HDPE in Medical Devices: Current Uses and Future Potential
HDPE in Medical Devices: Background and Objectives
High-density polyethylene (HDPE) has emerged as a versatile and indispensable material in the medical device industry, revolutionizing healthcare practices and patient outcomes. The journey of HDPE in medical applications began in the mid-20th century, with its initial use in simple medical containers and packaging. As material science advanced, HDPE's unique properties, including excellent chemical resistance, durability, and biocompatibility, became increasingly recognized and exploited in the medical field.
The evolution of HDPE in medical devices has been driven by the growing demand for safer, more efficient, and cost-effective healthcare solutions. From its early applications, HDPE has expanded into a wide range of medical products, including implants, prosthetics, surgical instruments, and drug delivery systems. This progression has been marked by continuous improvements in material formulation, processing techniques, and surface modifications, enhancing HDPE's performance and expanding its applicability.
In recent years, the medical device industry has witnessed a surge in research and development focused on HDPE, aiming to unlock its full potential in addressing complex medical challenges. The ongoing technological advancements are pushing the boundaries of HDPE's capabilities, exploring novel applications such as 3D-printed customized implants, smart medical devices incorporating HDPE, and enhanced drug-eluting systems.
The primary objective of this technical research report is to provide a comprehensive analysis of HDPE's current uses in medical devices and to explore its future potential. This involves examining the material's properties that make it suitable for medical applications, evaluating its performance in existing devices, and identifying emerging trends and innovations that could shape its future in healthcare.
Furthermore, this report aims to assess the challenges and limitations associated with HDPE in medical devices, including regulatory considerations, sterilization compatibility, and long-term biocompatibility. By addressing these aspects, we seek to provide valuable insights for researchers, manufacturers, and healthcare professionals to guide future developments and applications of HDPE in the medical field.
The scope of this investigation encompasses a wide range of medical devices utilizing HDPE, from disposable items to long-term implants. We will explore how HDPE compares to alternative materials, its environmental impact, and its potential role in advancing personalized medicine and improving patient care. Through this comprehensive analysis, we aim to illuminate the path forward for HDPE in medical devices, highlighting opportunities for innovation and areas requiring further research and development.
Market Analysis for HDPE-based Medical Products
The global market for HDPE-based medical products has been experiencing steady growth, driven by the increasing demand for safe, durable, and cost-effective medical devices and packaging solutions. HDPE's unique properties, including excellent chemical resistance, high impact strength, and low moisture absorption, make it an ideal material for various medical applications.
In recent years, the market has witnessed a significant surge in demand for HDPE-based medical products, particularly in disposable medical devices, pharmaceutical packaging, and laboratory equipment. The COVID-19 pandemic has further accelerated this trend, as healthcare facilities worldwide have increased their consumption of disposable medical supplies to maintain hygiene standards and prevent cross-contamination.
The pharmaceutical packaging segment represents a substantial portion of the HDPE medical products market. HDPE bottles and containers are widely used for storing and dispensing medications, vitamins, and other healthcare products due to their excellent barrier properties and resistance to moisture. The growing emphasis on drug safety and the rising prevalence of chronic diseases are expected to drive continued growth in this segment.
Another key area of growth is in surgical instruments and medical devices. HDPE's biocompatibility and ability to withstand sterilization processes make it suitable for manufacturing various medical tools, implants, and prosthetics. The aging population and increasing prevalence of chronic diseases are contributing to the expansion of this market segment.
Geographically, North America and Europe currently dominate the HDPE medical products market, owing to their advanced healthcare infrastructure and stringent regulatory standards. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, rising disposable incomes, and increasing awareness of healthcare hygiene.
The market is characterized by the presence of both large multinational corporations and smaller specialized manufacturers. Key players are focusing on product innovation, such as developing antimicrobial HDPE formulations or enhancing the material's barrier properties, to gain a competitive edge. Additionally, there is a growing trend towards sustainable and recyclable HDPE products in response to environmental concerns and regulatory pressures.
Looking ahead, the HDPE medical products market is projected to continue its growth trajectory. Factors such as technological advancements in HDPE manufacturing, increasing healthcare expenditure, and the ongoing shift towards home healthcare are expected to drive market expansion. However, challenges such as stringent regulatory requirements and competition from alternative materials may impact market dynamics in the coming years.
Current Applications and Challenges of HDPE in Healthcare
High-density polyethylene (HDPE) has become an increasingly important material in the healthcare industry, finding widespread applications in medical devices and equipment. Its unique properties, including high strength-to-density ratio, chemical resistance, and biocompatibility, make it an ideal choice for various medical applications.
In the realm of medical packaging, HDPE is extensively used for containers, bottles, and closures. Its excellent moisture barrier properties and resistance to chemicals make it suitable for storing pharmaceuticals, ensuring the integrity and shelf life of medications. HDPE containers are also commonly used for laboratory reagents and diagnostic kits, providing a safe and stable environment for sensitive materials.
HDPE's durability and impact resistance have led to its adoption in the manufacturing of medical equipment and devices. It is frequently used in the production of surgical instruments, such as forceps and clamps, offering a lightweight yet sturdy alternative to traditional metal tools. Additionally, HDPE is employed in the fabrication of prosthetic components, orthotics, and mobility aids, providing patients with comfortable and functional support devices.
In the field of implantable medical devices, HDPE has gained traction due to its biocompatibility and low friction properties. It is used in the production of artificial joints, particularly in hip and knee replacements, where its wear resistance and low coefficient of friction contribute to the longevity and performance of the implants. HDPE is also utilized in spinal fusion cages and other orthopedic applications, offering a balance of strength and flexibility.
Despite its numerous advantages, the use of HDPE in healthcare faces several challenges. One primary concern is the material's susceptibility to degradation when exposed to certain sterilization methods, particularly high-energy radiation. This limitation necessitates careful consideration of sterilization protocols for HDPE-based medical devices to ensure their integrity and safety.
Another challenge lies in the potential for HDPE to leach additives or undergo oxidation over time, which may impact the purity of stored substances or the long-term performance of medical devices. Researchers and manufacturers are continuously working to develop improved HDPE formulations and surface treatments to mitigate these issues and enhance the material's overall stability in medical applications.
The recyclability of HDPE presents both an opportunity and a challenge in the healthcare sector. While its potential for recycling aligns with sustainability goals, the strict regulations surrounding medical waste disposal often hinder the implementation of effective recycling programs for HDPE medical products. Balancing environmental concerns with safety and regulatory requirements remains an ongoing challenge for the industry.
Existing HDPE Solutions in Medical Devices
01 Composition and manufacturing of HDPE
High-Density Polyethylene (HDPE) is a thermoplastic polymer produced from ethylene monomers. Its manufacturing process involves various catalysts and polymerization techniques to achieve desired properties such as high density, strength, and chemical resistance. The composition can be modified with additives to enhance specific characteristics for different applications.- Composition and properties of HDPE: High-Density Polyethylene (HDPE) is a thermoplastic polymer with a high strength-to-density ratio. It is characterized by its long linear chains with minimal branching, resulting in higher tensile strength, stiffness, and chemical resistance compared to other polyethylene types. HDPE's properties make it suitable for various applications, including packaging, pipes, and industrial products.
- HDPE blends and composites: HDPE can be blended with other materials or reinforced with additives to enhance its properties. These blends and composites can improve characteristics such as impact resistance, thermal stability, or specific mechanical properties. The resulting materials find applications in areas where standard HDPE may not meet all requirements.
- HDPE processing techniques: Various processing techniques are used to manufacture HDPE products, including injection molding, extrusion, and blow molding. Each method offers specific advantages and is suited to different product types. Innovations in processing techniques focus on improving efficiency, reducing waste, and enhancing the final product quality.
- Recycling and sustainability of HDPE: HDPE is recyclable and efforts are being made to improve its recycling processes and increase the use of recycled HDPE in new products. Research focuses on enhancing the properties of recycled HDPE, developing more efficient recycling methods, and finding new applications for recycled materials to promote sustainability in the plastics industry.
- HDPE applications in specific industries: HDPE finds applications in various industries due to its versatile properties. It is used in packaging, construction, automotive, and medical industries, among others. Ongoing research and development focus on expanding HDPE's applications by tailoring its properties for specific industry needs, such as improved barrier properties for food packaging or enhanced durability for construction materials.
02 HDPE blends and composites
HDPE can be blended with other materials or reinforced with fillers to create composites with improved properties. These blends and composites often exhibit enhanced mechanical strength, thermal stability, or specific functional characteristics. The combination of HDPE with other polymers or additives allows for tailored material properties suitable for various industrial applications.Expand Specific Solutions03 HDPE recycling and sustainability
Recycling processes for HDPE have been developed to address environmental concerns and promote sustainability. These methods involve collecting, sorting, cleaning, and reprocessing HDPE products. Innovations in recycling technology aim to maintain or improve the quality of recycled HDPE, making it suitable for use in new products and reducing the environmental impact of plastic waste.Expand Specific Solutions04 HDPE applications in packaging and containers
HDPE is widely used in packaging and container manufacturing due to its excellent chemical resistance, durability, and moisture barrier properties. Applications include bottles for beverages and household chemicals, food packaging, and industrial containers. Innovations in this area focus on improving design, reducing material usage, and enhancing recyclability while maintaining product integrity.Expand Specific Solutions05 HDPE in construction and infrastructure
HDPE finds extensive use in construction and infrastructure projects due to its durability, corrosion resistance, and flexibility. Applications include pipes for water and gas distribution, geomembranes for landfills, and structural components in buildings. Research in this area focuses on improving long-term performance, ease of installation, and resistance to environmental factors.Expand Specific Solutions
Key Players in HDPE Medical Device Industry
The HDPE market in medical devices is in a growth phase, driven by increasing demand for biocompatible and durable materials. The global market size for HDPE in medical applications is expanding, with a projected CAGR of 6-8% over the next five years. Technologically, HDPE is mature but continues to evolve, with companies like Dow Global Technologies, DSM IP Assets, and Borealis AG leading innovations in material properties and processing techniques. Major medical device manufacturers such as Medtronic, Boston Scientific, and Becton, Dickinson & Co. are actively incorporating advanced HDPE formulations into their product lines. Research institutions like Sichuan University and the University of North Carolina at Charlotte are exploring novel applications, indicating potential for future breakthroughs in HDPE-based medical technologies.
Dow Global Technologies LLC
Becton, Dickinson & Co.
Innovations in HDPE for Medical Applications
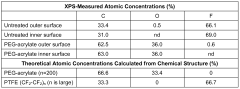
- Surface modification of ePTFE using a multi-step process involving radio frequency (RF)-generated plasmas to pre-treat, coat with a hydrophilic polymer, and crosslink the coating, specifically using argon and H2O plasmas with a PEG-acrylate coating to enhance wettability.
- A mixture comprising >70% high-density polyethylene (HDPE) and 5-25% cyclic olefin copolymer (COC) by weight, which reduces friction with metal surfaces through a unique blending process, producing a semi-crystalline HDPE structure within an amorphous COC framework, resulting in a lower friction coefficient.
Regulatory Framework for HDPE in Medical Devices
The regulatory framework for HDPE in medical devices is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of these products. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing medical devices, including those incorporating HDPE. The FDA classifies medical devices into three categories based on their risk level and intended use, with HDPE-containing devices falling into various classifications depending on their specific applications.
For Class I devices, which are considered low-risk, HDPE components may be subject to general controls, including good manufacturing practices and labeling requirements. Class II devices, which pose moderate risks, often require special controls in addition to general controls. These may include performance standards, post-market surveillance, and specific labeling requirements for HDPE components. Class III devices, which are high-risk and often implantable, undergo the most stringent regulatory scrutiny, including premarket approval (PMA) processes that may involve extensive clinical trials to demonstrate safety and effectiveness.
In the European Union, the regulatory framework for medical devices containing HDPE is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations establish a comprehensive system for device classification, conformity assessment, and post-market surveillance. HDPE-containing devices must comply with the Essential Requirements outlined in these regulations, which include demonstrating biocompatibility, chemical stability, and mechanical properties suitable for their intended use.
Globally, the International Organization for Standardization (ISO) provides standards that are widely recognized and often incorporated into regulatory frameworks. ISO 10993, for instance, outlines the biological evaluation of medical devices and is particularly relevant for assessing the biocompatibility of HDPE in medical applications. Manufacturers must conduct thorough testing to ensure compliance with these standards and to support regulatory submissions.
Regulatory bodies also focus on the manufacturing processes of HDPE medical devices. Good Manufacturing Practices (GMP) are essential, with requirements for quality management systems, process validation, and documentation. For HDPE specifically, regulations may address aspects such as material purity, additives, and potential leachables or extractables that could impact patient safety.
As the use of HDPE in medical devices continues to expand, regulatory frameworks are likely to evolve. Emerging areas of focus include the long-term stability of HDPE in implantable devices, its interaction with new sterilization methods, and its performance in combination products that may include drugs or biologics. Regulatory agencies are also increasingly considering the environmental impact of medical devices, which may lead to new requirements for the lifecycle management of HDPE-containing products.
Sustainability and Recycling of HDPE Medical Devices
The sustainability and recycling of HDPE medical devices have become increasingly important in the healthcare industry due to growing environmental concerns and the need for resource conservation. HDPE, being a thermoplastic material, offers significant potential for recycling and reuse, which can contribute to reducing the environmental impact of medical waste.
In the medical field, HDPE devices are often single-use items, leading to substantial waste generation. However, recent advancements in recycling technologies have opened up new possibilities for the sustainable management of these devices. Mechanical recycling processes can effectively break down HDPE medical devices into reusable plastic pellets, which can then be utilized in the production of new non-medical products.
Chemical recycling methods, such as pyrolysis and gasification, are also being explored for HDPE medical device recycling. These processes break down the plastic into its chemical components, allowing for the creation of new plastic materials or even fuel products. This approach offers the potential to close the loop in plastic production and reduce reliance on virgin materials.
One of the main challenges in recycling HDPE medical devices is the need for proper decontamination to ensure safety. Advanced sterilization techniques, including radiation and chemical treatments, are being developed to address this issue effectively. These methods aim to render the devices safe for recycling without compromising the material's integrity.
The implementation of recycling programs for HDPE medical devices requires collaboration between healthcare facilities, waste management companies, and recycling technology providers. Some hospitals have already initiated pilot programs to collect and recycle specific HDPE items, such as saline bottles and packaging materials. These efforts not only reduce waste but also demonstrate the feasibility of larger-scale recycling initiatives.
Looking to the future, there is a growing focus on designing HDPE medical devices with end-of-life considerations in mind. This approach, known as Design for Recycling (DfR), involves creating products that are easier to disassemble, separate, and recycle. By incorporating recycled HDPE into new medical device production, manufacturers can further enhance the sustainability of their products and contribute to a circular economy in healthcare.
As regulations and consumer demand for sustainable practices continue to evolve, the recycling of HDPE medical devices is likely to become more widespread. This shift towards sustainability not only addresses environmental concerns but also presents opportunities for cost savings and resource efficiency in the healthcare sector.