Graphene Oxide in Medicine: Enhancing Drug Delivery Systems
Graphene Oxide in Medicine: Background and Objectives
Graphene oxide (GO) has emerged as a revolutionary material in the field of medicine, particularly in enhancing drug delivery systems. This two-dimensional carbon-based nanomaterial, derived from graphene, has garnered significant attention due to its unique physicochemical properties and biocompatibility. The development of GO-based drug delivery systems represents a convergence of nanotechnology and medicine, aiming to address the limitations of conventional drug delivery methods.
The journey of graphene oxide in medicine began with the discovery of graphene in 2004, which led to a Nobel Prize in Physics in 2010. Since then, researchers have been exploring various modifications and applications of graphene, with GO emerging as a promising candidate for biomedical applications. The oxidation of graphene introduces oxygen-containing functional groups, enhancing its hydrophilicity and facilitating its integration with biological systems.
The evolution of GO in drug delivery systems has been driven by the need for more efficient and targeted therapeutic approaches. Traditional drug delivery methods often face challenges such as poor solubility, limited bioavailability, and off-target effects. GO offers potential solutions to these issues through its large surface area, which allows for high drug loading capacity, and its ability to be functionalized for targeted delivery.
The primary objectives of incorporating GO into drug delivery systems are multifaceted. Firstly, researchers aim to improve the solubility and stability of drugs, particularly those that are hydrophobic or prone to degradation. Secondly, there is a focus on enhancing the bioavailability of drugs by protecting them from premature metabolism and excretion. Thirdly, the development of GO-based systems seeks to achieve controlled and sustained drug release, optimizing therapeutic efficacy while minimizing side effects.
Another crucial objective is to leverage GO's potential for targeted drug delivery. By functionalizing GO with specific ligands or molecules, researchers aim to create smart delivery systems that can selectively target diseased cells or tissues. This approach holds promise for reducing systemic toxicity and improving the therapeutic index of various drugs, particularly in cancer treatment.
The technological trajectory of GO in medicine is closely aligned with the broader trends in nanomedicine and personalized healthcare. As research progresses, the integration of GO with other advanced technologies, such as stimuli-responsive materials and theranostic platforms, is expected to open new avenues for innovative therapeutic strategies. The ultimate goal is to develop highly efficient, safe, and versatile drug delivery systems that can revolutionize patient care across a wide range of medical conditions.
Market Analysis for Graphene Oxide-Based Drug Delivery
The market for graphene oxide-based drug delivery systems is experiencing significant growth, driven by the increasing demand for targeted and efficient drug delivery methods. Graphene oxide, with its unique properties such as large surface area, high drug loading capacity, and excellent biocompatibility, has emerged as a promising material for enhancing drug delivery systems.
The global market for graphene oxide in drug delivery is expected to expand rapidly in the coming years. This growth is fueled by the rising prevalence of chronic diseases, the need for improved therapeutic outcomes, and the continuous advancements in nanotechnology. The pharmaceutical industry, in particular, is showing keen interest in graphene oxide-based drug delivery systems due to their potential to overcome limitations associated with conventional drug delivery methods.
One of the key market drivers is the ability of graphene oxide to enhance drug solubility and bioavailability. This is particularly beneficial for drugs with poor water solubility, which is a common challenge in pharmaceutical development. By improving drug solubility, graphene oxide-based systems can potentially reduce dosage requirements and minimize side effects, leading to better patient outcomes.
The oncology segment is anticipated to be a major contributor to market growth. Graphene oxide's ability to target cancer cells specifically and deliver therapeutic agents more effectively has garnered significant attention from researchers and pharmaceutical companies. This targeted approach not only improves treatment efficacy but also reduces the systemic toxicity associated with traditional chemotherapy.
Geographically, North America and Europe are currently leading the market for graphene oxide-based drug delivery systems. This is attributed to the presence of well-established pharmaceutical industries, substantial research and development investments, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of advanced drug delivery technologies, and rising investments in nanotechnology research.
Despite the promising outlook, the market faces certain challenges. The high cost of production and the need for extensive clinical trials to ensure safety and efficacy are potential barriers to widespread adoption. Additionally, regulatory hurdles and concerns regarding the long-term effects of graphene oxide in the human body need to be addressed to gain broader market acceptance.
In conclusion, the market for graphene oxide-based drug delivery systems shows substantial promise, with significant growth potential across various therapeutic areas. As research progresses and more clinical data becomes available, it is expected that the adoption of these advanced drug delivery systems will accelerate, potentially revolutionizing the field of medicine and drug delivery.
Current Challenges in Graphene Oxide Drug Delivery Systems
Despite the promising potential of graphene oxide (GO) in drug delivery systems, several significant challenges persist in its practical application. One of the primary obstacles is the lack of standardization in GO production methods, leading to inconsistencies in material properties and performance across different batches. This variability hampers reproducibility in research and poses challenges for large-scale manufacturing and regulatory approval processes.
Another critical issue is the potential toxicity of GO, which remains a subject of ongoing debate and research. While some studies suggest minimal toxicity, others indicate potential adverse effects on cellular functions and organ systems. The long-term effects of GO accumulation in the body are not yet fully understood, raising concerns about its safety for prolonged or repeated use in drug delivery applications.
The stability of GO in physiological environments presents another challenge. GO tends to aggregate in biological fluids, potentially altering its drug-carrying capacity and cellular uptake efficiency. This aggregation can lead to reduced therapeutic efficacy and unpredictable pharmacokinetics, complicating dosage determinations and treatment protocols.
Furthermore, controlling the drug release profile from GO-based delivery systems remains a significant hurdle. While GO offers high drug loading capacity, achieving precise and sustained release of therapeutics at target sites is challenging. This is particularly problematic for drugs requiring specific release kinetics or those targeting chronic conditions that necessitate long-term controlled release.
The scalability of GO production for pharmaceutical applications is another major concern. Current synthesis methods are often limited in their ability to produce large quantities of high-quality, pharmaceutically grade GO. This limitation poses significant obstacles to the commercial viability and widespread adoption of GO-based drug delivery systems.
Additionally, the complexity of functionalizing GO for specific targeting and drug loading presents technical challenges. Developing efficient and reproducible methods for attaching targeting ligands and loading diverse drug molecules onto GO surfaces while maintaining their stability and activity is an ongoing area of research.
Lastly, the regulatory landscape for nanomaterials in medicine, including GO, is still evolving. The lack of clear guidelines and standardized testing protocols for GO-based drug delivery systems complicates the path to clinical trials and eventual market approval, potentially slowing down the translation of research findings into practical medical applications.
Existing Graphene Oxide Drug Delivery Mechanisms
01 Graphene oxide-based drug delivery systems
Graphene oxide is utilized as a carrier for drug delivery due to its unique properties. These systems can be designed to improve drug solubility, enhance targeted delivery, and control drug release. The large surface area and functional groups of graphene oxide allow for efficient drug loading and modification for specific applications.- Graphene oxide-based drug delivery systems: Graphene oxide is used as a carrier for drug delivery due to its large surface area and ability to be functionalized. These systems can improve drug solubility, stability, and targeted delivery. Various methods are employed to load drugs onto graphene oxide, including physical adsorption and chemical bonding.
- Functionalization of graphene oxide for enhanced drug delivery: Graphene oxide is modified with various functional groups or molecules to improve its drug delivery capabilities. This functionalization can enhance biocompatibility, increase drug loading capacity, and enable targeted delivery to specific cells or tissues. Common modifications include PEGylation and attachment of targeting ligands.
- Graphene oxide-based nanocomposites for drug delivery: Nanocomposites combining graphene oxide with other materials, such as polymers or inorganic nanoparticles, are developed for drug delivery applications. These composites can offer improved drug loading, controlled release, and enhanced therapeutic efficacy. The synergistic properties of the combined materials are exploited for better drug delivery performance.
- Stimuli-responsive graphene oxide drug delivery systems: Graphene oxide-based drug delivery systems are designed to respond to specific stimuli such as pH, temperature, light, or magnetic fields. These smart systems allow for controlled and targeted drug release at desired locations or under specific conditions, improving therapeutic efficacy and reducing side effects.
- Graphene oxide for gene delivery and theranostics: Graphene oxide is utilized for the delivery of genetic material, such as DNA or RNA, for gene therapy applications. Additionally, graphene oxide-based systems are developed for theranostic applications, combining both therapeutic and diagnostic functions in a single platform. These systems can simultaneously deliver drugs and enable imaging or monitoring of treatment progress.
02 Functionalization of graphene oxide for drug delivery
Various methods are employed to functionalize graphene oxide, enhancing its drug delivery capabilities. This includes surface modification with polymers, biomolecules, or other functional groups to improve biocompatibility, targeting efficiency, and drug loading capacity. Functionalization can also be used to create stimuli-responsive drug release mechanisms.Expand Specific Solutions03 Graphene oxide nanocomposites for drug delivery
Graphene oxide is combined with other materials to form nanocomposites with enhanced drug delivery properties. These composites can include polymers, metals, or other nanoparticles, resulting in improved stability, drug loading capacity, and controlled release characteristics. The synergistic effects of the combined materials offer unique advantages for specific therapeutic applications.Expand Specific Solutions04 Targeted drug delivery using graphene oxide
Graphene oxide-based systems are developed for targeted drug delivery to specific tissues or cells. This is achieved through surface modification with targeting ligands, antibodies, or other molecules that can recognize specific receptors or biomarkers. The targeted approach enhances therapeutic efficacy while reducing side effects by minimizing drug exposure to non-target tissues.Expand Specific Solutions05 Graphene oxide for combination therapy and theranostics
Graphene oxide platforms are designed for combination therapy, allowing the simultaneous delivery of multiple drugs or therapeutic agents. Additionally, these systems can integrate diagnostic and therapeutic functions, enabling theranostic applications. The versatility of graphene oxide allows for the incorporation of imaging agents, making it possible to monitor drug delivery and therapeutic efficacy in real-time.Expand Specific Solutions
Key Players in Graphene Oxide Medical Research
The field of graphene oxide in medicine, particularly for enhancing drug delivery systems, is in a rapidly evolving stage. The market size is expanding, driven by increasing research and potential applications in targeted drug delivery and cancer treatment. Technologically, it's progressing from early-stage research to more advanced development, with varying levels of maturity across different applications. Key players like Northwestern University, Gwangju Institute of Science & Technology, and Korea Advanced Institute of Science & Technology are at the forefront, conducting cutting-edge research. Companies such as NOF Corp. and Sierra Oncology, Inc. are also contributing to the advancement of this technology, indicating a growing interest from both academic and industrial sectors in harnessing graphene oxide's potential for medical applications.
Memorial Sloan Kettering Cancer Center
Osaka University
Innovative Graphene Oxide Functionalization Techniques
- Development of a graphene oxide loaded nanocarrier for targeted delivery of Gemcitabine, synthesized using a modified Hummer's method, which enables controlled loading and release of the drug through functionalization with EDC, enhancing biocompatibility and physiological stability.
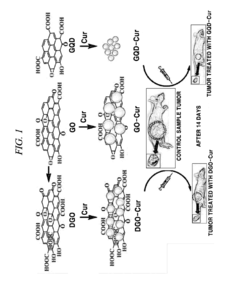
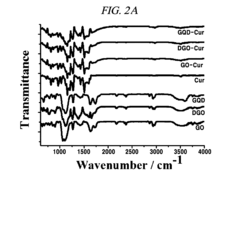
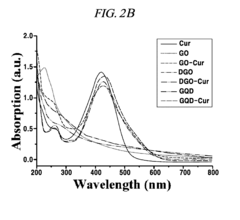
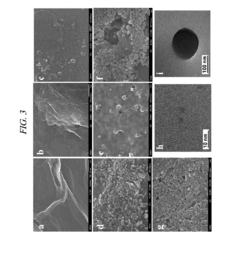
- A graphene derivative-based composition, including graphene oxide, double-oxidized graphene oxide, and graphene quantum dots, is developed for loading drugs like curcumin, doxorubicin, and paclitaxel, with controlled drug release based on pH levels and high oxygen-containing functional groups, enhancing drug loading capacity and stability.
Regulatory Framework for Nanomaterial-Based Pharmaceuticals
The regulatory framework for nanomaterial-based pharmaceuticals, including graphene oxide-enhanced drug delivery systems, is a complex and evolving landscape. Regulatory agencies worldwide are grappling with the unique challenges posed by nanomaterials in medicine, striving to ensure safety and efficacy while fostering innovation.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing guidelines for nanomedicine. The FDA's approach involves applying existing regulatory frameworks while adapting them to address the specific properties of nanomaterials. This includes considerations for particle size, surface characteristics, and potential interactions with biological systems. The agency has established the Nanotechnology Task Force to coordinate regulatory efforts across different centers within the FDA.
The European Medicines Agency (EMA) has also been proactive in addressing the regulatory challenges of nanomedicines. The EMA has published several reflection papers and guidelines specific to nanomaterial-based products, emphasizing the need for case-by-case evaluation due to the diverse nature of nanomaterials. These guidelines cover aspects such as quality, safety, and efficacy assessment of nanomedicines.
Internationally, the Organization for Economic Co-operation and Development (OECD) has been working on harmonizing regulatory approaches for nanomaterials. The OECD's Working Party on Manufactured Nanomaterials (WPMN) has developed testing guidelines and risk assessment methodologies specifically tailored for nanomaterials, which can be applied to pharmaceutical applications.
One of the key challenges in regulating nanomaterial-based pharmaceuticals is the lack of standardized testing methods. Regulatory bodies are actively collaborating with research institutions and industry to develop and validate new analytical techniques for characterizing nanomaterials in biological systems. This includes methods for assessing biodistribution, toxicity, and long-term effects of nanomaterials.
The regulatory framework also addresses the manufacturing processes of nanomedicines. Good Manufacturing Practices (GMP) for nanomaterial-based pharmaceuticals require additional considerations, such as ensuring batch-to-batch consistency in nanoparticle size, shape, and surface properties. Regulatory agencies are working on updating GMP guidelines to incorporate these nanomaterial-specific requirements.
As the field of nanomedicine rapidly evolves, regulatory frameworks are expected to continue adapting. There is a growing emphasis on developing predictive models and in silico tools to assess the safety and efficacy of nanomaterials, which could streamline the regulatory process. Additionally, international collaboration and harmonization efforts are likely to intensify to ensure consistent standards across different regions and facilitate global development and commercialization of nanomaterial-based pharmaceuticals.
Biocompatibility and Safety Considerations of Graphene Oxide
The biocompatibility and safety of graphene oxide (GO) are critical considerations for its application in drug delivery systems. GO's unique physicochemical properties, including its large surface area and high drug loading capacity, make it an attractive candidate for enhancing drug delivery. However, these same properties also raise concerns about potential toxicity and adverse effects on biological systems.
GO's interactions with biological entities are complex and depend on various factors such as size, shape, surface chemistry, and concentration. Studies have shown that GO can penetrate cell membranes and accumulate in different organs, potentially leading to oxidative stress and inflammatory responses. The sharp edges of GO nanosheets may also cause physical damage to cell membranes and organelles.
Despite these concerns, many studies have demonstrated the biocompatibility of GO at certain concentrations and in specific applications. Surface modifications, such as PEGylation or functionalization with biocompatible polymers, can significantly improve GO's safety profile by reducing its interactions with proteins and cellular components. These modifications also enhance GO's stability in biological fluids and prolong its circulation time.
The biodegradation of GO is another important aspect of its safety profile. Research has shown that GO can be degraded by enzymes such as myeloperoxidase and horseradish peroxidase, suggesting potential clearance mechanisms in the body. However, the long-term fate and potential accumulation of GO in tissues require further investigation.
Dose-dependent toxicity is a key consideration in GO-based drug delivery systems. While low concentrations of GO are generally well-tolerated, higher doses may lead to adverse effects. Therefore, careful dosage optimization is crucial for balancing therapeutic efficacy and safety. In vitro and in vivo studies have provided valuable insights into GO's toxicity profile, but more comprehensive long-term studies are needed to fully understand its safety implications.
The route of administration also plays a significant role in GO's biocompatibility and safety. Intravenous administration may pose different risks compared to topical or oral delivery. Understanding the pharmacokinetics and biodistribution of GO-based drug delivery systems is essential for assessing their safety and efficacy in various therapeutic applications.
In conclusion, while GO shows great promise in enhancing drug delivery systems, its biocompatibility and safety considerations remain paramount. Ongoing research focuses on optimizing GO's properties to minimize potential risks while maximizing its therapeutic benefits. As the field advances, regulatory guidelines and standardized safety assessments will be crucial for the successful translation of GO-based drug delivery systems into clinical applications.