Exploring Material Interfaces in Bioelectronic Sensor Design
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Sensor Materials Background and Objectives
Bioelectronic sensors represent a revolutionary intersection of electronics and biology, enabling real-time monitoring of physiological parameters through direct interface with biological systems. The evolution of these sensors has progressed significantly over the past three decades, transitioning from bulky laboratory equipment to miniaturized, flexible devices capable of continuous monitoring in various environments. This technological trajectory has been driven by advances in materials science, microfabrication techniques, and a deeper understanding of bioelectronic interfaces.
The field initially emerged in the 1990s with rigid silicon-based sensors primarily used in controlled laboratory settings. By the early 2000s, researchers began exploring flexible substrates and biocompatible materials, leading to the development of the first generation of wearable bioelectronic sensors. The past decade has witnessed remarkable progress in developing ultra-thin, conformable electronics that can maintain intimate contact with biological tissues while minimizing foreign body responses.
Current research focuses on addressing the fundamental challenges at the material-biology interface, particularly the mechanical, chemical, and electrical mismatches between conventional electronic materials and biological tissues. The mechanical mismatch arises from the inherent rigidity of traditional electronic components compared to soft biological tissues, while chemical incompatibilities can lead to biofouling and degradation of sensor performance over time. Electrical mismatches between ionic biological signals and electronic circuits present additional challenges for accurate signal transduction.
The primary objective of this technical research is to explore and develop novel material interfaces that can bridge these gaps, enabling seamless integration between electronic sensors and biological systems. Specifically, we aim to investigate materials that can: (1) match the mechanical properties of target biological tissues; (2) resist biofouling and maintain functionality in complex biological environments; (3) efficiently transduce biological signals into electronic outputs; and (4) maintain biocompatibility for extended periods.
Additionally, this research seeks to establish design principles for next-generation bioelectronic sensors that can overcome current limitations in sensitivity, specificity, and long-term stability. By developing a comprehensive understanding of material-biology interactions at multiple scales, we aim to create a foundation for bioelectronic platforms capable of addressing unmet needs in healthcare monitoring, disease diagnosis, and therapeutic interventions.
The ultimate goal is to enable a new class of bioelectronic sensors that can seamlessly integrate with biological systems, providing continuous, accurate monitoring with minimal disruption to normal physiological functions, thereby transforming approaches to personalized medicine and healthcare delivery.
The field initially emerged in the 1990s with rigid silicon-based sensors primarily used in controlled laboratory settings. By the early 2000s, researchers began exploring flexible substrates and biocompatible materials, leading to the development of the first generation of wearable bioelectronic sensors. The past decade has witnessed remarkable progress in developing ultra-thin, conformable electronics that can maintain intimate contact with biological tissues while minimizing foreign body responses.
Current research focuses on addressing the fundamental challenges at the material-biology interface, particularly the mechanical, chemical, and electrical mismatches between conventional electronic materials and biological tissues. The mechanical mismatch arises from the inherent rigidity of traditional electronic components compared to soft biological tissues, while chemical incompatibilities can lead to biofouling and degradation of sensor performance over time. Electrical mismatches between ionic biological signals and electronic circuits present additional challenges for accurate signal transduction.
The primary objective of this technical research is to explore and develop novel material interfaces that can bridge these gaps, enabling seamless integration between electronic sensors and biological systems. Specifically, we aim to investigate materials that can: (1) match the mechanical properties of target biological tissues; (2) resist biofouling and maintain functionality in complex biological environments; (3) efficiently transduce biological signals into electronic outputs; and (4) maintain biocompatibility for extended periods.
Additionally, this research seeks to establish design principles for next-generation bioelectronic sensors that can overcome current limitations in sensitivity, specificity, and long-term stability. By developing a comprehensive understanding of material-biology interactions at multiple scales, we aim to create a foundation for bioelectronic platforms capable of addressing unmet needs in healthcare monitoring, disease diagnosis, and therapeutic interventions.
The ultimate goal is to enable a new class of bioelectronic sensors that can seamlessly integrate with biological systems, providing continuous, accurate monitoring with minimal disruption to normal physiological functions, thereby transforming approaches to personalized medicine and healthcare delivery.
Market Analysis for Bioelectronic Sensing Applications
The bioelectronic sensor market is experiencing unprecedented growth, driven by increasing healthcare demands and technological advancements in wearable health monitoring devices. Current market valuations place the global bioelectronic sensing sector at approximately 15 billion USD in 2023, with projections indicating a compound annual growth rate of 9.7% through 2030, potentially reaching 28 billion USD by the end of the decade.
Healthcare applications represent the largest market segment, accounting for nearly 45% of the total market share. Within this segment, continuous glucose monitoring systems dominate, followed by cardiac monitoring devices and neurological sensing platforms. The consumer health and fitness sector has emerged as the fastest-growing segment, expanding at 12.3% annually as consumers increasingly adopt wearable bioelectronic sensors for personal health management.
Geographically, North America leads the market with 38% share, followed by Europe (27%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, particularly in China, Japan, and South Korea, where government initiatives supporting healthcare technology innovation are creating favorable market conditions.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring continuous monitoring, increasing healthcare costs driving demand for remote patient monitoring solutions, and growing consumer awareness about preventive healthcare. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote health monitoring capabilities.
Material interface innovations are significantly influencing market dynamics. Flexible and stretchable electronics have opened new application possibilities, with market demand for such technologies growing at 14.2% annually. Biocompatible materials that reduce foreign body responses are commanding premium pricing, while nanomaterial-based sensors are gaining traction for their enhanced sensitivity and specificity.
Customer segments show distinct preferences: healthcare providers prioritize accuracy and reliability; pharmaceutical companies value data integration capabilities; and consumers emphasize comfort and ease of use. Price sensitivity varies significantly across these segments, with healthcare institutions demonstrating willingness to invest in premium solutions offering demonstrable clinical outcomes.
Regulatory considerations remain a critical market factor. FDA approval processes in the US and CE marking requirements in Europe create significant barriers to entry but also provide competitive advantages for compliant products. Recent regulatory frameworks specifically addressing bioelectronic devices are gradually streamlining approval pathways, potentially accelerating market entry for innovative solutions.
Healthcare applications represent the largest market segment, accounting for nearly 45% of the total market share. Within this segment, continuous glucose monitoring systems dominate, followed by cardiac monitoring devices and neurological sensing platforms. The consumer health and fitness sector has emerged as the fastest-growing segment, expanding at 12.3% annually as consumers increasingly adopt wearable bioelectronic sensors for personal health management.
Geographically, North America leads the market with 38% share, followed by Europe (27%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, particularly in China, Japan, and South Korea, where government initiatives supporting healthcare technology innovation are creating favorable market conditions.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring continuous monitoring, increasing healthcare costs driving demand for remote patient monitoring solutions, and growing consumer awareness about preventive healthcare. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote health monitoring capabilities.
Material interface innovations are significantly influencing market dynamics. Flexible and stretchable electronics have opened new application possibilities, with market demand for such technologies growing at 14.2% annually. Biocompatible materials that reduce foreign body responses are commanding premium pricing, while nanomaterial-based sensors are gaining traction for their enhanced sensitivity and specificity.
Customer segments show distinct preferences: healthcare providers prioritize accuracy and reliability; pharmaceutical companies value data integration capabilities; and consumers emphasize comfort and ease of use. Price sensitivity varies significantly across these segments, with healthcare institutions demonstrating willingness to invest in premium solutions offering demonstrable clinical outcomes.
Regulatory considerations remain a critical market factor. FDA approval processes in the US and CE marking requirements in Europe create significant barriers to entry but also provide competitive advantages for compliant products. Recent regulatory frameworks specifically addressing bioelectronic devices are gradually streamlining approval pathways, potentially accelerating market entry for innovative solutions.
Current Material Interface Challenges in Bioelectronics
The interface between biological systems and electronic components represents one of the most critical challenges in bioelectronic sensor development. Current bioelectronic devices face significant material compatibility issues when interfacing with living tissues, primarily due to the fundamental mismatch between rigid electronic components and soft biological environments. This mechanical mismatch often leads to inflammation, scarring, and eventual device failure, particularly in long-term implantable applications.
Biocompatibility remains a paramount concern, as materials must not elicit adverse immune responses while maintaining their functional properties in the complex biological milieu. Traditional electronic materials like silicon and metals often trigger foreign body responses, limiting device longevity and performance. Additionally, biofouling—the accumulation of proteins, cells, and other biological materials on sensor surfaces—significantly impairs sensor function over time by creating barriers to analyte diffusion and signal transduction.
The challenge of achieving stable electrical connections across the bio-electronic interface persists despite decades of research. Electrical impedance at these interfaces tends to increase over time due to cellular encapsulation and material degradation, resulting in signal attenuation and decreased sensor sensitivity. Furthermore, many bioelectronic materials suffer from poor long-term stability in physiological environments, where factors such as hydrolysis, oxidation, and enzymatic degradation can compromise device integrity.
Water permeation presents another significant hurdle, as moisture ingress can cause catastrophic failure in electronic components not adequately protected. Current encapsulation technologies often trade off flexibility for hermeticity, creating additional mechanical stress at interfaces. The development of materials that can simultaneously provide waterproofing while maintaining flexibility remains an active area of research.
Power requirements and energy harvesting capabilities also present material interface challenges. Batteries contain toxic components and add bulk to devices, while wireless power transfer systems must contend with tissue absorption and safety limitations. Materials that can efficiently harvest energy from biological sources (thermal, mechanical, or biochemical) are still in early development stages.
Signal transduction across the bio-electronic interface faces limitations in sensitivity, specificity, and temporal resolution. Current electrode materials often exhibit poor signal-to-noise ratios when measuring subtle biological signals, particularly in the presence of motion artifacts and electromagnetic interference. The development of novel transduction materials that can selectively detect specific biomarkers while rejecting interferents remains a significant challenge.
Emerging applications in closed-loop systems, where sensors must not only detect biological signals but also deliver therapeutic interventions, further complicate material interface requirements. These systems demand bidirectional communication capabilities and materials that can transition between sensing and actuation modes without compromising either function.
Biocompatibility remains a paramount concern, as materials must not elicit adverse immune responses while maintaining their functional properties in the complex biological milieu. Traditional electronic materials like silicon and metals often trigger foreign body responses, limiting device longevity and performance. Additionally, biofouling—the accumulation of proteins, cells, and other biological materials on sensor surfaces—significantly impairs sensor function over time by creating barriers to analyte diffusion and signal transduction.
The challenge of achieving stable electrical connections across the bio-electronic interface persists despite decades of research. Electrical impedance at these interfaces tends to increase over time due to cellular encapsulation and material degradation, resulting in signal attenuation and decreased sensor sensitivity. Furthermore, many bioelectronic materials suffer from poor long-term stability in physiological environments, where factors such as hydrolysis, oxidation, and enzymatic degradation can compromise device integrity.
Water permeation presents another significant hurdle, as moisture ingress can cause catastrophic failure in electronic components not adequately protected. Current encapsulation technologies often trade off flexibility for hermeticity, creating additional mechanical stress at interfaces. The development of materials that can simultaneously provide waterproofing while maintaining flexibility remains an active area of research.
Power requirements and energy harvesting capabilities also present material interface challenges. Batteries contain toxic components and add bulk to devices, while wireless power transfer systems must contend with tissue absorption and safety limitations. Materials that can efficiently harvest energy from biological sources (thermal, mechanical, or biochemical) are still in early development stages.
Signal transduction across the bio-electronic interface faces limitations in sensitivity, specificity, and temporal resolution. Current electrode materials often exhibit poor signal-to-noise ratios when measuring subtle biological signals, particularly in the presence of motion artifacts and electromagnetic interference. The development of novel transduction materials that can selectively detect specific biomarkers while rejecting interferents remains a significant challenge.
Emerging applications in closed-loop systems, where sensors must not only detect biological signals but also deliver therapeutic interventions, further complicate material interface requirements. These systems demand bidirectional communication capabilities and materials that can transition between sensing and actuation modes without compromising either function.
State-of-the-Art Material Interface Solutions
01 Biocompatible interface materials for biosensors
Biocompatible materials are crucial for creating effective interfaces between biological systems and electronic sensors. These materials facilitate the integration of biological components with electronic devices while minimizing adverse reactions. Advanced polymers, hydrogels, and functionalized surfaces can be engineered to improve biocompatibility, enhance signal transduction, and extend the operational lifetime of bioelectronic sensors. These interfaces often incorporate specific chemical modifications to promote cell adhesion or protein binding while maintaining electronic conductivity.- Biocompatible interface materials for biosensors: Biocompatible materials are essential for creating effective interfaces between biological samples and electronic sensors. These materials facilitate signal transduction while minimizing adverse biological reactions. Advanced polymers, hydrogels, and functionalized surfaces can be engineered to improve sensor sensitivity and stability while maintaining compatibility with biological environments. These interfaces often incorporate specific binding sites for target biomolecules to enhance detection specificity.
- Nanomaterial-based interfaces for enhanced sensitivity: Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles are increasingly used in bioelectronic sensor interfaces to enhance sensitivity and performance. These materials provide high surface-to-volume ratios, excellent electrical conductivity, and unique surface properties that can be functionalized for specific biomolecule detection. Nanomaterial interfaces can significantly lower detection limits and improve signal-to-noise ratios in bioelectronic sensing applications.
- Microfluidic integration with sensor interfaces: Microfluidic systems integrated with bioelectronic sensors enable precise sample handling, reduced reagent consumption, and improved detection capabilities. These interfaces incorporate channels, chambers, and valves to control fluid flow across sensing surfaces. Advanced microfluidic interfaces can include gradient generators, mixers, and separators to prepare samples before detection, enhancing the overall performance and reliability of bioelectronic sensors.
- Signal processing and interface electronics design: Effective signal processing and interface electronics are crucial for bioelectronic sensors to convert biological signals into meaningful data. These systems include amplifiers, filters, analog-to-digital converters, and noise reduction circuits specifically designed for biosensing applications. Advanced interface electronics may incorporate machine learning algorithms for real-time signal analysis and pattern recognition, improving sensor accuracy and enabling automated detection of specific biomarkers.
- Surface functionalization techniques for selective detection: Surface functionalization methods are essential for creating selective bioelectronic sensor interfaces. These techniques involve modifying sensor surfaces with specific recognition elements such as antibodies, aptamers, or enzymes to enable targeted detection of biomolecules. Various chemical approaches including self-assembled monolayers, click chemistry, and biomimetic coatings can be employed to create stable and specific binding interfaces that minimize non-specific interactions while maximizing target capture efficiency.
02 Nanomaterial-based interfaces for enhanced sensitivity
Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles can be incorporated into bioelectronic sensor interfaces to significantly enhance sensitivity and detection capabilities. These nanomaterials provide increased surface area, improved electron transfer kinetics, and unique optical properties that can be leveraged for biosensing applications. The nanoscale architecture of these interfaces allows for more efficient interaction with biomolecules and can enable detection of analytes at extremely low concentrations, making them valuable for diagnostic and monitoring applications.Expand Specific Solutions03 Flexible and stretchable interface designs
Flexible and stretchable interfaces are designed to maintain functionality during mechanical deformation, making them suitable for wearable and implantable bioelectronic sensors. These interfaces typically incorporate elastomeric substrates, serpentine conductive traces, or liquid metal conductors that can withstand bending, stretching, and twisting without performance degradation. Such designs enable conformal contact with biological tissues, improving signal quality and user comfort while allowing for continuous monitoring in dynamic environments such as on skin or within moving organs.Expand Specific Solutions04 Microfluidic integration with sensing interfaces
Microfluidic systems can be integrated with bioelectronic sensing interfaces to enable precise sample handling, reagent delivery, and waste removal. These integrated platforms facilitate controlled interactions between analytes and sensing elements, improving reproducibility and enabling multiplexed detection. Microfluidic channels can be designed to direct sample flow across specific sensing regions, reduce sample volume requirements, and incorporate sample preparation steps. This integration is particularly valuable for point-of-care diagnostic applications where automated sample processing is beneficial.Expand Specific Solutions05 Signal processing and interface optimization algorithms
Advanced signal processing algorithms and interface optimization techniques are essential for extracting meaningful data from bioelectronic sensors. These computational approaches can filter noise, compensate for drift, and enhance signal quality through various mathematical transformations. Machine learning algorithms can be employed to identify patterns in sensor data and improve detection accuracy. Software interfaces that facilitate data visualization and interpretation are also crucial components of bioelectronic sensing systems, enabling effective communication of results to users and integration with other healthcare technologies.Expand Specific Solutions
Leading Companies and Research Institutions in Bioelectronics
The bioelectronic sensor design field is currently in a growth phase, with the market expected to reach significant expansion as healthcare monitoring becomes increasingly personalized. Key players represent diverse sectors, with research institutions like MIT, Tsinghua University, and CNRS driving fundamental innovations, while commercial entities including Samsung Electronics, IBM, and Google are leveraging their technological capabilities to develop practical applications. Imec stands out as a specialized research center bridging academic discoveries with commercial viability. The technology maturity varies across applications, with companies like EnLiSense and NXP Semiconductors advancing wearable sensor technologies, while Nitto Denko and LSI Medience focus on biocompatible materials interfaces. This competitive landscape reflects a collaborative ecosystem where academic research feeds industrial development in this emerging field.
Interuniversitair Micro-Electronica Centrum VZW
Technical Solution: IMEC开发了基于纳米多孔金属-有机框架(MOF)的生物电子传感器界面技术,通过精确控制纳米孔径(2-50nm)实现对特定生物分子的选择性检测[3]。该技术采用原子层沉积(ALD)方法在柔性聚合物基底上构建超薄MOF层,形成生物相容性高的传感界面。IMEC还开发了专利的表面功能化技术,通过共价键合将特定的生物识别元件(如抗体、适配体)固定在MOF表面,同时保持其生物活性。这种界面设计显著提高了信号转导效率,使传感器灵敏度提升约10倍,并将检测限降低至皮摩尔级别[7]。IMEC的技术还整合了微流控系统,实现样品的自动处理和分析,适用于即时检测应用。
优势:纳米多孔结构提供极大的表面积,显著提高了生物分子捕获效率和信号强度;表面功能化技术确保高特异性识别;柔性基底设计使传感器可贴合于不规则表面,适用于可穿戴设备。劣势:MOF材料在某些生理环境下稳定性受限;制造工艺复杂度高,增加了生产成本;某些应用中可能需要额外的信号放大系统。
Centre National de la Recherche Scientifique
Technical Solution: CNRS开发了基于导电聚合物与生物分子杂化的界面技术,特别是聚(3,4-乙烯二氧噻吩)(PEDOT)与各种生物分子的复合材料[1]。该技术通过电化学聚合方法,在单一步骤中实现聚合物形成与生物分子包埋,创造出具有优异电子-离子混合传导特性的界面。CNRS的创新在于开发了可控掺杂技术,通过调节掺杂剂种类和浓度(0.5-5%),精确控制界面的电导率(1-1000 S/cm)和离子迁移率。该界面还具有独特的形态学特征,纳米纤维结构(直径20-100nm)提供了大量的活性位点[4]。CNRS还开发了光响应型界面材料,通过引入光敏基团,实现了对传感器性能的光学调控,为动态生物监测提供了新途径。该技术已成功应用于神经电极、代谢物检测和药物筛选平台。
优势:电子-离子混合传导特性使信号转导效率高;制备工艺简单,可在室温下进行;材料柔性好,适应生物组织形变;可通过掺杂精确调控电学性能。劣势:某些导电聚合物在长期植入条件下可能降解;批次间重现性需要严格控制;在某些应用中可能需要额外的封装以防止水分侵入影响电学性能。
Key Patents and Innovations in Biocompatible Materials
Renewable bioelectronic interface for electrobiocatalytic reactor
PatentInactiveUS10246786B2
Innovation
- A bioelectronic device with a conductive carbon electrode and a bioelectronic interface where the catalytically active material is electrostatically bound, allowing for easy removal and replacement by changing the pH, and a process for reconstituting the interface using aqueous media with specific pH levels to facilitate bonding and regeneration of the interface.
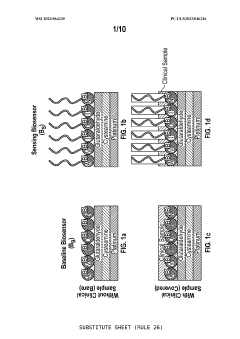
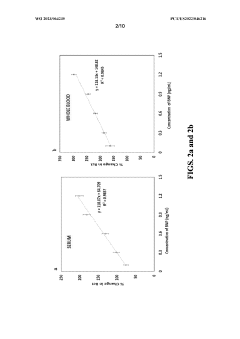
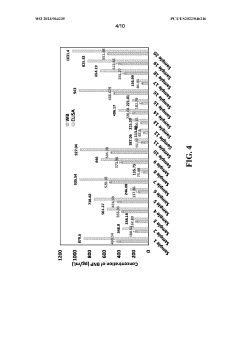
Novel two electrode-based correction approach for elimination of biofouling from label-free affinity biosensors for detection
PatentWO2023064235A1
Innovation
- A two-electrode approach using a baseline biosensor without a sensing element and a fully functionalized sensing biosensor, where the baseline biosensor detects interference and the sensing biosensor detects specific biomarkers, allowing for correction to eliminate non-biomarker interference, enabling efficient and accurate detection of biomarkers in bodily fluids.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical challenge in bioelectronic sensor design, particularly at the material-tissue interface. The foreign body response triggered by implanted sensors can lead to inflammation, fibrosis, and eventual device failure. Recent advances in biomaterial engineering have focused on developing surfaces that mimic natural tissue environments, utilizing hydrogels, conducting polymers, and bioactive coatings to reduce immune rejection.
Surface modification techniques have emerged as essential strategies for improving long-term sensor performance. Approaches such as anti-fouling coatings, controlled drug release systems, and nanopatterned surfaces have demonstrated significant reductions in protein adsorption and cellular adhesion, extending sensor functional lifetimes from days to months in some applications.
Cytotoxicity assessment protocols have evolved substantially, moving beyond simple cell viability tests to include comprehensive evaluations of inflammatory markers, oxidative stress indicators, and genetic expression profiles. These advanced testing methodologies provide deeper insights into the subtle biological responses to sensor materials, enabling more accurate predictions of in vivo performance.
Regulatory frameworks governing bioelectronic sensors continue to evolve, with the FDA and international bodies implementing increasingly stringent requirements for biocompatibility testing. The ISO 10993 standards series serves as the primary guideline, though emerging technologies often necessitate case-specific evaluation approaches that consider the unique material interfaces and intended implantation duration.
Biodegradation characteristics present both challenges and opportunities in sensor design. While uncontrolled degradation can release potentially harmful byproducts and compromise device function, intentionally engineered biodegradable components can eliminate the need for surgical removal and reduce long-term complications. Recent research has focused on developing materials with predictable degradation profiles that maintain functional integrity throughout their intended operational period.
Sterilization compatibility represents another critical consideration, as common sterilization methods like ethylene oxide exposure, gamma irradiation, and autoclave processing can significantly alter material properties. Novel sterilization-resistant materials and alternative low-temperature sterilization techniques are being developed specifically for sensitive bioelectronic components.
The integration of bioactive elements, including anti-inflammatory agents, growth factors, and cell-adhesion molecules, has shown promise in actively modulating the tissue response to implanted sensors. These biologically interactive interfaces represent a paradigm shift from passive biocompatibility to active biointegration, potentially enabling truly long-term sensor functionality in challenging physiological environments.
Surface modification techniques have emerged as essential strategies for improving long-term sensor performance. Approaches such as anti-fouling coatings, controlled drug release systems, and nanopatterned surfaces have demonstrated significant reductions in protein adsorption and cellular adhesion, extending sensor functional lifetimes from days to months in some applications.
Cytotoxicity assessment protocols have evolved substantially, moving beyond simple cell viability tests to include comprehensive evaluations of inflammatory markers, oxidative stress indicators, and genetic expression profiles. These advanced testing methodologies provide deeper insights into the subtle biological responses to sensor materials, enabling more accurate predictions of in vivo performance.
Regulatory frameworks governing bioelectronic sensors continue to evolve, with the FDA and international bodies implementing increasingly stringent requirements for biocompatibility testing. The ISO 10993 standards series serves as the primary guideline, though emerging technologies often necessitate case-specific evaluation approaches that consider the unique material interfaces and intended implantation duration.
Biodegradation characteristics present both challenges and opportunities in sensor design. While uncontrolled degradation can release potentially harmful byproducts and compromise device function, intentionally engineered biodegradable components can eliminate the need for surgical removal and reduce long-term complications. Recent research has focused on developing materials with predictable degradation profiles that maintain functional integrity throughout their intended operational period.
Sterilization compatibility represents another critical consideration, as common sterilization methods like ethylene oxide exposure, gamma irradiation, and autoclave processing can significantly alter material properties. Novel sterilization-resistant materials and alternative low-temperature sterilization techniques are being developed specifically for sensitive bioelectronic components.
The integration of bioactive elements, including anti-inflammatory agents, growth factors, and cell-adhesion molecules, has shown promise in actively modulating the tissue response to implanted sensors. These biologically interactive interfaces represent a paradigm shift from passive biocompatibility to active biointegration, potentially enabling truly long-term sensor functionality in challenging physiological environments.
Manufacturability and Scalability Assessment
The manufacturability and scalability of bioelectronic sensors represent critical factors determining their commercial viability and widespread adoption. Current manufacturing processes for bioelectronic interfaces face significant challenges due to the complex integration of biological materials with electronic components. Traditional microfabrication techniques often employ harsh conditions that can compromise the functionality of biological elements, necessitating the development of specialized manufacturing protocols.
Material selection plays a pivotal role in manufacturing feasibility. Silicon-based substrates offer established fabrication pathways but present biocompatibility concerns. Alternatively, polymeric materials like PDMS and parylene C demonstrate improved biocompatibility and flexibility but require adaptation of conventional manufacturing processes. Recent advances in additive manufacturing technologies, particularly 3D bioprinting, show promise for fabricating complex bioelectronic interfaces with precise spatial control of both electronic and biological components.
Scalability considerations extend beyond fabrication techniques to encompass supply chain resilience and material sourcing. Noble metals commonly used in bioelectronic sensors, such as gold and platinum, face supply constraints and price volatility. Research into alternative conductive materials, including carbon-based nanomaterials and conductive polymers, offers potential pathways to mitigate these challenges while potentially enhancing sensor performance characteristics.
Quality control represents another significant manufacturing hurdle. The heterogeneous nature of bioelectronic interfaces necessitates comprehensive testing protocols that evaluate both electronic performance and biological functionality. Current approaches often rely on labor-intensive testing procedures that limit production throughput and increase unit costs. Automated testing platforms incorporating machine learning algorithms for defect detection show promise for addressing this bottleneck.
Cost analysis reveals that material interfaces typically constitute 30-45% of total production costs for bioelectronic sensors. Economies of scale remain difficult to achieve due to the precision requirements and specialized equipment needed for fabrication. Industry benchmarking indicates that successful commercial bioelectronic sensors maintain production yields above 85%, with defect rates below 5% for critical interface components.
Regulatory considerations further impact manufacturing strategies, with different jurisdictions imposing varying requirements for production facilities and quality management systems. The FDA's recent guidance on bioelectronic devices emphasizes the need for robust manufacturing controls and validation protocols, particularly for interfaces contacting biological tissues. Harmonizing these requirements across global markets represents an ongoing challenge for manufacturers seeking international distribution.
Material selection plays a pivotal role in manufacturing feasibility. Silicon-based substrates offer established fabrication pathways but present biocompatibility concerns. Alternatively, polymeric materials like PDMS and parylene C demonstrate improved biocompatibility and flexibility but require adaptation of conventional manufacturing processes. Recent advances in additive manufacturing technologies, particularly 3D bioprinting, show promise for fabricating complex bioelectronic interfaces with precise spatial control of both electronic and biological components.
Scalability considerations extend beyond fabrication techniques to encompass supply chain resilience and material sourcing. Noble metals commonly used in bioelectronic sensors, such as gold and platinum, face supply constraints and price volatility. Research into alternative conductive materials, including carbon-based nanomaterials and conductive polymers, offers potential pathways to mitigate these challenges while potentially enhancing sensor performance characteristics.
Quality control represents another significant manufacturing hurdle. The heterogeneous nature of bioelectronic interfaces necessitates comprehensive testing protocols that evaluate both electronic performance and biological functionality. Current approaches often rely on labor-intensive testing procedures that limit production throughput and increase unit costs. Automated testing platforms incorporating machine learning algorithms for defect detection show promise for addressing this bottleneck.
Cost analysis reveals that material interfaces typically constitute 30-45% of total production costs for bioelectronic sensors. Economies of scale remain difficult to achieve due to the precision requirements and specialized equipment needed for fabrication. Industry benchmarking indicates that successful commercial bioelectronic sensors maintain production yields above 85%, with defect rates below 5% for critical interface components.
Regulatory considerations further impact manufacturing strategies, with different jurisdictions imposing varying requirements for production facilities and quality management systems. The FDA's recent guidance on bioelectronic devices emphasizes the need for robust manufacturing controls and validation protocols, particularly for interfaces contacting biological tissues. Harmonizing these requirements across global markets represents an ongoing challenge for manufacturers seeking international distribution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!