How Bioelectronic Interfaces Enhance Synthesized Tissue Compatibility
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, evolving significantly over the past three decades. Initially emerging in the 1990s as rudimentary electrode-based systems, these interfaces have transformed into sophisticated platforms capable of bidirectional communication with biological tissues. This evolution has been driven by advancements in materials science, microfabrication techniques, and a deeper understanding of cellular electrophysiology.
The trajectory of bioelectronic interface development has been marked by several pivotal milestones. Early interfaces primarily focused on neural recording capabilities, utilizing rigid metallic electrodes with limited biocompatibility. The 2000s witnessed the introduction of flexible substrates and conductive polymers, significantly enhancing tissue integration while reducing inflammatory responses. Recent years have seen the emergence of biodegradable electronics, wireless power transmission systems, and nanoscale interfaces that operate at the cellular level.
Current bioelectronic interfaces incorporate multiple functionalities beyond simple electrical stimulation, including chemical sensing, drug delivery, and mechanical property modulation. These multifunctional platforms enable more comprehensive interaction with synthesized tissues, facilitating improved integration and functionality. The miniaturization trend continues to drive development, with interfaces now approaching dimensions compatible with individual cell interaction.
The primary objective of modern bioelectronic interfaces in tissue engineering is to enhance the compatibility and functionality of synthesized tissues through precise electrical, mechanical, and biochemical modulation. These interfaces aim to recreate the native bioelectrical environment critical for proper tissue development and function. By providing controlled electrical stimulation, these systems can guide cell differentiation, promote organized tissue formation, and facilitate functional integration with host tissues.
Another crucial objective is developing interfaces that can dynamically adapt to changing tissue conditions during maturation. This adaptability requires responsive systems capable of adjusting stimulation parameters based on real-time feedback from the developing tissue. Such bidirectional communication represents a significant advancement over traditional static scaffolds.
Looking forward, the field is moving toward fully integrated, "smart" bioelectronic interfaces that can autonomously support tissue development without external intervention. These next-generation systems will likely incorporate artificial intelligence for optimized stimulation protocols, self-healing materials for extended durability, and advanced biosensing capabilities for precise monitoring of tissue development. The ultimate goal remains creating seamless integration between electronic components and biological tissues, effectively blurring the distinction between natural and synthetic systems.
The trajectory of bioelectronic interface development has been marked by several pivotal milestones. Early interfaces primarily focused on neural recording capabilities, utilizing rigid metallic electrodes with limited biocompatibility. The 2000s witnessed the introduction of flexible substrates and conductive polymers, significantly enhancing tissue integration while reducing inflammatory responses. Recent years have seen the emergence of biodegradable electronics, wireless power transmission systems, and nanoscale interfaces that operate at the cellular level.
Current bioelectronic interfaces incorporate multiple functionalities beyond simple electrical stimulation, including chemical sensing, drug delivery, and mechanical property modulation. These multifunctional platforms enable more comprehensive interaction with synthesized tissues, facilitating improved integration and functionality. The miniaturization trend continues to drive development, with interfaces now approaching dimensions compatible with individual cell interaction.
The primary objective of modern bioelectronic interfaces in tissue engineering is to enhance the compatibility and functionality of synthesized tissues through precise electrical, mechanical, and biochemical modulation. These interfaces aim to recreate the native bioelectrical environment critical for proper tissue development and function. By providing controlled electrical stimulation, these systems can guide cell differentiation, promote organized tissue formation, and facilitate functional integration with host tissues.
Another crucial objective is developing interfaces that can dynamically adapt to changing tissue conditions during maturation. This adaptability requires responsive systems capable of adjusting stimulation parameters based on real-time feedback from the developing tissue. Such bidirectional communication represents a significant advancement over traditional static scaffolds.
Looking forward, the field is moving toward fully integrated, "smart" bioelectronic interfaces that can autonomously support tissue development without external intervention. These next-generation systems will likely incorporate artificial intelligence for optimized stimulation protocols, self-healing materials for extended durability, and advanced biosensing capabilities for precise monitoring of tissue development. The ultimate goal remains creating seamless integration between electronic components and biological tissues, effectively blurring the distinction between natural and synthetic systems.
Market Analysis for Biocompatible Tissue Engineering
The biocompatible tissue engineering market is experiencing robust growth, driven by increasing demand for regenerative medicine solutions and advancements in bioelectronic interface technologies. Current market valuations indicate the global tissue engineering market reached approximately 12 billion USD in 2022, with projections suggesting a compound annual growth rate of 14.2% through 2030. This growth trajectory is particularly pronounced in regions with aging populations and high healthcare expenditure, notably North America, Europe, and increasingly in Asia-Pacific markets.
Demand analysis reveals several key market segments driving adoption of bioelectronic interfaces for enhanced tissue compatibility. The orthopedic and musculoskeletal segment currently dominates market share, accounting for roughly 35% of applications, followed by cardiovascular (22%), skin/integumentary (18%), and neurological applications (15%). The remaining market share is distributed across various specialized applications including dental, urological, and gastrointestinal tissue engineering.
Healthcare providers represent the largest end-user segment, particularly academic medical centers and specialized clinics focusing on regenerative medicine. Pharmaceutical and biotechnology companies constitute the second-largest market segment, increasingly investing in bioelectronic tissue engineering platforms to develop novel therapeutic approaches and drug testing models.
Market research indicates several factors accelerating demand for biocompatible tissue engineering solutions. The rising prevalence of chronic diseases and conditions requiring tissue replacement or regeneration serves as a primary driver. Additionally, the growing elderly population worldwide has increased the incidence of degenerative diseases, creating substantial demand for innovative tissue engineering solutions incorporating bioelectronic interfaces.
Regulatory landscapes significantly impact market dynamics across different regions. The FDA in the United States has established specific pathways for combination products involving bioelectronic components and engineered tissues, while the European Medicines Agency has implemented the Advanced Therapy Medicinal Products framework. These regulatory frameworks, while necessary for ensuring safety and efficacy, present market entry barriers that influence commercialization timelines.
Reimbursement policies represent another critical market factor. Currently, many bioelectronic tissue engineering applications face challenges in securing consistent reimbursement coverage, limiting widespread clinical adoption despite promising clinical outcomes. However, several countries are developing specialized reimbursement pathways for regenerative medicine technologies, potentially accelerating market penetration in the coming years.
Consumer awareness and acceptance of bioelectronic tissue engineering solutions continue to improve, particularly as successful clinical applications demonstrate improved outcomes compared to traditional approaches. This growing acceptance, coupled with increasing healthcare provider familiarity with these technologies, suggests a favorable environment for continued market expansion.
Demand analysis reveals several key market segments driving adoption of bioelectronic interfaces for enhanced tissue compatibility. The orthopedic and musculoskeletal segment currently dominates market share, accounting for roughly 35% of applications, followed by cardiovascular (22%), skin/integumentary (18%), and neurological applications (15%). The remaining market share is distributed across various specialized applications including dental, urological, and gastrointestinal tissue engineering.
Healthcare providers represent the largest end-user segment, particularly academic medical centers and specialized clinics focusing on regenerative medicine. Pharmaceutical and biotechnology companies constitute the second-largest market segment, increasingly investing in bioelectronic tissue engineering platforms to develop novel therapeutic approaches and drug testing models.
Market research indicates several factors accelerating demand for biocompatible tissue engineering solutions. The rising prevalence of chronic diseases and conditions requiring tissue replacement or regeneration serves as a primary driver. Additionally, the growing elderly population worldwide has increased the incidence of degenerative diseases, creating substantial demand for innovative tissue engineering solutions incorporating bioelectronic interfaces.
Regulatory landscapes significantly impact market dynamics across different regions. The FDA in the United States has established specific pathways for combination products involving bioelectronic components and engineered tissues, while the European Medicines Agency has implemented the Advanced Therapy Medicinal Products framework. These regulatory frameworks, while necessary for ensuring safety and efficacy, present market entry barriers that influence commercialization timelines.
Reimbursement policies represent another critical market factor. Currently, many bioelectronic tissue engineering applications face challenges in securing consistent reimbursement coverage, limiting widespread clinical adoption despite promising clinical outcomes. However, several countries are developing specialized reimbursement pathways for regenerative medicine technologies, potentially accelerating market penetration in the coming years.
Consumer awareness and acceptance of bioelectronic tissue engineering solutions continue to improve, particularly as successful clinical applications demonstrate improved outcomes compared to traditional approaches. This growing acceptance, coupled with increasing healthcare provider familiarity with these technologies, suggests a favorable environment for continued market expansion.
Current Challenges in Tissue-Electronics Integration
Despite significant advancements in bioelectronic interfaces for tissue integration, several critical challenges continue to impede progress in this rapidly evolving field. The fundamental issue remains the inherent incompatibility between rigid electronic components and soft biological tissues. This mechanical mismatch creates stress at the interface, leading to inflammation, scarring, and eventual device failure. Current materials used in bioelectronic devices typically possess elastic moduli in the gigapascal range, while most human tissues range from 1 kPa to 100 kPa, representing a disparity of several orders of magnitude.
Biocompatibility presents another significant hurdle, as foreign body responses to implanted electronic materials often trigger immune reactions that compromise device functionality and tissue health. The formation of fibrous encapsulation around implanted devices creates an insulating layer that increases impedance and reduces signal quality, particularly problematic for neural interfaces and biosensors requiring precise measurements.
Long-term stability remains elusive, with most current bioelectronic interfaces demonstrating performance degradation over time due to material deterioration, biofouling, and shifting tissue-electrode interfaces. Studies indicate that approximately 40% of implanted neural electrodes show significant signal deterioration within the first year of implantation, necessitating additional surgical interventions or rendering devices ineffective.
Power requirements pose another substantial challenge, as most active bioelectronic interfaces require external power sources or bulky batteries that limit implantation locations and increase infection risks. Wireless power transfer technologies show promise but face efficiency limitations when transmitting through biological tissues, with power transfer efficiency dropping by approximately 30-50% through just 1 cm of tissue.
Signal processing complexities further complicate integration efforts. Biological signals are inherently noisy, low-amplitude, and often require sophisticated algorithms to extract meaningful information. Current on-device processing capabilities remain limited by size and power constraints, necessitating external processing that introduces latency and reliability concerns.
Manufacturing scalability presents additional barriers, as many promising bioelectronic interfaces rely on complex fabrication techniques that are difficult to standardize and scale. The intricate nature of these devices, often requiring features at the micro or nanoscale, results in high production costs and limited accessibility for widespread clinical adoption.
Regulatory pathways for novel bioelectronic interfaces remain challenging to navigate, with unclear classification categories for hybrid biological-electronic systems. The FDA and similar international bodies continue to develop appropriate frameworks for evaluating the safety and efficacy of these innovative technologies, but the process remains time-consuming and expensive, delaying clinical translation.
Biocompatibility presents another significant hurdle, as foreign body responses to implanted electronic materials often trigger immune reactions that compromise device functionality and tissue health. The formation of fibrous encapsulation around implanted devices creates an insulating layer that increases impedance and reduces signal quality, particularly problematic for neural interfaces and biosensors requiring precise measurements.
Long-term stability remains elusive, with most current bioelectronic interfaces demonstrating performance degradation over time due to material deterioration, biofouling, and shifting tissue-electrode interfaces. Studies indicate that approximately 40% of implanted neural electrodes show significant signal deterioration within the first year of implantation, necessitating additional surgical interventions or rendering devices ineffective.
Power requirements pose another substantial challenge, as most active bioelectronic interfaces require external power sources or bulky batteries that limit implantation locations and increase infection risks. Wireless power transfer technologies show promise but face efficiency limitations when transmitting through biological tissues, with power transfer efficiency dropping by approximately 30-50% through just 1 cm of tissue.
Signal processing complexities further complicate integration efforts. Biological signals are inherently noisy, low-amplitude, and often require sophisticated algorithms to extract meaningful information. Current on-device processing capabilities remain limited by size and power constraints, necessitating external processing that introduces latency and reliability concerns.
Manufacturing scalability presents additional barriers, as many promising bioelectronic interfaces rely on complex fabrication techniques that are difficult to standardize and scale. The intricate nature of these devices, often requiring features at the micro or nanoscale, results in high production costs and limited accessibility for widespread clinical adoption.
Regulatory pathways for novel bioelectronic interfaces remain challenging to navigate, with unclear classification categories for hybrid biological-electronic systems. The FDA and similar international bodies continue to develop appropriate frameworks for evaluating the safety and efficacy of these innovative technologies, but the process remains time-consuming and expensive, delaying clinical translation.
State-of-the-Art Biocompatibility Solutions
01 Biocompatible materials for bioelectronic interfaces
Biocompatible materials are essential for creating effective bioelectronic interfaces that can integrate with biological tissues without causing adverse reactions. These materials include conductive polymers, hydrogels, and flexible substrates that minimize immune responses and tissue damage. The selection of appropriate biocompatible materials ensures long-term stability and functionality of bioelectronic devices when in contact with biological systems.- Biocompatible materials for neural interfaces: Biocompatible materials are essential for creating neural interfaces that can safely interact with biological tissues. These materials must minimize immune responses and tissue damage while maintaining functionality over extended periods. Advanced polymers, hydrogels, and flexible substrates are being developed to improve the long-term stability and performance of implantable neural devices. These materials can be engineered to match the mechanical properties of surrounding tissues, reducing inflammation and scar formation at the interface.
- Surface modification techniques for enhanced biocompatibility: Surface modification of bioelectronic interfaces can significantly improve their compatibility with biological systems. Techniques include coating with anti-inflammatory agents, cell-adhesive proteins, or bioactive molecules that promote integration with host tissue. Chemical functionalization and nanopatterning of electrode surfaces can reduce foreign body responses while enhancing signal transduction. These modifications aim to create a more favorable microenvironment at the tissue-device interface, improving long-term performance and reducing rejection.
- Wireless and flexible bioelectronic systems: Wireless and flexible bioelectronic interfaces offer improved compatibility by reducing mechanical mismatch with biological tissues. These systems can conform to curved biological surfaces and accommodate natural tissue movement, minimizing irritation and inflammation. Wireless power and data transmission eliminate the need for transcutaneous wires, reducing infection risk and improving patient comfort. Stretchable electronics and thin-film technologies enable devices that can flex and stretch with the body, maintaining stable connections during movement.
- Integration of biological components with electronic systems: Hybrid bioelectronic interfaces incorporate biological components to improve compatibility with living tissues. These systems may use living cells, biomolecules, or engineered tissues as intermediaries between electronic components and the host organism. Techniques include cell-seeded electrode arrays, protein-coated interfaces, and tissue-engineered constructs with embedded sensors. This biological integration can reduce foreign body responses while enhancing signal transduction and functional integration with target tissues.
- Software and signal processing for bioelectronic compatibility: Advanced software and signal processing techniques are crucial for ensuring compatibility between bioelectronic interfaces and biological systems. These computational approaches can adapt to changing biological conditions, filter noise, and optimize signal quality from implanted devices. Machine learning algorithms can personalize device function based on individual physiological responses, while real-time processing enables dynamic adjustment of stimulation parameters. These software solutions complement hardware improvements to create more responsive and adaptable bioelectronic systems.
02 Integration of electronic components with biological systems
The integration of electronic components with biological systems involves designing interfaces that can effectively communicate between electronic devices and living tissues. This includes developing sensors, electrodes, and signal processing systems that can accurately detect and interpret biological signals while maintaining compatibility with the host tissue. Advanced integration techniques focus on minimizing invasiveness and maximizing signal quality for applications in neural interfaces, prosthetics, and health monitoring.Expand Specific Solutions03 Surface modification techniques for enhanced biocompatibility
Surface modification techniques are employed to enhance the biocompatibility of bioelectronic interfaces. These techniques include coating with bioactive molecules, plasma treatment, chemical functionalization, and nanopatterning to improve cell adhesion, reduce foreign body responses, and enhance integration with host tissues. Modified surfaces can promote specific cellular interactions while preventing unwanted immune responses, leading to improved long-term performance of implanted bioelectronic devices.Expand Specific Solutions04 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to match the mechanical properties of biological tissues, reducing mechanical mismatch and improving comfort and durability. These interfaces utilize materials and structural designs that can conform to the dynamic nature of biological surfaces while maintaining electrical functionality. Technologies include serpentine interconnects, mesh electronics, and elastomeric substrates that allow the interface to move with the tissue without causing damage or losing connectivity.Expand Specific Solutions05 Software and communication protocols for bioelectronic interfaces
Software systems and communication protocols are crucial for ensuring compatibility between bioelectronic devices and external systems. These include specialized algorithms for signal processing, data interpretation, and device control that can adapt to biological variability. Advanced software frameworks enable real-time monitoring, feedback control, and integration with existing medical systems while ensuring data security and reliability in bioelectronic applications.Expand Specific Solutions
Leading Organizations in Bioelectronic Tissue Engineering
The bioelectronic interfaces market for enhanced synthesized tissue compatibility is currently in its growth phase, with an estimated market size of $3-5 billion and projected annual growth of 15-20%. The technology is advancing from experimental to early commercial applications, with varying degrees of maturity across different applications. Leading academic institutions like MIT, Carnegie Mellon, and Northwestern University are driving fundamental research, while companies such as Infineon Technologies and W.L. Gore & Associates are developing commercial applications. Medical device manufacturers including Covidien and Cardiac Pacemakers are integrating bioelectronic interfaces into existing product lines. The competitive landscape features collaboration between research institutions and industry partners, with increasing interest from pharmaceutical companies like Mochida Pharmaceutical seeking to enhance drug delivery systems through bioelectronic interface technologies.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered bioelectronic interfaces that enhance tissue compatibility through their "tissue-electronics" platform. This approach integrates flexible, stretchable electronic materials with biological tissues to create seamless interfaces. Their technology utilizes hydrogel-based electronics that match the mechanical properties of human tissues, reducing foreign body responses and inflammation. MIT researchers have developed conductive polymers with tissue-like elasticity that can be embedded with growth factors to promote cell adhesion and proliferation at the interface. Their recent innovations include 3D-printed scaffolds with integrated electronic components that guide tissue regeneration while simultaneously monitoring cellular activity. The electronic components are designed to degrade at controlled rates that match tissue integration timelines, ensuring long-term biocompatibility[1][3]. MIT's platform also incorporates microfluidic channels for delivering therapeutics directly to the tissue-electrode interface, maintaining tissue health and reducing fibrotic encapsulation that typically degrades signal quality over time.
Strengths: Superior mechanical matching with host tissues, reducing immune rejection; integrated sensing and stimulation capabilities in a single platform; controlled degradation profiles aligned with tissue integration. Weaknesses: Complex manufacturing processes limit scalability; higher costs compared to conventional interfaces; potential long-term stability issues in dynamic tissue environments.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed advanced bioelectronic interfaces focusing on neural tissue compatibility through their proprietary "bio-inspired electrode" technology. Their approach utilizes ultrasoft polymer composites that mimic the mechanical properties of neural tissue, with elastic moduli below 100 kPa to minimize mechanical mismatch. These interfaces incorporate anti-inflammatory drug-eluting coatings that actively suppress foreign body responses during the critical initial implantation period. Michigan's research team has pioneered "stealth" surface modifications using zwitterionic polymers that significantly reduce protein adsorption and subsequent immune cell attachment at the tissue-electrode interface[2]. Their most recent innovation involves a dual-mode interface that combines electrical recording/stimulation with optical interrogation capabilities, allowing for multimodal interaction with synthesized tissues. The electrodes feature nanoporous surfaces that encourage controlled tissue ingrowth while maintaining electrical properties, creating a more stable long-term interface with reduced impedance fluctuations[4]. Michigan's technology has demonstrated sustained functionality in vivo for over 12 months, significantly outperforming conventional rigid electrode systems.
Strengths: Exceptional mechanical compatibility with neural tissues; integrated anti-inflammatory capabilities reduce scarring; multimodal interaction capabilities enable more comprehensive tissue monitoring and modulation. Weaknesses: Limited to primarily neural applications; complex fabrication processes increase production costs; requires specialized implantation techniques to prevent damage to the ultrasoft materials.
Critical Patents in Tissue-Electronic Interface Design
Conformable neural interface device with hydrogel adhesion and methods of using the same
PatentWO2020018120A1
Innovation
- A conformable neural interface device featuring a suture-like anchor device with a gel polymer network, comprising a crosslinkable polymer precursor crosslinked with a redox active metal, that adheres to peripheral nerves using an adhesive hydrogel layer, allowing for enhanced mechanical compliance and selective tissue contact without invasive penetration.
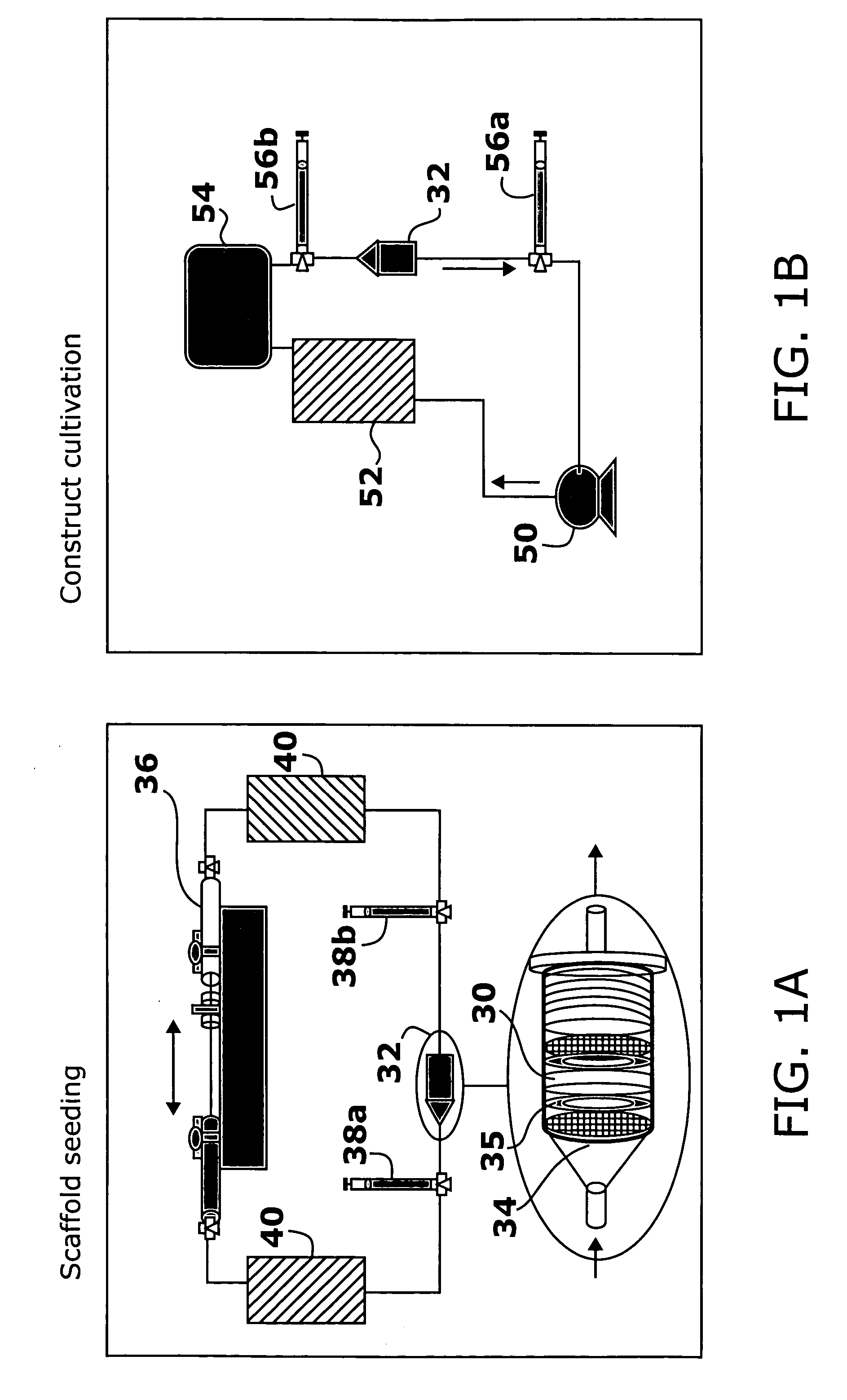
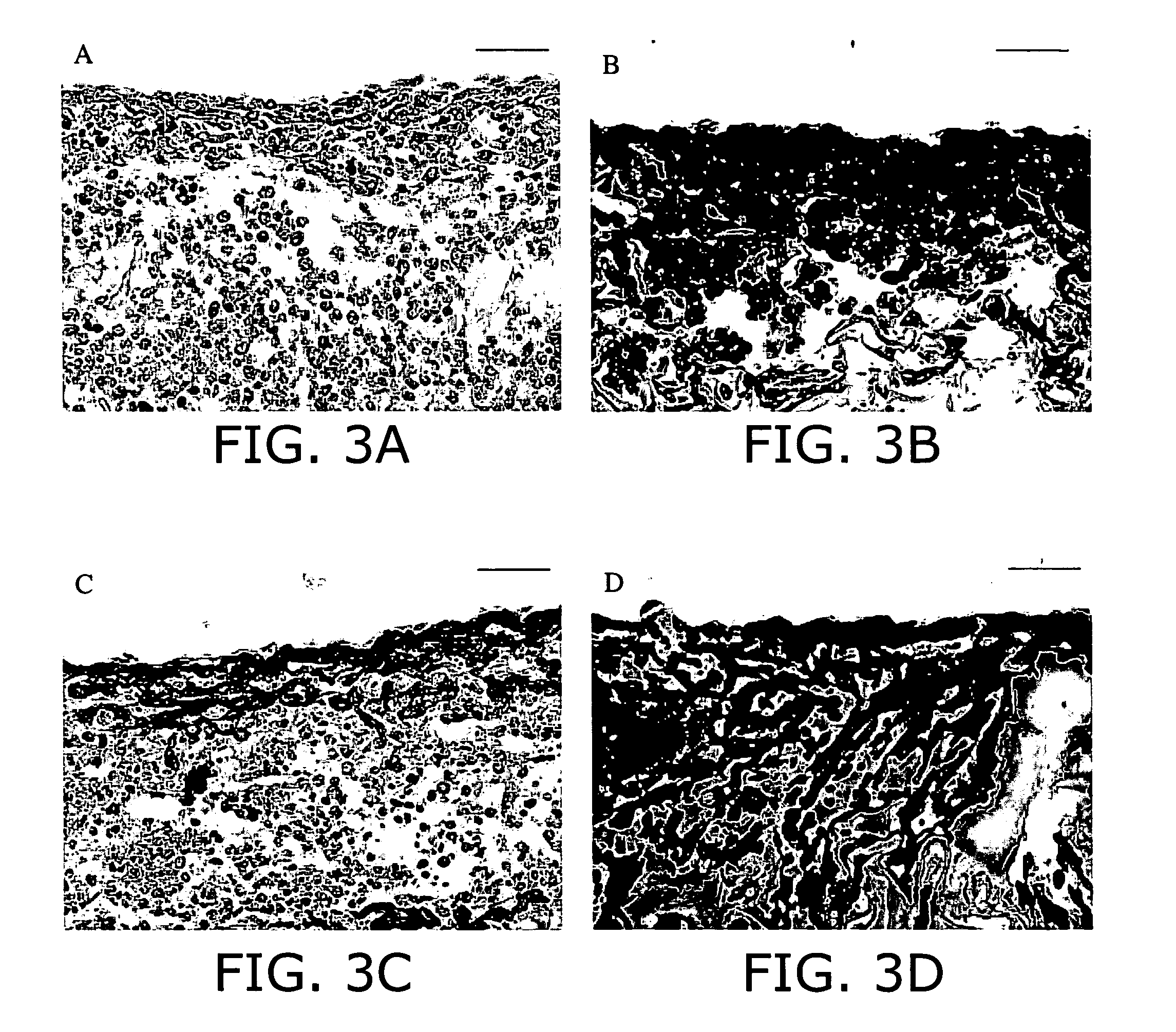
Application of electrical stimulation for functional tissue engineering in vitro and in vivo
PatentInactiveUS20050112759A1
Innovation
- The development of methods involving cell-seeded constructs cultivated in the presence of biomimetic electrical stimulation, using biocompatible substrates, and incorporating biologically active agents to promote cell differentiation and integration, aiming to create tissue-engineered constructs with defined orientation and enhanced functionality.
Regulatory Framework for Implantable Bioelectronics
The regulatory landscape for implantable bioelectronic devices represents a complex framework that spans multiple jurisdictions and oversight bodies. In the United States, the Food and Drug Administration (FDA) classifies most bioelectronic interfaces as Class III medical devices, requiring premarket approval (PMA) with extensive clinical trials demonstrating both safety and efficacy. The FDA's Center for Devices and Radiological Health (CDRH) has recently established specialized guidelines for tissue-integrated electronics, emphasizing long-term biocompatibility testing protocols.
The European Union, under the Medical Device Regulation (MDR 2017/745), imposes stringent requirements for CE marking of implantable bioelectronics, particularly those interfacing with synthesized tissues. These regulations mandate comprehensive technical documentation, risk management procedures, and post-market surveillance systems that specifically address the unique challenges of tissue-electronic interfaces.
International standards organizations have developed critical frameworks governing bioelectronic implants. ISO 14708 specifically addresses active implantable medical devices, while ISO 10993 series provides essential guidance on biological evaluation of medical devices. The IEC 60601 standards family covers electrical safety aspects crucial for bioelectronic interfaces. These standards are continuously evolving to address emerging technologies in tissue-electronic integration.
Regulatory bodies increasingly recognize the unique challenges posed by bioelectronic-tissue interfaces. The FDA's Digital Health Innovation Action Plan and the EU's guidance on software as a medical device (SaMD) are expanding to include provisions for devices that incorporate both biological and electronic components. These frameworks are developing specialized pathways for approval of hybrid technologies that don't fit traditional device classifications.
A significant regulatory challenge involves determining appropriate testing methodologies for tissue-integrated electronics. Current frameworks struggle with establishing standardized protocols for evaluating long-term compatibility between synthetic tissues and electronic components. Regulatory agencies are working with research institutions to develop new testing paradigms that better predict in vivo performance of these complex systems.
Global harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), are working to standardize approaches to bioelectronic device regulation across major markets. These initiatives aim to reduce regulatory barriers while maintaining rigorous safety standards, potentially accelerating innovation in bioelectronic interfaces for tissue engineering applications.
The European Union, under the Medical Device Regulation (MDR 2017/745), imposes stringent requirements for CE marking of implantable bioelectronics, particularly those interfacing with synthesized tissues. These regulations mandate comprehensive technical documentation, risk management procedures, and post-market surveillance systems that specifically address the unique challenges of tissue-electronic interfaces.
International standards organizations have developed critical frameworks governing bioelectronic implants. ISO 14708 specifically addresses active implantable medical devices, while ISO 10993 series provides essential guidance on biological evaluation of medical devices. The IEC 60601 standards family covers electrical safety aspects crucial for bioelectronic interfaces. These standards are continuously evolving to address emerging technologies in tissue-electronic integration.
Regulatory bodies increasingly recognize the unique challenges posed by bioelectronic-tissue interfaces. The FDA's Digital Health Innovation Action Plan and the EU's guidance on software as a medical device (SaMD) are expanding to include provisions for devices that incorporate both biological and electronic components. These frameworks are developing specialized pathways for approval of hybrid technologies that don't fit traditional device classifications.
A significant regulatory challenge involves determining appropriate testing methodologies for tissue-integrated electronics. Current frameworks struggle with establishing standardized protocols for evaluating long-term compatibility between synthetic tissues and electronic components. Regulatory agencies are working with research institutions to develop new testing paradigms that better predict in vivo performance of these complex systems.
Global harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), are working to standardize approaches to bioelectronic device regulation across major markets. These initiatives aim to reduce regulatory barriers while maintaining rigorous safety standards, potentially accelerating innovation in bioelectronic interfaces for tissue engineering applications.
Ethical Implications of Human-Machine Integration
The integration of bioelectronic interfaces with synthesized tissues raises profound ethical questions about the boundaries between humans and machines. As these technologies advance, society must confront fundamental philosophical questions about human identity and what constitutes "natural" versus "enhanced" humanity. The blurring of these boundaries challenges traditional ethical frameworks and necessitates new approaches to bioethics that can address the unique considerations of human-machine integration.
Privacy and autonomy concerns emerge prominently in this domain. Bioelectronic interfaces that monitor, record, or influence biological processes may compromise individual privacy in unprecedented ways. The potential for unauthorized access to neural data or biological information stored within these interfaces presents significant ethical challenges. Furthermore, questions arise regarding who maintains control over these integrated systems—the individual, healthcare providers, or the manufacturers of the technology.
Informed consent takes on new dimensions when considering permanent or semi-permanent integration of bioelectronic components with human tissues. Traditional consent models may prove inadequate when addressing technologies that could potentially alter an individual's sense of self or agency. The long-term implications of such integration, which may not be fully understood at the time of implementation, further complicate the consent process.
Social justice considerations cannot be overlooked, as access to advanced bioelectronic interfaces may create new forms of inequality. If these technologies significantly enhance quality of life or extend lifespan, disparities in access could exacerbate existing social divides. The potential emergence of a "bioelectronically enhanced" class versus those without such enhancements raises serious ethical concerns about fairness and human dignity.
Regulatory frameworks currently lag behind technological developments in this field. The unique nature of bioelectronic-tissue interfaces challenges existing medical device regulations, human subject research protocols, and healthcare delivery systems. International coordination will be essential to prevent regulatory arbitrage and ensure consistent ethical standards across borders.
The potential for dual-use applications presents additional ethical challenges. Technologies developed to enhance tissue compatibility for medical purposes could potentially be repurposed for military applications or human enhancement beyond therapeutic needs. Establishing appropriate boundaries for the application of these technologies requires ongoing ethical deliberation involving diverse stakeholders.
As we advance in this field, establishing ethics committees specifically focused on human-machine integration will be crucial. These committees should include not only scientists and ethicists but also representatives from patient advocacy groups, disability rights organizations, and the broader public to ensure comprehensive ethical oversight of these transformative technologies.
Privacy and autonomy concerns emerge prominently in this domain. Bioelectronic interfaces that monitor, record, or influence biological processes may compromise individual privacy in unprecedented ways. The potential for unauthorized access to neural data or biological information stored within these interfaces presents significant ethical challenges. Furthermore, questions arise regarding who maintains control over these integrated systems—the individual, healthcare providers, or the manufacturers of the technology.
Informed consent takes on new dimensions when considering permanent or semi-permanent integration of bioelectronic components with human tissues. Traditional consent models may prove inadequate when addressing technologies that could potentially alter an individual's sense of self or agency. The long-term implications of such integration, which may not be fully understood at the time of implementation, further complicate the consent process.
Social justice considerations cannot be overlooked, as access to advanced bioelectronic interfaces may create new forms of inequality. If these technologies significantly enhance quality of life or extend lifespan, disparities in access could exacerbate existing social divides. The potential emergence of a "bioelectronically enhanced" class versus those without such enhancements raises serious ethical concerns about fairness and human dignity.
Regulatory frameworks currently lag behind technological developments in this field. The unique nature of bioelectronic-tissue interfaces challenges existing medical device regulations, human subject research protocols, and healthcare delivery systems. International coordination will be essential to prevent regulatory arbitrage and ensure consistent ethical standards across borders.
The potential for dual-use applications presents additional ethical challenges. Technologies developed to enhance tissue compatibility for medical purposes could potentially be repurposed for military applications or human enhancement beyond therapeutic needs. Establishing appropriate boundaries for the application of these technologies requires ongoing ethical deliberation involving diverse stakeholders.
As we advance in this field, establishing ethics committees specifically focused on human-machine integration will be crucial. These committees should include not only scientists and ethicists but also representatives from patient advocacy groups, disability rights organizations, and the broader public to ensure comprehensive ethical oversight of these transformative technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!