Role of Bioelectronic Interfaces in Strengthening Public Health Systems
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interfaces Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of this field traces back to the 1780s when Luigi Galvani discovered that electricity could stimulate muscle movement in frogs, establishing the foundation for bioelectricity. The modern era of bioelectronic interfaces began in the mid-20th century with the development of the first implantable cardiac pacemakers, demonstrating the potential for electronic devices to interact with human physiology.
Over the past two decades, bioelectronic interfaces have undergone remarkable advancement, transitioning from bulky, invasive devices to miniaturized, flexible, and even wireless systems. This evolution has been driven by breakthroughs in materials science, particularly the development of biocompatible materials and flexible electronics that can conform to biological tissues without causing significant immune responses or tissue damage.
The primary objective of bioelectronic interfaces in public health systems is to establish seamless communication channels between electronic devices and biological systems for monitoring, diagnosis, and therapeutic interventions. These interfaces aim to provide real-time, continuous health monitoring capabilities that can detect physiological changes before they manifest as clinical symptoms, enabling preventive interventions rather than reactive treatments.
Another critical objective is to democratize healthcare access through portable, user-friendly bioelectronic devices that can function in resource-limited settings. This includes developing low-cost, point-of-care diagnostic tools that can operate without sophisticated laboratory infrastructure, making advanced healthcare technologies accessible to underserved populations globally.
The integration of bioelectronic interfaces with artificial intelligence represents a significant technological goal, enabling systems that can not only collect biological data but also interpret it meaningfully. This combination aims to create predictive health models that can anticipate disease outbreaks, identify at-risk populations, and optimize resource allocation within public health systems.
Looking forward, the field is moving toward creating closed-loop systems that can both monitor biological signals and deliver appropriate therapeutic responses autonomously. These systems would represent a paradigm shift in disease management, particularly for chronic conditions like diabetes and neurological disorders, by providing personalized, responsive care without constant human intervention.
The ultimate technological trajectory points toward bioelectronic interfaces becoming integral components of comprehensive public health infrastructure, forming networks of interconnected devices that collectively monitor population health trends while simultaneously providing individualized care. This vision aligns with the broader trend toward precision public health, where interventions are tailored to specific populations based on their unique biological, environmental, and social determinants of health.
Over the past two decades, bioelectronic interfaces have undergone remarkable advancement, transitioning from bulky, invasive devices to miniaturized, flexible, and even wireless systems. This evolution has been driven by breakthroughs in materials science, particularly the development of biocompatible materials and flexible electronics that can conform to biological tissues without causing significant immune responses or tissue damage.
The primary objective of bioelectronic interfaces in public health systems is to establish seamless communication channels between electronic devices and biological systems for monitoring, diagnosis, and therapeutic interventions. These interfaces aim to provide real-time, continuous health monitoring capabilities that can detect physiological changes before they manifest as clinical symptoms, enabling preventive interventions rather than reactive treatments.
Another critical objective is to democratize healthcare access through portable, user-friendly bioelectronic devices that can function in resource-limited settings. This includes developing low-cost, point-of-care diagnostic tools that can operate without sophisticated laboratory infrastructure, making advanced healthcare technologies accessible to underserved populations globally.
The integration of bioelectronic interfaces with artificial intelligence represents a significant technological goal, enabling systems that can not only collect biological data but also interpret it meaningfully. This combination aims to create predictive health models that can anticipate disease outbreaks, identify at-risk populations, and optimize resource allocation within public health systems.
Looking forward, the field is moving toward creating closed-loop systems that can both monitor biological signals and deliver appropriate therapeutic responses autonomously. These systems would represent a paradigm shift in disease management, particularly for chronic conditions like diabetes and neurological disorders, by providing personalized, responsive care without constant human intervention.
The ultimate technological trajectory points toward bioelectronic interfaces becoming integral components of comprehensive public health infrastructure, forming networks of interconnected devices that collectively monitor population health trends while simultaneously providing individualized care. This vision aligns with the broader trend toward precision public health, where interventions are tailored to specific populations based on their unique biological, environmental, and social determinants of health.
Public Health System Needs Analysis
Public health systems worldwide face unprecedented challenges that require innovative technological solutions. The integration of bioelectronic interfaces presents a transformative opportunity to address critical gaps in healthcare delivery, disease surveillance, and emergency response. Current public health infrastructures struggle with data fragmentation, delayed response times, and limited resources for continuous monitoring of population health metrics.
Analysis of global health systems reveals several key needs that bioelectronic interfaces could potentially address. First, there is an urgent requirement for real-time health monitoring capabilities that can detect disease outbreaks earlier than traditional surveillance methods. The COVID-19 pandemic demonstrated how delayed detection can lead to catastrophic public health consequences, with economic costs estimated in trillions of dollars globally.
Second, public health agencies need improved data integration systems that can synthesize information from multiple sources. Current systems often operate in silos, preventing the holistic analysis necessary for effective public health decision-making. Bioelectronic interfaces could serve as crucial data collection points that feed into unified health information networks.
Third, resource allocation remains a persistent challenge, particularly in underserved and rural communities. Health systems require technologies that can extend their reach without proportional increases in personnel or infrastructure costs. Wearable and implantable bioelectronic devices offer potential solutions by enabling remote monitoring and intervention.
Fourth, preventive care capabilities are inadequately developed in most public health systems, which remain predominantly reactive rather than proactive. Early intervention systems powered by continuous biomonitoring could significantly reduce the burden of chronic diseases, which account for approximately 70% of deaths worldwide according to WHO data.
Fifth, emergency preparedness demands rapid deployment capabilities for health monitoring during crises. Current systems often rely on manual reporting mechanisms that become overwhelmed during large-scale emergencies. Bioelectronic networks could provide automated, scalable monitoring solutions during natural disasters, disease outbreaks, or other public health emergencies.
Finally, public health systems face growing challenges in managing non-communicable diseases at population scale. Bioelectronic interfaces offer promising approaches for monitoring key physiological parameters across large populations, potentially enabling earlier interventions and more personalized public health strategies.
Analysis of global health systems reveals several key needs that bioelectronic interfaces could potentially address. First, there is an urgent requirement for real-time health monitoring capabilities that can detect disease outbreaks earlier than traditional surveillance methods. The COVID-19 pandemic demonstrated how delayed detection can lead to catastrophic public health consequences, with economic costs estimated in trillions of dollars globally.
Second, public health agencies need improved data integration systems that can synthesize information from multiple sources. Current systems often operate in silos, preventing the holistic analysis necessary for effective public health decision-making. Bioelectronic interfaces could serve as crucial data collection points that feed into unified health information networks.
Third, resource allocation remains a persistent challenge, particularly in underserved and rural communities. Health systems require technologies that can extend their reach without proportional increases in personnel or infrastructure costs. Wearable and implantable bioelectronic devices offer potential solutions by enabling remote monitoring and intervention.
Fourth, preventive care capabilities are inadequately developed in most public health systems, which remain predominantly reactive rather than proactive. Early intervention systems powered by continuous biomonitoring could significantly reduce the burden of chronic diseases, which account for approximately 70% of deaths worldwide according to WHO data.
Fifth, emergency preparedness demands rapid deployment capabilities for health monitoring during crises. Current systems often rely on manual reporting mechanisms that become overwhelmed during large-scale emergencies. Bioelectronic networks could provide automated, scalable monitoring solutions during natural disasters, disease outbreaks, or other public health emergencies.
Finally, public health systems face growing challenges in managing non-communicable diseases at population scale. Bioelectronic interfaces offer promising approaches for monitoring key physiological parameters across large populations, potentially enabling earlier interventions and more personalized public health strategies.
Current Bioelectronic Technology Landscape
The bioelectronic interface landscape has evolved significantly over the past decade, with remarkable advancements in materials science, miniaturization, and wireless technologies. Current bioelectronic interfaces span a wide spectrum from non-invasive wearable devices to implantable sensors and neural interfaces. Wearable health monitors have reached mainstream adoption, with devices capable of tracking multiple physiological parameters simultaneously, including heart rate, blood oxygen levels, skin conductance, and even preliminary electrocardiogram readings.
Implantable bioelectronic systems represent the cutting edge of the field, with devices such as continuous glucose monitors and cardiac pacemakers becoming increasingly sophisticated. Recent innovations include biodegradable electronics that dissolve after their functional period, reducing the need for surgical removal and minimizing long-term foreign body responses. Neural interfaces have progressed from rigid electrode arrays to flexible, biocompatible materials that better match the mechanical properties of biological tissues, significantly improving long-term stability and reducing inflammatory responses.
Wireless communication capabilities have transformed bioelectronic interfaces from isolated monitoring tools to integrated components of health information systems. Modern devices utilize Bluetooth Low Energy, Near Field Communication, and emerging protocols specifically designed for medical device communication, enabling real-time data transmission while maintaining energy efficiency. Power management remains a critical challenge, though recent advances in energy harvesting from body heat, motion, and ambient radio frequency signals are extending device operational lifetimes.
Data processing capabilities have shifted from simple data collection to edge computing architectures, where preliminary analysis occurs directly on the device before transmission. This approach reduces bandwidth requirements and enables immediate feedback for time-sensitive applications. Machine learning algorithms increasingly operate directly on bioelectronic platforms, allowing for personalized baseline establishment and anomaly detection without continuous cloud connectivity.
Materials innovation has yielded stretchable electronics that conform to body contours and maintain functionality during movement. Conductive polymers, carbon-based nanomaterials, and liquid metal alloys have enabled devices that better integrate with biological systems while maintaining electronic performance. Biocompatibility has improved through surface modifications and encapsulation techniques that mitigate foreign body responses.
Standardization efforts are gradually addressing interoperability challenges, though fragmentation remains across proprietary systems. Several international consortia are working toward unified communication protocols and data formats specifically for bioelectronic health interfaces, which will be crucial for public health system integration. Security and privacy protection mechanisms have become standard features, with encryption and access controls implemented at hardware and software levels to safeguard sensitive health information.
Implantable bioelectronic systems represent the cutting edge of the field, with devices such as continuous glucose monitors and cardiac pacemakers becoming increasingly sophisticated. Recent innovations include biodegradable electronics that dissolve after their functional period, reducing the need for surgical removal and minimizing long-term foreign body responses. Neural interfaces have progressed from rigid electrode arrays to flexible, biocompatible materials that better match the mechanical properties of biological tissues, significantly improving long-term stability and reducing inflammatory responses.
Wireless communication capabilities have transformed bioelectronic interfaces from isolated monitoring tools to integrated components of health information systems. Modern devices utilize Bluetooth Low Energy, Near Field Communication, and emerging protocols specifically designed for medical device communication, enabling real-time data transmission while maintaining energy efficiency. Power management remains a critical challenge, though recent advances in energy harvesting from body heat, motion, and ambient radio frequency signals are extending device operational lifetimes.
Data processing capabilities have shifted from simple data collection to edge computing architectures, where preliminary analysis occurs directly on the device before transmission. This approach reduces bandwidth requirements and enables immediate feedback for time-sensitive applications. Machine learning algorithms increasingly operate directly on bioelectronic platforms, allowing for personalized baseline establishment and anomaly detection without continuous cloud connectivity.
Materials innovation has yielded stretchable electronics that conform to body contours and maintain functionality during movement. Conductive polymers, carbon-based nanomaterials, and liquid metal alloys have enabled devices that better integrate with biological systems while maintaining electronic performance. Biocompatibility has improved through surface modifications and encapsulation techniques that mitigate foreign body responses.
Standardization efforts are gradually addressing interoperability challenges, though fragmentation remains across proprietary systems. Several international consortia are working toward unified communication protocols and data formats specifically for bioelectronic health interfaces, which will be crucial for public health system integration. Security and privacy protection mechanisms have become standard features, with encryption and access controls implemented at hardware and software levels to safeguard sensitive health information.
Existing Bioelectronic Applications in Public Health
01 Neural interfaces for bioelectronic applications
Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and immune response.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neural prosthetics, brain-machine interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and inflammatory responses.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic and curved surfaces of biological tissues. These interfaces incorporate elastic materials, serpentine structures, or mesh designs to achieve mechanical compliance while maintaining electronic functionality. Such flexibility reduces mechanical mismatch between rigid electronics and soft tissues, improving long-term biocompatibility and enabling applications in wearable health monitoring, implantable devices, and soft robotics.
- Biosensing and molecular detection interfaces: Bioelectronic interfaces for biosensing and molecular detection incorporate biological recognition elements with electronic transduction mechanisms to detect specific biomolecules, cells, or physiological parameters. These interfaces may utilize electrochemical, optical, or mechanical sensing principles to convert biological interactions into measurable electronic signals. Applications include point-of-care diagnostics, continuous health monitoring, environmental sensing, and fundamental biological research.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices are designed to function within the body for extended periods, providing therapeutic interventions or monitoring physiological parameters. These devices incorporate biocompatible materials, hermetic packaging, and wireless power and communication capabilities. Advanced fabrication techniques ensure miniaturization while maintaining functionality. Applications include cardiac pacemakers, neurostimulators, drug delivery systems, and continuous glucose monitors.
- Nanomaterial-based bioelectronic interfaces: Nanomaterial-based bioelectronic interfaces leverage the unique properties of nanomaterials such as carbon nanotubes, graphene, quantum dots, and nanoparticles to enhance the performance of bioelectronic devices. These materials offer advantages including high surface-to-volume ratio, tunable electronic properties, and dimensions comparable to biological entities. Applications include highly sensitive biosensors, neural interfaces with improved signal-to-noise ratios, and advanced drug delivery systems with electronic control capabilities.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces utilize elastic materials, serpentine structures, or mesh designs to achieve mechanical compliance with soft tissues. The flexibility allows for better contact with biological surfaces, reduced mechanical mismatch, and improved long-term stability. These interfaces find applications in wearable health monitoring, implantable devices, and soft robotics.Expand Specific Solutions03 Biosensing and molecular detection interfaces
Bioelectronic interfaces for biosensing and molecular detection incorporate biological recognition elements with electronic transduction mechanisms. These interfaces can detect specific biomolecules, pathogens, or physiological parameters with high sensitivity and selectivity. Various transduction methods including electrochemical, optical, and piezoelectric are employed to convert biological recognition events into measurable electronic signals. Applications include point-of-care diagnostics, environmental monitoring, and continuous health tracking.Expand Specific Solutions04 Implantable bioelectronic medical devices
Implantable bioelectronic medical devices are designed to function within the body for extended periods. These interfaces incorporate biocompatible materials, hermetic packaging, and wireless communication capabilities. They can monitor physiological parameters, deliver therapeutic stimulation, or dispense medications. Advanced power management strategies, including wireless power transfer and energy harvesting, are employed to extend device lifetime. These interfaces are used in cardiac pacemakers, neurostimulators, and smart drug delivery systems.Expand Specific Solutions05 Nanomaterial-based bioelectronic interfaces
Nanomaterial-based bioelectronic interfaces leverage the unique properties of nanomaterials such as carbon nanotubes, graphene, and quantum dots to enhance interface performance. These materials offer high surface-to-volume ratios, excellent electrical conductivity, and tunable surface chemistry. Nanomaterial interfaces can achieve higher signal-to-noise ratios, improved spatial resolution, and enhanced biocompatibility. Applications include high-density neural recording arrays, ultrasensitive biosensors, and targeted drug delivery systems.Expand Specific Solutions
Leading Organizations in Bioelectronic Health Solutions
The bioelectronic interfaces market for public health systems is in its early growth phase, characterized by significant research activity but limited commercial deployment. The market is projected to expand rapidly, reaching approximately $5-7 billion by 2027 with a CAGR of 15-20%. Academic institutions like MIT, University of California, and Northwestern University are leading fundamental research, while commercial players including Apple, Samsung, and Qualcomm are developing practical applications. Medical technology specialists such as Cochlear and Aviana Molecular Technologies are creating specialized bioelectronic health monitoring devices. The ecosystem shows a collaborative pattern between academia and industry, with increasing investment in miniaturization, biocompatibility, and wireless connectivity to enhance public health monitoring capabilities.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced bioelectronic interfaces that integrate seamlessly with human tissue for continuous health monitoring. Their platform combines flexible electronics with biocompatible materials to create minimally invasive sensors that can detect biomarkers in real-time. The technology employs wireless power transfer and data transmission capabilities, enabling remote monitoring of patient vital signs and biochemical markers without requiring frequent hospital visits. MIT's system incorporates machine learning algorithms that analyze collected data to identify patterns indicative of disease progression or treatment efficacy, allowing for early intervention in public health scenarios[1]. Their recent innovations include stretchable electronic sensors that conform to skin and tissue surfaces while maintaining functionality, significantly improving patient comfort and compliance in long-term monitoring applications[3].
Strengths: Superior integration of flexible electronics with biological systems; exceptional data analytics capabilities; strong focus on wireless functionality for remote monitoring. Weaknesses: Higher implementation costs compared to conventional monitoring systems; requires specialized expertise for deployment and maintenance; potential privacy concerns with continuous data collection.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a comprehensive bioelectronic interface system focused on democratizing access to advanced health monitoring. Their platform combines low-cost fabrication techniques with sophisticated sensing capabilities to create accessible bioelectronic devices suitable for widespread public health deployment. The technology utilizes screen-printed electrodes on flexible substrates that can detect multiple biomarkers simultaneously, including infectious disease antigens, stress hormones, and metabolic indicators. Michigan's system incorporates energy harvesting components that extend device lifespan by capturing energy from body movement or temperature differentials, making it particularly suitable for continuous monitoring in resource-limited settings[6]. Their recent innovations include point-of-care diagnostic platforms that integrate with smartphone technology for data analysis and transmission, creating distributed networks of health monitoring that can inform public health decision-making in real-time[8].
Strengths: Exceptional cost-effectiveness for widespread deployment; strong focus on accessibility and usability in diverse settings; excellent integration with existing mobile technology infrastructure. Weaknesses: Potential trade-offs between affordability and sensing precision; challenges in ensuring data security across distributed networks; requires careful calibration for diverse user populations.
Key Patents and Research in Bioelectronic Interfaces
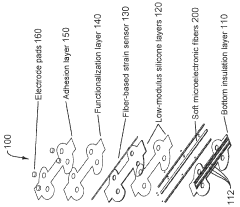
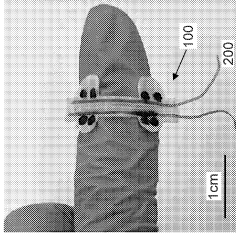
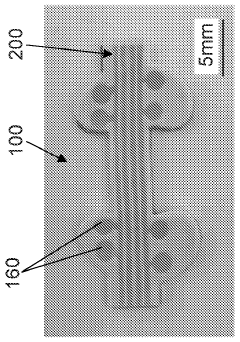
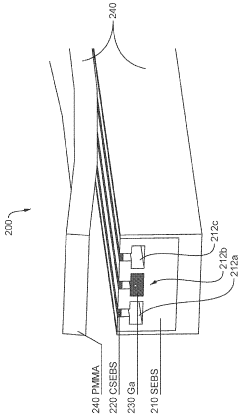
Stretchable microelectronic fibers and assemblies as multifunctional bioelectronic interfaces for whole organs
PatentWO2023201131A1
Innovation
- Development of soft and stretchable bioelectronic interfaces using elastic microelectronic fibers with liquid metal conductors and integrated microelectronic components, such as micro LEDs and sensors, which are fabricated through thermal drawing and integrated into a low-modulus elastomeric substrate for scalable, multifunctional bioelectronic therapies.
Magnetoelectric data and power to miniature biodevices with tunable amplitude and waveform
PatentPendingEP4356953A2
Innovation
- The integration of magnetoelectric (ME) materials with CMOS chips to create a wireless power and data transfer technology that uses low-frequency alternating magnetic fields, converting them into electric potentials for efficient power delivery and data transmission, enabling miniaturized, reliable, and portable neural stimulators.
Regulatory Framework for Bioelectronic Health Devices
The regulatory landscape for bioelectronic health devices represents a complex and evolving framework that balances innovation with patient safety. Currently, major regulatory bodies such as the FDA in the United States, the EMA in Europe, and the NMPA in China have established specific pathways for bioelectronic medical devices, though these frameworks often struggle to keep pace with rapid technological advancements.
In the United States, bioelectronic devices typically fall under the FDA's medical device regulatory framework, with classification depending on risk level and intended use. Class II and Class III designations are common for bioelectronic interfaces that interact directly with the nervous system or other critical physiological functions, requiring premarket approval processes that can take 3-7 years.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence and post-market surveillance, particularly affecting novel bioelectronic technologies. These regulations emphasize the importance of real-world performance data and continuous monitoring throughout the product lifecycle.
Regulatory challenges specific to bioelectronic health devices include the classification of hybrid products that combine drug delivery with electronic monitoring, the validation of AI algorithms integrated into bioelectronic systems, and establishing appropriate clinical trial designs for devices that may adapt or "learn" over time.
Privacy and data security regulations present additional compliance requirements, with GDPR in Europe and HIPAA in the US imposing strict standards on the handling of health data generated by bioelectronic interfaces. These regulations necessitate robust data protection measures, transparent consent processes, and clear policies on data ownership and sharing.
Emerging regulatory trends include the development of "regulatory sandboxes" that allow controlled testing of innovative bioelectronic solutions, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF), and the creation of expedited approval pathways for breakthrough technologies addressing unmet public health needs.
For manufacturers and developers, navigating this regulatory environment requires early engagement with regulatory bodies, robust quality management systems, and comprehensive risk management strategies that address both technical performance and cybersecurity vulnerabilities. The development of consensus standards specific to bioelectronic interfaces remains an ongoing priority for industry stakeholders and standards organizations.
In the United States, bioelectronic devices typically fall under the FDA's medical device regulatory framework, with classification depending on risk level and intended use. Class II and Class III designations are common for bioelectronic interfaces that interact directly with the nervous system or other critical physiological functions, requiring premarket approval processes that can take 3-7 years.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence and post-market surveillance, particularly affecting novel bioelectronic technologies. These regulations emphasize the importance of real-world performance data and continuous monitoring throughout the product lifecycle.
Regulatory challenges specific to bioelectronic health devices include the classification of hybrid products that combine drug delivery with electronic monitoring, the validation of AI algorithms integrated into bioelectronic systems, and establishing appropriate clinical trial designs for devices that may adapt or "learn" over time.
Privacy and data security regulations present additional compliance requirements, with GDPR in Europe and HIPAA in the US imposing strict standards on the handling of health data generated by bioelectronic interfaces. These regulations necessitate robust data protection measures, transparent consent processes, and clear policies on data ownership and sharing.
Emerging regulatory trends include the development of "regulatory sandboxes" that allow controlled testing of innovative bioelectronic solutions, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF), and the creation of expedited approval pathways for breakthrough technologies addressing unmet public health needs.
For manufacturers and developers, navigating this regulatory environment requires early engagement with regulatory bodies, robust quality management systems, and comprehensive risk management strategies that address both technical performance and cybersecurity vulnerabilities. The development of consensus standards specific to bioelectronic interfaces remains an ongoing priority for industry stakeholders and standards organizations.
Data Security and Ethics in Bioelectronic Health Systems
The integration of bioelectronic interfaces into public health systems introduces significant data security and ethical challenges that must be addressed comprehensively. Patient data collected through bioelectronic devices contains highly sensitive information, including physiological parameters, medication adherence patterns, and potentially genetic data, requiring robust encryption protocols and secure storage solutions to prevent unauthorized access and breaches.
Healthcare organizations implementing bioelectronic health monitoring systems must comply with regulatory frameworks such as HIPAA in the United States, GDPR in Europe, and equivalent regulations globally. These frameworks establish minimum standards for data protection, but the rapidly evolving nature of bioelectronic technologies often outpaces regulatory development, creating compliance challenges for healthcare providers and technology developers.
Consent management represents a critical ethical consideration in bioelectronic health systems. Patients must be fully informed about what data is being collected, how it will be used, who will have access to it, and the potential implications of data sharing. The complexity of bioelectronic systems may make truly informed consent difficult to achieve, particularly for vulnerable populations or those with limited technological literacy.
The potential for algorithmic bias in bioelectronic health systems raises significant ethical concerns. If these systems are trained on non-representative data sets, they may perform differently across demographic groups, potentially exacerbating existing healthcare disparities. Continuous monitoring and validation of algorithms against diverse populations is essential to ensure equitable health outcomes.
Data ownership and control present ongoing challenges in bioelectronic health systems. Questions about who owns the data generated by implantable or wearable bioelectronic devices—patients, healthcare providers, device manufacturers, or third-party data processors—remain contentious. Transparent data governance frameworks that prioritize patient autonomy while enabling beneficial research and public health applications are needed.
The increasing connectivity of bioelectronic health systems expands the attack surface for potential cybersecurity threats. Vulnerabilities in these systems could lead not only to data breaches but also to direct patient harm if device functionality is compromised. Implementing security-by-design principles, regular security audits, and incident response protocols is essential for maintaining system integrity and patient trust.
Healthcare organizations implementing bioelectronic health monitoring systems must comply with regulatory frameworks such as HIPAA in the United States, GDPR in Europe, and equivalent regulations globally. These frameworks establish minimum standards for data protection, but the rapidly evolving nature of bioelectronic technologies often outpaces regulatory development, creating compliance challenges for healthcare providers and technology developers.
Consent management represents a critical ethical consideration in bioelectronic health systems. Patients must be fully informed about what data is being collected, how it will be used, who will have access to it, and the potential implications of data sharing. The complexity of bioelectronic systems may make truly informed consent difficult to achieve, particularly for vulnerable populations or those with limited technological literacy.
The potential for algorithmic bias in bioelectronic health systems raises significant ethical concerns. If these systems are trained on non-representative data sets, they may perform differently across demographic groups, potentially exacerbating existing healthcare disparities. Continuous monitoring and validation of algorithms against diverse populations is essential to ensure equitable health outcomes.
Data ownership and control present ongoing challenges in bioelectronic health systems. Questions about who owns the data generated by implantable or wearable bioelectronic devices—patients, healthcare providers, device manufacturers, or third-party data processors—remain contentious. Transparent data governance frameworks that prioritize patient autonomy while enabling beneficial research and public health applications are needed.
The increasing connectivity of bioelectronic health systems expands the attack surface for potential cybersecurity threats. Vulnerabilities in these systems could lead not only to data breaches but also to direct patient harm if device functionality is compromised. Implementing security-by-design principles, regular security audits, and incident response protocols is essential for maintaining system integrity and patient trust.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!