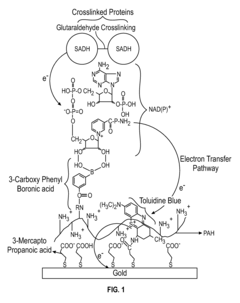
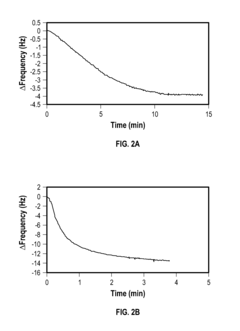
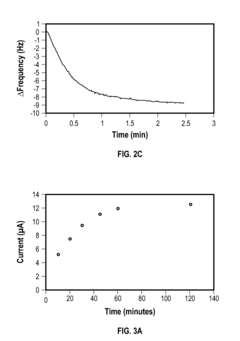
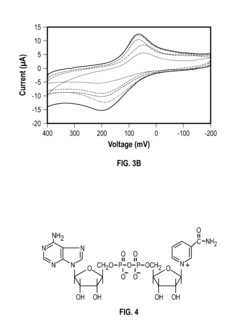
Regulations Affecting Bioelectronic Interface in Pharmaceuticals
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Regulatory Landscape and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biological systems, particularly in pharmaceutical applications. The regulatory landscape governing these technologies has evolved significantly over the past decade, reflecting both technological advancements and growing safety concerns. Initially, these interfaces were primarily regulated under traditional medical device frameworks, but their unique characteristics have necessitated more specialized regulatory approaches.
The global regulatory environment for bioelectronic interfaces in pharmaceuticals remains fragmented, with significant variations across jurisdictions. The FDA in the United States has established the Center for Devices and Radiological Health (CDRH) to oversee combination products that integrate drugs and electronic components. Meanwhile, the European Medicines Agency (EMA) has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which include specific provisions for bioelectronic technologies used in pharmaceutical applications.
In Asia, regulatory frameworks are developing at varying paces. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has introduced expedited approval pathways for innovative bioelectronic technologies, while China's National Medical Products Administration (NMPA) has recently strengthened its oversight of emerging bioelectronic pharmaceutical applications.
The primary objective of these regulatory frameworks is to ensure patient safety while fostering innovation. This includes establishing clear guidelines for biocompatibility testing, electronic component safety, data integrity, and long-term reliability. Regulatory bodies are increasingly focusing on the unique challenges posed by bioelectronic interfaces, such as tissue-electrode interactions, potential electromagnetic interference, and cybersecurity concerns.
Another critical objective is standardization across the industry. Organizations like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) are developing specific standards for bioelectronic interfaces in pharmaceutical applications. These efforts aim to harmonize testing methodologies, performance requirements, and safety parameters globally.
Looking forward, regulatory frameworks are expected to evolve toward more adaptive approaches that can accommodate rapid technological innovation. This includes the development of regulatory science initiatives focused specifically on bioelectronic interfaces and their integration with pharmaceutical products. The goal is to create pathways that balance rigorous safety evaluation with the need for timely market access.
Achieving regulatory compliance remains a significant challenge for developers of bioelectronic interfaces in pharmaceuticals. The interdisciplinary nature of these technologies often means navigating multiple regulatory domains simultaneously, requiring expertise in both pharmaceutical regulations and electronic device standards. This complexity underscores the importance of early engagement with regulatory authorities and strategic planning throughout the development process.
The global regulatory environment for bioelectronic interfaces in pharmaceuticals remains fragmented, with significant variations across jurisdictions. The FDA in the United States has established the Center for Devices and Radiological Health (CDRH) to oversee combination products that integrate drugs and electronic components. Meanwhile, the European Medicines Agency (EMA) has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which include specific provisions for bioelectronic technologies used in pharmaceutical applications.
In Asia, regulatory frameworks are developing at varying paces. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has introduced expedited approval pathways for innovative bioelectronic technologies, while China's National Medical Products Administration (NMPA) has recently strengthened its oversight of emerging bioelectronic pharmaceutical applications.
The primary objective of these regulatory frameworks is to ensure patient safety while fostering innovation. This includes establishing clear guidelines for biocompatibility testing, electronic component safety, data integrity, and long-term reliability. Regulatory bodies are increasingly focusing on the unique challenges posed by bioelectronic interfaces, such as tissue-electrode interactions, potential electromagnetic interference, and cybersecurity concerns.
Another critical objective is standardization across the industry. Organizations like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) are developing specific standards for bioelectronic interfaces in pharmaceutical applications. These efforts aim to harmonize testing methodologies, performance requirements, and safety parameters globally.
Looking forward, regulatory frameworks are expected to evolve toward more adaptive approaches that can accommodate rapid technological innovation. This includes the development of regulatory science initiatives focused specifically on bioelectronic interfaces and their integration with pharmaceutical products. The goal is to create pathways that balance rigorous safety evaluation with the need for timely market access.
Achieving regulatory compliance remains a significant challenge for developers of bioelectronic interfaces in pharmaceuticals. The interdisciplinary nature of these technologies often means navigating multiple regulatory domains simultaneously, requiring expertise in both pharmaceutical regulations and electronic device standards. This complexity underscores the importance of early engagement with regulatory authorities and strategic planning throughout the development process.
Pharmaceutical Market Demand for Bioelectronic Interfaces
The pharmaceutical industry is witnessing a significant shift toward bioelectronic interfaces, driven by the increasing need for precision medicine and personalized healthcare solutions. Market research indicates that the global bioelectronics market, including pharmaceutical applications, is projected to grow substantially over the next decade, with bioelectronic interfaces representing a critical segment of this expansion.
Patient monitoring and drug delivery systems incorporating bioelectronic interfaces are experiencing particularly strong demand. Healthcare providers and patients alike are seeking solutions that can provide real-time data on drug efficacy, physiological responses, and potential adverse reactions. This demand is further amplified by the aging global population and the corresponding increase in chronic conditions requiring continuous monitoring and treatment.
Pharmaceutical companies are increasingly investing in bioelectronic interface technologies to enhance drug development processes. These interfaces enable more precise measurement of drug interactions within the body, potentially reducing development timelines and costs while improving success rates in clinical trials. The market for such research applications is expected to grow as regulatory bodies increasingly require comprehensive data on drug safety and efficacy.
The integration of bioelectronic interfaces with traditional pharmaceutical products is creating new market categories and revenue streams. Smart pills, bioelectronic patches, and implantable drug delivery systems represent emerging product segments with substantial growth potential. These hybrid pharmaceutical-device products address unmet medical needs and command premium pricing in the marketplace.
Geographically, North America currently leads in market demand for pharmaceutical bioelectronic interfaces, followed by Europe and Asia-Pacific. However, the fastest growth is anticipated in emerging markets as healthcare infrastructure improves and access to advanced medical technologies expands. China and India, in particular, are expected to become significant markets due to their large populations and increasing healthcare expenditures.
Therapeutic areas demonstrating the highest demand include neurology, cardiology, and diabetes management. The ability of bioelectronic interfaces to provide continuous monitoring and responsive treatment for these chronic conditions addresses a substantial market need. Oncology applications are also gaining traction, with bioelectronic interfaces enabling more precise administration of potent therapeutics.
Consumer demand for greater involvement in personal healthcare management is further driving market growth. Patients increasingly expect access to their health data and prefer treatments that offer minimal disruption to daily life. Bioelectronic interfaces that can be integrated with consumer electronics and provide user-friendly interfaces are particularly well-positioned to capitalize on this trend.
Despite strong market signals, adoption barriers remain, including concerns about data privacy, cybersecurity, and the need for healthcare provider education. Addressing these concerns represents both a challenge and an opportunity for companies developing pharmaceutical bioelectronic interface technologies.
Patient monitoring and drug delivery systems incorporating bioelectronic interfaces are experiencing particularly strong demand. Healthcare providers and patients alike are seeking solutions that can provide real-time data on drug efficacy, physiological responses, and potential adverse reactions. This demand is further amplified by the aging global population and the corresponding increase in chronic conditions requiring continuous monitoring and treatment.
Pharmaceutical companies are increasingly investing in bioelectronic interface technologies to enhance drug development processes. These interfaces enable more precise measurement of drug interactions within the body, potentially reducing development timelines and costs while improving success rates in clinical trials. The market for such research applications is expected to grow as regulatory bodies increasingly require comprehensive data on drug safety and efficacy.
The integration of bioelectronic interfaces with traditional pharmaceutical products is creating new market categories and revenue streams. Smart pills, bioelectronic patches, and implantable drug delivery systems represent emerging product segments with substantial growth potential. These hybrid pharmaceutical-device products address unmet medical needs and command premium pricing in the marketplace.
Geographically, North America currently leads in market demand for pharmaceutical bioelectronic interfaces, followed by Europe and Asia-Pacific. However, the fastest growth is anticipated in emerging markets as healthcare infrastructure improves and access to advanced medical technologies expands. China and India, in particular, are expected to become significant markets due to their large populations and increasing healthcare expenditures.
Therapeutic areas demonstrating the highest demand include neurology, cardiology, and diabetes management. The ability of bioelectronic interfaces to provide continuous monitoring and responsive treatment for these chronic conditions addresses a substantial market need. Oncology applications are also gaining traction, with bioelectronic interfaces enabling more precise administration of potent therapeutics.
Consumer demand for greater involvement in personal healthcare management is further driving market growth. Patients increasingly expect access to their health data and prefer treatments that offer minimal disruption to daily life. Bioelectronic interfaces that can be integrated with consumer electronics and provide user-friendly interfaces are particularly well-positioned to capitalize on this trend.
Despite strong market signals, adoption barriers remain, including concerns about data privacy, cybersecurity, and the need for healthcare provider education. Addressing these concerns represents both a challenge and an opportunity for companies developing pharmaceutical bioelectronic interface technologies.
Global Regulatory Challenges for Bioelectronic Pharmaceutical Devices
The bioelectronic interface in pharmaceuticals represents a convergence of electronic technology and biological systems, creating novel therapeutic approaches. However, this innovative field faces a complex global regulatory landscape that varies significantly across regions, creating substantial challenges for developers and manufacturers.
In the United States, the FDA has established a regulatory framework that often classifies bioelectronic pharmaceutical devices as combination products, requiring compliance with both drug and device regulations. The 21st Century Cures Act has attempted to streamline approval processes, but regulatory pathways remain complex for these hybrid technologies that don't fit neatly into traditional categories.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Additionally, the EU's GDPR imposes strict data protection requirements that affect bioelectronic devices collecting patient data, adding another layer of compliance complexity.
In Asia, regulatory approaches vary dramatically. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has implemented the Sakigake designation to accelerate innovative medical technologies, while China's National Medical Products Administration (NMPA) has been reforming its regulatory framework to expedite approvals while maintaining stringent control over foreign technologies.
A significant challenge across all jurisdictions is the lack of harmonized standards specifically designed for bioelectronic interfaces. International bodies like the International Medical Device Regulators Forum (IMDRF) are working toward greater harmonization, but progress remains slow relative to technological advancement.
Cybersecurity regulations present another critical challenge, as bioelectronic devices often incorporate wireless connectivity and data transmission capabilities. Regulatory bodies increasingly require robust security measures to prevent unauthorized access and protect patient safety, though specific requirements vary by jurisdiction.
Reimbursement pathways represent a non-traditional regulatory hurdle, with health technology assessment bodies in different countries applying varying criteria to determine coverage. This economic aspect of regulation can significantly impact market access despite regulatory approval.
The rapidly evolving nature of bioelectronic technology often outpaces regulatory frameworks, creating situations where novel technologies must navigate outdated regulatory pathways. Regulatory science initiatives are attempting to address this gap, but significant challenges remain in creating adaptive regulatory approaches that ensure safety while enabling innovation.
In the United States, the FDA has established a regulatory framework that often classifies bioelectronic pharmaceutical devices as combination products, requiring compliance with both drug and device regulations. The 21st Century Cures Act has attempted to streamline approval processes, but regulatory pathways remain complex for these hybrid technologies that don't fit neatly into traditional categories.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Additionally, the EU's GDPR imposes strict data protection requirements that affect bioelectronic devices collecting patient data, adding another layer of compliance complexity.
In Asia, regulatory approaches vary dramatically. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has implemented the Sakigake designation to accelerate innovative medical technologies, while China's National Medical Products Administration (NMPA) has been reforming its regulatory framework to expedite approvals while maintaining stringent control over foreign technologies.
A significant challenge across all jurisdictions is the lack of harmonized standards specifically designed for bioelectronic interfaces. International bodies like the International Medical Device Regulators Forum (IMDRF) are working toward greater harmonization, but progress remains slow relative to technological advancement.
Cybersecurity regulations present another critical challenge, as bioelectronic devices often incorporate wireless connectivity and data transmission capabilities. Regulatory bodies increasingly require robust security measures to prevent unauthorized access and protect patient safety, though specific requirements vary by jurisdiction.
Reimbursement pathways represent a non-traditional regulatory hurdle, with health technology assessment bodies in different countries applying varying criteria to determine coverage. This economic aspect of regulation can significantly impact market access despite regulatory approval.
The rapidly evolving nature of bioelectronic technology often outpaces regulatory frameworks, creating situations where novel technologies must navigate outdated regulatory pathways. Regulatory science initiatives are attempting to address this gap, but significant challenges remain in creating adaptive regulatory approaches that ensure safety while enabling innovation.
Current Compliance Frameworks for Bioelectronic Pharmaceuticals
01 Neural-electronic interfaces for biosensing
Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for monitoring of neural activity and detection of biomarkers. The technology incorporates specialized electrodes and transducers that can detect electrical signals from neurons and translate them into electronic data for analysis and interpretation.- Neural-electronic interfaces for biosensing: Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, providing valuable data for medical diagnostics and neural research.
- Implantable bioelectronic devices: Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices are engineered with biocompatible materials and specialized coatings to minimize immune response and enhance tissue integration. They can monitor physiological parameters, deliver therapeutic stimulation, or restore lost biological functions. Advanced designs incorporate wireless power and data transmission capabilities to eliminate the need for transcutaneous connections.
- Molecular bioelectronic interfaces: Interfaces that utilize molecular components to bridge the gap between biological systems and electronic devices. These interfaces employ biomolecules such as proteins, enzymes, or DNA as functional elements to facilitate signal transduction between biological and electronic domains. The molecular components can be engineered to respond to specific biological signals and convert them into electronic outputs, enabling highly selective and sensitive detection systems.
- Flexible and wearable bioelectronic interfaces: Bioelectronic interfaces designed with flexible, stretchable materials that conform to biological tissues for non-invasive monitoring. These interfaces can be integrated into wearable devices that adhere to the skin or incorporated into textiles. The flexibility allows for continuous monitoring of physiological parameters while minimizing discomfort and enabling natural movement. Advanced materials and fabrication techniques ensure stable electrical performance despite mechanical deformation.
- Nanomaterial-based bioelectronic interfaces: Bioelectronic interfaces that incorporate nanomaterials such as carbon nanotubes, graphene, or quantum dots to enhance performance and functionality. These nanomaterials provide improved electrical conductivity, increased surface area for biological interaction, and enhanced signal transduction capabilities. The nanoscale dimensions enable interfaces with individual cells or even subcellular components, allowing for unprecedented spatial resolution in biological sensing and stimulation applications.
02 Implantable bioelectronic devices
Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices are engineered with biocompatible materials and specialized coatings to minimize immune response and enhance integration with surrounding tissues. They can be used for continuous monitoring of physiological parameters, drug delivery, or stimulation of specific tissues, with applications in neurological disorders, cardiac monitoring, and other medical conditions requiring persistent interaction with biological systems.Expand Specific Solutions03 Flexible and wearable bioelectronic sensors
Flexible and wearable bioelectronic interfaces that conform to the body's contours for non-invasive monitoring. These sensors utilize stretchable electronics, conductive polymers, and thin-film technologies to create comfortable, skin-adherent devices that can detect various physiological signals. The flexibility allows for better contact with biological tissues, improving signal quality while maintaining user comfort for extended periods of monitoring.Expand Specific Solutions04 Molecular bioelectronic interfaces
Bioelectronic interfaces at the molecular level that utilize biomolecules as functional components. These interfaces incorporate proteins, enzymes, DNA, or other biological molecules as active elements in electronic circuits. The biomolecules can serve as recognition elements, signal transducers, or catalysts, enabling highly specific detection of target analytes or biological processes with enhanced sensitivity and selectivity compared to conventional electronic sensors.Expand Specific Solutions05 Nanomaterial-based bioelectronic interfaces
Bioelectronic interfaces utilizing nanomaterials such as carbon nanotubes, graphene, and quantum dots to enhance performance. These nanomaterials provide increased surface area, improved electrical conductivity, and unique optical properties that enhance signal transduction between biological systems and electronic components. The nanoscale dimensions of these materials enable interaction with biological systems at the cellular and subcellular levels, improving sensitivity and spatial resolution of bioelectronic devices.Expand Specific Solutions
Key Regulatory Bodies and Pharmaceutical Companies
The bioelectronic interface in pharmaceuticals regulatory landscape is evolving rapidly, currently positioned at an early growth stage with significant market expansion potential. The market is characterized by increasing collaboration between technology and pharmaceutical sectors, with key players including MIT, Apple, and Robert Bosch driving innovation alongside pharmaceutical companies like Wyeth, Hoffmann-La Roche, and Alkermes. Academic institutions (Harvard, Michigan, California) are contributing crucial research, while healthcare providers (Brigham & Women's Hospital, General Hospital Corp) bridge clinical implementation gaps. The regulatory framework remains complex, with varying maturity across regions, requiring companies to navigate FDA, EMA, and international standards while addressing privacy, safety, and ethical considerations in this emerging bioelectronic medicine field.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced bioelectronic interfaces that integrate seamlessly with pharmaceutical delivery systems, focusing on regulatory-compliant microelectronic devices. Their platform includes implantable microchips capable of controlled drug release through electrical stimulation, with built-in biosensors for real-time monitoring of physiological responses. MIT's technology incorporates secure wireless communication protocols that meet FDA cybersecurity requirements for medical devices, allowing remote monitoring while maintaining data integrity. Their systems feature closed-loop feedback mechanisms that automatically adjust drug delivery based on patient response, with all components designed to meet ISO 10993 biocompatibility standards and IEC 60601 medical electrical equipment safety standards. MIT researchers have pioneered miniaturized electronics that minimize tissue inflammation while maintaining therapeutic efficacy, addressing key regulatory concerns about long-term implantable devices.
Strengths: Superior integration of microelectronics with biological systems; strong compliance with FDA device regulations; extensive intellectual property portfolio. Weaknesses: Higher manufacturing costs compared to conventional drug delivery systems; potential challenges with long-term biocompatibility despite engineering efforts; complex regulatory pathway requiring both pharmaceutical and medical device approvals.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive bioelectronic interface platform for pharmaceutical applications that addresses multiple regulatory challenges. Their system incorporates flexible, biodegradable electronics that conform to tissue surfaces while minimizing foreign body responses, thereby addressing FDA concerns about biocompatibility. The platform features multiplexed sensing capabilities that simultaneously monitor drug concentrations, metabolites, and physiological parameters, providing the comprehensive data required by regulatory bodies for precision medicine applications. UC researchers have implemented advanced encryption protocols that protect patient data while enabling secure transmission to healthcare providers, meeting HIPAA requirements and FDA cybersecurity guidelines. Their technology includes self-calibrating circuits that maintain measurement accuracy over extended periods, addressing regulatory concerns about long-term reliability of implantable sensors. The system's modular architecture allows components to be independently validated against specific regulatory standards, streamlining the approval process for complex bioelectronic pharmaceutical systems.
Strengths: Exceptional biocompatibility through biodegradable components; comprehensive data collection capabilities supporting regulatory submissions; modular design facilitating incremental regulatory approvals. Weaknesses: Complex manufacturing processes potentially limiting scalability; challenges in standardizing performance across diverse patient populations; potential regulatory hurdles for novel biodegradable electronic materials.
Critical Regulatory Standards and Guidance Documents
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
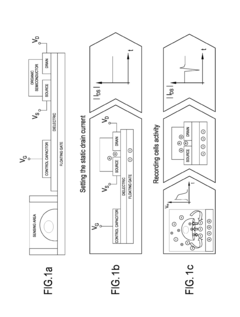
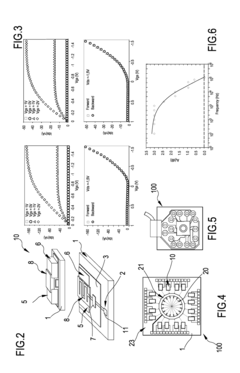
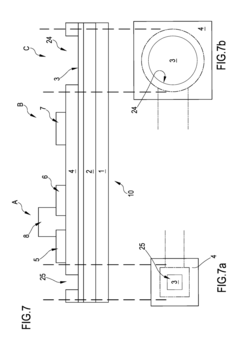
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
Customizable and renewable nanostructured interface for bioelectronic applications
PatentInactiveUS8435773B2
Innovation
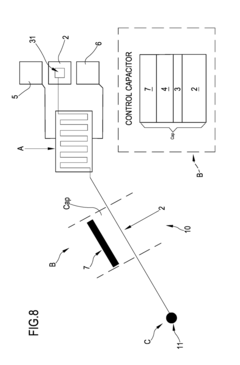
- A biomimetic device with a polyelectrolyte-bound enzyme system that allows reversible binding to a treated substrate electrode, facilitating efficient electron transfer and regeneration by electrostatic attraction, using charged polyelectrolytes like poly(allylamine hydrochloride) and poly(ethyleneimine) to maintain the enzyme, cofactor, and mediator in proper orientation, enabling efficient electron transfer and easy regeneration.
Patient Safety and Data Privacy Considerations
The integration of bioelectronic interfaces in pharmaceutical applications introduces significant patient safety and data privacy considerations that must be addressed through comprehensive regulatory frameworks. These devices, which interact directly with biological systems, collect sensitive health data that requires stringent protection measures.
Patient safety remains paramount when implementing bioelectronic interfaces in pharmaceutical contexts. These devices must undergo rigorous clinical testing to ensure they do not cause adverse physiological reactions or tissue damage during extended use. The FDA and EMA have established specific safety protocols for implantable electronic devices, requiring manufacturers to demonstrate biocompatibility, electrical safety, and long-term stability. Recent incidents involving bioelectronic implants have prompted regulatory bodies to implement more stringent post-market surveillance requirements.
Data privacy considerations present equally complex challenges as bioelectronic interfaces continuously monitor physiological parameters and potentially transmit this information to external systems. This data flow creates vulnerabilities that could compromise patient confidentiality if not properly secured. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe provide baseline requirements for handling such sensitive health information.
Emerging regulations increasingly focus on the concept of "privacy by design," requiring manufacturers to incorporate data protection measures from the earliest stages of product development. This includes implementing end-to-end encryption, secure authentication protocols, and data minimization strategies. The International Medical Device Regulators Forum (IMDRF) has published guidance specifically addressing cybersecurity requirements for connected medical devices, which applies directly to bioelectronic pharmaceutical interfaces.
Informed consent processes are evolving to address the unique nature of bioelectronic data collection. Patients must understand not only the immediate medical implications but also how their data will be stored, processed, and potentially shared. Regulatory frameworks increasingly require dynamic consent models that allow patients to modify their data sharing preferences over time as the use of their information evolves.
Cross-border data transfer presents additional regulatory challenges, particularly for multinational pharmaceutical companies deploying bioelectronic solutions globally. Different jurisdictions maintain varying standards for data protection, necessitating complex compliance strategies. The EU-US Data Privacy Framework and similar arrangements attempt to facilitate compliant data transfers while maintaining protection standards.
As artificial intelligence becomes more integrated with bioelectronic interfaces, regulations are beginning to address algorithmic transparency and accountability. Patients and healthcare providers must understand how AI-driven decisions are made when these systems automatically adjust drug delivery or treatment parameters based on bioelectronic monitoring.
Patient safety remains paramount when implementing bioelectronic interfaces in pharmaceutical contexts. These devices must undergo rigorous clinical testing to ensure they do not cause adverse physiological reactions or tissue damage during extended use. The FDA and EMA have established specific safety protocols for implantable electronic devices, requiring manufacturers to demonstrate biocompatibility, electrical safety, and long-term stability. Recent incidents involving bioelectronic implants have prompted regulatory bodies to implement more stringent post-market surveillance requirements.
Data privacy considerations present equally complex challenges as bioelectronic interfaces continuously monitor physiological parameters and potentially transmit this information to external systems. This data flow creates vulnerabilities that could compromise patient confidentiality if not properly secured. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe provide baseline requirements for handling such sensitive health information.
Emerging regulations increasingly focus on the concept of "privacy by design," requiring manufacturers to incorporate data protection measures from the earliest stages of product development. This includes implementing end-to-end encryption, secure authentication protocols, and data minimization strategies. The International Medical Device Regulators Forum (IMDRF) has published guidance specifically addressing cybersecurity requirements for connected medical devices, which applies directly to bioelectronic pharmaceutical interfaces.
Informed consent processes are evolving to address the unique nature of bioelectronic data collection. Patients must understand not only the immediate medical implications but also how their data will be stored, processed, and potentially shared. Regulatory frameworks increasingly require dynamic consent models that allow patients to modify their data sharing preferences over time as the use of their information evolves.
Cross-border data transfer presents additional regulatory challenges, particularly for multinational pharmaceutical companies deploying bioelectronic solutions globally. Different jurisdictions maintain varying standards for data protection, necessitating complex compliance strategies. The EU-US Data Privacy Framework and similar arrangements attempt to facilitate compliant data transfers while maintaining protection standards.
As artificial intelligence becomes more integrated with bioelectronic interfaces, regulations are beginning to address algorithmic transparency and accountability. Patients and healthcare providers must understand how AI-driven decisions are made when these systems automatically adjust drug delivery or treatment parameters based on bioelectronic monitoring.
Cross-Border Regulatory Harmonization Strategies
The global nature of pharmaceutical development and bioelectronic interface technologies necessitates a coordinated approach to regulatory frameworks across different jurisdictions. Currently, significant regulatory divergences exist between major markets such as the FDA (US), EMA (Europe), PMDA (Japan), and NMPA (China), creating substantial barriers for companies developing bioelectronic pharmaceutical interfaces.
Harmonization efforts are increasingly focusing on establishing common technical documents and shared evaluation standards. The International Medical Device Regulators Forum (IMDRF) has emerged as a critical platform for advancing regulatory convergence specifically for bioelectronic devices, building upon the foundation established by its predecessor, the Global Harmonization Task Force (GHTF).
Strategic approaches to cross-border harmonization include the implementation of mutual recognition agreements (MRAs) between regulatory authorities. These agreements enable regulators to rely on each other's inspection findings and quality assessments, significantly reducing duplicative efforts. The US-EU MRA represents a landmark achievement in this domain, with potential expansion to include bioelectronic interface technologies in its scope.
Regulatory sandboxes are being established in progressive jurisdictions to facilitate controlled testing of novel bioelectronic interfaces. These experimental regulatory frameworks allow companies to test innovative products in real-world settings under regulatory supervision but with modified compliance requirements. Singapore's Health Sciences Authority and the UK's MHRA have pioneered such approaches, creating models that other nations are beginning to adopt.
The development of international standards through organizations like ISO and IEC provides another pathway toward harmonization. The recent establishment of ISO/TC 276 (Biotechnology) and IEC 62304 (Medical device software) standards demonstrates progress in creating unified technical specifications that can be referenced across different regulatory frameworks.
Pharmaceutical and medical device companies are increasingly adopting "regulatory by design" approaches, incorporating global regulatory considerations into early development phases. This strategy involves mapping regulatory requirements across target markets and designing development programs that can simultaneously satisfy multiple authorities' requirements, thereby streamlining the path to multi-market approval for bioelectronic interface technologies.
Looking forward, artificial intelligence-powered regulatory intelligence systems are emerging as tools to navigate complex cross-border requirements. These systems can analyze regulatory trends, predict policy changes, and identify optimal regulatory pathways across multiple jurisdictions, potentially revolutionizing how companies approach global compliance for bioelectronic pharmaceutical interfaces.
Harmonization efforts are increasingly focusing on establishing common technical documents and shared evaluation standards. The International Medical Device Regulators Forum (IMDRF) has emerged as a critical platform for advancing regulatory convergence specifically for bioelectronic devices, building upon the foundation established by its predecessor, the Global Harmonization Task Force (GHTF).
Strategic approaches to cross-border harmonization include the implementation of mutual recognition agreements (MRAs) between regulatory authorities. These agreements enable regulators to rely on each other's inspection findings and quality assessments, significantly reducing duplicative efforts. The US-EU MRA represents a landmark achievement in this domain, with potential expansion to include bioelectronic interface technologies in its scope.
Regulatory sandboxes are being established in progressive jurisdictions to facilitate controlled testing of novel bioelectronic interfaces. These experimental regulatory frameworks allow companies to test innovative products in real-world settings under regulatory supervision but with modified compliance requirements. Singapore's Health Sciences Authority and the UK's MHRA have pioneered such approaches, creating models that other nations are beginning to adopt.
The development of international standards through organizations like ISO and IEC provides another pathway toward harmonization. The recent establishment of ISO/TC 276 (Biotechnology) and IEC 62304 (Medical device software) standards demonstrates progress in creating unified technical specifications that can be referenced across different regulatory frameworks.
Pharmaceutical and medical device companies are increasingly adopting "regulatory by design" approaches, incorporating global regulatory considerations into early development phases. This strategy involves mapping regulatory requirements across target markets and designing development programs that can simultaneously satisfy multiple authorities' requirements, thereby streamlining the path to multi-market approval for bioelectronic interface technologies.
Looking forward, artificial intelligence-powered regulatory intelligence systems are emerging as tools to navigate complex cross-border requirements. These systems can analyze regulatory trends, predict policy changes, and identify optimal regulatory pathways across multiple jurisdictions, potentially revolutionizing how companies approach global compliance for bioelectronic pharmaceutical interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!