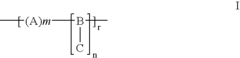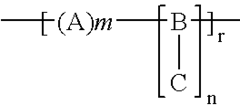Cellophane in Biomedical Applications: A Future Outlook
JUL 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cellophane Biomedical Evolution and Objectives
Cellophane, a transparent sheet made from regenerated cellulose, has a rich history in biomedical applications dating back to the early 20th century. Initially used as a packaging material, its potential in the medical field was recognized due to its unique properties such as transparency, flexibility, and biocompatibility. The evolution of cellophane in biomedical applications has been marked by continuous innovation and adaptation to meet the changing needs of the healthcare industry.
In the 1930s, cellophane found its first significant biomedical application as a material for wound dressings. Its ability to maintain a moist environment while allowing gas exchange proved beneficial for wound healing. This discovery paved the way for further exploration of cellophane's potential in various medical contexts.
The 1950s and 1960s saw a surge in cellophane's use in dialysis membranes, revolutionizing the treatment of kidney failure. Its semi-permeable nature allowed for the efficient filtration of blood, removing waste products while retaining essential proteins. This application significantly improved the quality of life for patients with renal disorders and remains a crucial use of cellophane in modern medicine.
As the field of tissue engineering emerged in the late 20th century, cellophane found new applications as a scaffold material for cell culture and tissue regeneration. Its biocompatibility and ability to be modified at the molecular level made it an attractive option for creating artificial tissues and organs.
In recent years, the focus has shifted towards developing advanced cellophane-based materials with enhanced properties. Researchers are exploring ways to incorporate antimicrobial agents, growth factors, and other bioactive compounds into cellophane matrices to create multifunctional biomaterials.
Looking ahead, the objectives for cellophane in biomedical applications are multifaceted. One primary goal is to develop cellophane-based materials with improved mechanical properties and controlled degradation rates, tailored for specific medical applications. This includes creating stronger, more durable cellophane derivatives for long-term implants and biodegradable versions for temporary medical devices.
Another objective is to enhance the bioactivity of cellophane-based materials. This involves developing techniques to functionalize cellophane surfaces with biomolecules that can promote cell adhesion, proliferation, and differentiation. Such advancements could lead to more effective tissue engineering scaffolds and drug delivery systems.
Furthermore, there is a growing interest in exploring cellophane's potential in emerging fields such as 3D bioprinting and personalized medicine. The goal is to create cellophane-based bioinks that can be precisely deposited to form complex, patient-specific tissue constructs.
In the 1930s, cellophane found its first significant biomedical application as a material for wound dressings. Its ability to maintain a moist environment while allowing gas exchange proved beneficial for wound healing. This discovery paved the way for further exploration of cellophane's potential in various medical contexts.
The 1950s and 1960s saw a surge in cellophane's use in dialysis membranes, revolutionizing the treatment of kidney failure. Its semi-permeable nature allowed for the efficient filtration of blood, removing waste products while retaining essential proteins. This application significantly improved the quality of life for patients with renal disorders and remains a crucial use of cellophane in modern medicine.
As the field of tissue engineering emerged in the late 20th century, cellophane found new applications as a scaffold material for cell culture and tissue regeneration. Its biocompatibility and ability to be modified at the molecular level made it an attractive option for creating artificial tissues and organs.
In recent years, the focus has shifted towards developing advanced cellophane-based materials with enhanced properties. Researchers are exploring ways to incorporate antimicrobial agents, growth factors, and other bioactive compounds into cellophane matrices to create multifunctional biomaterials.
Looking ahead, the objectives for cellophane in biomedical applications are multifaceted. One primary goal is to develop cellophane-based materials with improved mechanical properties and controlled degradation rates, tailored for specific medical applications. This includes creating stronger, more durable cellophane derivatives for long-term implants and biodegradable versions for temporary medical devices.
Another objective is to enhance the bioactivity of cellophane-based materials. This involves developing techniques to functionalize cellophane surfaces with biomolecules that can promote cell adhesion, proliferation, and differentiation. Such advancements could lead to more effective tissue engineering scaffolds and drug delivery systems.
Furthermore, there is a growing interest in exploring cellophane's potential in emerging fields such as 3D bioprinting and personalized medicine. The goal is to create cellophane-based bioinks that can be precisely deposited to form complex, patient-specific tissue constructs.
Market Analysis for Biomedical Cellophane
The biomedical cellophane market is experiencing significant growth, driven by the increasing demand for sustainable and biodegradable materials in healthcare applications. Cellophane, a thin transparent sheet made from regenerated cellulose, has gained traction in the biomedical field due to its unique properties and eco-friendly nature.
The global market for biomedical cellophane is projected to expand at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026. This growth is primarily attributed to the rising awareness of environmental concerns and the shift towards sustainable healthcare solutions. The market size is expected to reach $1.2 billion by 2026, up from $875 million in 2021.
Key factors driving the market include the increasing adoption of cellophane in wound dressings, drug delivery systems, and tissue engineering applications. The material's biocompatibility, transparency, and barrier properties make it an attractive option for various medical devices and packaging solutions.
Geographically, North America and Europe currently dominate the biomedical cellophane market, accounting for over 60% of the global share. However, the Asia-Pacific region is anticipated to witness the fastest growth during the forecast period, driven by rapid industrialization, improving healthcare infrastructure, and growing investments in research and development.
The wound care segment holds the largest market share, with cellophane-based dressings gaining popularity due to their ability to maintain a moist wound environment and promote healing. The drug delivery segment is expected to exhibit the highest growth rate, as cellophane's controlled release properties make it suitable for transdermal patches and oral drug delivery systems.
Challenges in the market include the high production costs associated with biomedical-grade cellophane and the competition from synthetic polymers. However, ongoing research and development efforts are focused on improving the material's properties and reducing production costs, which is expected to drive further market growth.
The increasing focus on personalized medicine and the growing demand for advanced wound care products are creating new opportunities for biomedical cellophane applications. Additionally, the rising prevalence of chronic diseases and the aging population in developed countries are expected to fuel market growth in the coming years.
The global market for biomedical cellophane is projected to expand at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026. This growth is primarily attributed to the rising awareness of environmental concerns and the shift towards sustainable healthcare solutions. The market size is expected to reach $1.2 billion by 2026, up from $875 million in 2021.
Key factors driving the market include the increasing adoption of cellophane in wound dressings, drug delivery systems, and tissue engineering applications. The material's biocompatibility, transparency, and barrier properties make it an attractive option for various medical devices and packaging solutions.
Geographically, North America and Europe currently dominate the biomedical cellophane market, accounting for over 60% of the global share. However, the Asia-Pacific region is anticipated to witness the fastest growth during the forecast period, driven by rapid industrialization, improving healthcare infrastructure, and growing investments in research and development.
The wound care segment holds the largest market share, with cellophane-based dressings gaining popularity due to their ability to maintain a moist wound environment and promote healing. The drug delivery segment is expected to exhibit the highest growth rate, as cellophane's controlled release properties make it suitable for transdermal patches and oral drug delivery systems.
Challenges in the market include the high production costs associated with biomedical-grade cellophane and the competition from synthetic polymers. However, ongoing research and development efforts are focused on improving the material's properties and reducing production costs, which is expected to drive further market growth.
The increasing focus on personalized medicine and the growing demand for advanced wound care products are creating new opportunities for biomedical cellophane applications. Additionally, the rising prevalence of chronic diseases and the aging population in developed countries are expected to fuel market growth in the coming years.
Current Challenges in Cellophane Biomedical Applications
Despite the promising potential of cellophane in biomedical applications, several significant challenges currently hinder its widespread adoption and optimal utilization. One of the primary obstacles is the limited biocompatibility of traditional cellophane. While it is generally considered non-toxic, its interaction with living tissues and biological systems can sometimes trigger unwanted immune responses or inflammation, particularly in long-term implantation scenarios.
Another critical challenge lies in the mechanical properties of cellophane. Although it possesses good flexibility and transparency, its strength and durability may not meet the rigorous demands of certain biomedical applications, especially those involving load-bearing or high-stress environments. This limitation restricts its use in areas such as orthopedic implants or cardiovascular devices where mechanical robustness is crucial.
The biodegradability of cellophane presents a double-edged sword in biomedical applications. While biodegradability can be advantageous for temporary implants or drug delivery systems, controlling the degradation rate to match the desired therapeutic timeline remains a significant challenge. Inconsistent or unpredictable degradation can lead to premature device failure or uncontrolled release of encapsulated drugs.
Surface modification and functionalization of cellophane pose another hurdle. Enhancing its surface properties to improve cell adhesion, promote tissue integration, or incorporate bioactive molecules is essential for many biomedical applications. However, achieving stable and uniform surface modifications without compromising the bulk properties of cellophane is technically challenging.
The sterilization of cellophane-based medical devices is another area of concern. Traditional sterilization methods such as high-temperature autoclaving or ethylene oxide treatment can potentially alter the physical and chemical properties of cellophane, affecting its performance and safety in biomedical applications.
Scalability and cost-effectiveness in manufacturing cellophane-based biomedical products present additional challenges. While cellophane itself is relatively inexpensive, the processes required to modify and adapt it for specific biomedical applications can be complex and costly, potentially limiting its commercial viability.
Regulatory hurdles also pose significant challenges. As a relatively new material in the biomedical field, cellophane-based devices may face stringent regulatory scrutiny, requiring extensive testing and validation to ensure safety and efficacy. This process can be time-consuming and expensive, potentially deterring investment and innovation in this area.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians to develop innovative solutions that can unlock the full potential of cellophane in biomedical applications.
Another critical challenge lies in the mechanical properties of cellophane. Although it possesses good flexibility and transparency, its strength and durability may not meet the rigorous demands of certain biomedical applications, especially those involving load-bearing or high-stress environments. This limitation restricts its use in areas such as orthopedic implants or cardiovascular devices where mechanical robustness is crucial.
The biodegradability of cellophane presents a double-edged sword in biomedical applications. While biodegradability can be advantageous for temporary implants or drug delivery systems, controlling the degradation rate to match the desired therapeutic timeline remains a significant challenge. Inconsistent or unpredictable degradation can lead to premature device failure or uncontrolled release of encapsulated drugs.
Surface modification and functionalization of cellophane pose another hurdle. Enhancing its surface properties to improve cell adhesion, promote tissue integration, or incorporate bioactive molecules is essential for many biomedical applications. However, achieving stable and uniform surface modifications without compromising the bulk properties of cellophane is technically challenging.
The sterilization of cellophane-based medical devices is another area of concern. Traditional sterilization methods such as high-temperature autoclaving or ethylene oxide treatment can potentially alter the physical and chemical properties of cellophane, affecting its performance and safety in biomedical applications.
Scalability and cost-effectiveness in manufacturing cellophane-based biomedical products present additional challenges. While cellophane itself is relatively inexpensive, the processes required to modify and adapt it for specific biomedical applications can be complex and costly, potentially limiting its commercial viability.
Regulatory hurdles also pose significant challenges. As a relatively new material in the biomedical field, cellophane-based devices may face stringent regulatory scrutiny, requiring extensive testing and validation to ensure safety and efficacy. This process can be time-consuming and expensive, potentially deterring investment and innovation in this area.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians to develop innovative solutions that can unlock the full potential of cellophane in biomedical applications.
Existing Biomedical Cellophane Solutions
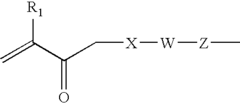
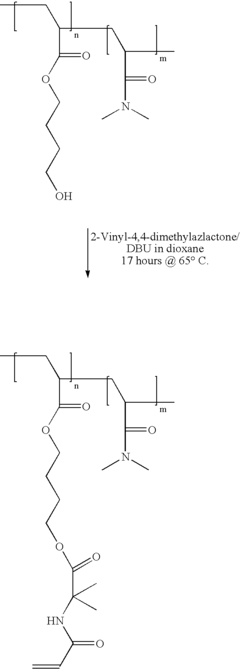
01 Cellophane production and modification
Various methods and processes for producing and modifying cellophane are described. These include techniques for improving the properties of cellophane, such as its strength, flexibility, and barrier characteristics. Modifications may involve chemical treatments or the incorporation of additives to enhance specific qualities of the material.- Cellophane production methods: Various methods for producing cellophane are described, including improvements in the manufacturing process to enhance the quality and properties of the final product. These methods may involve specific chemical treatments, extrusion techniques, or modifications to the cellulose raw material.
- Packaging applications of cellophane: Cellophane is widely used in packaging applications due to its transparency, flexibility, and barrier properties. Innovations in this area include improved packaging designs, combinations with other materials, and specialized coatings to enhance functionality for various products.
- Biodegradable cellophane alternatives: Research into biodegradable alternatives to traditional cellophane is ongoing, with a focus on developing materials that maintain similar properties while being more environmentally friendly. These alternatives may incorporate natural polymers or modified cellulose derivatives.
- Cellophane-based composite materials: Composite materials incorporating cellophane are being developed for various applications. These composites may combine cellophane with other materials to create products with enhanced strength, flexibility, or specific functional properties.
- Cellophane in medical and pharmaceutical applications: Cellophane finds use in medical and pharmaceutical applications due to its biocompatibility and barrier properties. Innovations in this area include drug delivery systems, wound dressings, and specialized packaging for medical devices or pharmaceuticals.
02 Packaging applications of cellophane
Cellophane is widely used in packaging applications due to its transparency, flexibility, and barrier properties. Innovations in this area focus on developing cellophane-based packaging solutions for various industries, including food, pharmaceuticals, and consumer goods. These developments aim to improve product preservation, extend shelf life, and enhance visual appeal.Expand Specific Solutions03 Biodegradable and eco-friendly cellophane
Research and development efforts are directed towards creating biodegradable and environmentally friendly versions of cellophane. These innovations aim to address sustainability concerns by developing cellophane-like materials that can decompose naturally without harming the environment. Such materials may incorporate plant-based components or utilize novel production processes to achieve biodegradability.Expand Specific Solutions04 Cellophane-based composite materials
Cellophane is used as a component in various composite materials to enhance their properties. These composites may combine cellophane with other materials such as polymers, fibers, or nanoparticles to create products with improved strength, flexibility, or functionality. Applications of these composites range from packaging to industrial uses.Expand Specific Solutions05 Cellophane processing and handling equipment
Specialized equipment and machinery are developed for processing, handling, and manipulating cellophane in industrial settings. These innovations focus on improving efficiency, reducing waste, and enhancing the quality of cellophane-based products. Equipment may include cutting, sealing, and packaging machines designed specifically for cellophane materials.Expand Specific Solutions
Key Players in Biomedical Cellophane Industry
The cellophane biomedical applications market is in its early growth stage, with increasing research and development activities. The market size is relatively small but expanding, driven by the material's biocompatibility and versatility. Technologically, cellophane is moderately mature, but its biomedical applications are still evolving. Key players like The University of Michigan, Harvard College, and The University of California are leading academic research, while companies such as Plasticell Ltd. and Otsuka Pharmaceutical Factory, Inc. are exploring commercial applications. The involvement of diverse institutions, including Shanghai Institute of Ceramics and Commonwealth Scientific & Industrial Research Organisation, indicates a global interest in advancing cellophane's biomedical potential.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed innovative approaches for using cellophane in biomedical applications. They have created a cellophane-based microfluidic device for drug screening and toxicity testing[1]. This platform utilizes the transparent and biocompatible nature of cellophane to create channels and chambers for cell culture and analysis. The researchers have also explored cellophane as a substrate for tissue engineering, leveraging its mechanical properties and ability to be functionalized with biomolecules[2]. Additionally, they have investigated the use of cellophane as a barrier membrane in controlled drug delivery systems, taking advantage of its semi-permeable nature[3].
Strengths: Cost-effective, easily available material; Excellent optical properties for imaging; Biocompatible and biodegradable. Weaknesses: Limited mechanical strength compared to synthetic polymers; Potential batch-to-batch variability in properties.
President & Fellows of Harvard College
Technical Solution: Harvard College has made significant strides in utilizing cellophane for biomedical applications. They have developed a cellophane-based wearable sensor for continuous health monitoring[4]. This sensor exploits the flexibility and breathability of cellophane to create a comfortable, skin-friendly device capable of measuring various physiological parameters. Harvard researchers have also engineered cellophane-based scaffolds for 3D cell culture and tissue regeneration[5]. These scaffolds leverage the biodegradability and customizable porosity of cellophane to mimic natural extracellular matrices. Furthermore, they have explored the use of modified cellophane as a wound dressing material, incorporating antimicrobial agents for enhanced healing properties[6].
Strengths: Excellent biocompatibility; Versatile material for various biomedical applications; Environmentally friendly and sustainable. Weaknesses: May require surface modifications for specific applications; Limited long-term stability in certain physiological conditions.
Innovative Cellophane Biomedical Patents
Hydrophilic biomedical compositions
PatentInactiveUS20050004255A1
Innovation
- Hydrophilic ethylenically unsaturated macromonomers with specific structures and reaction conditions, including the use of acrylamide derivatives and photoinitiators, allow for rapid crosslinking with minimal oxygen inhibition, forming biocompatible polymers with controlled mechanical properties and refractive indices, enabling in situ formation of intraocular lenses with improved incision size and reaction efficiency.
Hydrophilic biomedical composition
PatentInactiveUS6774197B1
Innovation
- Development of hydrophilic ethylenically unsaturated macromonomers that can be injected as a flowable composition and polymerized in situ, using acrylamide derivatives and specific photoinitiators to achieve rapid crosslinking with minimal oxygen inhibition, resulting in biocompatible polymers with controlled mechanical properties.
Regulatory Framework for Biomedical Materials
The regulatory framework for biomedical materials plays a crucial role in ensuring the safety and efficacy of cellophane and other materials used in biomedical applications. As cellophane gains traction in this field, it is essential to understand the current regulatory landscape and anticipate future developments.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing biomedical materials. The FDA classifies medical devices into three categories based on their risk level and intended use. Cellophane-based products would likely fall under Class II or III, requiring premarket notification (510(k)) or premarket approval (PMA), respectively. The FDA's guidance on biocompatibility testing for medical devices would be applicable to cellophane products, necessitating thorough evaluation of their biological safety.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern biomedical materials. These regulations emphasize a risk-based approach and require manufacturers to demonstrate compliance with essential safety and performance requirements. Cellophane products would need to undergo conformity assessment procedures and obtain CE marking before entering the European market.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates biomedical materials under the Pharmaceutical and Medical Device Act. The PMDA's approval process involves rigorous safety and efficacy evaluations, with a focus on quality management systems and post-market surveillance.
As the use of cellophane in biomedical applications expands, regulatory bodies may need to develop specific guidelines addressing its unique properties and potential risks. This could include standards for manufacturing processes, sterilization methods, and long-term biocompatibility assessments. Additionally, the increasing focus on sustainability in healthcare may lead to regulations promoting the use of biodegradable materials like cellophane, potentially offering a competitive advantage in the regulatory landscape.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. As cellophane gains prominence in biomedical applications, it may become a focus area for such harmonization initiatives, facilitating global market access for innovative cellophane-based products.
Regulatory agencies are also likely to adapt their frameworks to accommodate emerging technologies and manufacturing methods associated with cellophane in biomedical applications. This may include guidelines for 3D printing of cellophane-based structures or the use of nanotechnology to enhance cellophane's properties. As the field evolves, close collaboration between researchers, manufacturers, and regulatory bodies will be essential to ensure that the regulatory framework keeps pace with technological advancements while maintaining stringent safety standards.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing biomedical materials. The FDA classifies medical devices into three categories based on their risk level and intended use. Cellophane-based products would likely fall under Class II or III, requiring premarket notification (510(k)) or premarket approval (PMA), respectively. The FDA's guidance on biocompatibility testing for medical devices would be applicable to cellophane products, necessitating thorough evaluation of their biological safety.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern biomedical materials. These regulations emphasize a risk-based approach and require manufacturers to demonstrate compliance with essential safety and performance requirements. Cellophane products would need to undergo conformity assessment procedures and obtain CE marking before entering the European market.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates biomedical materials under the Pharmaceutical and Medical Device Act. The PMDA's approval process involves rigorous safety and efficacy evaluations, with a focus on quality management systems and post-market surveillance.
As the use of cellophane in biomedical applications expands, regulatory bodies may need to develop specific guidelines addressing its unique properties and potential risks. This could include standards for manufacturing processes, sterilization methods, and long-term biocompatibility assessments. Additionally, the increasing focus on sustainability in healthcare may lead to regulations promoting the use of biodegradable materials like cellophane, potentially offering a competitive advantage in the regulatory landscape.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. As cellophane gains prominence in biomedical applications, it may become a focus area for such harmonization initiatives, facilitating global market access for innovative cellophane-based products.
Regulatory agencies are also likely to adapt their frameworks to accommodate emerging technologies and manufacturing methods associated with cellophane in biomedical applications. This may include guidelines for 3D printing of cellophane-based structures or the use of nanotechnology to enhance cellophane's properties. As the field evolves, close collaboration between researchers, manufacturers, and regulatory bodies will be essential to ensure that the regulatory framework keeps pace with technological advancements while maintaining stringent safety standards.
Environmental Impact of Cellophane in Healthcare
The environmental impact of cellophane in healthcare is a critical consideration as the biomedical industry increasingly adopts this versatile material. Cellophane, a biodegradable and renewable resource derived from cellulose, offers potential advantages over traditional plastics in terms of sustainability. However, its widespread use in healthcare applications also raises concerns about waste management and ecological consequences.
One of the primary environmental benefits of cellophane in healthcare is its biodegradability. Unlike conventional petroleum-based plastics, cellophane can decompose naturally, reducing long-term environmental pollution. This characteristic is particularly valuable in medical settings, where single-use items are prevalent. The ability of cellophane to break down in landfills or composting facilities can significantly decrease the volume of persistent medical waste.
However, the production process of cellophane involves chemical treatments that may have negative environmental implications. The use of carbon disulfide and other chemicals in manufacturing can lead to air and water pollution if not properly managed. Additionally, the energy-intensive nature of cellophane production contributes to greenhouse gas emissions, potentially offsetting some of its eco-friendly attributes.
In healthcare applications, cellophane's barrier properties against moisture and bacteria make it an excellent choice for packaging medical supplies and pharmaceuticals. This can extend the shelf life of products, reducing waste from expired items. However, the increased use of cellophane in healthcare packaging may lead to a surge in overall material consumption, necessitating careful consideration of lifecycle assessments and waste reduction strategies.
The disposal of cellophane-based medical products presents both opportunities and challenges. While biodegradable, cellophane may not decompose efficiently in all waste management systems. Healthcare facilities must implement proper sorting and disposal protocols to ensure that cellophane waste is directed to appropriate treatment facilities. This may require additional infrastructure and staff training, potentially increasing the overall environmental footprint of healthcare operations.
As the healthcare industry strives for sustainability, the role of cellophane in reducing plastic pollution is gaining attention. Its potential to replace non-biodegradable plastics in various medical applications could lead to significant reductions in persistent environmental contaminants. However, a comprehensive analysis of cellophane's environmental impact throughout its lifecycle is essential to fully understand its ecological implications in healthcare settings.
One of the primary environmental benefits of cellophane in healthcare is its biodegradability. Unlike conventional petroleum-based plastics, cellophane can decompose naturally, reducing long-term environmental pollution. This characteristic is particularly valuable in medical settings, where single-use items are prevalent. The ability of cellophane to break down in landfills or composting facilities can significantly decrease the volume of persistent medical waste.
However, the production process of cellophane involves chemical treatments that may have negative environmental implications. The use of carbon disulfide and other chemicals in manufacturing can lead to air and water pollution if not properly managed. Additionally, the energy-intensive nature of cellophane production contributes to greenhouse gas emissions, potentially offsetting some of its eco-friendly attributes.
In healthcare applications, cellophane's barrier properties against moisture and bacteria make it an excellent choice for packaging medical supplies and pharmaceuticals. This can extend the shelf life of products, reducing waste from expired items. However, the increased use of cellophane in healthcare packaging may lead to a surge in overall material consumption, necessitating careful consideration of lifecycle assessments and waste reduction strategies.
The disposal of cellophane-based medical products presents both opportunities and challenges. While biodegradable, cellophane may not decompose efficiently in all waste management systems. Healthcare facilities must implement proper sorting and disposal protocols to ensure that cellophane waste is directed to appropriate treatment facilities. This may require additional infrastructure and staff training, potentially increasing the overall environmental footprint of healthcare operations.
As the healthcare industry strives for sustainability, the role of cellophane in reducing plastic pollution is gaining attention. Its potential to replace non-biodegradable plastics in various medical applications could lead to significant reductions in persistent environmental contaminants. However, a comprehensive analysis of cellophane's environmental impact throughout its lifecycle is essential to fully understand its ecological implications in healthcare settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!