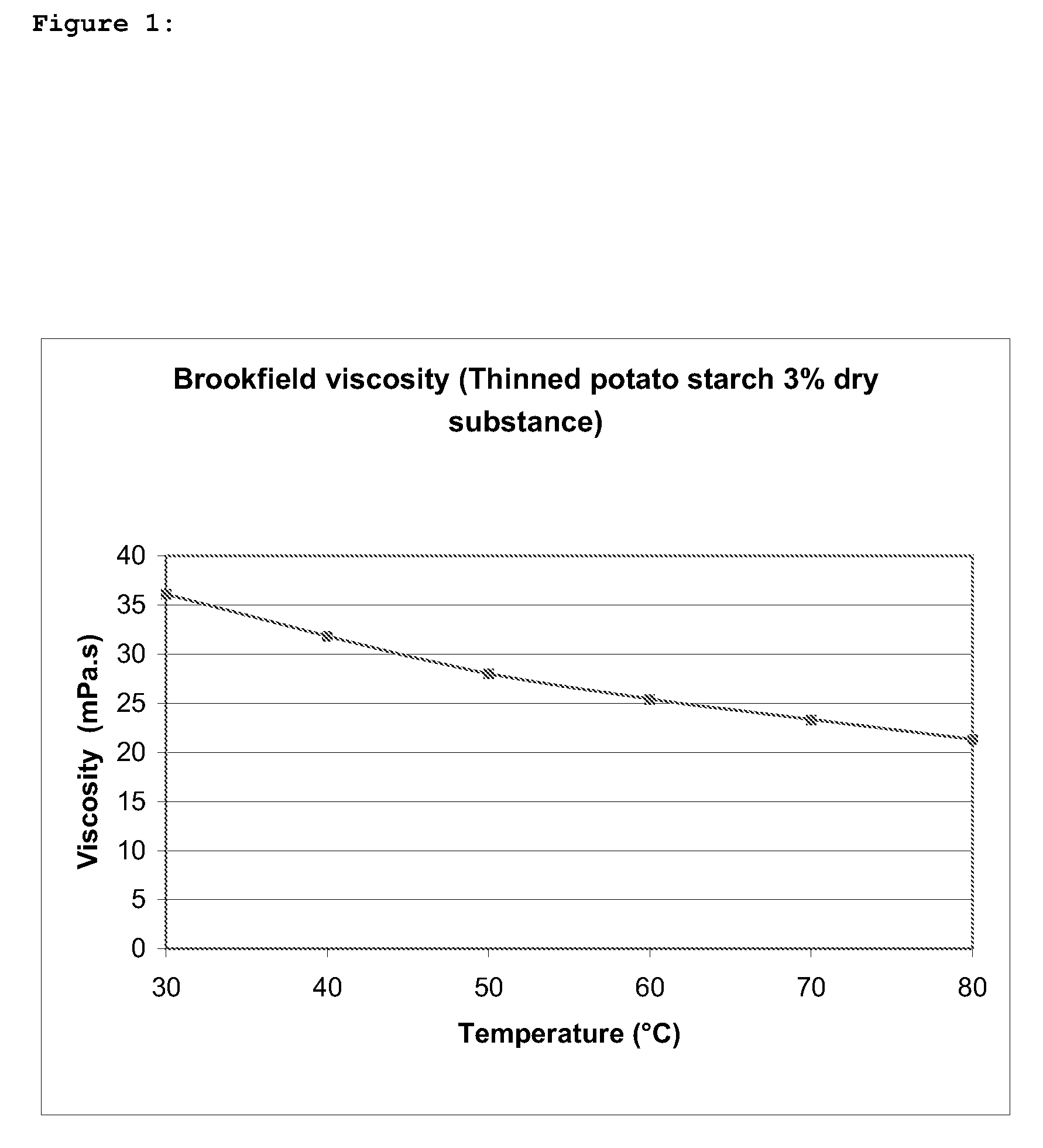
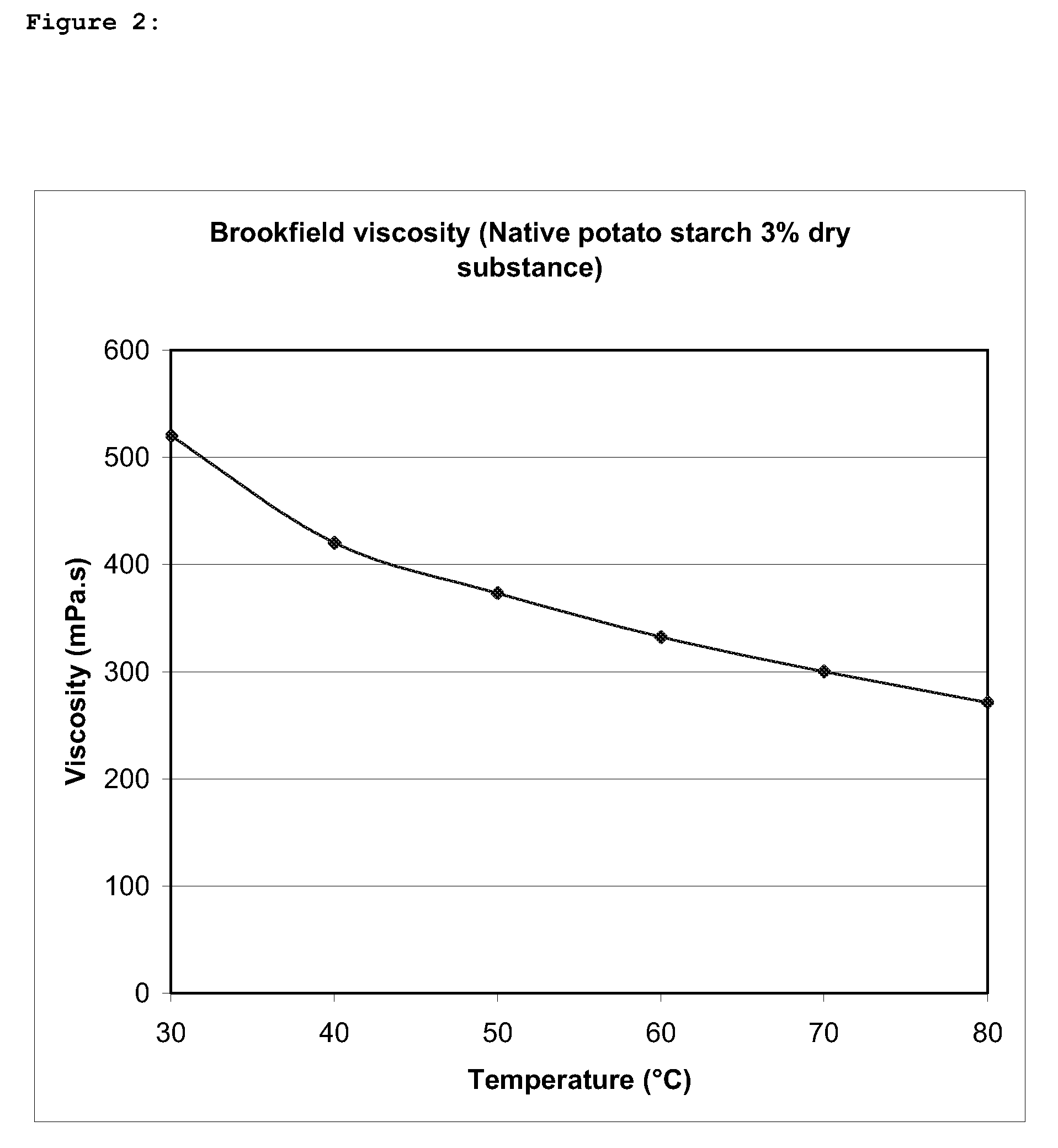
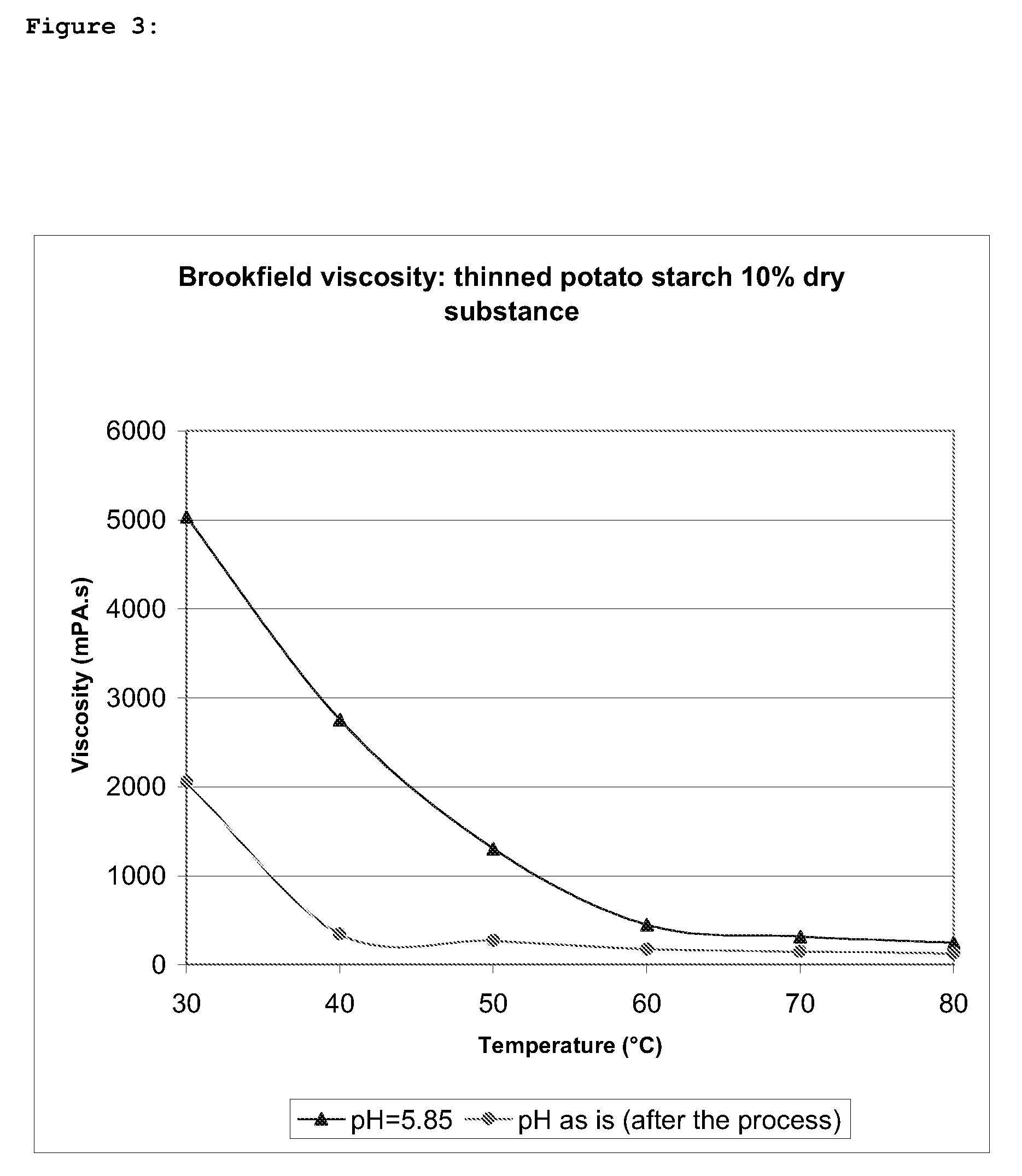
A biopolymer thinning process is provided comprising the steps of (a) mixing a biopolymer substrate with a thinning agent and an alkalizing agent; and (b) drying the mixture of step (a), wherein the thinning agent consists of one or more hypochlorites; and step (a) is carried out at a neutral to alkaline pH and does not involve any artificial heating.
Owner:CARGILL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com