Exploring the Mechanisms of Bioelectronic Interface Signal Transduction
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary frontier where biology meets electronics, enabling direct communication between biological systems and electronic devices. The evolution of this field traces back to the mid-20th century with rudimentary neural recordings, but has accelerated dramatically in recent decades due to advances in materials science, microelectronics, and biotechnology. This convergence has transformed our ability to interact with biological systems at unprecedented levels of precision and sophistication.
The progression of bioelectronic interfaces has followed several distinct phases. Initially, research focused on basic electrode designs capable of recording neural activity. This evolved into more sophisticated implantable devices in the 1980s and 1990s, such as cochlear implants and deep brain stimulators. The early 2000s witnessed the emergence of flexible electronics and biocompatible materials, addressing previous limitations of rigid interfaces. Most recently, wireless capabilities and miniaturization have pushed the boundaries of what these interfaces can achieve.
Current technological trends point toward increasingly seamless integration between biological tissues and electronic components. This includes the development of ultra-thin, flexible electronics that conform to biological surfaces, biodegradable components that eliminate the need for removal surgeries, and self-powered systems that harvest energy from biological processes. The field is also witnessing significant advances in signal processing algorithms and machine learning techniques that enhance signal interpretation.
The primary objective of bioelectronic interface research is to establish reliable, long-term bidirectional communication between electronic devices and biological systems. This involves overcoming significant challenges in biocompatibility, signal fidelity, power requirements, and longevity. Specifically for signal transduction mechanisms, objectives include improving signal-to-noise ratios, developing more sensitive detection methods, and creating interfaces that minimize tissue damage and immune responses.
Looking forward, the field aims to develop interfaces capable of real-time monitoring and modulation of biological signals with minimal invasiveness. This includes advancing closed-loop systems that can automatically respond to detected biological signals, enhancing spatial and temporal resolution of recordings, and expanding the types of biological signals that can be reliably detected and interpreted. The ultimate goal remains creating truly symbiotic relationships between electronic systems and biological organisms, opening new frontiers in medicine, human augmentation, and our understanding of complex biological systems.
The progression of bioelectronic interfaces has followed several distinct phases. Initially, research focused on basic electrode designs capable of recording neural activity. This evolved into more sophisticated implantable devices in the 1980s and 1990s, such as cochlear implants and deep brain stimulators. The early 2000s witnessed the emergence of flexible electronics and biocompatible materials, addressing previous limitations of rigid interfaces. Most recently, wireless capabilities and miniaturization have pushed the boundaries of what these interfaces can achieve.
Current technological trends point toward increasingly seamless integration between biological tissues and electronic components. This includes the development of ultra-thin, flexible electronics that conform to biological surfaces, biodegradable components that eliminate the need for removal surgeries, and self-powered systems that harvest energy from biological processes. The field is also witnessing significant advances in signal processing algorithms and machine learning techniques that enhance signal interpretation.
The primary objective of bioelectronic interface research is to establish reliable, long-term bidirectional communication between electronic devices and biological systems. This involves overcoming significant challenges in biocompatibility, signal fidelity, power requirements, and longevity. Specifically for signal transduction mechanisms, objectives include improving signal-to-noise ratios, developing more sensitive detection methods, and creating interfaces that minimize tissue damage and immune responses.
Looking forward, the field aims to develop interfaces capable of real-time monitoring and modulation of biological signals with minimal invasiveness. This includes advancing closed-loop systems that can automatically respond to detected biological signals, enhancing spatial and temporal resolution of recordings, and expanding the types of biological signals that can be reliably detected and interpreted. The ultimate goal remains creating truly symbiotic relationships between electronic systems and biological organisms, opening new frontiers in medicine, human augmentation, and our understanding of complex biological systems.
Market Applications and Demand Analysis
The bioelectronic interface market is experiencing unprecedented growth, driven by increasing demand for advanced medical devices, neural interfaces, and wearable health monitoring systems. Current market valuations indicate the global bioelectronic medicine sector reached approximately 25 billion USD in 2022, with projections suggesting a compound annual growth rate of 13-15% through 2030. This rapid expansion reflects the growing recognition of bioelectronic interfaces as transformative technologies across multiple sectors.
Healthcare represents the primary market for bioelectronic interface signal transduction technologies, with applications spanning neural prosthetics, deep brain stimulation systems, cochlear implants, and retinal implants. The aging global population and rising prevalence of neurological disorders have significantly increased demand for these technologies. Particularly notable is the expanding market for closed-loop neuromodulation systems, which provide real-time signal monitoring and therapeutic response.
Consumer electronics constitutes another rapidly growing market segment, with bioelectronic interfaces increasingly integrated into wearable fitness trackers, emotion recognition systems, and augmented reality devices. Market research indicates consumer willingness to adopt these technologies has increased by approximately 30% over the past five years, particularly among younger demographics and technology early adopters.
The military and defense sector represents a specialized but high-value market for bioelectronic interfaces, focusing on enhanced soldier performance monitoring, stress detection systems, and advanced human-machine interfaces for weapon systems and vehicles. This sector often drives innovation in signal processing algorithms and miniaturization techniques that eventually transfer to civilian applications.
Industrial applications are emerging as a significant growth area, with bioelectronic interfaces being developed for worker safety monitoring, fatigue detection, and enhanced human-robot collaboration in manufacturing environments. The industrial Internet of Things (IoT) integration with bioelectronic interfaces represents a particularly promising market opportunity.
Market barriers include concerns regarding data privacy, regulatory approval timelines, and the high cost of advanced bioelectronic systems. Consumer adoption remains constrained by these factors, particularly in regions with less developed healthcare infrastructure. Additionally, interoperability challenges between different bioelectronic platforms limit market expansion potential.
Regional analysis reveals North America currently dominates the bioelectronic interface market, accounting for approximately 45% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region, particularly China and South Korea, demonstrates the fastest growth rate, supported by significant government investment in bioelectronic research and manufacturing capabilities.
Healthcare represents the primary market for bioelectronic interface signal transduction technologies, with applications spanning neural prosthetics, deep brain stimulation systems, cochlear implants, and retinal implants. The aging global population and rising prevalence of neurological disorders have significantly increased demand for these technologies. Particularly notable is the expanding market for closed-loop neuromodulation systems, which provide real-time signal monitoring and therapeutic response.
Consumer electronics constitutes another rapidly growing market segment, with bioelectronic interfaces increasingly integrated into wearable fitness trackers, emotion recognition systems, and augmented reality devices. Market research indicates consumer willingness to adopt these technologies has increased by approximately 30% over the past five years, particularly among younger demographics and technology early adopters.
The military and defense sector represents a specialized but high-value market for bioelectronic interfaces, focusing on enhanced soldier performance monitoring, stress detection systems, and advanced human-machine interfaces for weapon systems and vehicles. This sector often drives innovation in signal processing algorithms and miniaturization techniques that eventually transfer to civilian applications.
Industrial applications are emerging as a significant growth area, with bioelectronic interfaces being developed for worker safety monitoring, fatigue detection, and enhanced human-robot collaboration in manufacturing environments. The industrial Internet of Things (IoT) integration with bioelectronic interfaces represents a particularly promising market opportunity.
Market barriers include concerns regarding data privacy, regulatory approval timelines, and the high cost of advanced bioelectronic systems. Consumer adoption remains constrained by these factors, particularly in regions with less developed healthcare infrastructure. Additionally, interoperability challenges between different bioelectronic platforms limit market expansion potential.
Regional analysis reveals North America currently dominates the bioelectronic interface market, accounting for approximately 45% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region, particularly China and South Korea, demonstrates the fastest growth rate, supported by significant government investment in bioelectronic research and manufacturing capabilities.
Current Signal Transduction Technologies and Barriers
The field of bioelectronic interface signal transduction has witnessed significant technological advancements in recent years, yet continues to face substantial challenges. Current signal transduction technologies primarily fall into three categories: electrical, optical, and chemical methods, each with distinct advantages and limitations in bioelectronic applications.
Electrical signal transduction technologies dominate the field, utilizing electrodes made from various materials including platinum, gold, and conductive polymers. These systems measure changes in electrical potential, impedance, or current flow at the biological interface. While offering high temporal resolution and direct integration with electronic systems, electrical methods often suffer from poor spatial resolution and signal degradation due to biofouling—the accumulation of proteins and cells on electrode surfaces that diminishes signal quality over time.
Optical transduction methods have gained prominence, employing techniques such as fluorescence imaging, surface plasmon resonance, and optogenetics. These approaches provide superior spatial resolution and can be less invasive than electrical methods. However, they typically require complex instrumentation, face challenges with light scattering in tissues, and often necessitate genetic modification or introduction of exogenous markers into biological systems.
Chemical transduction technologies, including enzyme-based biosensors and ion-selective field-effect transistors (ISFETs), convert biological signals into measurable electrical outputs through biochemical reactions. While highly specific, these methods frequently encounter issues with response time, stability, and reproducibility in complex biological environments.
A significant barrier across all transduction modalities is the fundamental mismatch between biological and electronic systems. Biological signals are predominantly ionic, operating in wet, dynamic environments with complex three-dimensional structures, while electronic systems function via electron flow in static, dry conditions. This bio-electronic interface presents challenges in signal fidelity, long-term stability, and biocompatibility.
Material limitations further constrain current technologies. Ideal interface materials must simultaneously exhibit excellent electrical properties, biocompatibility, flexibility, and durability—a combination that remains elusive. Novel materials such as graphene, carbon nanotubes, and conducting polymers show promise but face challenges in manufacturing scalability and long-term performance.
Signal processing represents another critical barrier. Biological signals are inherently noisy, with low signal-to-noise ratios and significant variability. Current algorithms struggle to effectively filter relevant information from background noise, particularly in real-time applications where computational efficiency is paramount.
Miniaturization and power requirements present additional challenges, especially for implantable or wearable bioelectronic devices. The need for compact, energy-efficient systems that can operate autonomously for extended periods remains a significant technological hurdle.
Electrical signal transduction technologies dominate the field, utilizing electrodes made from various materials including platinum, gold, and conductive polymers. These systems measure changes in electrical potential, impedance, or current flow at the biological interface. While offering high temporal resolution and direct integration with electronic systems, electrical methods often suffer from poor spatial resolution and signal degradation due to biofouling—the accumulation of proteins and cells on electrode surfaces that diminishes signal quality over time.
Optical transduction methods have gained prominence, employing techniques such as fluorescence imaging, surface plasmon resonance, and optogenetics. These approaches provide superior spatial resolution and can be less invasive than electrical methods. However, they typically require complex instrumentation, face challenges with light scattering in tissues, and often necessitate genetic modification or introduction of exogenous markers into biological systems.
Chemical transduction technologies, including enzyme-based biosensors and ion-selective field-effect transistors (ISFETs), convert biological signals into measurable electrical outputs through biochemical reactions. While highly specific, these methods frequently encounter issues with response time, stability, and reproducibility in complex biological environments.
A significant barrier across all transduction modalities is the fundamental mismatch between biological and electronic systems. Biological signals are predominantly ionic, operating in wet, dynamic environments with complex three-dimensional structures, while electronic systems function via electron flow in static, dry conditions. This bio-electronic interface presents challenges in signal fidelity, long-term stability, and biocompatibility.
Material limitations further constrain current technologies. Ideal interface materials must simultaneously exhibit excellent electrical properties, biocompatibility, flexibility, and durability—a combination that remains elusive. Novel materials such as graphene, carbon nanotubes, and conducting polymers show promise but face challenges in manufacturing scalability and long-term performance.
Signal processing represents another critical barrier. Biological signals are inherently noisy, with low signal-to-noise ratios and significant variability. Current algorithms struggle to effectively filter relevant information from background noise, particularly in real-time applications where computational efficiency is paramount.
Miniaturization and power requirements present additional challenges, especially for implantable or wearable bioelectronic devices. The need for compact, energy-efficient systems that can operate autonomously for extended periods remains a significant technological hurdle.
Contemporary Signal Transduction Mechanisms
01 Bioelectronic interfaces for neural signal transduction
Bioelectronic interfaces designed specifically for neural signal transduction enable direct communication between electronic devices and the nervous system. These interfaces incorporate specialized materials and structures that can detect, process, and transmit neural signals with high fidelity. The technology allows for both recording neural activity and stimulating neural tissues, creating bidirectional communication channels that can be used for therapeutic applications, neural prosthetics, and brain-computer interfaces.- Bioelectronic interfaces for neural signal transduction: Bioelectronic interfaces designed specifically for neural signal transduction enable direct communication between electronic devices and the nervous system. These interfaces incorporate specialized materials and structures that can detect, process, and transmit neural signals with high fidelity. The technology allows for both recording neural activity and stimulating neural tissues, creating bidirectional communication channels that can be used for therapeutic interventions or neural prosthetics.
- Molecular and cellular signal transduction mechanisms: Research on molecular and cellular mechanisms underlying signal transduction focuses on understanding how biological signals are converted and transmitted at the cellular level. This includes studies of receptor-ligand interactions, intracellular signaling cascades, and the molecular components involved in signal amplification and regulation. These fundamental biological processes provide inspiration and targets for developing bioelectronic interfaces that can effectively interact with living systems.
- Nanomaterial-based bioelectronic sensors: Advanced nanomaterials are being incorporated into bioelectronic interfaces to enhance signal detection and transduction capabilities. These materials include carbon nanotubes, graphene, quantum dots, and various nanocomposites that offer unique electrical, optical, and mechanical properties. Nanomaterial-based sensors provide improved sensitivity, specificity, and spatial resolution for detecting biological signals, while their small size allows for minimally invasive integration with biological tissues.
- Implantable bioelectronic devices for signal monitoring: Implantable bioelectronic devices are designed for long-term in vivo monitoring of physiological signals. These devices incorporate biocompatible materials, wireless power and data transmission capabilities, and miniaturized electronics to ensure stable performance within the body. The technology enables continuous monitoring of various biological parameters and can be used for diagnostic purposes, therapeutic interventions, or closed-loop control systems that respond to physiological changes in real-time.
- Biomolecular components for signal amplification and processing: Biomolecular components such as engineered proteins, DNA circuits, and enzymatic systems are being integrated into bioelectronic interfaces to enhance signal amplification and processing capabilities. These biological elements can perform complex computational functions, provide signal gain, or act as specific recognition elements. By combining the specificity and efficiency of biological systems with electronic components, these hybrid interfaces achieve improved performance in detecting and processing biological signals.
02 Molecular and cellular signal transduction mechanisms
This technology focuses on understanding and manipulating the molecular and cellular mechanisms involved in biological signal transduction. It includes methods for detecting and analyzing signaling pathways, protein interactions, and cellular responses to various stimuli. By elucidating these fundamental biological processes, researchers can develop more effective bioelectronic interfaces that mimic or interact with natural signaling systems, leading to improved biocompatibility and functionality of implantable devices.Expand Specific Solutions03 Nanomaterial-based bioelectronic sensors
Nanomaterials such as carbon nanotubes, graphene, and quantum dots are incorporated into bioelectronic interfaces to enhance signal detection and transduction. These materials offer unique electrical, optical, and mechanical properties that improve sensitivity, specificity, and response time of biosensors. The nanoscale dimensions of these materials allow for intimate contact with biological entities, facilitating more efficient signal transfer and enabling real-time monitoring of biological processes with minimal invasiveness.Expand Specific Solutions04 Implantable bioelectronic devices for therapeutic applications
Implantable bioelectronic devices designed for therapeutic applications can modulate physiological functions through targeted electrical stimulation. These devices incorporate biocompatible materials, miniaturized electronics, and sophisticated signal processing algorithms to deliver precise therapeutic interventions. Applications include neuromodulation for pain management, treatment of neurological disorders, regulation of organ function, and management of chronic conditions through controlled electrical stimulation of specific tissues or neural pathways.Expand Specific Solutions05 Signal processing and data analysis for bioelectronic interfaces
Advanced signal processing and data analysis techniques are essential for interpreting the complex data generated by bioelectronic interfaces. These methods include noise reduction algorithms, feature extraction, pattern recognition, and machine learning approaches that can identify meaningful signals within biological noise. Real-time processing capabilities allow for immediate feedback and adaptive responses in bioelectronic systems, enabling more sophisticated control mechanisms and improved performance in applications ranging from prosthetics to diagnostic devices.Expand Specific Solutions
Leading Research Institutions and Industry Players
The bioelectronic interface signal transduction field is currently in a growth phase, with the market expected to reach significant expansion as healthcare applications increase. The competitive landscape features a mix of established pharmaceutical companies (Merck), technology leaders (Rambus, MediaTek), and academic research powerhouses (MIT, University of Michigan, Arizona State). Technical maturity varies across applications, with academic institutions driving fundamental research while companies focus on commercialization. Leading players like Illumina and Merck are advancing clinical applications, while universities such as MIT and Carnegie Mellon are pioneering next-generation interfaces. The integration of semiconductor expertise from companies like MediaTek and Rambus with biological research capabilities positions cross-disciplinary collaborators at a competitive advantage in this emerging field.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed sophisticated bioelectronic interfaces focusing on neural signal transduction mechanisms. Their technology utilizes ultra-small, high-density microelectrode arrays fabricated using advanced semiconductor processing techniques. These arrays incorporate novel materials such as conducting polymers and carbon-based composites to enhance charge transfer capacity while maintaining biocompatibility. Michigan's approach includes specialized surface treatments that promote controlled protein adsorption at the electrode-tissue interface, improving long-term stability. Their signal transduction pathway incorporates custom integrated circuits for amplification and digitization directly at the recording site, minimizing noise introduction. The university has pioneered wireless telemetry systems specifically designed for neural interfaces, enabling high-bandwidth data transmission while maintaining low power consumption. Recent innovations include adaptive recording systems that can automatically adjust parameters based on signal quality and neural activity patterns, optimizing performance across varying physiological conditions.
Strengths: Exceptional microelectrode density allows for high spatial resolution recording. Their integrated signal processing architecture significantly reduces noise and improves signal fidelity. Weaknesses: The sophisticated fabrication processes increase production costs, and the high-density arrays require complex surgical implantation techniques that may limit widespread clinical adoption.
Arizona State University
Technical Solution: Arizona State University has developed innovative bioelectronic interfaces focusing on flexible, biocompatible materials for enhanced signal transduction. Their approach utilizes stretchable electronics platforms that can conform to dynamic biological tissues while maintaining electrical performance. ASU researchers have pioneered the use of liquid metal alloys (primarily gallium-based) encapsulated in elastomeric substrates to create deformable conductive pathways that can withstand mechanical strain while maintaining excellent electrical properties. Their signal transduction mechanisms incorporate novel impedance matching techniques that optimize the interface between biological tissues and electronic components. ASU has developed specialized surface modification protocols that enhance biocompatibility while reducing biofouling, resulting in more stable long-term recordings. Recent innovations include self-healing electronic materials that can recover from mechanical damage, addressing one of the primary failure modes in implantable bioelectronic systems. Their platforms have demonstrated particular success in epidermal sensing applications where conformability to skin surfaces is critical for signal quality.
Strengths: Superior mechanical compliance allows for applications on dynamic tissues with minimal mechanical mismatch. Their liquid metal technology provides exceptional conductivity even under significant mechanical deformation. Weaknesses: Liquid metal encapsulation presents manufacturing challenges for mass production, and long-term stability of these materials in biological environments requires further validation.
Critical Patents and Breakthroughs in Bioelectronic Interfaces
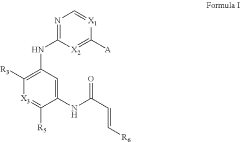
Heteroaryl compounds for kinase inhibition
PatentActiveUS11958850B2
Innovation
- Development of compounds that selectively inhibit mutant EGFR and HER2 proteins with exon 20 insertions, including specific chemical structures represented by Formula I, which can effectively target and modulate these proteins, thereby inhibiting their kinase activity and associated cancerous signaling.
Protein kinase inhibitors and methods for identifying same
PatentWO2005079300A2
Innovation
- The use of combinatorial libraries with consensus sequences for protein kinases, incorporating amino acid mimetics and chemical moieties, to create potent and selective inhibitors of protein kinase C (PKC) isoforms, such as PKCα, PKCβl, PKCδ, and PKCζ, without requiring structural information of the enzyme.
Biocompatibility and Safety Considerations
The integration of bioelectronic interfaces with biological systems necessitates rigorous consideration of biocompatibility and safety factors. Material selection represents a critical determinant of biocompatibility, with noble metals like gold and platinum demonstrating favorable characteristics due to their chemical inertness and resistance to corrosion in physiological environments. Alternatively, conductive polymers such as PEDOT:PSS offer flexibility and structural similarity to biological tissues, potentially reducing foreign body responses.
Inflammatory responses present significant challenges to long-term device functionality. Upon implantation, the body's immune system typically initiates a foreign body reaction characterized by protein adsorption, leukocyte recruitment, and eventual fibrous encapsulation. This cascade not only compromises signal quality but may also damage surrounding tissues. Advanced surface modifications incorporating anti-inflammatory agents or biomimetic coatings have shown promise in mitigating these responses while maintaining electrical performance.
Cytotoxicity assessment protocols must evaluate both acute and chronic effects of bioelectronic materials and their degradation products. Standardized in vitro assays examining cell viability, proliferation, and morphological changes provide initial safety profiles, while in vivo models offer more comprehensive insights into tissue-level responses. The potential for electrochemical reactions at the tissue-electrode interface demands particular attention, as charge injection beyond safe limits can generate toxic byproducts or induce irreversible tissue damage.
Sterilization compatibility represents another crucial consideration, as common sterilization methods may compromise device integrity or alter surface properties. Gamma irradiation, ethylene oxide treatment, and autoclave procedures each present distinct advantages and limitations depending on material composition and device architecture. Developers must validate that sterilization protocols maintain both device functionality and biocompatibility profiles.
Long-term stability assessment requires accelerated aging studies and chronic implantation models to predict performance degradation and biological responses over the intended device lifetime. Particular attention must focus on material delamination, corrosion resistance, and mechanical stability under physiological conditions. The development of non-invasive monitoring techniques for implanted devices would significantly enhance safety surveillance capabilities.
Regulatory frameworks governing bioelectronic interfaces continue to evolve, with agencies like the FDA and EMA establishing specific guidance for combination products that incorporate both electronic and biological components. Comprehensive documentation of biocompatibility testing according to ISO 10993 standards remains essential for regulatory approval, with particular emphasis on genotoxicity, carcinogenicity, and systemic toxicity evaluations for long-term implantable systems.
Inflammatory responses present significant challenges to long-term device functionality. Upon implantation, the body's immune system typically initiates a foreign body reaction characterized by protein adsorption, leukocyte recruitment, and eventual fibrous encapsulation. This cascade not only compromises signal quality but may also damage surrounding tissues. Advanced surface modifications incorporating anti-inflammatory agents or biomimetic coatings have shown promise in mitigating these responses while maintaining electrical performance.
Cytotoxicity assessment protocols must evaluate both acute and chronic effects of bioelectronic materials and their degradation products. Standardized in vitro assays examining cell viability, proliferation, and morphological changes provide initial safety profiles, while in vivo models offer more comprehensive insights into tissue-level responses. The potential for electrochemical reactions at the tissue-electrode interface demands particular attention, as charge injection beyond safe limits can generate toxic byproducts or induce irreversible tissue damage.
Sterilization compatibility represents another crucial consideration, as common sterilization methods may compromise device integrity or alter surface properties. Gamma irradiation, ethylene oxide treatment, and autoclave procedures each present distinct advantages and limitations depending on material composition and device architecture. Developers must validate that sterilization protocols maintain both device functionality and biocompatibility profiles.
Long-term stability assessment requires accelerated aging studies and chronic implantation models to predict performance degradation and biological responses over the intended device lifetime. Particular attention must focus on material delamination, corrosion resistance, and mechanical stability under physiological conditions. The development of non-invasive monitoring techniques for implanted devices would significantly enhance safety surveillance capabilities.
Regulatory frameworks governing bioelectronic interfaces continue to evolve, with agencies like the FDA and EMA establishing specific guidance for combination products that incorporate both electronic and biological components. Comprehensive documentation of biocompatibility testing according to ISO 10993 standards remains essential for regulatory approval, with particular emphasis on genotoxicity, carcinogenicity, and systemic toxicity evaluations for long-term implantable systems.
Regulatory Framework for Bioelectronic Devices
The regulatory landscape for bioelectronic devices presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies bioelectronic interfaces primarily under medical device regulations, with specific pathways depending on risk classification. Class III devices, which include many invasive neural interfaces, require premarket approval (PMA) with extensive clinical trials demonstrating safety and efficacy. Meanwhile, less invasive monitoring devices may qualify for the less stringent 510(k) clearance process.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more rigorous requirements for clinical evidence, post-market surveillance, and unique device identification. These regulations specifically address active implantable medical devices, creating additional hurdles for bioelectronic interface developers seeking European market access.
In Asia, regulatory approaches differ substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system to accelerate approval for innovative medical technologies. China's National Medical Products Administration (NMPA) has recently reformed its regulatory framework to expedite review processes while maintaining stringent safety standards for implantable electronic devices.
International standards organizations play a crucial role in harmonizing these diverse regulatory frameworks. The International Organization for Standardization (ISO) has developed ISO 14708 specifically for active implantable medical devices, while the International Electrotechnical Commission (IEC) provides standards for electrical safety and electromagnetic compatibility through IEC 60601 series.
Emerging regulatory considerations are increasingly focusing on cybersecurity requirements for connected bioelectronic devices. The FDA has issued guidance on managing cybersecurity in medical devices, requiring manufacturers to implement security measures throughout the product lifecycle. Similarly, the EU's MDR includes provisions for information security and data protection.
Privacy regulations such as GDPR in Europe and HIPAA in the US create additional compliance requirements for bioelectronic interfaces that collect, process, or transmit personal health data. These regulations mandate strict data protection measures, informed consent protocols, and limitations on data usage.
The evolving nature of bioelectronic technology presents ongoing regulatory challenges. Regulatory bodies worldwide are developing adaptive frameworks to accommodate rapid technological advancement while ensuring patient safety. Initiatives like the FDA's Digital Health Software Precertification Program represent efforts to create more flexible regulatory approaches for software-driven medical technologies, including many modern bioelectronic interfaces.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more rigorous requirements for clinical evidence, post-market surveillance, and unique device identification. These regulations specifically address active implantable medical devices, creating additional hurdles for bioelectronic interface developers seeking European market access.
In Asia, regulatory approaches differ substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system to accelerate approval for innovative medical technologies. China's National Medical Products Administration (NMPA) has recently reformed its regulatory framework to expedite review processes while maintaining stringent safety standards for implantable electronic devices.
International standards organizations play a crucial role in harmonizing these diverse regulatory frameworks. The International Organization for Standardization (ISO) has developed ISO 14708 specifically for active implantable medical devices, while the International Electrotechnical Commission (IEC) provides standards for electrical safety and electromagnetic compatibility through IEC 60601 series.
Emerging regulatory considerations are increasingly focusing on cybersecurity requirements for connected bioelectronic devices. The FDA has issued guidance on managing cybersecurity in medical devices, requiring manufacturers to implement security measures throughout the product lifecycle. Similarly, the EU's MDR includes provisions for information security and data protection.
Privacy regulations such as GDPR in Europe and HIPAA in the US create additional compliance requirements for bioelectronic interfaces that collect, process, or transmit personal health data. These regulations mandate strict data protection measures, informed consent protocols, and limitations on data usage.
The evolving nature of bioelectronic technology presents ongoing regulatory challenges. Regulatory bodies worldwide are developing adaptive frameworks to accommodate rapid technological advancement while ensuring patient safety. Initiatives like the FDA's Digital Health Software Precertification Program represent efforts to create more flexible regulatory approaches for software-driven medical technologies, including many modern bioelectronic interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!