Integrative Approaches to Advancing Bioelectronic Interface Design Techniques
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary frontier where biology meets electronics, enabling direct communication between biological systems and electronic devices. The evolution of these interfaces has been marked by significant milestones over the past several decades, transitioning from rudimentary electrode-based systems to sophisticated, multifunctional platforms capable of bidirectional communication with living tissues.
The field emerged in the 1970s with the development of basic neural recording electrodes, primarily focused on understanding brain activity. By the 1990s, advancements in materials science and microfabrication techniques led to the creation of more refined microelectrode arrays, enabling higher resolution recordings and stimulation capabilities. The early 2000s witnessed the integration of flexible electronics, addressing the mechanical mismatch between rigid electronic components and soft biological tissues.
A paradigm shift occurred around 2010 with the introduction of organic electronics and conducting polymers, which offered improved biocompatibility and charge transfer characteristics. This period also saw the emergence of wireless power and data transmission systems, eliminating the need for transcutaneous connections and reducing infection risks. Recent years have been characterized by the development of biodegradable electronics, self-healing materials, and nanoscale interfaces that minimize foreign body responses.
The current technological trajectory is moving toward fully integrated, multifunctional bioelectronic systems that can simultaneously sense, process, and modulate biological signals with minimal invasiveness. Machine learning algorithms are increasingly being incorporated to interpret complex biological data patterns and optimize therapeutic interventions in real-time.
The primary objectives of advancing bioelectronic interface design techniques include enhancing long-term stability and biocompatibility to enable chronic implantation without tissue damage or performance degradation. Improving spatial and temporal resolution remains crucial for precise interaction with specific cellular populations. Miniaturization efforts aim to reduce invasiveness while maintaining functionality, particularly important for neural applications.
Energy efficiency represents another critical goal, as bioelectronic devices often operate under strict power constraints, especially for implantable applications. Developing self-powered systems that harvest energy from biological processes could eliminate the need for external power sources or battery replacements. Additionally, creating closed-loop systems capable of autonomous operation based on physiological feedback would enable more responsive and personalized therapeutic interventions.
The ultimate vision for bioelectronic interfaces encompasses seamless integration with biological systems, functioning as natural extensions of the body rather than foreign implants. This requires interdisciplinary approaches combining expertise from materials science, electrical engineering, neuroscience, and medicine to overcome current limitations and realize the full potential of this transformative technology.
The field emerged in the 1970s with the development of basic neural recording electrodes, primarily focused on understanding brain activity. By the 1990s, advancements in materials science and microfabrication techniques led to the creation of more refined microelectrode arrays, enabling higher resolution recordings and stimulation capabilities. The early 2000s witnessed the integration of flexible electronics, addressing the mechanical mismatch between rigid electronic components and soft biological tissues.
A paradigm shift occurred around 2010 with the introduction of organic electronics and conducting polymers, which offered improved biocompatibility and charge transfer characteristics. This period also saw the emergence of wireless power and data transmission systems, eliminating the need for transcutaneous connections and reducing infection risks. Recent years have been characterized by the development of biodegradable electronics, self-healing materials, and nanoscale interfaces that minimize foreign body responses.
The current technological trajectory is moving toward fully integrated, multifunctional bioelectronic systems that can simultaneously sense, process, and modulate biological signals with minimal invasiveness. Machine learning algorithms are increasingly being incorporated to interpret complex biological data patterns and optimize therapeutic interventions in real-time.
The primary objectives of advancing bioelectronic interface design techniques include enhancing long-term stability and biocompatibility to enable chronic implantation without tissue damage or performance degradation. Improving spatial and temporal resolution remains crucial for precise interaction with specific cellular populations. Miniaturization efforts aim to reduce invasiveness while maintaining functionality, particularly important for neural applications.
Energy efficiency represents another critical goal, as bioelectronic devices often operate under strict power constraints, especially for implantable applications. Developing self-powered systems that harvest energy from biological processes could eliminate the need for external power sources or battery replacements. Additionally, creating closed-loop systems capable of autonomous operation based on physiological feedback would enable more responsive and personalized therapeutic interventions.
The ultimate vision for bioelectronic interfaces encompasses seamless integration with biological systems, functioning as natural extensions of the body rather than foreign implants. This requires interdisciplinary approaches combining expertise from materials science, electrical engineering, neuroscience, and medicine to overcome current limitations and realize the full potential of this transformative technology.
Market Analysis for Bioelectronic Interface Applications
The bioelectronic interface market is experiencing unprecedented growth, driven by advancements in materials science, miniaturization technologies, and increasing applications across healthcare and consumer electronics. Current market valuations place the global bioelectronic interface sector at approximately 5.7 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 14.3% through 2030.
Healthcare applications represent the largest market segment, accounting for nearly 60% of current demand. Within this segment, neural interfaces for managing neurological disorders such as Parkinson's disease, epilepsy, and chronic pain domination, followed by cardiac monitoring devices and glucose sensors for diabetes management. The aging global population and rising prevalence of chronic diseases are significant demand drivers in this sector.
Consumer applications are emerging as the fastest-growing segment, with wearable bioelectronic interfaces for fitness tracking, stress management, and sleep monitoring gaining substantial market traction. This segment is projected to grow at a CAGR of 17.8% over the next five years, outpacing the overall market growth rate.
Regionally, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (23%). However, the Asia-Pacific region is demonstrating the highest growth potential, particularly in countries like China, Japan, and South Korea, where substantial investments in healthcare infrastructure and bioelectronic research are being made.
Key market challenges include regulatory hurdles, with FDA and CE approval processes often extending development timelines by 2-3 years. Additionally, reimbursement uncertainties in healthcare systems and concerns regarding data privacy present significant market barriers.
Customer adoption patterns reveal increasing acceptance of invasive bioelectronic interfaces for medical applications when significant therapeutic benefits are demonstrated. Meanwhile, non-invasive interfaces are gaining wider consumer acceptance as design aesthetics improve and form factors become less obtrusive.
Market consolidation is evident with major medical device manufacturers acquiring innovative startups to expand their bioelectronic portfolios. Venture capital investment in the sector reached 1.2 billion USD in 2022, a 35% increase from the previous year, indicating strong investor confidence in future market growth.
Emerging market opportunities include brain-computer interfaces for rehabilitation, bioelectronic medicine for inflammatory conditions, and closed-loop systems that can both monitor and deliver therapeutic interventions. These applications represent potential new market segments with projected values exceeding 3 billion USD by 2028.
Healthcare applications represent the largest market segment, accounting for nearly 60% of current demand. Within this segment, neural interfaces for managing neurological disorders such as Parkinson's disease, epilepsy, and chronic pain domination, followed by cardiac monitoring devices and glucose sensors for diabetes management. The aging global population and rising prevalence of chronic diseases are significant demand drivers in this sector.
Consumer applications are emerging as the fastest-growing segment, with wearable bioelectronic interfaces for fitness tracking, stress management, and sleep monitoring gaining substantial market traction. This segment is projected to grow at a CAGR of 17.8% over the next five years, outpacing the overall market growth rate.
Regionally, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (23%). However, the Asia-Pacific region is demonstrating the highest growth potential, particularly in countries like China, Japan, and South Korea, where substantial investments in healthcare infrastructure and bioelectronic research are being made.
Key market challenges include regulatory hurdles, with FDA and CE approval processes often extending development timelines by 2-3 years. Additionally, reimbursement uncertainties in healthcare systems and concerns regarding data privacy present significant market barriers.
Customer adoption patterns reveal increasing acceptance of invasive bioelectronic interfaces for medical applications when significant therapeutic benefits are demonstrated. Meanwhile, non-invasive interfaces are gaining wider consumer acceptance as design aesthetics improve and form factors become less obtrusive.
Market consolidation is evident with major medical device manufacturers acquiring innovative startups to expand their bioelectronic portfolios. Venture capital investment in the sector reached 1.2 billion USD in 2022, a 35% increase from the previous year, indicating strong investor confidence in future market growth.
Emerging market opportunities include brain-computer interfaces for rehabilitation, bioelectronic medicine for inflammatory conditions, and closed-loop systems that can both monitor and deliver therapeutic interventions. These applications represent potential new market segments with projected values exceeding 3 billion USD by 2028.
Current Challenges in Bioelectronic Interface Technology
Bioelectronic interfaces represent a critical nexus between biological systems and electronic devices, enabling unprecedented capabilities in healthcare monitoring, neural prosthetics, and therapeutic interventions. Despite significant advancements, the field faces substantial technical challenges that impede widespread clinical adoption and optimal functionality. The biocompatibility of materials remains a paramount concern, as long-term implantation often triggers foreign body responses, leading to device encapsulation by scar tissue and subsequent performance degradation. Current materials struggle to balance mechanical compliance with biological tissues while maintaining electronic functionality, creating a fundamental mismatch at the biotic-abiotic interface.
Signal quality and stability present another significant hurdle. Bioelectronic interfaces must detect extremely weak biological signals amid considerable noise, requiring sophisticated signal processing algorithms and hardware solutions. The signal-to-noise ratio deteriorates over time due to electrode degradation, cellular remodeling, and micro-motion at the tissue-device interface, compromising long-term recording fidelity and stimulation precision.
Power management constitutes a critical limitation, particularly for implantable devices. Conventional batteries necessitate periodic surgical replacement, while wireless power transfer systems face efficiency challenges through biological tissues. Energy harvesting approaches from the body remain insufficient for power-intensive applications, creating a technological bottleneck for chronic implantable systems.
Miniaturization and integration challenges persist as bioelectronic interfaces incorporate increasingly complex functionality into smaller form factors. The integration of sensing, stimulation, processing, and communication capabilities within biocompatible packages demands advanced fabrication techniques and novel architectural approaches. Current manufacturing methods struggle to achieve the necessary precision at scales compatible with biological structures.
Data processing and interpretation represent emerging challenges as interfaces generate unprecedented volumes of biological data. Real-time analysis requires edge computing capabilities within severe power and size constraints. Machine learning algorithms must be adapted to operate within these limitations while maintaining accuracy in biological signal interpretation.
Regulatory and standardization issues further complicate advancement, with unclear pathways for novel bioelectronic technologies. The interdisciplinary nature of the field necessitates collaboration across traditionally separate domains, including materials science, electrical engineering, neuroscience, and medicine. This integration challenge extends to manufacturing, where scalable production of high-precision, biocompatible devices remains problematic.
Addressing these multifaceted challenges requires coordinated efforts across scientific disciplines and industrial sectors, with particular emphasis on materials innovation, system-level integration approaches, and translational research methodologies.
Signal quality and stability present another significant hurdle. Bioelectronic interfaces must detect extremely weak biological signals amid considerable noise, requiring sophisticated signal processing algorithms and hardware solutions. The signal-to-noise ratio deteriorates over time due to electrode degradation, cellular remodeling, and micro-motion at the tissue-device interface, compromising long-term recording fidelity and stimulation precision.
Power management constitutes a critical limitation, particularly for implantable devices. Conventional batteries necessitate periodic surgical replacement, while wireless power transfer systems face efficiency challenges through biological tissues. Energy harvesting approaches from the body remain insufficient for power-intensive applications, creating a technological bottleneck for chronic implantable systems.
Miniaturization and integration challenges persist as bioelectronic interfaces incorporate increasingly complex functionality into smaller form factors. The integration of sensing, stimulation, processing, and communication capabilities within biocompatible packages demands advanced fabrication techniques and novel architectural approaches. Current manufacturing methods struggle to achieve the necessary precision at scales compatible with biological structures.
Data processing and interpretation represent emerging challenges as interfaces generate unprecedented volumes of biological data. Real-time analysis requires edge computing capabilities within severe power and size constraints. Machine learning algorithms must be adapted to operate within these limitations while maintaining accuracy in biological signal interpretation.
Regulatory and standardization issues further complicate advancement, with unclear pathways for novel bioelectronic technologies. The interdisciplinary nature of the field necessitates collaboration across traditionally separate domains, including materials science, electrical engineering, neuroscience, and medicine. This integration challenge extends to manufacturing, where scalable production of high-precision, biocompatible devices remains problematic.
Addressing these multifaceted challenges requires coordinated efforts across scientific disciplines and industrial sectors, with particular emphasis on materials innovation, system-level integration approaches, and translational research methodologies.
Contemporary Bioelectronic Interface Design Solutions
01 Flexible and stretchable bioelectronic interfaces
Development of flexible and stretchable materials for bioelectronic interfaces that can conform to biological tissues. These interfaces incorporate elastic substrates, conductive polymers, and novel fabrication techniques to create devices that maintain functionality during movement and deformation. Such flexibility enables better integration with living systems, improved signal quality, and reduced tissue damage for long-term implantation.- Flexible and stretchable bioelectronic interfaces: Design techniques for creating flexible and stretchable bioelectronic interfaces that can conform to biological tissues. These interfaces utilize specialized materials and fabrication methods to achieve mechanical compliance with soft tissues while maintaining electrical functionality. Such designs enable better signal acquisition and stimulation by improving the contact between electronic devices and biological systems, reducing mechanical mismatch, and allowing for natural movement without disrupting the interface.
- Nanomaterial-based bioelectronic interfaces: Integration of nanomaterials such as carbon nanotubes, graphene, and nanoparticles in bioelectronic interfaces to enhance performance. These nanomaterials provide improved electrical conductivity, increased surface area for biological interaction, and enhanced biocompatibility. Design techniques focus on controlled deposition, patterning, and functionalization of nanomaterials to create high-performance sensing and stimulation interfaces at the cellular and molecular levels.
- Implantable bioelectronic systems: Design techniques for implantable bioelectronic interfaces that can function reliably within the body for extended periods. These designs address challenges such as biocompatibility, hermeticity, power management, and wireless communication. Specialized encapsulation methods, electrode materials, and circuit designs are employed to ensure long-term stability and functionality in the physiological environment while minimizing foreign body responses.
- Neural interface technologies: Specialized bioelectronic interfaces designed specifically for interfacing with neural tissue. These designs incorporate microelectrode arrays, neural probes, and signal processing systems optimized for recording and stimulating neural activity. Advanced fabrication techniques enable high-density electrode arrangements with minimal tissue damage, while specialized materials and coatings improve biocompatibility and reduce inflammatory responses at the neural interface.
- Biohybrid interface systems: Design approaches that combine living biological components with electronic systems to create hybrid bioelectronic interfaces. These designs incorporate cells, tissues, or biomolecules directly into the electronic interface to improve biocompatibility and functionality. Techniques include surface functionalization with bioactive molecules, incorporation of living cells into device structures, and development of biomimetic materials that better integrate with biological systems.
02 Nanomaterial-based bioelectronic sensors
Integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles into bioelectronic interfaces to enhance sensitivity and specificity. These nanomaterials provide high surface-to-volume ratios, unique electrical properties, and can be functionalized with biomolecules for targeted sensing applications. The resulting sensors offer improved detection limits, faster response times, and capabilities for real-time monitoring of biological signals.Expand Specific Solutions03 Neural interface technologies
Design techniques for creating direct communication pathways between electronic devices and the nervous system. These interfaces incorporate microelectrode arrays, signal processing algorithms, and biocompatible materials to record and stimulate neural activity. Advanced fabrication methods enable high-density electrode arrangements while minimizing tissue damage, allowing for applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders.Expand Specific Solutions04 Biofunctionalization of electronic surfaces
Methods for modifying electronic surfaces with biological molecules to improve biocompatibility and functionality. These techniques include immobilization of proteins, antibodies, or nucleic acids onto electrode surfaces through chemical crosslinking, self-assembled monolayers, or polymer coatings. Biofunctionalization enhances specificity of detection, reduces biofouling, and improves the long-term stability of implanted devices by mediating the interface between electronic components and biological systems.Expand Specific Solutions05 Wireless and implantable bioelectronic systems
Development of miniaturized, wireless bioelectronic interfaces for implantable and wearable applications. These systems integrate power harvesting technologies, low-power electronics, and wireless communication protocols to enable remote monitoring and control of bioelectronic devices. Advanced packaging techniques provide hermetic sealing against biological fluids while maintaining small form factors, making these interfaces suitable for long-term in vivo applications and continuous health monitoring.Expand Specific Solutions
Leading Organizations in Bioelectronic Interface Development
The bioelectronic interface design field is currently in a growth phase, with an estimated market size of $3-5 billion and projected annual growth of 15-20%. The competitive landscape features a mix of academic institutions (MIT, Northwestern University, University of Michigan) leading fundamental research, alongside established industrial players (Infineon Technologies, Philips, Otsuka Pharmaceutical) commercializing applications. Technical maturity varies across applications, with medical implants being more advanced than neural interfaces. Key technology leaders include Fraunhofer-Gesellschaft and Draper Laboratory developing conductive polymers, Biotectix pioneering bioactive coatings, and MIT advancing flexible electronics. The field is characterized by increasing cross-sector collaboration between academia, medical device manufacturers, and electronics companies to overcome biocompatibility and longevity challenges.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered integrative approaches to bioelectronic interfaces through their development of flexible, stretchable electronic systems that can seamlessly integrate with biological tissues. Their technology utilizes novel materials including conducting polymers and carbon nanotubes to create interfaces with enhanced biocompatibility and long-term stability. MIT researchers have developed a platform that combines soft electronics with hydrogel-based interfaces to minimize mechanical mismatch between electronic devices and biological tissues[1]. Their recent innovations include tissue-like electronics that can be injected through needles into specific brain regions, enabling minimally invasive neural recording and stimulation[2]. MIT has also advanced wireless power transfer technologies for implantable bioelectronic devices, eliminating the need for batteries and reducing device size while extending operational lifetimes[3]. Their integrated approach combines materials science, electrical engineering, and neuroscience to create multifunctional interfaces capable of both recording and modulating biological activity with unprecedented precision.
Strengths: Superior materials engineering capabilities allowing for flexible, tissue-compatible interfaces; strong interdisciplinary collaboration between engineering and biological sciences; advanced fabrication techniques for microscale devices. Weaknesses: Some technologies remain in early research phases with significant translational challenges; higher manufacturing costs compared to conventional rigid electronics; potential long-term reliability issues in physiological environments.
Northwestern University
Technical Solution: Northwestern University has developed groundbreaking bioelectronic interfaces through their "skin-interfaced bioelectronics" platform. Their technology features ultrathin, soft electronic systems that conform to the skin's surface and provide wireless, battery-free operation for continuous health monitoring[1]. Northwestern's approach integrates advanced materials science with innovative fabrication techniques to create devices with thickness less than 100 micrometers that can stretch, bend, and twist without performance degradation[2]. Their bioelectronic interfaces incorporate microfluidic channels alongside electronic components, enabling simultaneous collection of biofluids and electrophysiological signals. Northwestern researchers have pioneered transient electronics that can dissolve harmlessly in the body after their functional lifetime, eliminating the need for surgical removal of implanted devices[3]. Their latest innovations include epidermal VR systems that provide haptic feedback through programmable patterns of localized mechanical vibrations, creating new possibilities for human-machine interfaces in healthcare applications[4].
Strengths: Exceptional expertise in materials and manufacturing for skin-interfaced electronics; strong focus on wireless, battery-free operation enhancing usability; innovative approaches to biodegradable electronics. Weaknesses: Some technologies face challenges in scaling to commercial production; potential limitations in power delivery for more energy-intensive applications; current interfaces primarily focus on surface measurements rather than deep tissue integration.
Critical Patents and Research in Bioelectronic Interfaces
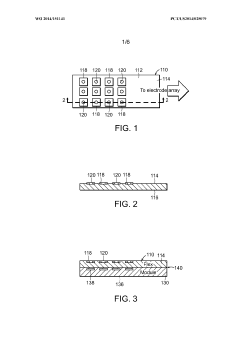
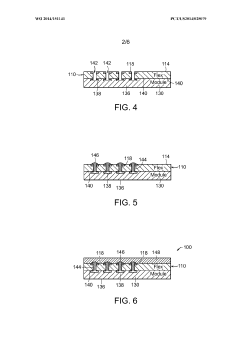
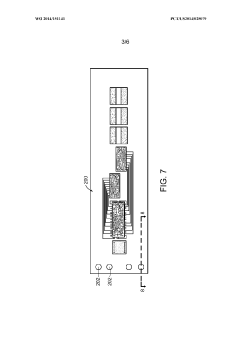
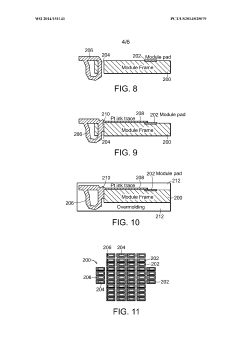
Methods for bonding a hermetic module to an electrode array
PatentWO2014151141A1
Innovation
- The use of micro-ink-jet or aerojet printing of biocompatible conductive ink, combined with laser-formed vias and overmolding, to create a robust and high-density connection system between hermetic modules, electrode arrays, and lead wires, allowing for increased connection density and reliability.
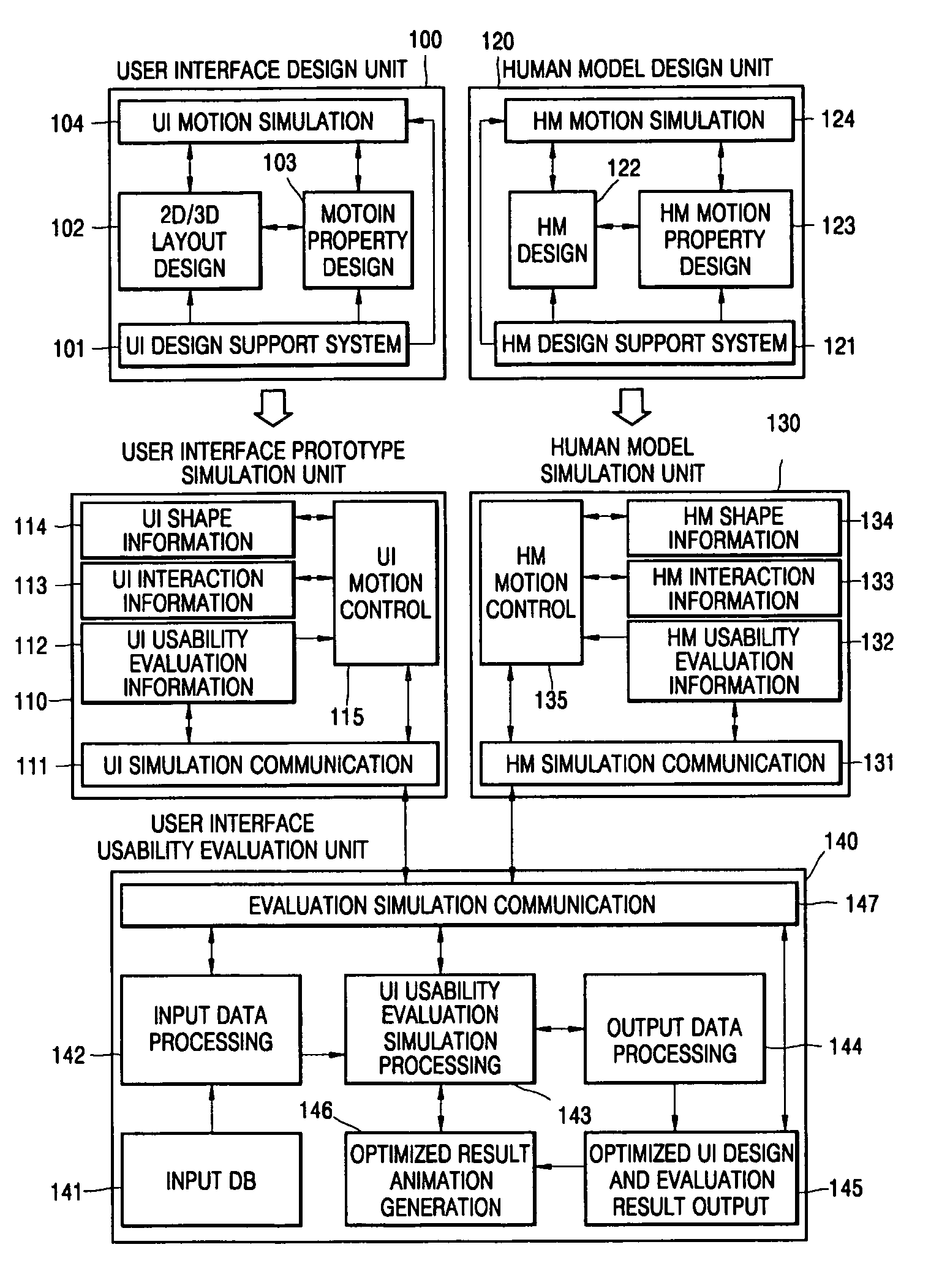
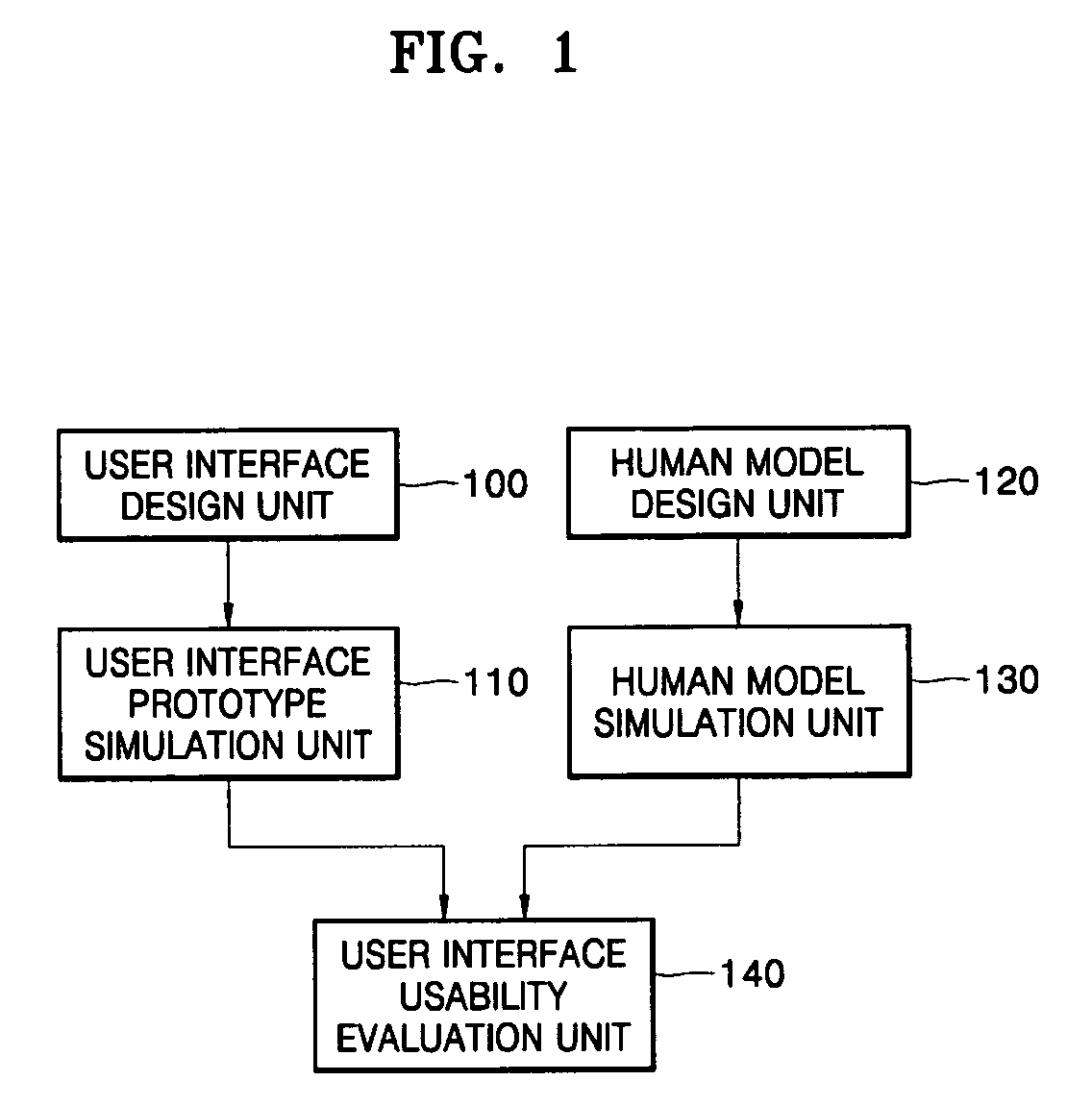
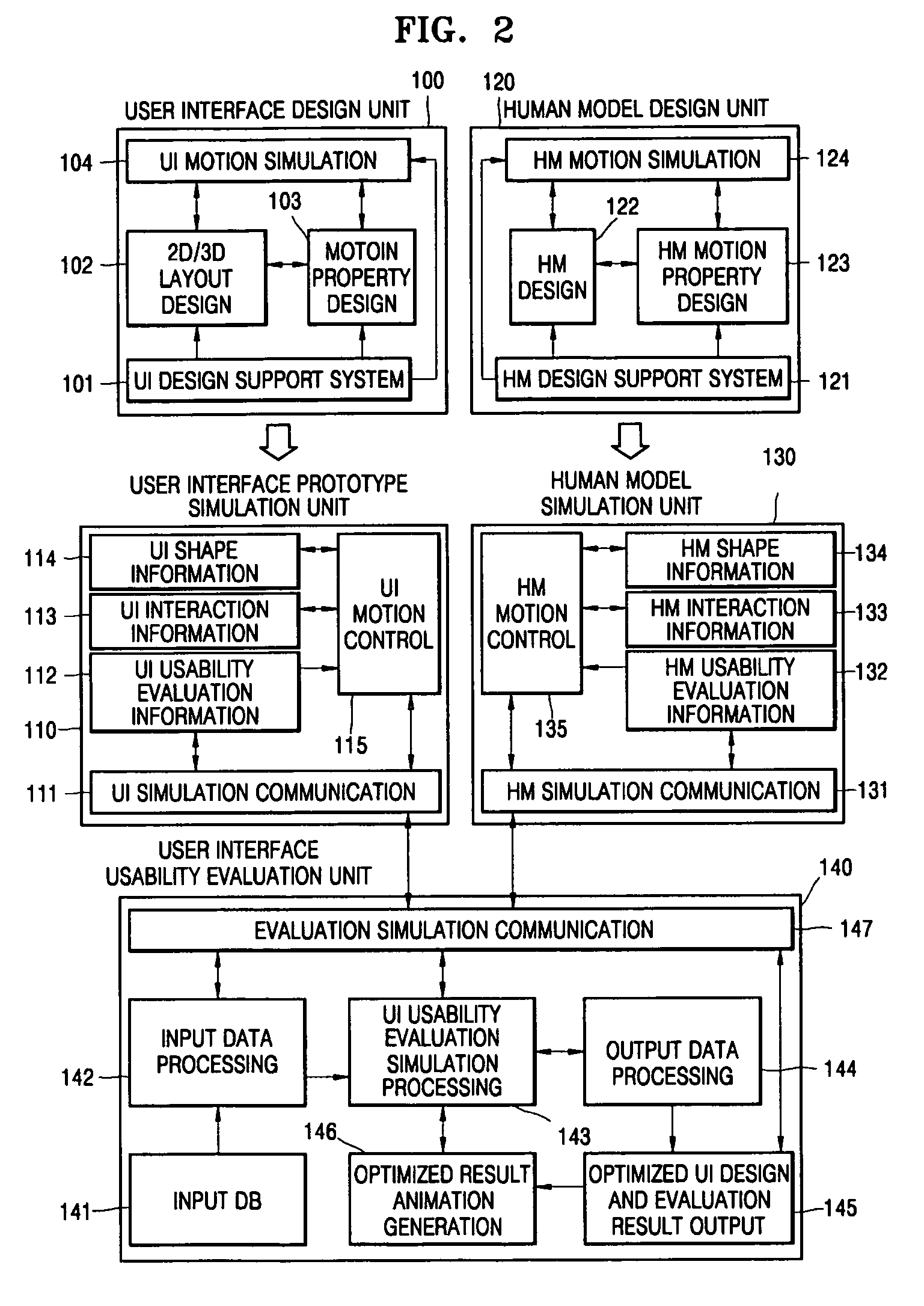
User interface design and evaluation system and hand interaction based user interface design and evaluation system
PatentActiveUS7464010B2
Innovation
- A user interface design and evaluation system that includes a user interface design unit, human model design unit, prototype simulation unit, and usability evaluation unit, which simulates user interactions and evaluates usability through motion properties and feedback data to optimize interface design for various user environments.
Biocompatibility and Safety Standards
Biocompatibility and safety standards represent critical pillars in the advancement of bioelectronic interface technologies. As these devices establish intimate contact with biological tissues, stringent regulatory frameworks have evolved to ensure patient safety while enabling innovation. The FDA in the United States and the European Medicines Agency have established comprehensive guidelines specifically addressing bioelectronic implants, with ISO 10993 serving as the international benchmark for biological evaluation of medical devices.
These standards evaluate multiple aspects of biocompatibility, including cytotoxicity, sensitization, irritation, acute systemic toxicity, and long-term implantation effects. For bioelectronic interfaces, particular attention is paid to electrochemical stability, corrosion resistance, and leaching potential of electrode materials. Recent updates to these standards have incorporated specific provisions for devices utilizing novel nanomaterials and conductive polymers, reflecting the rapid evolution of the field.
Material selection represents a fundamental consideration in meeting these standards. Traditional metallic electrodes (platinum, iridium oxide, and titanium nitride) have established safety profiles, while newer materials such as PEDOT:PSS and graphene derivatives require more extensive validation. The standards now mandate comprehensive leachable and extractable testing protocols to identify potential toxic byproducts resulting from material degradation in the biological environment.
Long-term safety assessment methodologies have also evolved significantly. Accelerated aging studies, combined with computational models predicting material degradation, now complement traditional animal studies. These approaches help predict device performance over extended implantation periods, addressing concerns about chronic foreign body responses and material fatigue. The standards increasingly emphasize the importance of real-world performance data collected through post-market surveillance.
Emerging bioelectronic interfaces incorporating drug delivery capabilities or biodegradable components face additional regulatory scrutiny. Combination products that merge electronic and pharmaceutical elements must satisfy requirements from multiple regulatory frameworks. Standards organizations are actively developing specialized protocols for these hybrid technologies, recognizing their unique risk profiles and potential benefits.
International harmonization efforts are underway to streamline the regulatory pathway for innovative bioelectronic interfaces. The Medical Device Single Audit Program (MDSAP) allows manufacturers to undergo a single audit satisfying requirements of multiple regulatory jurisdictions. This approach reduces redundancy while maintaining rigorous safety standards, potentially accelerating the translation of laboratory innovations to clinical applications.
These standards evaluate multiple aspects of biocompatibility, including cytotoxicity, sensitization, irritation, acute systemic toxicity, and long-term implantation effects. For bioelectronic interfaces, particular attention is paid to electrochemical stability, corrosion resistance, and leaching potential of electrode materials. Recent updates to these standards have incorporated specific provisions for devices utilizing novel nanomaterials and conductive polymers, reflecting the rapid evolution of the field.
Material selection represents a fundamental consideration in meeting these standards. Traditional metallic electrodes (platinum, iridium oxide, and titanium nitride) have established safety profiles, while newer materials such as PEDOT:PSS and graphene derivatives require more extensive validation. The standards now mandate comprehensive leachable and extractable testing protocols to identify potential toxic byproducts resulting from material degradation in the biological environment.
Long-term safety assessment methodologies have also evolved significantly. Accelerated aging studies, combined with computational models predicting material degradation, now complement traditional animal studies. These approaches help predict device performance over extended implantation periods, addressing concerns about chronic foreign body responses and material fatigue. The standards increasingly emphasize the importance of real-world performance data collected through post-market surveillance.
Emerging bioelectronic interfaces incorporating drug delivery capabilities or biodegradable components face additional regulatory scrutiny. Combination products that merge electronic and pharmaceutical elements must satisfy requirements from multiple regulatory frameworks. Standards organizations are actively developing specialized protocols for these hybrid technologies, recognizing their unique risk profiles and potential benefits.
International harmonization efforts are underway to streamline the regulatory pathway for innovative bioelectronic interfaces. The Medical Device Single Audit Program (MDSAP) allows manufacturers to undergo a single audit satisfying requirements of multiple regulatory jurisdictions. This approach reduces redundancy while maintaining rigorous safety standards, potentially accelerating the translation of laboratory innovations to clinical applications.
Interdisciplinary Collaboration Frameworks
The advancement of bioelectronic interface design techniques requires robust interdisciplinary collaboration frameworks that systematically integrate expertise from diverse fields. These frameworks serve as structured approaches for coordinating efforts across disciplines such as materials science, electrical engineering, neuroscience, and medicine. Effective collaboration models typically establish common terminologies, shared research objectives, and standardized protocols that facilitate seamless knowledge transfer between specialists.
Cross-disciplinary research centers represent a primary framework model, where dedicated facilities house experts from multiple fields working under unified administrative structures. These centers often implement matrix management systems that balance discipline-specific depth with project-focused integration. Notable examples include the Center for Bioelectronic Medicine at the Feinstein Institute and MIT's Media Lab, which have pioneered breakthrough bioelectronic interfaces through structured interdisciplinary engagement.
Virtual collaboration networks offer an alternative framework that leverages digital platforms to connect geographically dispersed experts. These networks typically employ cloud-based research management systems, regular virtual symposia, and collaborative document environments to maintain cohesion across institutional boundaries. The International Bioelectronics Consortium exemplifies this approach, connecting researchers across 17 countries through a standardized digital collaboration infrastructure.
Industry-academia partnership frameworks provide another critical model, establishing formal mechanisms for knowledge exchange between commercial R&D teams and university researchers. These partnerships often utilize phased development processes with clear transition points between fundamental research and applied development. Intellectual property sharing agreements and co-development protocols form the legal foundation for these collaborations, ensuring equitable distribution of innovation benefits.
Translational research pipelines represent specialized frameworks designed to accelerate the movement of bioelectronic innovations from laboratory to clinical application. These frameworks typically incorporate staged validation processes, regulatory navigation protocols, and clinical testing partnerships. The DARPA-funded Bioelectronics for Tissue Regeneration program demonstrates this approach through its structured progression from material development to preclinical testing and eventual human trials.
Convergence education programs constitute a forward-looking framework type that develops future bioelectronics researchers through integrated training across traditionally separate disciplines. These programs feature co-taught courses, multidisciplinary laboratory rotations, and team-based capstone projects that prepare students to navigate the complex interdisciplinary landscape of bioelectronic interface development.
Cross-disciplinary research centers represent a primary framework model, where dedicated facilities house experts from multiple fields working under unified administrative structures. These centers often implement matrix management systems that balance discipline-specific depth with project-focused integration. Notable examples include the Center for Bioelectronic Medicine at the Feinstein Institute and MIT's Media Lab, which have pioneered breakthrough bioelectronic interfaces through structured interdisciplinary engagement.
Virtual collaboration networks offer an alternative framework that leverages digital platforms to connect geographically dispersed experts. These networks typically employ cloud-based research management systems, regular virtual symposia, and collaborative document environments to maintain cohesion across institutional boundaries. The International Bioelectronics Consortium exemplifies this approach, connecting researchers across 17 countries through a standardized digital collaboration infrastructure.
Industry-academia partnership frameworks provide another critical model, establishing formal mechanisms for knowledge exchange between commercial R&D teams and university researchers. These partnerships often utilize phased development processes with clear transition points between fundamental research and applied development. Intellectual property sharing agreements and co-development protocols form the legal foundation for these collaborations, ensuring equitable distribution of innovation benefits.
Translational research pipelines represent specialized frameworks designed to accelerate the movement of bioelectronic innovations from laboratory to clinical application. These frameworks typically incorporate staged validation processes, regulatory navigation protocols, and clinical testing partnerships. The DARPA-funded Bioelectronics for Tissue Regeneration program demonstrates this approach through its structured progression from material development to preclinical testing and eventual human trials.
Convergence education programs constitute a forward-looking framework type that develops future bioelectronics researchers through integrated training across traditionally separate disciplines. These programs feature co-taught courses, multidisciplinary laboratory rotations, and team-based capstone projects that prepare students to navigate the complex interdisciplinary landscape of bioelectronic interface development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!