Impact of Bioelectronic Interfaces on Genetic Engineering Applications
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic-Genetic Integration Background and Objectives
The convergence of bioelectronics and genetic engineering represents one of the most promising interdisciplinary frontiers in modern biotechnology. This integration has evolved significantly over the past two decades, transitioning from theoretical concepts to practical applications with transformative potential across medicine, agriculture, and environmental science. Historically, these fields developed separately: genetic engineering focused on manipulating DNA to alter organism traits, while bioelectronics concentrated on creating devices that interface with biological systems to monitor or influence physiological processes.
The technological trajectory began with rudimentary biosensors in the early 2000s and has now advanced to sophisticated implantable devices capable of real-time genetic monitoring and modulation. This progression has been accelerated by parallel developments in materials science, particularly the creation of biocompatible electronics and nanomaterials that minimize rejection responses while maximizing signal fidelity at the biological interface.
Recent breakthroughs in CRISPR-Cas9 technology have dramatically expanded the possibilities for precise genetic manipulation, creating new opportunities for bioelectronic interfaces to serve as delivery mechanisms or triggers for genetic modifications. The miniaturization of electronic components has further enabled the development of microscale and nanoscale devices capable of interacting with cellular structures at unprecedented resolution.
The primary objective of bioelectronic-genetic integration is to establish bidirectional communication channels between electronic systems and genetic material. This aims to enable real-time monitoring of genetic expression, targeted delivery of genetic payloads, and dynamic modulation of genetic activity in response to environmental or physiological changes. Such capabilities could revolutionize personalized medicine through continuous health monitoring and precisely timed therapeutic interventions.
Secondary objectives include developing self-powered bioelectronic systems that can operate autonomously within biological environments, improving the biocompatibility of electronic materials to enable long-term implantation, and creating standardized interfaces that facilitate broader adoption across research and clinical applications. These objectives align with the broader trend toward precision medicine and targeted therapies that minimize side effects while maximizing efficacy.
The technical evolution in this field is increasingly focused on wireless capabilities, allowing for non-invasive control and data collection from implanted devices. This direction presents both opportunities for patient comfort and challenges related to security and privacy of biological data. As these technologies mature, establishing appropriate regulatory frameworks and ethical guidelines will become increasingly important to ensure responsible development and deployment.
The technological trajectory began with rudimentary biosensors in the early 2000s and has now advanced to sophisticated implantable devices capable of real-time genetic monitoring and modulation. This progression has been accelerated by parallel developments in materials science, particularly the creation of biocompatible electronics and nanomaterials that minimize rejection responses while maximizing signal fidelity at the biological interface.
Recent breakthroughs in CRISPR-Cas9 technology have dramatically expanded the possibilities for precise genetic manipulation, creating new opportunities for bioelectronic interfaces to serve as delivery mechanisms or triggers for genetic modifications. The miniaturization of electronic components has further enabled the development of microscale and nanoscale devices capable of interacting with cellular structures at unprecedented resolution.
The primary objective of bioelectronic-genetic integration is to establish bidirectional communication channels between electronic systems and genetic material. This aims to enable real-time monitoring of genetic expression, targeted delivery of genetic payloads, and dynamic modulation of genetic activity in response to environmental or physiological changes. Such capabilities could revolutionize personalized medicine through continuous health monitoring and precisely timed therapeutic interventions.
Secondary objectives include developing self-powered bioelectronic systems that can operate autonomously within biological environments, improving the biocompatibility of electronic materials to enable long-term implantation, and creating standardized interfaces that facilitate broader adoption across research and clinical applications. These objectives align with the broader trend toward precision medicine and targeted therapies that minimize side effects while maximizing efficacy.
The technical evolution in this field is increasingly focused on wireless capabilities, allowing for non-invasive control and data collection from implanted devices. This direction presents both opportunities for patient comfort and challenges related to security and privacy of biological data. As these technologies mature, establishing appropriate regulatory frameworks and ethical guidelines will become increasingly important to ensure responsible development and deployment.
Market Analysis for Bioelectronic-Genetic Applications
The bioelectronic-genetic engineering market is experiencing unprecedented growth, driven by convergence of electronic systems with genetic manipulation technologies. Current market valuations indicate this sector reached approximately $3.2 billion in 2022, with projections suggesting a compound annual growth rate of 18.7% through 2030, potentially reaching $14.5 billion. This growth trajectory significantly outpaces traditional biotechnology sectors, reflecting the transformative potential of integrated bioelectronic-genetic solutions.
Demand is particularly strong in three key segments: medical therapeutics, agricultural applications, and industrial biotechnology. The medical sector represents the largest market share at 62%, driven by applications in targeted gene therapy delivery, real-time genetic monitoring systems, and personalized medicine platforms. Notable growth is observed in neural interface technologies that enable direct genetic modification in response to physiological signals, creating new treatment paradigms for neurological disorders.
Agricultural applications constitute 24% of the current market, with significant investment in crop optimization systems that utilize bioelectronic sensors to trigger genetic modifications in response to environmental stressors. This segment is projected to grow at 22.3% annually as climate adaptation concerns intensify globally.
Industrial biotechnology applications represent the remaining 14%, focused primarily on biomanufacturing processes where bioelectronic interfaces enable precise control of genetically engineered microorganisms for chemical and pharmaceutical production.
Regionally, North America dominates with 45% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (5%). However, the Asia-Pacific region demonstrates the fastest growth rate at 24.6% annually, driven by substantial government investments in China, South Korea, and Singapore.
Consumer and regulatory acceptance represents a significant market determinant. Public perception studies indicate growing acceptance of bioelectronic-genetic technologies for medical applications (73% approval), while agricultural applications face more resistance (41% approval). Regulatory frameworks are evolving rapidly, with the FDA establishing a dedicated Bioelectronic Technologies Division in 2021, and the European Medicines Agency developing specialized approval pathways for hybrid bioelectronic-genetic therapies.
Market barriers include high development costs, complex regulatory pathways, and technical challenges in interface biocompatibility. Despite these challenges, venture capital funding in this sector reached $4.7 billion in 2022, a 34% increase from the previous year, indicating strong investor confidence in long-term market potential.
Demand is particularly strong in three key segments: medical therapeutics, agricultural applications, and industrial biotechnology. The medical sector represents the largest market share at 62%, driven by applications in targeted gene therapy delivery, real-time genetic monitoring systems, and personalized medicine platforms. Notable growth is observed in neural interface technologies that enable direct genetic modification in response to physiological signals, creating new treatment paradigms for neurological disorders.
Agricultural applications constitute 24% of the current market, with significant investment in crop optimization systems that utilize bioelectronic sensors to trigger genetic modifications in response to environmental stressors. This segment is projected to grow at 22.3% annually as climate adaptation concerns intensify globally.
Industrial biotechnology applications represent the remaining 14%, focused primarily on biomanufacturing processes where bioelectronic interfaces enable precise control of genetically engineered microorganisms for chemical and pharmaceutical production.
Regionally, North America dominates with 45% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (5%). However, the Asia-Pacific region demonstrates the fastest growth rate at 24.6% annually, driven by substantial government investments in China, South Korea, and Singapore.
Consumer and regulatory acceptance represents a significant market determinant. Public perception studies indicate growing acceptance of bioelectronic-genetic technologies for medical applications (73% approval), while agricultural applications face more resistance (41% approval). Regulatory frameworks are evolving rapidly, with the FDA establishing a dedicated Bioelectronic Technologies Division in 2021, and the European Medicines Agency developing specialized approval pathways for hybrid bioelectronic-genetic therapies.
Market barriers include high development costs, complex regulatory pathways, and technical challenges in interface biocompatibility. Despite these challenges, venture capital funding in this sector reached $4.7 billion in 2022, a 34% increase from the previous year, indicating strong investor confidence in long-term market potential.
Current Bioelectronic Interface Technologies and Limitations
Bioelectronic interfaces represent a critical intersection between electronic systems and biological entities, enabling direct communication between technological devices and living organisms. Current bioelectronic interface technologies have evolved significantly over the past decade, yet still face substantial limitations that hinder their full potential in genetic engineering applications.
The state-of-the-art in bioelectronic interfaces includes several key technologies. Microelectrode arrays (MEAs) have become increasingly sophisticated, allowing for high-resolution recording and stimulation of cellular activity. These arrays can now achieve sub-cellular spatial resolution and millisecond temporal precision, enabling precise monitoring of genetic expression in real-time. However, they remain limited by issues of biocompatibility, with foreign body responses often degrading signal quality over extended periods.
Organic bioelectronics represent another promising frontier, utilizing conductive polymers that offer improved mechanical compatibility with biological tissues. These materials can transduce ionic signals from cells into electronic signals for processing, creating more natural interfaces. Despite these advantages, organic bioelectronics still struggle with long-term stability in physiological environments and often exhibit lower signal-to-noise ratios compared to traditional electronic components.
Optogenetic interfaces have revolutionized the field by enabling light-controlled manipulation of genetically modified cells. This technology allows unprecedented specificity in targeting individual cell types within complex tissues. The major limitations include the need for genetic modification of target cells and challenges in delivering light to deep tissues without invasive procedures.
Nanoscale bioelectronic interfaces, including carbon nanotubes and graphene-based devices, offer exceptional electrical properties and dimensions compatible with cellular structures. These materials can achieve intimate contact with cell membranes and even intracellular components, potentially enabling direct monitoring and manipulation of genetic material. However, concerns regarding cytotoxicity, reproducibility in manufacturing, and long-term stability remain significant barriers to widespread adoption.
Wireless bioelectronic systems have emerged to address the limitations of tethered interfaces, allowing for less invasive monitoring of biological systems. These technologies face challenges in power delivery, miniaturization, and achieving sufficient bandwidth for complex genetic engineering applications.
A fundamental limitation across all bioelectronic interface technologies is the "biotic-abiotic" interface challenge – the difficulty in creating stable, long-term connections between electronic components and living tissues. This interface is particularly problematic for genetic engineering applications, where sustained monitoring and precise control are essential for successful outcomes.
Signal processing and data interpretation represent another significant hurdle, as the complex signals generated by biological systems often require sophisticated algorithms to extract meaningful genetic information. The integration of artificial intelligence and machine learning approaches shows promise in addressing these challenges but remains in early developmental stages.
The state-of-the-art in bioelectronic interfaces includes several key technologies. Microelectrode arrays (MEAs) have become increasingly sophisticated, allowing for high-resolution recording and stimulation of cellular activity. These arrays can now achieve sub-cellular spatial resolution and millisecond temporal precision, enabling precise monitoring of genetic expression in real-time. However, they remain limited by issues of biocompatibility, with foreign body responses often degrading signal quality over extended periods.
Organic bioelectronics represent another promising frontier, utilizing conductive polymers that offer improved mechanical compatibility with biological tissues. These materials can transduce ionic signals from cells into electronic signals for processing, creating more natural interfaces. Despite these advantages, organic bioelectronics still struggle with long-term stability in physiological environments and often exhibit lower signal-to-noise ratios compared to traditional electronic components.
Optogenetic interfaces have revolutionized the field by enabling light-controlled manipulation of genetically modified cells. This technology allows unprecedented specificity in targeting individual cell types within complex tissues. The major limitations include the need for genetic modification of target cells and challenges in delivering light to deep tissues without invasive procedures.
Nanoscale bioelectronic interfaces, including carbon nanotubes and graphene-based devices, offer exceptional electrical properties and dimensions compatible with cellular structures. These materials can achieve intimate contact with cell membranes and even intracellular components, potentially enabling direct monitoring and manipulation of genetic material. However, concerns regarding cytotoxicity, reproducibility in manufacturing, and long-term stability remain significant barriers to widespread adoption.
Wireless bioelectronic systems have emerged to address the limitations of tethered interfaces, allowing for less invasive monitoring of biological systems. These technologies face challenges in power delivery, miniaturization, and achieving sufficient bandwidth for complex genetic engineering applications.
A fundamental limitation across all bioelectronic interface technologies is the "biotic-abiotic" interface challenge – the difficulty in creating stable, long-term connections between electronic components and living tissues. This interface is particularly problematic for genetic engineering applications, where sustained monitoring and precise control are essential for successful outcomes.
Signal processing and data interpretation represent another significant hurdle, as the complex signals generated by biological systems often require sophisticated algorithms to extract meaningful genetic information. The integration of artificial intelligence and machine learning approaches shows promise in addressing these challenges but remains in early developmental stages.
Existing Bioelectronic Solutions for Genetic Engineering
01 Neural interfaces for bioelectronic applications
Neural interfaces are a key component in bioelectronic systems, enabling direct communication between electronic devices and the nervous system. These interfaces can record neural activity and deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create biocompatible neural electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces find applications in neuroprosthetics, brain-computer interfaces, and therapeutic devices for neurological disorders.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between the nervous system and electronic devices. These interfaces can record neural activity, stimulate neural tissue, or both. They are crucial for applications such as brain-computer interfaces, neuroprosthetics, and neuromodulation therapies. Advanced materials and fabrication techniques are employed to create biocompatible interfaces that minimize tissue damage and maintain long-term functionality within the body.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces utilize elastic materials, serpentine structures, or mesh designs to accommodate movement while maintaining electrical functionality. They reduce mechanical mismatch between rigid electronics and soft tissues, minimizing inflammation and improving long-term biocompatibility. Applications include skin-mounted sensors, implantable devices, and wearable health monitors that can bend and stretch with the body.
- Biosensing and biofeedback systems: Biosensing and biofeedback systems utilize bioelectronic interfaces to detect biological signals and provide responsive feedback. These systems incorporate various sensing modalities including electrochemical, optical, and electrical sensors to monitor physiological parameters such as glucose levels, neural activity, or cardiac function. The collected data can be processed in real-time to deliver appropriate feedback or therapeutic interventions. Advanced signal processing algorithms and machine learning techniques are employed to improve the accuracy and reliability of these systems.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices are designed for long-term integration within the body to monitor health conditions or deliver therapeutic interventions. These devices incorporate biocompatible materials, hermetic packaging, and wireless communication capabilities to ensure functionality and safety within the biological environment. Power management strategies, including wireless power transfer and energy harvesting techniques, are implemented to extend device lifetime. Applications include cardiac pacemakers, neurostimulators, drug delivery systems, and continuous health monitoring implants.
- Nanomaterial-based bioelectronic interfaces: Nanomaterial-based bioelectronic interfaces leverage the unique properties of nanomaterials to enhance the performance of bioelectronic devices. Materials such as carbon nanotubes, graphene, and metal nanoparticles offer excellent electrical conductivity, high surface-to-volume ratio, and tunable surface chemistry. These properties enable improved signal transduction, reduced impedance, and enhanced biocompatibility. Nanomaterials can be functionalized with biomolecules to increase specificity for target analytes or tissues, making them valuable for biosensing, neural recording, and targeted drug delivery applications.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces incorporate elastic materials, serpentine structures, and thin-film technologies to achieve mechanical compliance with soft tissues. The flexibility reduces mechanical mismatch between rigid electronics and soft biological tissues, minimizing inflammation and improving long-term stability. These interfaces are particularly valuable for epidermal electronics, implantable sensors, and wearable health monitoring devices that must maintain functionality during body movement.Expand Specific Solutions03 Biosensing and molecular detection interfaces
Bioelectronic interfaces for molecular detection incorporate various sensing elements such as electrochemical sensors, field-effect transistors, and optical transducers to detect biological molecules. These interfaces often utilize functionalized surfaces with recognition elements like antibodies, aptamers, or enzymes to achieve specificity. Signal processing components are integrated to amplify and interpret the biological signals. Applications include point-of-care diagnostics, continuous health monitoring, environmental sensing, and rapid detection of pathogens or biomarkers.Expand Specific Solutions04 Implantable bioelectronic medical devices
Implantable bioelectronic medical devices integrate electronic components with biological systems for therapeutic or diagnostic purposes. These devices incorporate biocompatible materials, hermetic packaging, and wireless power and data transmission capabilities to ensure long-term functionality within the body. Advanced fabrication techniques are employed to miniaturize components while maintaining reliability. These interfaces are used in cardiac pacemakers, neurostimulators, drug delivery systems, and continuous glucose monitors, providing real-time monitoring and intervention for various medical conditions.Expand Specific Solutions05 Nanomaterial-based bioelectronic interfaces
Nanomaterial-based bioelectronic interfaces leverage unique properties of nanomaterials such as carbon nanotubes, graphene, quantum dots, and metal nanoparticles to enhance interface performance. These materials provide increased surface area, improved electrical conductivity, and enhanced biocompatibility. Nanomaterials can be functionalized with biomolecules to increase specificity and sensitivity in sensing applications. These advanced interfaces enable higher resolution neural recording, more efficient energy harvesting, and novel sensing modalities for next-generation bioelectronic devices.Expand Specific Solutions
Leading Organizations in Bioelectronic-Genetic Engineering
The bioelectronic interfaces and genetic engineering market is currently in a growth phase, with an expanding ecosystem of academic institutions and commercial players driving innovation. The market is projected to reach significant scale as these technologies converge to enable precise genetic manipulations with real-time monitoring capabilities. Leading companies like CRISPR Therapeutics and Vertex Pharmaceuticals are advancing clinical applications, while research powerhouses including MIT, Broad Institute, and Tsinghua University are developing foundational technologies. The field's technical maturity varies across applications, with companies like Amyris leveraging AI and machine learning to enhance bioengineering processes, while Samsung and Infineon contribute electronics expertise. This interdisciplinary landscape demonstrates increasing integration between biological systems and electronic interfaces, creating new therapeutic and industrial opportunities.
CRISPR Therapeutics AG
Technical Solution: CRISPR Therapeutics has developed a proprietary bioelectronic interface platform called "ElectroPore" that combines CRISPR gene editing technology with advanced bioelectronic delivery systems. Their approach utilizes precisely controlled electrical fields to create temporary pores in cell membranes (electroporation) while simultaneously guiding charged CRISPR-Cas9 ribonucleoprotein complexes into target cells. This system achieves significantly higher editing efficiency compared to traditional delivery methods. The company has further refined this technology with their "Pulse-Sequence Optimization" protocol that applies specific electrical pulse patterns tailored to different cell types and tissues, minimizing cellular damage while maximizing transfection efficiency. Their latest innovation includes implantable bioelectronic devices that can be remotely controlled to activate CRISPR systems in vivo, allowing for temporal control of gene editing in therapeutic applications. This technology has shown promising results in preclinical models for treating genetic blood disorders and certain cancers[4][7].
Strengths: Enhanced delivery efficiency for CRISPR components; reduced off-target effects through precise control; potential for in vivo applications with remote activation; compatibility with various cell types including hard-to-transfect cells. Weaknesses: Requires specialized equipment; potential tissue damage from electrical stimulation; challenges in targeting specific tissues in complex organisms; regulatory hurdles for implantable electronic devices.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered bioelectronic interfaces for genetic engineering through their development of the "Electro-Genetic Device" platform. This technology uses electrical signals to precisely control gene expression in engineered cells, enabling real-time regulation of genetic circuits without chemical inducers. MIT researchers have created bioelectronic systems that can trigger CRISPR-based gene editing through electrical stimulation, allowing for spatiotemporal control of genetic modifications. Their platform incorporates redox-responsive transcription factors that respond to electrical signals, creating a direct interface between electronic devices and genetic machinery. Recent advancements include the development of electrogenetic promoters that can be fine-tuned with different voltage inputs, creating analog-like control over gene expression levels. This technology has been demonstrated in both prokaryotic and eukaryotic systems, showing potential for applications ranging from bioproduction to medical therapeutics[1][3].
Strengths: Precise temporal control over gene expression; non-invasive regulation without chemical inducers; compatibility with existing genetic engineering tools; scalable for high-throughput applications. Weaknesses: Requires specialized equipment for electrical stimulation; potential cytotoxicity from electrical currents; limited to certain cell types that can withstand electrical stimulation.
Key Technical Innovations in Bioelectronic-Genetic Integration
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
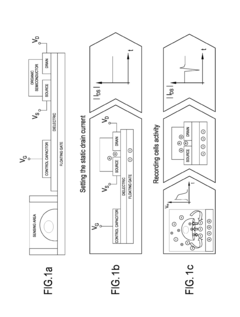
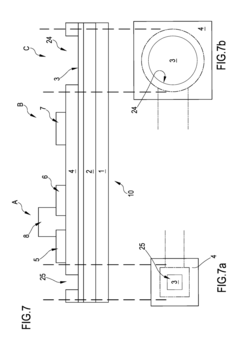
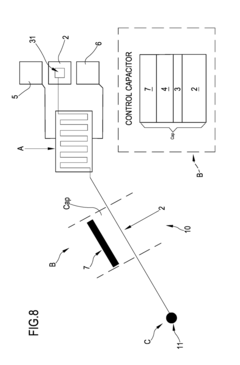
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
In vivo delivery system of the genome DNA modifying enzymes and the use thereof
PatentWO2020021312A1
Innovation
- The use of stem cells-derived extracellular vesicles (EVs) characterized by specific surface markers (CD90, CD105, CD147, and lack of CD45) for delivering DNA modifying enzymes like ZFN, TALEN, and CRISPR/Cas9 in serum-free conditions, which are safer and more effective for precise genome editing.
Biosafety and Regulatory Considerations
The integration of bioelectronic interfaces with genetic engineering applications presents significant biosafety and regulatory challenges that require careful consideration. Current regulatory frameworks were largely established before the emergence of these hybrid technologies, creating a governance gap that must be addressed. Regulatory bodies such as the FDA in the United States, the EMA in Europe, and the NMPA in China are actively developing guidelines specific to bioelectronic-genetic interfaces, though these remain in preliminary stages.
Risk assessment protocols for these technologies must consider both the electronic components and genetic modifications simultaneously, requiring new evaluation methodologies. The potential for unintended genetic alterations triggered by bioelectronic signals represents a novel biosafety concern that traditional genetic engineering safety protocols do not adequately address. Additionally, the long-term stability of bioelectronic interfaces within biological systems raises questions about chronic exposure effects and genetic drift over time.
Containment strategies for bioelectronic-genetic systems present unique challenges, as traditional physical containment methods may be insufficient when dealing with remotely activated or autonomous systems. The possibility of wireless communication with implanted bioelectronic devices creates potential cybersecurity vulnerabilities that could impact genetic functions, necessitating new security protocols and safeguards against unauthorized access.
International harmonization of regulations remains fragmented, with significant variations in approach between jurisdictions. While the International Council for Harmonisation of Technical Requirements (ICH) has begun preliminary discussions on bioelectronic-genetic interfaces, consensus standards are still years away. This regulatory uncertainty has created barriers to clinical translation and commercial development, with many companies hesitant to invest heavily in technologies with unclear approval pathways.
Ethical considerations further complicate the regulatory landscape, particularly regarding human enhancement applications versus therapeutic uses. Several countries have established specialized ethics committees focused specifically on bioelectronic-genetic technologies, recognizing the unique questions these hybrid systems raise about human identity and autonomy. Public perception and acceptance will significantly influence regulatory approaches, with transparency in risk communication being essential for building trust.
Moving forward, adaptive regulatory frameworks that can evolve alongside technological developments will be crucial. Regulatory sandboxes and phased approval processes are being explored as potential models to balance innovation with safety concerns. Multi-stakeholder engagement, including scientists, ethicists, patient advocates, and industry representatives, will be essential in developing appropriate governance structures for this rapidly evolving field.
Risk assessment protocols for these technologies must consider both the electronic components and genetic modifications simultaneously, requiring new evaluation methodologies. The potential for unintended genetic alterations triggered by bioelectronic signals represents a novel biosafety concern that traditional genetic engineering safety protocols do not adequately address. Additionally, the long-term stability of bioelectronic interfaces within biological systems raises questions about chronic exposure effects and genetic drift over time.
Containment strategies for bioelectronic-genetic systems present unique challenges, as traditional physical containment methods may be insufficient when dealing with remotely activated or autonomous systems. The possibility of wireless communication with implanted bioelectronic devices creates potential cybersecurity vulnerabilities that could impact genetic functions, necessitating new security protocols and safeguards against unauthorized access.
International harmonization of regulations remains fragmented, with significant variations in approach between jurisdictions. While the International Council for Harmonisation of Technical Requirements (ICH) has begun preliminary discussions on bioelectronic-genetic interfaces, consensus standards are still years away. This regulatory uncertainty has created barriers to clinical translation and commercial development, with many companies hesitant to invest heavily in technologies with unclear approval pathways.
Ethical considerations further complicate the regulatory landscape, particularly regarding human enhancement applications versus therapeutic uses. Several countries have established specialized ethics committees focused specifically on bioelectronic-genetic technologies, recognizing the unique questions these hybrid systems raise about human identity and autonomy. Public perception and acceptance will significantly influence regulatory approaches, with transparency in risk communication being essential for building trust.
Moving forward, adaptive regulatory frameworks that can evolve alongside technological developments will be crucial. Regulatory sandboxes and phased approval processes are being explored as potential models to balance innovation with safety concerns. Multi-stakeholder engagement, including scientists, ethicists, patient advocates, and industry representatives, will be essential in developing appropriate governance structures for this rapidly evolving field.
Ethical Implications and Societal Impact
The integration of bioelectronic interfaces with genetic engineering raises profound ethical questions that society must address proactively. These technologies enable unprecedented manipulation of biological systems, creating a responsibility to establish robust ethical frameworks before widespread implementation. Key concerns include informed consent for individuals whose genetic material may be modified through bioelectronic interfaces, especially when considering long-term and potentially heritable changes that could affect future generations.
Privacy considerations are particularly acute as bioelectronic interfaces generate vast amounts of genetic and neurological data. This information is exceptionally personal and could be vulnerable to misuse, discrimination, or unauthorized access. The potential for creating biological surveillance systems necessitates stringent data protection protocols and transparent governance structures to maintain public trust.
Equity of access represents another critical ethical dimension. Without deliberate intervention, these technologies risk exacerbating existing socioeconomic disparities in healthcare. The high costs associated with cutting-edge bioelectronic genetic engineering applications could create a two-tiered system where only privileged populations benefit from potentially life-changing treatments, widening the global health divide.
The dual-use potential of bioelectronic genetic engineering technologies presents significant security concerns. Capabilities developed for therapeutic purposes could be repurposed for enhancement, biological weapons, or other applications that threaten public safety or international security. This necessitates international cooperation on regulatory frameworks that balance innovation with appropriate safeguards.
Public perception and acceptance will ultimately determine the trajectory of these technologies. Current research indicates mixed attitudes, with enthusiasm for medical applications tempered by concerns about unintended consequences and misuse. Engaging diverse stakeholders in transparent dialogue about risks, benefits, and governance is essential for responsible development.
Regulatory frameworks must evolve to address these novel ethical challenges. Current regulations designed for conventional genetic engineering or medical devices may prove inadequate for the unique convergence represented by bioelectronic genetic interfaces. Adaptive governance approaches that can respond to rapidly evolving capabilities while upholding core ethical principles will be necessary to guide responsible innovation in this transformative field.
Privacy considerations are particularly acute as bioelectronic interfaces generate vast amounts of genetic and neurological data. This information is exceptionally personal and could be vulnerable to misuse, discrimination, or unauthorized access. The potential for creating biological surveillance systems necessitates stringent data protection protocols and transparent governance structures to maintain public trust.
Equity of access represents another critical ethical dimension. Without deliberate intervention, these technologies risk exacerbating existing socioeconomic disparities in healthcare. The high costs associated with cutting-edge bioelectronic genetic engineering applications could create a two-tiered system where only privileged populations benefit from potentially life-changing treatments, widening the global health divide.
The dual-use potential of bioelectronic genetic engineering technologies presents significant security concerns. Capabilities developed for therapeutic purposes could be repurposed for enhancement, biological weapons, or other applications that threaten public safety or international security. This necessitates international cooperation on regulatory frameworks that balance innovation with appropriate safeguards.
Public perception and acceptance will ultimately determine the trajectory of these technologies. Current research indicates mixed attitudes, with enthusiasm for medical applications tempered by concerns about unintended consequences and misuse. Engaging diverse stakeholders in transparent dialogue about risks, benefits, and governance is essential for responsible development.
Regulatory frameworks must evolve to address these novel ethical challenges. Current regulations designed for conventional genetic engineering or medical devices may prove inadequate for the unique convergence represented by bioelectronic genetic interfaces. Adaptive governance approaches that can respond to rapidly evolving capabilities while upholding core ethical principles will be necessary to guide responsible innovation in this transformative field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!