Patents Focused on the Future of Bioelectronic Interface Technology
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Technology Background and Objectives
Bioelectronic interface technology represents the convergence of electronics and biology, enabling direct communication between biological systems and electronic devices. This field has evolved significantly over the past three decades, transitioning from rudimentary neural implants to sophisticated, minimally invasive interfaces capable of bidirectional communication with nervous systems at unprecedented resolution.
The historical trajectory of bioelectronic interfaces began with basic electrode arrays in the 1970s, progressing through microelectrode arrays in the 1990s, and now advancing toward flexible, biocompatible materials that minimize tissue damage while maximizing signal quality. Recent breakthroughs in materials science, particularly the development of ultra-thin polymers and carbon-based electronics, have dramatically improved the longevity and functionality of these interfaces.
Current technological trends indicate a shift toward wireless, self-powering systems that can operate autonomously within biological environments. The miniaturization of electronic components continues to push the boundaries of what's possible, with nanoscale interfaces emerging as a promising frontier. Additionally, advances in machine learning algorithms have significantly enhanced signal processing capabilities, allowing for more accurate interpretation of neural signals.
The primary objectives in this field center on developing interfaces that are biocompatible, durable, and capable of high-fidelity signal transduction. Researchers aim to create systems that can seamlessly integrate with biological tissues, minimize foreign body responses, and maintain functionality over extended periods without degradation or tissue damage.
Long-term goals include the development of fully implantable systems for treating neurological disorders, restoring sensory and motor functions, and establishing direct brain-computer interfaces for enhanced human capabilities. The therapeutic potential spans conditions from Parkinson's disease and epilepsy to spinal cord injuries and sensory impairments.
The convergence with other emerging technologies, particularly artificial intelligence and nanotechnology, is accelerating progress in this field. AI algorithms are increasingly capable of decoding complex neural patterns, while nanofabrication techniques enable the creation of interfaces that operate at the cellular level.
Patent activity in bioelectronic interfaces has grown exponentially in the past decade, with particular concentration in neural recording and stimulation technologies, flexible electronics for biological integration, and wireless power and data transmission systems. This surge in intellectual property development underscores the field's transition from primarily academic research to commercially viable technologies with significant market potential.
The historical trajectory of bioelectronic interfaces began with basic electrode arrays in the 1970s, progressing through microelectrode arrays in the 1990s, and now advancing toward flexible, biocompatible materials that minimize tissue damage while maximizing signal quality. Recent breakthroughs in materials science, particularly the development of ultra-thin polymers and carbon-based electronics, have dramatically improved the longevity and functionality of these interfaces.
Current technological trends indicate a shift toward wireless, self-powering systems that can operate autonomously within biological environments. The miniaturization of electronic components continues to push the boundaries of what's possible, with nanoscale interfaces emerging as a promising frontier. Additionally, advances in machine learning algorithms have significantly enhanced signal processing capabilities, allowing for more accurate interpretation of neural signals.
The primary objectives in this field center on developing interfaces that are biocompatible, durable, and capable of high-fidelity signal transduction. Researchers aim to create systems that can seamlessly integrate with biological tissues, minimize foreign body responses, and maintain functionality over extended periods without degradation or tissue damage.
Long-term goals include the development of fully implantable systems for treating neurological disorders, restoring sensory and motor functions, and establishing direct brain-computer interfaces for enhanced human capabilities. The therapeutic potential spans conditions from Parkinson's disease and epilepsy to spinal cord injuries and sensory impairments.
The convergence with other emerging technologies, particularly artificial intelligence and nanotechnology, is accelerating progress in this field. AI algorithms are increasingly capable of decoding complex neural patterns, while nanofabrication techniques enable the creation of interfaces that operate at the cellular level.
Patent activity in bioelectronic interfaces has grown exponentially in the past decade, with particular concentration in neural recording and stimulation technologies, flexible electronics for biological integration, and wireless power and data transmission systems. This surge in intellectual property development underscores the field's transition from primarily academic research to commercially viable technologies with significant market potential.
Market Demand Analysis for Bioelectronic Interfaces
The bioelectronic interface technology market is experiencing unprecedented growth, driven by increasing applications in healthcare, neuroscience research, and human-machine interaction. Current market valuations indicate the global bioelectronic medicine market reached approximately 19 billion USD in 2022, with projections suggesting a compound annual growth rate of 7-8% through 2030. This growth trajectory is supported by substantial investments from both private and public sectors, with venture capital funding for bioelectronic startups exceeding 1.5 billion USD in 2022 alone.
Healthcare applications represent the largest market segment, with neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management showing significant clinical adoption. The aging global population and rising prevalence of neurological disorders are creating sustained demand, with an estimated 50 million people worldwide potentially benefiting from neural interface technologies for therapeutic purposes.
Consumer applications are emerging as a rapidly expanding segment, particularly in areas of human augmentation and performance enhancement. Market research indicates that approximately 30% of consumers in developed economies express interest in non-invasive bioelectronic interfaces for cognitive enhancement, stress management, and improved physical performance. This represents a potential consumer base of hundreds of millions of users globally.
Military and defense sectors are also significant drivers of market demand, with investments focusing on enhanced soldier capabilities, remote operation of equipment, and rehabilitation technologies for injured personnel. Government funding for these applications has seen a 15% increase year-over-year since 2020.
Industrial applications for bioelectronic interfaces are gaining traction in high-precision manufacturing, hazardous environment operations, and advanced robotics control. The industrial segment is projected to be the fastest-growing application area, with an anticipated growth rate of 12% annually through 2028.
Geographically, North America currently dominates the market with approximately 45% share, followed by Europe (25%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to show the highest growth rate in the coming decade, driven by increasing healthcare expenditure, technological adoption, and government initiatives in countries like China, Japan, and South Korea.
Key market challenges include regulatory hurdles, with approval processes for implantable devices taking 3-7 years in major markets, as well as concerns regarding data privacy, security, and ethical considerations. Despite these challenges, market sentiment remains highly positive, with industry surveys indicating that 85% of healthcare providers believe bioelectronic interfaces will become standard treatment options for numerous conditions within the next decade.
Healthcare applications represent the largest market segment, with neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management showing significant clinical adoption. The aging global population and rising prevalence of neurological disorders are creating sustained demand, with an estimated 50 million people worldwide potentially benefiting from neural interface technologies for therapeutic purposes.
Consumer applications are emerging as a rapidly expanding segment, particularly in areas of human augmentation and performance enhancement. Market research indicates that approximately 30% of consumers in developed economies express interest in non-invasive bioelectronic interfaces for cognitive enhancement, stress management, and improved physical performance. This represents a potential consumer base of hundreds of millions of users globally.
Military and defense sectors are also significant drivers of market demand, with investments focusing on enhanced soldier capabilities, remote operation of equipment, and rehabilitation technologies for injured personnel. Government funding for these applications has seen a 15% increase year-over-year since 2020.
Industrial applications for bioelectronic interfaces are gaining traction in high-precision manufacturing, hazardous environment operations, and advanced robotics control. The industrial segment is projected to be the fastest-growing application area, with an anticipated growth rate of 12% annually through 2028.
Geographically, North America currently dominates the market with approximately 45% share, followed by Europe (25%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to show the highest growth rate in the coming decade, driven by increasing healthcare expenditure, technological adoption, and government initiatives in countries like China, Japan, and South Korea.
Key market challenges include regulatory hurdles, with approval processes for implantable devices taking 3-7 years in major markets, as well as concerns regarding data privacy, security, and ethical considerations. Despite these challenges, market sentiment remains highly positive, with industry surveys indicating that 85% of healthcare providers believe bioelectronic interfaces will become standard treatment options for numerous conditions within the next decade.
Current Status and Challenges in Bioelectronic Interface Development
Bioelectronic interface technology has witnessed remarkable advancements globally, with significant progress in both academic research and commercial applications. Currently, the field is characterized by a diverse ecosystem of technologies ranging from implantable neural interfaces to non-invasive wearable systems. Leading research institutions in North America, Europe, and Asia have established specialized centers dedicated to bioelectronic research, creating geographical clusters of innovation particularly around major medical technology hubs in the United States, Germany, Switzerland, and increasingly in China and South Korea.
Despite impressive progress, the field faces substantial technical challenges that limit widespread clinical adoption. Material biocompatibility remains a critical issue, as long-term implantation often triggers foreign body responses leading to signal degradation over time. Current materials struggle to maintain stable interfaces with biological tissues beyond a few years, necessitating replacement surgeries or resulting in diminished functionality. This challenge is particularly acute for neural interfaces where glial scarring can isolate electrodes from target neurons.
Signal quality and resolution present another significant hurdle. While recent advances in microelectrode arrays have improved spatial resolution, the signal-to-noise ratio remains problematic for many applications, especially in noisy physiological environments. Power requirements for implantable devices continue to constrain functionality, with batteries often determining device size and operational lifespan. Wireless power transmission technologies show promise but face efficiency and safety limitations when scaling to clinical applications.
Data processing capabilities represent both a challenge and opportunity. The enormous volume of biological data collected requires sophisticated algorithms for real-time analysis, particularly for closed-loop systems that must respond to physiological changes. Edge computing solutions are emerging but face power and size constraints in implantable formats.
Regulatory pathways present non-technical but equally significant barriers. The novel nature of many bioelectronic interfaces means regulatory frameworks are still evolving, creating uncertainty for developers. Clinical trials for implantable technologies are particularly complex, requiring long-term safety data that can extend development timelines significantly.
Manufacturing scalability remains problematic, with many cutting-edge interfaces relying on fabrication techniques that are difficult to standardize for mass production. This contributes to high costs that limit accessibility beyond specialized medical centers. The interdisciplinary nature of the field also creates workforce challenges, requiring professionals with expertise spanning electronics, materials science, biology, and clinical medicine.
Despite impressive progress, the field faces substantial technical challenges that limit widespread clinical adoption. Material biocompatibility remains a critical issue, as long-term implantation often triggers foreign body responses leading to signal degradation over time. Current materials struggle to maintain stable interfaces with biological tissues beyond a few years, necessitating replacement surgeries or resulting in diminished functionality. This challenge is particularly acute for neural interfaces where glial scarring can isolate electrodes from target neurons.
Signal quality and resolution present another significant hurdle. While recent advances in microelectrode arrays have improved spatial resolution, the signal-to-noise ratio remains problematic for many applications, especially in noisy physiological environments. Power requirements for implantable devices continue to constrain functionality, with batteries often determining device size and operational lifespan. Wireless power transmission technologies show promise but face efficiency and safety limitations when scaling to clinical applications.
Data processing capabilities represent both a challenge and opportunity. The enormous volume of biological data collected requires sophisticated algorithms for real-time analysis, particularly for closed-loop systems that must respond to physiological changes. Edge computing solutions are emerging but face power and size constraints in implantable formats.
Regulatory pathways present non-technical but equally significant barriers. The novel nature of many bioelectronic interfaces means regulatory frameworks are still evolving, creating uncertainty for developers. Clinical trials for implantable technologies are particularly complex, requiring long-term safety data that can extend development timelines significantly.
Manufacturing scalability remains problematic, with many cutting-edge interfaces relying on fabrication techniques that are difficult to standardize for mass production. This contributes to high costs that limit accessibility beyond specialized medical centers. The interdisciplinary nature of the field also creates workforce challenges, requiring professionals with expertise spanning electronics, materials science, biology, and clinical medicine.
Current Bioelectronic Interface Technical Solutions
01 Neural-electronic interfaces for biosensing
Neural-electronic interfaces enable direct communication between biological neural systems and electronic devices. These interfaces incorporate biocompatible materials and microelectrode arrays that can detect and record neural signals with high sensitivity. Advanced biosensing capabilities allow for real-time monitoring of neural activity, which has applications in neurological research, diagnostics, and therapeutic interventions for various neurological disorders.- Neural-electronic interfaces for biosensing: Neural-electronic interfaces enable direct communication between biological neural systems and electronic devices. These interfaces incorporate biocompatible materials and microelectrode arrays that can detect and record neural signals with high sensitivity. Advanced biosensing capabilities allow for real-time monitoring of neural activity, which has applications in neurological research, diagnostics, and the development of neural prosthetics.
- Implantable bioelectronic devices: Implantable bioelectronic devices integrate with biological tissues to provide therapeutic functions or monitor physiological parameters. These devices utilize biocompatible materials and specialized coatings to minimize immune responses and ensure long-term functionality within the body. The technology incorporates wireless power transmission and data communication capabilities, enabling remote monitoring and control of the implanted devices without invasive procedures.
- Flexible and wearable bioelectronic interfaces: Flexible and wearable bioelectronic interfaces are designed to conform to the contours of the human body, providing comfortable and non-invasive monitoring of physiological signals. These interfaces utilize stretchable electronics, conductive polymers, and thin-film technologies to create devices that can bend and flex with body movements. Applications include continuous health monitoring, athletic performance tracking, and personalized medicine.
- Molecular-level bioelectronic interfaces: Molecular-level bioelectronic interfaces facilitate direct interaction between electronic components and biological molecules such as proteins, DNA, or cells. These interfaces employ functionalized surfaces, nanomaterials, and biomolecular recognition elements to achieve high specificity and sensitivity. The technology enables applications in rapid diagnostics, drug discovery, and fundamental research on biological processes at the molecular level.
- Advanced materials for bioelectronic interfaces: Advanced materials play a crucial role in improving the performance and biocompatibility of bioelectronic interfaces. These materials include conducting polymers, carbon-based nanomaterials, hydrogels, and composite structures that mimic the mechanical and electrical properties of biological tissues. The development of these materials focuses on enhancing signal transduction, reducing biofouling, and improving long-term stability in biological environments.
02 Implantable bioelectronic devices
Implantable bioelectronic devices integrate with biological tissues to provide therapeutic functions or monitoring capabilities. These devices utilize biocompatible materials and specialized coatings to minimize immune responses and ensure long-term functionality within the body. The technology includes miniaturized electronics, power management systems, and wireless communication capabilities for data transmission, enabling applications in neural stimulation, drug delivery, and continuous health monitoring.Expand Specific Solutions03 Molecular bioelectronic interfaces
Molecular bioelectronic interfaces utilize biomolecules such as proteins, enzymes, or DNA to create functional connections between biological systems and electronic components. These interfaces employ molecular recognition principles to achieve high specificity and sensitivity in signal transduction. The technology enables the development of advanced biosensors, biofuel cells, and molecular computing systems that can operate at the nanoscale with minimal power requirements.Expand Specific Solutions04 Flexible and wearable bioelectronic systems
Flexible and wearable bioelectronic systems incorporate stretchable electronics, conductive polymers, and thin-film technologies to create interfaces that conform to biological tissues. These systems can be applied to the skin or integrated into textiles to monitor physiological parameters non-invasively. The technology enables continuous health monitoring, human-machine interfaces, and therapeutic applications while maintaining user comfort and mobility.Expand Specific Solutions05 Bioelectronic signal processing and data analysis
Advanced signal processing and data analysis techniques are essential components of bioelectronic interface technology. These methods include noise reduction algorithms, feature extraction, pattern recognition, and machine learning approaches to interpret complex biological signals. Real-time processing capabilities enable immediate feedback for closed-loop systems, while sophisticated data analysis supports diagnostic applications and personalized therapeutic interventions based on individual bioelectronic signatures.Expand Specific Solutions
Key Industry Players and Patent Holders Analysis
Bioelectronic interface technology is currently in an early growth phase, with the market expected to expand significantly as applications in healthcare and consumer electronics mature. The competitive landscape features a diverse mix of established tech giants like Apple and Panasonic alongside specialized biotech innovators such as Precision Neuroscience Corp. and academic powerhouses including Harvard, Columbia, and King's College London. Major research institutions like Lawrence Livermore National Security and Electronics & Telecommunications Research Institute are advancing fundamental research, while companies like Meta Platforms Technologies are exploring consumer applications. The technology remains in developmental stages with varying degrees of maturity across applications, with medical implementations generally more advanced than consumer-facing solutions. Cross-sector collaborations between academia, healthcare institutions, and technology companies are increasingly defining the innovation landscape.
Precision Neuroscience Corp.
Technical Solution: Precision Neuroscience has developed an ultra-thin, flexible neural interface technology called the Layer 7 Cortical Interface. This system consists of microelectrode arrays that can be inserted into the brain through a minimally invasive procedure without requiring traditional open-brain surgery. The interface utilizes a film-like array of micron-scale electrodes that conform to the brain's surface, allowing for high-resolution neural recording and stimulation. Their patented technology enables the interface to be inserted through a small cranial slit and unfurled onto the brain surface, significantly reducing surgical complexity and recovery time. The system is designed to be removable and upgradable, addressing a key limitation of many implanted neural interfaces. Precision's approach focuses on high-density electrode arrays that can record from and stimulate thousands of neurons simultaneously, providing unprecedented spatial resolution for brain-computer interfaces.
Strengths: Minimally invasive insertion technique reduces surgical risks and recovery time; ultra-thin flexible design conforms to brain tissue, potentially reducing foreign body response; high-density electrode arrays provide superior spatial resolution. Weaknesses: As with all invasive neural interfaces, long-term biocompatibility remains a challenge; signal stability over extended periods may degrade; regulatory approval pathway for brain implants is complex and lengthy.
Lawrence Livermore National Security LLC
Technical Solution: Lawrence Livermore National Laboratory has developed advanced bioelectronic interface technologies through their neural technology group, focusing on high-density microelectrode arrays for both recording and stimulation applications. Their patented technologies include the "LLNL Neural Interface Platform," which features flexible polymer-based electrode arrays with thousands of individually addressable microelectrodes. These arrays utilize novel microfabrication techniques to create ultra-small electrode sites (< 10 μm diameter) while maintaining low impedance through specialized coatings and surface treatments. A distinguishing feature of LLNL's approach is their development of hermetically sealed packaging solutions that protect sensitive electronics while remaining biocompatible and minimally invasive. Their patents also cover innovative signal processing architectures integrated directly into the implantable devices, enabling on-board spike sorting and data compression to reduce bandwidth requirements for wireless transmission. Recent developments include "neural dust" concepts - microscale, wireless sensor nodes that can be distributed throughout neural tissue to create widely distributed recording networks without the tissue displacement issues of traditional arrays.
Strengths: High-channel count systems enable large-scale neural recording; advanced packaging solutions address a critical challenge in long-term implantable electronics; integrated signal processing reduces power requirements and enhances wireless operation; national laboratory resources enable sophisticated fabrication capabilities. Weaknesses: Complex fabrication processes may limit scalability and increase costs; high-density arrays face challenges in heat dissipation that could damage surrounding tissue; wireless power delivery to deeply implanted devices remains challenging; security concerns for wireless neural interfaces require additional protective measures.
Core Patent Analysis and Technical Innovations
Hybrid bioelectrical interface device
PatentInactiveUS20090292325A1
Innovation
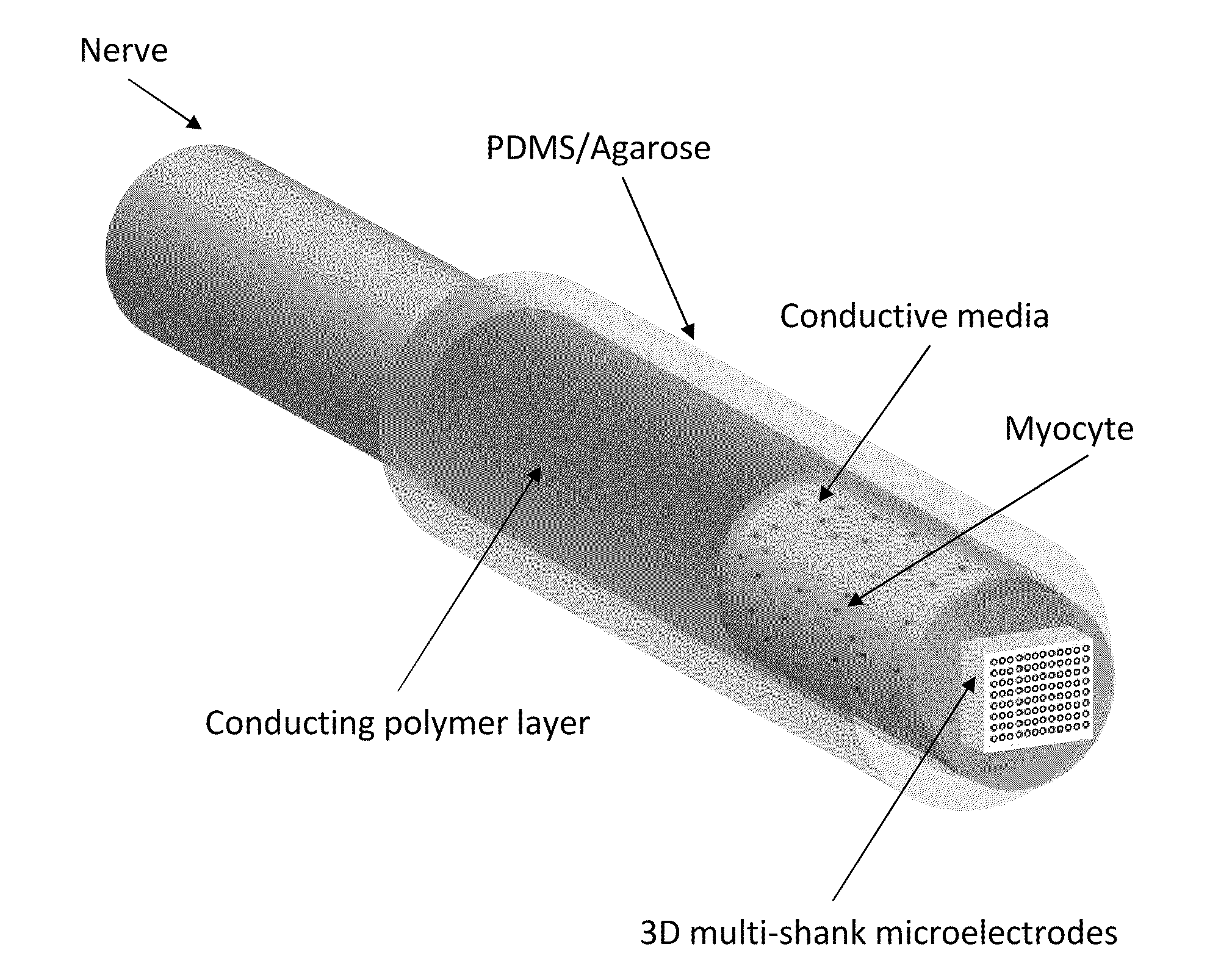
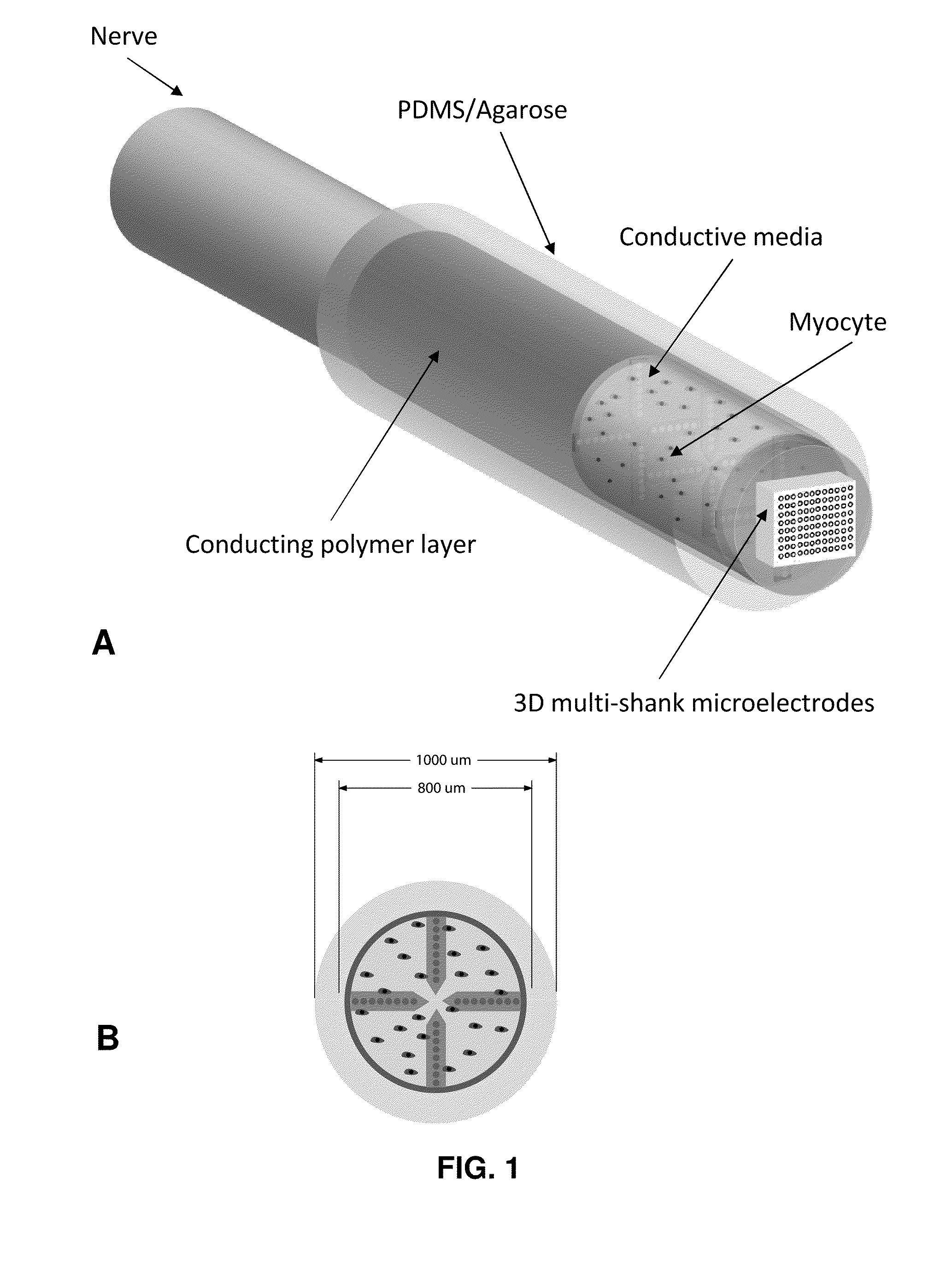
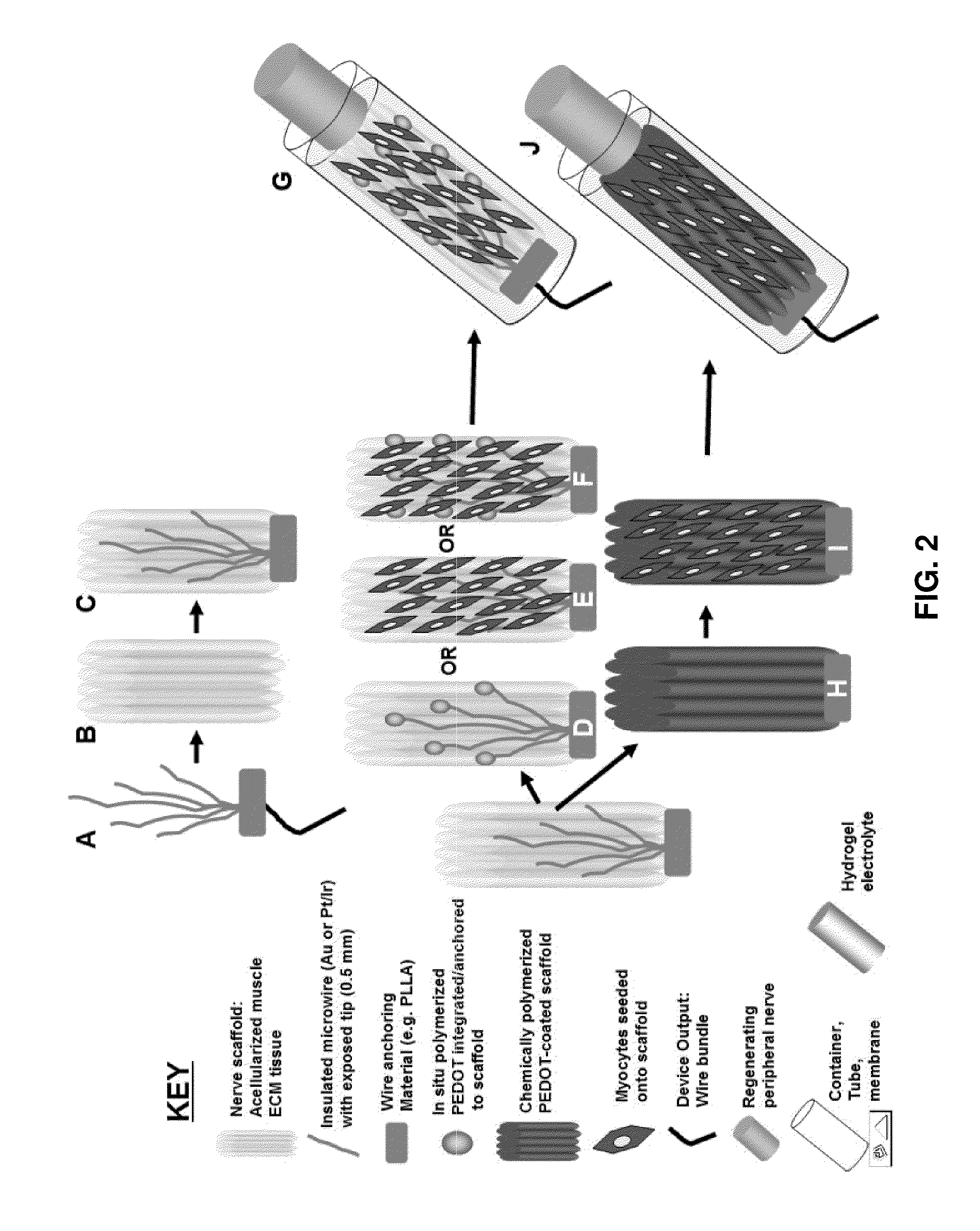
- The development of hybrid bioelectrical interface devices that combine abiotic components for charge transmission with biologically-derived or bio-functionalized materials, using conjugated polymers to facilitate electronic to ionic charge transfer, and a housing structure for nerve interfacing with synthetic neural devices and prostheses, such as polydimethylsiloxane or hydrogel materials.
Interface material for virtual reality interaction and preparation method therefor
PatentPendingUS20230211019A1
Innovation
- A hydrogel-based interface material with reversible chemical bonds for improved elasticity and biocompatibility, using an organic solvent for water retention and alkali metal salts for ionic conductivity, along with tannic acid for viscosity, is developed, allowing for reusable and high-sensitivity bioelectrical signal detection.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical challenge in bioelectronic interface development, as these devices must maintain long-term functionality while minimizing adverse biological responses. Current research focuses on developing materials that mimic biological tissues' mechanical properties, with hydrogels and elastomers showing promising results in reducing inflammatory responses. Patents from companies like Medtronic and Boston Scientific highlight innovations in coating technologies that incorporate anti-inflammatory agents and bioactive molecules to improve tissue integration.
Safety considerations extend beyond material selection to encompass electrical safety parameters. Recent patents address the challenge of maintaining stable electrical connections while preventing tissue damage from current leakage or heat generation. Innovations include self-regulating circuits that automatically adjust stimulation parameters based on tissue impedance measurements, reducing the risk of thermal injury during long-term implantation.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with the FDA and European regulatory bodies developing specialized guidelines for these hybrid medical technologies. Patent applications increasingly include comprehensive safety testing protocols that address both immediate biocompatibility and long-term stability concerns, reflecting the growing importance of regulatory compliance in commercialization strategies.
Infection risk management represents another critical safety consideration, with recent patents focusing on antimicrobial surface modifications and drug-eluting coatings. These innovations aim to prevent biofilm formation—a common cause of implant failure—without compromising electrical performance or tissue integration. Companies like Medtronic and Abbott have filed patents for interfaces incorporating controlled-release antimicrobial systems that maintain effectiveness over extended periods.
Emerging patents also address the challenge of degradation products from bioelectronic interfaces. Novel encapsulation strategies aim to contain potentially harmful breakdown products while maintaining device functionality. These approaches include multi-layered barrier systems and bioresorbable components designed to degrade into non-toxic byproducts that can be safely metabolized or excreted.
The intersection of biocompatibility and functionality presents unique challenges, as materials optimized for tissue integration often have suboptimal electrical properties. Recent patent applications describe composite materials that segregate functions—providing soft, biocompatible tissue interfaces while maintaining efficient signal transduction through specialized conductive elements. These innovations represent significant progress toward resolving the fundamental tension between biological acceptance and technical performance in bioelectronic interfaces.
Safety considerations extend beyond material selection to encompass electrical safety parameters. Recent patents address the challenge of maintaining stable electrical connections while preventing tissue damage from current leakage or heat generation. Innovations include self-regulating circuits that automatically adjust stimulation parameters based on tissue impedance measurements, reducing the risk of thermal injury during long-term implantation.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with the FDA and European regulatory bodies developing specialized guidelines for these hybrid medical technologies. Patent applications increasingly include comprehensive safety testing protocols that address both immediate biocompatibility and long-term stability concerns, reflecting the growing importance of regulatory compliance in commercialization strategies.
Infection risk management represents another critical safety consideration, with recent patents focusing on antimicrobial surface modifications and drug-eluting coatings. These innovations aim to prevent biofilm formation—a common cause of implant failure—without compromising electrical performance or tissue integration. Companies like Medtronic and Abbott have filed patents for interfaces incorporating controlled-release antimicrobial systems that maintain effectiveness over extended periods.
Emerging patents also address the challenge of degradation products from bioelectronic interfaces. Novel encapsulation strategies aim to contain potentially harmful breakdown products while maintaining device functionality. These approaches include multi-layered barrier systems and bioresorbable components designed to degrade into non-toxic byproducts that can be safely metabolized or excreted.
The intersection of biocompatibility and functionality presents unique challenges, as materials optimized for tissue integration often have suboptimal electrical properties. Recent patent applications describe composite materials that segregate functions—providing soft, biocompatible tissue interfaces while maintaining efficient signal transduction through specialized conductive elements. These innovations represent significant progress toward resolving the fundamental tension between biological acceptance and technical performance in bioelectronic interfaces.
Ethical and Privacy Implications of Neural Interfaces
The rapid advancement of bioelectronic interface technology, particularly neural interfaces, raises significant ethical and privacy concerns that must be addressed proactively. As these technologies evolve from experimental to clinical and potentially consumer applications, they create unprecedented access to neural data that was previously inaccessible. This fundamental shift demands careful consideration of the ethical frameworks governing their development and deployment.
Privacy concerns stand at the forefront of ethical considerations. Neural interfaces capture extremely sensitive biological data that may reveal not only physical health metrics but potentially thoughts, emotions, and cognitive processes. Recent patent applications from major technology companies demonstrate increasing sophistication in data extraction capabilities, raising questions about data ownership, consent mechanisms, and the potential for unauthorized access or surveillance. The intimate nature of neural data necessitates protection standards that exceed conventional data privacy frameworks.
Informed consent presents another critical challenge. As neural interfaces grow more complex, ensuring users fully understand the implications of granting access to their neural data becomes increasingly difficult. Patents focusing on seamless, minimally intrusive interfaces may inadvertently reduce user awareness of ongoing data collection. This creates tension between user experience design and ethical transparency requirements, particularly for vulnerable populations or those with cognitive impairments who may benefit most from these technologies.
The potential for algorithmic bias represents a significant concern in neural interface development. Patents analyzing neural signals must account for neurological diversity across populations. Evidence suggests that current algorithms may not perform equally across different demographic groups, potentially leading to disparate outcomes in medical applications or accessibility features. This raises questions about testing protocols and representation in development phases.
Autonomy and identity considerations emerge as neural interfaces advance toward capabilities for modulating brain activity. Patents describing therapeutic applications that can influence mood, attention, or cognitive function blur the line between treatment and enhancement. This creates complex questions about authenticity of experience, cognitive liberty, and the right to mental privacy that existing regulatory frameworks are ill-equipped to address.
The dual-use potential of neural interface technology presents additional ethical challenges. Patents originally developed for medical rehabilitation could potentially be repurposed for military applications, workplace monitoring, or deception detection without additional consent. This necessitates careful consideration of licensing agreements and technology transfer policies that incorporate ethical safeguards throughout the innovation pipeline.
Privacy concerns stand at the forefront of ethical considerations. Neural interfaces capture extremely sensitive biological data that may reveal not only physical health metrics but potentially thoughts, emotions, and cognitive processes. Recent patent applications from major technology companies demonstrate increasing sophistication in data extraction capabilities, raising questions about data ownership, consent mechanisms, and the potential for unauthorized access or surveillance. The intimate nature of neural data necessitates protection standards that exceed conventional data privacy frameworks.
Informed consent presents another critical challenge. As neural interfaces grow more complex, ensuring users fully understand the implications of granting access to their neural data becomes increasingly difficult. Patents focusing on seamless, minimally intrusive interfaces may inadvertently reduce user awareness of ongoing data collection. This creates tension between user experience design and ethical transparency requirements, particularly for vulnerable populations or those with cognitive impairments who may benefit most from these technologies.
The potential for algorithmic bias represents a significant concern in neural interface development. Patents analyzing neural signals must account for neurological diversity across populations. Evidence suggests that current algorithms may not perform equally across different demographic groups, potentially leading to disparate outcomes in medical applications or accessibility features. This raises questions about testing protocols and representation in development phases.
Autonomy and identity considerations emerge as neural interfaces advance toward capabilities for modulating brain activity. Patents describing therapeutic applications that can influence mood, attention, or cognitive function blur the line between treatment and enhancement. This creates complex questions about authenticity of experience, cognitive liberty, and the right to mental privacy that existing regulatory frameworks are ill-equipped to address.
The dual-use potential of neural interface technology presents additional ethical challenges. Patents originally developed for medical rehabilitation could potentially be repurposed for military applications, workplace monitoring, or deception detection without additional consent. This necessitates careful consideration of licensing agreements and technology transfer policies that incorporate ethical safeguards throughout the innovation pipeline.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!