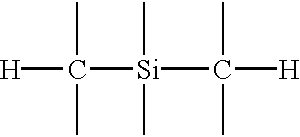Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1614 results about "Oxygen rich" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
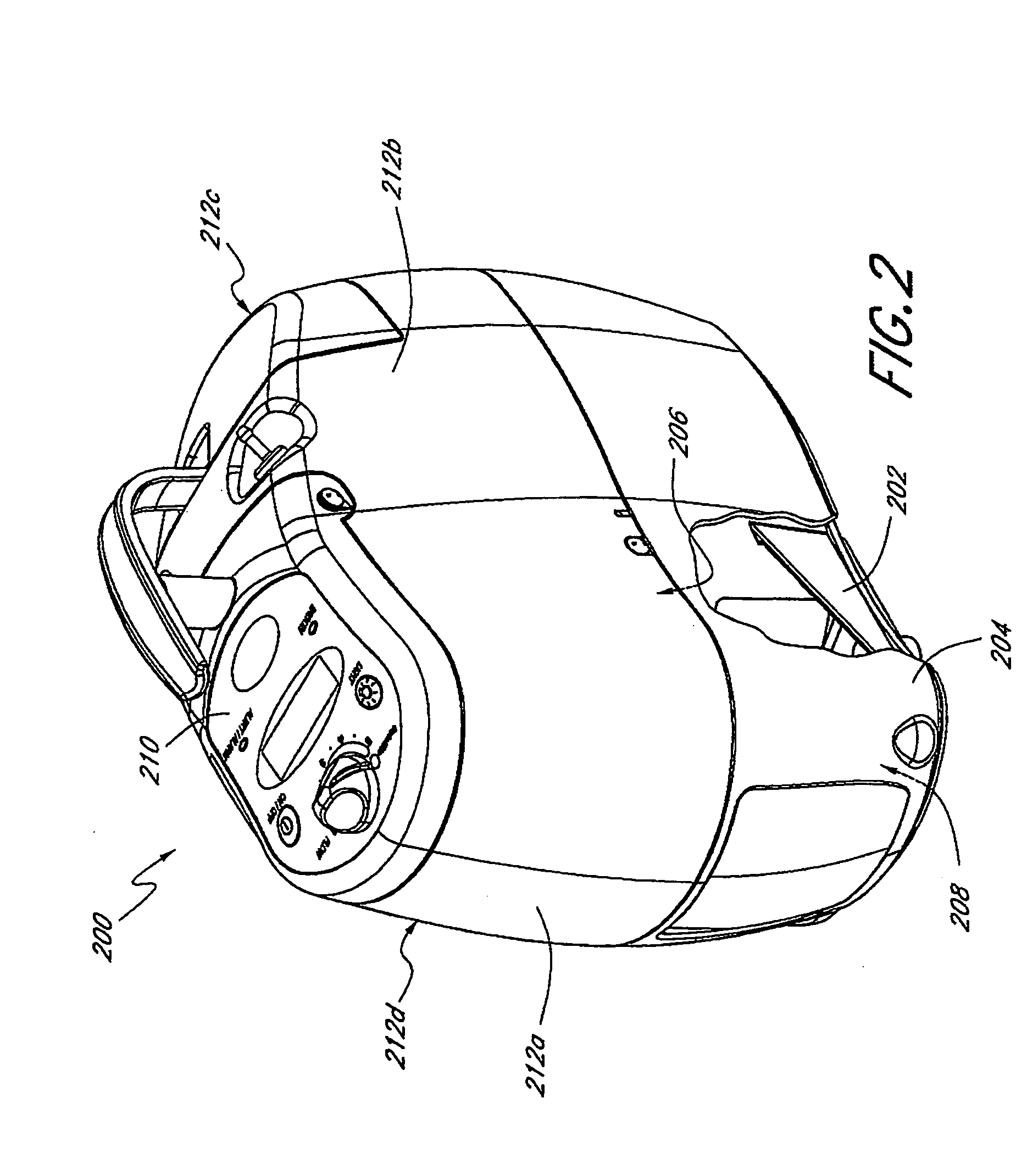
Microsensors for glucose and insulin monitoring
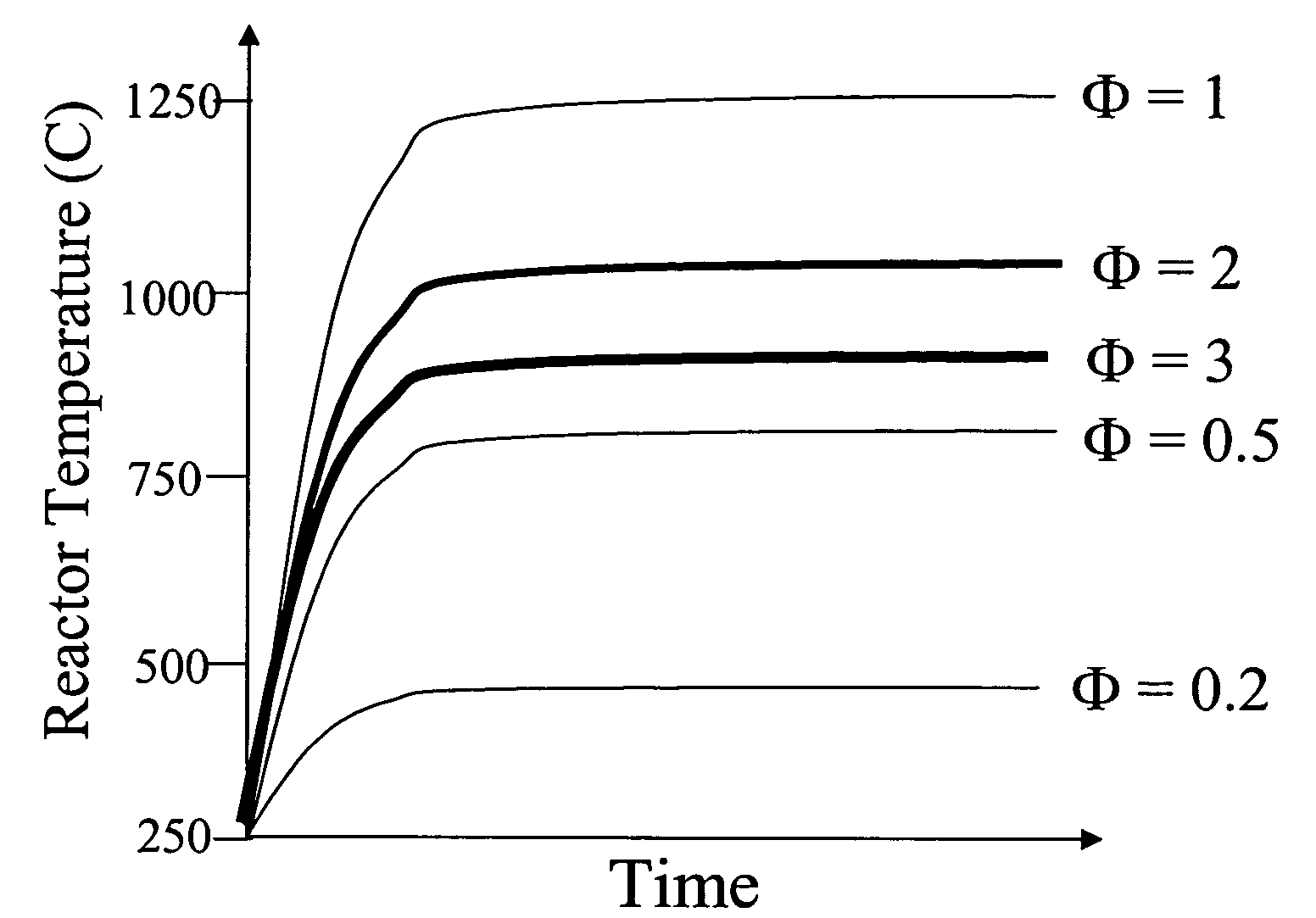
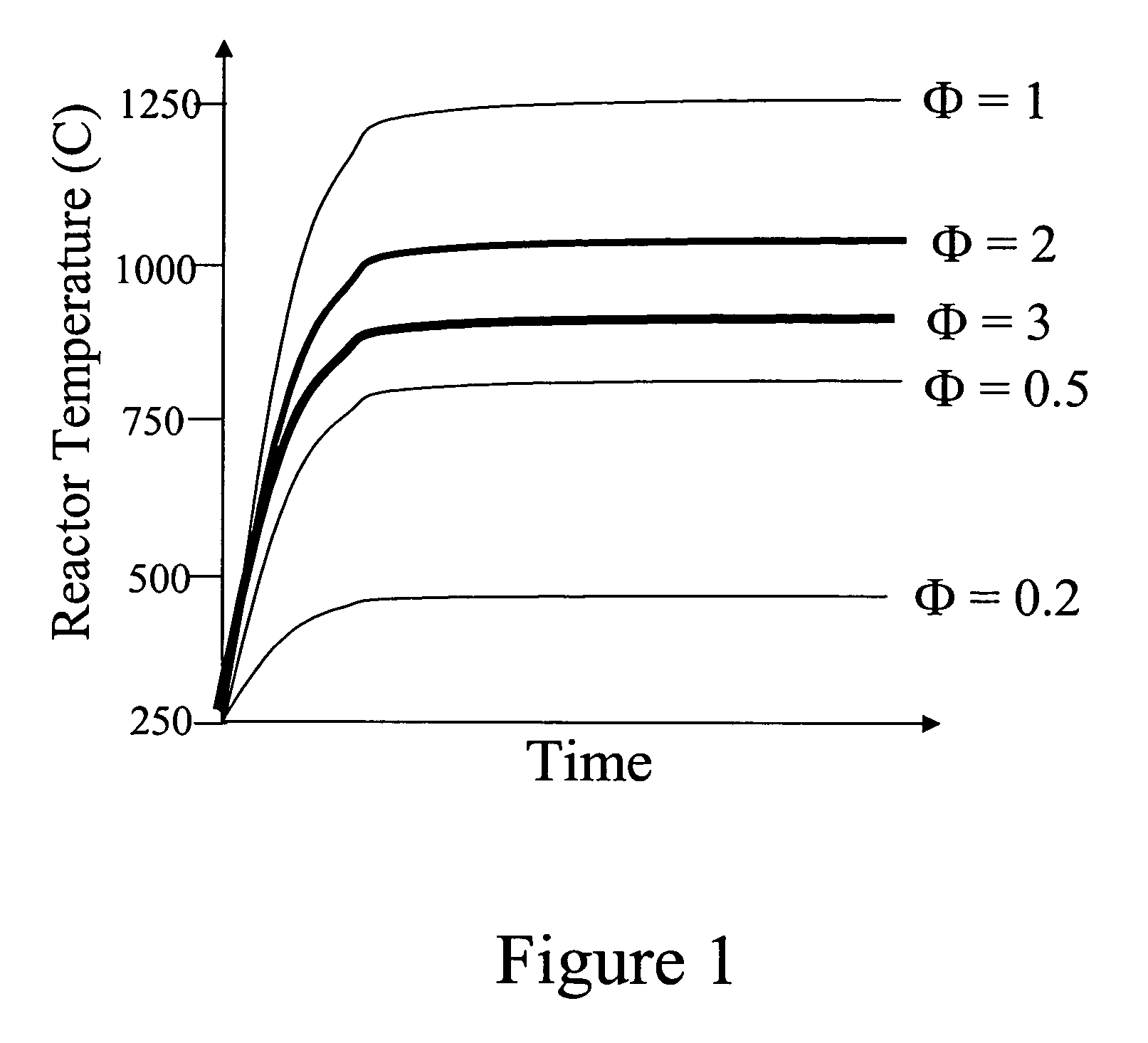
InactiveUS6893552B1Simultaneous measurementLacking and neededImmobilised enzymesBioreactor/fermenter combinationsOxidative enzymeD-Glucose
A dual sensor for the simultaneous amperometric monitoring of glucose and insulin, wherein the glucose probe is based on the biocatalytic action of glucose oxidase, and the insulin probe is based on the electrocatalytic activity of metal oxide. Further provided is an oxidase enzyme composite electrode with an internal oxygen-rich binder. The present invention also optionally includes metallizing components within the carbon paste to eliminate signals from interfering compounds. The present invention includes embodiments for both in vitro and in vivo uses.
Owner:ARROWHEAD CENT
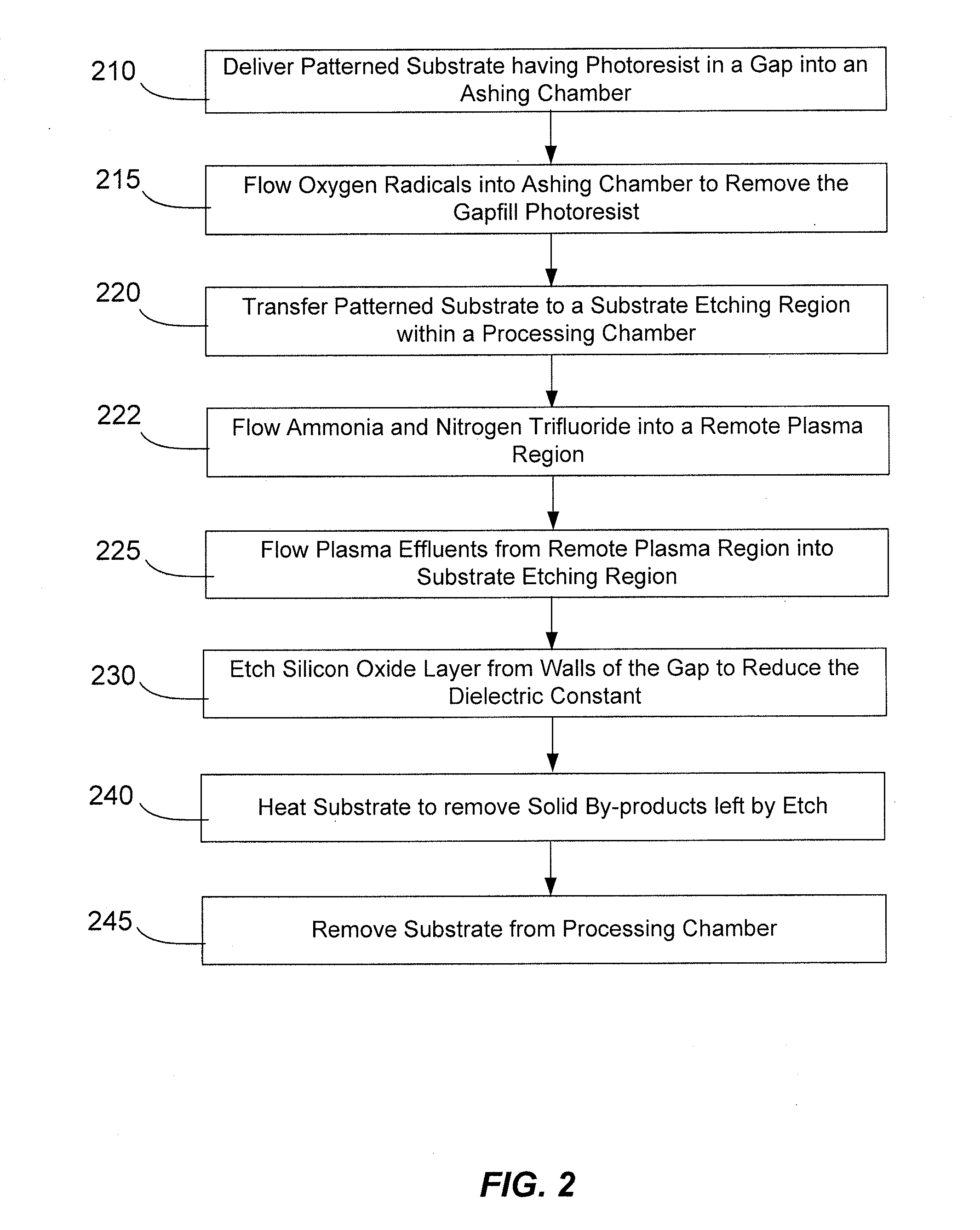
Post-ash sidewall healing
InactiveUS20120009796A1Easy and timelyEffective dielectric constantSemiconductor/solid-state device manufacturingGas phaseOxygen rich
Methods of decreasing the effective dielectric constant present between two conducting components of an integrated circuit are described. The methods involve the use of a gas phase etch which is selective towards the oxygen-rich portion of the low-K dielectric layer. The etch rate attenuates as the etch process passes through the relatively high-K oxygen-rich portion and reaches the low-K portion. The etch process may be easily timed since the gas phase etch process does not readily remove the desirable low-K portion.
Owner:APPLIED MATERIALS INC
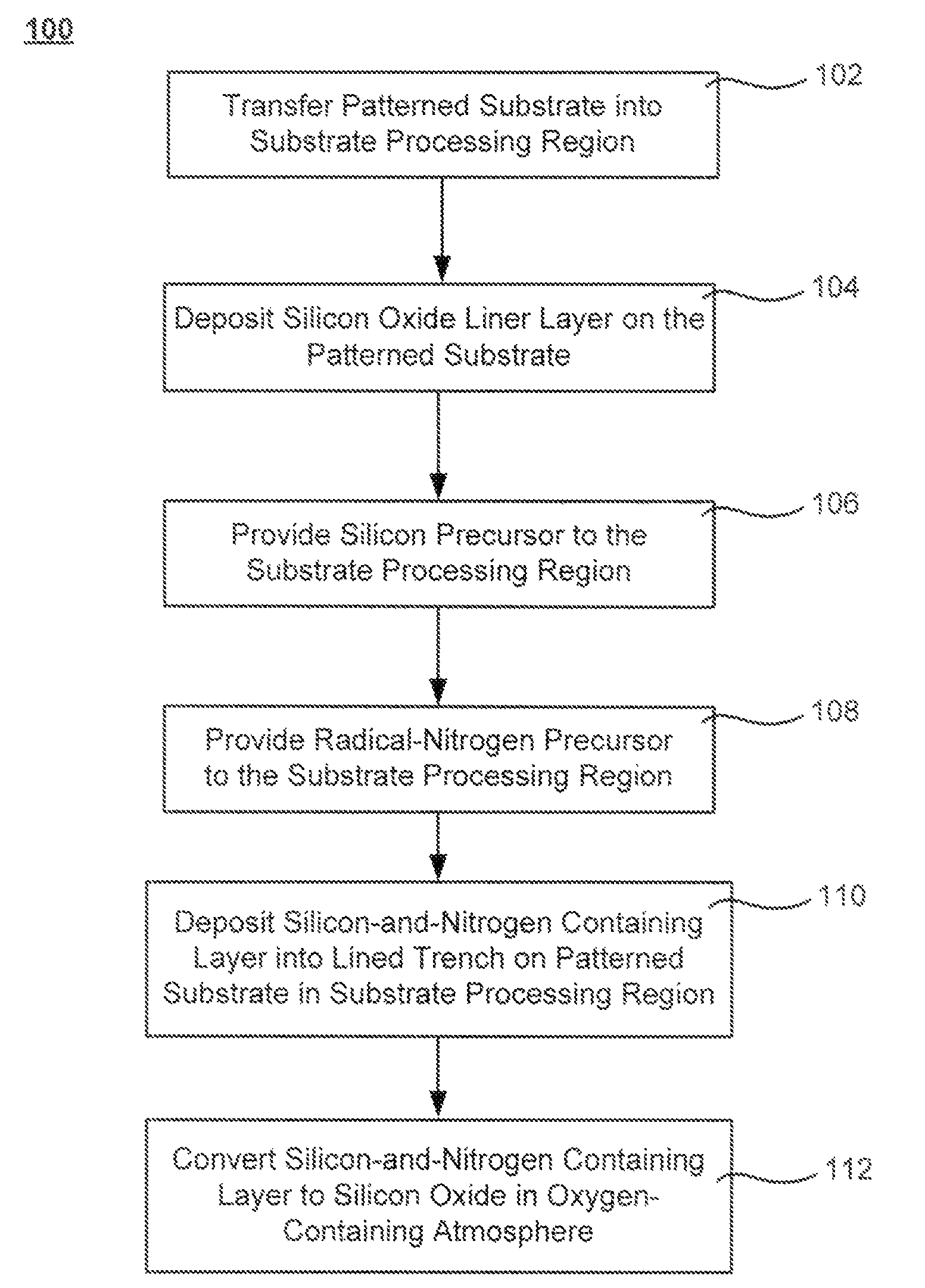
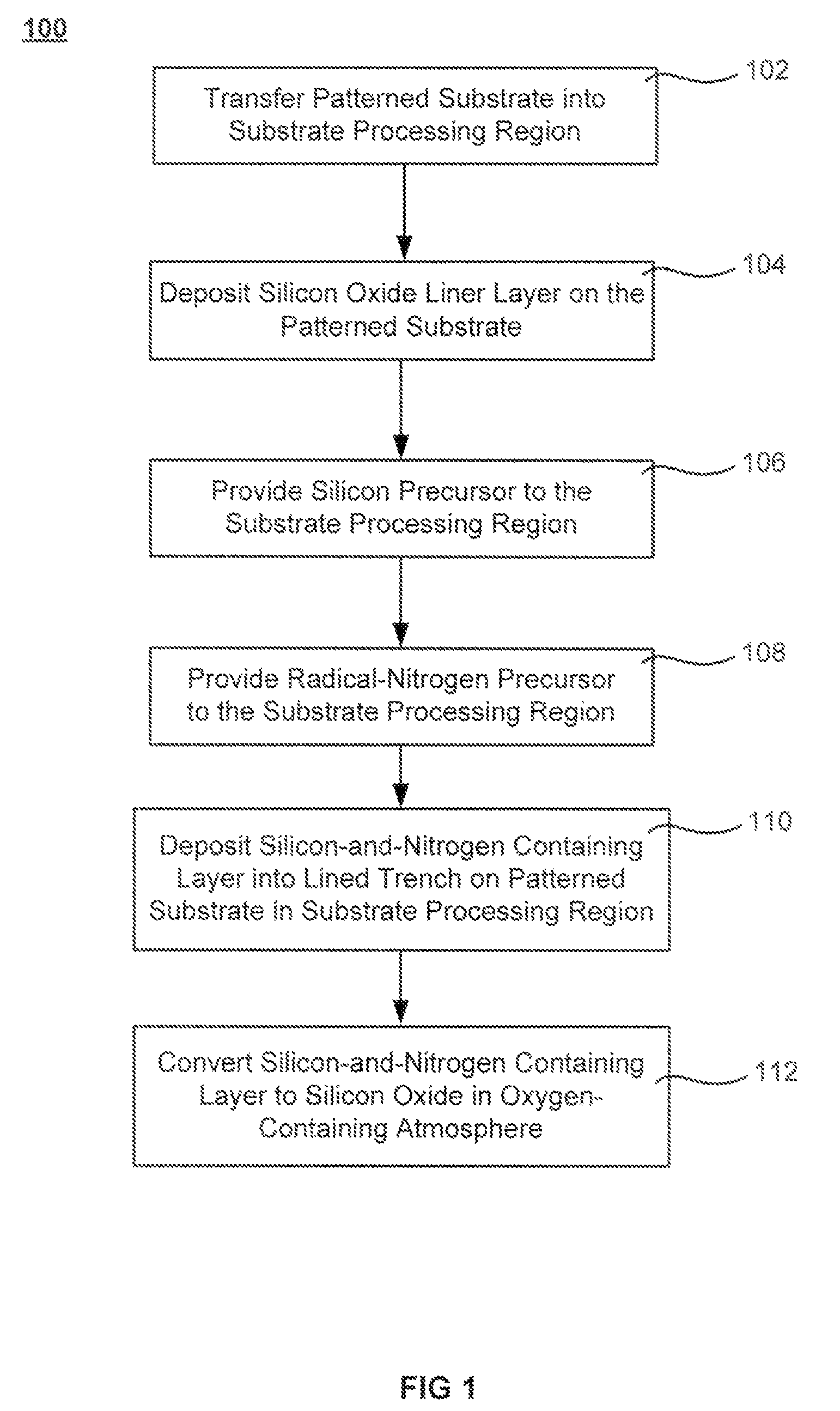
Oxide-rich liner layer for flowable CVD gapfill
The formation of a gap-filling silicon oxide layer with reduced volume fraction of voids is described. The deposition involves the formation of an oxygen-rich less-flowable liner layer before an oxygen-poor more-flowable gapfill layer. However, the liner layer is deposited within the same chamber as the gapfill layer. The liner layer and the gapfill layer may both be formed by combining a radical component with an unexcited silicon-containing precursor (i.e. not directly excited by application of plasma power). The liner layer has more oxygen content than the gapfill layer and deposits more conformally. The deposition rate of the gapfill layer may be increased by the presence of the liner layer. The gapfill layer may contain silicon, oxygen and nitrogen and be converted at elevated temperature to contain more oxygen and less nitrogen. The presence of the gapfill liner provides a source of oxygen underneath the gapfill layer to augment the gas phase oxygen introduced during the conversion.
Owner:APPLIED MATERIALS INC
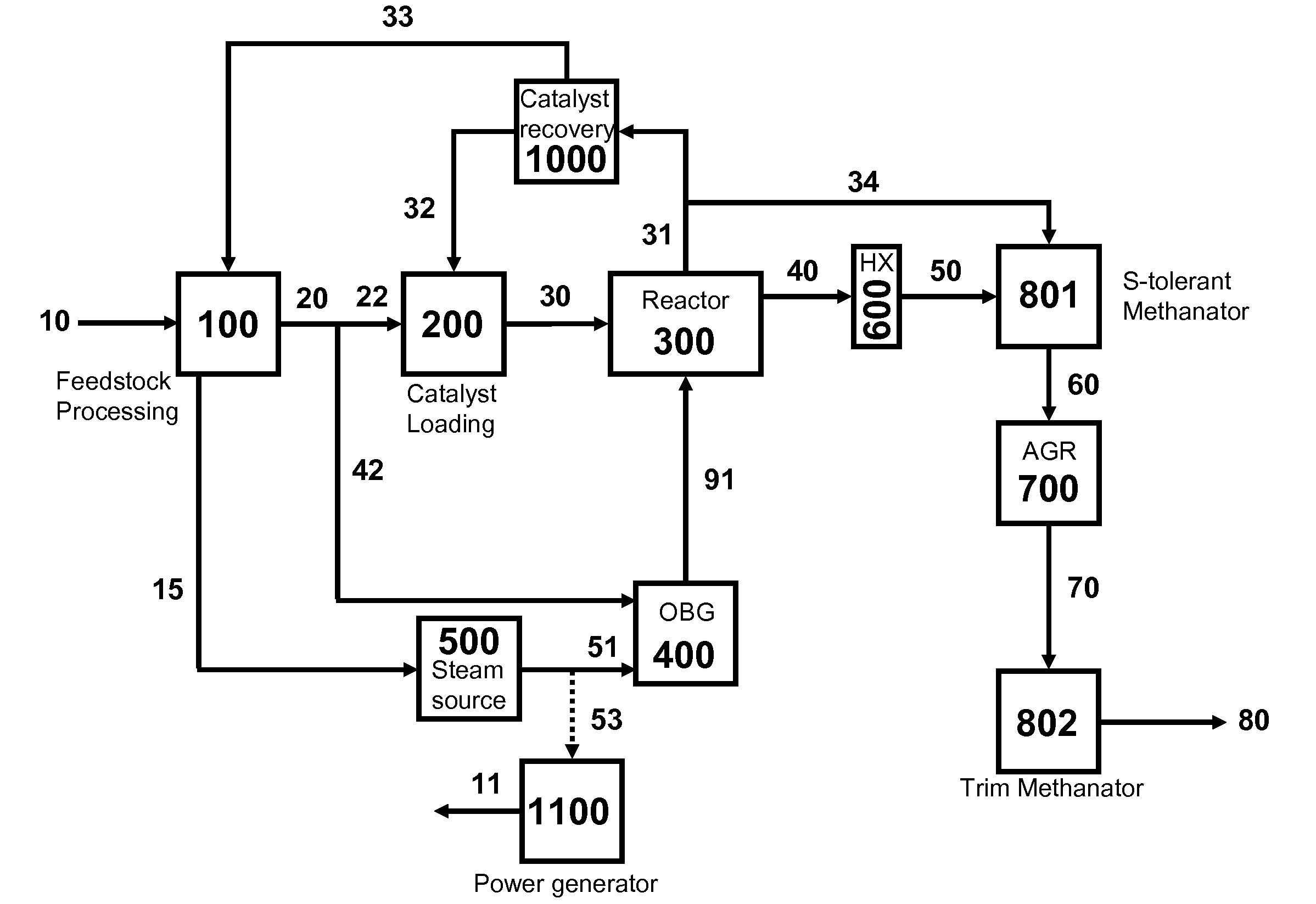
Processes for Gasification of a Carbonaceous Feedstock
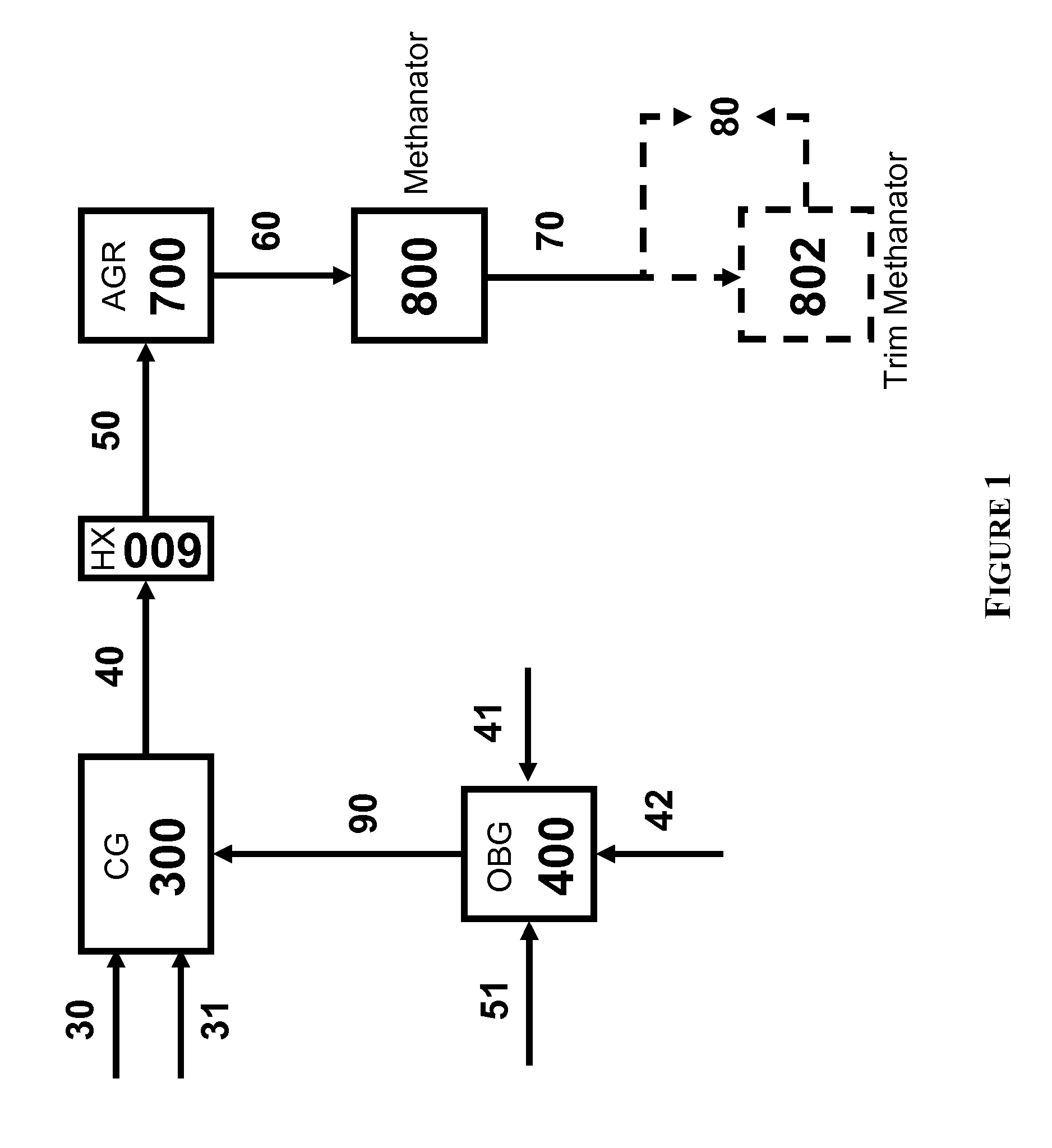
The present invention relates to processes and continuous processes for preparing gaseous products, and in particular, methane via the catalytic gasification of carbonaceous feedstocks in the presence of steam. In one aspect of the invention, the processes comprise at least partially combusting a first carbonaceous feedstock with an oxygen-rich gas stream in an oxygen-blown gasifier, under suitable temperature and pressure, to generate a first gas stream comprising hydrogen, carbon monoxide and superheated steam; and reacting a second carbonaceous feedstock and the first gas stream in a catalytic gasifier in the presence of a gasification catalyst under suitable temperature and pressure to form a second gas stream comprising a plurality of gaseous products comprising methane, carbon dioxide, hydrogen, carbon monoxide and hydrogen sulfide. The processes can comprise using at least one catalytic methanator to convert carbon monoxide and hydrogen in the gaseous products to methane and in certain embodiments do not recycle carbon monoxide or hydrogen to the gasifier.
Owner:SURE CHAMPION INVESTMENT LTD
Processes for Gasification of a Carbonaceous Feedstock
The present invention relates to processes for preparing gaseous products, and in particular, methane via the catalytic gasification of carbonaceous feedstocks in the presence of steam and an oxygen-rich gas stream. The processes comprise using at least one catalytic methanator to convert carbon monoxide and hydrogen in the gaseous products to methane and do not recycle carbon monoxide or hydrogen to the catalytic gasifier.
Owner:SURE CHAMPION INVESTMENT LTD
Plant and a method for increased oil recovery
Owner:DEN NORSKE STATS OLJESELSKAP AS
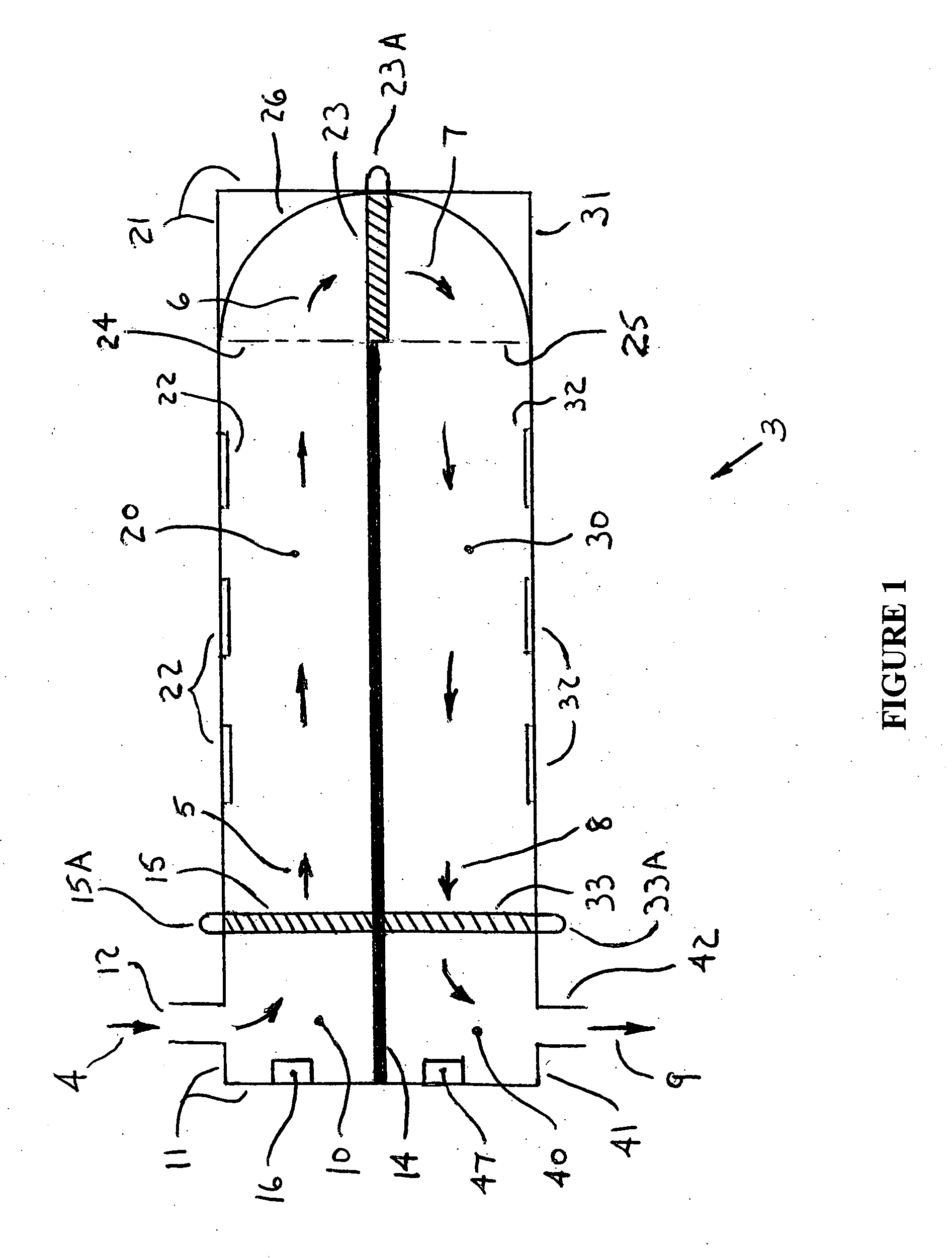
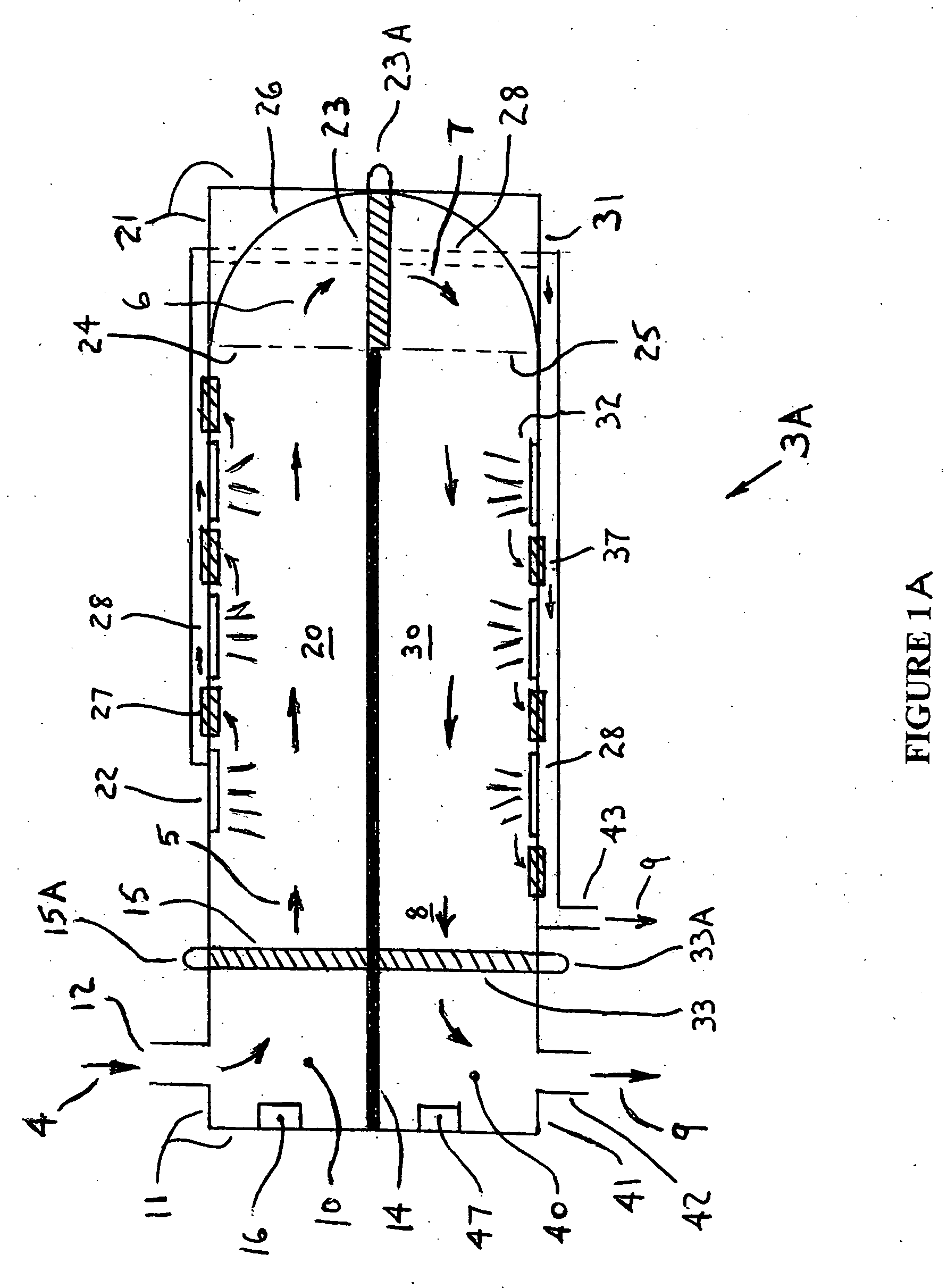
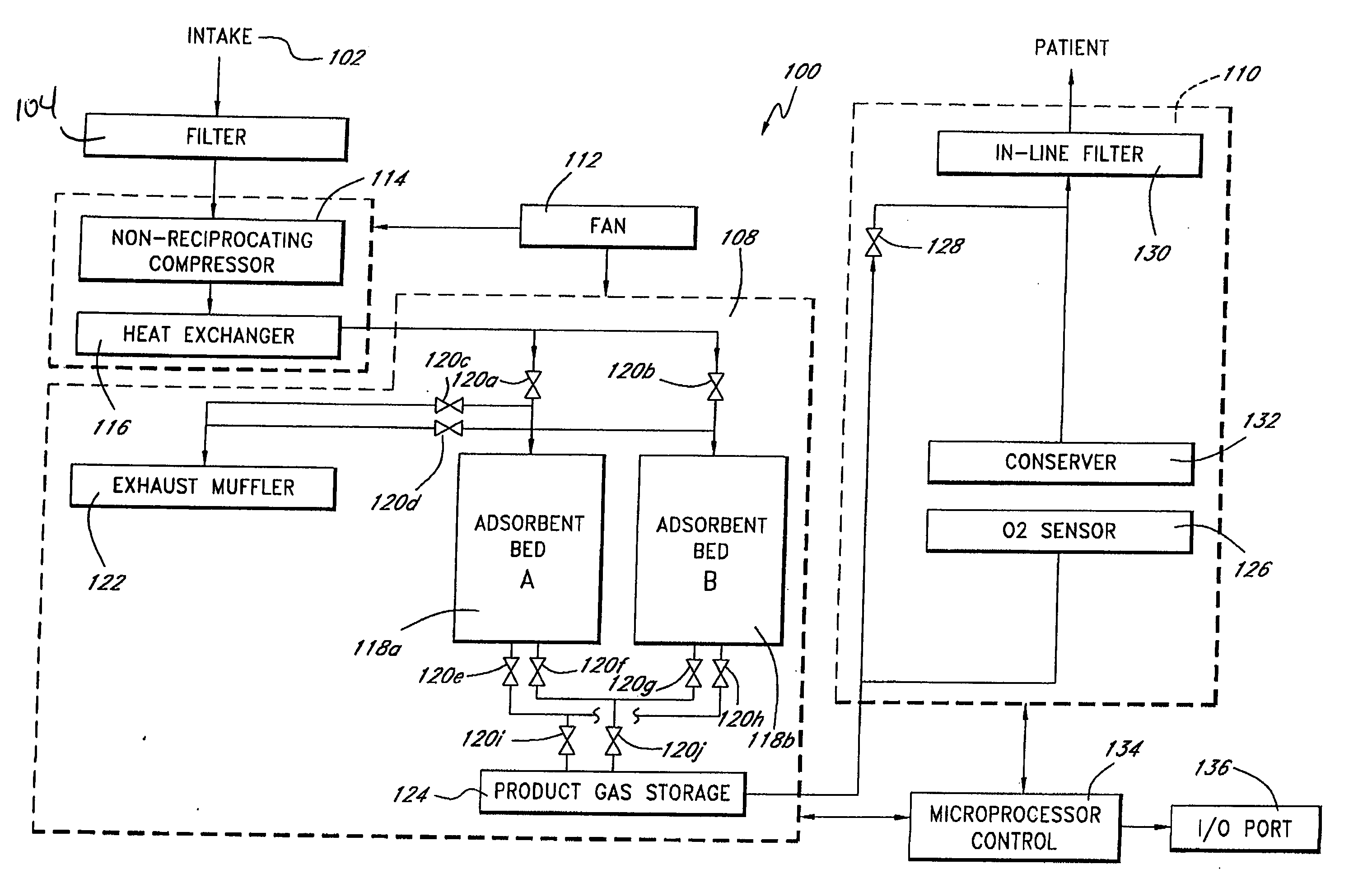
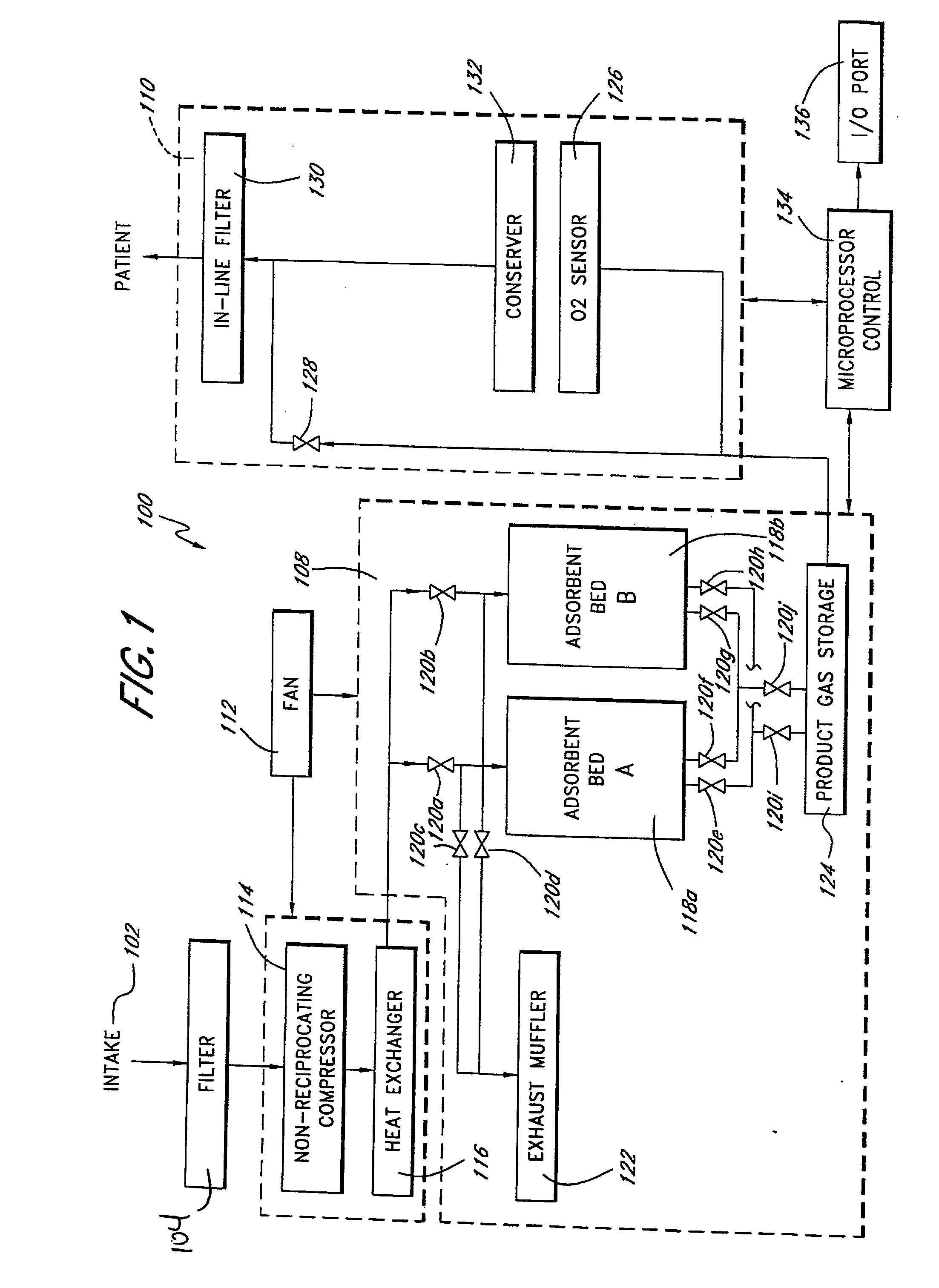
Portable gas fractionalization system
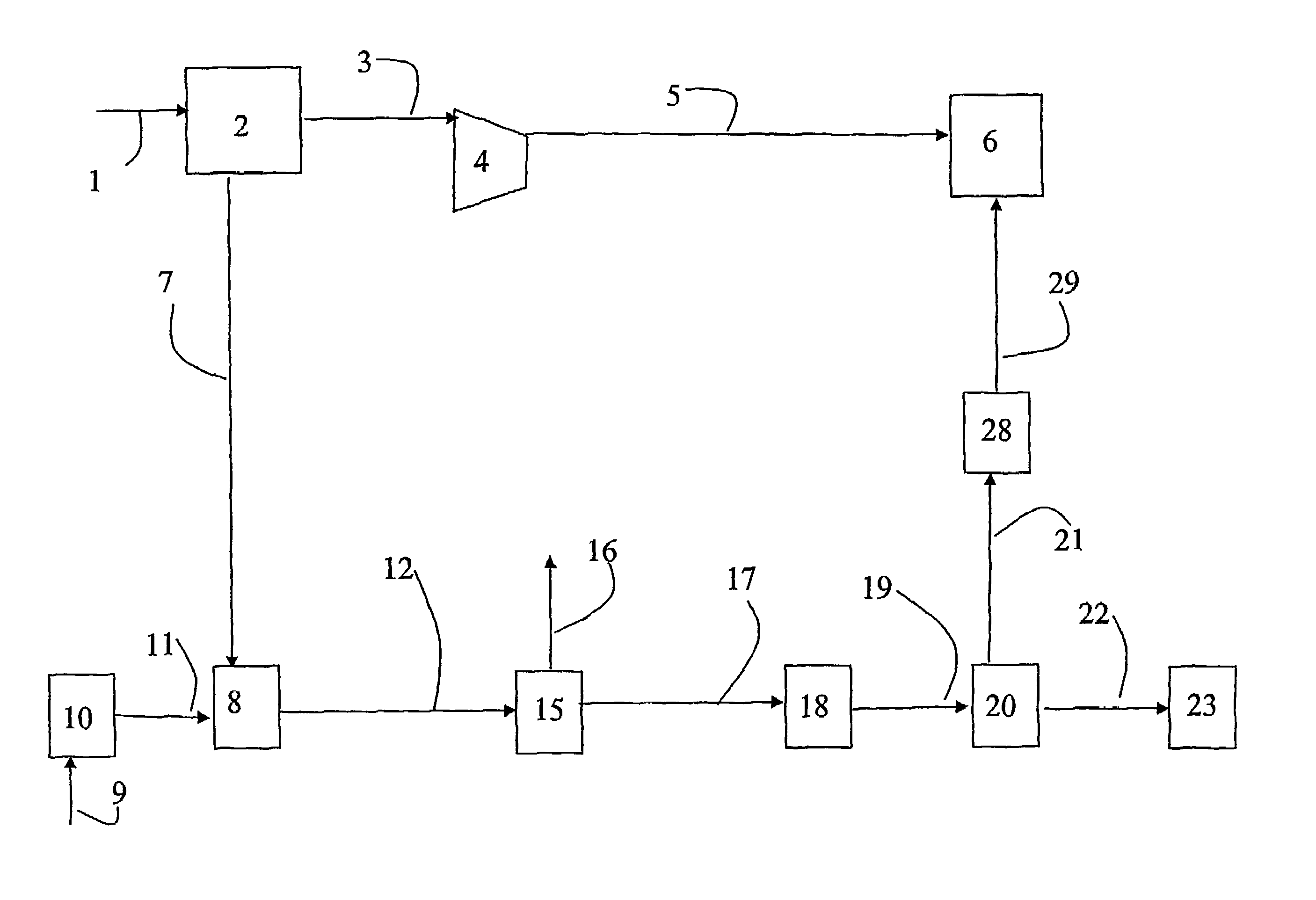
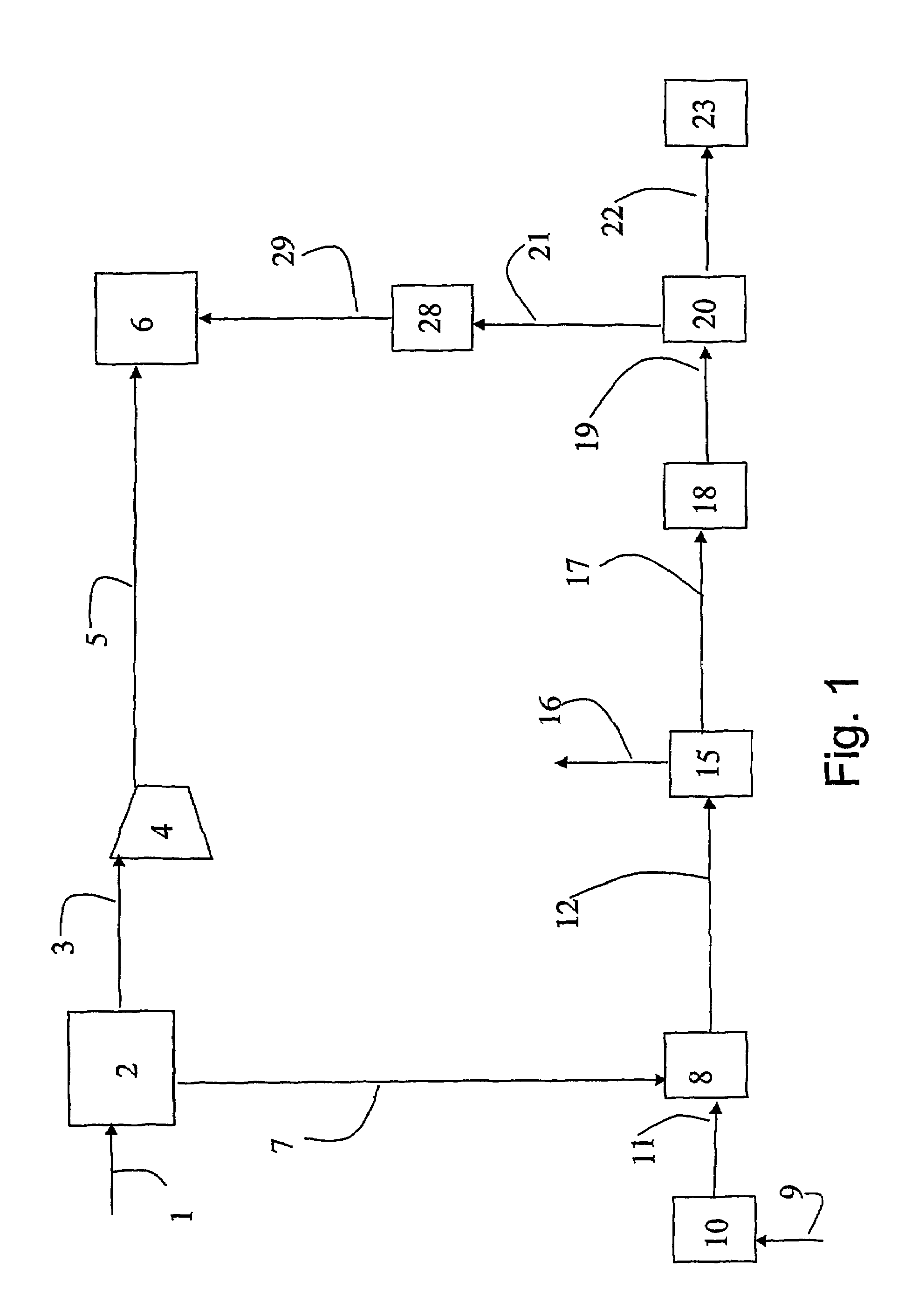
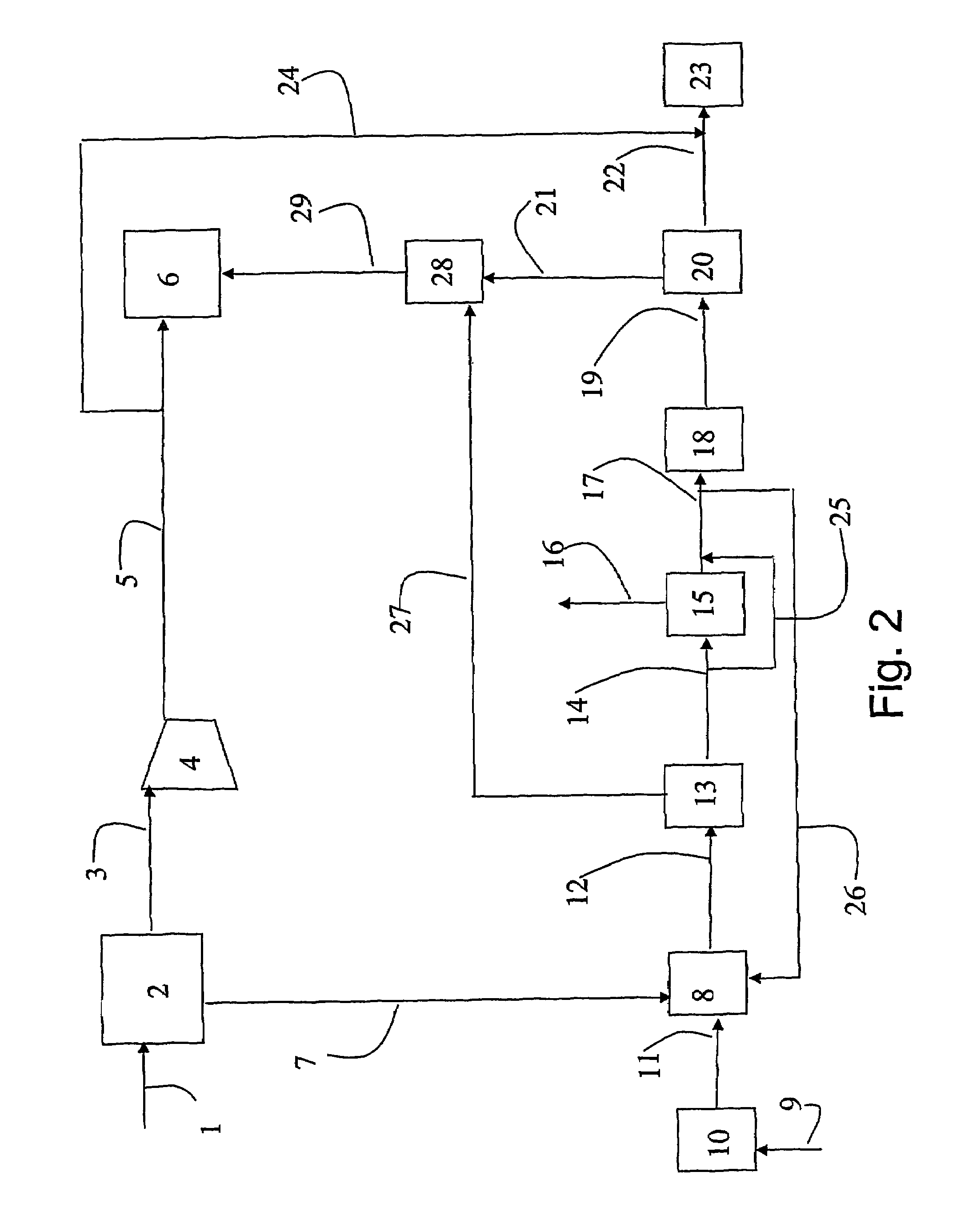
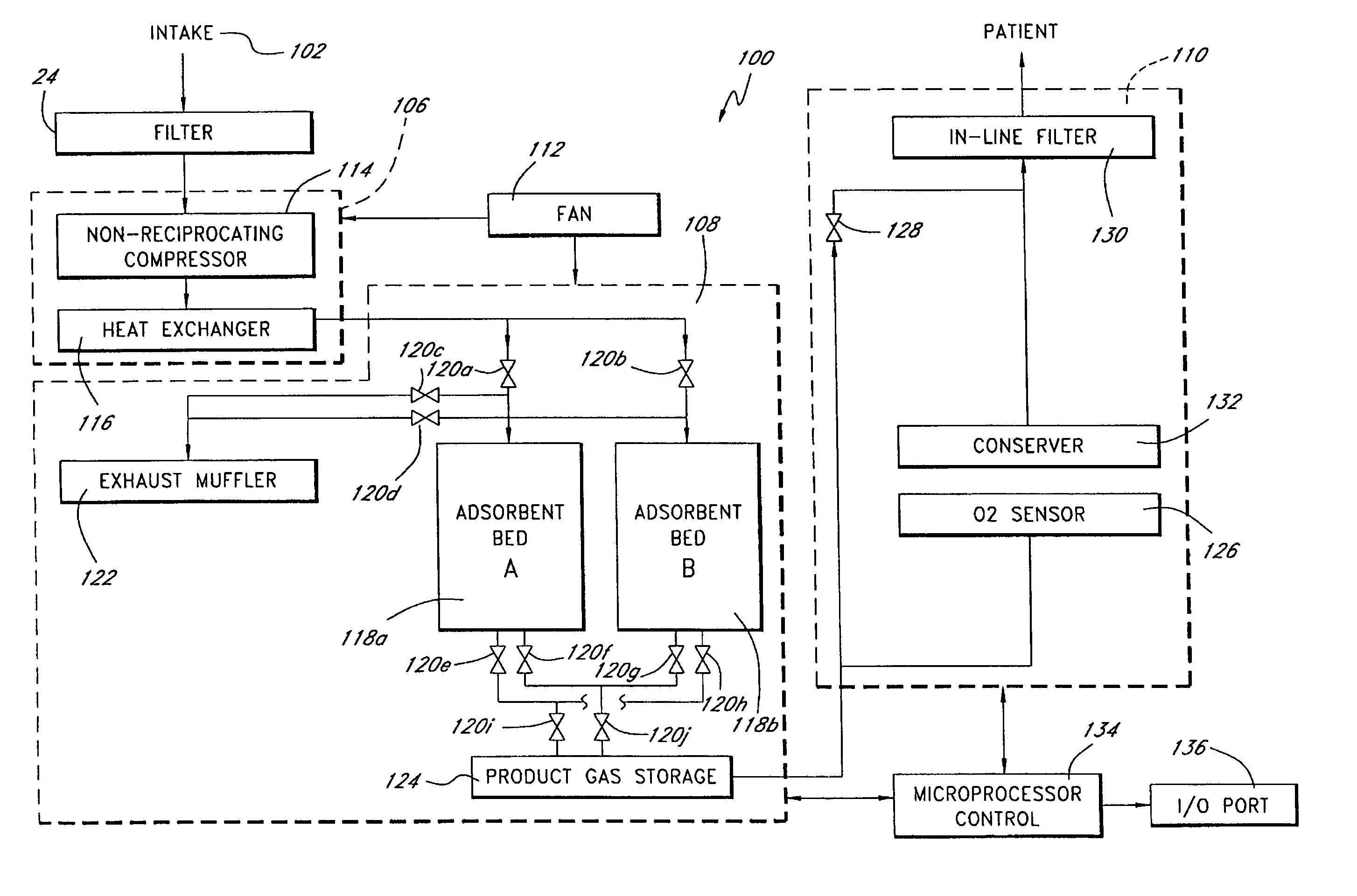
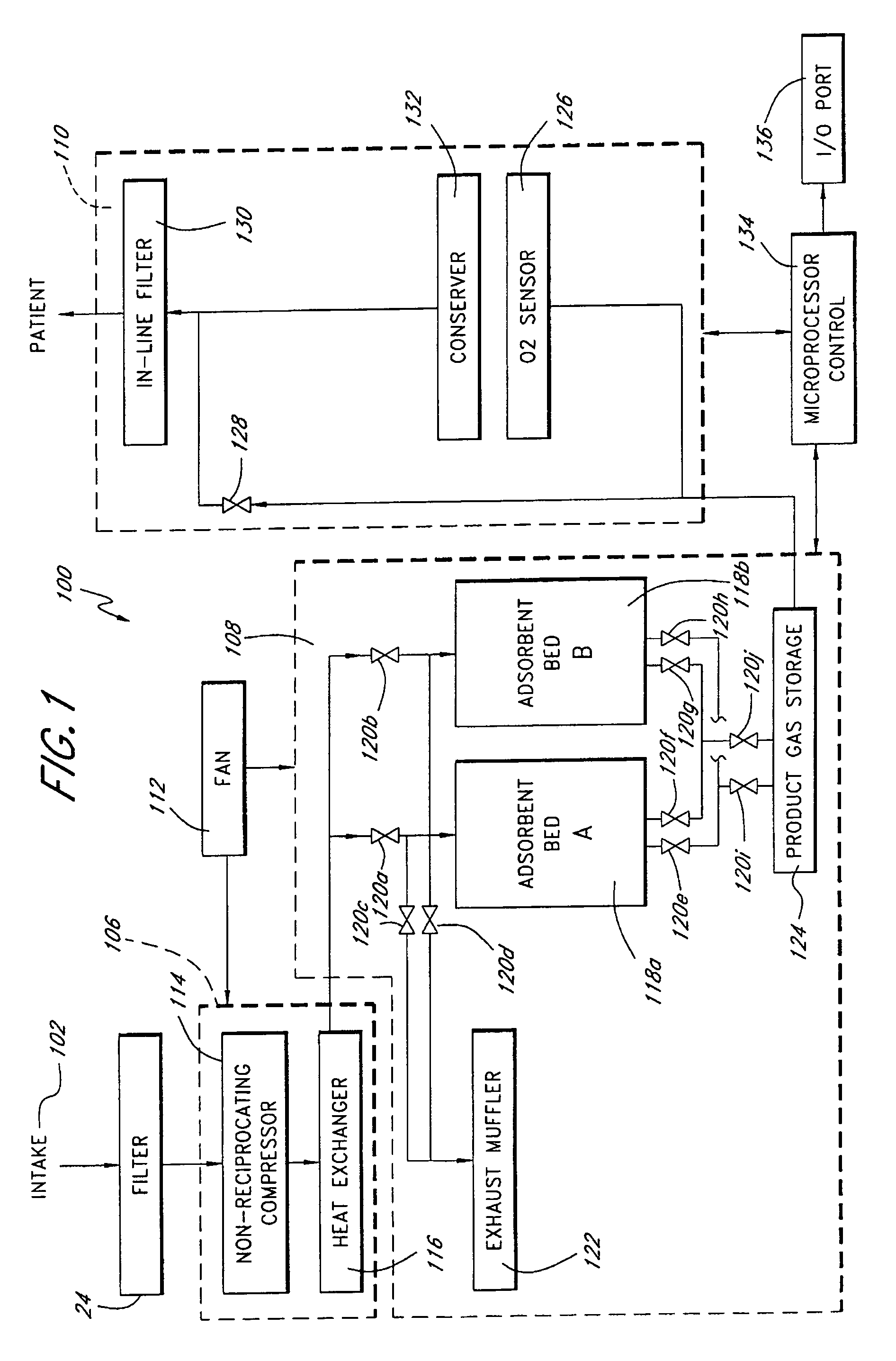
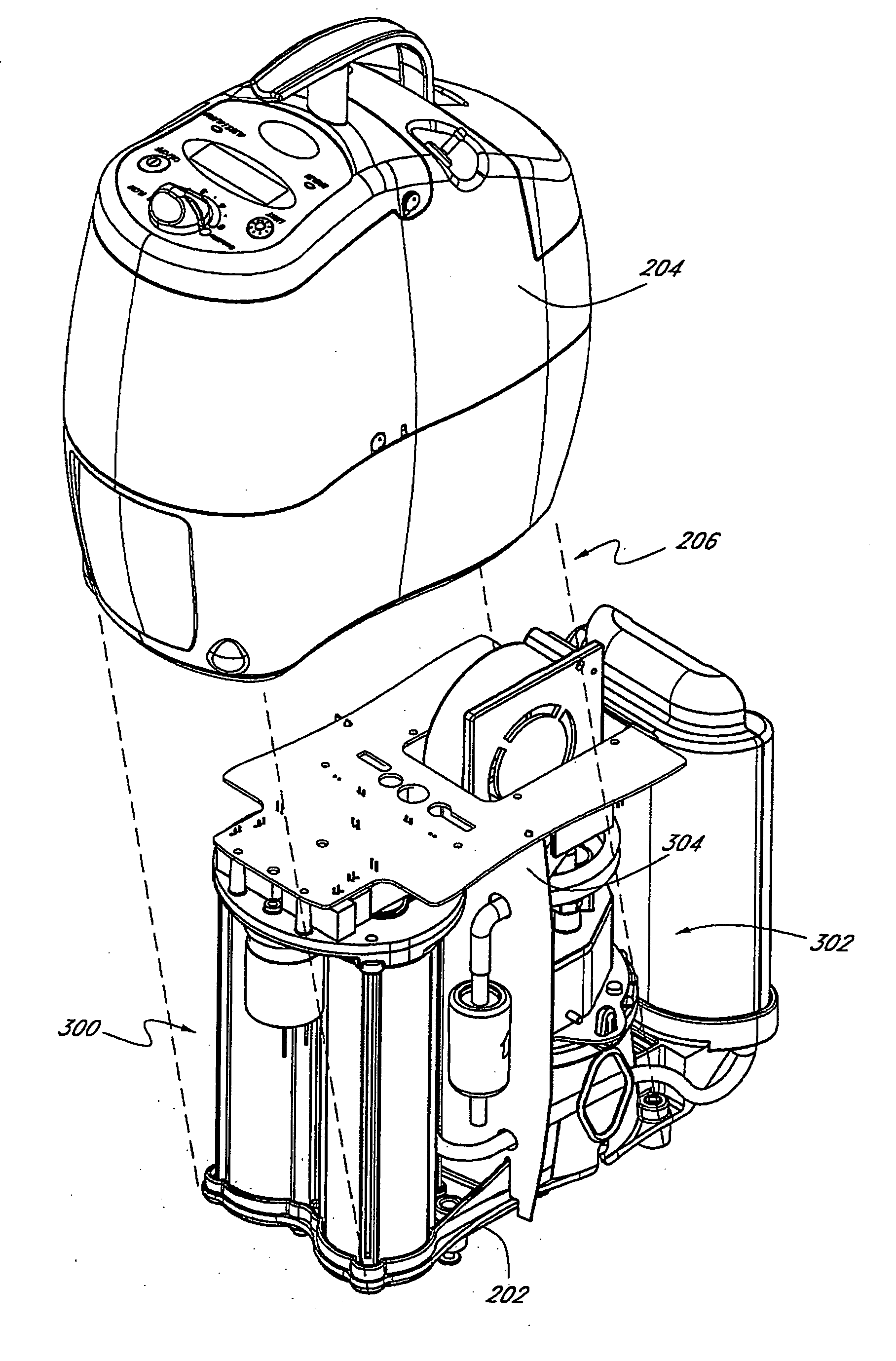
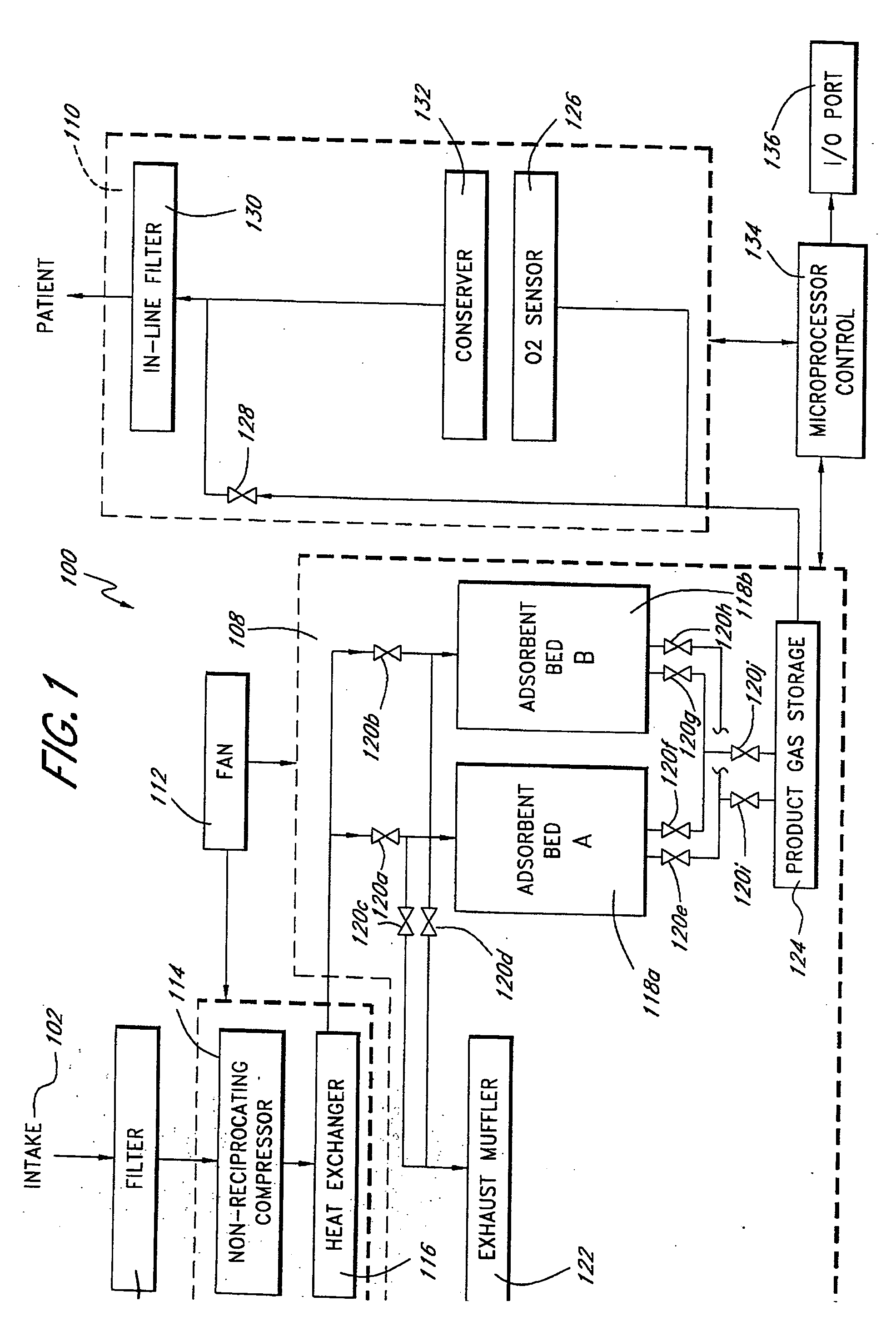
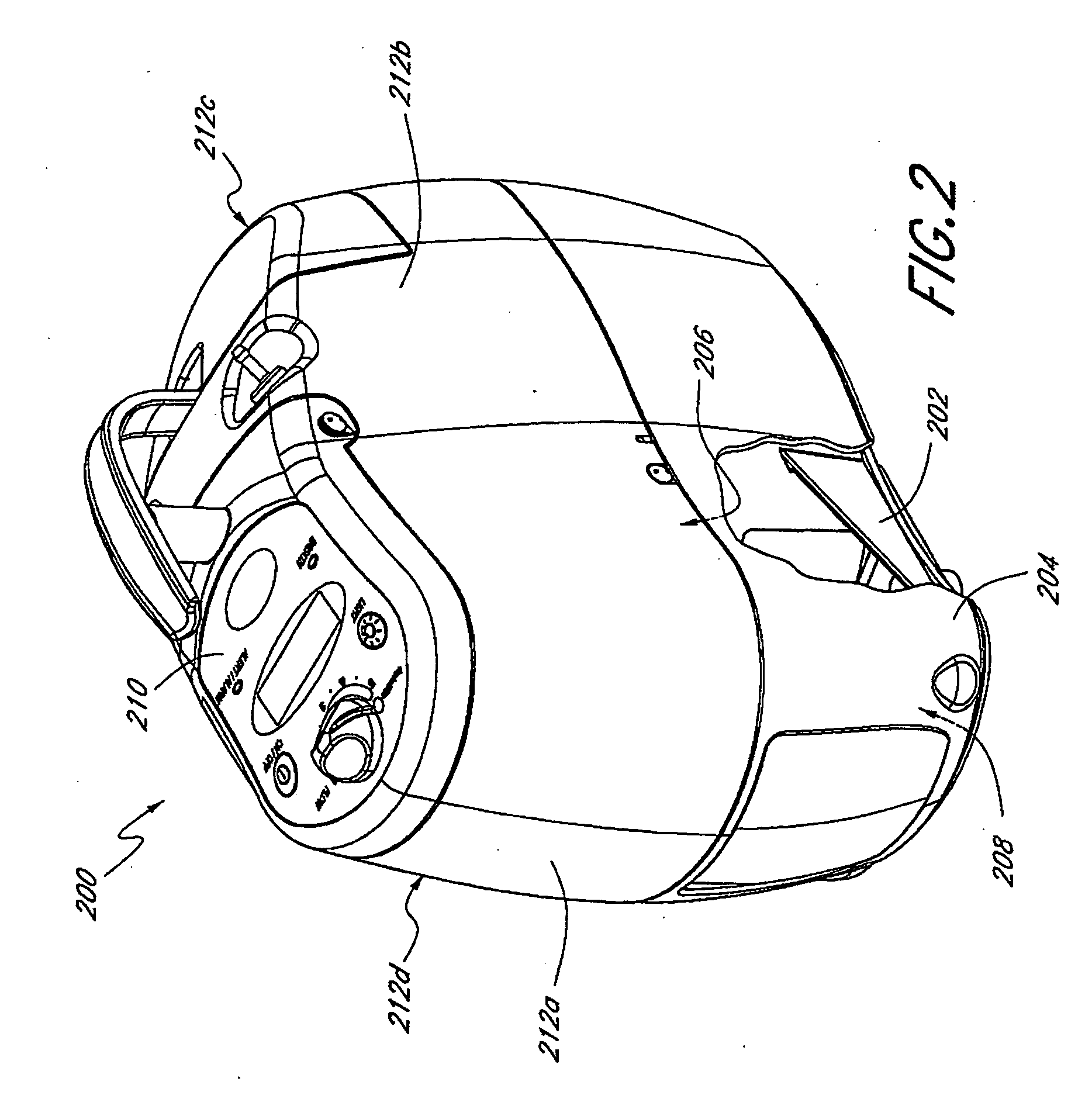
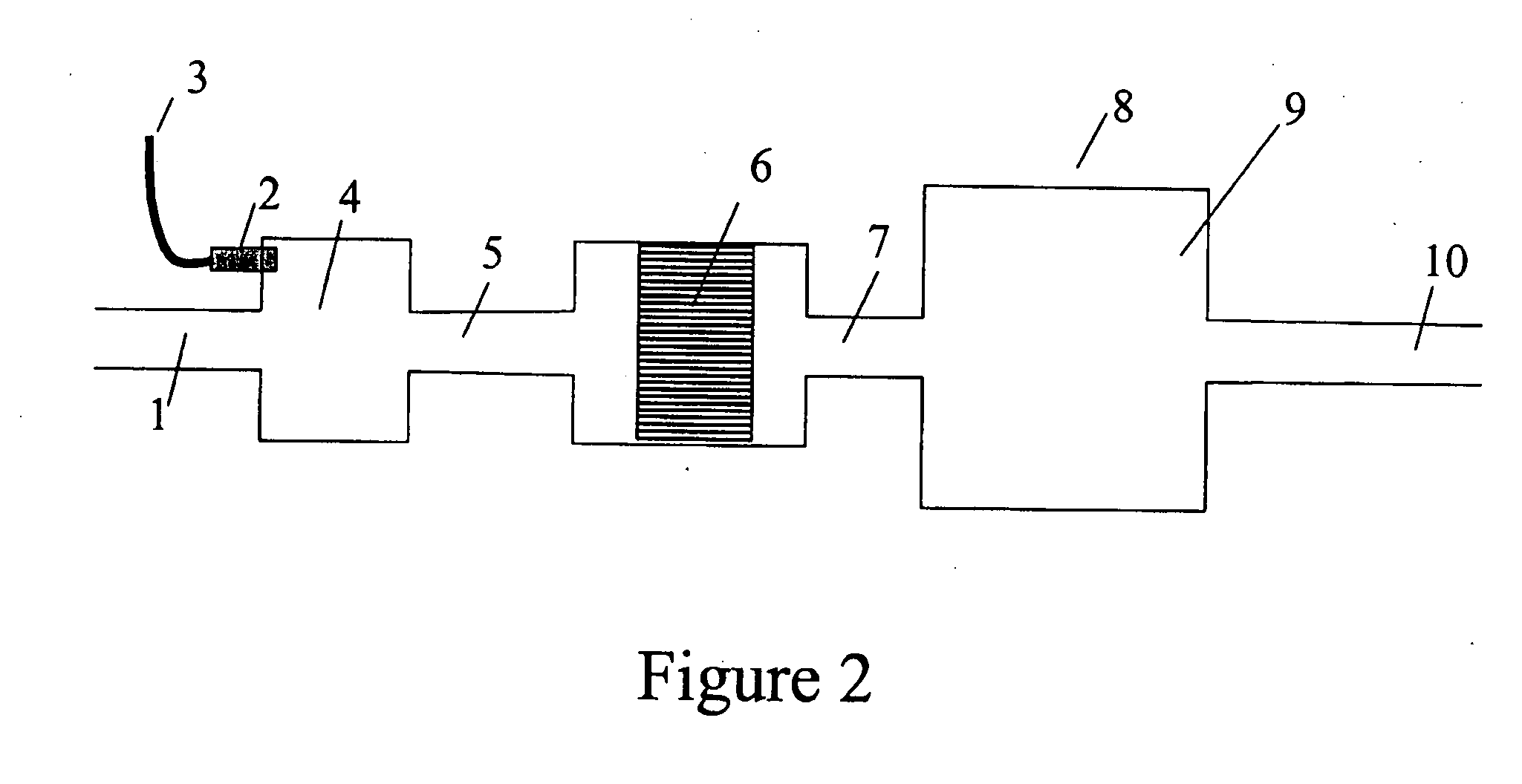
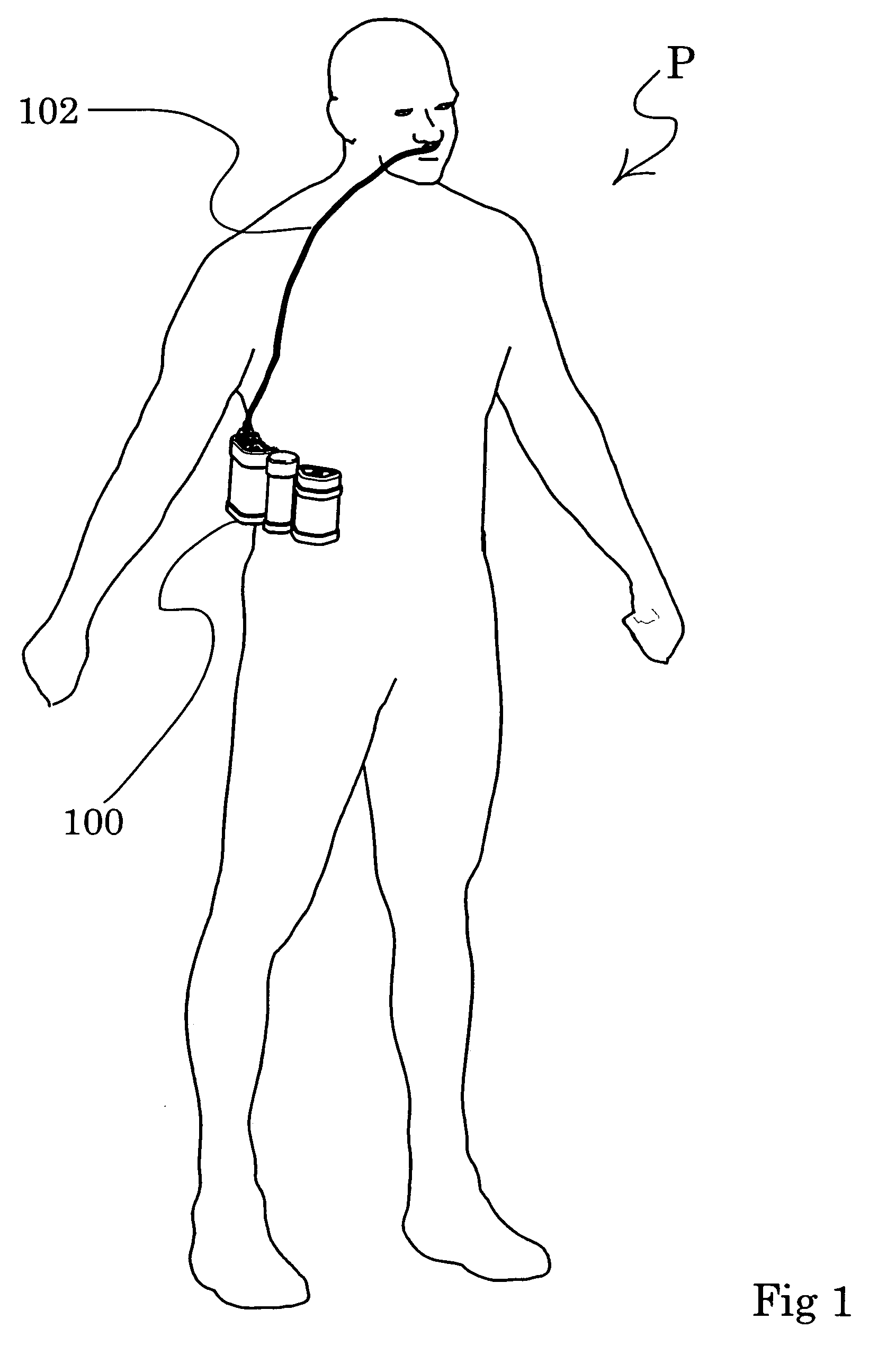
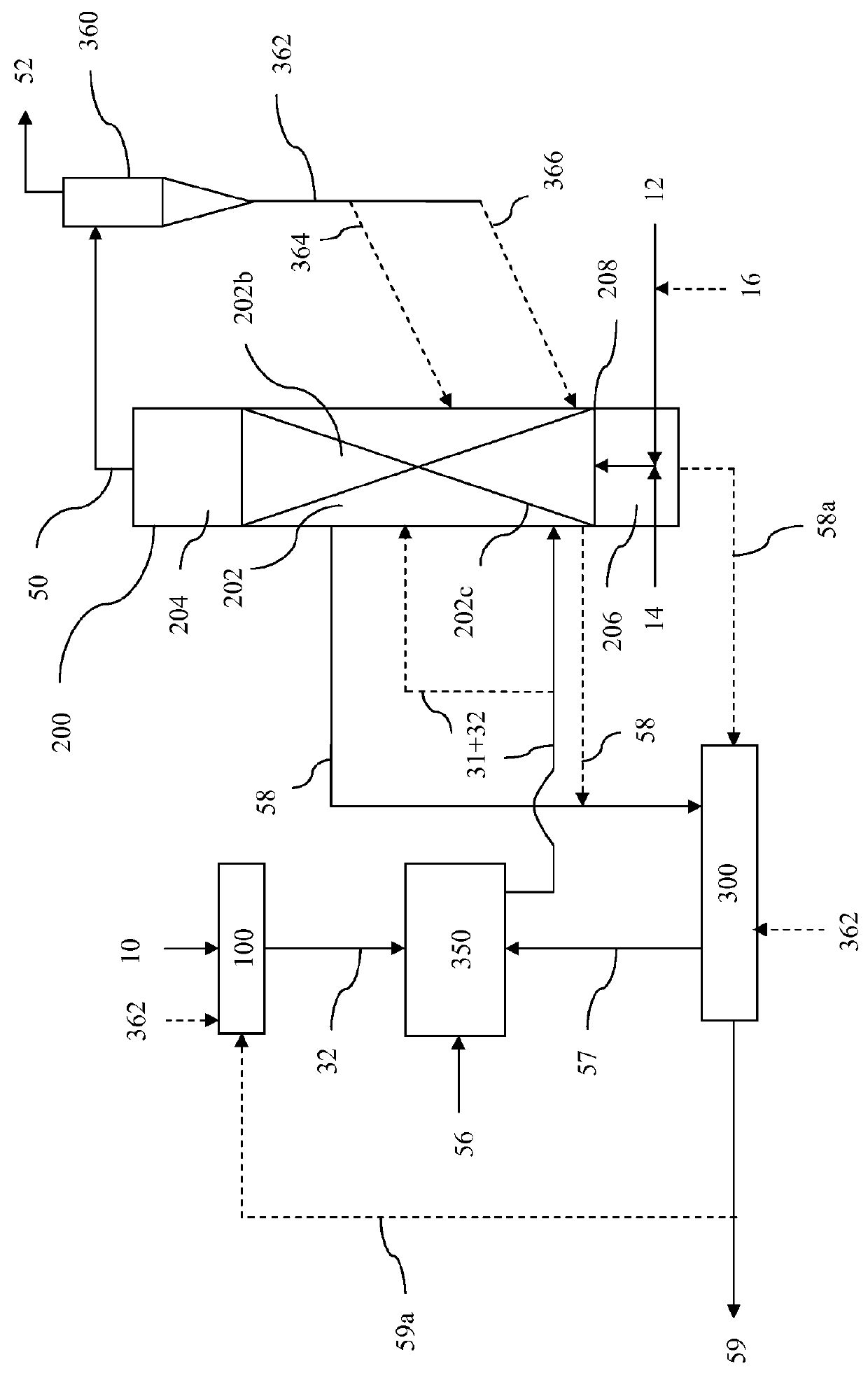
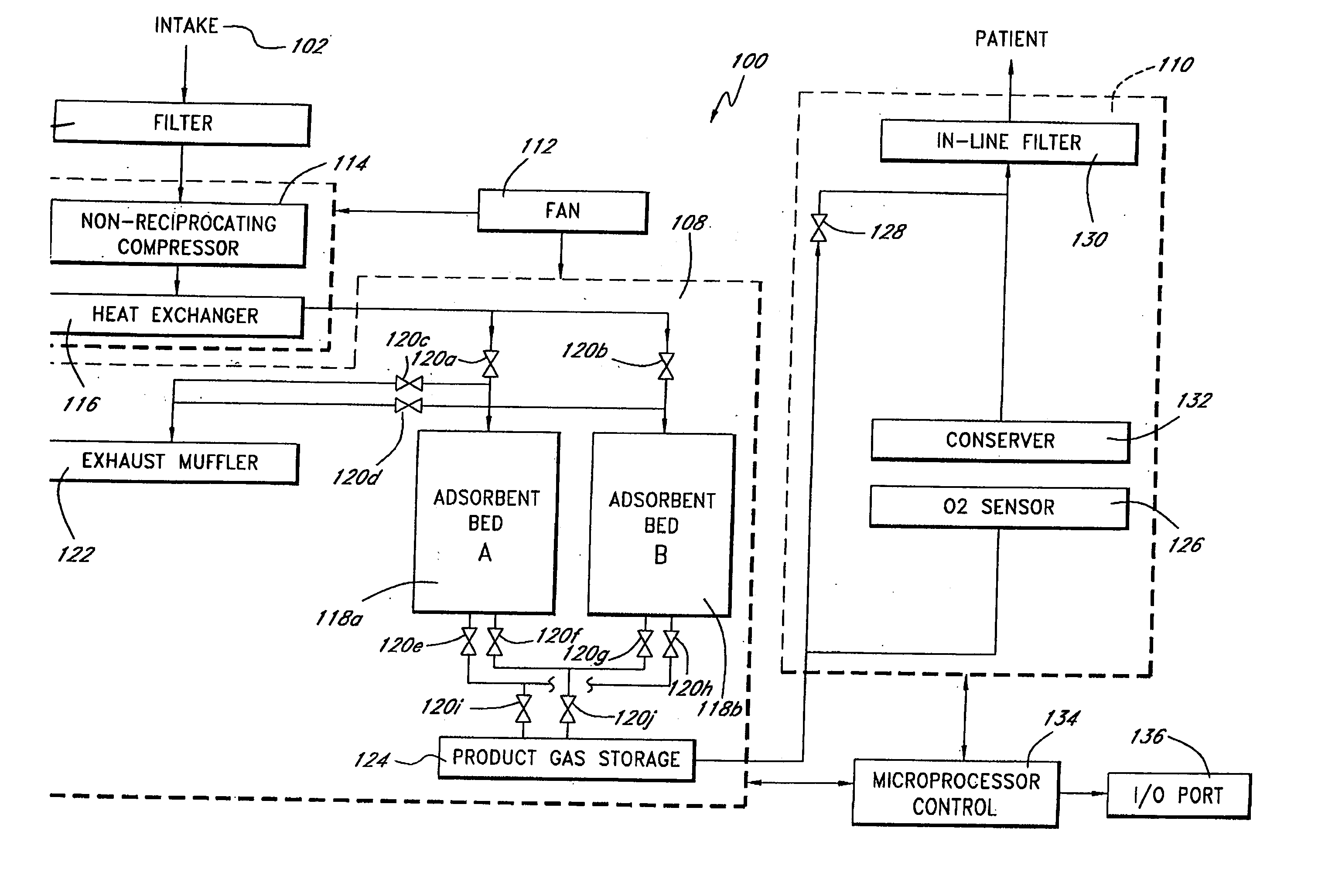
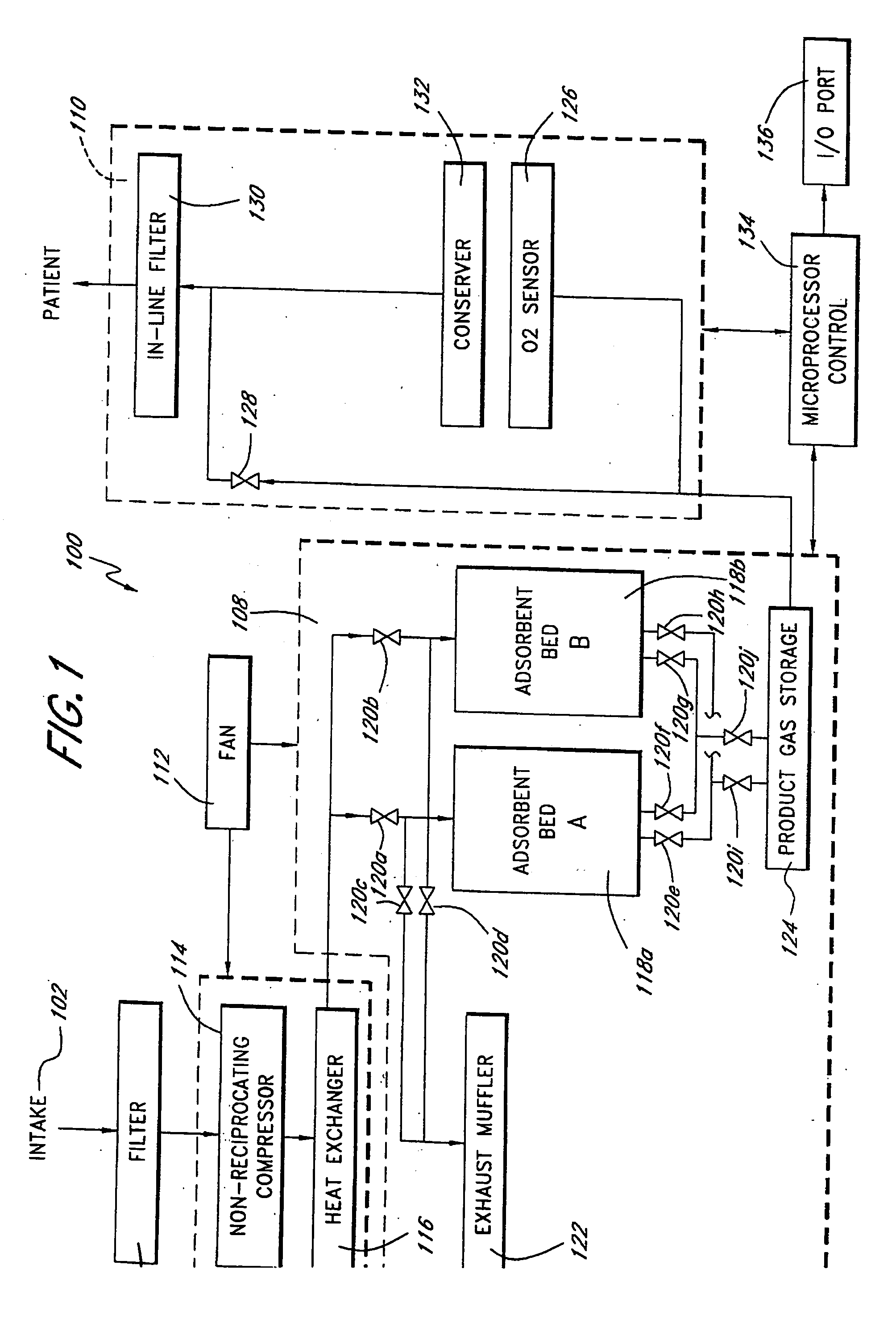
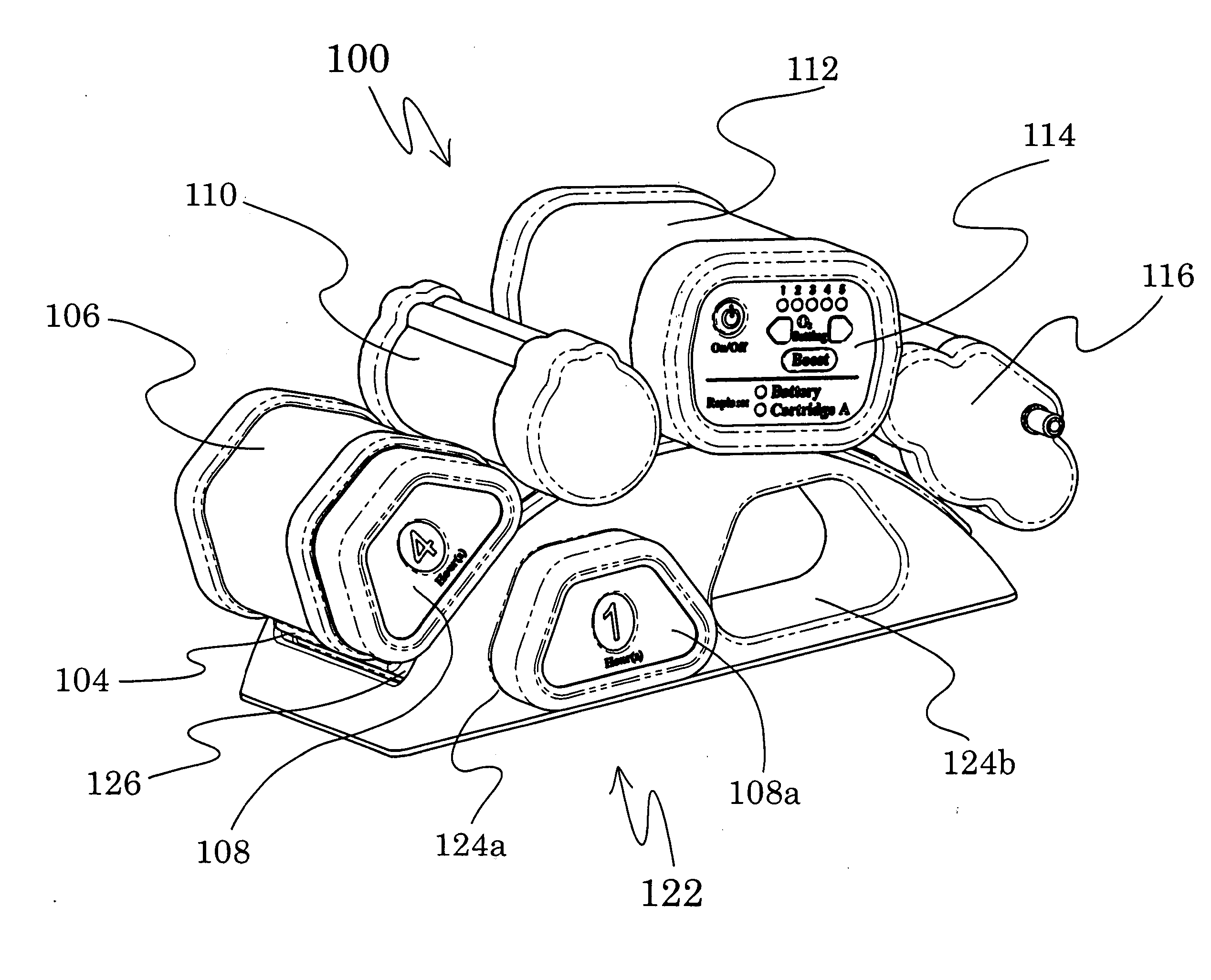
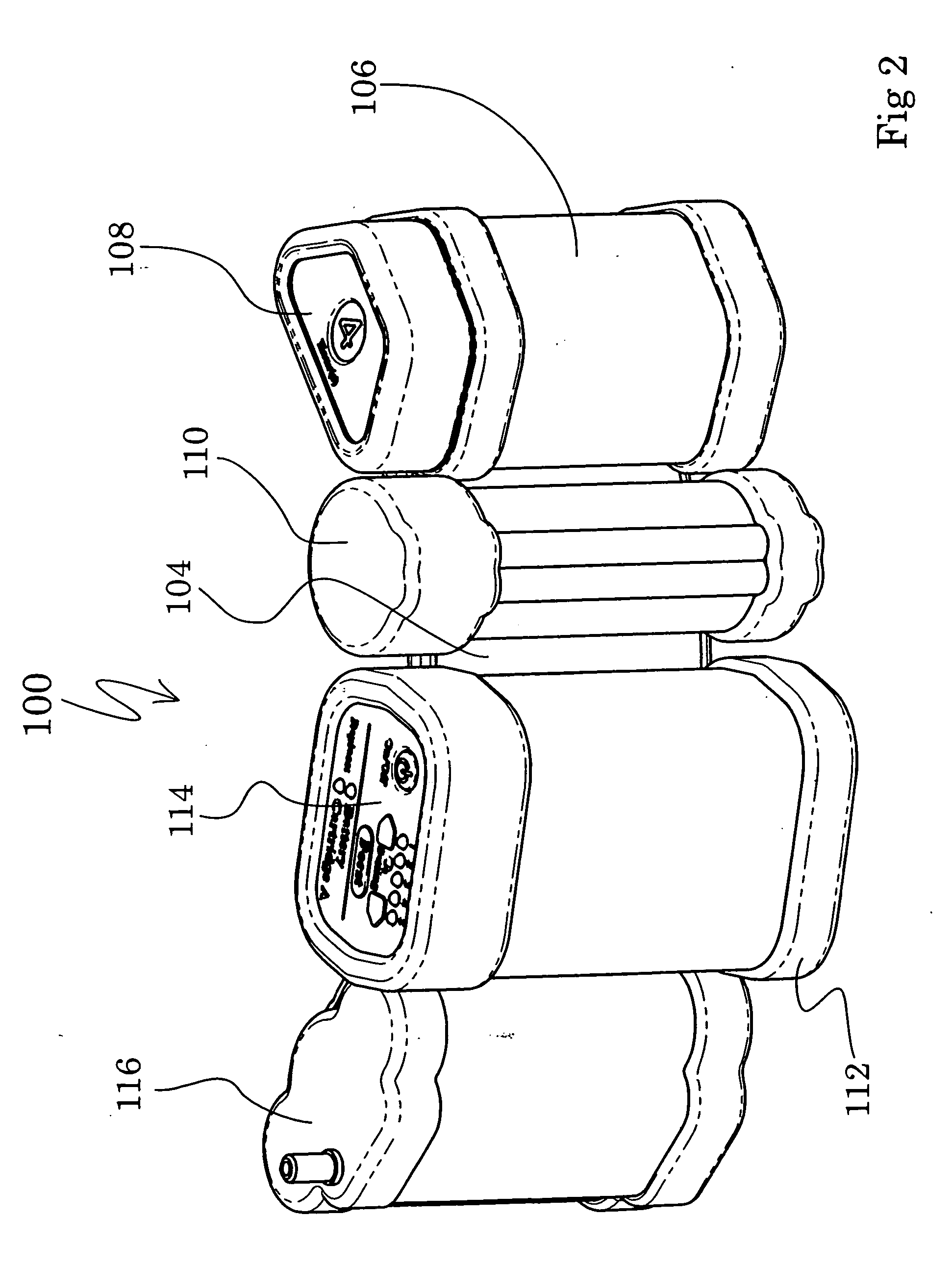
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
Method of depositing low k films using an oxidizing plasma
InactiveUS6593247B1Semiconductor/solid-state device detailsSolid-state devicesTrimethylsilaneSilicon oxide
A silicon oxide layer is produced by plasma enhanced oxidation of an organosilicon compound to deposit films having a carbon content of at least 1% by atomic weight. Films having low moisture content and resistance to cracking are deposited by introducing oxygen into the processing chamber at a flow rate of less than or equal to the flow rate of the organosilicon compounds, and generating a plasma at a power density ranging between 0.9 W / cm2 and about 3.2 W / cm2. An optional carrier gas may be introduced to facilitate the deposition process at a flow rate less than or equal to the flow rate of the organosilicon compounds. The organosilicon compound preferably has 2 or 3 carbon atoms bonded to each silicon atom, such as trimethylsilane, (CH3)3SiH. An oxygen rich surface may be formed adjacent the silicon oxide layer by temporarily increasing oxidation of the organosilicon compound.
Owner:APPLIED MATERIALS INC
Portable gas fractionalization system
InactiveUS7066985B2Reduce heat loadReduce noiseCombination devicesAuxillary pretreatmentLow noiseProcess engineering
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
Portable gas fractionalization system
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
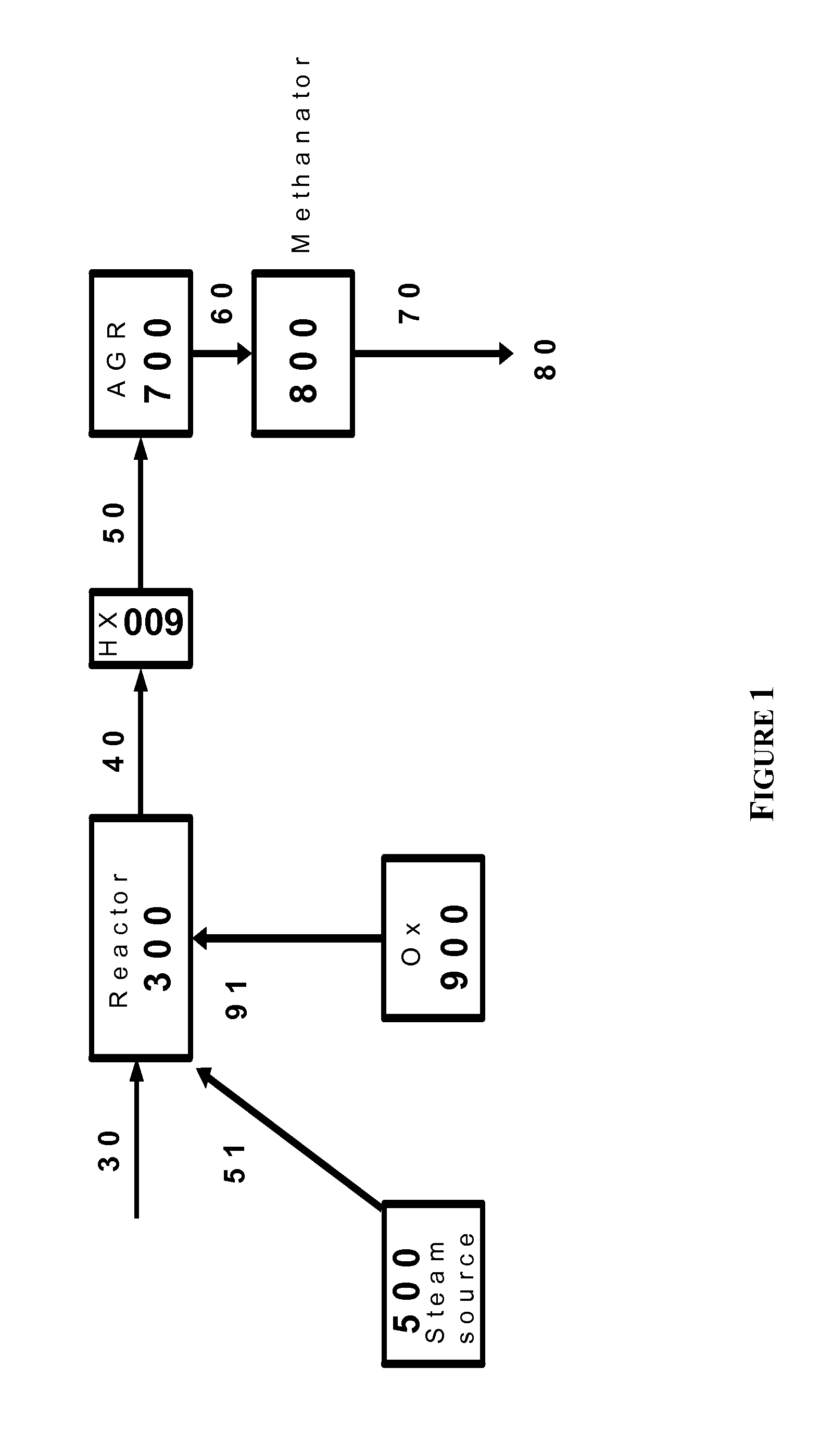
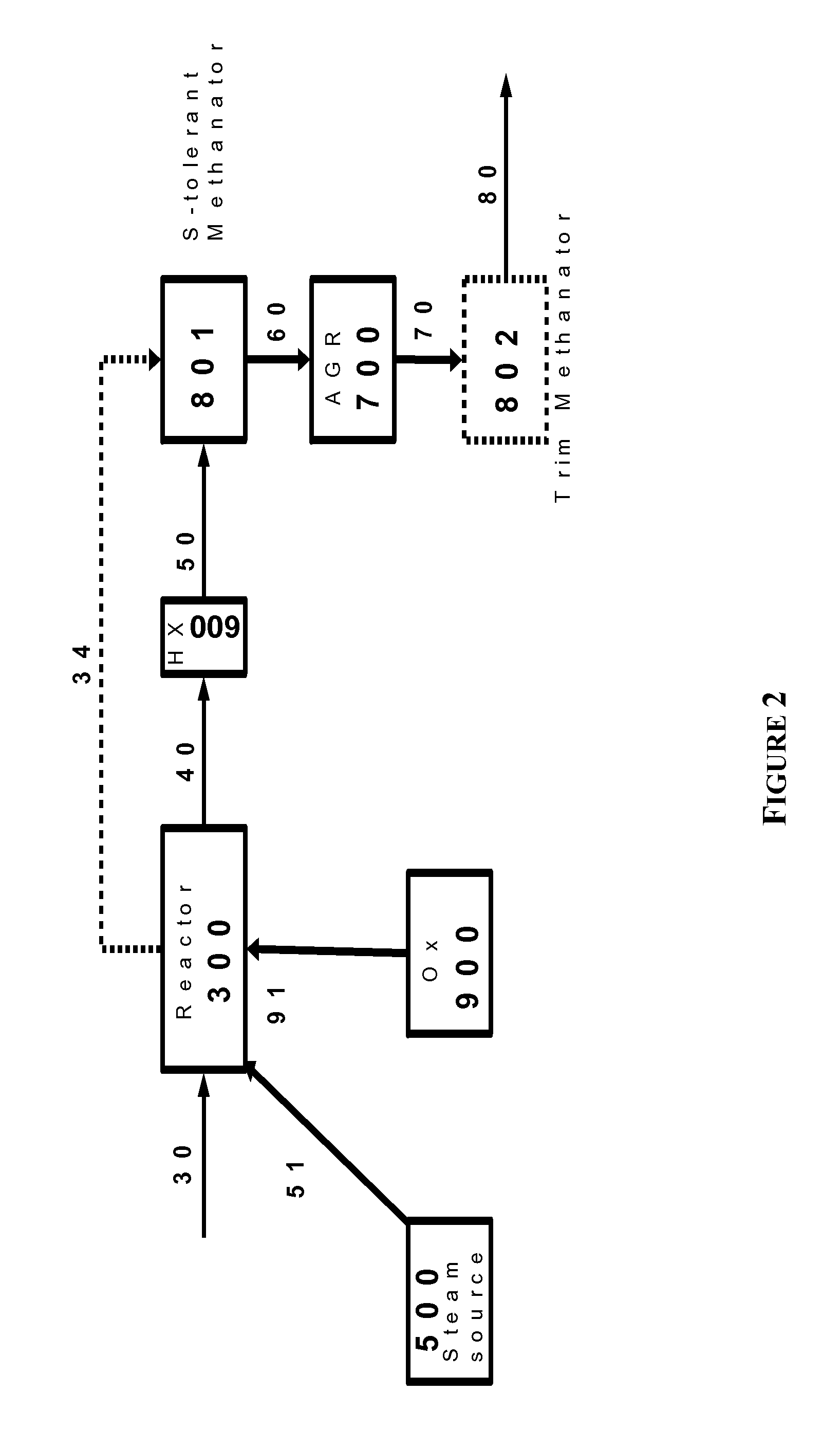
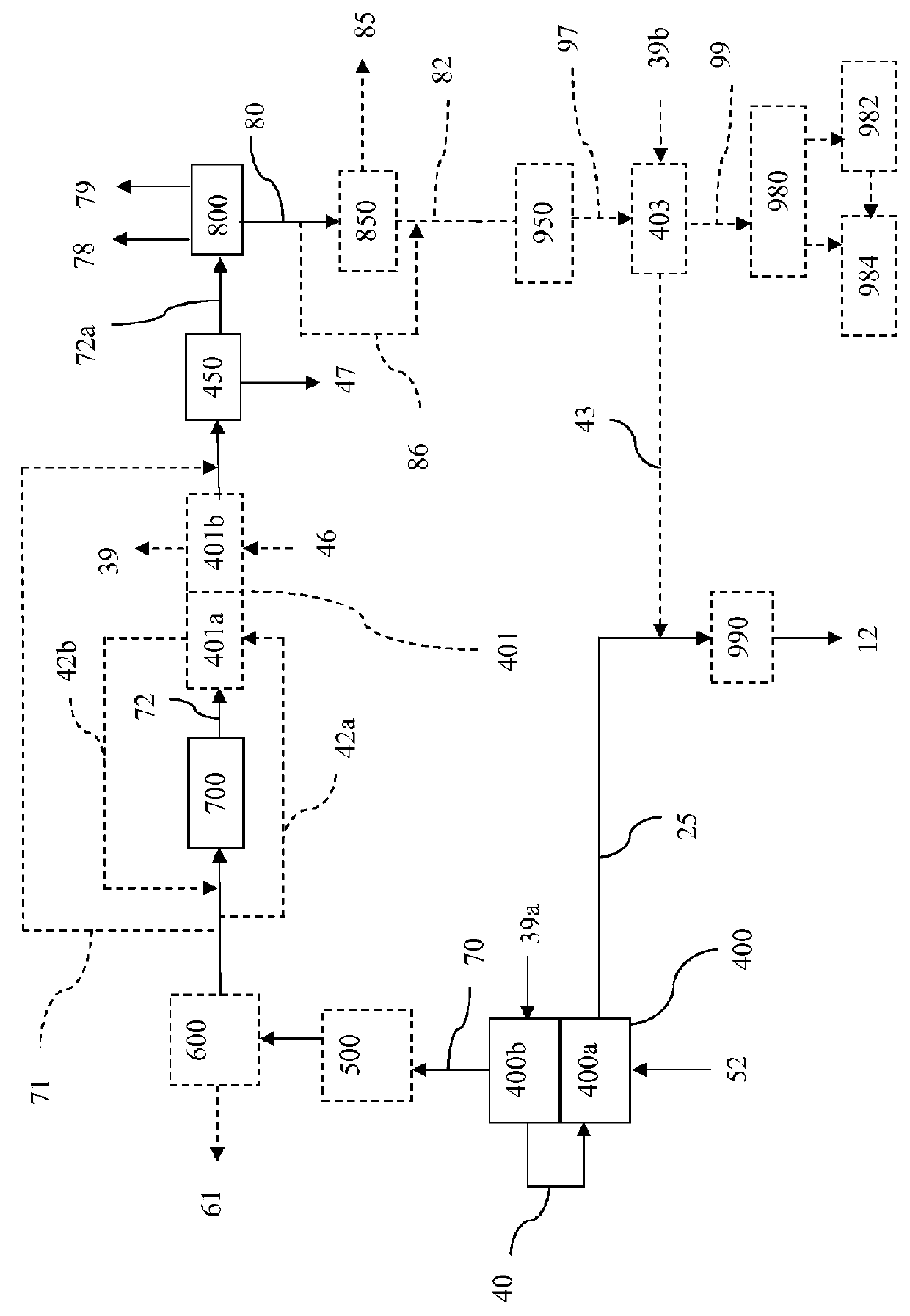
Processes for Hydromethanation of a Carbonaceous Feedstock
ActiveUS20100287835A1Increase the amount of carbonIncrease volumeHydrocarbon by isomerisationHydrogen separationMethanationOrganic chemistry
The present invention relates to processes for preparing gaseous products, and in particular methane, via the hydromethanation of a carbonaceous feedstock in the presence of steam, syngas, a hydromethanation catalyst and an oxygen-rich gas stream.
Owner:SURE CHAMPION INVESTMENT LTD
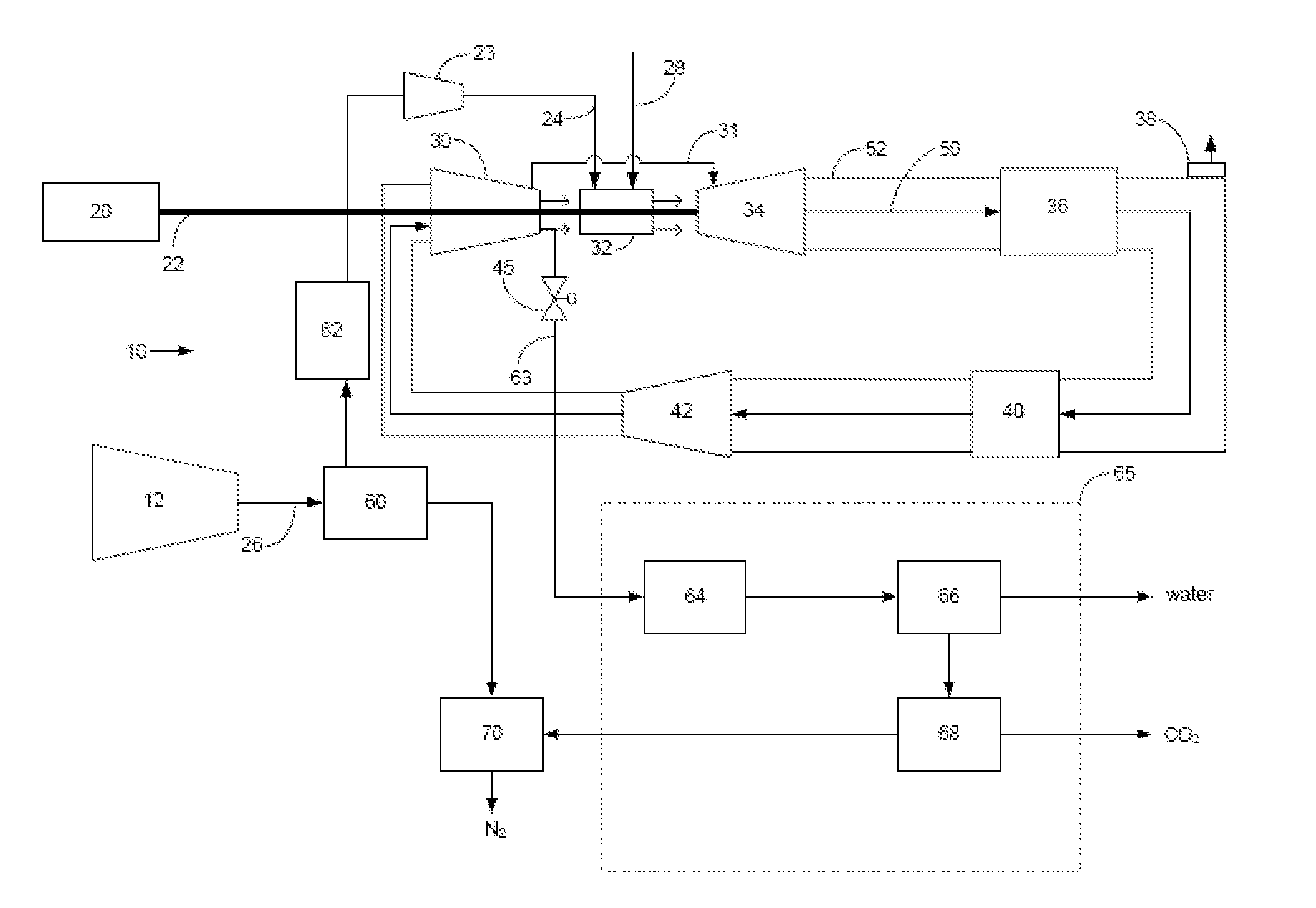
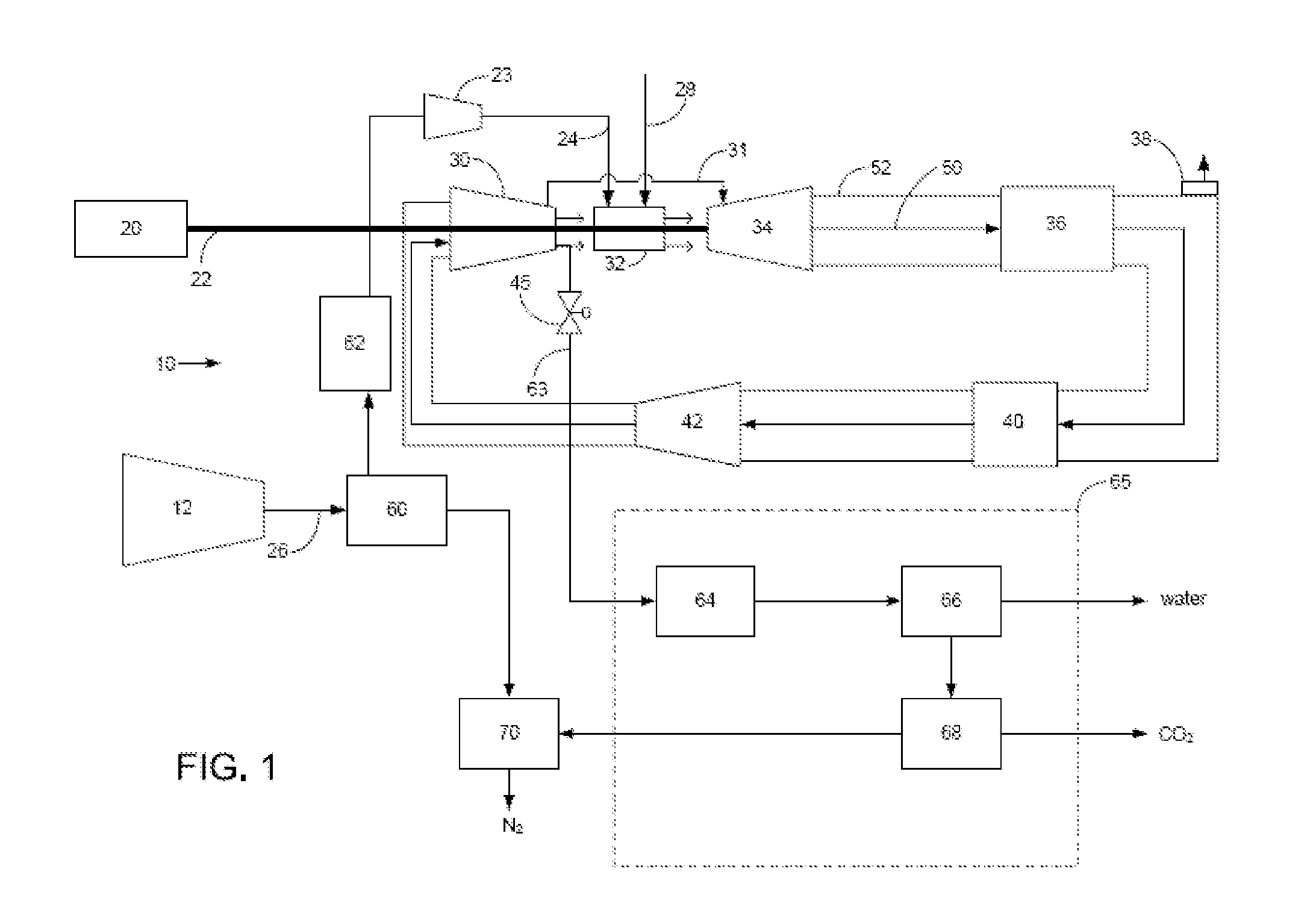
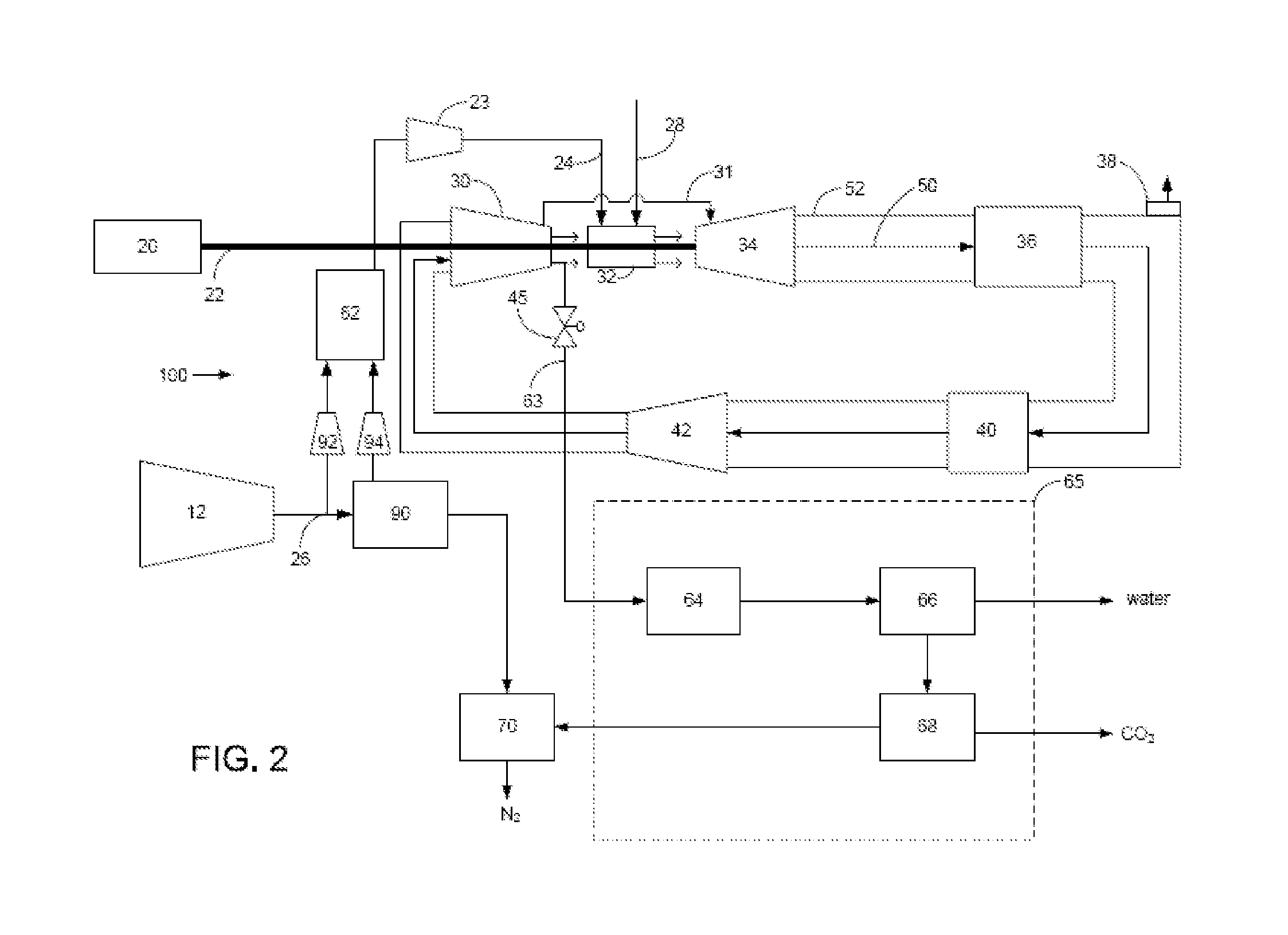
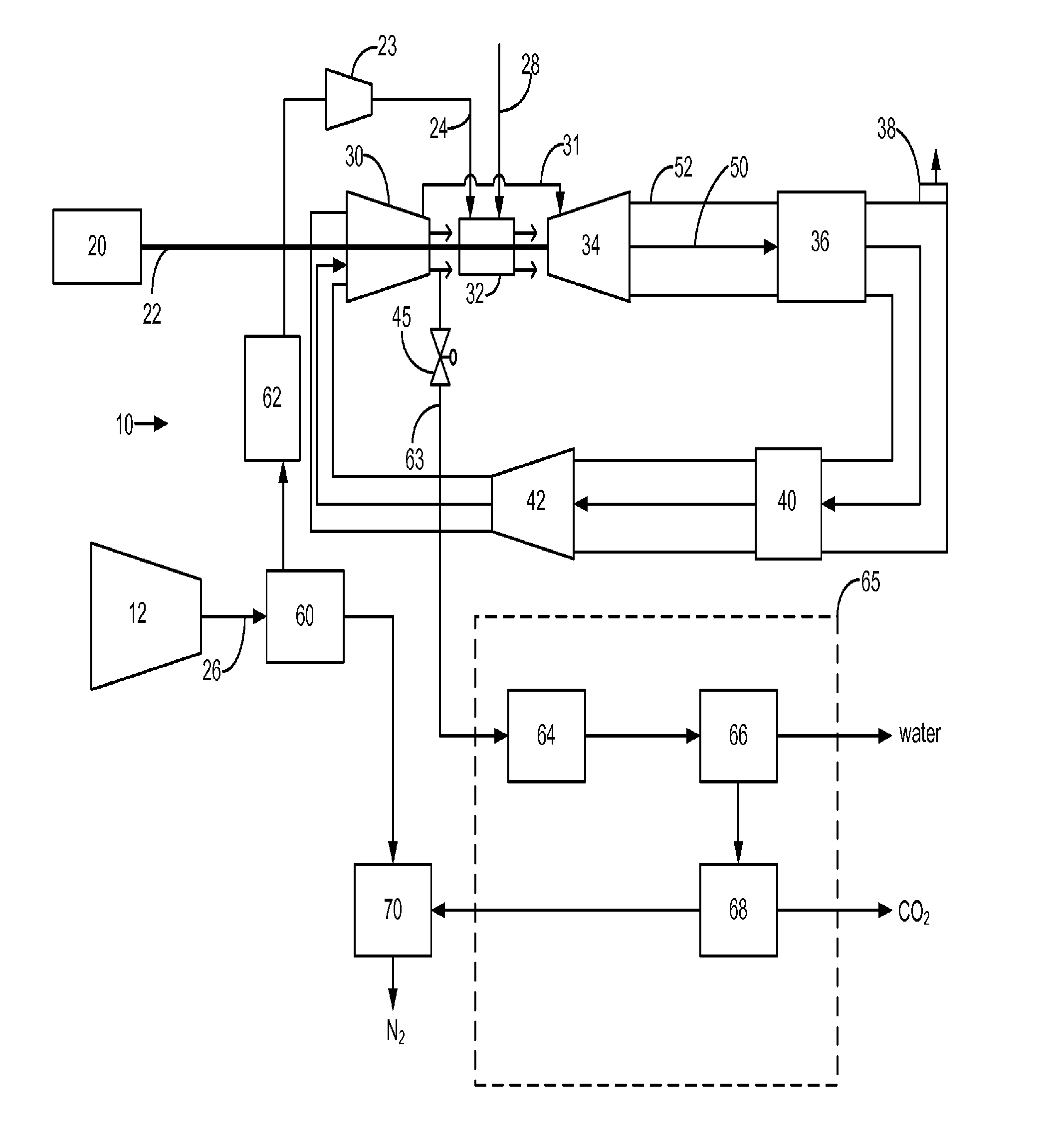
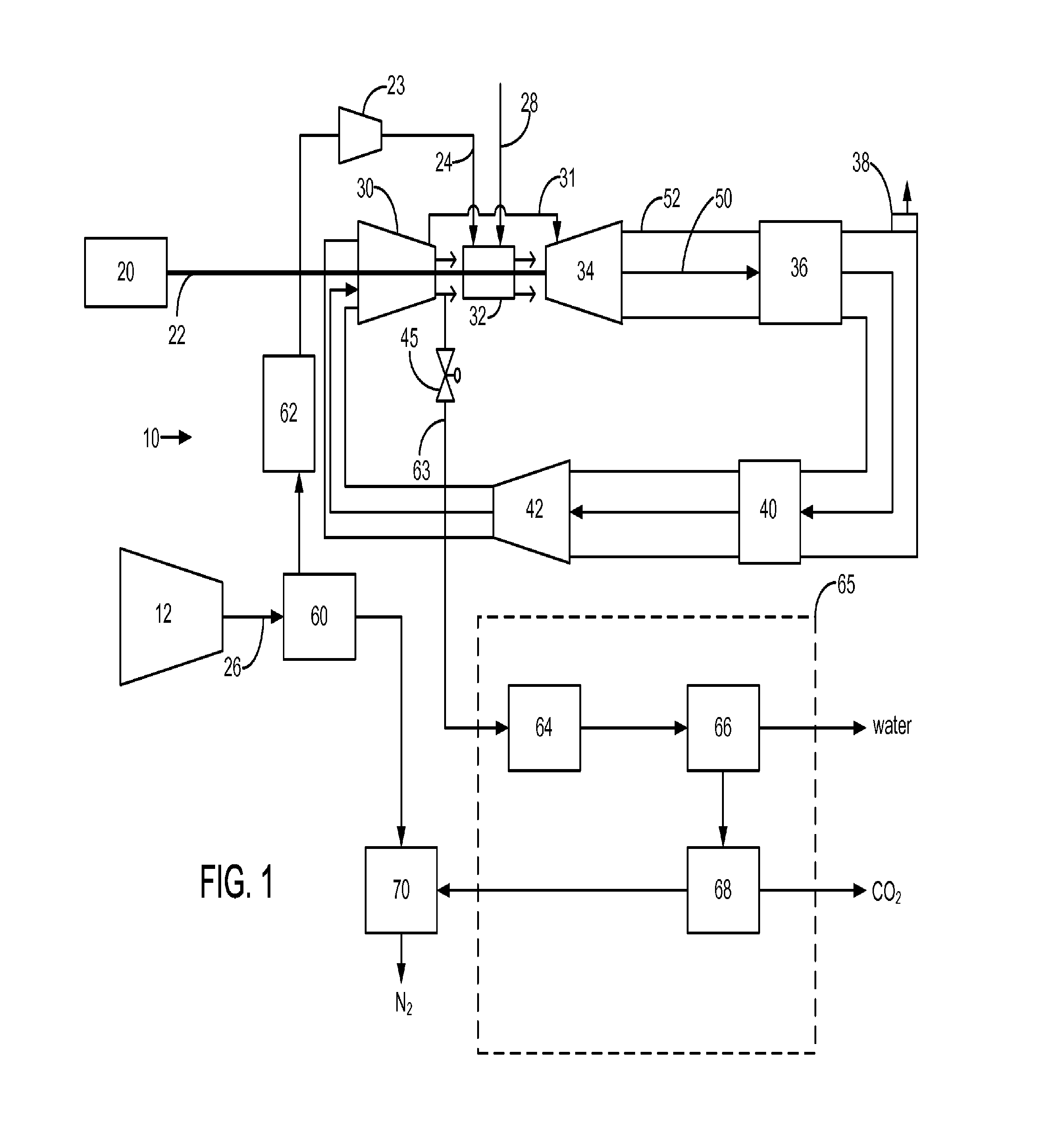
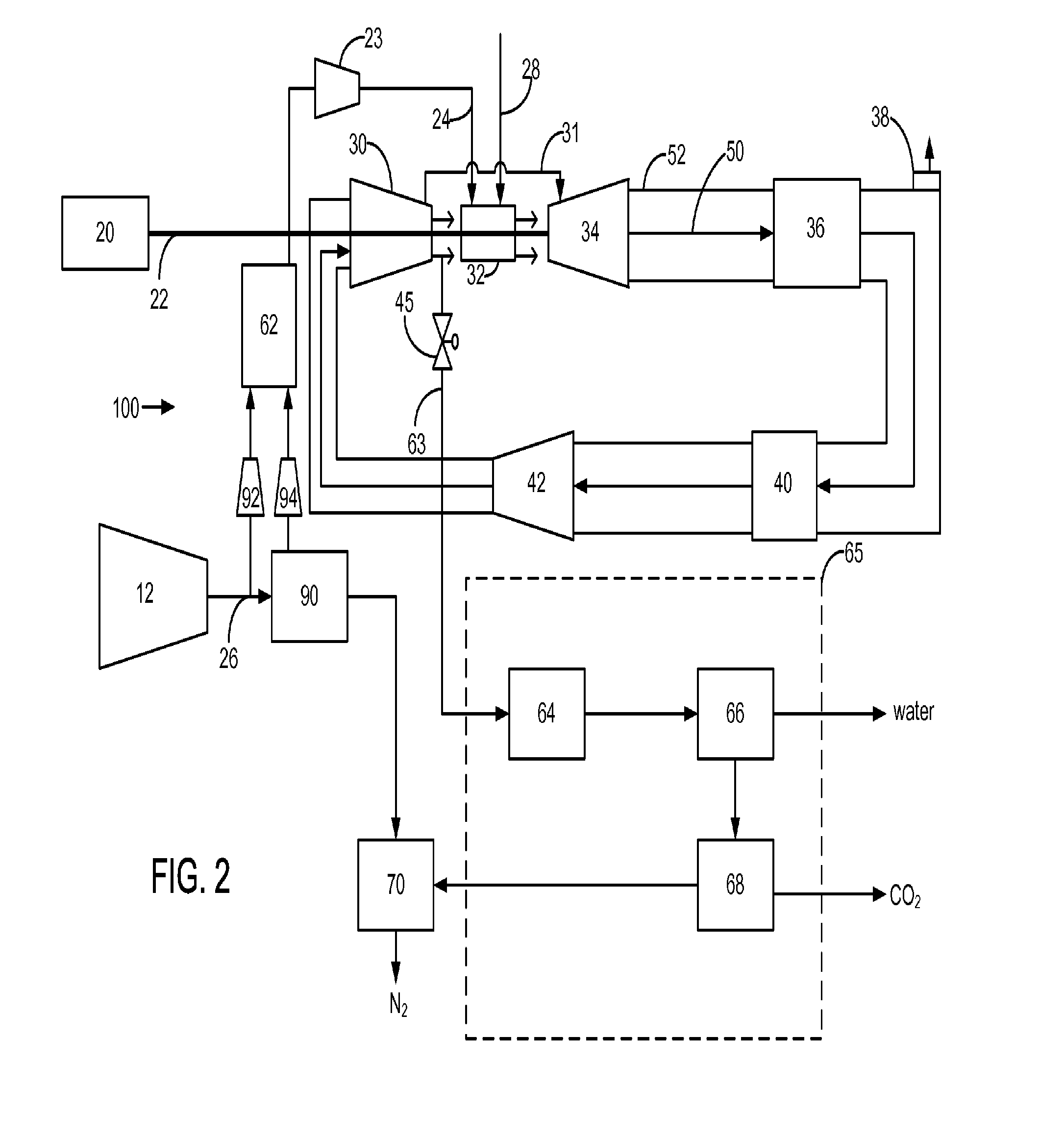
Power plant and method of operation
A power plant and method of operation is provided. The power plant comprises at least one main air compressor, an oxidizer unit configured to deliver a compressed oxygen-rich gas flow to at least one gas turbine assembly. Each assembly comprises a turbine combustor for mixing the compressed oxygen-rich gas flow with a recirculated gas flow and a fuel stream to burn a combustible mixture and form the recirculated gas flow. The assembly also comprises a recirculation loop for recirculating the recirculated gas flow from a turbine to a turbine compressor. The assembly further comprises a recirculated gas flow extraction path for extracting a portion of the recirculated gas flow from the assembly and delivering this to a gas separation system. The gas separation system separates the portion of the recirculated gas flow into a nitrogen portion and a carbon dioxide portion.
Owner:GENERAL ELECTRIC CO
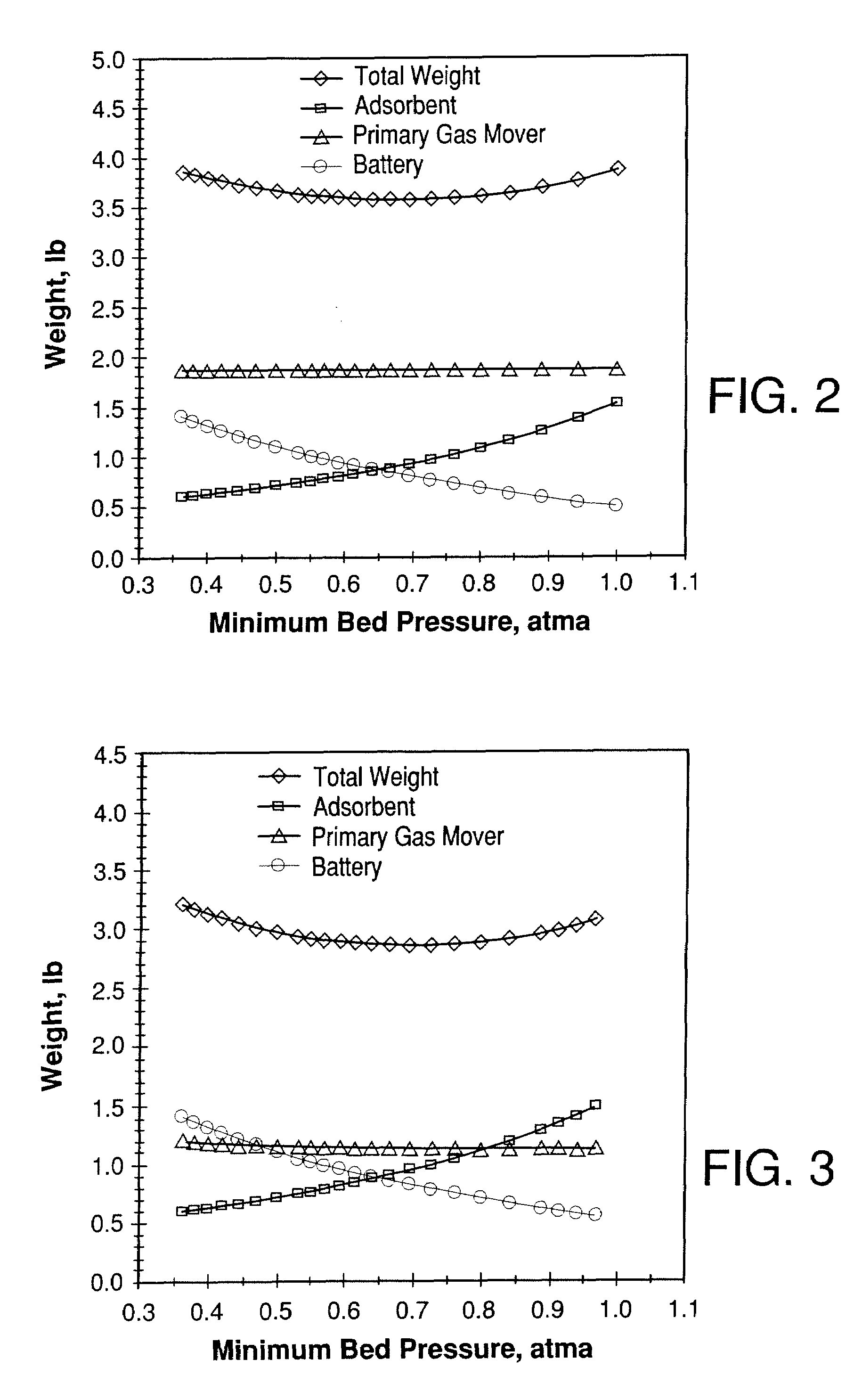
Weight-optimized portable oxygen concentrator
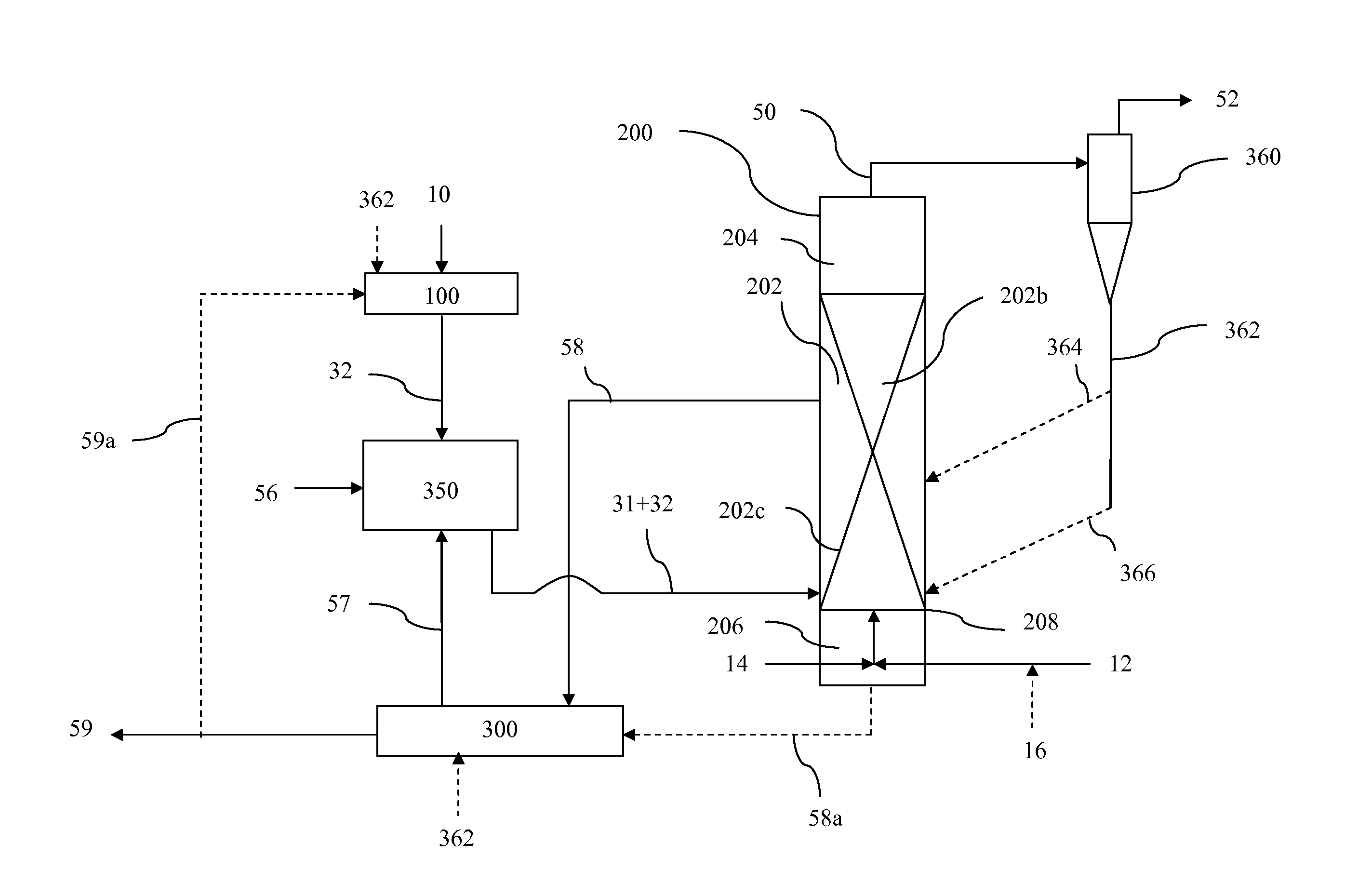
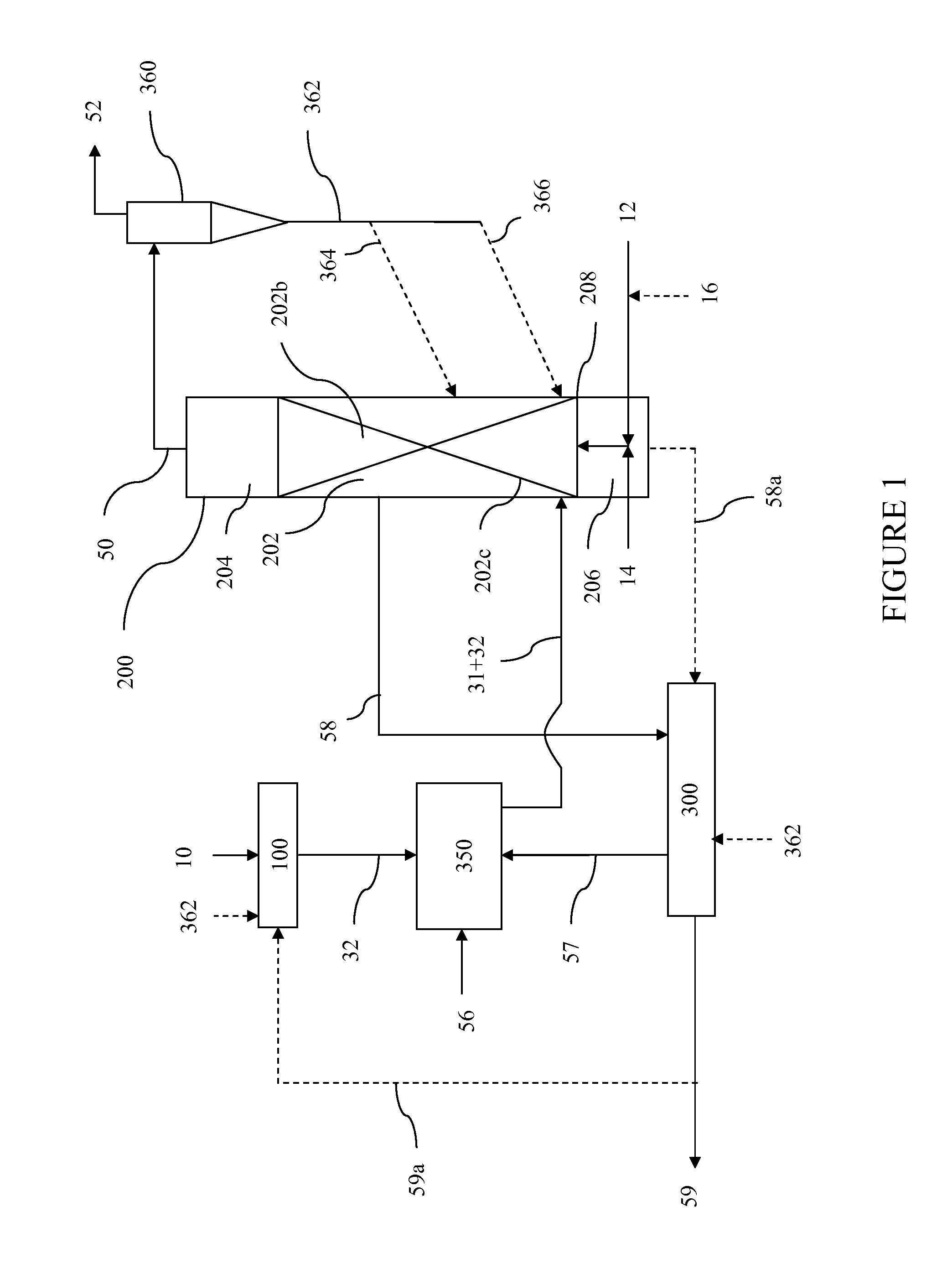
System for producing an oxygen-rich gas comprising (a) a primary gas mover including a first and a second compressor, wherein the primary gas mover is characterized by a weight Wp; (b) a drive motor adapted to drive the first and second compressors; (c) a rechargeable power supply characterized by a weight, Wb; and (d) a pressure / vacuum swing adsorption unit adapted to separate the pressurized feed air into an oxygen-rich product at a product flow rate Fp and an oxygen-depleted waste gas, wherein the adsorption unit comprises a plurality of adsorber beds containing an adsorbent and characterized by a total adsorbent weight Wa; and wherein the combined weight, Wt, of the adsorbent, the primary gas mover, and the rechargeable power supply is characterized by the expression0.75 Fp<Wt<2.02 Fpwhere Fp is in liters per min (at 23° C. and 1 atma pressure), and Wa, Wp, and Wb are in pounds.
Owner:INOGEN INC
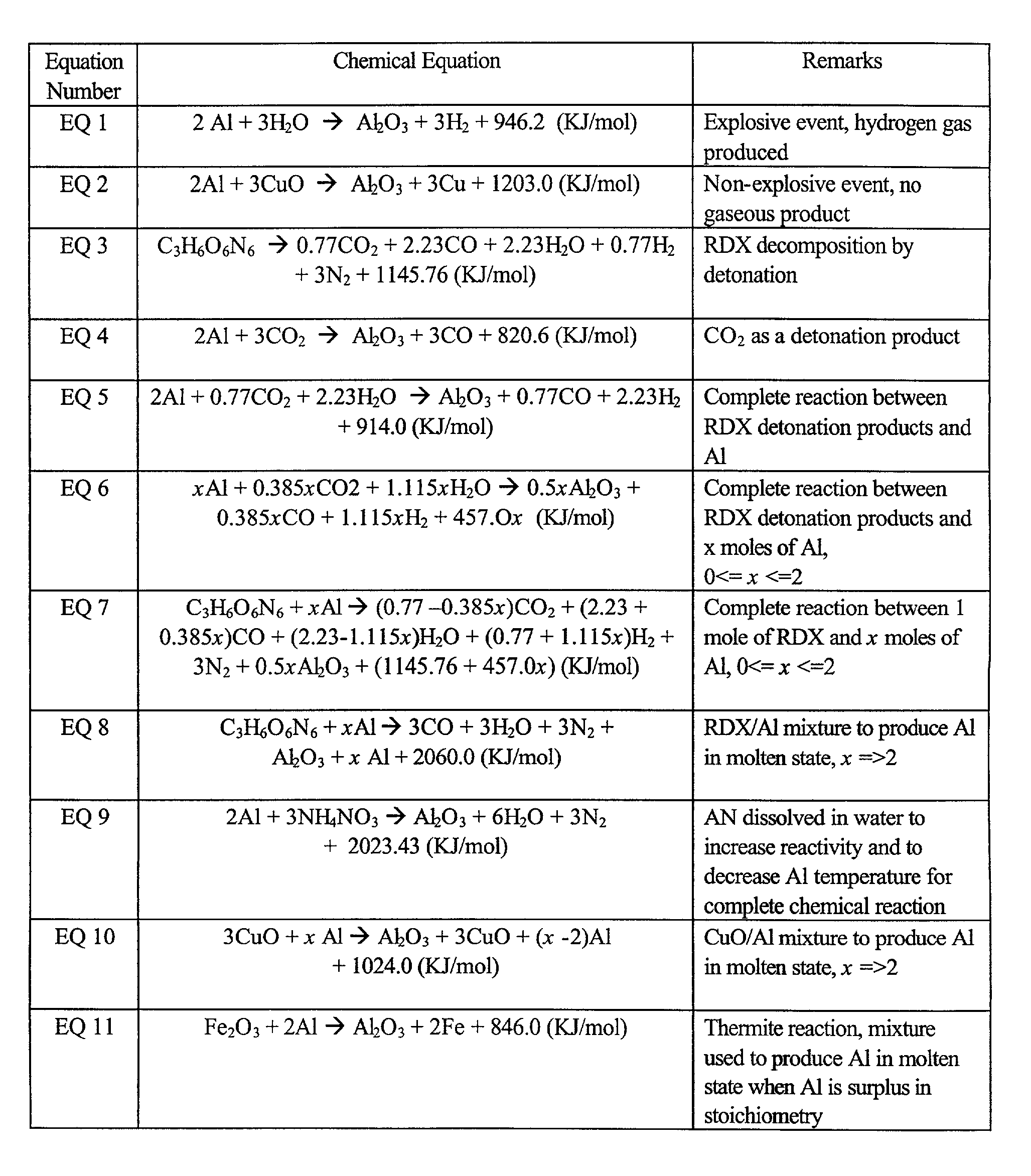
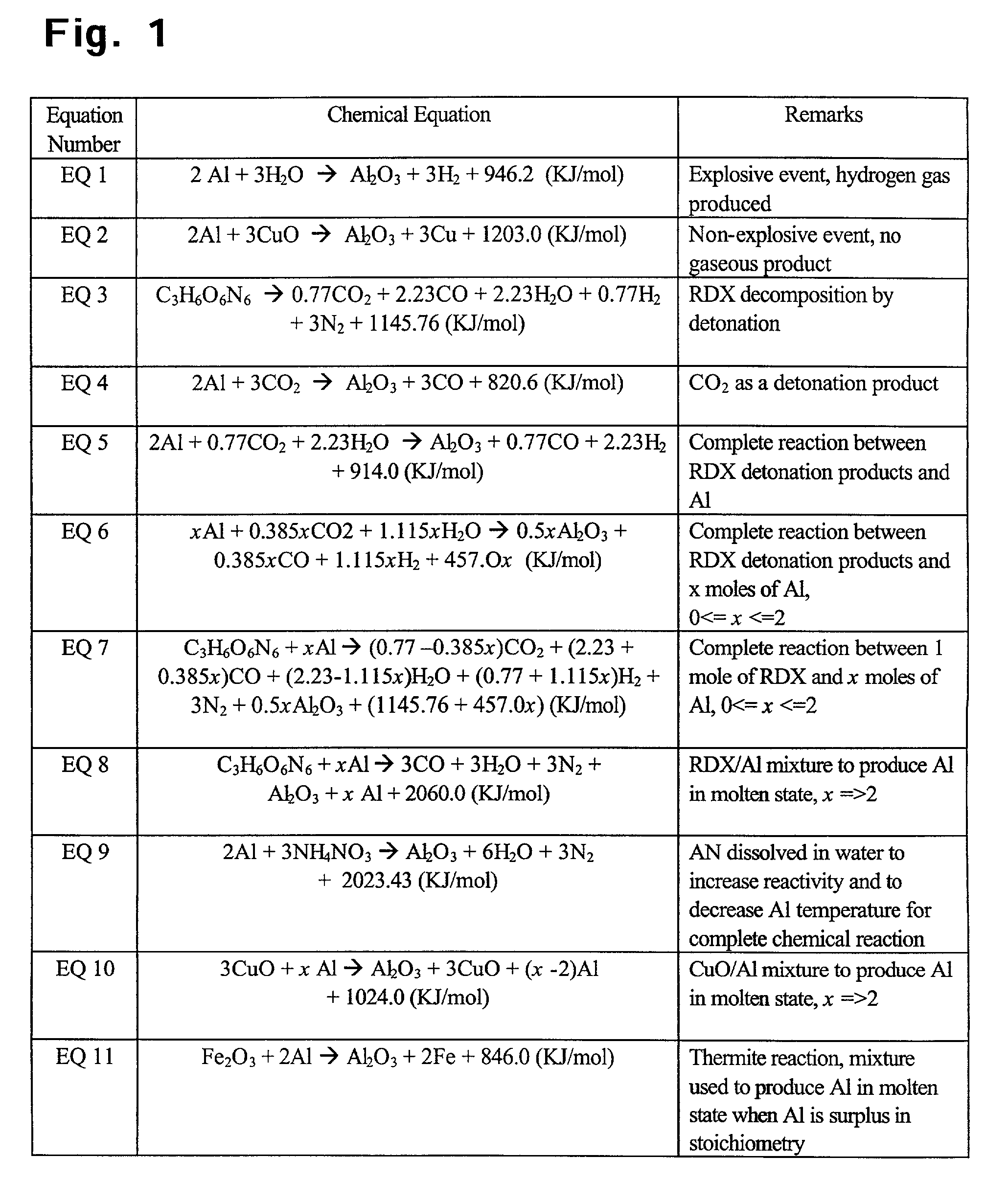
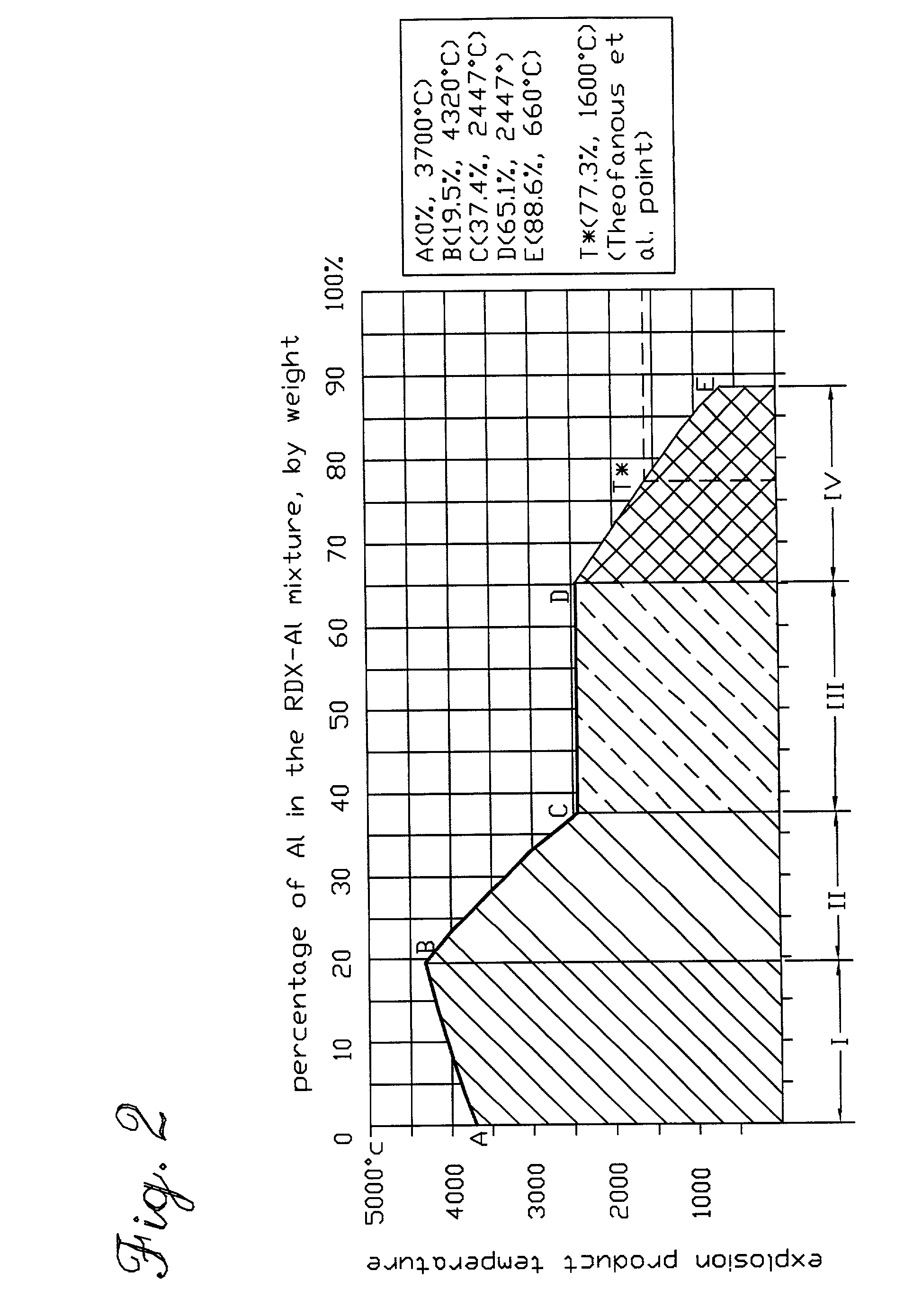
Use of aluminum in perforating and stimulating a subterranean formation and other engineering applications
InactiveUS7393423B2More energy outputImprove mechanical propertiesExplosive chargesBlasting cartridgesMolten stateThermal energy
A chemical reaction between molten aluminum and an oxygen carrier such as water to do useful work is disclosed, and in particular two chemical methods to obtain aluminum in its molten state. One is to detonate a HE / Al mixture with surplus Al in stoichiometry, and the other is to use an oxidizer / Al mixture with surplus Al in stoichiometry. Additionally, there is a physical method of shocking and heating Al using high temperature reaction products. The produced Al in its liquid form is forced to react with an oxygen carrying liquid (e.g. water), giving off heat and releasing hydrogen gas or other gaseous material. A water solution of some oxygen-rich chemicals (e.g. ammonium nitrate) can be advantageously used in place of water. A shaped charge is also disclosed having a liner that contains aluminum, propelled by a high explosive such as RDX or its mixture with aluminum powder. Some aluminum in its molten state is projected into the perforation and forced to react with water that also enters the perforation, creating another explosion, fracturing the crushed zone of the perforation and initializing cracks. Another shaped charge is shown having a liner of energetic material such as a mixture of aluminum powder and a metal oxide. Upon detonation, the collapsed liner carries kinetic and thermal energy. Also shown are methods to build and to detonate or fire explosive devices in an oxygen carrying liquid (e.g. water) to perforate and stimulate a hydrocarbon-bearing formation.
Owner:GEODYNAMICS
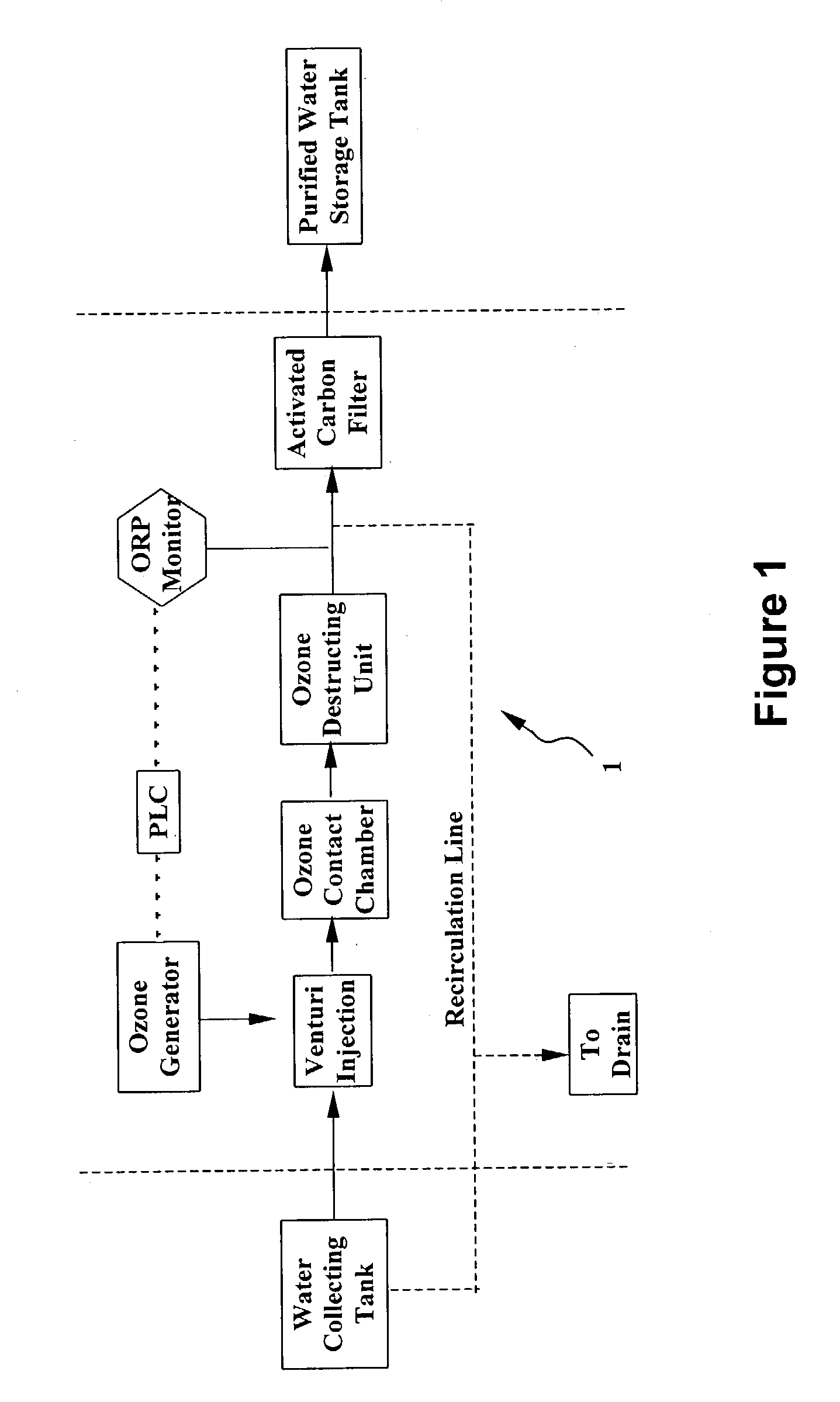
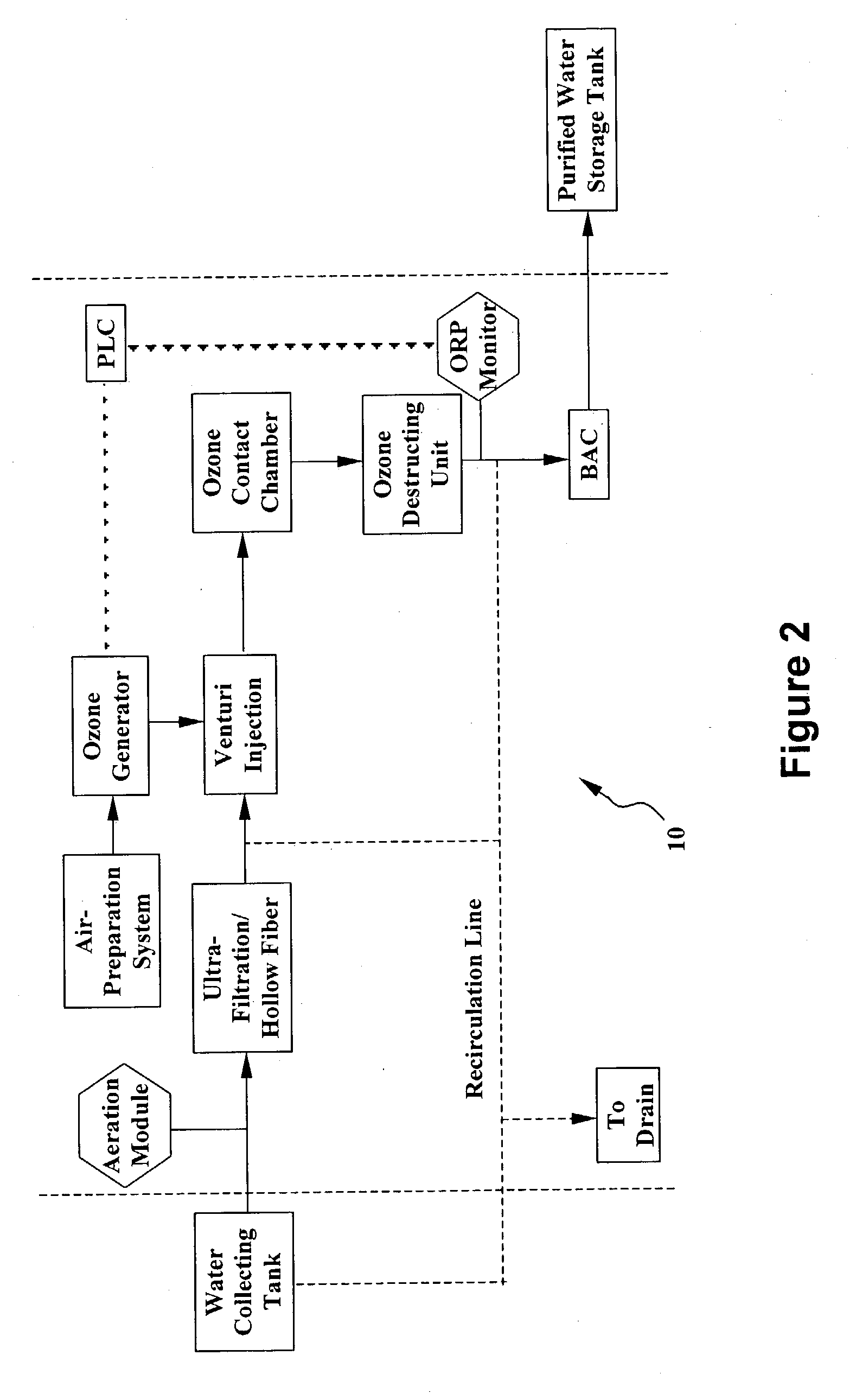
System and method for water purification
InactiveUS20040168989A1Treatment using aerobic processesSedimentation separationBiological activated carbonActivated carbon filtration
A self-contained, portable water purification system, including (a) an ozone supply, (b) an ozone contact chamber for mixing a contaminated or potentially contaminated water stream with ozone generated by such ozone supply, (c) an ozone destruction unit for destructing ozone contained in the water stream and converting said water stream into an oxygen-rich and ozone-depleted water stream, and (d) a downstream biologically active carbon filter, for receiving such oxygen-rich and ozone-depleted water stream and biologically destructing at least a portion of contaminants contained therein.
Owner:TEMPEST ENVIRONMENTAL SYST
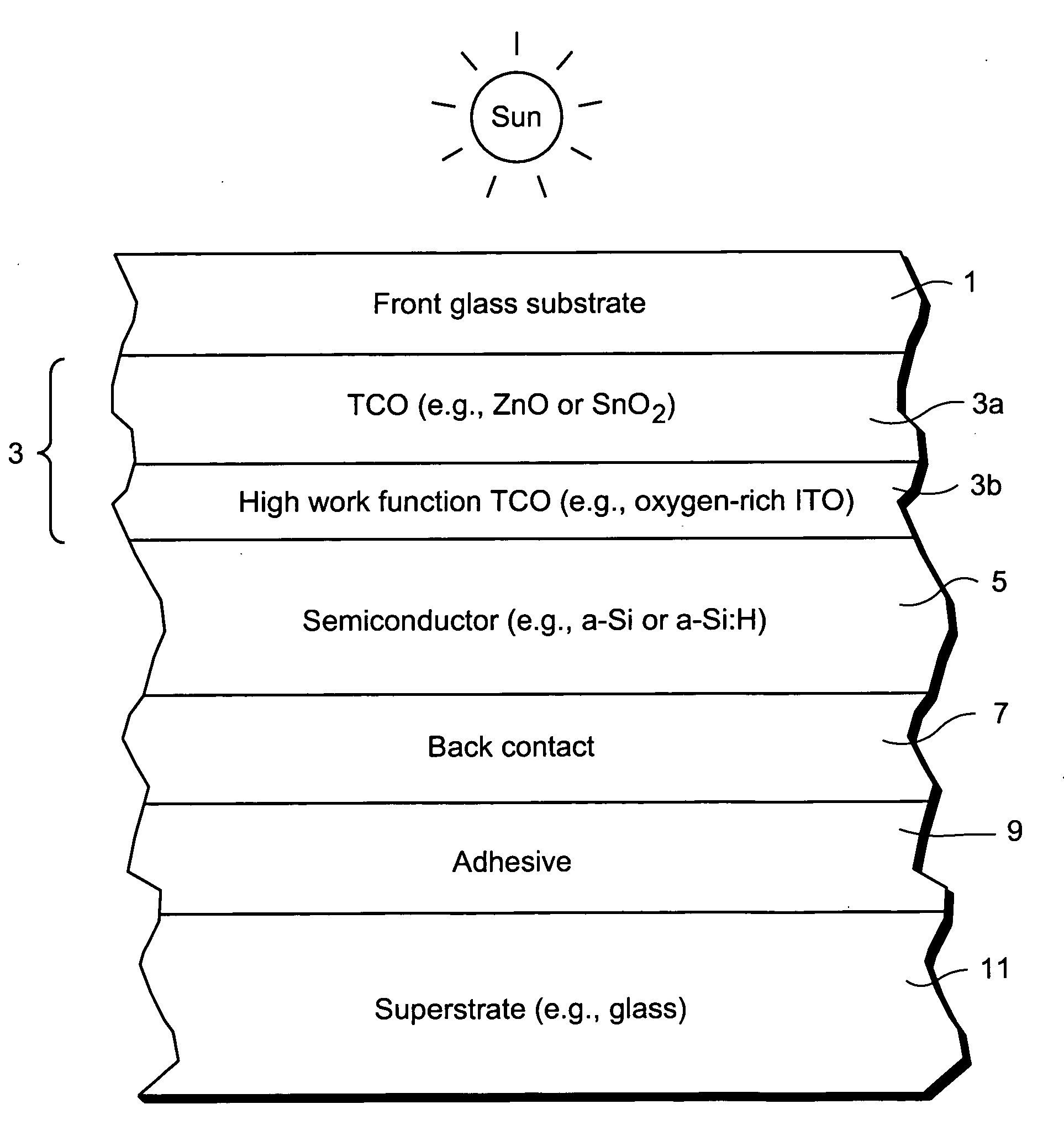
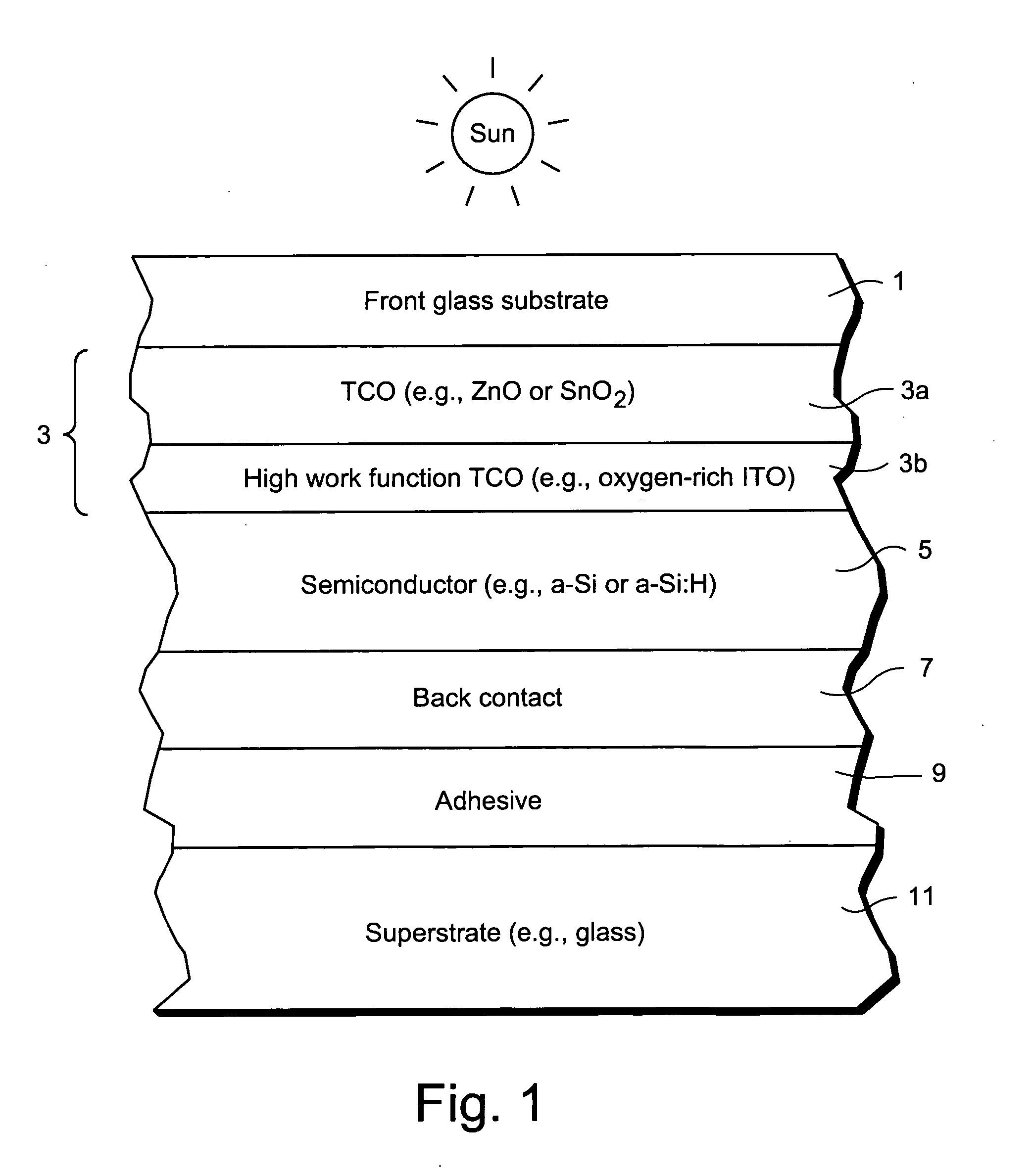
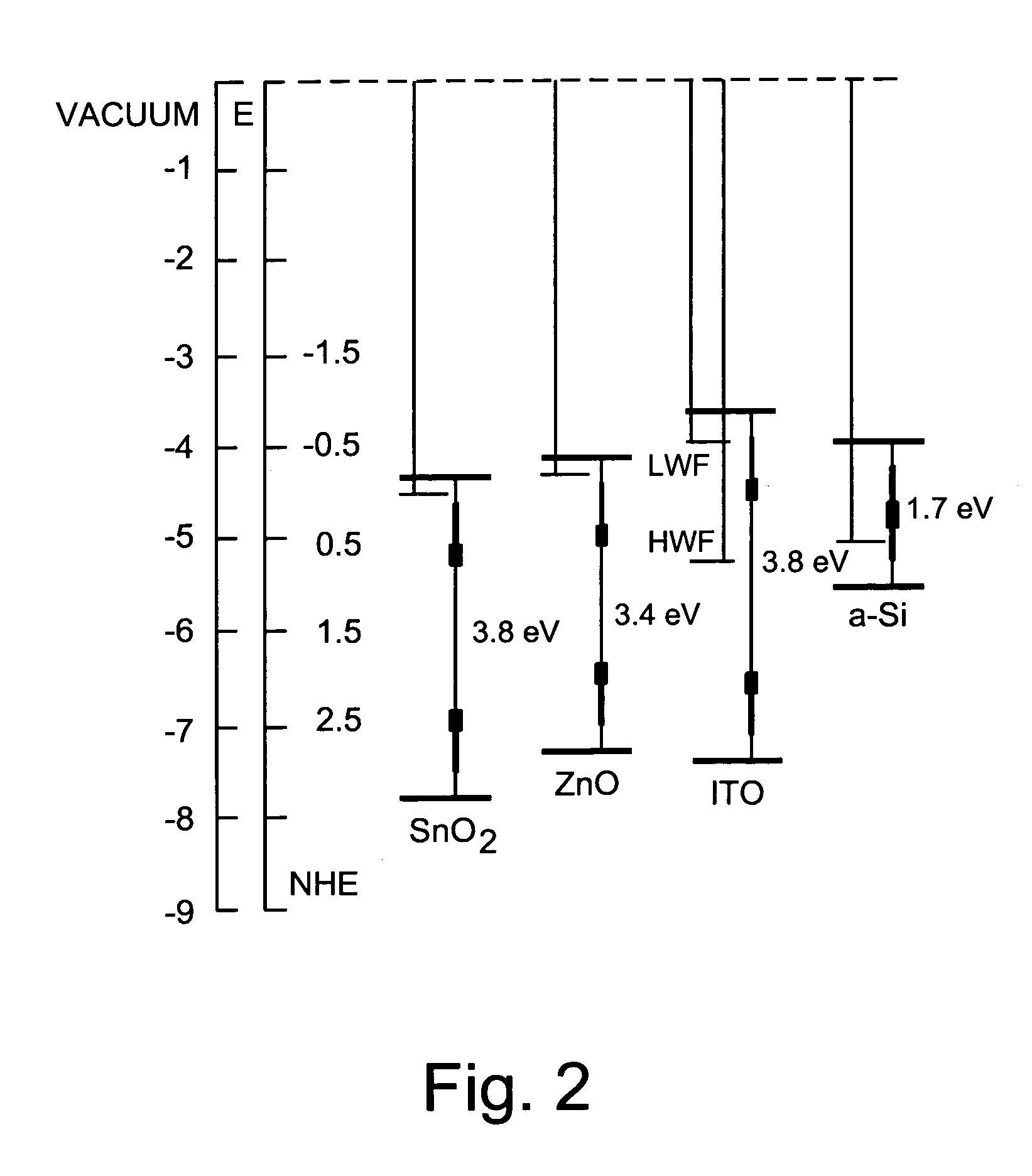
Front contact with high-function TCO for use in photovoltaic device and method of making same
InactiveUS20080047602A1Reduce potential barrierLower work functionSemiconductor devicesIndium tin oxideZinc
This invention relates to a front contact for use in an electronic device such as a photovoltaic device. In certain example embodiments, the front contact of the photovoltaic device includes a low work-function transparent conductive oxide (TCO) of a material such as tin oxide, zinc oxide, or the like, and a thin high work-function TCO of a material such as oxygen-rich ITO (indium tin oxide) or the like. The high-work function TCO is located between the low work-function TCO and the uppermost semiconductor layer of the photovoltaic device so as to provide for substantial work-function matching between the low work-function TCO and the high work-function uppermost semiconductor layer of the device in order to reduce a potential barrier for holes extracted from the device by the front contact.
Owner:GUARDIAN GLASS LLC
Power plant and method of operation
A power plant and method of operation is provided. The power plant comprises at least one main air compressor, an oxidizer unit configured to deliver a compressed oxygen-rich gas flow to at least one gas turbine assembly. Each assembly comprises a turbine combustor for mixing the compressed oxygen-rich gas flow with a recirculated gas flow and a fuel stream to burn a combustible mixture and form the recirculated gas flow. The assembly also comprises a recirculation loop for recirculating the recirculated gas flow from a turbine to a turbine compressor. The assembly further comprises a recirculated gas flow extraction path for extracting a portion of the recirculated gas flow from the assembly and delivering this to a gas separation system. The gas separation system separates the portion of the recirculated gas flow into a nitrogen portion and a carbon dioxide portion.
Owner:GENERAL ELECTRIC CO
Devices and methods for reduction of NOx emissions from lean burn engines
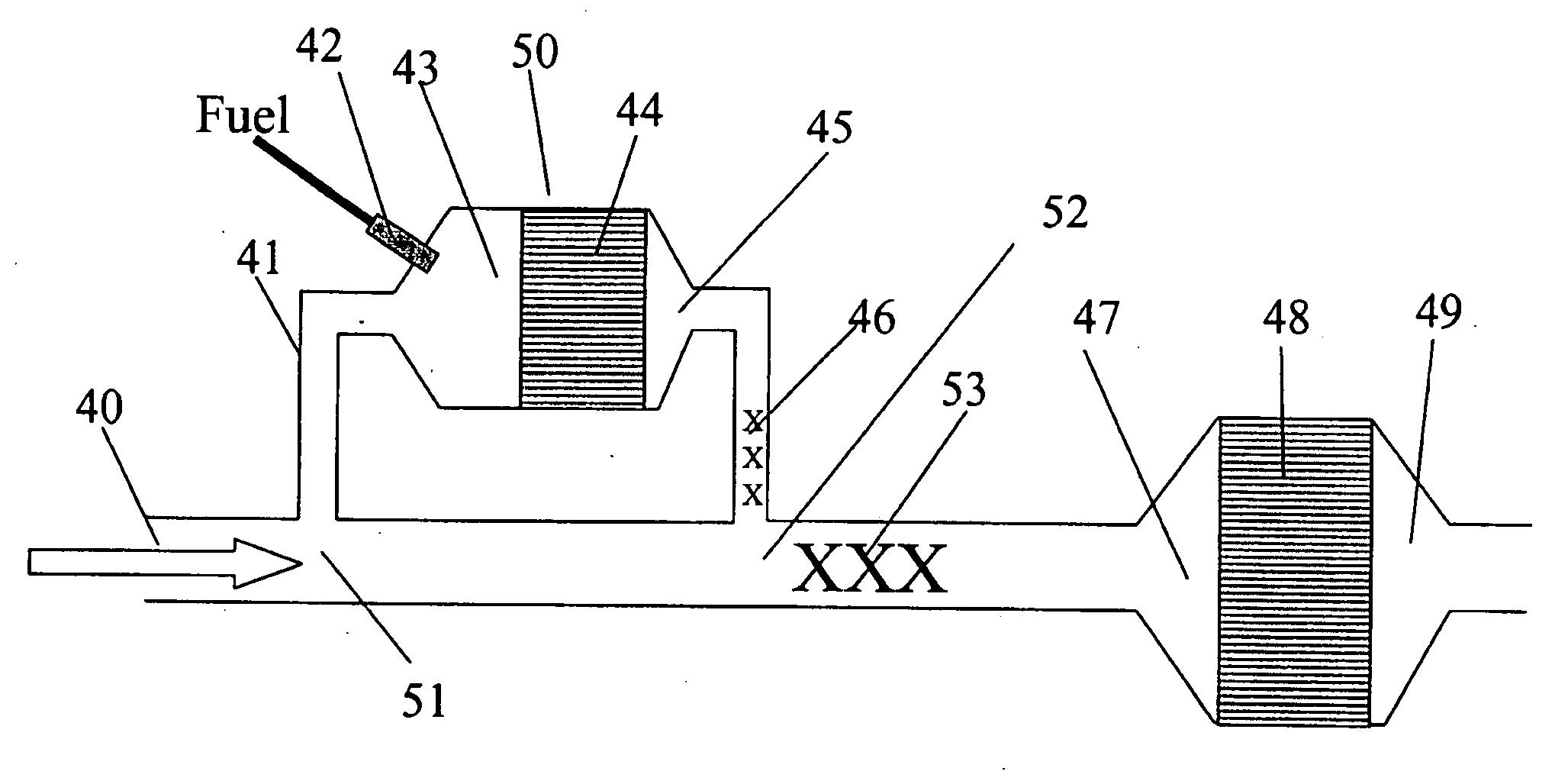
The invention provides devices and methods for generating H2 and CO in an O2 containing gas stream. The invention also provides devices and methods for removal of NOX from an O2 containing gas stream, particularly the oxygen-rich exhaust stream from a lean-burning engine, such as a diesel engine. The invention includes a fuel processor that efficiently converts added hydrocarbon fuel to a reducing mixture of H2 and CO. The added fuel may be a portion of the onboard fuel on a vehicle. The H2 and CO are incorporated into the exhaust stream and reacted over a selective lean NOX catalyst to convert NOX to N2. thereby providing an efficient means of NOX emission control.
Owner:INT ENGINE INTPROP CO LLC
Portable gas fractionalization system
InactiveUS7135059B2Facilitate mounting and removalIncrease the lengthRespiratorsCombination devicesProduct gasProcess engineering
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
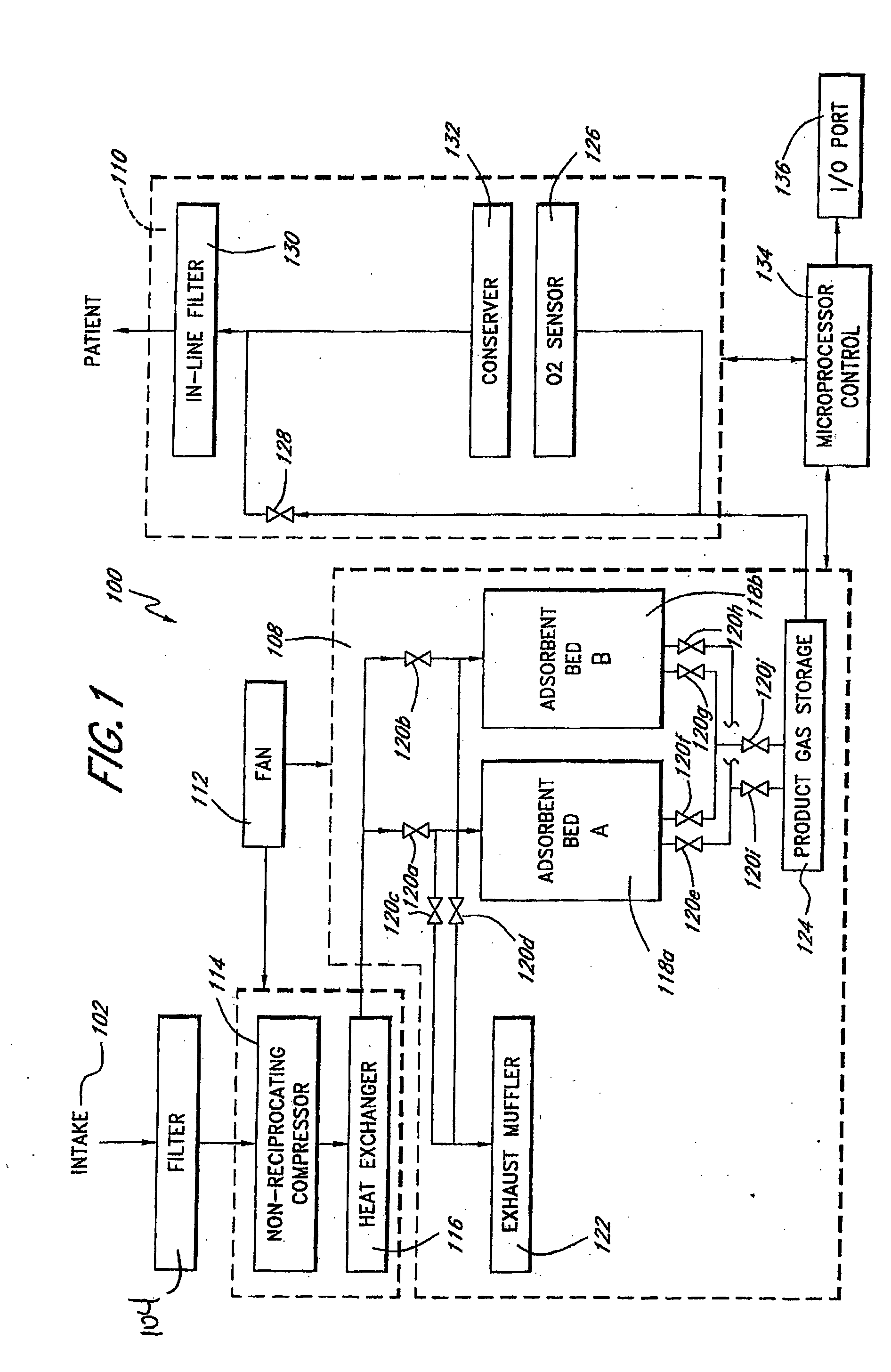
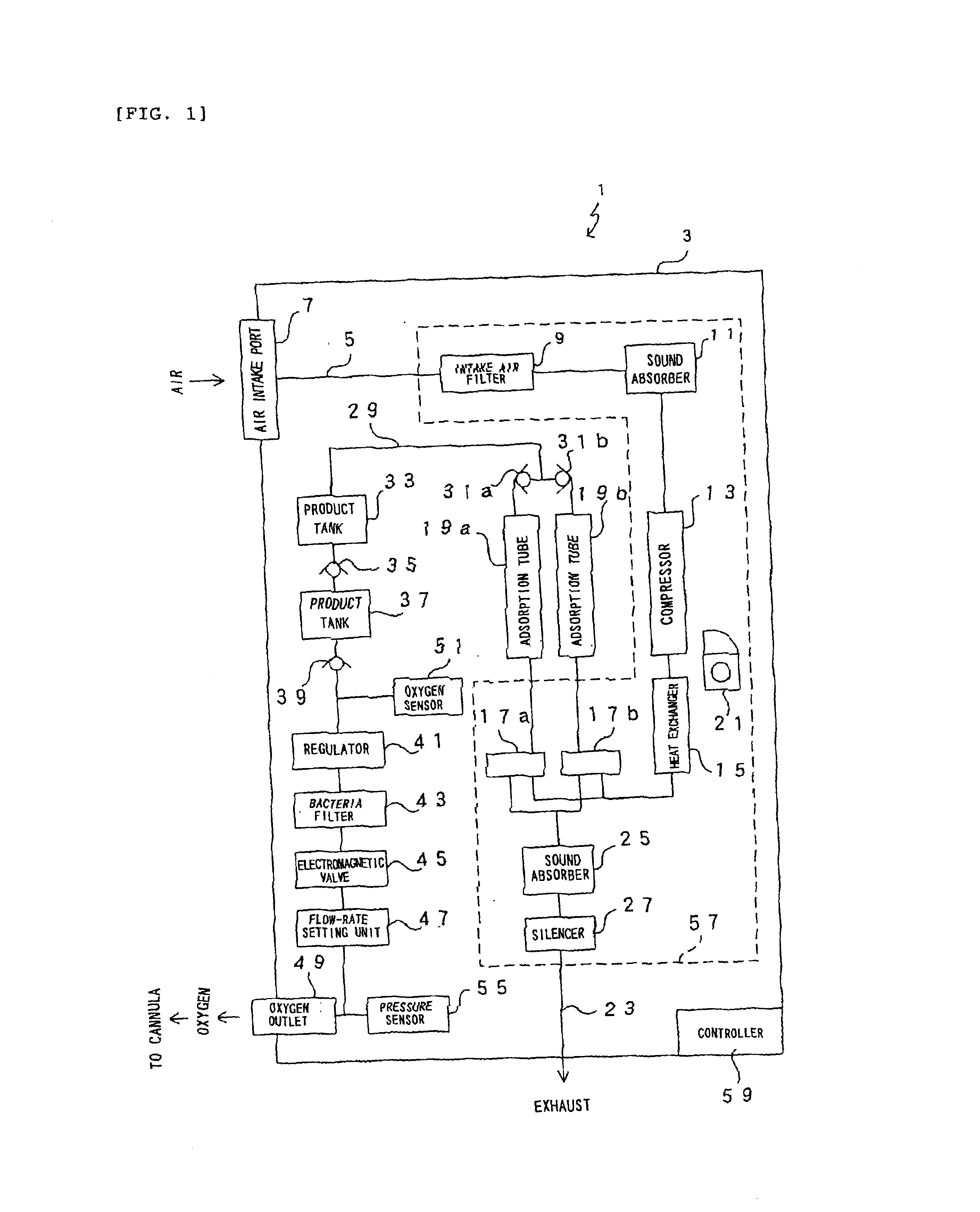
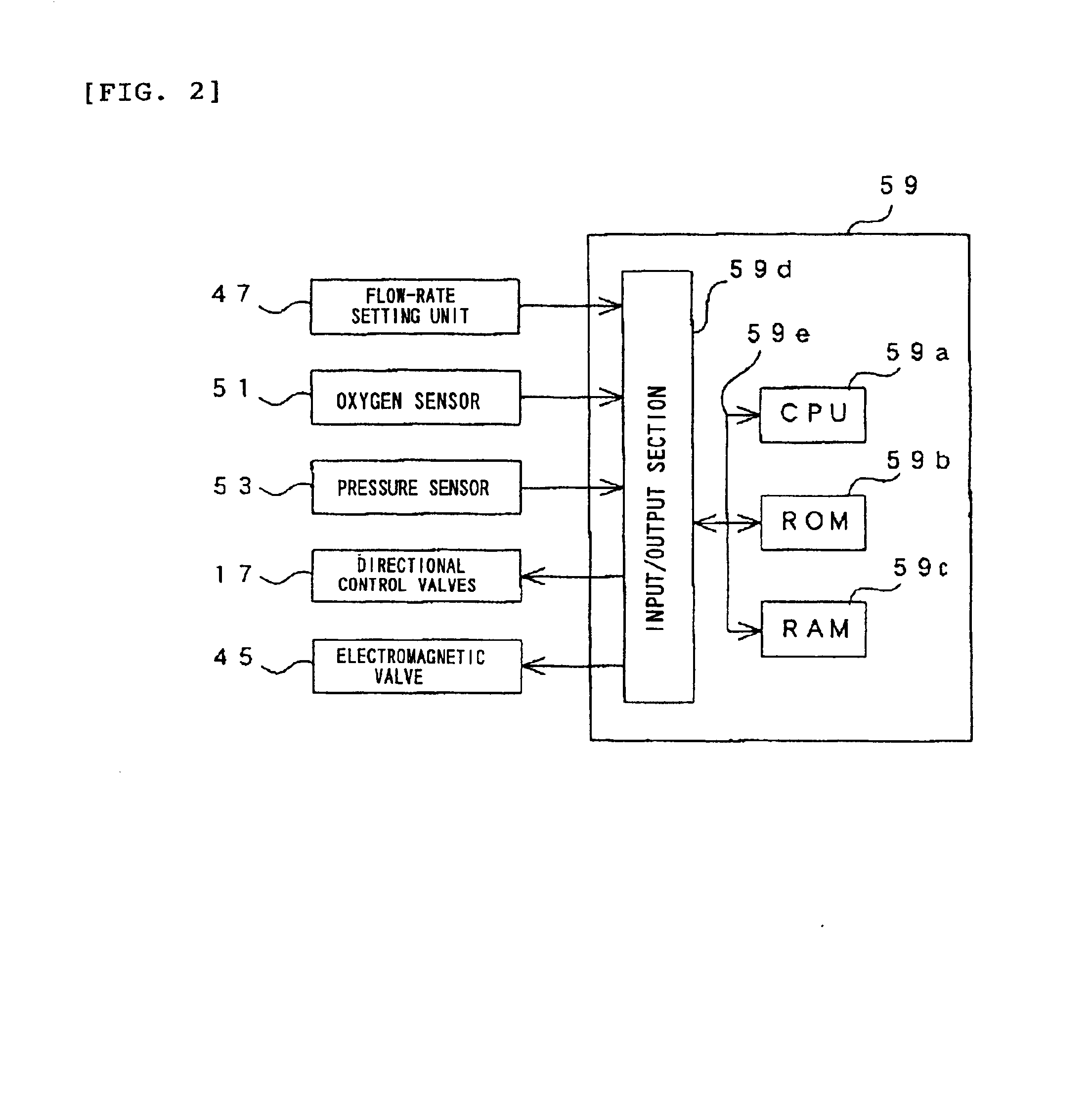
Oxygen enriching apparatus, controller for the oxygen enriching apparatus, and recording medium for the controller
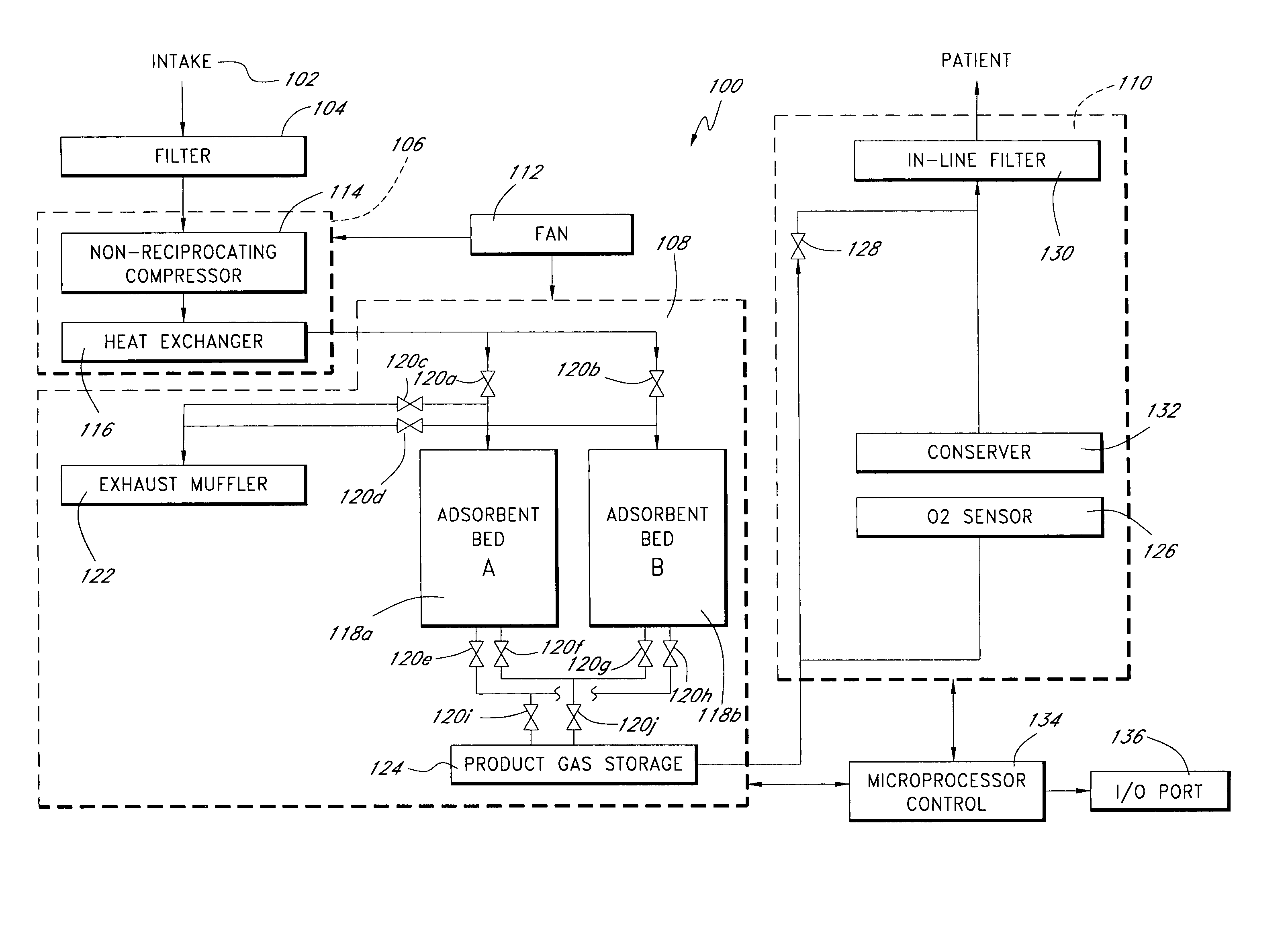
InactiveUS6837244B2Increase flow rateAdverse effect of pressure can be preventedOperating means/releasing devices for valvesRespiratory masksInhalationProcess engineering
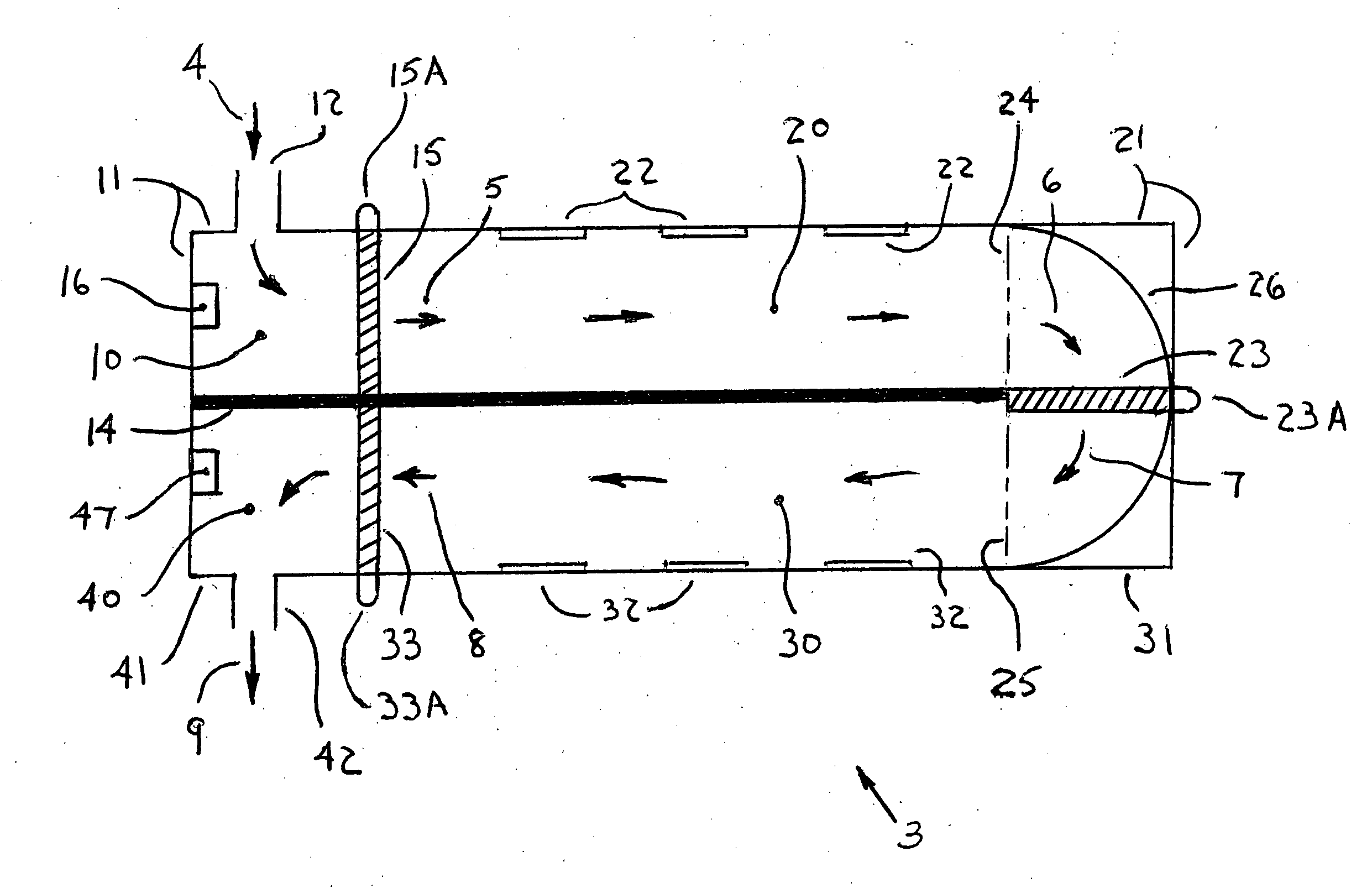
A small oxygen enriching apparatus which can supply oxygen-enriched gas at high flow rate without imparting unnatural sensation to a user, as well as a controller and recording medium therefore. In step 100, a judgment is made as to whether a flow rate set by use of a flow-rate setting unit 47 is equal to or less than a continuous base flow rate (2 liters / min). When the set flow rate is a low flow rate of not greater than 2 liters / min, breath-synchronized operation is not performed (continuous supply is to be performed), and therefore, in step 110, oxygen-enriched gas is supplied continuously at the set flow rate. When the set flow rate is a high flow rate of greater than 2 liters / min, breath-synchronized operation is to be performed (supply during the inhalation period only of each breathing cycle), and therefore, in step 120, oxygen-enriched gas is continuously supplied at the continuous base flow rate. In step 140, in order to perform breath-synchronized operation, control for opening and closing an electromagnetic valve 45 is performed. That is, oxygen-enriched gas is supplied during the inhalation period only of each breathing cycle, and supply of oxygen-enriched gas is stopped during the exhalation period.
Owner:NGK SPARK PLUG CO LTD
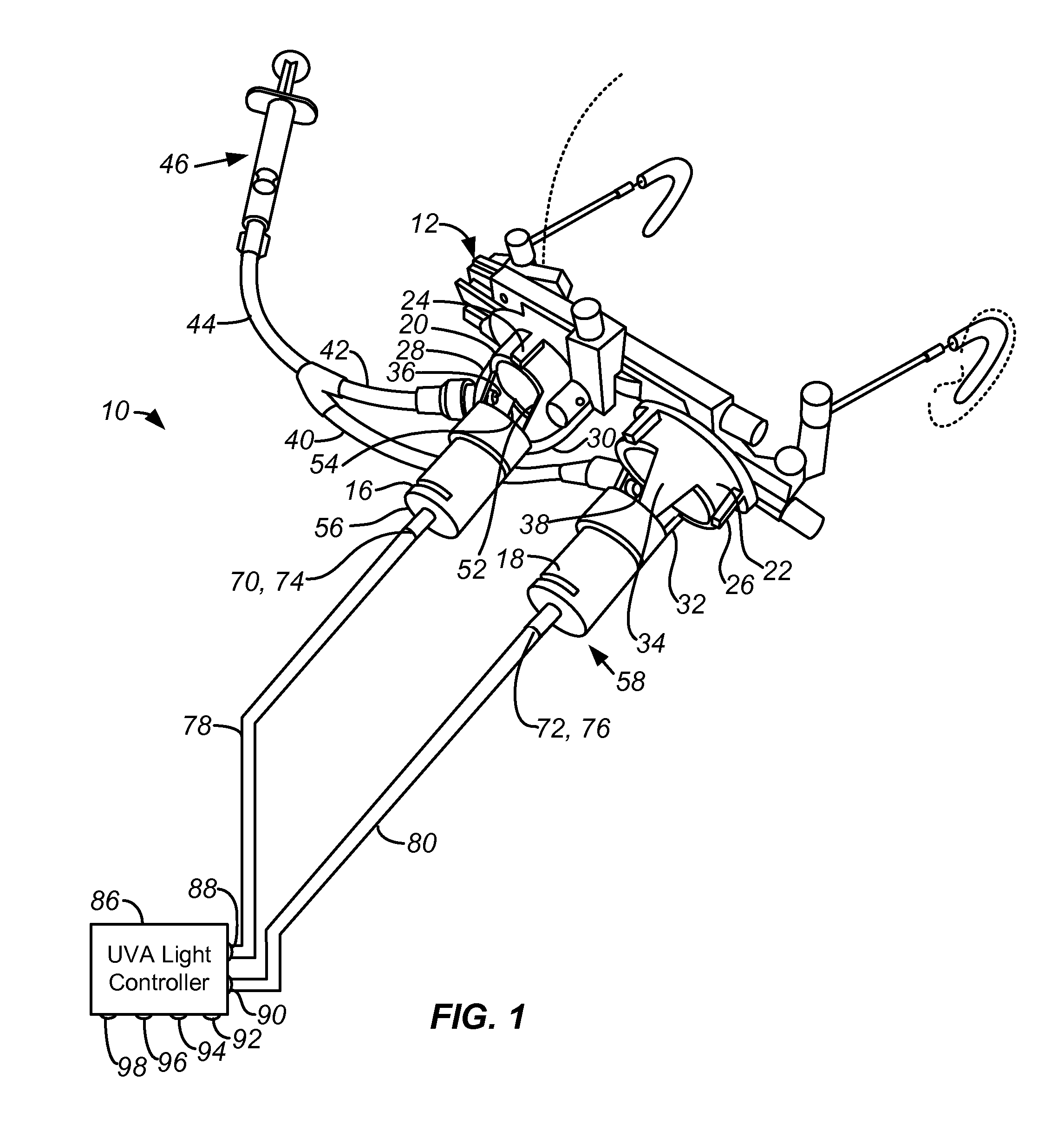
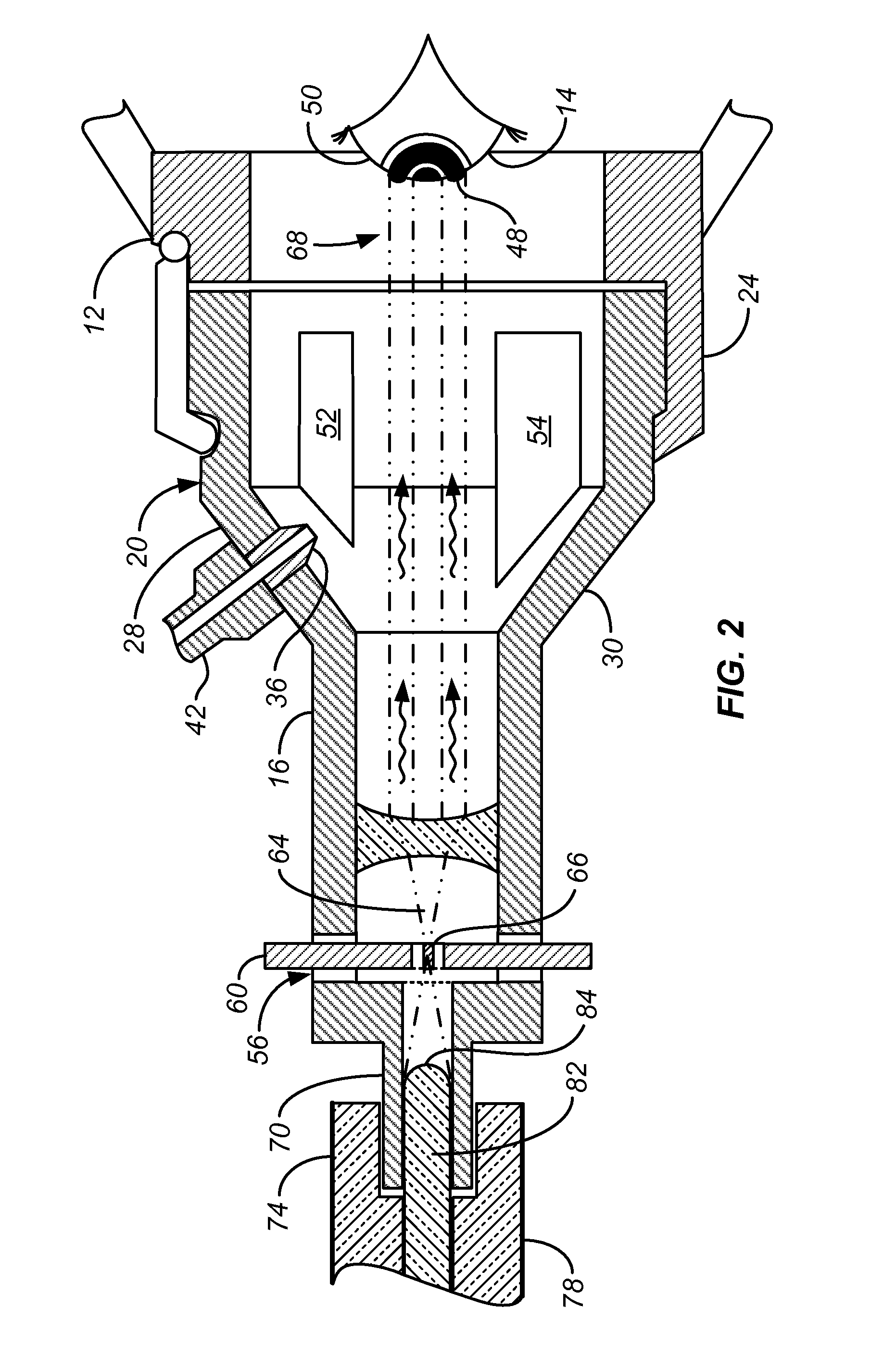
In Situ UV/Riboflavin Ocular Treatment System
InactiveUS20100057060A1Guarantee stable progressSafer collagen cross-linkingLaser surgerySurgical instrument detailsTrial frameControl objective
A system for accurately delivering bilateral simultaneous equi-dosed time-fractionated pulsed UVA to irradiate a class of riboflavin / collagen mixture in the presence of oxygen for treatment of ocular tissue such as scleral and corneal tissue. The system employs ocular trial frames for mounting on the face that are fitted with 1) a nozzle for introducing Riboflavin in solution to collagen on the surface of the ocular tissue, 2) a port for introducing oxygen-rich gas to the ocular tissue, and 3) a pair of optical collimator inserts mounted in the lens holders, wherein the collimator inserts have a mask in the optical path at an aperture on focal point to control the pattern of UVA radiation at the ocular target, the collimator inserts further having optical input ports coupled to a controlled source of UVA radiation that is operative in accordance with the related inventive method.
Owner:SEROS MEDICAL
Product pump for an oxygen concentrator
Owner:VBOX
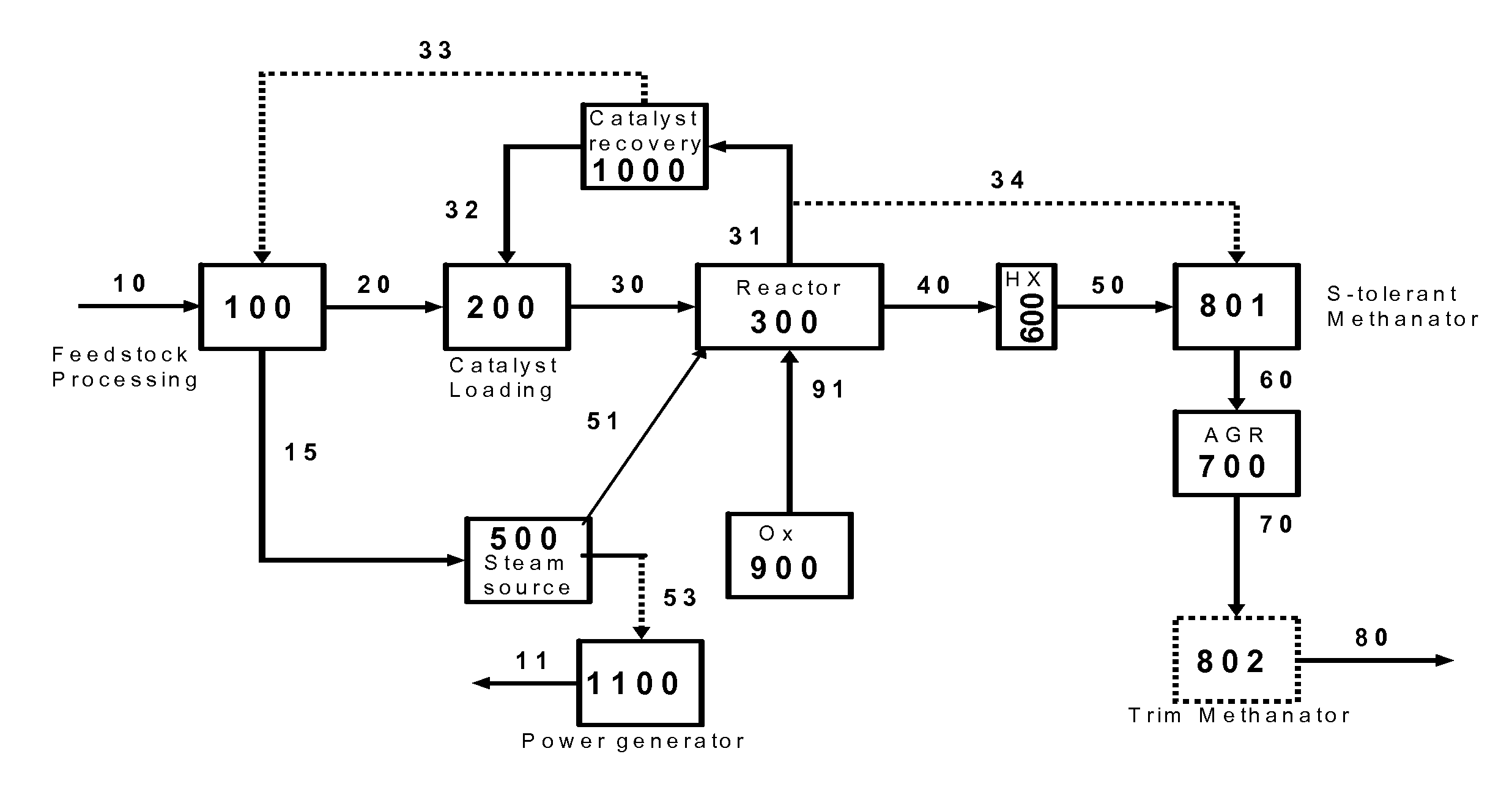
Hydromethanation Of A Carbonaceous Feedstock
The present invention relates to processes for hydromethanating a carbonaceous feedstock to a methane-enriched synthesis gas, where an oxygen-rich gas stream and the carbonaceous feedstock are fed into a fluidized-bed hydromethanation reactor at a specified zone in order to assist in heat management within the hydromethanation reactor.
Owner:SURE CHAMPION INVESTMENT LTD
Hydromethanation Of A Carbonaceous Feedstock
InactiveUS20120102837A1Improve processing efficiencyIncrease moisture contentGaseous fuelsGasification processes detailsHeat managementFluidized bed
The present invention relates to processes for hydromethanating a carbonaceous feedstock to a methane-enriched synthesis gas, where an oxygen-rich gas stream and the carbonaceous feedstock are fed into a fluidized-bed hydromethanation reactor, and where the carbonaceous feedstock as fed into the hydromethanation reactor has an elevated moisture content in order, for example, to assist in heat management within the hydromethanation reactor.
Owner:SURE CHAMPION INVESTMENT LTD
Portable gas fractionalization system
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
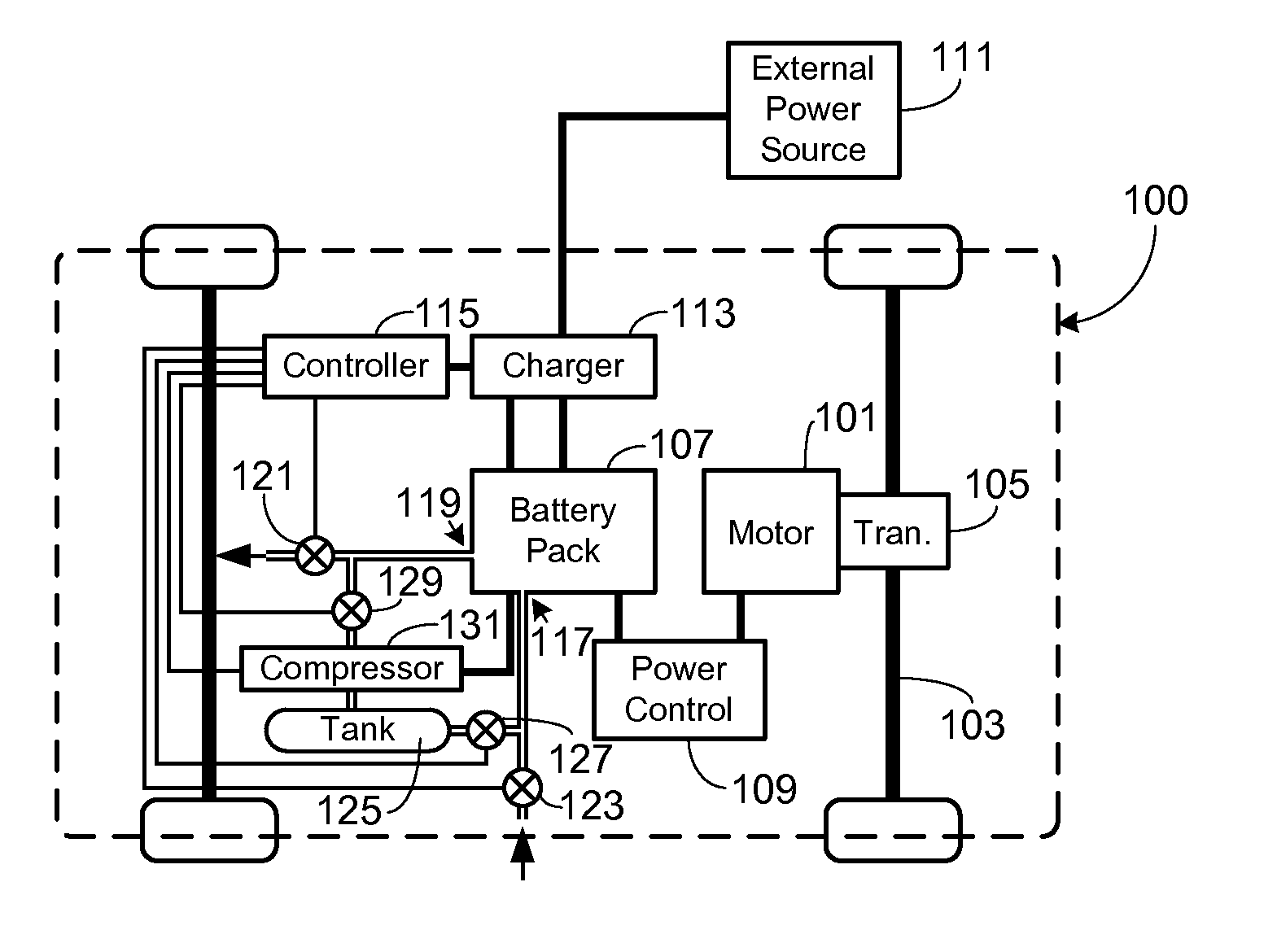
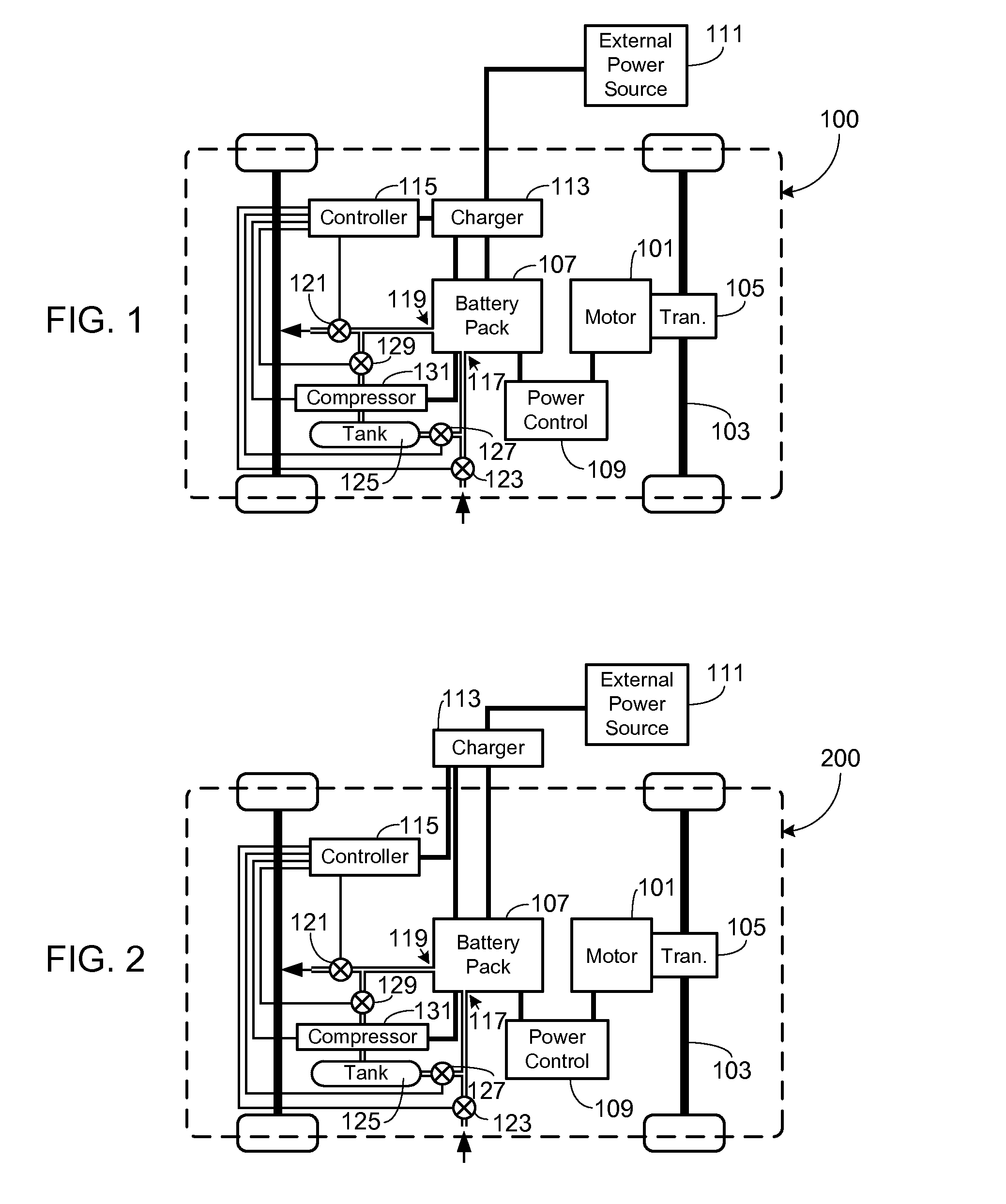
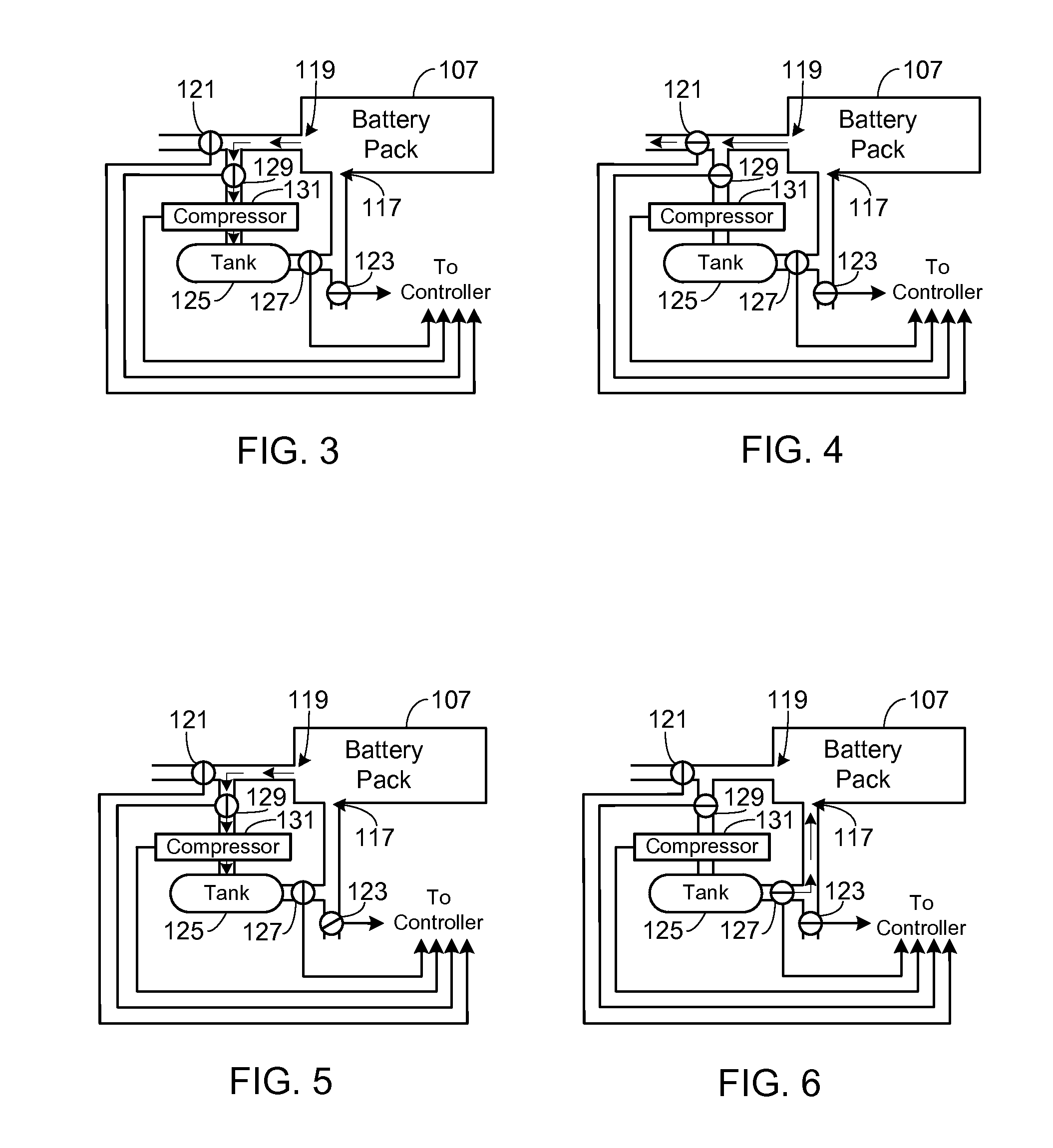
Collection, Storage and Use of Metal-Air Battery Pack Effluent
A system and method for collecting, storing and using the oxygen-rich effluent generated when charging a metal-air battery pack is provided.
Owner:TESLA INC
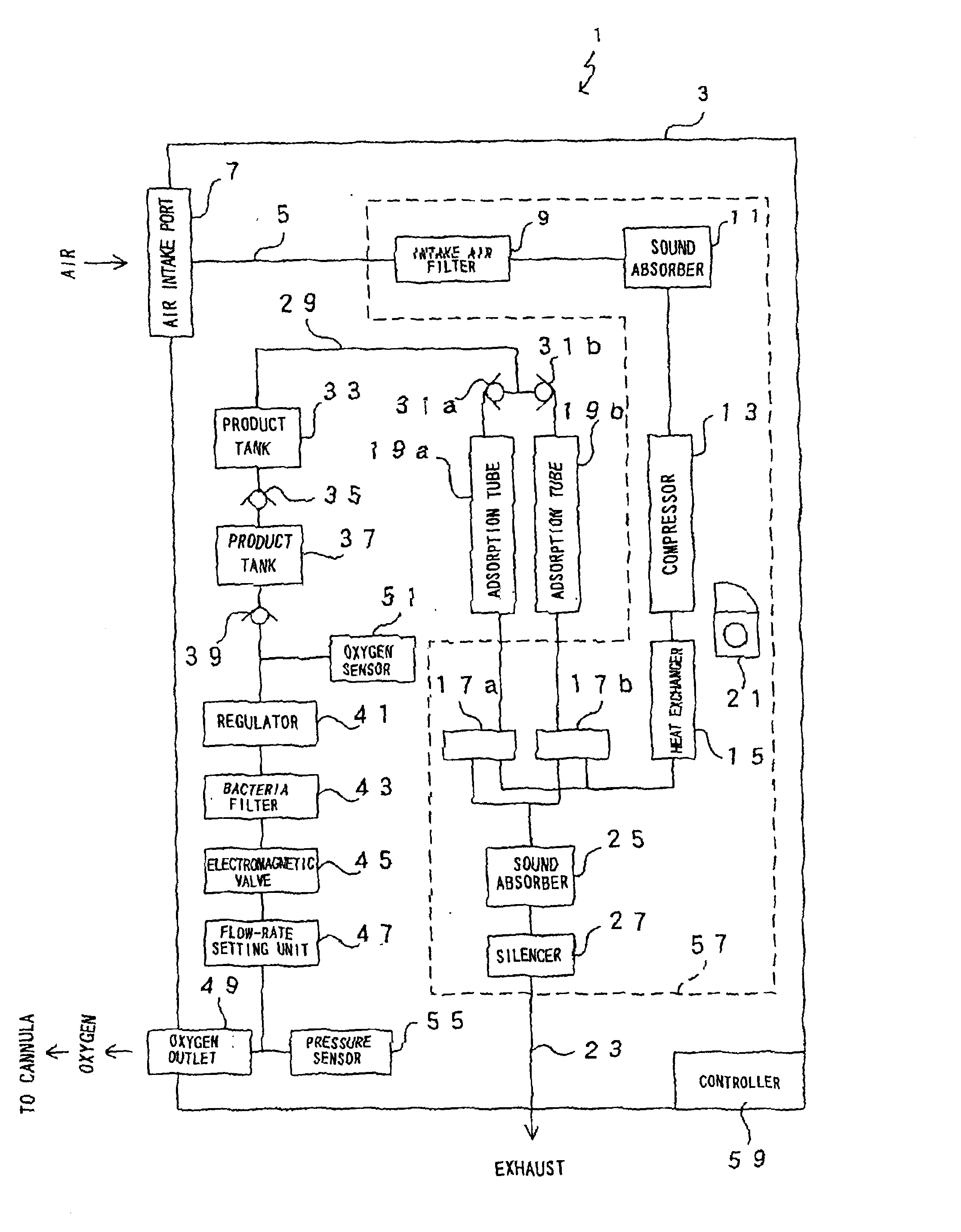
Ambulatory oxygen concentrator containing a power pack
An oxygen concentrator for providing ambulatory oxygen containing a vacuum swing adsorption (VSA) oxygen separator powered by a power pack. The separator has a plurality of nitrogen-selective adsorbent beds, each operating in VSA cycles including feed, evacuation and repressurization phases. The concentrator also contains a reservoir for storing oxygen-rich product gas produced by the VSA oxygen separator.
Owner:VBOX
Atmospheric molecular respirator
InactiveUS20100111792A1Purifying substantial volumes of room airRobust methodProductsGas treatmentActivated carbon filtrationAtmospherics
An apparatus for removing contaminants from air, including nitrogen oxides, carbon monoxide, carbon dioxide, and sulphur dioxide. In one of the chambers of a multi-chambered enclosure, polluted inlet air is exposed to one or more first light sources emitting light at wavelengths less than or equal to 242.3 nm to cause dissociation of contaminant molecules, creating ozone plus remaining atoms. The remaining atoms are largely filtered by activated charcoal filters having an appropriate thickness which is sized to achieve suitable dwell times, and which also serves as an oxygen rich medium permitting the ozone generated to undergo atomic rearrangement, whereby ozone molecules (O3) and atomic oxygen atoms (O) form oxygen molecules (O2). In another downstream chamber, the air flow is exposed to one or more second light sources emitting light at wavelengths greater than 242.3 nm but less than 280 nm, causing conversion of remaining ozone into oxygen molecules.
Owner:NELSON EDWARD D
Portable gas fractionalization system
ActiveUS20050072298A1Reduce heat loadReduce noiseCombination devicesAuxillary pretreatmentLow noiseCombined use
A portable gas fractionalization apparatus that provides oxygen rich air to patients is provided. The apparatus is compact, lightweight, and low-noise. The components are assembled in a housing that is divided into two compartments. One compartment is maintained at a lower temperature than the other compartment. The lower temperature compartment is configured for mounting components that can be damaged by heat. The higher temperature compartment is configured for mounting heat generating components. An air stream is directed to flow from an ambient air inlet to an air outlet constantly so that there is always a fresh source of cooling air. The apparatus utilizes a PSA unit to produce an oxygen enriched product. The PSA unit incorporates a novel single ended column design in which all flow paths and valves can be co-located on a single integrated manifold. The apparatus also can be used in conjunction with a satellite conserver and a mobility cart.
Owner:INOGEN INC
Devices and methods for reduction of NOx emissions from lean burn engines
ActiveUS7181906B2Reduce nitrogen oxide emissionsHydrogenInternal combustion piston enginesDiesel engineOxygen rich
The invention provides devices and methods for generating H2 and CO in an O2 containing gas stream. The invention also provides devices and methods for removal of NOX from an O2 containing gas stream, particularly the oxygen-rich exhaust stream from a lean-burning engine, such as a diesel engine. The invention includes a fuel processor that efficiently converts added hydrocarbon fuel to a reducing mixture of H2 and CO. The added fuel may be a portion of the onboard fuel on a vehicle. The H2 and CO are incorporated into the exhaust stream and reacted over a selective lean NOX catalyst to convert NOX to N2. thereby providing an efficient means of NOX emission control.
Owner:INT ENGINE INTPROP CO LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com