Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
172 results about "Good manufacturing practice" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS).
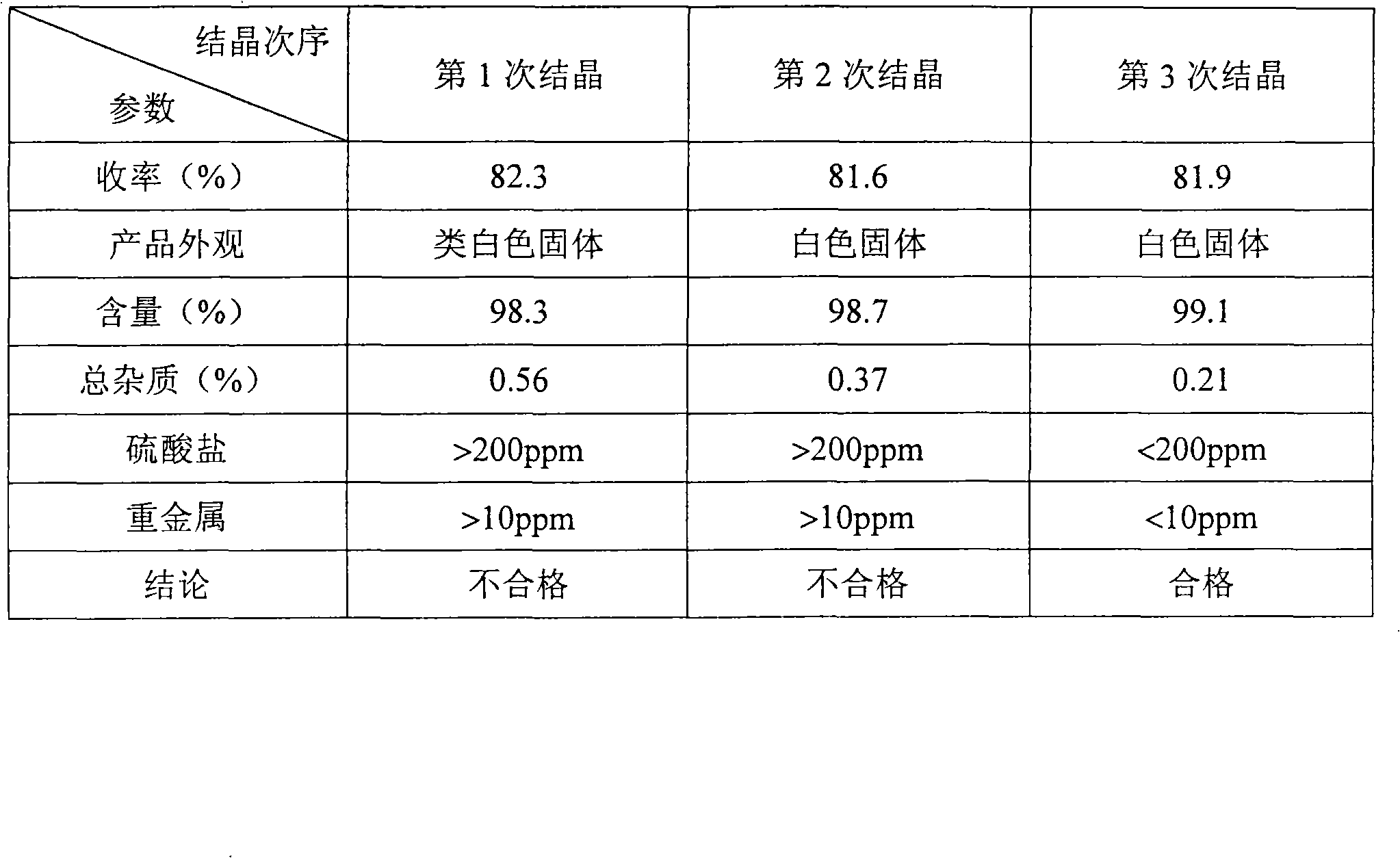
Prevalidated, modular good manufacturing practice-compliant facility
InactiveUS20090305626A1Precious timeShorten the timeApparatus sterilizationDust-free enclosuresInterior spaceGood laboratory practice
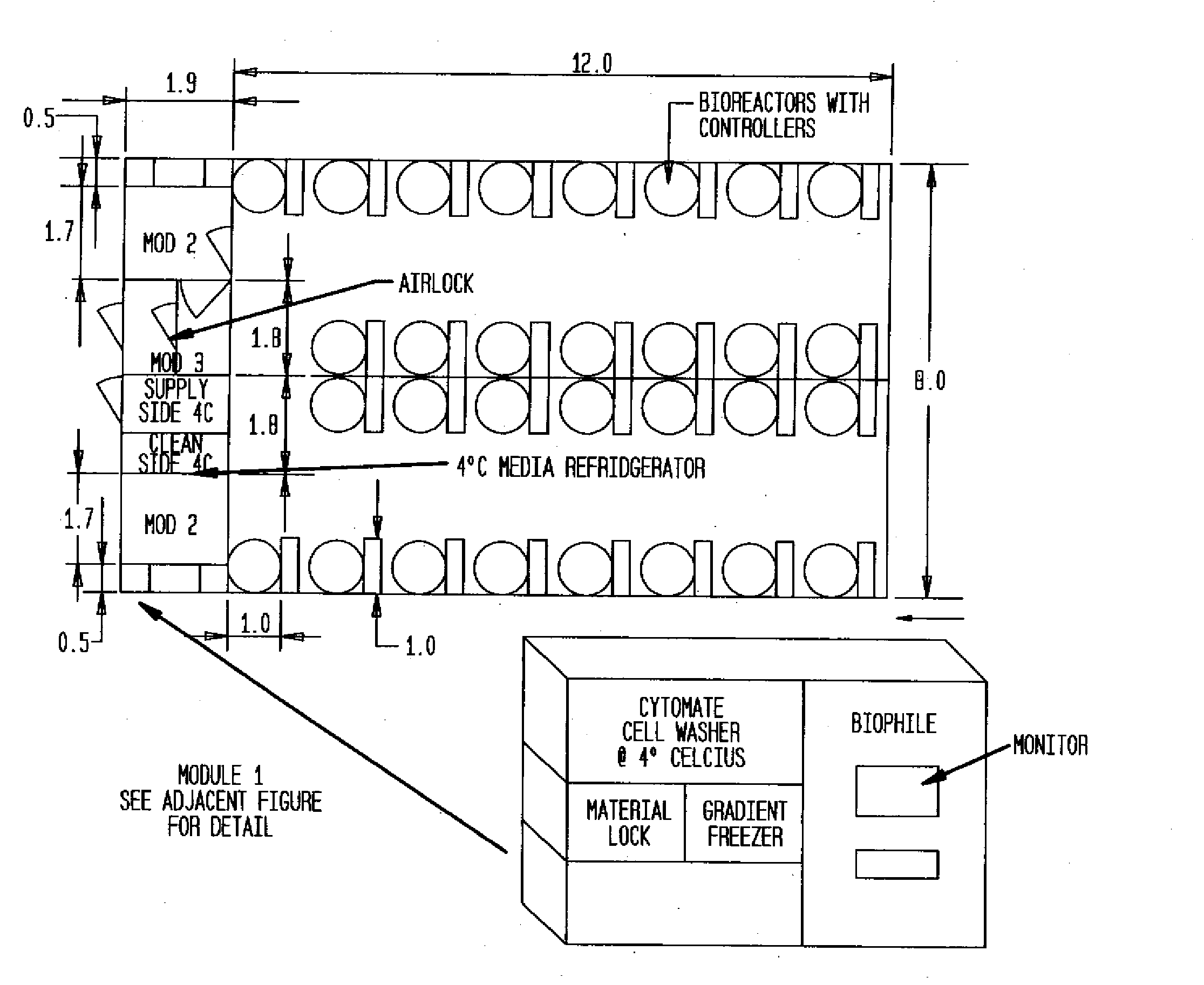
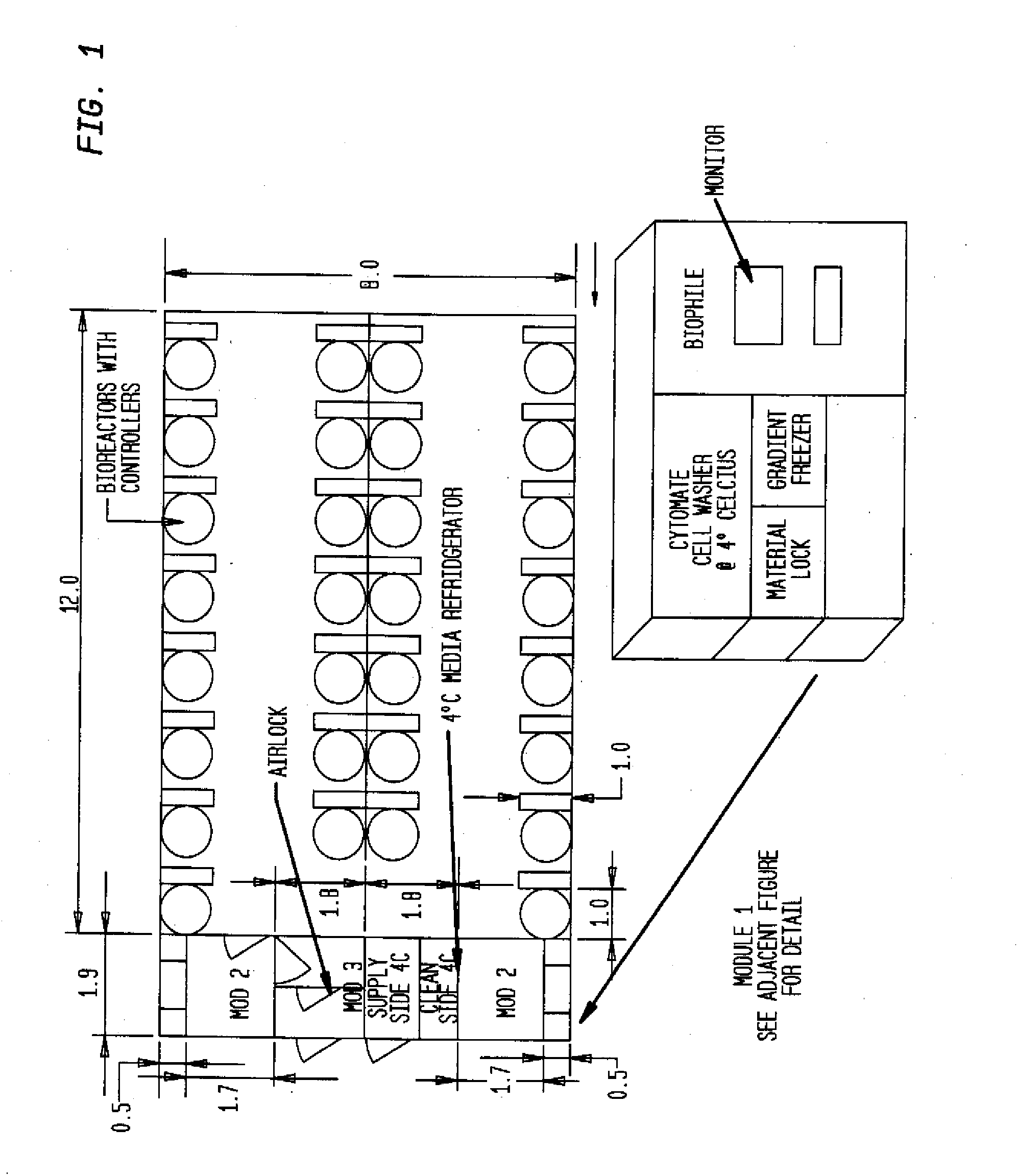
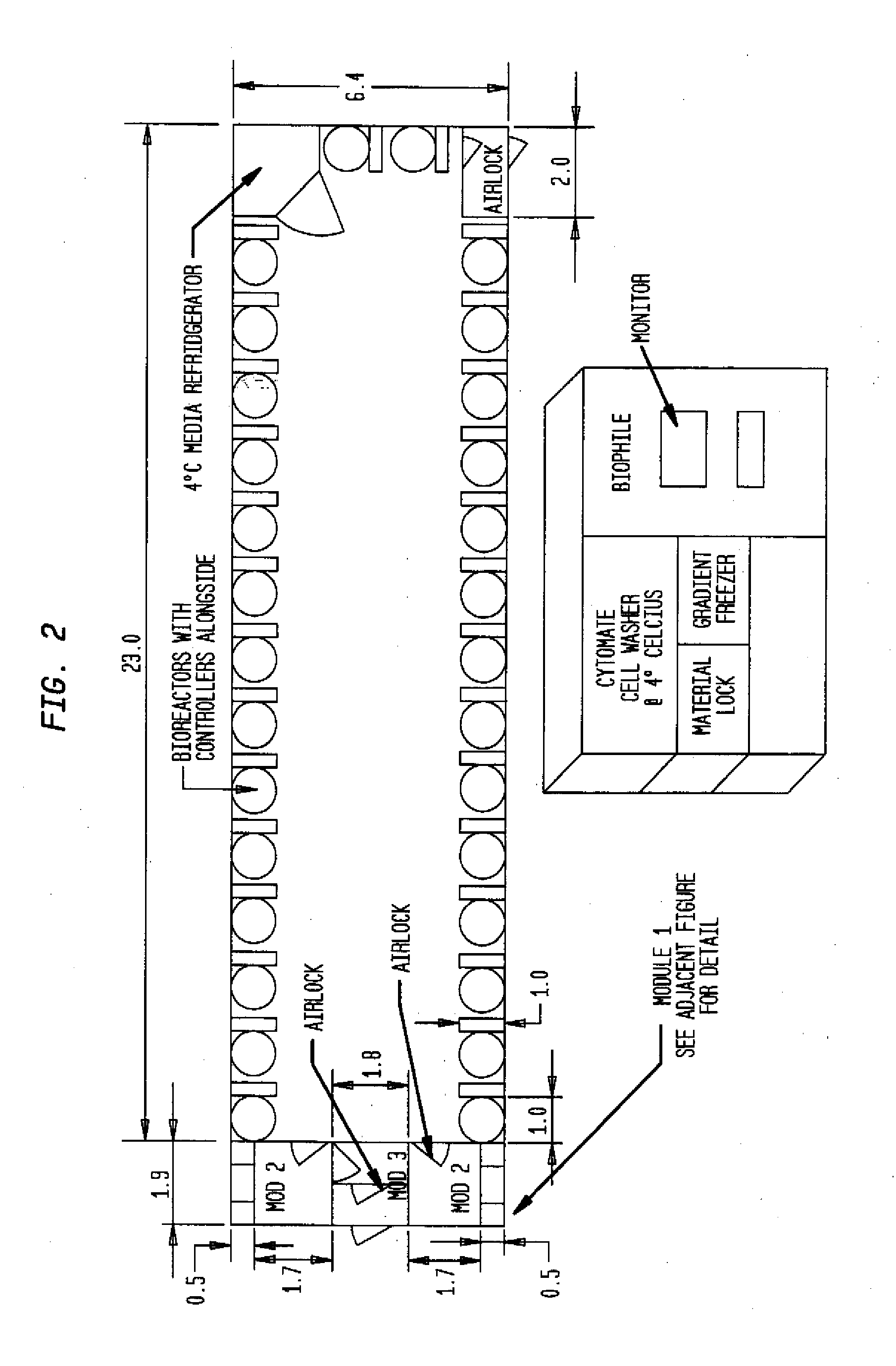
The invention is directed to a ready-to-use modular cleanroom and facility, in particular for the production of drugs and biological substances, which is equipped with pre-approved manufacturing equipment cores. The modular cleanroom is implemented in the interior space of a container, such as a standard shipping container, and includes at least one bioreactor station. The modular facility can be installed on-site from pre-approved cleanroom modules without further regulatory approval. The cleanroom and facility comply with FDA-approved good manufacturing practices (GMP) and good laboratory practices (GLP).
Owner:HOPE ERNEST G
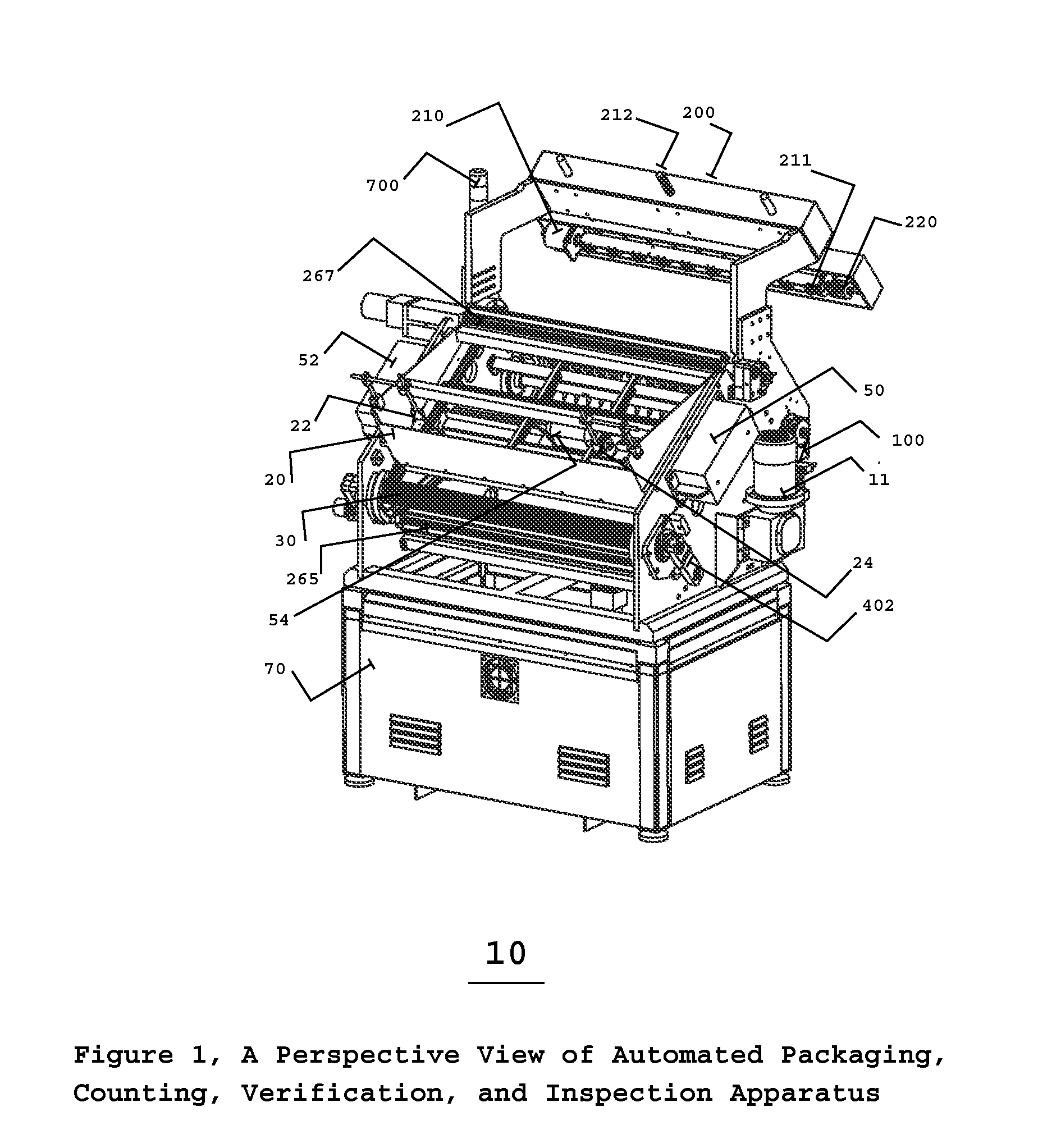
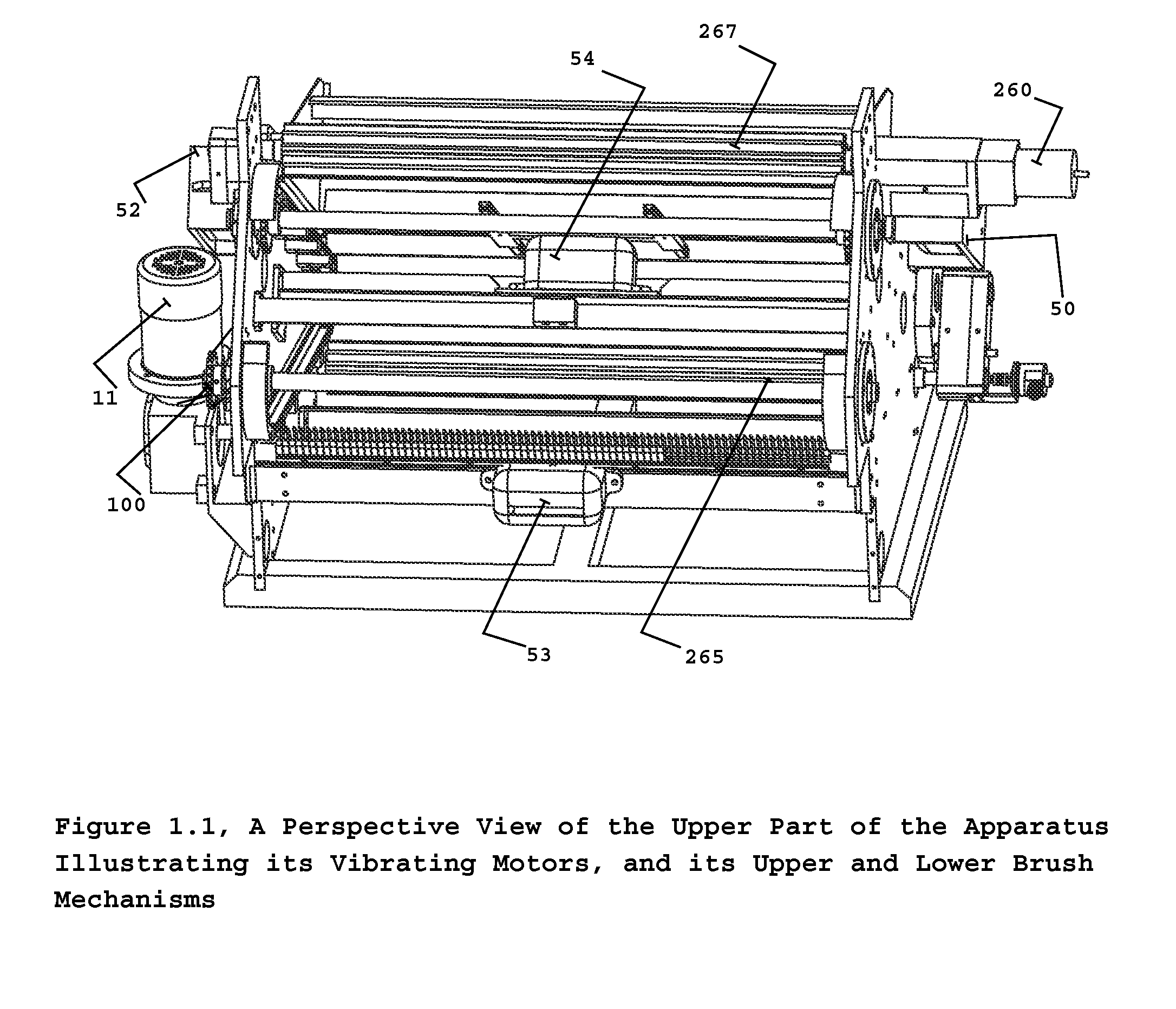
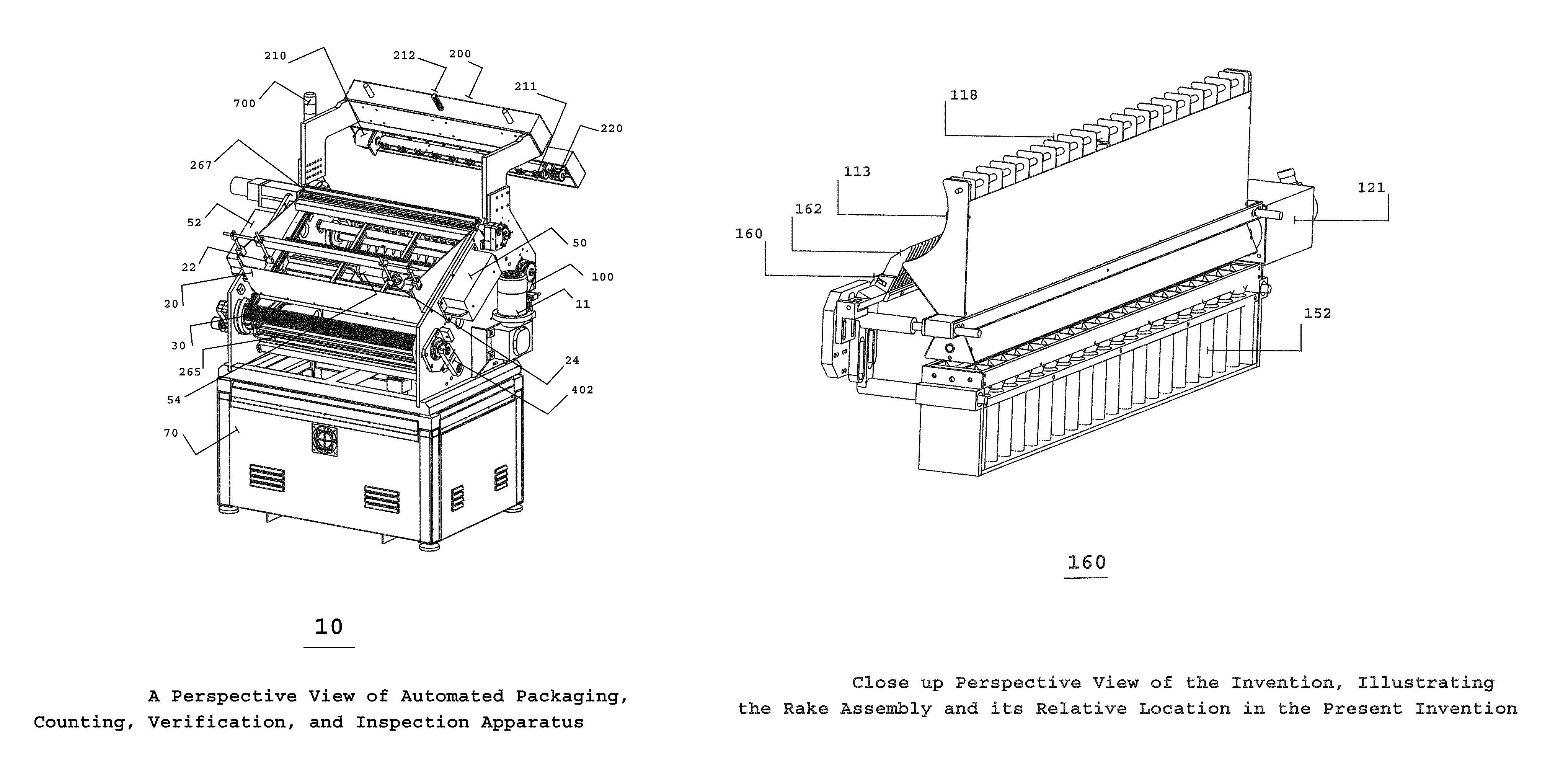
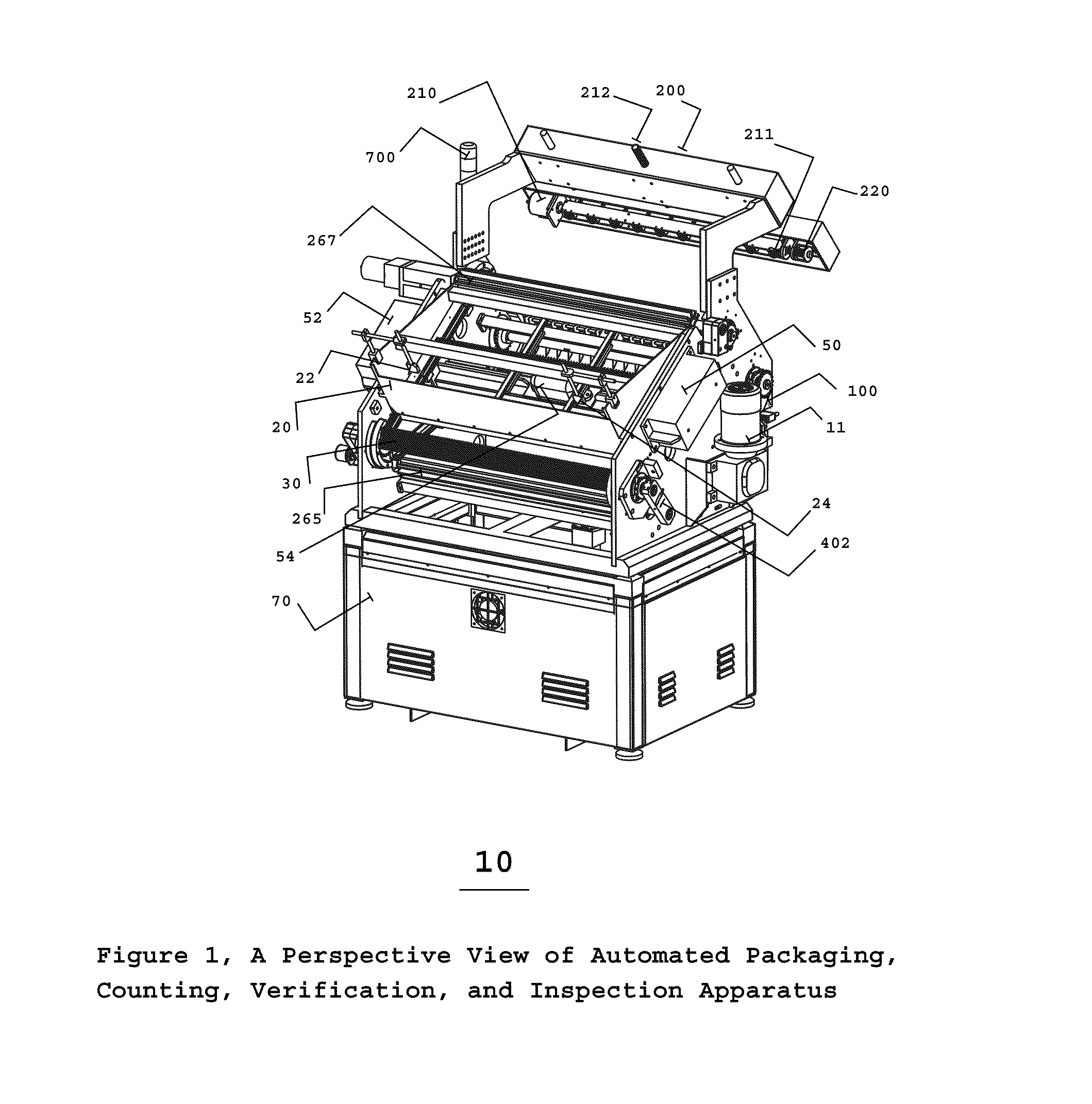
Automated pharmaceutical product packaging, inspection, verification, and counting apparatus
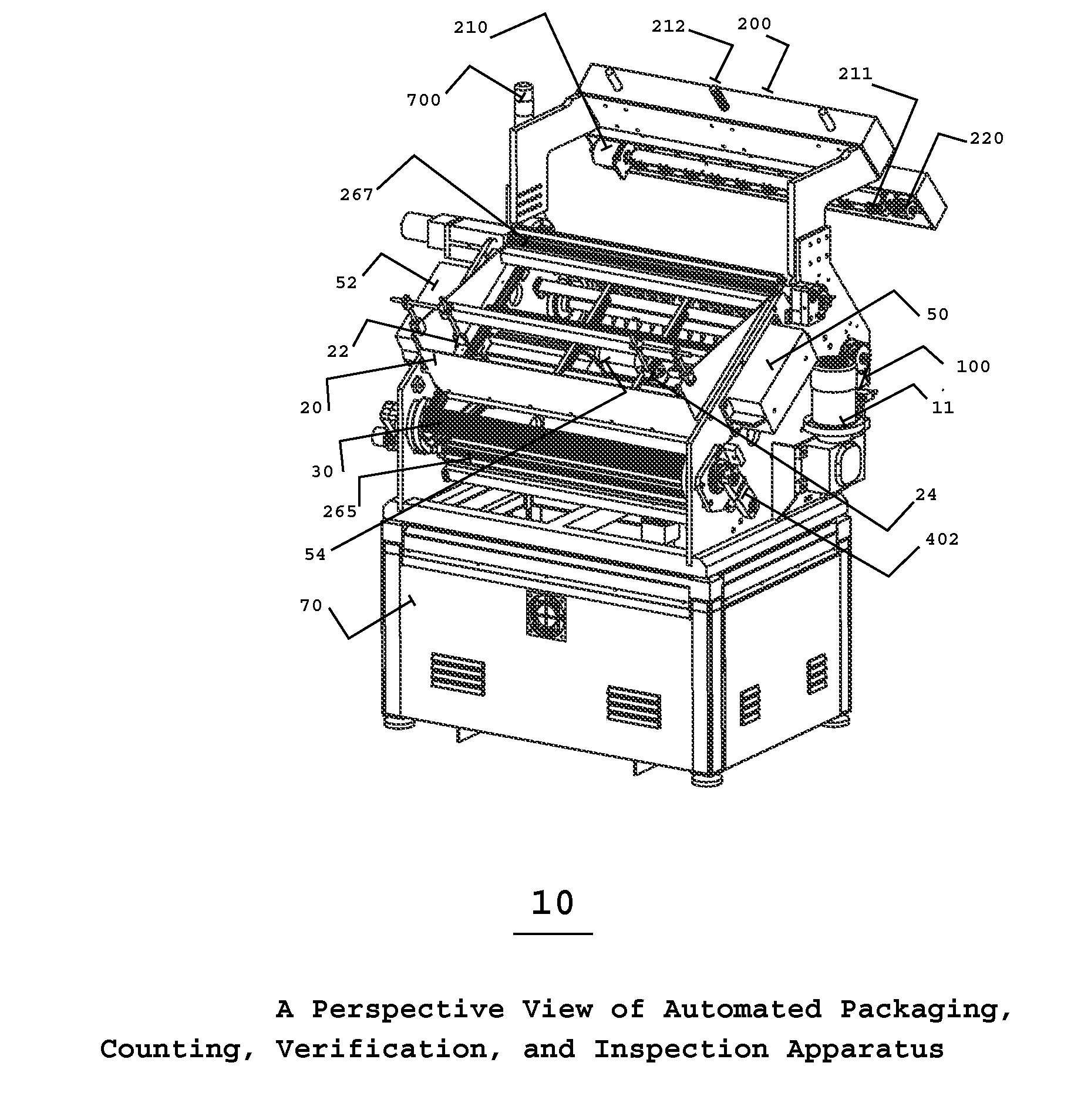
InactiveUS20100175352A1High container filling speedFast filling speedCapsCapping machinery safety/controlProcess engineeringEngineering
The present invention is an automated packaging apparatus utilizing a rotating assembly of elongated slats containing cavities to receive discrete pharmaceutical, vitamin, or food products. Quantities of discrete products such as tablets, capsules, or gels are deposited into the hopper of the apparatus. The apparatus then dispenses the discrete products into containers moving on a conveyor system such that each container receives a predetermined quality and quantity of pharmaceutical, vitamin, or food products. While operating at high speed, the apparatus inspects, counts, identifies and analyzes each product deposited into the containers and maintains electronic records describing the status of each product. In the event any errors occur the apparatus produces various alerts to inform the operator. The presence of foreign products or objects may cause the apparatus to instantly stop the entire system including peripheral equipment. A series of Good Manufacturing Practice protocols can then be enforced as per FDA requirements.
Owner:SOLOMAN SABRIE
Treatment of vocal cords with autologous dermal fibroblast formulation
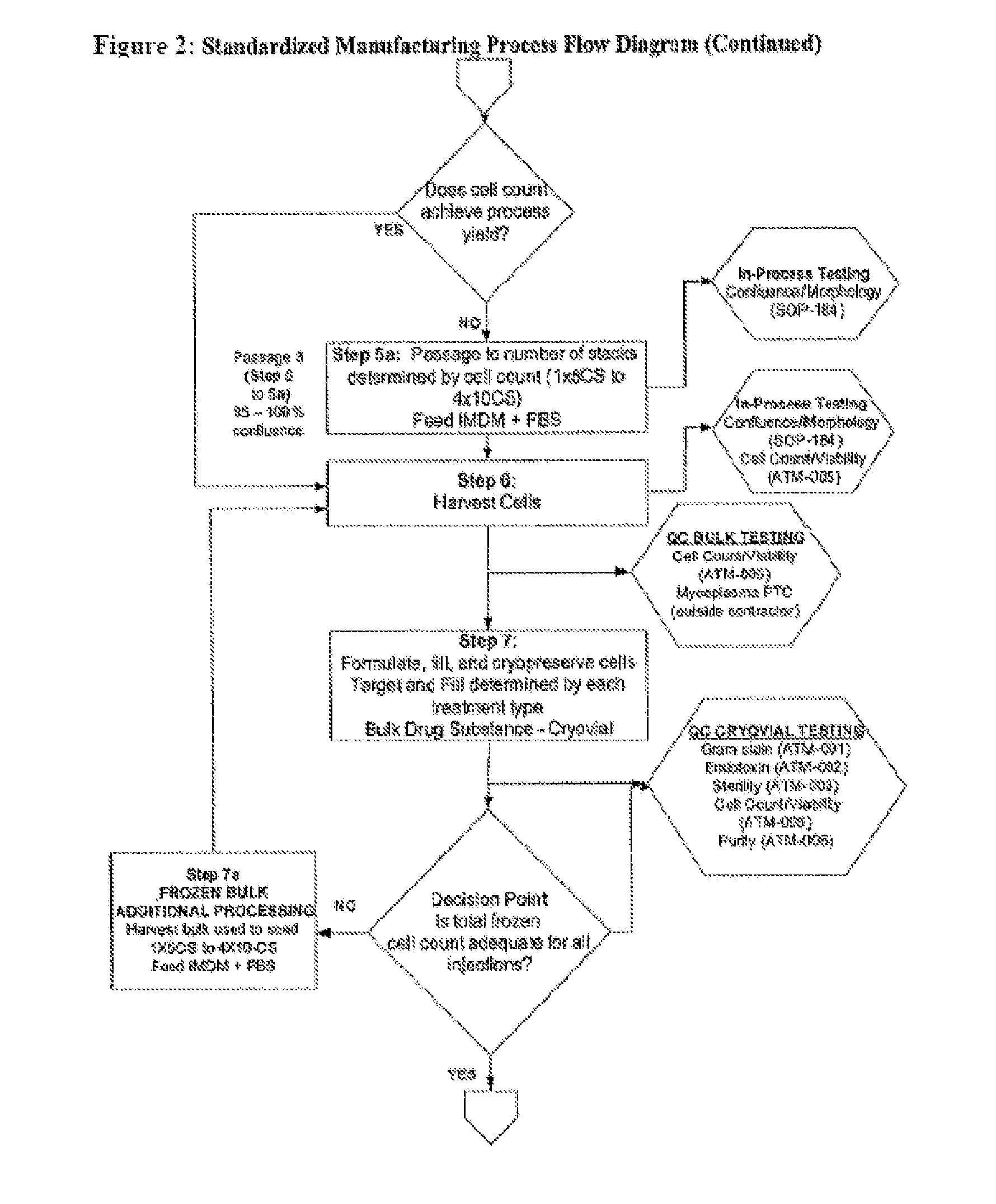
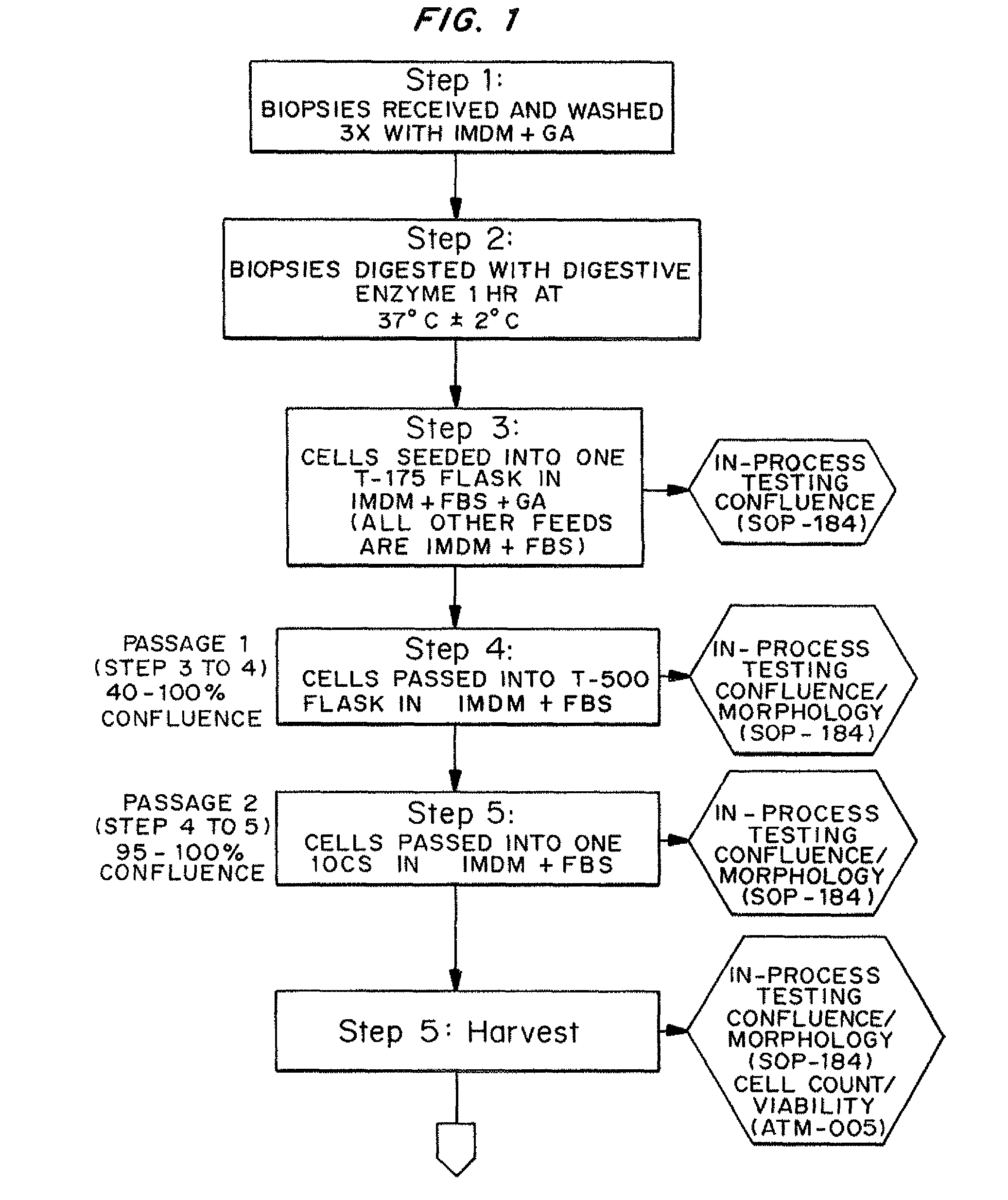
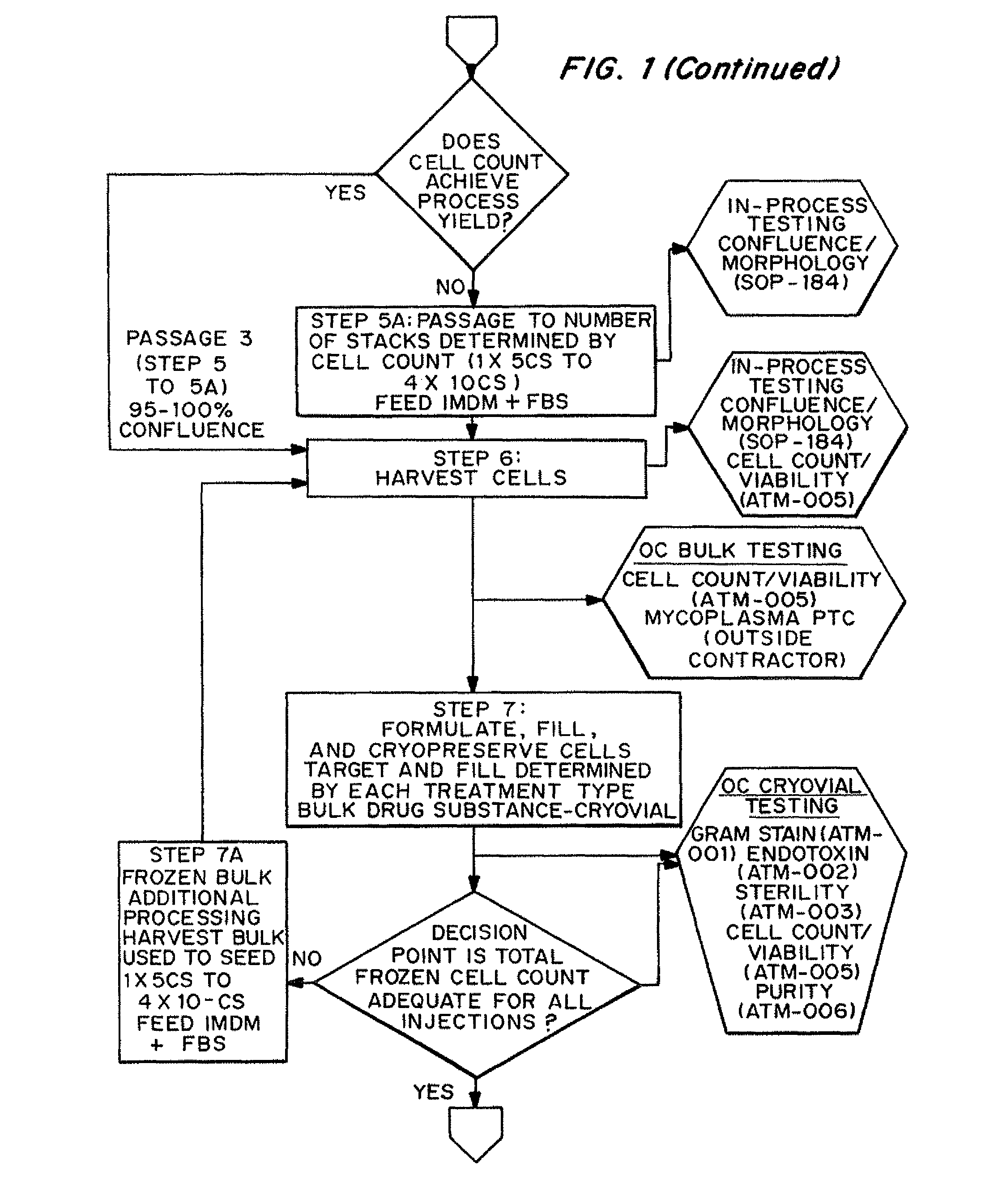
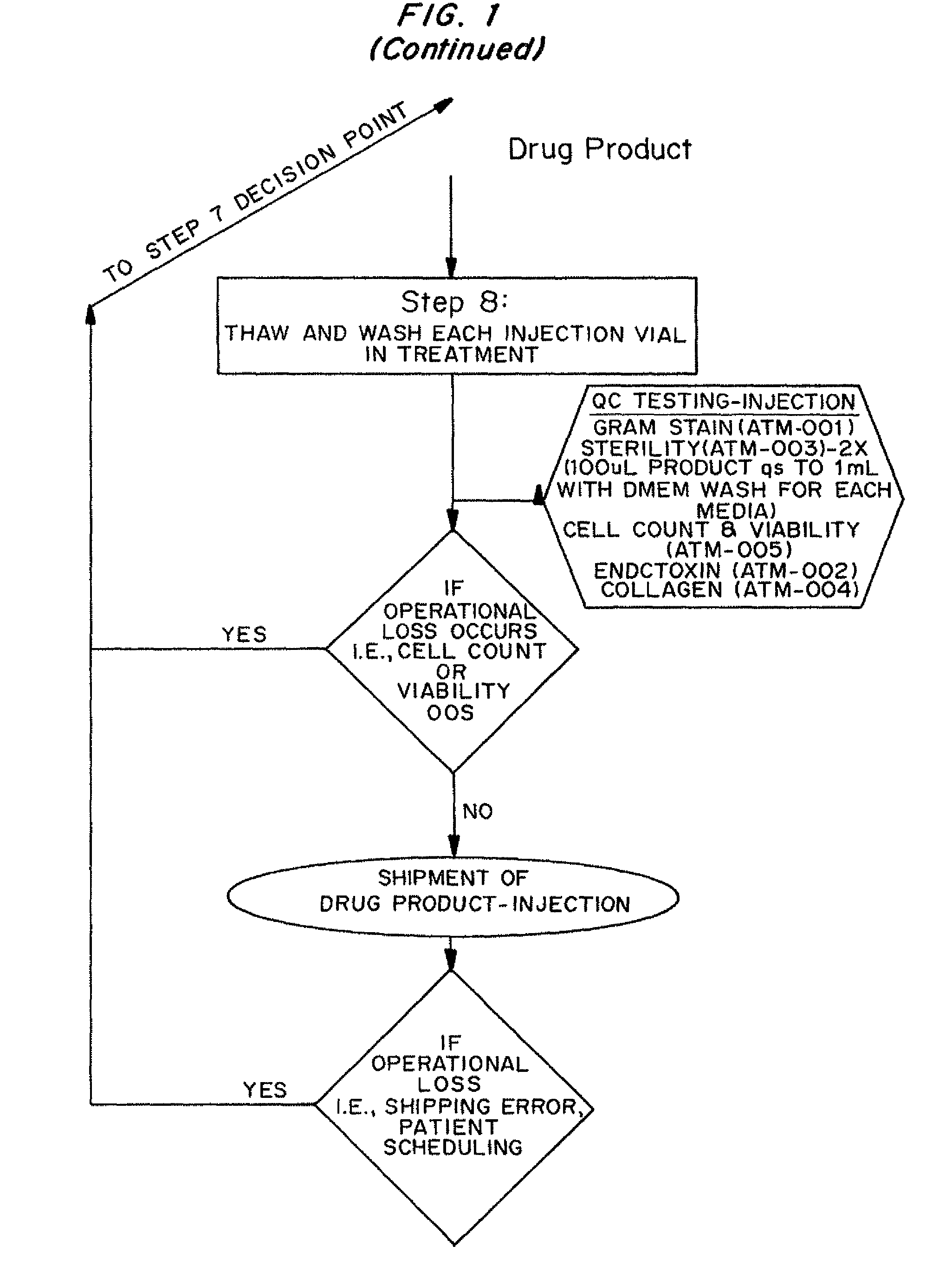
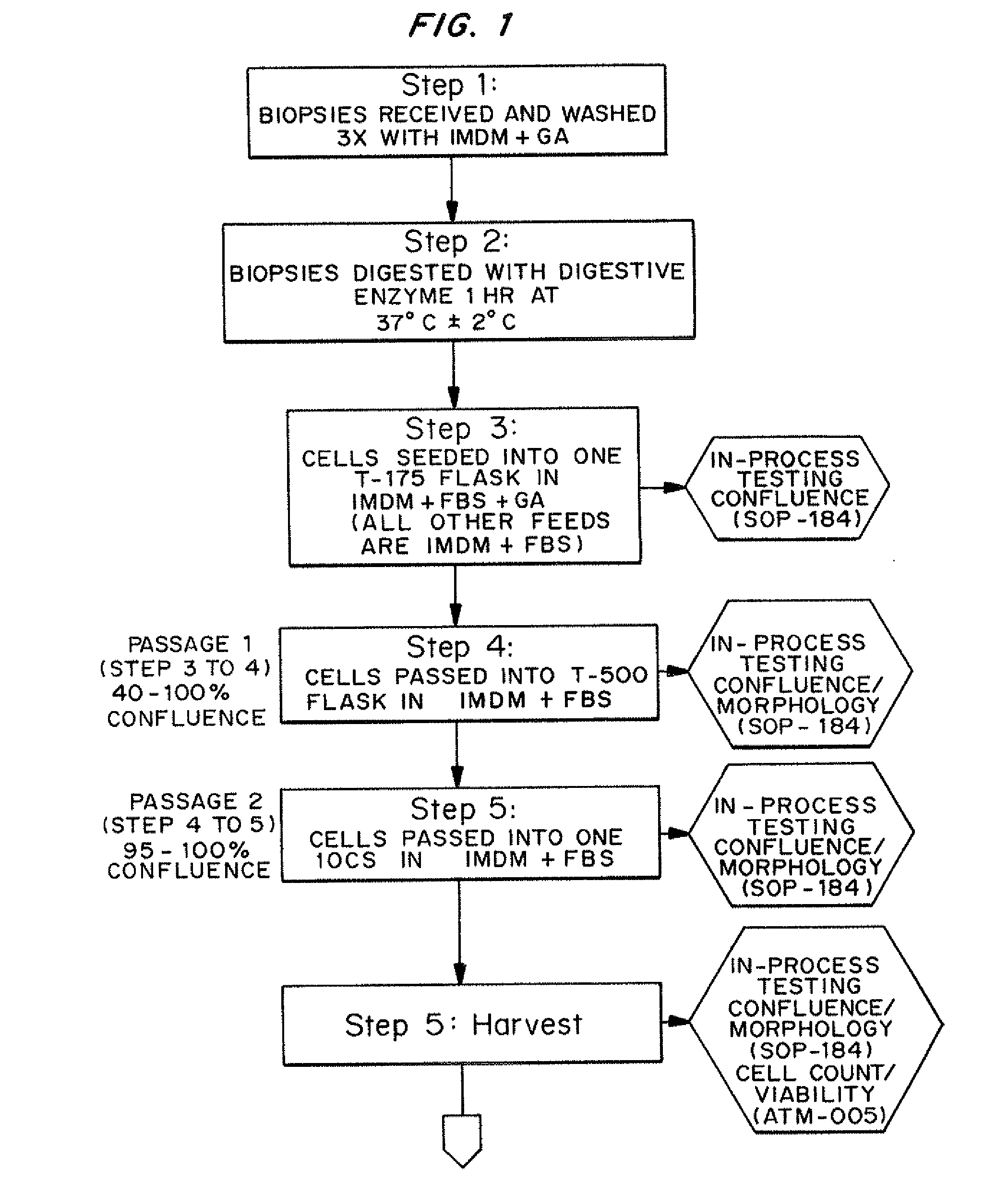
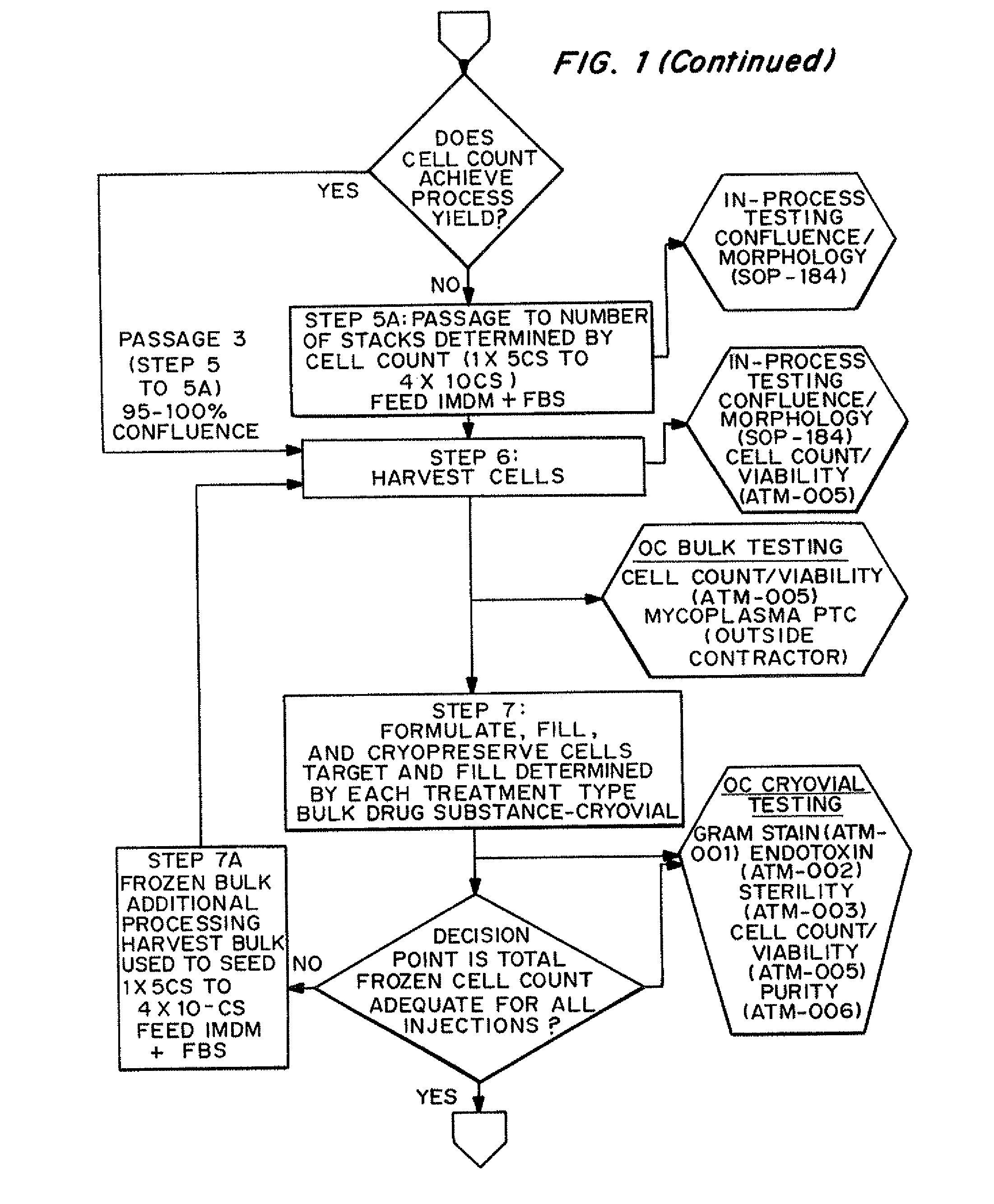
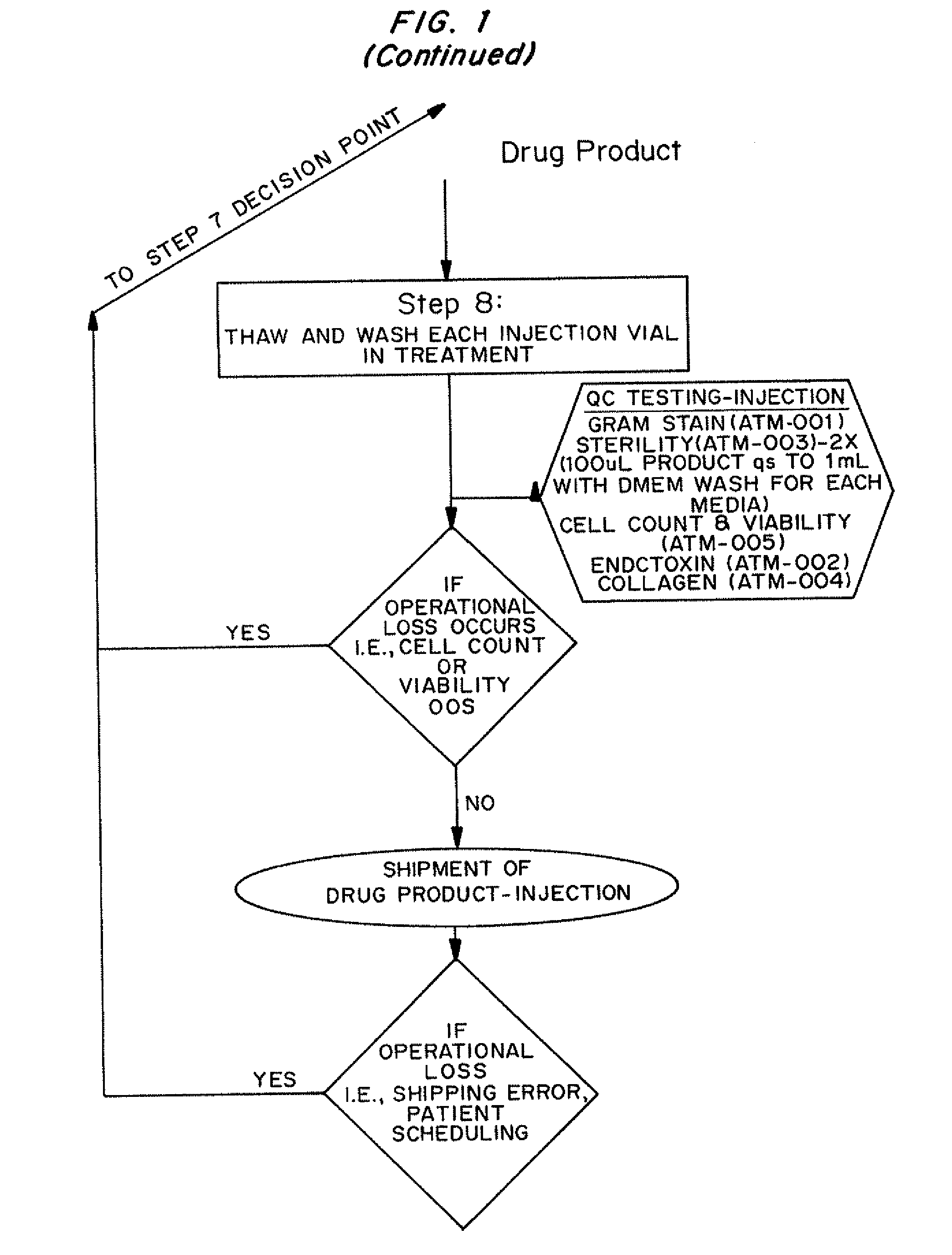
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated for augmentation or regeneration of vocal cords. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own buccal mucosa or skin using current good manufacturing practices (CGMP) and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL administered three times approximately three to six weeks apart, of cells at a concentration of from 1.0-2.0×107 cells / mL.
Owner:CASTLE CREEK BIOSCIENCES LLC
Dosage unit formulations of autologous dermal fibroblasts
ActiveUS8529883B2Reduce severityAdditional componentBiocideArtificial cell constructsWrinkle skinNasolabial fold
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own skin using current good manufacturing practices (CGMP), and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL, of cells at a concentration of from 1.0-2.0×107 cells / mL. When injected into the nasolabial fold wrinkles (creases on the sides of the nose that extend to the corners of the mouth), the autologous fibroblasts are thought to increase the synthesis of extracellular matrix components, including collagen, reducing the severity of these wrinkles. Dosage and timing of administration have been demonstrated to be critical to achieving clinically significant outcomes.
Owner:CASTLE CREEK BIOSCIENCES LLC
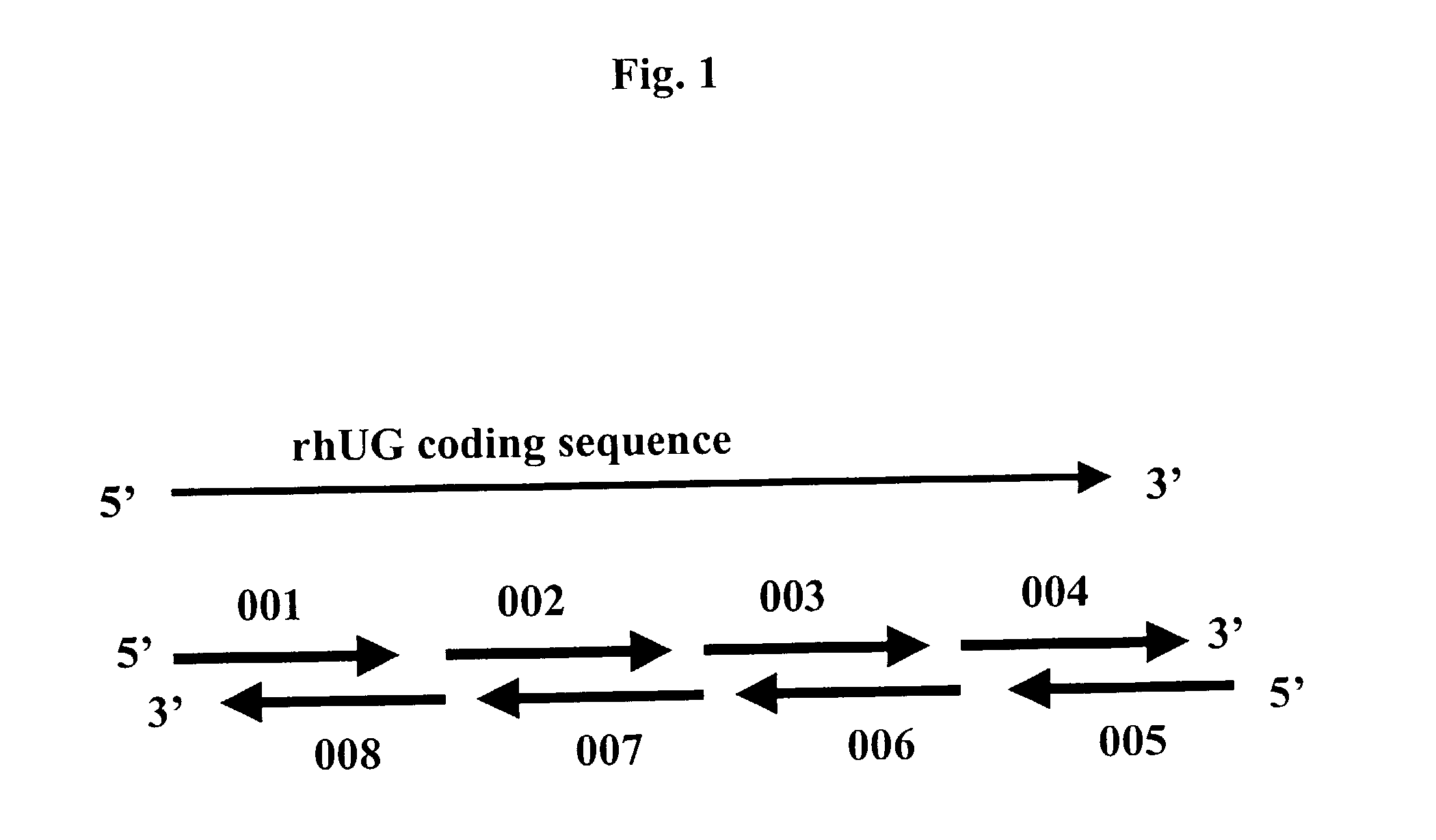
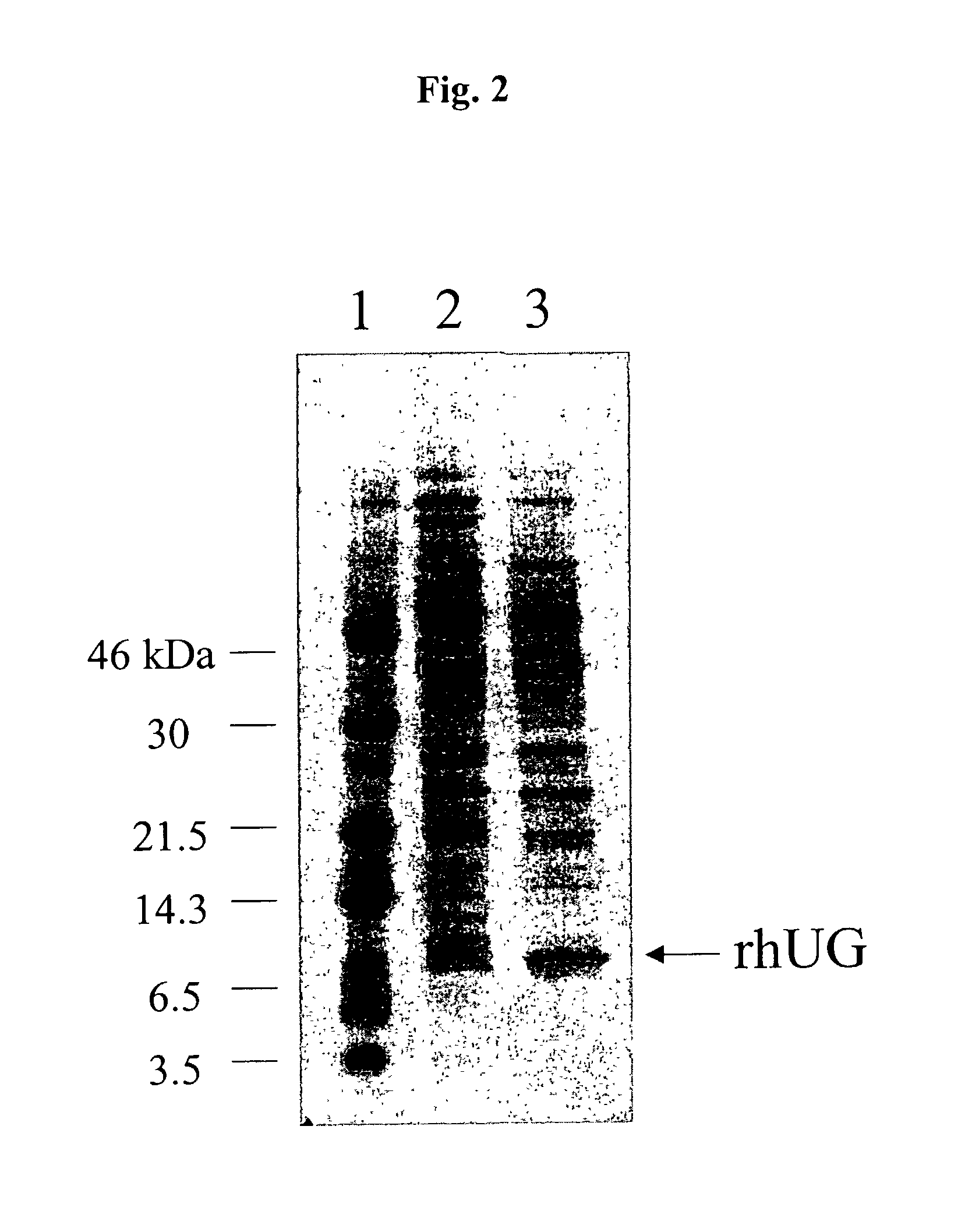
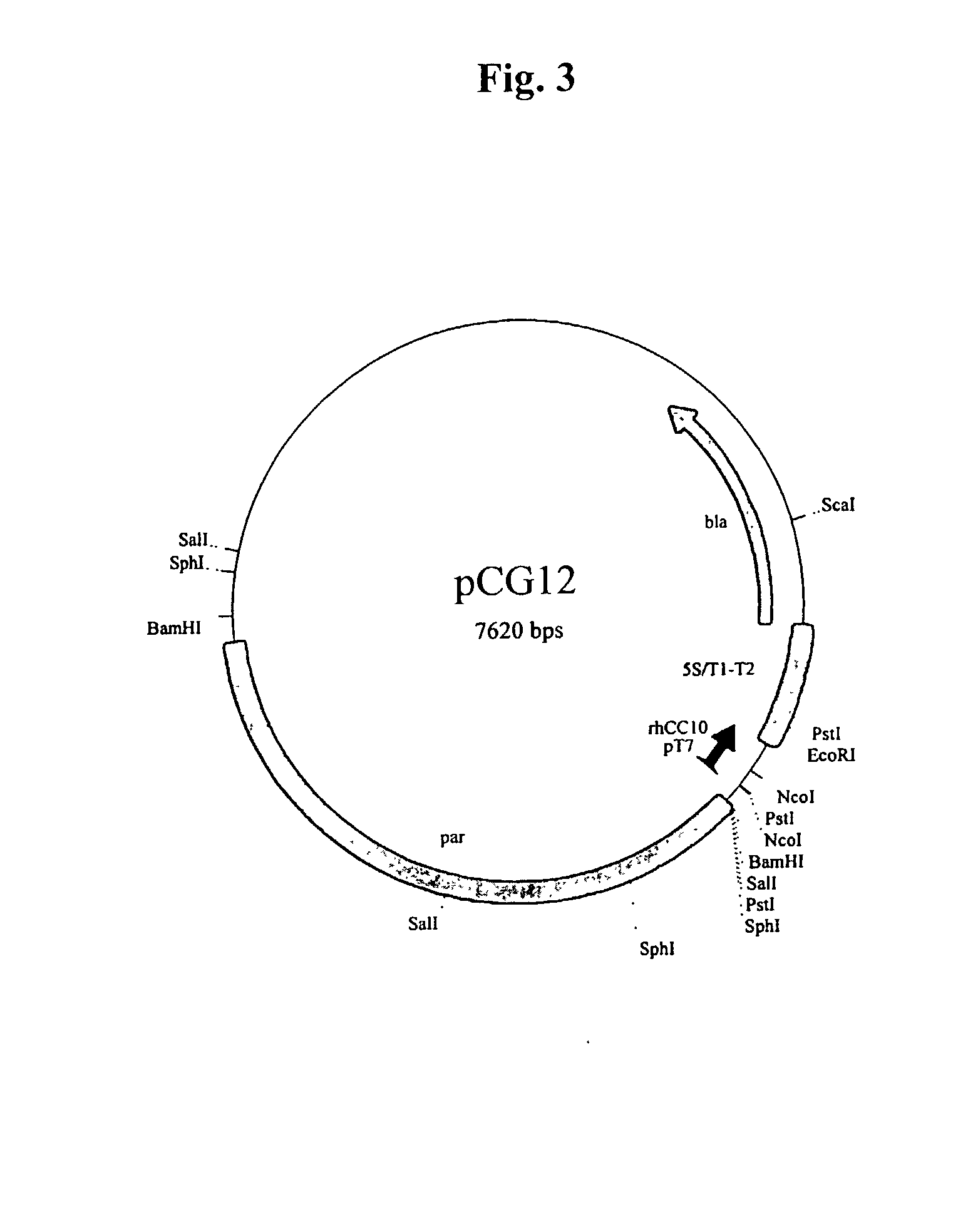
Methods for the production of purified recombinant human uteroglobin for the treatment of inflammatory and fibrotic conditions
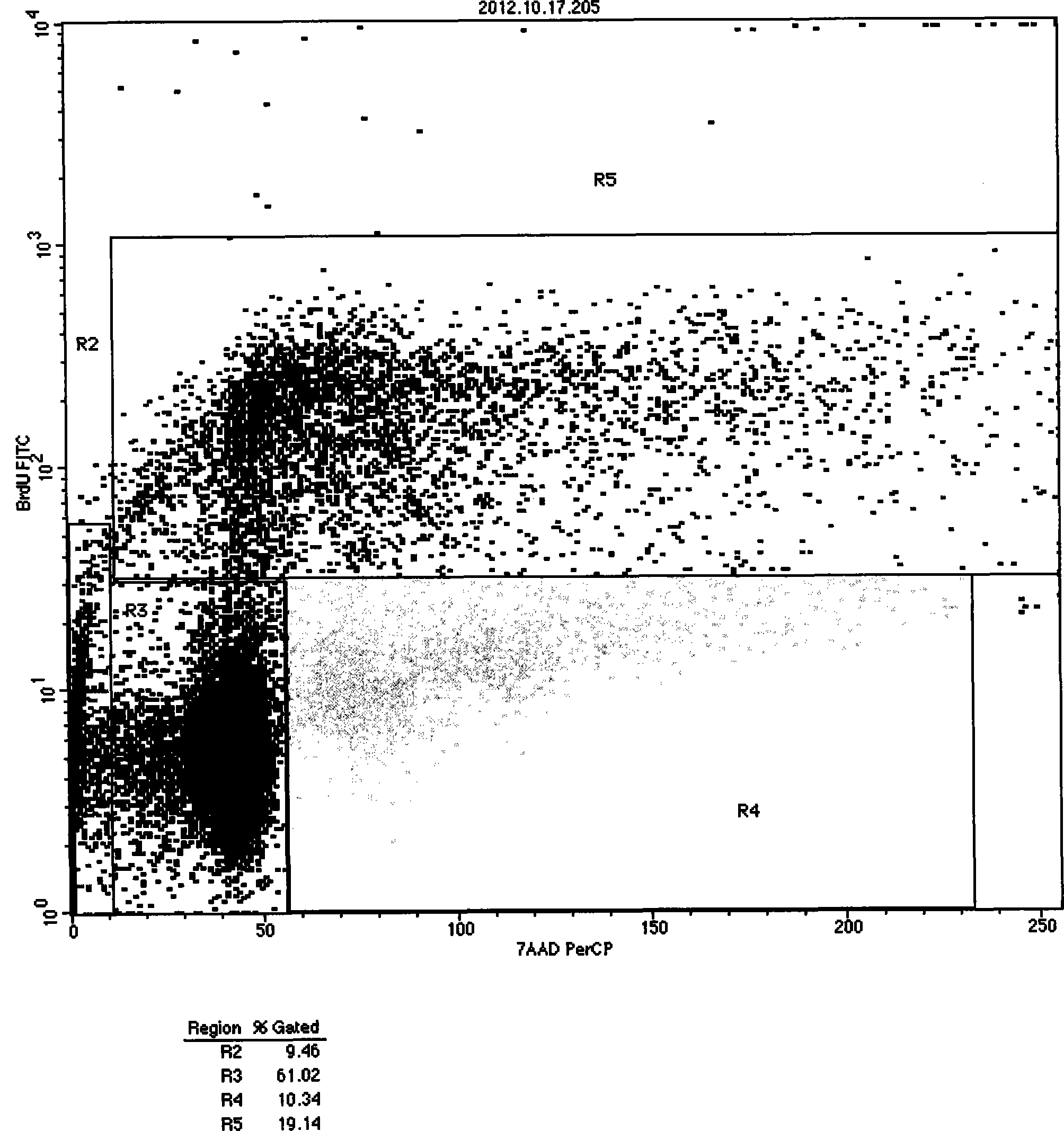
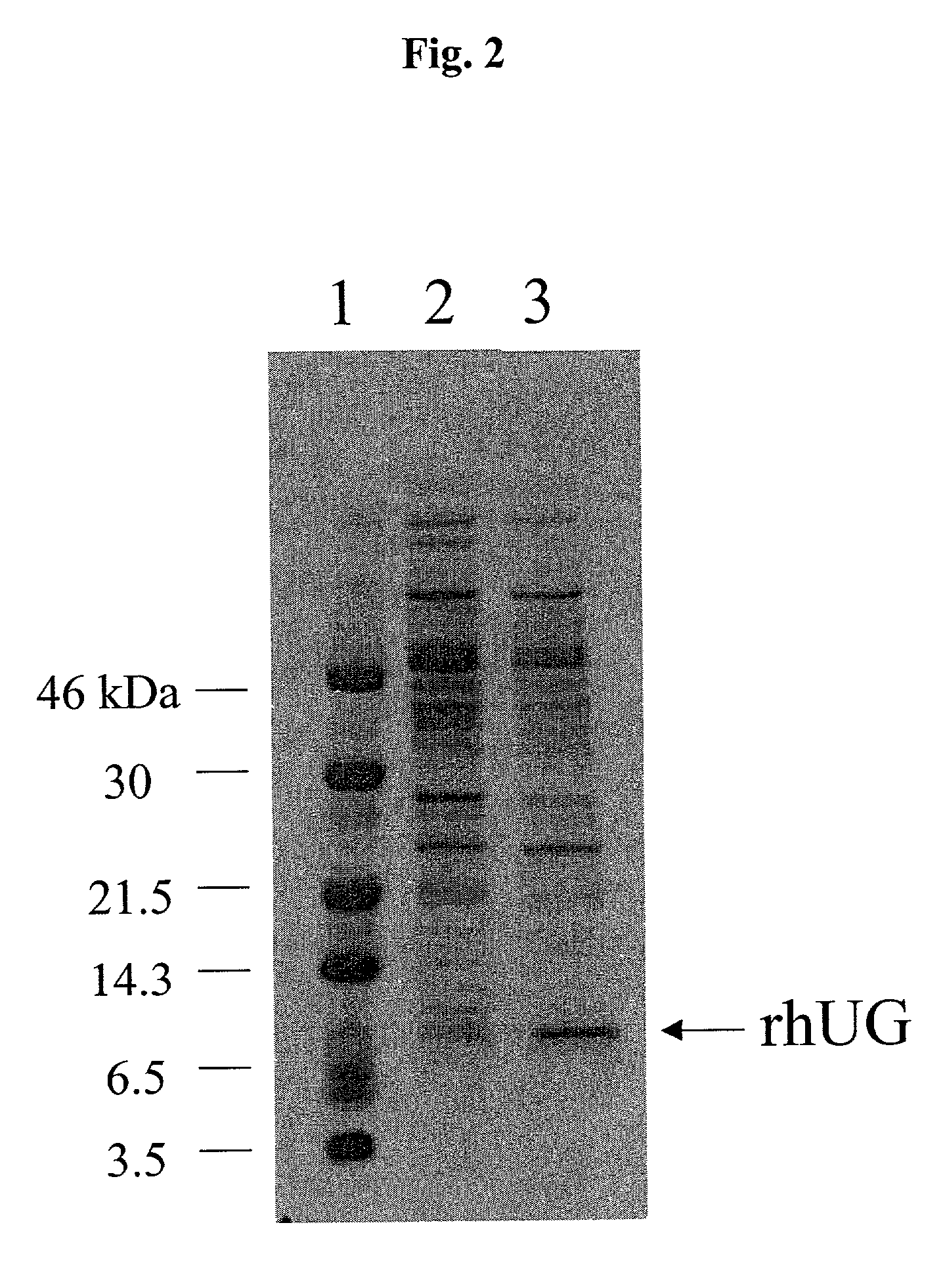
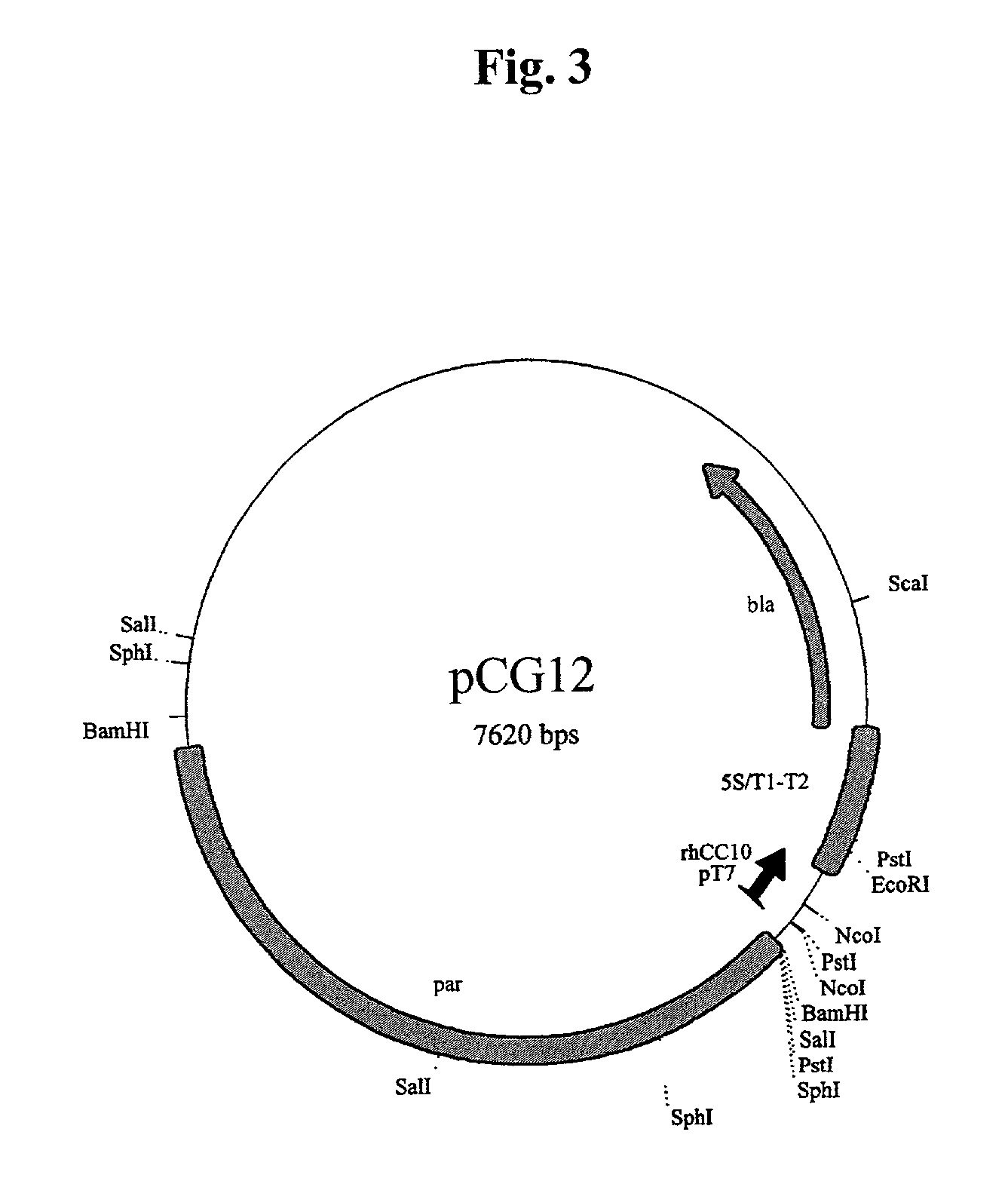
The present invention relates generally to the production of recombinant human uteroglobin (rhUG) for use as a therapeutic in the treatment of inflammation and fibrotic diseases. More particularly, the invention provides processes, including broadly the steps of bacterial expression and protein purification, for the scaled-up production of rhUG according to current Good Manufacturing Practices (cGMP). The invention further provides analytical assays for evaluating the relative strength of in vivo biological activity of rhUG produced via the scaled-up cGMP processes.
Owner:CC10 SWEDEN +4
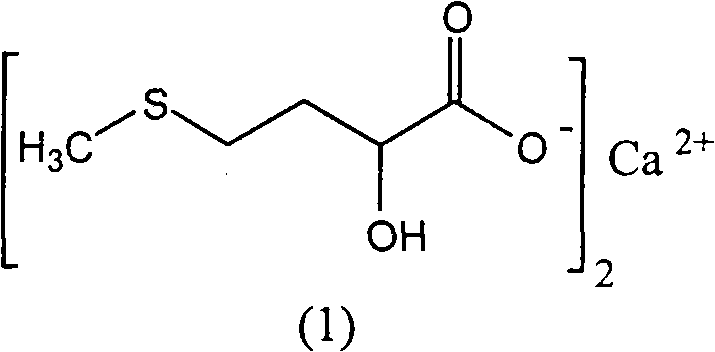
Preparation of medicinal D,L-2-hydroxy-4-methylthio calcium butyrate
The invention discloses a method for preparing a D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose. The method comprises following steps of: a. using a D,L-2-hydroxyl-4-methylthio butanoic acid and an alcohol with a general formula of ROH as the raw materials to carry out esterification to obtain a D, L-2-hydroxyl-4-methylthio butyrate, and; b. hydrolyzing the D, L-2-hydroxyl-4-methylthio butyrate in step a and calcium oxide in a solvent to produce the D, L-2-hydroxyl-4-methylthio butanoic calcium salt. The method provided by the invention for the production of the D, L-2-hydroxyl-4-methylthio butanoic calcium salt has the advantages of short course, readily available the raw materials, low cost, easy control over the quality of the product, and more importantly, the method can prepare high-purity D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose, thereby satisfying the requirements of State Food and Drug Administration Bureau and Good Manufacturing Practice (GMP) for drug production, and facilitating the preparation of pharmaceutical preparations.
Owner:NANJING LIFENERGY R & D +1
Methods of producing carbon-13 labeled biomass
InactiveUS6872516B2Preventing oxygen buildupHigh saturationCompounds screening/testingBiocideVolumetric Mass DensityWater soluble
A method and apparatus for preparing uniform carbon-13 labeled biomass using a water soluble carbon-13 labeled carbon source, such as a [13C]-bicarbonate or [13C]-carbonate salt, is disclosed. The biomass is prepared in one or more sterile carboys filled with growth medium, in which acidity, oxygen, and biomass density are carefully monitored and maintained. By using a solid, water-soluble [13C]-bicarbonate or [13C]-carbonate salt as the sole carbon source, a biomass is provided which is uniformly and efficiently labeled with carbon-13. This method and apparatus is particularly useful for the growth of an edible carbon-13 labeled algal mass, with Spirulina platensis being a specific alga species. The biomass may be prepared in conformance with FDA current good manufacturing practice regulations, and may be harvested and formed into lyophilized bulk drug powder which may be further processed into various drug product forms which are useful for diagnostic tests or in pharmaceutical compositions.
Owner:ADVANCED BREATH DIAGNOSTICS
Preparation method of Muscovy duck parvo novel vaccines
ActiveCN101880651AGenetically stableImprove securityMicroorganism based processesAntiviralsFibroblastCells fibroblast
The invention relates to a preparation method of Muscovy duck parvo novel vaccines, which comprises the following steps of: using Muscovy duck parvovirus attenuated virus P1 strain (MPV-P1 strain with a preservation number of CCTCC NO:V201013) and Muscovy duck origin goose parvovirus attenuated virus D strain ( GPV-D strain with a preservation number of CCTCC NO:V201014) as seed viruses; proliferating viruses by applying the muscovy duck embryone fibroblast spinner culture technology to obtain cell toxic liquids; mixing the cell toxic liquids according to the proper proportion and freezing a dry protective agent to freeze and dry to research safe and effective Muscovy duck parvo novel vaccines. The young Muscovy ducks are inoculated once so as to prevent and control the Muscovy duck parvovirus infection and the Muscovy duck gosling blast dieases at the same time, thereby the stress reaction of the Muscovy ducks caused by immunization for many times is solved. The invention is suitable for scale production under the GMP (Good Manufacturing Practices) condition, and saves the production cost of the vaccines.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
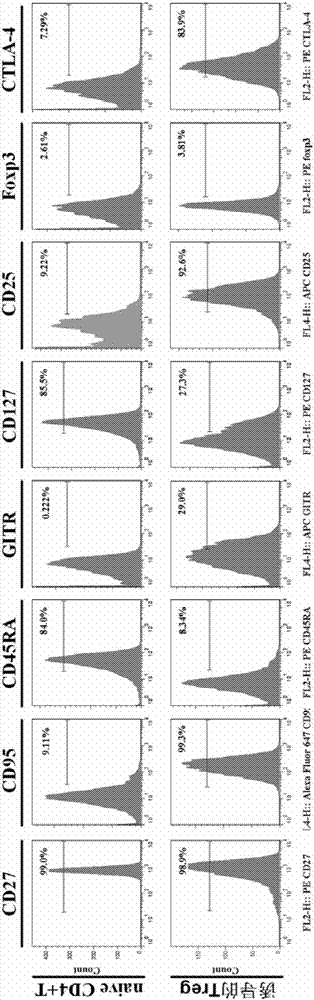
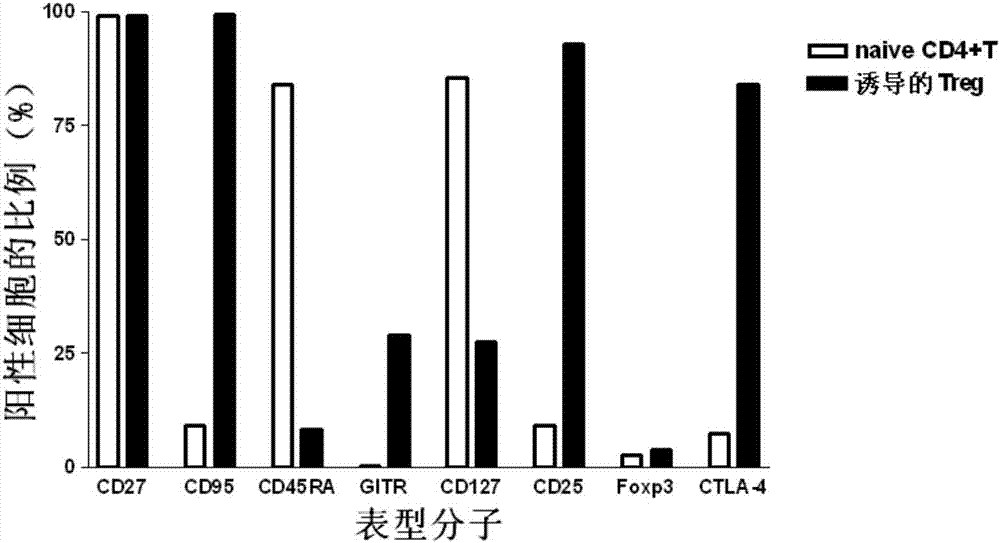
Method for in vitro induction and amplification of human antigen nonspecific regulatory T cell
InactiveCN107083360APhenotype stableStability adjustment functionBlood/immune system cellsCell culture active agentsAntigenMicroorganism
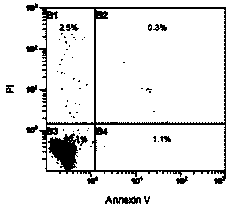
The invention provides a method for in vitro induction and amplification of a human antigen nonspecific regulatory T cell. A human peripheral blood CD4<+> T cell with an abundant source is separated, and a CD4<+>CD25<-> T cell is induced and amplified into a CD4<+>CD25<+>CD127<dim> antigen nonspecific regulatory T cell with high purity and high regulation efficiency within a short period through a simple induction method meeting the Clinical Good Manufacturing Practice (GMP). An enough therapeutic dose of Treg can be obtained through induced cultivation of a week (6-7d), the phenotype and regulation function of the induced Treg are stable, furthermore, the incidence rate of events, such as cell activity reduction and microbial contamination due to long-term culture in vitro is greatly reduced, and the quality control method is specific and fast and is very suitable for clinical application.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH +1
Establishment method and application of clinical-grade neural stem cell line
InactiveCN103451153ALow tumorigenicityAvoid Tumor RiskNervous disorderMicroorganism based processesClinical gradeNeurulation
The invention belongs to the field of cytobiology and neurobiology, and provides an establishment method and application of a clinical-grade neural stem cell line. The method under GMP (Good Manufacturing Practice) control comprises two steps, namely, primary culturing of neural stem cells, and subculturing of neural stem cells. The identification result shows that the neural stem cell line established by the method can express a plurality of stem cell markers, can be differentiated into nerve cells, astrocyte and oligodendrocyte, has high stability, and is suitable for long-term culturing; a large amount of sufficient clinical-grade neural stem cells can be obtained by the method. The method can be used for establishing the neural stem cell line based on a plurality of neural stem cells and even single neural stem cell, and thus the error in study can be reduced as far as possible. By adopting the neural stem cell line established by the method and the cells obtained by differentiating the neural stem cell line, the problems of the safety and effectiveness risk in cell therapy of nervous system disease can be solved effectively, and safe and effective treatment on such diseases becomes possible.
Owner:栾佐
Methods for the production of purified recombinant human uteroglobin for the treatment of inflammatory and fibrotic conditions
The present invention relates generally to the production of recombinant human uteroglobin (rhUG) for use as a therapeutic in the treatment of inflammation and fibrotic diseases. More particularly, the invention provides processes, including broadly the steps of bacterial expression and protein purification, for the scaled-up production of rhUG according to current Good Manufacturing Practices (cGMP). The invention further provides analytical assays for evaluating the relative strength of in vivo biological activity of rhUG produced via the scaled-up cGMP processes.
Owner:APRILE PILON +3
Preparation method of pickled pepper type poultry product
The invention discloses a preparation method of pickled peppery type poultry product, comprising the following steps: sterilizing and disinfecting a multi-stage GMP (Good manufacturing practice) workshop, then washing, unfreezing, selecting and slitting poultry raw materials, washing again, then boiling the cleaned raw materials, washing the raw materials after being boiled, cooling, putting the raw materials into pre-prepared material water added with compound fresh-keeping color fixative, then taking out for vacuum packaging, and finally, radiating and sterilizing the materials. The pickledpeppery type poultry product prepared by the method is rich in nutrition, keeps the special mouthfeeling and flavor of the poultry product, the nutrition content and the flavor of the pickled pepperytype poultry product can be kept to the greatest degree, the shelf life of the product is prolonged, and as for the method, the process is simple and the investment cost is low.
Owner:ANHUI TRUELOVE FOODS
Automated packaging, inspection, verification, and counting apparatus
InactiveUS8146331B2Fast filling speedCapsCapping machinery safety/controlEngineeringProcess engineering
The present invention is an automated packaging apparatus utilizing a rotating assembly of elongated slats containing cavities to receive discrete pharmaceutical, vitamin, or food products. Quantities of discrete products such as tablets, capsules, or gels are deposited into the hopper of the apparatus. The apparatus then dispenses the discrete products into containers moving on a conveyor system such that each container receives a predetermined quality and quantity of pharmaceutical, vitamin, or food products. While operating at high speed, the apparatus inspects, counts, identifies and analyzes each product deposited into the containers and maintains electronic records describing the status of each product. In the event any errors occur the apparatus produces various alerts to inform the operator. The presence of foreign products or objects may cause the apparatus to instantly stop the entire system including peripheral equipment. A series of Good Manufacturing Practice protocols can then be enforced as per FDA requirements.
Owner:SOLOMAN SABRIE
Magnetic navigation feeding trolley
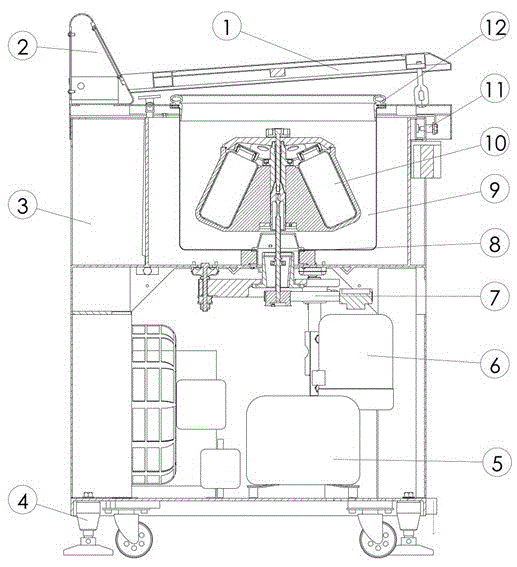
The invention provides a magnetic navigation feeding trolley which comprises a trolley driving chassis. A chassis control system controls the trolley driving chassis to run along a preset route under guide of a magnet or a magnetic strip pre-laid on the ground. The magnetic navigation feeding trolley is characterized by comprising a butt connection system, a propulsion system, a lifting system, an isolation system, a control system and a power supply system. The magnetic navigation feeding trolley can replace the traditional mode of manual pushing of the trolley, is suitable for large-scale feeding and discharging occasions, has the advantages of self-service, automation, flexible transfer and the like, emancipates workers from the mode of manual trolley pushing, and is suitable for feeding and discharging of a novel freezer dryer and also suitable for novel good manufacturing practice (GMP) modification of an old freeze dryer.
Owner:SHANGHAI TOFFLON SCI & TECH CO LTD
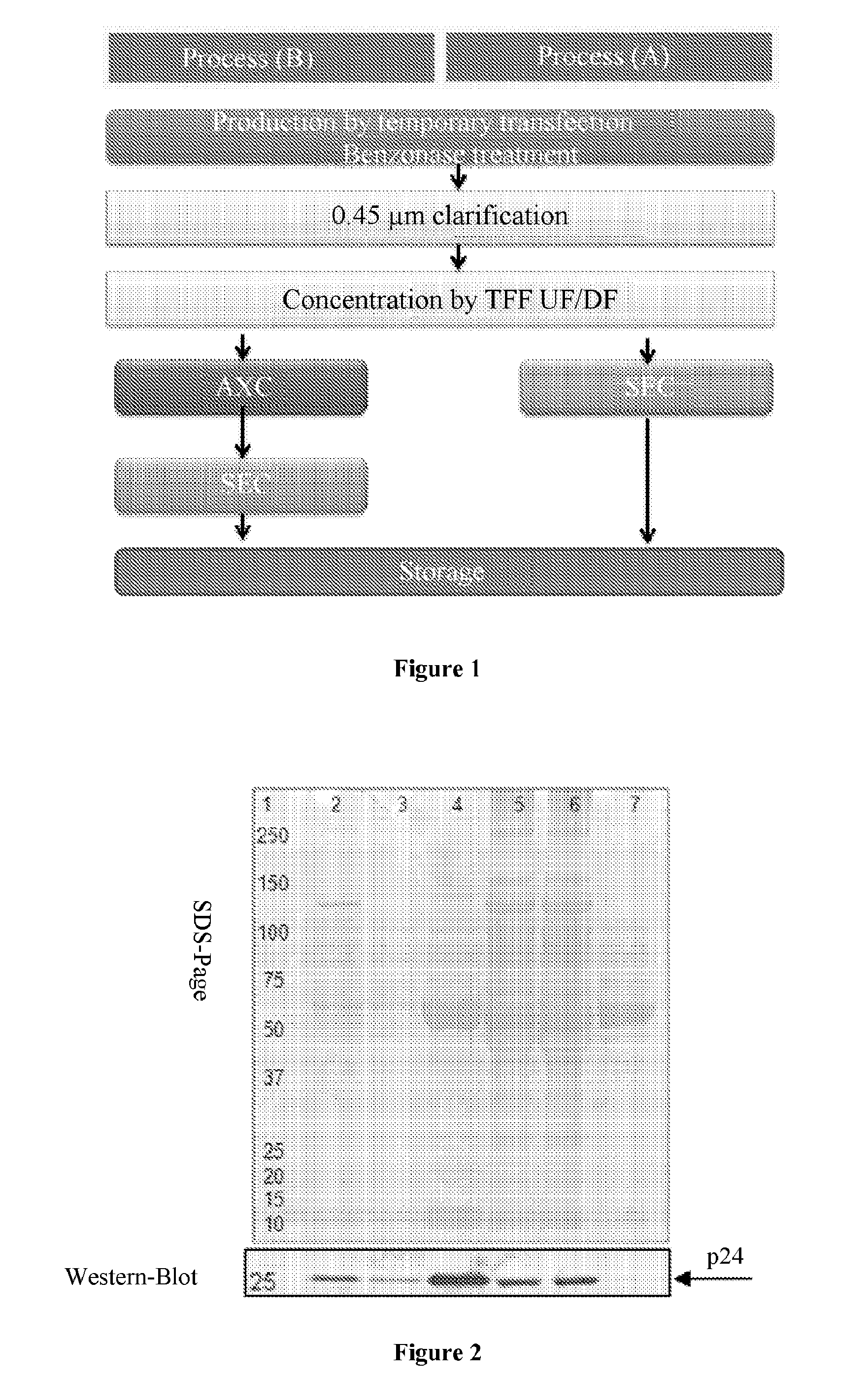
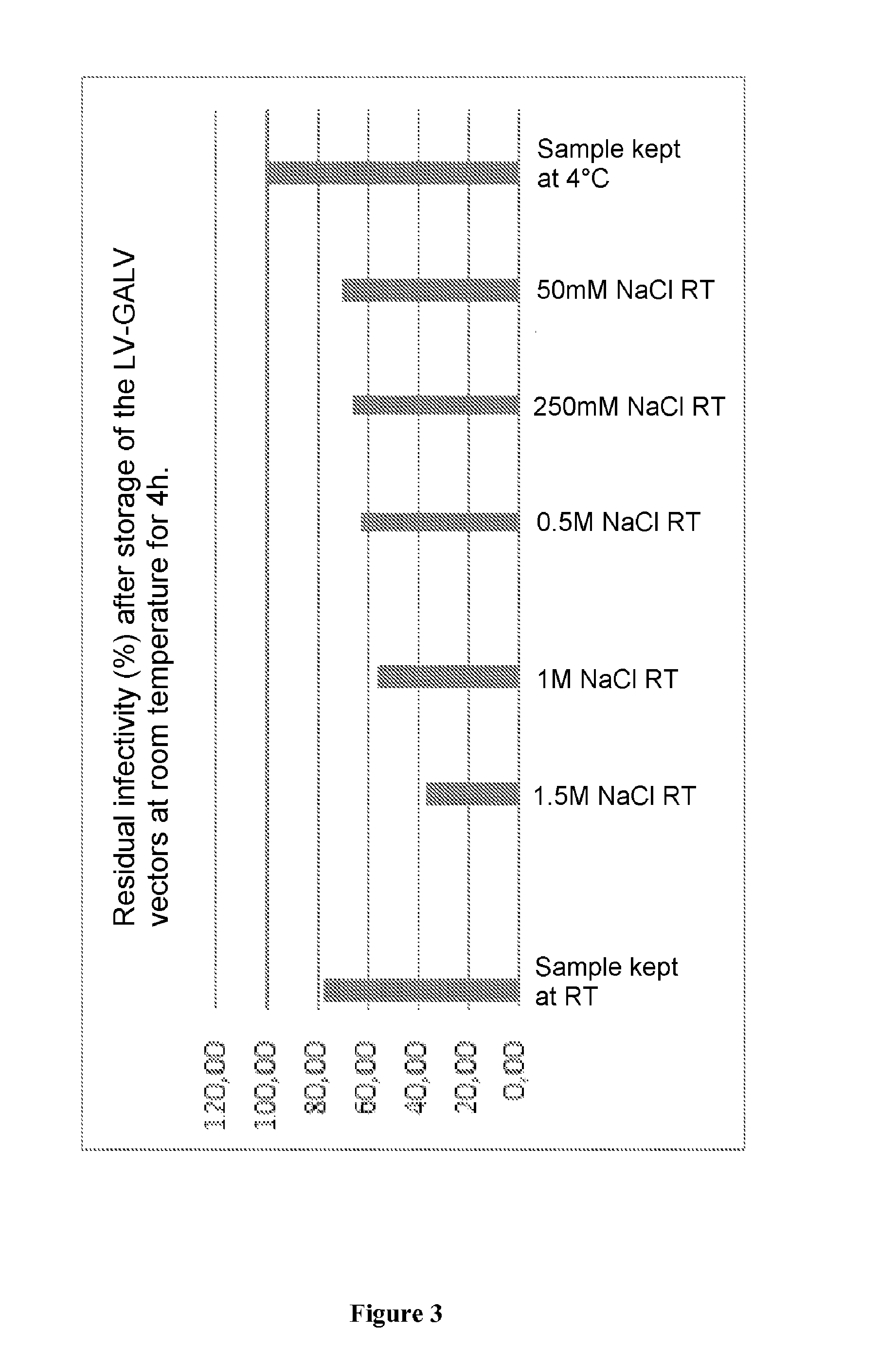
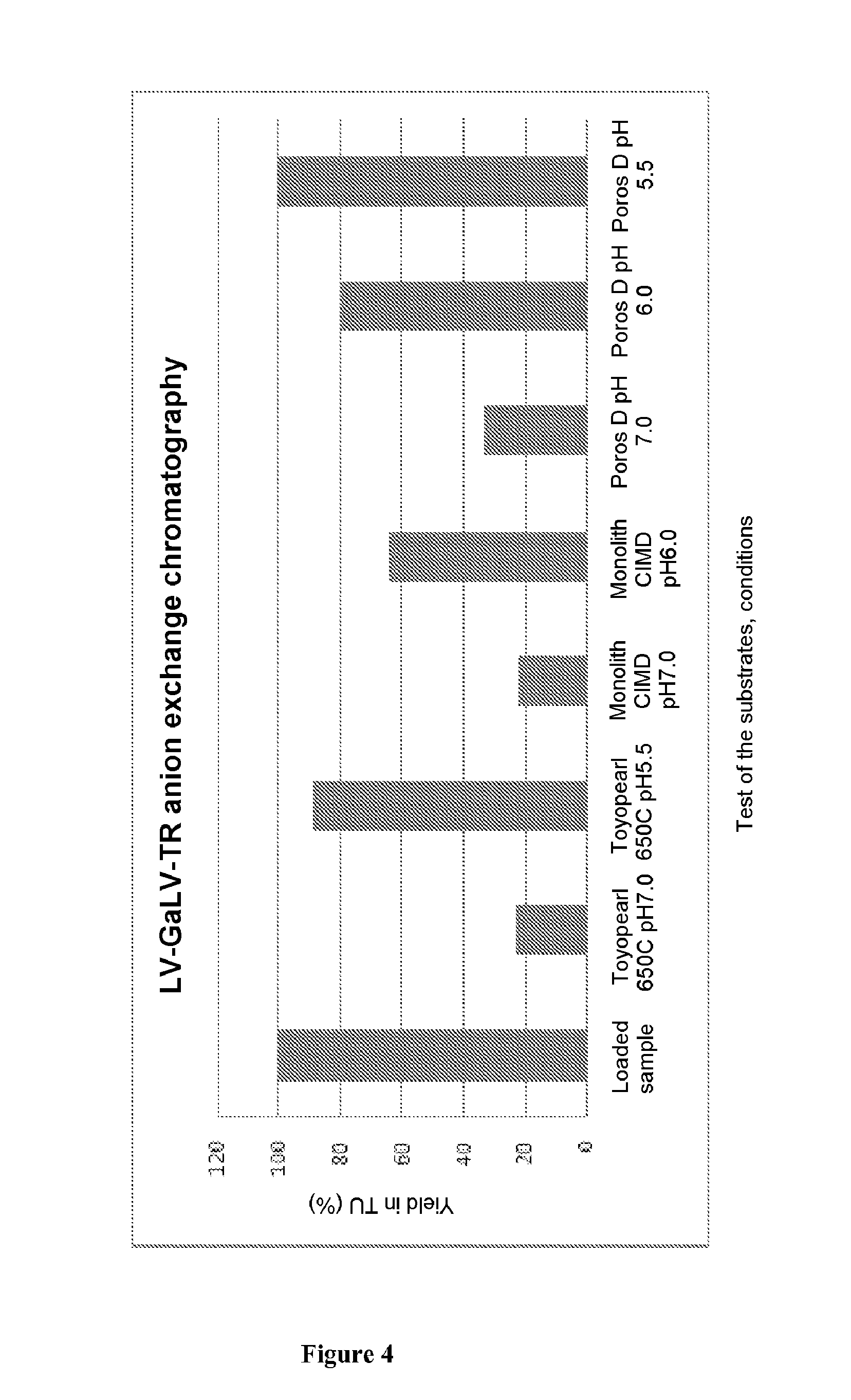
Method for purifying enveloped viruses or viral vectors
ActiveUS20170002332A1High purification yieldIncrease productionVector-based foreign material introductionReverse transcribing RNA virusesClinical gradeViral vector
The invention relates to a process for purifying enveloped viruses. The process of the invention is useful for recovering at a large scale enveloped viruses under conditions complying with good manufacturing practices and allowing viruses of a clinical grade to be obtained.
Owner:GENETHON
High-speed refrigerated centrifuge
The invention discloses a high-speed refrigerated centrifuge. The entire machine is located in a shell; the bottom surface in the shell is fixedly provided with a refrigerator and a high-speed motor respectively; the high-speed motor is connected with a driving main shaft through a driving belt; the driving main shaft is connected with a rotor mechanism in a cavity and is communicated with the cavity through a pipe system refrigerator; the upper side face is provided with an upper cover and a control key surface, and feet are fixedly arranged on the lower side for supporting; a door cover lock is arranged between the upper cover and the shell; a seal ring is fixedly arranged on the surrounding shell between the upper cover and the shell. Compared with the prior art, temperature set can be performed before separation, and parameters can also be adjusted in the separation process to perform timely automatic control. The refrigerated centrifuge is convenient to operate, has a refrigeration function, and can meet the requirements on material separation, thinning and refining of higher requirements so as to excellently and precisely control the material quality and meet the requirement of GMP (Good Manufacturing Practice).
Owner:SHANGHAI ZHIZHENG CENTRIFUGE
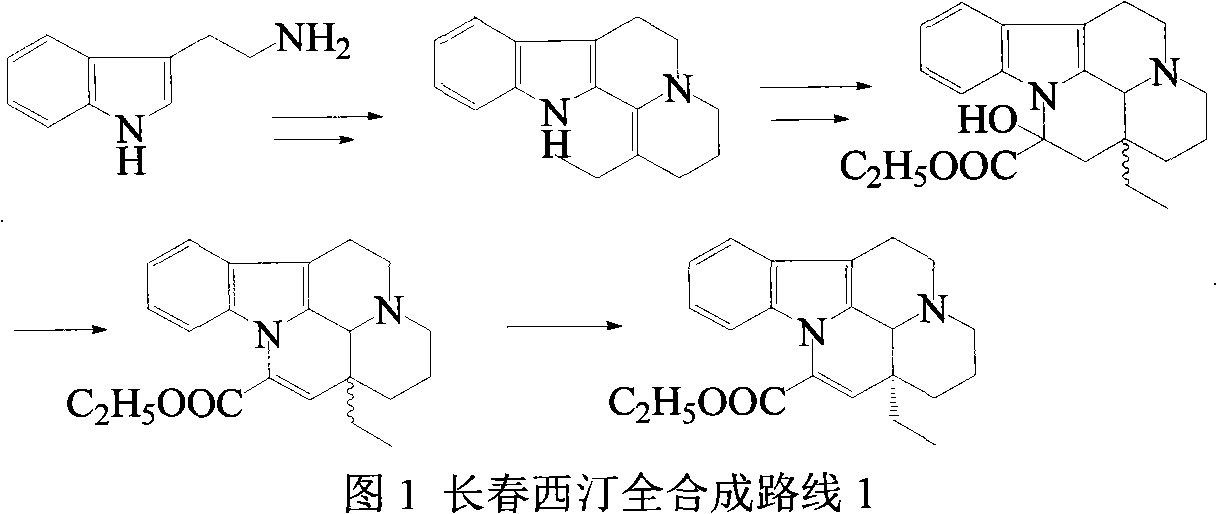
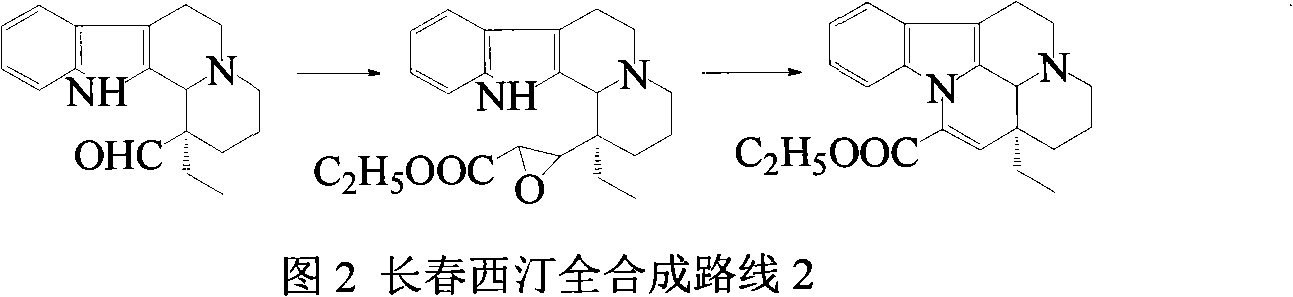
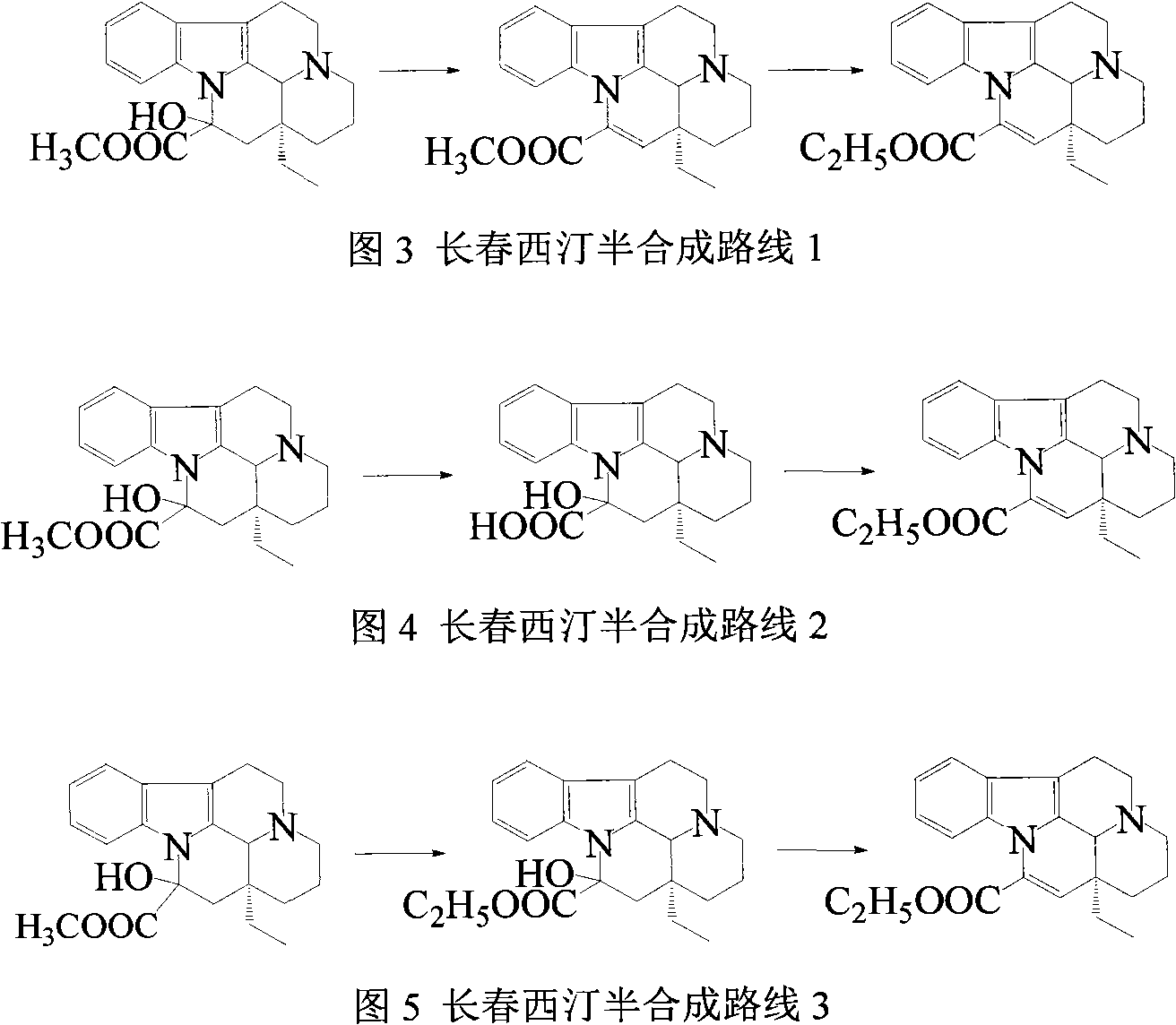
Semi-synthesis of vinpocetine through one kettle way and preparation of water-soluble vinpocetine salt
InactiveCN102485723AConducive to large-scale industrial productionAtom economy is highOrganic chemistryFood additiveVinpocetine
The invention relates to a semi-synthesis of vinpocetine and a preparation of a vinpocetine salt, wherein vincamine is used as a raw material. The one kettle way for the synthesis of vinpocetine allows the production efficiency to be improved and the total yield to be above 85%; and a method of the preparation of the vinpocetine salt is simple, and the yield is above 85%. Compared with original production methods, the method of the invention, which has the advantages of high atom economy, and concise and controllable process flow, is in favor of the production of GMP (good manufacturing practice) grade bulk medicines and food additives.
Owner:江苏斯威森生物医药工程研究中心有限公司
Improved expansion culture medium for regulatory T cells of human cord blood origin and application method of expansion culture medium
ActiveCN104357389AReduce riskPromote growth rateBlood/immune system cellsCulture mediumsClinical disease
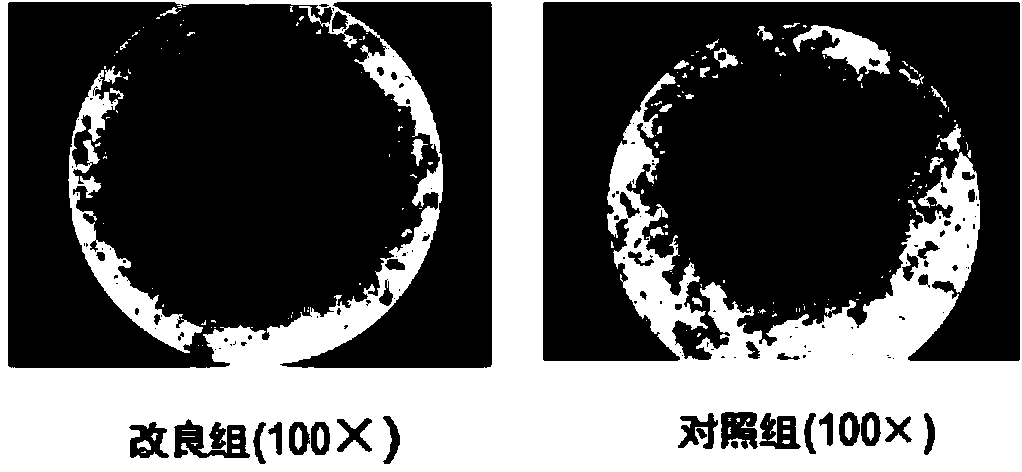
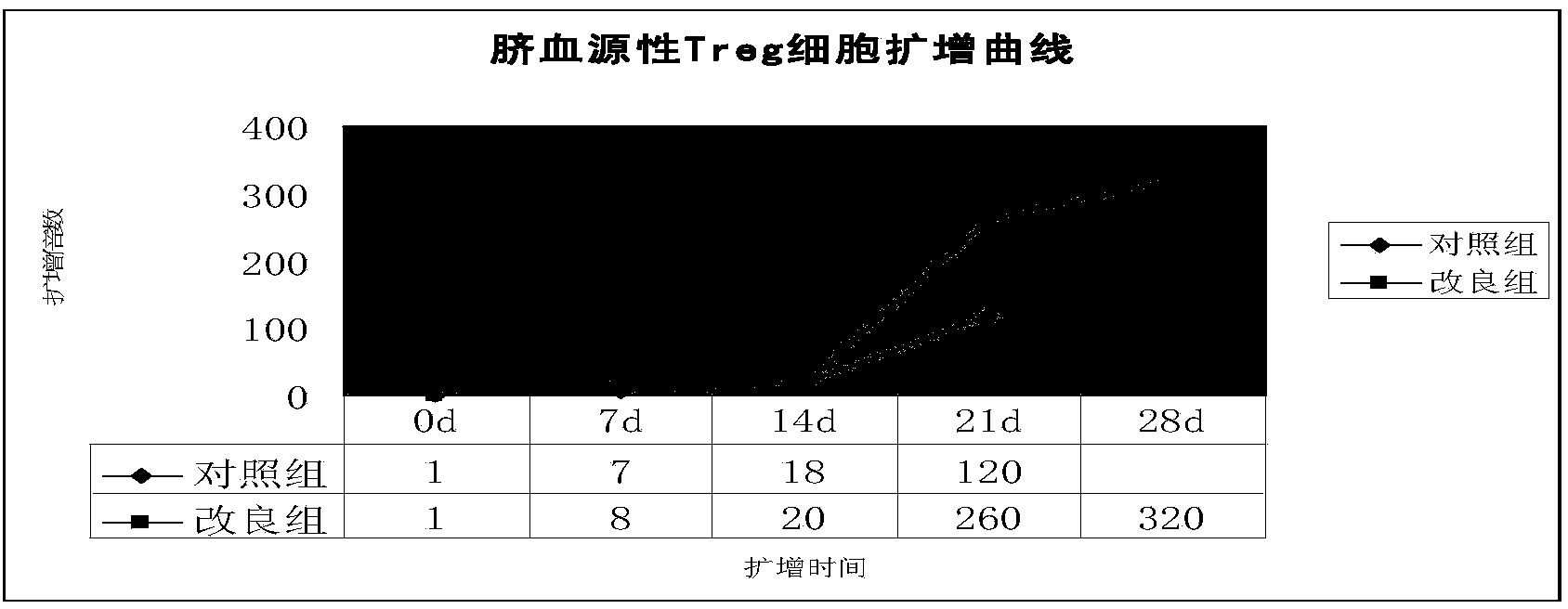
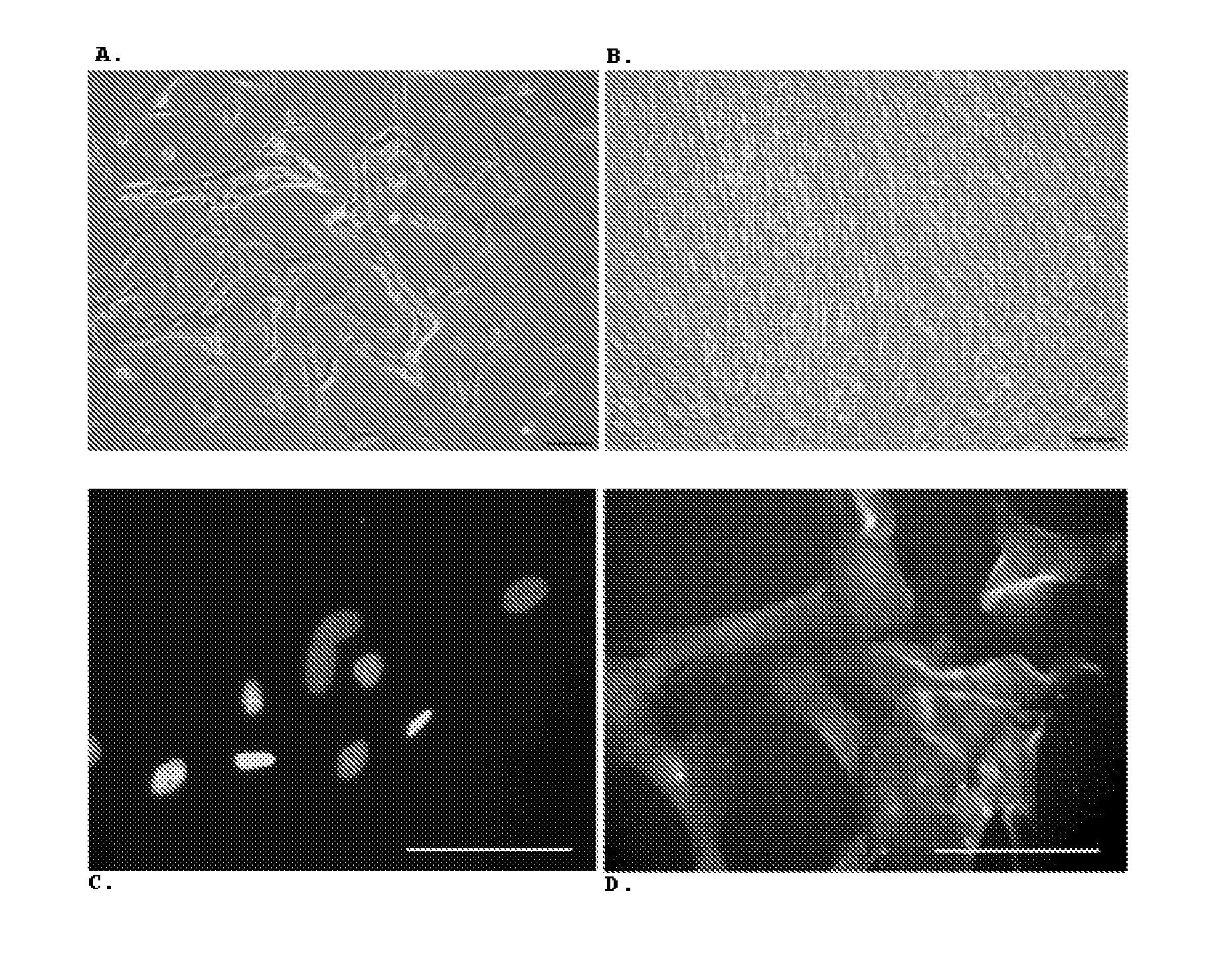
The invention relates to an improved expansion culture medium for regulatory T cells of human cord blood origin and an application method of the expansion culture medium. According to the expansion culture medium, heparin anticoagulated autologous cord blood plasma accounting for 10%-12% of the volume of a culture medium, CD3-CD28 antibody co-expressed immunomagnetic beads, recombinant human interleukin 2, 2-mercaptoethanol, rapamycin, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and gentamicin are added into the RPMI (Roswell Park Memorial Institute)1640 culture medium; and then separated cell suspension is inoculated in a 96-well plates with a U-shaped bottom, hole-division expansion can be performed every 1-2 days, and the expansion period is 3-4 weeks. All reagents in the culture system reach the GMP (good manufacturing practice) level or are originated from autologous cord blood, so that risks caused by ingredients of animal origin are avoided, and the regulatory T cells can be used for a third-party unrelated donor and directly applied to clinical disease treatment; and compared with a traditional culture system, Treg cells (the regulatory T cells) expanded by the improved culture medium is excellent in aspects of growth speed, purity, activity, lymphocyte inhibition function and the like, and the Treg cells are expected to be used as the regulatory T cells of the third-party unrelated donor and applied to the clinical disease treatment.
Owner:HUNAN XENO LIFE SCI
Optimized and defined method for isolation and preservation of precursor cells from human umbilical cord
ActiveUS20100216237A1Promote resultsImprove efficiencyCell dissociation methodsArtificial cell constructsCell FractionCell phenotype
The present invention refers to an optimized and defined method for isolation and preservation of precursor cells from human umbilical cord. Besides being reproducible and 100% reliable, in terms of the number of samples processed, the method results in a high and defined number of precursor cells, being the majority obtained after a single adhesion and expansion / multiplication phase ex vivo (thus granting cell phenotype), in a shorter time frame than what was previously described in the state-of-the-art. With this method, it is possible to obtain, in 9 days, after direct freezing of a cell fraction, and after one expansion / multiplication phase ex vivo (end of P0) of the majority of the cells, about 8.6(±0.1)×105 cells / gram of processed umbilical cord. In turn, the characteristics of the cells allow, for example, after 35 days, obtaining an average of 7.7×1015 cells, with precursor phenotype, from 100% of processed umbilical cord samples. The method, because it is simple, robust and 100% reliable, can be performed under good manufacturing practices (GMP) in laboratories dedicated to cell therapy in humans. Furthermore, the method has applications in the pharmaceutical, cosmetic and biotechnology areas.
Owner:LAB MEDINFAR PROD FARMS
Automatic small-capacity penicillin bottle feeder
ActiveCN104044890AImprove equipment automation levelMeet production requirementsConveyor partsPenicillinEconomic benefits
The invention provides an automatic small-capacity penicillin bottle feeder. The feeder at least comprises a material neatening disc used for placing penicillin bottles, a material neatening conveyor belt and a mechanical arm, wherein the material neatening disc is of a disc shape which is horizontally placed; a rotating shaft is connected to the bottom of the material neatening disc; the rotating shaft is connected with an adjustable-speed motor and enables the material neatening disc to contrarotate; a guide mechanism is arranged on the upper surface of the material neatening disc and is used for guiding the movement of the penicillin bottles; the inlet of the guide mechanism is connetced with the material neatening disc; the outlet of the guide mechanism is connected with the material neatening conveyor belt; the upstream part of the material neatening conveyor belt is connected with the outlet of the guide mechanism; the downstream part of the material neatening conveyor belt is connected with the mechanical arm; the mechanical arm is provided with a sucking disc and is capable of sucking the penicillin bottles at the downstream part of the conveyor belt up and placing the penicillin bottles into a PVC (polyvinyl chloride) box support used for packaging. According to the automatic small-capacity penicillin bottle feeder, the automation equipment level of automatic feeding of the penicillin bottles is improved, the labor intensity is reduced, the production requirement of GMP (Good Manufacturing Practice) is met, the production efficiency is greatly improved, and the economic benefit of an enterprise is improved.
Owner:SHAN DONG DONG E E JIAO
Method for automatically transferring material drug freeze-drying tray to freeze drier ply
InactiveCN102305517AGuaranteed sterilitySolve manual transferLiquid flow controllersLiquid transferring devicesBiochemical engineeringPharmaceutical industry
The invention provides a method for automatically transferring a material drug freeze-drying tray to a freeze drier ply. The method is characterized by comprising the following steps: taking the tray out of a tray sterilizing tunnel oven by using an automatic tray feeding and discharging device; putting the tray into a freeze drier, putting while feeding until all the plies of the freeze drier are filled; and then starting the freeze drier to carry out freeze-drying. The method provided by the invention has the advantages of solving the high-risk problem of manually tray transferring or tray loading in the traditional method, guaranteeing that the whole tray transferring process is automatically completed under the asepsis protection state and conforming to the requirements of international pharmaceutical industry CGMP (current good manufacturing practice), and has high sterility.
Owner:SHANGHAI TOFFLON SCI & TECH CO LTD
Method for preparing nattokinase by solid state fermentation method
ActiveCN106995810AGood material basisIncrease productionHydrolasesMicroorganism based processesUnit operationSecreted substance
The invention discloses a method for preparing nattokinase by a solid state fermentation method. The method comprises unit operations such as soybean pretreatment, culture medium preparation, seed preparation, inoculation, temperature-variable fermentation, drying and smashing. The method is characterized in that the techniques such as raw material pretreatment, culture medium and seed preparation, inoculation, fermentation, drying and smashing are integrated; the whole process is performed in clean or bacteria-free environment; poor phenomena such as flowing, overflowing, dripping and leaking cannot be generated; the GMP (good manufacturing practice) requirements can be easily met. Compared with a traditional method, the method has the following characteristics that the ingenious raw material pretreatment and multicomponent culture medium composition design provides a good material base for the growth of nattokinase producing bacteria; a surfactant is used, so that the generation and the secretion of the nattokinase are obviously improved; through the application of a temperature-variable fermentation method and process, the nattokinase yield is improved by 10 to 45 percent; the method is scientific; the process is reasonable and advanced; the environment protection is facilitated. The method can be widely applied to development and production of nattokinase and other relative products.
Owner:郑州金百合生物工程有限公司
Dust removing device with air curtain separation and self-circulation purification
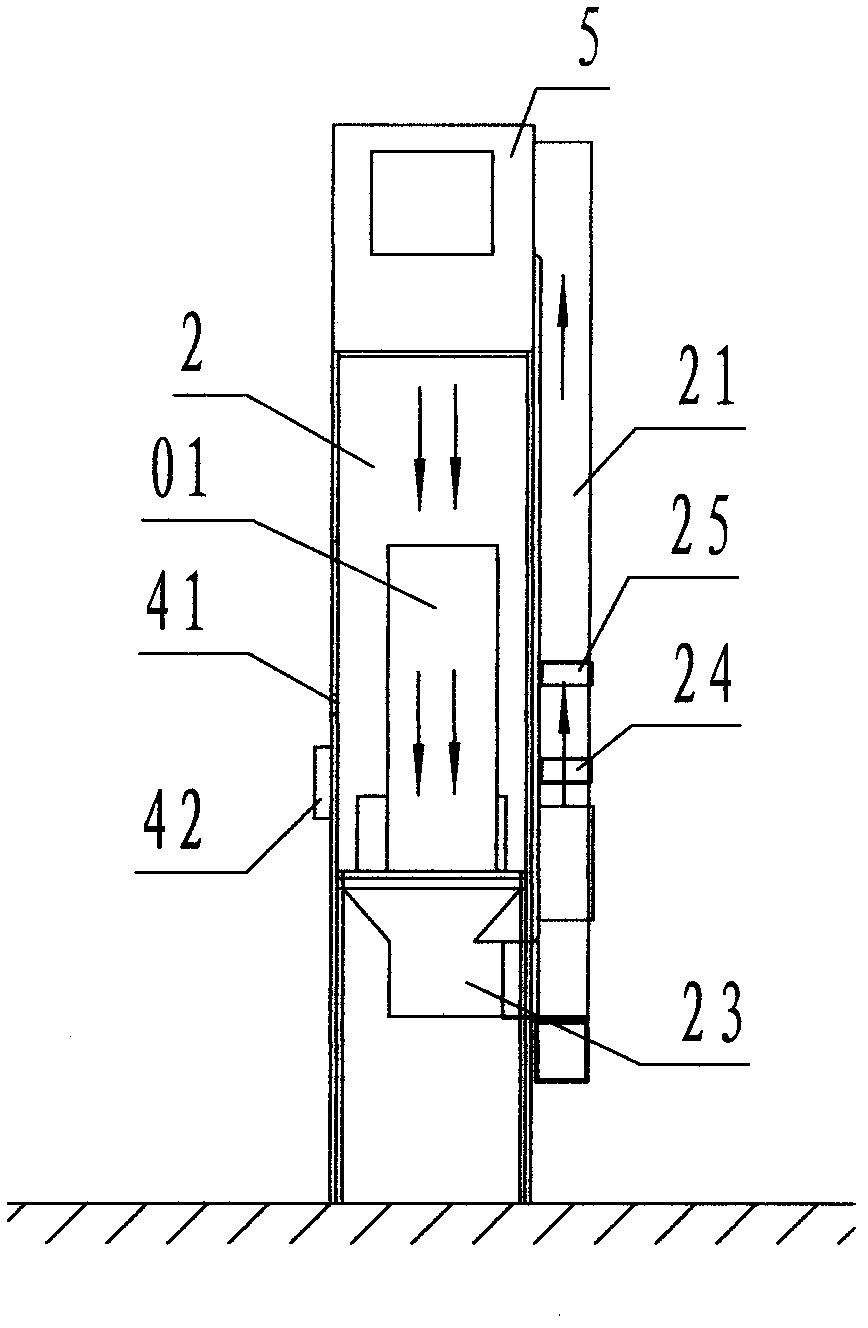
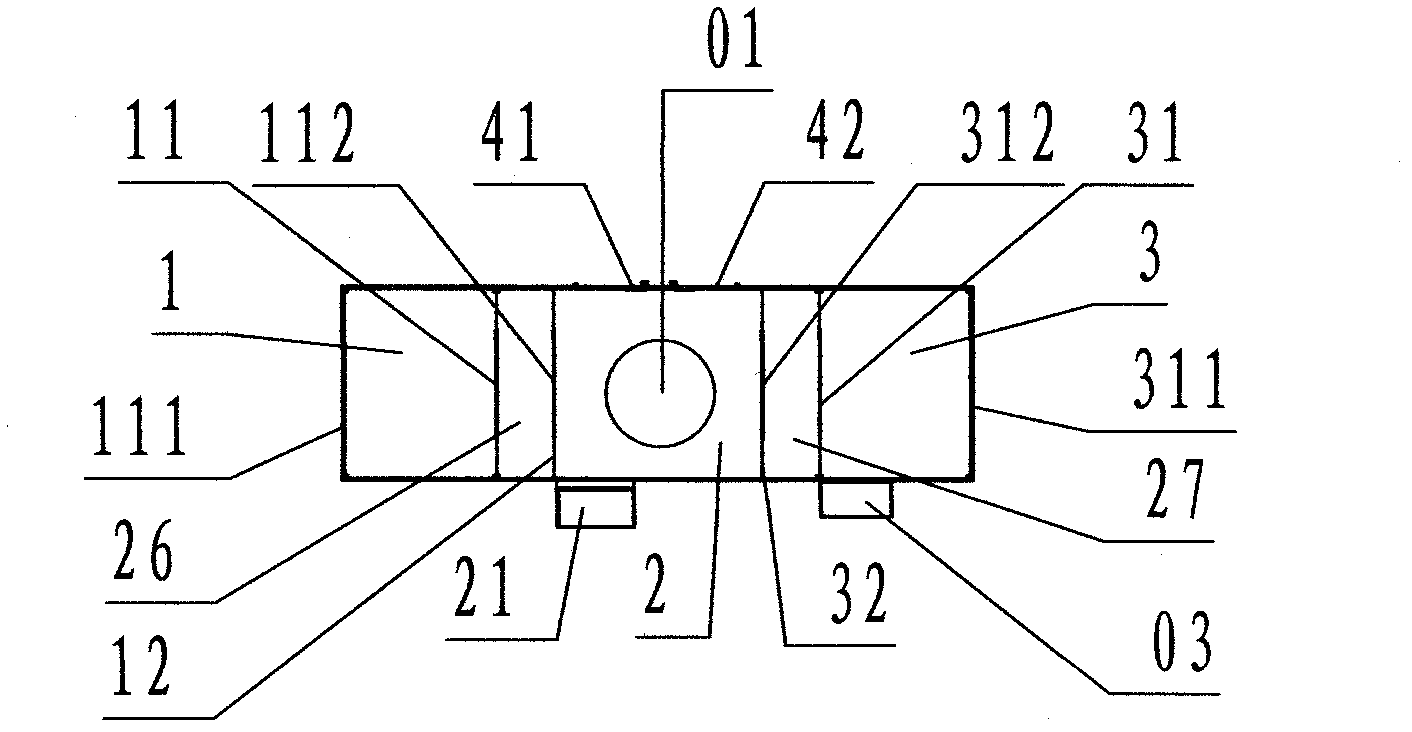
InactiveCN102219174AGuarantee air qualityGuaranteed service lifeMechanical apparatusDispersed particle filtrationProcess equipmentDevice form
The invention discloses a dust removing device with air curtain separation and self-circulation purification, comprising material input transmission cabin (1), main separation cabin (2), material output transmission cabin (3) and a control cabinet (03). The dust removing device adopts the following technical scheme: three independent interlayer separation cabins consist of the main separation cabin, the material input transmission cabin and the material output transmission cabin, a self-circulation purification device formed by arranging a self-circulation air tube with an inserting plate type coarse-efficiency filter and an inserting plate type medium-efficiency filter on the main separation cabin, and split-flow cleaning air curtains formed by inlet split-flow air curtain channels and outlet split-flow air curtain channels are respectively arranged between the main separation cabin and the material input transmission cabin, and between the main separation cabin and the material output transmission cabin. By the modes of air curtain separation and self-circulation purification, dust generated by processing equipment is separated into a dust generating area, and is removed when being generated instantly, so that the cleaning degree of the medicine processing equipment can achieve the standard requirement of Chinese 2010-section GMP (Good Manufacturing Practice).
Owner:ZHEJIANG VACIN BIO PHARMA LTD
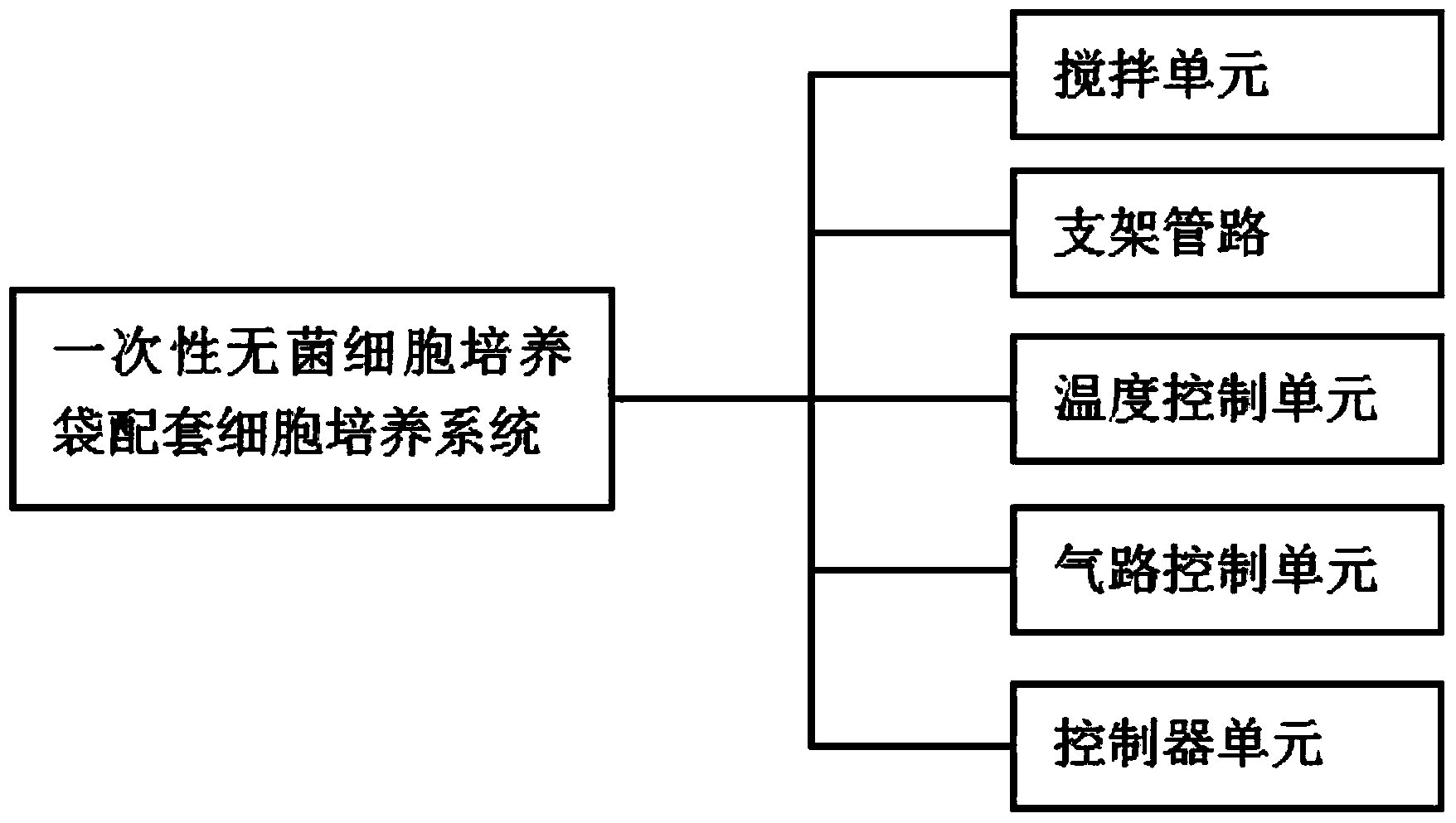
Disposable supporting cell culture system for sterile cell culture bag
ActiveCN104017727AHigh gas conduction rateLow mechanical shearBioreactor/fermenter combinationsBiological substance pretreatmentsTemperature control3D cell culture
The invention provides a disposable supporting cell culture system for a sterile cell culture bag. The disposable supporting cell culture system comprises a stirring unit, a bracket pipeline, a temperature control unit, a gas path control circuit and a controller unit for controlling the four units, wherein the bracket pipeline is respectively connected with the stirring unit, the temperature control unit and the gas path control unit, and used for providing a channel for gas and liquid needed for the system; the stirring unit is provided with a stirring paddle which is integrated with a hollow gas distributor; the hollow gas distributor is connected with the gas path control unit; the hollow gas distributor comprises a micro air bubble distributor arranged on the lower part of the stirring paddle and a ventilating pipe arranged inside the stirring paddle. The system is used for lowering the labor intensity, staff needed for production and GMP (good manufacturing practice) production workshop area are greatly lower than those of the conventional production equipment, thereby facilitating realizing quicker refit for conveniently operating novel flow.
Owner:奥星制药设备(石家庄)有限公司
Dosage unit formulations of autologous dermal fibroblasts
ActiveUS20110274665A1Reduce severityAdditional componentCosmetic preparationsBiocideWrinkle skinNasolabial fold
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own skin using current good manufacturing practices (CGMP), and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL, of cells at a concentration of from 1.0-2.0×107 cells / mL. When injected into the nasolabial fold wrinkles (creases on the sides of the nose that extend to the corners of the mouth), the autologous fibroblasts are thought to increase the synthesis of extracellular matrix components, including collagen, reducing the severity of these wrinkles. Dosage and timing of administration have been demonstrated to be critical to achieving clinically significant outcomes.
Owner:CASTLE CREEK BIOSCIENCES LLC
Continuous reverse flow extracting tank
InactiveCN102019099ACompact structureReduce unit consumptionSolid solvent extractionSlagEnvironmental quality
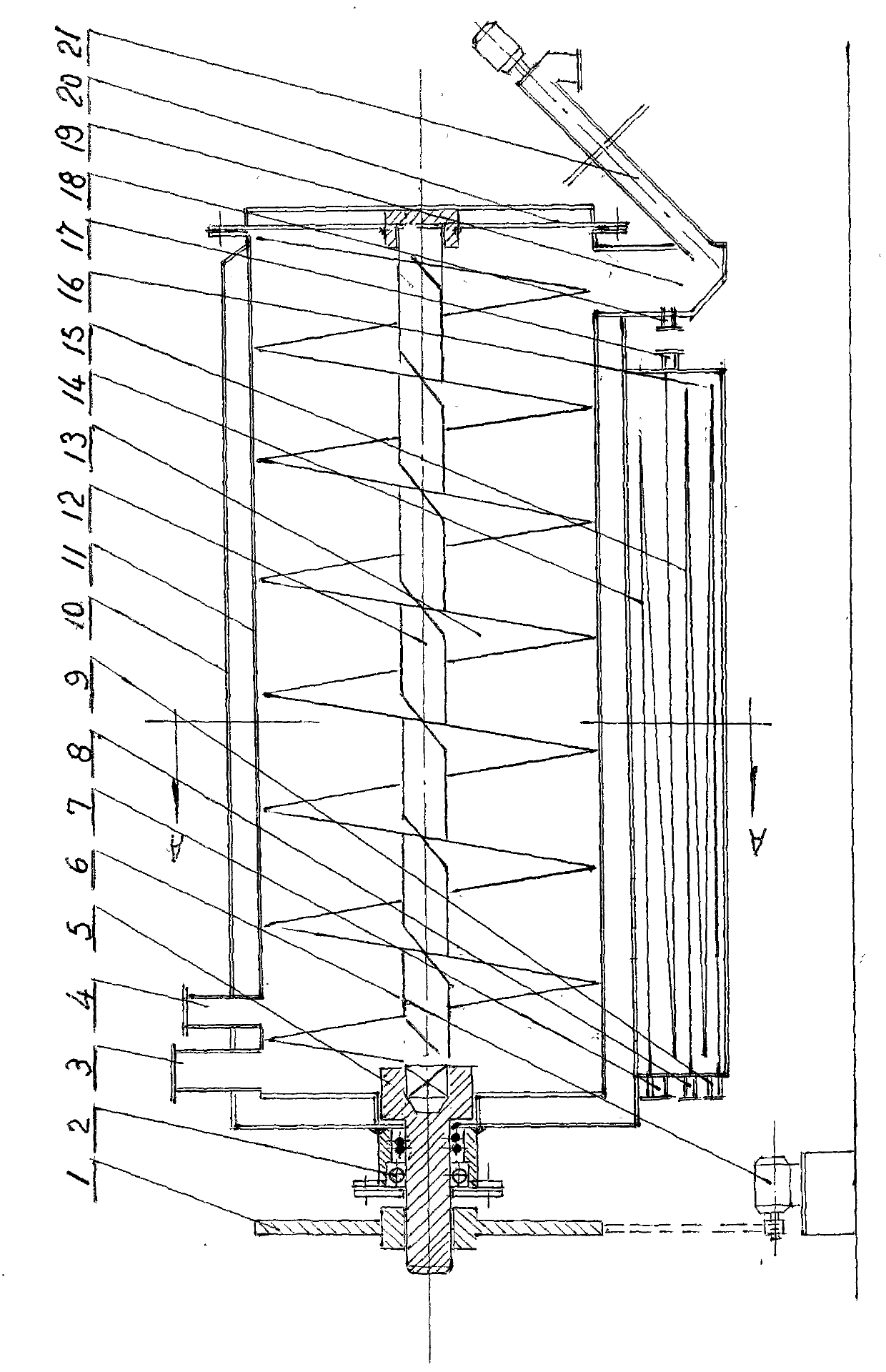
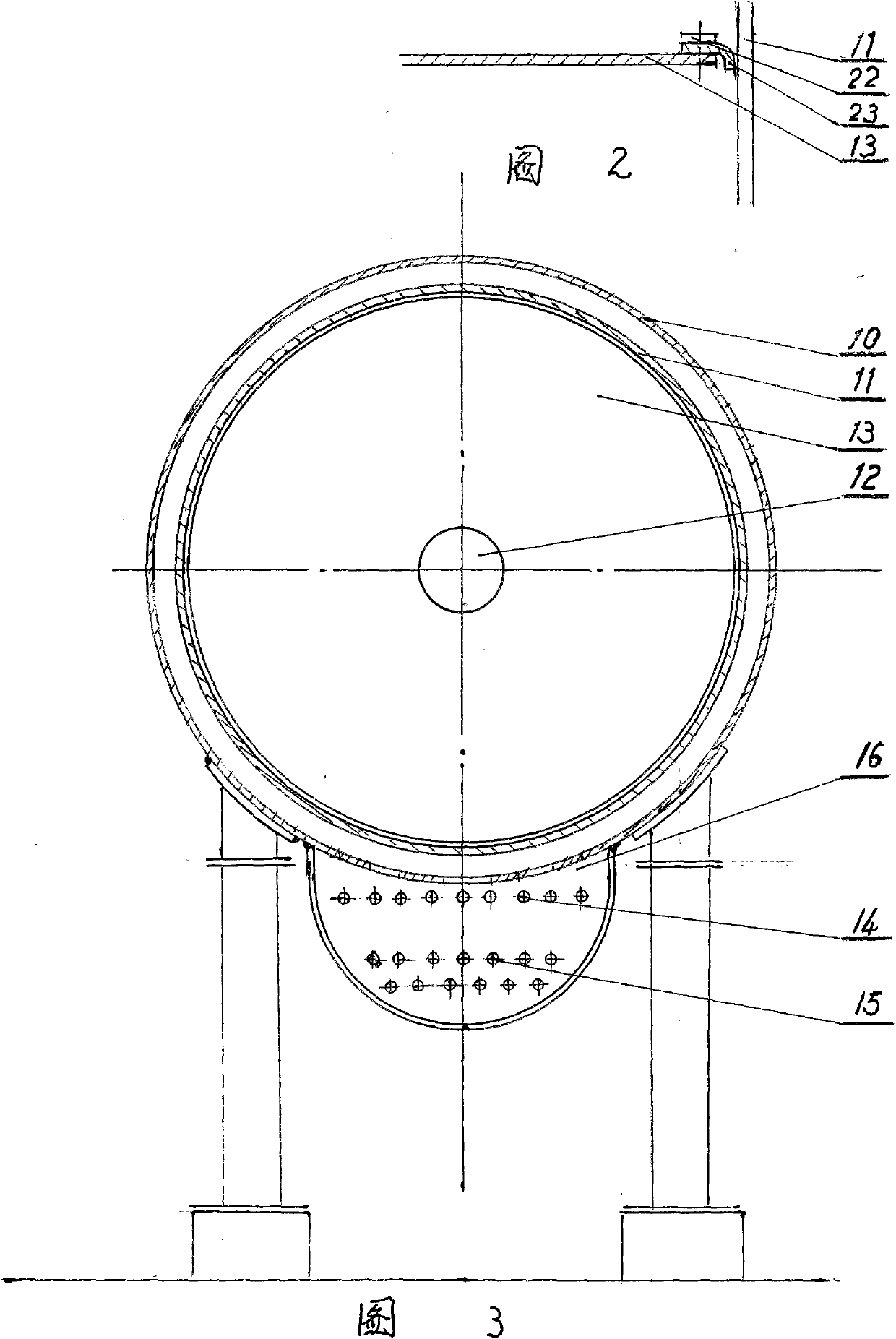
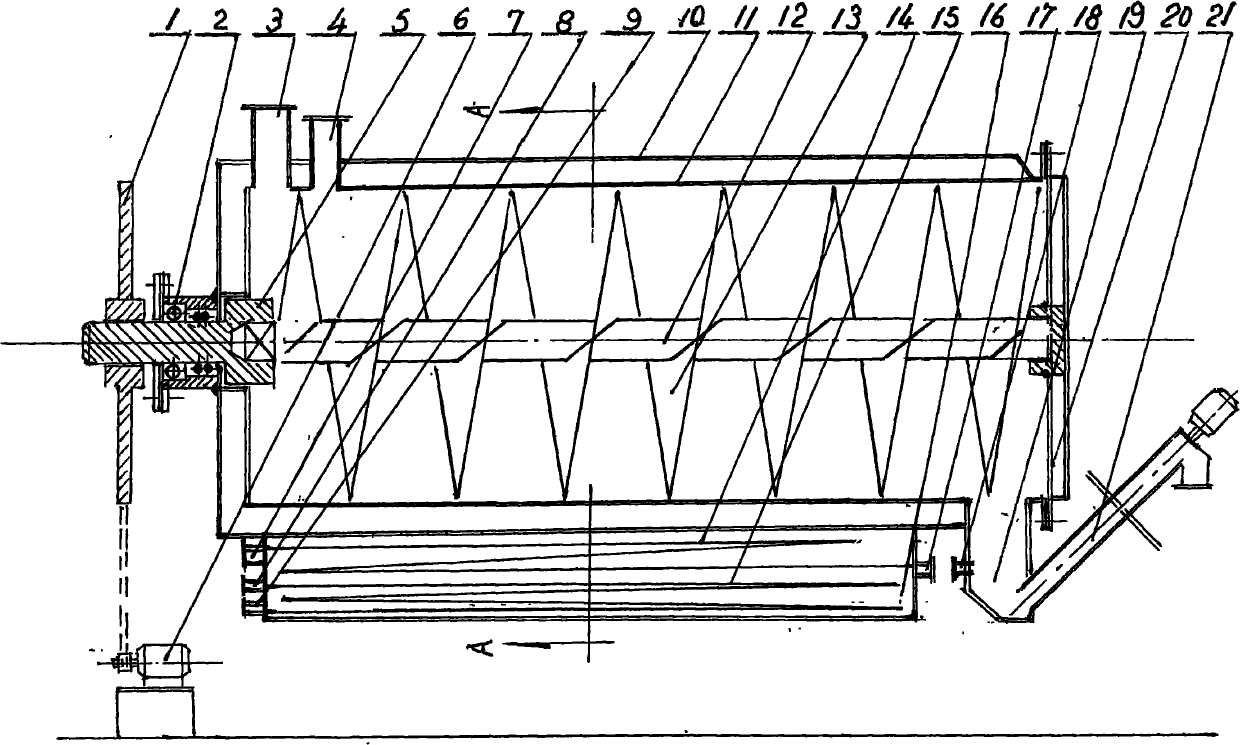
The invention relates to a continuous reverse flow extracting tank, which is used for extracting traditional Chinese medicines. The extracting tank comprises a heating jacket, a horizontal barrel, a material pushing screw, a driver, a low-temperature steam generator, a medicinal material inlet, a solvent inlet, an extracting solution outlet, a spiral slag extractor and the like, wherein the volume of the barrel is partitioned into a long continuous spiral groove by the material pushing screw so as to create condition for reverse flow of extracting solution and medicinal materials as well as uniform contact, dispersion and permeation of the extracting solution and the medicinal materials in mobile flow; and the driver drives the material pushing screw to rotate along one direction so as to push the medicinal materials from one end to the other end and continuously and hermetically discharge waste dregs of decoction. The continuous reverse flow extracting tank has a simple and compact structure. Hundreds of the conventional multifunctional extracting tanks which are not accordant with the requirements of the good manufacturing practice (GMP) are reformed by the technology of the continuous reverse flow extracting tank, so that continuous operation is realized, yield is increased by multiple times, energy consumption is saved greatly, the environmental quality of a production field is changed completely, the cost on continuous reverse flow extraction is lowered greatly and the popularization of continuous reverse flow extracting technology is promoted greatly.
Owner:沈善明
NK (Natural Killer) cell new technology for treating tumor
InactiveCN103372029AEasy accessLarge sourceMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthLymphatic Spread
Hematopoietic stem cells of healthy volunteers are separated and purified in a 100 grade GMP (Good Manufacturing Practice) plant, and are induced to divide to NK cells under a special condition of culture. Qualified NK cells are put in storage or KIR-matched with HLA (Human Leukocyte Antigen) and KN cells of the patients. The qualified NK cells are intravenously infused to tumor patients to prevent recurrence and metastasis of cancer cells of the tumor patients. The hematopoietic stem cells used by the method have the advantages of abundant source, material object storage and the like, are free from toxic and side effects and adverse reaction for treating tumor, and remarkably improve the survival quality of the tumor patients.
Owner:孙勇
Industrialized preparing process for traditional Chinese medicine decoction pieces simmered monkshood and automatic temperature-control simmering furnace
InactiveCN103585017AAdapt to GMP requirementsGMP requirements are metPharmaceutical product form changeTemperature controlMonkshoods
The invention discloses an industrialized preparing process for traditional Chinese medicine decoction pieces simmered monkshood and an automatic temperature-control simmering furnace. The industrialized preparing process is characterized by including taking salted aconite roots, selecting and grading, placing the salted aconite roots in a container, adding water until the salted aconite roots are completely submerged, changing the water 2-4 times each day during soaking, taking out the salted aconite roots when conductivity of soaked water at 25 DEG C is lowered to be below 6000 microsecond per centimeter, enabling the top ends of the salted aconite roots to be up and the pointed ends of the salted aconite roots to be down, placing the salted aconite roots on a shelf of the automatic temperature-control simmering furnace, covering a plurality of layers of clean ginger pieces on the salted aconite roots, adding fine chaff ash untile a furnace body of the simmering furnace is fully filled, setting heating temperature of a lower heating component to be 70-130 DEG C and that of an upper heating component to be 110-180 DEG C, heating and simmering for 24-48 hours, taking out the salted aconite roots until the ash is cold, steaming, slicing and drying. Production process parameters are accurate and controllable, product difference between batches is obviously reduced, labor intensity is remarkably lowered, production cost is lowered, production environment meets GMP (good manufacturing practice) requirements, and a solid foundation is laid for standardized and industrialized production of the simmered monkshood.
Owner:王小平
Compositions, uses, and method of making wound care products from naturally occurring food ingredients
InactiveUS20090036413A1Good effectReduce excessSalicyclic acid active ingredientsBiocideFood additiveGuideline
Rationally designed wound care products made entirely of naturally occurring food ingredients that can be standardized and made available for the mass market using good manufacturing practice (GMP) guidelines, optionally, a safe food additive can be added. These products: are safe and effective; have an osmotic pressure compatible with optimal healing; are buffered to maintain optimal pH throughout the healing process; provide a protective barrier from further irritation and insult; control bacteria, viruses and fungi found in the skin and mucosa; nourish wounds; control excessive prolonged inflammation and thereby minimize scarring; minimize allergenic and irritation potential; are easy to use or apply; pass the preservative challenge test required for products intended for multiple use; contain fragrant essential oils to take advantage of the benefits provided by aromatherapy; can be individually optimized based on the diet of an individual or group of people.
Owner:BILL MCANALLEY & ASSOCS
Optimised and defined method for isolation and preservation of precursor cells from human umbilical cord
InactiveCN101868536ACell dissociation methodsSkeletal/connective tissue cellsCell phenotypeCell Fraction
The present invention refers to an optimized and defined method for isolation and preservation of precursor cells from human umbilical cord. Besides being reproducible and 100% reliable, in terms of the number of samples processed, the method results in a high and defined number of precursor cells, being the majority obtained after a single adhesion and expansion / multiplication phase ex vivo (thus granting cell phenotype), in a shorter time frame than what was previously described in the state-of-the-art. With this method, it is possible to obtain, in 9 days, after direct freezing of a cell fraction, and after one expansion / multiplication phase ex vivo (end of PO) of the majority of the cells, about 8, 6, 6 (+ / -0, 1) x l05 cells / gram of processed umbilical cord. In turn, the characteristics of the cells allow, for example, after 35 days, obtaining an average of 7.7xlO15 cells, with precursor phenotype, from 100% of processed umbilical cord samples. The method, because it is simple, robust and 100% reliable, can be performed under good manufacturing practices (GMP) in laboratories dedicated to cell therapy in humans. Furthermore, the method has applications in the pharmaceutical, cosmetic and biotechnology areas.
Owner:MEDINFAR PROD FARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com