Evaluating the Coexistence of Bioelectronic Interfaces with Traditional Devices
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronic engineering and biological systems, marking a significant paradigm shift in how humans interact with technology. The evolution of these interfaces can be traced back to the 1970s with the development of basic neural implants, progressing through significant advancements in materials science, miniaturization, and wireless communication technologies over subsequent decades.
The 1990s witnessed the emergence of more sophisticated neural interfaces capable of recording and stimulating neural activity with greater precision. By the early 2000s, researchers had developed the first generation of practical brain-computer interfaces (BCIs), enabling limited control of external devices through neural signals. The 2010s brought remarkable progress in flexible electronics and biocompatible materials, allowing for less invasive and more durable interfaces.
Current bioelectronic interfaces encompass a diverse range of technologies, including neural implants, epidermal electronics, smart contact lenses, and ingestible sensors. These technologies are increasingly moving beyond laboratory settings into clinical applications and consumer products, signaling a transition from experimental to practical implementation.
The primary technical objectives in this field include enhancing biocompatibility to reduce foreign body responses and extend device longevity, improving signal quality and resolution for more precise biological data acquisition, and developing more efficient power solutions to enable long-term operation without frequent recharging or replacement.
Another critical objective is achieving seamless integration with existing electronic ecosystems. As bioelectronic interfaces become more prevalent, they must coexist and communicate effectively with traditional electronic devices such as smartphones, computers, and IoT devices. This integration presents unique challenges in terms of data protocols, security standards, and user experience design.
Looking forward, the field aims to develop truly symbiotic interfaces that adapt to biological systems while maintaining compatibility with conventional electronics. Research is increasingly focused on self-healing materials, biodegradable components, and adaptive algorithms that can optimize performance based on biological feedback.
The ultimate goal is to create bioelectronic interfaces that function as natural extensions of biological systems while maintaining full interoperability with traditional electronic infrastructure. This vision requires addressing fundamental questions about the long-term implications of human-technology integration and establishing appropriate regulatory frameworks to ensure safety, privacy, and ethical use of these powerful technologies.
The 1990s witnessed the emergence of more sophisticated neural interfaces capable of recording and stimulating neural activity with greater precision. By the early 2000s, researchers had developed the first generation of practical brain-computer interfaces (BCIs), enabling limited control of external devices through neural signals. The 2010s brought remarkable progress in flexible electronics and biocompatible materials, allowing for less invasive and more durable interfaces.
Current bioelectronic interfaces encompass a diverse range of technologies, including neural implants, epidermal electronics, smart contact lenses, and ingestible sensors. These technologies are increasingly moving beyond laboratory settings into clinical applications and consumer products, signaling a transition from experimental to practical implementation.
The primary technical objectives in this field include enhancing biocompatibility to reduce foreign body responses and extend device longevity, improving signal quality and resolution for more precise biological data acquisition, and developing more efficient power solutions to enable long-term operation without frequent recharging or replacement.
Another critical objective is achieving seamless integration with existing electronic ecosystems. As bioelectronic interfaces become more prevalent, they must coexist and communicate effectively with traditional electronic devices such as smartphones, computers, and IoT devices. This integration presents unique challenges in terms of data protocols, security standards, and user experience design.
Looking forward, the field aims to develop truly symbiotic interfaces that adapt to biological systems while maintaining compatibility with conventional electronics. Research is increasingly focused on self-healing materials, biodegradable components, and adaptive algorithms that can optimize performance based on biological feedback.
The ultimate goal is to create bioelectronic interfaces that function as natural extensions of biological systems while maintaining full interoperability with traditional electronic infrastructure. This vision requires addressing fundamental questions about the long-term implications of human-technology integration and establishing appropriate regulatory frameworks to ensure safety, privacy, and ethical use of these powerful technologies.
Market Analysis for Bioelectronic-Traditional Device Integration
The bioelectronic interface market is experiencing unprecedented growth, with projections indicating a compound annual growth rate of 15.2% from 2023 to 2030. This surge is primarily driven by increasing applications in healthcare, consumer electronics, and industrial automation sectors. The integration of bioelectronic interfaces with traditional devices represents a significant market opportunity estimated at $12.7 billion by 2025, as organizations seek to enhance human-machine interaction capabilities.
Healthcare remains the dominant sector for bioelectronic-traditional device integration, accounting for approximately 45% of the current market share. The demand is particularly strong for neural interfaces, smart prosthetics, and continuous health monitoring systems that seamlessly integrate with conventional medical equipment. Hospitals and rehabilitation centers are increasingly adopting hybrid systems that combine bioelectronic sensing with traditional diagnostic tools, creating a market segment growing at 18.3% annually.
Consumer electronics represents the fastest-growing application area, with wearable technology leading the integration efforts. The market for bioelectronic-enhanced consumer devices is expected to reach $5.4 billion by 2026, driven by demand for more intuitive and responsive user experiences. Products combining electroencephalography (EEG) sensors with conventional smartphones and computers are gaining significant traction, particularly among early technology adopters and gaming enthusiasts.
Regional analysis reveals North America currently dominates the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the highest growth rate at 19.7% annually, primarily due to increasing investments in bioelectronic research and manufacturing capabilities in China, Japan, and South Korea.
Market challenges include high integration costs, with bioelectronic components typically increasing device production expenses by 30-40%. Regulatory hurdles also present significant barriers, particularly for medical applications where approval processes for hybrid devices can take 40% longer than for traditional alternatives. Consumer adoption remains selective, with market penetration currently limited to high-end products and specialized applications.
Investment trends show venture capital funding for bioelectronic-traditional device integration startups reached $2.1 billion in 2022, a 35% increase from the previous year. Major technology corporations are actively pursuing acquisition strategies, with 14 significant mergers and acquisitions completed in the past 24 months, signaling industry consolidation and maturation.
The market demonstrates strong potential for cross-industry applications, with emerging opportunities in automotive (driver monitoring systems), security (advanced biometric authentication), and workplace safety (fatigue detection systems). These diversification trends suggest the integration market will continue expanding beyond its current primary applications, potentially reaching new sectors worth an additional $3.8 billion by 2027.
Healthcare remains the dominant sector for bioelectronic-traditional device integration, accounting for approximately 45% of the current market share. The demand is particularly strong for neural interfaces, smart prosthetics, and continuous health monitoring systems that seamlessly integrate with conventional medical equipment. Hospitals and rehabilitation centers are increasingly adopting hybrid systems that combine bioelectronic sensing with traditional diagnostic tools, creating a market segment growing at 18.3% annually.
Consumer electronics represents the fastest-growing application area, with wearable technology leading the integration efforts. The market for bioelectronic-enhanced consumer devices is expected to reach $5.4 billion by 2026, driven by demand for more intuitive and responsive user experiences. Products combining electroencephalography (EEG) sensors with conventional smartphones and computers are gaining significant traction, particularly among early technology adopters and gaming enthusiasts.
Regional analysis reveals North America currently dominates the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the highest growth rate at 19.7% annually, primarily due to increasing investments in bioelectronic research and manufacturing capabilities in China, Japan, and South Korea.
Market challenges include high integration costs, with bioelectronic components typically increasing device production expenses by 30-40%. Regulatory hurdles also present significant barriers, particularly for medical applications where approval processes for hybrid devices can take 40% longer than for traditional alternatives. Consumer adoption remains selective, with market penetration currently limited to high-end products and specialized applications.
Investment trends show venture capital funding for bioelectronic-traditional device integration startups reached $2.1 billion in 2022, a 35% increase from the previous year. Major technology corporations are actively pursuing acquisition strategies, with 14 significant mergers and acquisitions completed in the past 24 months, signaling industry consolidation and maturation.
The market demonstrates strong potential for cross-industry applications, with emerging opportunities in automotive (driver monitoring systems), security (advanced biometric authentication), and workplace safety (fatigue detection systems). These diversification trends suggest the integration market will continue expanding beyond its current primary applications, potentially reaching new sectors worth an additional $3.8 billion by 2027.
Technical Challenges in Bioelectronic Coexistence
The integration of bioelectronic interfaces with traditional electronic devices presents significant technical challenges that must be addressed for successful coexistence. Bioelectronic systems operate at the intersection of biology and electronics, requiring unique considerations beyond conventional electronic design principles. These challenges span multiple domains including material compatibility, signal processing, power management, and system integration.
Material biocompatibility represents a primary obstacle, as materials that interface directly with biological tissues must not trigger immune responses or cause tissue damage while maintaining electronic functionality. Current biocompatible materials often compromise on electrical performance, creating a fundamental engineering trade-off. Additionally, the wet, ion-based biological environment contrasts sharply with the electron-based operation of traditional electronics, necessitating specialized interface designs.
Signal transduction between biological and electronic systems presents another significant challenge. Biological signals are typically low-amplitude, noisy, and operate at different timescales compared to electronic signals. Converting between ionic biological signals and electronic currents requires sophisticated transducers that can maintain signal fidelity while operating in physiologically relevant conditions. The signal-to-noise ratio is particularly problematic in implantable or wearable bioelectronic systems where environmental interference is common.
Power management poses unique difficulties in bioelectronic interfaces. Traditional batteries contain toxic components unsuitable for biomedical applications, while energy harvesting from biological sources yields limited power. The development of miniaturized, biocompatible power sources that can sustain long-term operation remains an unresolved challenge, particularly for implantable devices where battery replacement requires invasive procedures.
Durability and longevity concerns are heightened in bioelectronic applications. The harsh biological environment, with its corrosive properties and constant movement, accelerates device degradation. Traditional electronic encapsulation methods often fail to provide adequate protection without compromising device functionality or increasing bulk beyond acceptable limits for biological integration.
Wireless communication capabilities, essential for bioelectronic devices to interface with external systems, face unique constraints. Signal attenuation through biological tissues, power limitations, and security concerns all complicate the implementation of reliable wireless protocols. Traditional RF approaches may cause tissue heating or require power levels incompatible with biological applications.
Regulatory and safety considerations further complicate bioelectronic development. These devices must meet both electronic performance standards and stringent biomedical safety requirements, creating a complex approval pathway that traditional electronic devices don't face. The dual-domain nature of these technologies often results in regulatory uncertainty and extended development timelines.
Material biocompatibility represents a primary obstacle, as materials that interface directly with biological tissues must not trigger immune responses or cause tissue damage while maintaining electronic functionality. Current biocompatible materials often compromise on electrical performance, creating a fundamental engineering trade-off. Additionally, the wet, ion-based biological environment contrasts sharply with the electron-based operation of traditional electronics, necessitating specialized interface designs.
Signal transduction between biological and electronic systems presents another significant challenge. Biological signals are typically low-amplitude, noisy, and operate at different timescales compared to electronic signals. Converting between ionic biological signals and electronic currents requires sophisticated transducers that can maintain signal fidelity while operating in physiologically relevant conditions. The signal-to-noise ratio is particularly problematic in implantable or wearable bioelectronic systems where environmental interference is common.
Power management poses unique difficulties in bioelectronic interfaces. Traditional batteries contain toxic components unsuitable for biomedical applications, while energy harvesting from biological sources yields limited power. The development of miniaturized, biocompatible power sources that can sustain long-term operation remains an unresolved challenge, particularly for implantable devices where battery replacement requires invasive procedures.
Durability and longevity concerns are heightened in bioelectronic applications. The harsh biological environment, with its corrosive properties and constant movement, accelerates device degradation. Traditional electronic encapsulation methods often fail to provide adequate protection without compromising device functionality or increasing bulk beyond acceptable limits for biological integration.
Wireless communication capabilities, essential for bioelectronic devices to interface with external systems, face unique constraints. Signal attenuation through biological tissues, power limitations, and security concerns all complicate the implementation of reliable wireless protocols. Traditional RF approaches may cause tissue heating or require power levels incompatible with biological applications.
Regulatory and safety considerations further complicate bioelectronic development. These devices must meet both electronic performance standards and stringent biomedical safety requirements, creating a complex approval pathway that traditional electronic devices don't face. The dual-domain nature of these technologies often results in regulatory uncertainty and extended development timelines.
Current Coexistence Solutions and Architectures
01 Wireless communication protocols for bioelectronic interfaces
Various wireless communication protocols are implemented to enable coexistence of bioelectronic interfaces with other wireless systems. These protocols include mechanisms for interference mitigation, channel selection, and adaptive frequency hopping to ensure reliable data transmission in crowded spectrum environments. Advanced algorithms allow bioelectronic devices to operate alongside other wireless technologies while maintaining signal integrity and minimizing cross-interference.- Wireless communication protocols for bioelectronic interfaces: Various wireless communication protocols are implemented to enable coexistence of bioelectronic interfaces in crowded spectrum environments. These protocols include adaptive frequency hopping, channel selection algorithms, and interference mitigation techniques that allow multiple bioelectronic devices to operate simultaneously without signal degradation. Advanced scheduling mechanisms and spectrum sharing approaches ensure reliable data transmission while minimizing power consumption for implantable and wearable bioelectronic systems.
- Integration of bioelectronic interfaces with biological systems: Techniques for seamless integration of bioelectronic interfaces with biological systems focus on biocompatibility and long-term stability. These approaches include development of flexible, stretchable materials that conform to biological tissues, surface modifications to reduce foreign body responses, and controlled release of anti-inflammatory agents. The integration strategies enable coexistence between electronic components and living tissues, facilitating applications in neural recording, stimulation, and biosensing with minimal tissue damage or immune rejection.
- Multi-modal sensing and stimulation systems: Multi-modal bioelectronic interfaces combine different sensing and stimulation modalities to enhance functionality while maintaining coexistence between various operational modes. These systems integrate electrical, optical, chemical, and mechanical transduction mechanisms within a single platform. Advanced signal processing algorithms and multiplexing techniques enable simultaneous operation of different modalities without cross-interference, allowing for comprehensive monitoring and modulation of biological processes for therapeutic and diagnostic applications.
- Power management for coexisting bioelectronic systems: Power management strategies enable the coexistence of multiple bioelectronic interfaces by optimizing energy harvesting, storage, and consumption. These approaches include adaptive duty cycling, context-aware power allocation, and wireless power transfer techniques that minimize electromagnetic interference between devices. Energy-efficient communication protocols and low-power circuit designs extend device lifetime while ensuring reliable operation of coexisting bioelectronic systems, particularly important for implantable devices with limited battery capacity.
- Interference management in bioelectronic networks: Specialized interference management techniques are developed for bioelectronic interface networks to ensure stable coexistence of multiple devices. These include adaptive filtering algorithms, cross-talk cancellation methods, and intelligent frequency coordination. Advanced shielding designs and signal isolation techniques minimize electromagnetic interference between closely positioned bioelectronic components. Machine learning approaches dynamically adjust transmission parameters based on the electromagnetic environment, enabling reliable operation of dense bioelectronic networks in clinical and consumer applications.
02 Integration of bioelectronic interfaces with biological systems
Techniques for integrating electronic interfaces with biological systems focus on biocompatibility and long-term stability. These approaches include specialized materials and surface modifications that reduce immune responses and promote tissue integration. The interfaces are designed to maintain functionality while coexisting with living cells and tissues, enabling applications in neural recording, stimulation, and biosensing without disrupting normal biological processes.Expand Specific Solutions03 Spectrum sharing and coexistence mechanisms
Advanced spectrum sharing techniques enable bioelectronic interfaces to coexist with other wireless systems in congested frequency bands. These mechanisms include cognitive radio approaches, dynamic spectrum access, and cooperative interference management. By implementing these technologies, bioelectronic devices can adaptively select optimal transmission parameters based on real-time environmental sensing, ensuring reliable operation while minimizing interference with other systems.Expand Specific Solutions04 Multi-modal sensing and communication systems
Multi-modal approaches combine different sensing and communication technologies to enhance the coexistence capabilities of bioelectronic interfaces. These systems integrate various signal modalities such as electrical, optical, and chemical sensing to provide redundancy and complementary information. By leveraging multiple communication channels, these interfaces can maintain reliable operation even when one modality experiences interference, ensuring continuous functionality in complex environments.Expand Specific Solutions05 Power management for coexisting bioelectronic systems
Specialized power management techniques enable multiple bioelectronic interfaces to coexist while optimizing energy consumption. These approaches include adaptive duty cycling, power scaling based on proximity to other devices, and coordinated transmission scheduling. Advanced energy harvesting methods are also implemented to extend device lifetime while maintaining operational compatibility with nearby electronic and biological systems, reducing the need for battery replacement or external power sources.Expand Specific Solutions
Industry Leaders in Bioelectronic Integration
The bioelectronic interfaces market is in its early growth phase, characterized by increasing convergence between traditional electronics and biological systems. The global market is projected to expand significantly, driven by healthcare applications and wearable technology integration. From a technological maturity perspective, established electronics giants like Samsung, Sony, and Philips are leveraging their consumer electronics expertise to develop commercial bioelectronic solutions, while specialized research institutions such as University of Michigan, CEA, and Fraunhofer-Gesellschaft are advancing fundamental technologies. Companies like Google and MediaTek are focusing on data integration aspects, while healthcare-focused entities like BioMerieux are exploring clinical applications. The competitive landscape reflects a blend of traditional device manufacturers adapting to biological interfaces and research institutions pioneering new technological approaches.
Koninklijke Philips NV
Technical Solution: Philips has developed an integrated bioelectronic interface system that combines traditional medical devices with bioelectronic sensors. Their approach focuses on creating seamless connectivity between implantable bioelectronic devices and conventional healthcare monitoring systems. The technology utilizes specialized electromagnetic shielding and adaptive frequency hopping to minimize interference between bioelectronic implants and traditional electronic devices. Philips' solution incorporates a proprietary signal processing algorithm that can distinguish between biological signals and electronic noise from nearby devices, allowing for reliable data collection even in electromagnetically crowded environments. Their platform includes a middleware layer that standardizes data formats across different device types, enabling interoperability between bioelectronic interfaces and traditional hospital equipment. This integration is particularly valuable in critical care settings where multiple monitoring devices must function simultaneously without interference.
Strengths: Strong integration with existing healthcare infrastructure; robust electromagnetic compatibility design; extensive clinical validation in hospital environments. Weaknesses: Higher implementation costs compared to standalone solutions; requires specialized training for healthcare providers; system complexity may limit adoption in resource-constrained settings.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has pioneered a comprehensive ecosystem for bioelectronic and traditional device coexistence through their "Bio-Digital Convergence Platform." This technology enables seamless integration between wearable bioelectronic sensors and consumer electronics. Samsung's approach utilizes a multi-layered communication protocol that allows bioelectronic interfaces to operate on separate frequency bands from traditional wireless devices, minimizing cross-interference. Their system incorporates adaptive power management that dynamically adjusts signal strength based on proximity to other electronic devices, optimizing both performance and battery life. Samsung has developed specialized microprocessors with dedicated bioelectronic signal processing cores that can filter out electromagnetic interference from nearby consumer electronics. The platform includes cloud-based synchronization that enables data from bioelectronic interfaces to be securely integrated with information from traditional devices, creating a comprehensive health monitoring ecosystem that spans from implantable sensors to smartphone applications.
Strengths: Exceptional consumer device integration; strong ecosystem approach connecting multiple device types; advanced power optimization for extended battery life in bioelectronic components. Weaknesses: Consumer-focused approach may limit applicability in specialized medical settings; proprietary standards could restrict interoperability with non-Samsung devices; higher cost compared to single-purpose medical solutions.
Key Patents in Bioelectronic-Traditional Device Compatibility
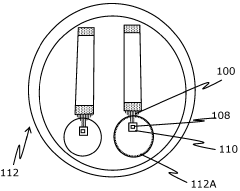
Implantable bioelectronic device and method of using same
PatentActiveGB2617100A
Innovation
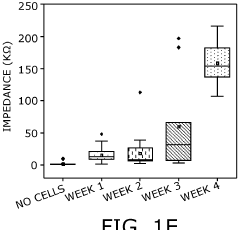
- An implantable bioelectronic device with a flexible base material, seeded with a biological sample and coated with biodegradable hydrogel, allowing for controlled synaptic integration and reduced scar tissue formation, enabling chronic implantation and improved electrophysiology recordings by mimicking nerve stiffness and promoting vascularization for nutrient diffusion.
Biocompatibility and Safety Standards
The integration of bioelectronic interfaces with traditional electronic devices necessitates rigorous biocompatibility and safety standards to ensure both user protection and device functionality. Current regulatory frameworks, including ISO 10993 for biological evaluation of medical devices and IEC 60601 for medical electrical equipment, provide foundational guidelines but require significant adaptation to address the unique challenges posed by bioelectronic interfaces.
Material selection represents a critical aspect of biocompatibility standards, with emphasis on non-toxic, non-immunogenic materials that maintain stability in biological environments. Recent advancements have introduced novel biocompatible polymers, ceramics, and composite materials specifically engineered for long-term implantation and skin contact applications, reducing inflammatory responses and tissue damage.
Electrical safety standards for bioelectronic interfaces must address both conventional electrical hazards and biological-specific concerns. These include leakage current limitations, electrical isolation requirements, and protection against electromagnetic interference. The FDA's guidance on electromagnetic compatibility for implantable medical devices offers valuable direction, though industry-specific standards for consumer bioelectronic interfaces remain underdeveloped.
Sterilization and contamination control protocols constitute another crucial dimension of safety standards. While traditional medical device sterilization methods (ethylene oxide, gamma radiation, steam) are well-established, their application to sensitive bioelectronic components presents unique challenges, necessitating the development of specialized low-temperature sterilization techniques that preserve electronic functionality.
Long-term biocompatibility testing protocols have evolved significantly, incorporating in vitro cell culture assays, animal implantation studies, and increasingly sophisticated computational modeling approaches. The emergence of organ-on-chip technologies offers promising new avenues for biocompatibility assessment without extensive animal testing, though regulatory acceptance remains limited.
International harmonization efforts, led by organizations such as the International Medical Device Regulators Forum (IMDRF), aim to standardize biocompatibility requirements across global markets. However, significant regional variations persist, creating compliance challenges for manufacturers targeting multiple markets. The European Union's Medical Device Regulation (MDR) and the FDA's regulatory framework represent the most comprehensive approaches but differ in key aspects of biocompatibility assessment.
Future standardization efforts must address emerging concerns including nanomaterial biocompatibility, biodegradable electronics, and the long-term effects of chronic low-level electrical stimulation on tissues. Additionally, standards must evolve to accommodate personalized bioelectronic interfaces, where device specifications may vary based on individual patient characteristics.
Material selection represents a critical aspect of biocompatibility standards, with emphasis on non-toxic, non-immunogenic materials that maintain stability in biological environments. Recent advancements have introduced novel biocompatible polymers, ceramics, and composite materials specifically engineered for long-term implantation and skin contact applications, reducing inflammatory responses and tissue damage.
Electrical safety standards for bioelectronic interfaces must address both conventional electrical hazards and biological-specific concerns. These include leakage current limitations, electrical isolation requirements, and protection against electromagnetic interference. The FDA's guidance on electromagnetic compatibility for implantable medical devices offers valuable direction, though industry-specific standards for consumer bioelectronic interfaces remain underdeveloped.
Sterilization and contamination control protocols constitute another crucial dimension of safety standards. While traditional medical device sterilization methods (ethylene oxide, gamma radiation, steam) are well-established, their application to sensitive bioelectronic components presents unique challenges, necessitating the development of specialized low-temperature sterilization techniques that preserve electronic functionality.
Long-term biocompatibility testing protocols have evolved significantly, incorporating in vitro cell culture assays, animal implantation studies, and increasingly sophisticated computational modeling approaches. The emergence of organ-on-chip technologies offers promising new avenues for biocompatibility assessment without extensive animal testing, though regulatory acceptance remains limited.
International harmonization efforts, led by organizations such as the International Medical Device Regulators Forum (IMDRF), aim to standardize biocompatibility requirements across global markets. However, significant regional variations persist, creating compliance challenges for manufacturers targeting multiple markets. The European Union's Medical Device Regulation (MDR) and the FDA's regulatory framework represent the most comprehensive approaches but differ in key aspects of biocompatibility assessment.
Future standardization efforts must address emerging concerns including nanomaterial biocompatibility, biodegradable electronics, and the long-term effects of chronic low-level electrical stimulation on tissues. Additionally, standards must evolve to accommodate personalized bioelectronic interfaces, where device specifications may vary based on individual patient characteristics.
Regulatory Framework for Hybrid Device Systems
The regulatory landscape for hybrid bioelectronic-traditional device systems presents significant complexity due to the convergence of multiple technological domains. Current regulatory frameworks were primarily designed for either standalone medical devices or consumer electronics, creating substantial gaps when addressing integrated systems that combine both bioelectronic interfaces and conventional electronic devices.
In the United States, the FDA has established the Digital Health Center of Excellence to address emerging technologies, but specific guidelines for hybrid systems remain underdeveloped. The FDA's premarket approval process typically categorizes devices based on risk levels, with bioelectronic interfaces often falling under Class II or III, requiring more stringent oversight than traditional consumer electronics.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide more comprehensive approaches to software as a medical device (SaMD) and connected health technologies. However, these frameworks still struggle with clearly defining regulatory pathways for devices that serve both medical and non-medical functions simultaneously.
International standards organizations such as ISO and IEC have developed standards like ISO 13485 for quality management systems and IEC 60601 for medical electrical equipment safety. These standards are increasingly incorporating provisions for connected devices, but integration points between bioelectronic and traditional systems remain inadequately addressed.
Regulatory challenges specific to hybrid systems include data privacy concerns, cybersecurity vulnerabilities at integration points, and unclear liability frameworks when multiple components from different manufacturers interact. The FDA's recent guidance on cybersecurity for networked medical devices represents progress but does not fully address the unique security challenges of bioelectronic-traditional device interfaces.
Emerging regulatory trends include the development of "regulatory sandboxes" in jurisdictions like Singapore and the UK, allowing controlled testing of hybrid systems before formal regulatory frameworks are established. Additionally, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working toward consistent approaches to software validation and artificial intelligence in medical devices.
For manufacturers developing hybrid systems, a risk-based approach to regulatory compliance is recommended, with particular attention to documentation of system boundaries, interface specifications, and comprehensive risk management across the entire integrated system rather than individual components in isolation.
In the United States, the FDA has established the Digital Health Center of Excellence to address emerging technologies, but specific guidelines for hybrid systems remain underdeveloped. The FDA's premarket approval process typically categorizes devices based on risk levels, with bioelectronic interfaces often falling under Class II or III, requiring more stringent oversight than traditional consumer electronics.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide more comprehensive approaches to software as a medical device (SaMD) and connected health technologies. However, these frameworks still struggle with clearly defining regulatory pathways for devices that serve both medical and non-medical functions simultaneously.
International standards organizations such as ISO and IEC have developed standards like ISO 13485 for quality management systems and IEC 60601 for medical electrical equipment safety. These standards are increasingly incorporating provisions for connected devices, but integration points between bioelectronic and traditional systems remain inadequately addressed.
Regulatory challenges specific to hybrid systems include data privacy concerns, cybersecurity vulnerabilities at integration points, and unclear liability frameworks when multiple components from different manufacturers interact. The FDA's recent guidance on cybersecurity for networked medical devices represents progress but does not fully address the unique security challenges of bioelectronic-traditional device interfaces.
Emerging regulatory trends include the development of "regulatory sandboxes" in jurisdictions like Singapore and the UK, allowing controlled testing of hybrid systems before formal regulatory frameworks are established. Additionally, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working toward consistent approaches to software validation and artificial intelligence in medical devices.
For manufacturers developing hybrid systems, a risk-based approach to regulatory compliance is recommended, with particular attention to documentation of system boundaries, interface specifications, and comprehensive risk management across the entire integrated system rather than individual components in isolation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!