How to Implement Graphene Oxide in Biocompatible Designs?
Graphene Oxide Biocompatibility: Background and Objectives
Graphene oxide (GO) has emerged as a revolutionary material in the field of biomedical engineering, offering unique properties that make it highly attractive for biocompatible designs. This two-dimensional carbon nanomaterial, derived from graphene, possesses exceptional mechanical strength, electrical conductivity, and thermal properties. The journey of GO in biomedical applications began in the early 2000s, with researchers recognizing its potential for drug delivery, tissue engineering, and biosensing.
The evolution of GO in biocompatible designs has been driven by its remarkable characteristics, including large surface area, abundant functional groups, and excellent biocompatibility. These properties have opened up numerous possibilities for tailoring GO-based materials to meet specific biological requirements. As research progressed, scientists discovered that GO could be easily functionalized with various biomolecules, enhancing its interaction with biological systems and expanding its potential applications.
One of the primary objectives in implementing GO in biocompatible designs is to develop advanced drug delivery systems. GO's large surface area and ability to carry high drug payloads make it an ideal candidate for targeted drug delivery. Researchers aim to create GO-based nanocarriers that can efficiently transport therapeutic agents to specific sites in the body, minimizing side effects and improving treatment efficacy.
Another crucial goal is to utilize GO in tissue engineering applications. The material's mechanical properties and ability to support cell adhesion and proliferation make it promising for developing scaffolds for tissue regeneration. Scientists are working towards creating GO-based composites that can mimic the extracellular matrix and promote tissue growth, particularly in bone and cartilage regeneration.
Biosensing is yet another area where GO implementation is being actively pursued. The material's high electrical conductivity and sensitivity to environmental changes make it suitable for developing highly sensitive and selective biosensors. Researchers are focusing on creating GO-based sensors for early disease detection, monitoring of biological processes, and environmental sensing.
As the field progresses, addressing potential toxicity concerns and optimizing GO's long-term biocompatibility remain critical objectives. Scientists are investigating various surface modifications and functionalization techniques to enhance GO's safety profile and reduce any potential adverse effects on biological systems.
The implementation of GO in biocompatible designs is expected to revolutionize various aspects of healthcare and biotechnology. From advanced drug delivery systems to regenerative medicine and next-generation biosensors, GO holds immense potential to address current limitations in biomedical applications and pave the way for innovative therapeutic approaches.
Market Analysis for Biocompatible Graphene Oxide Applications
The market for biocompatible graphene oxide applications is experiencing rapid growth, driven by increasing demand in healthcare, biotechnology, and advanced materials sectors. Graphene oxide's unique properties, including its high surface area, excellent mechanical strength, and biocompatibility, make it a promising material for various biomedical applications.
In the healthcare sector, graphene oxide is finding applications in drug delivery systems, biosensors, and tissue engineering. The global drug delivery market, which is a key area for graphene oxide applications, is projected to reach $2.2 trillion by 2026, with a compound annual growth rate (CAGR) of 7.2%. Graphene oxide-based drug delivery systems offer enhanced targeting and controlled release, addressing critical challenges in pharmaceutical development.
The biosensor market, another significant area for graphene oxide applications, is expected to grow at a CAGR of 8.3% from 2021 to 2028. Graphene oxide-based biosensors provide improved sensitivity and specificity for detecting various biomarkers, pathogens, and environmental contaminants. This technology is particularly relevant in the context of point-of-care diagnostics and personalized medicine.
Tissue engineering and regenerative medicine represent another promising market for biocompatible graphene oxide. The global tissue engineering market is forecasted to reach $28.9 billion by 2027, growing at a CAGR of 13.2%. Graphene oxide scaffolds offer enhanced cell adhesion, proliferation, and differentiation, making them valuable in developing advanced tissue engineering solutions.
In the advanced materials sector, graphene oxide is being explored for applications in wound healing, antimicrobial coatings, and implantable devices. The global wound care market, which can benefit from graphene oxide-based materials, is expected to reach $24.8 billion by 2024, with a CAGR of 4.6%.
Geographically, North America and Europe are currently leading in research and development of biocompatible graphene oxide applications. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure and government initiatives to promote advanced materials research.
Despite the promising market outlook, challenges remain in scaling up production, ensuring consistent quality, and addressing regulatory concerns. The successful commercialization of graphene oxide-based biocompatible products will depend on overcoming these hurdles and demonstrating clear clinical benefits over existing solutions.
Current Challenges in Graphene Oxide Biocompatibility
Despite the promising potential of graphene oxide (GO) in biomedical applications, several significant challenges persist in achieving optimal biocompatibility. One of the primary concerns is the potential cytotoxicity of GO, which can vary depending on its size, shape, and surface functionalization. Studies have shown that GO can induce oxidative stress and inflammatory responses in cells, leading to potential damage or cell death. This toxicity is particularly pronounced with smaller GO particles, which can more easily penetrate cell membranes and accumulate in tissues.
Another challenge lies in the long-term fate and biodegradation of GO in biological systems. While some studies suggest that GO can be degraded by certain enzymes, the complete elimination of GO from the body remains a concern. The potential for GO to accumulate in organs such as the liver and spleen raises questions about its long-term effects on organ function and overall health.
The interaction of GO with proteins and other biomolecules in the body presents another hurdle. GO's large surface area and abundant functional groups can lead to non-specific adsorption of proteins, potentially altering their structure and function. This protein corona formation can not only affect the intended functionality of GO-based materials but also influence their biodistribution and cellular uptake, complicating their use in targeted drug delivery or imaging applications.
Surface modification of GO to enhance biocompatibility is a double-edged sword. While functionalization can reduce toxicity and improve stability in biological environments, it may also alter the unique properties of GO that make it desirable for biomedical applications. Striking the right balance between biocompatibility and functionality remains a significant challenge for researchers.
The scalability and reproducibility of GO production for biomedical applications also pose challenges. Ensuring consistent quality, size distribution, and surface properties of GO across different batches is crucial for reliable and safe biomedical applications. However, achieving this consistency at a large scale while maintaining cost-effectiveness is an ongoing challenge in the field.
Lastly, the regulatory landscape for GO-based biomedical products remains complex and evolving. The unique properties of nanomaterials like GO necessitate careful consideration of safety and efficacy standards, which are still being developed. Navigating these regulatory challenges and establishing clear guidelines for the use of GO in biomedical applications is essential for translating research findings into clinical practice.
Existing Biocompatible Graphene Oxide Design Strategies
01 Surface modification of graphene oxide for biocompatibility
Various surface modification techniques are employed to enhance the biocompatibility of graphene oxide. These modifications can include functionalization with biocompatible polymers or molecules, which can reduce toxicity and improve cellular interactions. Such modifications can also enhance the dispersibility of graphene oxide in biological environments, making it more suitable for biomedical applications.- Surface modification of graphene oxide for improved biocompatibility: Various surface modification techniques are employed to enhance the biocompatibility of graphene oxide. These modifications can include functionalization with biocompatible polymers or molecules, which can reduce toxicity and improve cellular interactions. Such modifications can also enhance the dispersibility of graphene oxide in biological environments, making it more suitable for biomedical applications.
- Graphene oxide-based composites for biomedical applications: Graphene oxide is incorporated into various composite materials to create biocompatible structures for medical use. These composites can combine the unique properties of graphene oxide with other biocompatible materials, resulting in improved mechanical strength, electrical conductivity, or drug delivery capabilities. Such composites find applications in tissue engineering, wound healing, and biosensing.
- Evaluation of graphene oxide toxicity and biocompatibility: Extensive studies are conducted to assess the toxicity and biocompatibility of graphene oxide in various biological systems. These evaluations include in vitro cell culture studies, in vivo animal models, and long-term exposure assessments. The results help in understanding the potential risks and safe dosage levels of graphene oxide for biomedical applications.
- Size and shape-dependent biocompatibility of graphene oxide: The biocompatibility of graphene oxide is influenced by its size and shape. Research focuses on optimizing these parameters to enhance biocompatibility while maintaining desired properties. Nano-sized graphene oxide sheets or specific shapes like nanoribbons may exhibit different interactions with biological systems, affecting their overall biocompatibility and potential applications.
- Graphene oxide-based drug delivery systems: Graphene oxide is utilized as a platform for developing biocompatible drug delivery systems. Its large surface area and ability to be functionalized make it suitable for carrying various therapeutic agents. The biocompatibility of these systems is crucial for their successful application in targeted drug delivery, controlled release, and enhanced therapeutic efficacy.
02 Graphene oxide-based biocompatible composites
Graphene oxide is incorporated into various composite materials to create biocompatible structures. These composites can combine the unique properties of graphene oxide with other biocompatible materials, resulting in improved mechanical strength, electrical conductivity, or drug delivery capabilities. Such composites find applications in tissue engineering, wound healing, and biosensors.Expand Specific Solutions03 Evaluation of graphene oxide toxicity and biocompatibility
Research focuses on assessing the toxicity and biocompatibility of graphene oxide through various in vitro and in vivo studies. These evaluations consider factors such as size, shape, surface chemistry, and concentration of graphene oxide. Understanding these aspects is crucial for determining safe usage levels and potential applications in biomedical fields.Expand Specific Solutions04 Graphene oxide in drug delivery systems
Graphene oxide is utilized in the development of drug delivery systems due to its large surface area and ability to be functionalized. These systems can improve drug loading capacity, controlled release, and targeted delivery. The biocompatibility of graphene oxide in this context is crucial for ensuring safe and effective drug delivery without adverse effects on biological systems.Expand Specific Solutions05 Reduction and deoxygenation of graphene oxide for biocompatibility
Methods for reducing or deoxygenating graphene oxide are explored to enhance its biocompatibility. These processes can alter the surface properties and reactivity of graphene oxide, potentially reducing its toxicity and improving its interaction with biological systems. The resulting reduced graphene oxide may exhibit different biocompatibility profiles compared to its oxidized counterpart.Expand Specific Solutions
Key Players in Graphene Oxide Biomedical Research
The implementation of graphene oxide in biocompatible designs is currently in a rapidly evolving phase, with significant market potential in biomedical applications. The global market for graphene-based products is expanding, driven by increasing research and development efforts. Technologically, the field is progressing from early-stage research to more advanced applications, with varying levels of maturity across different sectors. Key players like Northwestern University, Tsinghua University, and the Centre National de la Recherche Scientifique are at the forefront of research, while companies such as Novartis AG are exploring commercial applications. Universities in China, including Peking University and Sichuan University, are also making substantial contributions, indicating a competitive and collaborative international landscape in this emerging field.
Northwestern University
Tsinghua University
Innovative Approaches to Graphene Oxide Biocompatibility
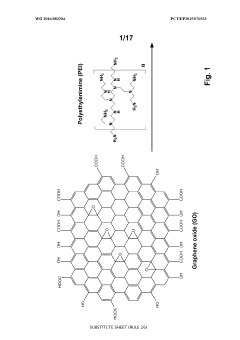
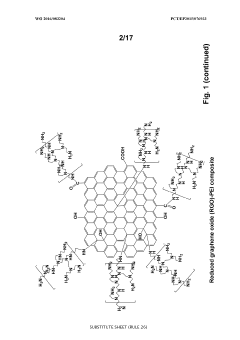
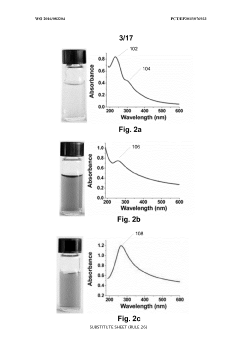
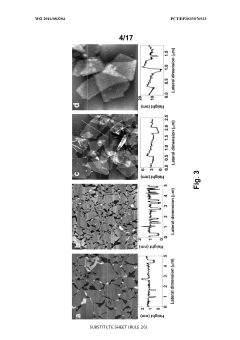
- A one-step green reduction method using polyethylenimine (PEI) forms stable and biocompatible reduced graphene oxide (RGO) nanosheets with covalent bonds, creating a biocompatible matrix that maintains enzyme activity and stability.
- Using soluble organic polymers such as wheat starch or corn starch as a support, the nano-iron-manganese oxide copolymer precursor is prepared through a redox reaction and aged under ultrasonic oscillation conditions to form a dispersed composite nano-adsorbent. Such organic polymer supports prevent agglomeration and play a bridging role during flocculation and sedimentation, achieving rapid separation.
Safety and Toxicology Considerations
The implementation of graphene oxide in biocompatible designs necessitates a thorough examination of safety and toxicology considerations. Graphene oxide's unique properties, while promising for various biomedical applications, also raise concerns about potential adverse effects on biological systems.
One primary consideration is the potential cytotoxicity of graphene oxide. Studies have shown that the toxicity of graphene oxide can vary depending on factors such as size, shape, surface functionalization, and concentration. At higher concentrations, graphene oxide has been observed to induce oxidative stress, membrane damage, and DNA damage in cells. However, at lower concentrations and with appropriate surface modifications, the cytotoxicity can be significantly reduced.
The biodistribution and accumulation of graphene oxide in various organs and tissues are crucial factors to consider. Research has indicated that graphene oxide can accumulate in the liver, spleen, and lungs following systemic administration. This accumulation may lead to long-term toxicity concerns and potential organ dysfunction. Therefore, strategies to enhance the clearance of graphene oxide from the body or to target specific tissues are essential for improving its safety profile.
Immune system interactions are another critical aspect of graphene oxide safety. Some studies have reported that graphene oxide can trigger inflammatory responses and activate the complement system. These immune reactions may lead to undesired side effects and compromise the biocompatibility of graphene oxide-based materials. Developing methods to modulate or mitigate these immune responses is crucial for successful implementation in biomedical applications.
The potential genotoxicity of graphene oxide is an area that requires careful investigation. While some studies have reported DNA damage and chromosomal aberrations in cells exposed to graphene oxide, others have found no significant genotoxic effects. The conflicting results highlight the need for standardized testing protocols and long-term studies to assess the genotoxic potential of graphene oxide accurately.
Environmental toxicity is an additional concern, particularly in the context of the potential release of graphene oxide into ecosystems. Studies have shown that graphene oxide can have adverse effects on aquatic organisms and may persist in the environment. Developing strategies for the safe disposal and degradation of graphene oxide-containing materials is essential to minimize environmental risks.
To address these safety and toxicology considerations, researchers are exploring various approaches. Surface functionalization of graphene oxide with biocompatible molecules, such as polyethylene glycol or natural polymers, has shown promise in reducing toxicity and improving biocompatibility. Additionally, the development of green synthesis methods and the use of bio-based precursors for graphene oxide production may lead to safer and more environmentally friendly materials.
Regulatory Framework for Graphene-Based Biomedical Materials
The regulatory framework for graphene-based biomedical materials is a critical aspect of implementing graphene oxide in biocompatible designs. As the field of graphene-based biomaterials rapidly evolves, regulatory bodies worldwide are working to establish comprehensive guidelines to ensure the safety and efficacy of these innovative materials.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating graphene-based biomedical materials. The FDA has developed specific guidance documents for nanomaterials, which include graphene oxide. These guidelines focus on the characterization, toxicology, and risk assessment of nanomaterials in medical devices and drug delivery systems. Manufacturers must demonstrate the safety and effectiveness of their graphene-based products through rigorous testing and clinical trials before obtaining FDA approval.
The European Medicines Agency (EMA) has also implemented regulations for nanomaterials, including graphene oxide, in biomedical applications. The EMA's guidelines emphasize the importance of thorough physicochemical characterization, in vitro and in vivo toxicity studies, and long-term safety assessments. Additionally, the European Union has established the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which requires manufacturers to register and provide safety data for nanomaterials, including graphene-based products.
In Asia, countries like Japan and South Korea have developed their own regulatory frameworks for nanomaterials in biomedical applications. The Japanese Ministry of Health, Labour and Welfare has established guidelines for the safety assessment of nanomaterials in pharmaceuticals and medical devices. Similarly, the Korean Ministry of Food and Drug Safety has implemented regulations specific to nanomaterials, including graphene oxide, in biomedical products.
International organizations, such as the International Organization for Standardization (ISO), have also contributed to the regulatory landscape by developing standards for the characterization and toxicity assessment of graphene-based materials. These standards provide a common framework for researchers and manufacturers to ensure consistency in testing and reporting.
As the field of graphene-based biomedical materials continues to advance, regulatory bodies are adapting their frameworks to address emerging challenges. Key areas of focus include the development of standardized protocols for assessing the long-term safety of graphene oxide in biological systems, establishing guidelines for the biodegradation and clearance of graphene-based materials, and addressing potential environmental impacts.
Researchers and manufacturers working on implementing graphene oxide in biocompatible designs must navigate this complex regulatory landscape to ensure compliance and facilitate the translation of their innovations from the laboratory to clinical applications. Collaboration between regulatory agencies, academic institutions, and industry stakeholders is essential to refine and harmonize the regulatory framework for graphene-based biomedical materials, ultimately promoting the safe and effective implementation of these promising materials in healthcare applications.