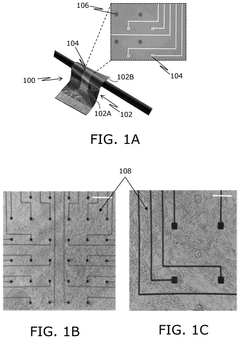
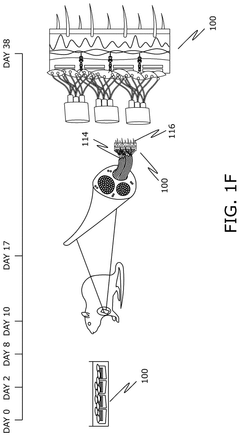
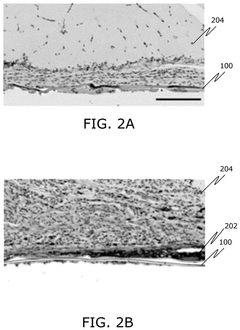
Bioelectronic Interface and the Evolution of Implantable Biochips
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Research Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of this field traces back to the 1950s with the development of the first cardiac pacemakers, which marked the beginning of electronic devices interfacing with the human body. Over subsequent decades, significant advancements in materials science, microelectronics, and biotechnology have dramatically expanded the capabilities and applications of bioelectronic interfaces.
The 1990s witnessed a paradigm shift with the miniaturization of electronic components and the emergence of microelectromechanical systems (MEMS), allowing for more sophisticated and less invasive implantable devices. By the early 2000s, researchers began developing neural interfaces capable of recording and stimulating neural activity, opening new frontiers in treating neurological disorders and developing brain-computer interfaces.
Recent technological breakthroughs have accelerated progress in this field, particularly in flexible and stretchable electronics, which conform better to biological tissues, reducing immune responses and improving long-term stability. Advances in wireless power transfer and communication have eliminated the need for transcutaneous wires, significantly reducing infection risks and enhancing patient comfort.
The current trajectory of bioelectronic interfaces is moving toward increasingly sophisticated implantable biochips that integrate multiple functions, including sensing, drug delivery, and tissue stimulation. These "smart" implants represent the next generation of medical devices, capable of autonomous operation and real-time adaptation to physiological changes.
The primary objectives of current research in this field include enhancing biocompatibility to minimize foreign body responses, extending device longevity through improved power management and materials, and developing bidirectional communication capabilities for closed-loop systems that can both monitor and respond to biological signals.
Another critical research goal is the development of high-density electrode arrays that can interface with individual neurons or small neural populations, enabling unprecedented precision in neural recording and stimulation. This capability is essential for advanced neuroprosthetics and brain-computer interfaces that aim to restore lost sensory or motor functions.
Looking forward, the field is trending toward fully integrated systems that combine sensing, computation, and actuation within miniaturized, biocompatible packages. The ultimate vision encompasses self-regulating implants that can diagnose conditions, deliver targeted therapies, and communicate wirelessly with external monitoring systems, fundamentally transforming how we approach healthcare and human-machine interaction.
The 1990s witnessed a paradigm shift with the miniaturization of electronic components and the emergence of microelectromechanical systems (MEMS), allowing for more sophisticated and less invasive implantable devices. By the early 2000s, researchers began developing neural interfaces capable of recording and stimulating neural activity, opening new frontiers in treating neurological disorders and developing brain-computer interfaces.
Recent technological breakthroughs have accelerated progress in this field, particularly in flexible and stretchable electronics, which conform better to biological tissues, reducing immune responses and improving long-term stability. Advances in wireless power transfer and communication have eliminated the need for transcutaneous wires, significantly reducing infection risks and enhancing patient comfort.
The current trajectory of bioelectronic interfaces is moving toward increasingly sophisticated implantable biochips that integrate multiple functions, including sensing, drug delivery, and tissue stimulation. These "smart" implants represent the next generation of medical devices, capable of autonomous operation and real-time adaptation to physiological changes.
The primary objectives of current research in this field include enhancing biocompatibility to minimize foreign body responses, extending device longevity through improved power management and materials, and developing bidirectional communication capabilities for closed-loop systems that can both monitor and respond to biological signals.
Another critical research goal is the development of high-density electrode arrays that can interface with individual neurons or small neural populations, enabling unprecedented precision in neural recording and stimulation. This capability is essential for advanced neuroprosthetics and brain-computer interfaces that aim to restore lost sensory or motor functions.
Looking forward, the field is trending toward fully integrated systems that combine sensing, computation, and actuation within miniaturized, biocompatible packages. The ultimate vision encompasses self-regulating implants that can diagnose conditions, deliver targeted therapies, and communicate wirelessly with external monitoring systems, fundamentally transforming how we approach healthcare and human-machine interaction.
Market Analysis for Implantable Biochip Technologies
The global market for implantable biochip technologies is experiencing robust growth, driven by increasing prevalence of chronic diseases, rising geriatric population, and growing demand for minimally invasive procedures. The market was valued at approximately 3.67 billion USD in 2022 and is projected to reach 11.25 billion USD by 2030, representing a compound annual growth rate (CAGR) of 15.1% during the forecast period.
North America currently dominates the implantable biochip market, accounting for nearly 42% of global market share. This dominance is attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. Europe follows with approximately 28% market share, while Asia-Pacific represents the fastest-growing regional market with an estimated CAGR of 17.3% through 2030, primarily driven by improving healthcare access in China and India.
By application segment, medical diagnostics leads the market with 38% share, followed by drug delivery systems (27%), patient monitoring (21%), and others (14%). The therapeutic segment is witnessing the highest growth rate due to increasing applications in neurostimulation and cardiac rhythm management.
Consumer demand is increasingly shifting toward multifunctional biochips that can simultaneously monitor multiple parameters and deliver therapeutic interventions. Market research indicates that 73% of healthcare providers express interest in biochips with integrated sensing and drug delivery capabilities, while 65% of patients surveyed would consider implantable devices for chronic disease management if proven safe and effective.
Key market restraints include high development and implementation costs, with average R&D investment for a new implantable biochip platform exceeding 50 million USD. Regulatory hurdles present significant challenges, with approval timelines averaging 3-5 years in major markets. Additionally, privacy concerns and cybersecurity risks remain prominent, with 78% of potential users expressing data security concerns in recent surveys.
Emerging market opportunities include integration with artificial intelligence for predictive healthcare, expansion into mental health applications, and development of biodegradable implants. The veterinary biochip segment also shows promising growth potential, with market projections indicating a 12.8% CAGR through 2030.
Strategic partnerships between technology companies and healthcare providers are becoming increasingly common, with over 35 major collaborations announced in the past two years, signaling a trend toward ecosystem-based innovation rather than standalone product development.
North America currently dominates the implantable biochip market, accounting for nearly 42% of global market share. This dominance is attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. Europe follows with approximately 28% market share, while Asia-Pacific represents the fastest-growing regional market with an estimated CAGR of 17.3% through 2030, primarily driven by improving healthcare access in China and India.
By application segment, medical diagnostics leads the market with 38% share, followed by drug delivery systems (27%), patient monitoring (21%), and others (14%). The therapeutic segment is witnessing the highest growth rate due to increasing applications in neurostimulation and cardiac rhythm management.
Consumer demand is increasingly shifting toward multifunctional biochips that can simultaneously monitor multiple parameters and deliver therapeutic interventions. Market research indicates that 73% of healthcare providers express interest in biochips with integrated sensing and drug delivery capabilities, while 65% of patients surveyed would consider implantable devices for chronic disease management if proven safe and effective.
Key market restraints include high development and implementation costs, with average R&D investment for a new implantable biochip platform exceeding 50 million USD. Regulatory hurdles present significant challenges, with approval timelines averaging 3-5 years in major markets. Additionally, privacy concerns and cybersecurity risks remain prominent, with 78% of potential users expressing data security concerns in recent surveys.
Emerging market opportunities include integration with artificial intelligence for predictive healthcare, expansion into mental health applications, and development of biodegradable implants. The veterinary biochip segment also shows promising growth potential, with market projections indicating a 12.8% CAGR through 2030.
Strategic partnerships between technology companies and healthcare providers are becoming increasingly common, with over 35 major collaborations announced in the past two years, signaling a trend toward ecosystem-based innovation rather than standalone product development.
Current Bioelectronic Interface Challenges and Limitations
Despite significant advancements in bioelectronic interfaces and implantable biochips, the field faces several critical challenges that impede widespread clinical adoption and technological maturation. Biocompatibility remains a fundamental obstacle, as foreign materials introduced into the body often trigger immune responses, leading to inflammation, fibrosis, and eventual device failure. Current materials used in implantable devices, while improving, still struggle to achieve long-term stability in the dynamic biological environment.
Power management presents another significant limitation. Implantable biochips require sustainable power sources that can function reliably within the body for extended periods. Traditional battery technologies pose size constraints and safety concerns, while wireless power transfer methods suffer from efficiency losses and potential tissue heating issues. Energy harvesting from biological sources shows promise but remains insufficient for many high-demand applications.
Signal quality and stability represent persistent technical challenges. The bioelectronic interface must maintain consistent signal acquisition capabilities despite tissue movement, changes in hydration, and natural biological processes. Current electrode technologies experience signal degradation over time, with decreasing signal-to-noise ratios that compromise data reliability for long-term implants.
Miniaturization constraints continue to challenge designers of implantable systems. As functionality requirements increase, accommodating complex circuitry, sensors, and communication components within biocompatible, minimally invasive form factors becomes increasingly difficult. This limitation particularly affects applications requiring distributed sensing or stimulation across multiple anatomical sites.
Data security and privacy concerns present growing challenges as implantable biochips become more sophisticated and connected. Wireless communication capabilities, while necessary for data transmission and device control, create potential vulnerabilities to unauthorized access or interference. Current encryption methods often demand computational resources that strain the limited power budgets of implantable devices.
Regulatory hurdles and standardization gaps further complicate advancement in this field. The novel nature of many bioelectronic interfaces means regulatory frameworks are still evolving, creating uncertainty in development pathways. The lack of standardized testing protocols and performance metrics makes comparative evaluation difficult and slows industry-wide progress.
Surgical implementation and retrieval procedures present practical limitations. Current implantation techniques often require invasive surgery, with associated risks and recovery periods. Additionally, the potential need for device removal or replacement is complicated by tissue integration and the risk of additional trauma during extraction procedures.
Power management presents another significant limitation. Implantable biochips require sustainable power sources that can function reliably within the body for extended periods. Traditional battery technologies pose size constraints and safety concerns, while wireless power transfer methods suffer from efficiency losses and potential tissue heating issues. Energy harvesting from biological sources shows promise but remains insufficient for many high-demand applications.
Signal quality and stability represent persistent technical challenges. The bioelectronic interface must maintain consistent signal acquisition capabilities despite tissue movement, changes in hydration, and natural biological processes. Current electrode technologies experience signal degradation over time, with decreasing signal-to-noise ratios that compromise data reliability for long-term implants.
Miniaturization constraints continue to challenge designers of implantable systems. As functionality requirements increase, accommodating complex circuitry, sensors, and communication components within biocompatible, minimally invasive form factors becomes increasingly difficult. This limitation particularly affects applications requiring distributed sensing or stimulation across multiple anatomical sites.
Data security and privacy concerns present growing challenges as implantable biochips become more sophisticated and connected. Wireless communication capabilities, while necessary for data transmission and device control, create potential vulnerabilities to unauthorized access or interference. Current encryption methods often demand computational resources that strain the limited power budgets of implantable devices.
Regulatory hurdles and standardization gaps further complicate advancement in this field. The novel nature of many bioelectronic interfaces means regulatory frameworks are still evolving, creating uncertainty in development pathways. The lack of standardized testing protocols and performance metrics makes comparative evaluation difficult and slows industry-wide progress.
Surgical implementation and retrieval procedures present practical limitations. Current implantation techniques often require invasive surgery, with associated risks and recovery periods. Additionally, the potential need for device removal or replacement is complicated by tissue integration and the risk of additional trauma during extraction procedures.
Existing Bioelectronic Interface Implementation Approaches
01 Neural interface technologies for implantable biochips
Neural interface technologies enable direct communication between electronic devices and the nervous system. These interfaces can be implanted into the brain or peripheral nerves to record neural activity or deliver stimulation. Advanced bioelectronic interfaces incorporate microelectrode arrays, signal processing capabilities, and wireless communication to monitor and modulate neural activity. These technologies have applications in treating neurological disorders, controlling prosthetic devices, and brain-computer interfaces.- Neural interface technologies for bioelectronic implants: Advanced neural interface technologies enable direct communication between electronic devices and the nervous system. These interfaces incorporate microelectrode arrays and signal processing capabilities to record neural activity and deliver targeted stimulation. The technology allows for bidirectional communication with neural tissue, facilitating applications in neuroprosthetics, neuromodulation therapies, and brain-computer interfaces. These systems typically feature biocompatible materials to minimize tissue reaction and ensure long-term functionality within the body.
- Biochip fabrication and integration technologies: Specialized fabrication techniques are employed to create implantable biochips with microscale features. These methods include photolithography, soft lithography, and various deposition techniques to pattern bioactive components onto substrates. The integration of biological elements with electronic components requires precise manufacturing processes to maintain functionality in physiological environments. Advanced packaging solutions protect sensitive electronics while allowing interaction with surrounding tissues through specialized interfaces.
- Biosensing and biomarker detection systems: Implantable biochips incorporate various sensing modalities to detect biological markers and physiological parameters. These systems utilize electrochemical, optical, or mechanical transduction mechanisms to convert biological signals into measurable electronic outputs. Real-time monitoring capabilities allow for continuous assessment of health status and early detection of pathological conditions. The integration of multiple sensing elements enables comprehensive physiological monitoring from a single implanted device.
- Wireless power and data transmission for implantable devices: Wireless technologies enable power delivery and data communication with implanted bioelectronic devices without physical connections through the skin. These systems utilize radiofrequency, inductive coupling, or ultrasonic methods to transmit energy and information. Advanced protocols ensure efficient power transfer while minimizing tissue heating and maintaining data integrity. Bidirectional communication capabilities allow for device programming and retrieval of recorded physiological data without requiring surgical access to the implant.
- Biocompatible materials and coatings for long-term implantation: Specialized materials and surface modifications enhance the biocompatibility of implantable biochips. These include hydrogels, conducting polymers, and various nanomaterials designed to mimic natural tissue properties. Anti-fouling coatings prevent protein adsorption and cellular adhesion that could compromise device function. Drug-eluting coatings can deliver anti-inflammatory agents locally to reduce foreign body response. These approaches collectively extend device longevity and maintain performance in the challenging in vivo environment.
02 Biocompatible materials and coatings for implantable biochips
Biocompatible materials and specialized coatings are essential for implantable biochips to minimize immune responses and ensure long-term functionality within the body. These materials include biocompatible polymers, hydrogels, and ceramics that can safely interface with biological tissues. Advanced coatings may incorporate anti-inflammatory agents, growth factors, or cell-adhesion molecules to promote integration with surrounding tissues and reduce fibrosis. The development of flexible, stretchable substrates allows implants to better conform to biological tissues.Expand Specific Solutions03 Wireless power and data transmission for implantable biochips
Wireless power and data transmission technologies enable implantable biochips to operate without physical connections through the skin, reducing infection risk and improving patient comfort. These systems use techniques such as radiofrequency induction, ultrasonic energy transfer, or near-field communication to power the implants and transmit data to external receivers. Advanced designs incorporate energy harvesting from biological sources, power management circuits to optimize energy usage, and secure communication protocols to protect sensitive health data.Expand Specific Solutions04 Biosensing capabilities in implantable biochips
Implantable biochips with biosensing capabilities can continuously monitor physiological parameters and biomarkers in real-time. These sensors can detect various analytes including glucose, electrolytes, proteins, and specific disease markers. The integration of nanomaterials, enzymatic recognition elements, and electrochemical detection methods enhances sensitivity and specificity. Advanced biosensing platforms incorporate multiple sensing modalities and automated calibration systems to maintain accuracy over extended periods in the biological environment.Expand Specific Solutions05 Therapeutic and drug delivery applications of implantable biochips
Implantable biochips can be designed for therapeutic interventions and controlled drug delivery based on real-time physiological monitoring. These systems integrate drug reservoirs, microfluidic channels, and release mechanisms that can be triggered electronically or biochemically. Smart drug delivery systems can adjust dosing based on biosensor feedback, enabling personalized medicine approaches. Applications include localized chemotherapy, chronic pain management, hormone regulation, and treatment of neurodegenerative diseases with precise spatial and temporal control of therapeutic agents.Expand Specific Solutions
Leading Organizations in Bioelectronic Interface Research
The bioelectronic interface and implantable biochips market is currently in a growth phase, transitioning from early development to commercial applications. The global market is expanding rapidly, estimated to reach $25-30 billion by 2028, driven by healthcare applications and wearable technologies. Leading companies like DexCom and Infineon Technologies are advancing glucose monitoring systems and semiconductor solutions, while academic institutions including MIT, Tsinghua University, and Rice University are pioneering fundamental research. The technology maturity varies across applications - continuous glucose monitoring systems (DexCom) are relatively mature, while neural interfaces (developed by universities and Koninklijke Philips) remain in early stages. The competitive landscape features collaboration between established medical device manufacturers, semiconductor companies, and research institutions, creating a dynamic ecosystem for innovation.
DexCom, Inc.
Technical Solution: DexCom has established itself as a leader in implantable continuous glucose monitoring (CGM) systems, representing one of the most commercially successful applications of bioelectronic interfaces. Their technology utilizes subcutaneously implanted sensors featuring enzyme-based electrochemical detection methods to continuously monitor interstitial glucose levels. DexCom's latest generation devices incorporate advanced biocompatible materials and specialized membrane technologies that significantly extend sensor lifetime while maintaining accuracy. Their systems feature sophisticated signal processing algorithms that filter biological noise and compensate for sensor drift, enabling reliable glucose measurements for up to 10 days without calibration. DexCom has pioneered wireless communication protocols optimized for implantable devices, balancing power efficiency with reliable data transmission to external receivers or smartphones. Their research extends beyond glucose monitoring to explore multi-analyte sensing capabilities and integration with automated insulin delivery systems, creating closed-loop solutions for diabetes management. DexCom's iterative design approach has progressively miniaturized their implantable components while improving biocompatibility and reducing foreign body responses.
Strengths: Market-leading position in implantable glucose sensors with proven clinical efficacy; sophisticated signal processing algorithms that enhance measurement reliability; strong commercialization pathway and regulatory expertise. Weaknesses: Current technology limited primarily to glucose monitoring rather than broader bioelectronic applications; sensors require periodic replacement rather than offering truly long-term implantation solutions.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered significant advancements in bioelectronic interfaces through their development of flexible, ultra-thin electronic systems that can seamlessly integrate with biological tissues. Their research includes the creation of conformable electronic devices that can be implanted in the brain and other organs with minimal invasiveness. MIT's engineers have developed a novel fabrication technique for creating nanoscale electronic components on flexible substrates that can withstand the harsh biological environment while maintaining functionality over extended periods. Their research teams have successfully demonstrated wireless power transfer and data communication capabilities for implantable biochips, enabling real-time monitoring of physiological parameters without the need for battery replacement. MIT has also made breakthroughs in biocompatible materials that reduce foreign body responses and tissue inflammation, significantly improving the long-term viability of implanted devices. Their interdisciplinary approach combines expertise in electrical engineering, materials science, and biology to create next-generation neural interfaces capable of both recording and stimulating neural activity with unprecedented precision.
Strengths: Exceptional interdisciplinary research capabilities combining electronics, materials science, and biology; strong focus on minimally invasive, flexible electronics that conform to biological tissues; advanced wireless power and data transmission technologies. Weaknesses: Some technologies remain in early research phases with significant challenges for clinical translation; high manufacturing costs for specialized materials and fabrication techniques may limit widespread adoption.
Key Patents and Innovations in Implantable Biochip Design
Implantable bioelectronic device and method of using same
PatentWO2023187526A1
Innovation
- A flexible bioelectronic device with a biodegradable hydrogel and human stem-cell derived cells is used, allowing for controlled synaptic integration and reduced scar tissue formation, enabling intuitive control and chronic implantation by mimicking nerve stiffness and promoting vascularization for nutrient and waste diffusion.
Implantable bioelectronic device and method of using same
PatentPendingUS20250058117A1
Innovation
- An implantable bioelectronic device with a flexible base material and a biological sample seeded on its surface, allowing for controlled synaptic integration and reduced scar tissue formation, thereby enhancing the restoration of neurological functions.
Biocompatibility and Long-term Stability Considerations
Biocompatibility and long-term stability represent critical challenges in the development of implantable biochips. The human body presents a harsh environment for electronic devices, with immune responses, protein adsorption, and corrosive fluids potentially compromising device functionality. Current research focuses on developing materials that minimize foreign body responses while maintaining operational integrity over extended periods.
Advanced biocompatible materials such as medical-grade silicones, polyimides, and parylene-C have emerged as promising candidates for device encapsulation. These materials demonstrate reduced inflammatory responses and protein adhesion compared to earlier generations. Notably, diamond-like carbon coatings and zwitterionic polymer surfaces have shown exceptional resistance to biofouling, extending device longevity significantly.
The interface between electronic components and biological tissues presents unique challenges. Recent innovations include hydrogel-based interfaces that mimic tissue mechanical properties, reducing mechanical stress and subsequent inflammatory responses. These soft interfaces help distribute forces more evenly, minimizing tissue damage during natural body movements and reducing fibrotic encapsulation that can isolate devices from target tissues.
Long-term stability testing protocols have evolved substantially, with accelerated aging studies now incorporating physiologically relevant conditions. Research indicates that electrical performance degradation often precedes visible material deterioration, highlighting the need for comprehensive monitoring approaches. Impedance spectroscopy has emerged as a valuable tool for detecting early signs of interface degradation before clinical failure occurs.
Biofouling remains a significant obstacle, with protein adsorption initiating cascades of biological responses that compromise device function. Anti-fouling strategies now incorporate both passive (surface chemistry modifications) and active approaches (localized drug delivery systems releasing anti-inflammatory or anti-fibrotic agents). Controlled-release systems using biodegradable polymers have demonstrated efficacy in maintaining clear interfaces for up to 18 months in preclinical models.
Hermetic sealing technologies have advanced considerably, with atomic layer deposition techniques creating nanometer-thick barriers against moisture ingress. Multilayer encapsulation approaches combining organic and inorganic materials have shown superior performance compared to single-material solutions. Self-healing polymers represent a promising frontier, potentially addressing microfractures that develop during long-term implantation.
Regulatory frameworks increasingly emphasize long-term biocompatibility testing, requiring evidence of stability beyond the traditional 90-day implantation period. This shift acknowledges the intended multi-year deployment of modern implantable biochips and has accelerated research into degradation mechanisms and failure modes under physiological conditions.
Advanced biocompatible materials such as medical-grade silicones, polyimides, and parylene-C have emerged as promising candidates for device encapsulation. These materials demonstrate reduced inflammatory responses and protein adhesion compared to earlier generations. Notably, diamond-like carbon coatings and zwitterionic polymer surfaces have shown exceptional resistance to biofouling, extending device longevity significantly.
The interface between electronic components and biological tissues presents unique challenges. Recent innovations include hydrogel-based interfaces that mimic tissue mechanical properties, reducing mechanical stress and subsequent inflammatory responses. These soft interfaces help distribute forces more evenly, minimizing tissue damage during natural body movements and reducing fibrotic encapsulation that can isolate devices from target tissues.
Long-term stability testing protocols have evolved substantially, with accelerated aging studies now incorporating physiologically relevant conditions. Research indicates that electrical performance degradation often precedes visible material deterioration, highlighting the need for comprehensive monitoring approaches. Impedance spectroscopy has emerged as a valuable tool for detecting early signs of interface degradation before clinical failure occurs.
Biofouling remains a significant obstacle, with protein adsorption initiating cascades of biological responses that compromise device function. Anti-fouling strategies now incorporate both passive (surface chemistry modifications) and active approaches (localized drug delivery systems releasing anti-inflammatory or anti-fibrotic agents). Controlled-release systems using biodegradable polymers have demonstrated efficacy in maintaining clear interfaces for up to 18 months in preclinical models.
Hermetic sealing technologies have advanced considerably, with atomic layer deposition techniques creating nanometer-thick barriers against moisture ingress. Multilayer encapsulation approaches combining organic and inorganic materials have shown superior performance compared to single-material solutions. Self-healing polymers represent a promising frontier, potentially addressing microfractures that develop during long-term implantation.
Regulatory frameworks increasingly emphasize long-term biocompatibility testing, requiring evidence of stability beyond the traditional 90-day implantation period. This shift acknowledges the intended multi-year deployment of modern implantable biochips and has accelerated research into degradation mechanisms and failure modes under physiological conditions.
Ethical and Regulatory Framework for Implantable Technologies
The ethical and regulatory landscape surrounding implantable biochips and bioelectronic interfaces presents complex challenges that require careful consideration as these technologies advance. Current regulatory frameworks across major jurisdictions like the FDA in the United States, the EMA in Europe, and NMPA in China have established pathways for medical device approval, but these frameworks were primarily designed for traditional medical devices rather than sophisticated bioelectronic implants that interface directly with neural tissue.
A significant regulatory gap exists in addressing the unique characteristics of implantable biochips, particularly regarding long-term safety monitoring, data privacy, and the potential for unauthorized access or manipulation. The FDA's recent guidance on "Software as a Medical Device" (SaMD) begins to address some digital aspects, but comprehensive regulations specific to neural interfaces remain underdeveloped.
Ethical considerations surrounding these technologies are multifaceted and evolve alongside technological capabilities. Patient autonomy represents a primary concern, particularly regarding informed consent for devices that may alter neural function or collect sensitive biological data. The potential for cognitive enhancement through neural interfaces raises questions about equitable access and the creation of a "neural divide" between enhanced and non-enhanced individuals.
Data security and privacy frameworks for implantable technologies require substantial strengthening. Current regulations like GDPR in Europe and HIPAA in the US provide general data protection principles but lack specific provisions for the continuous, intimate biological data collection enabled by implantable biochips. The potential for unauthorized access to neural interfaces presents unprecedented security vulnerabilities that extend beyond traditional data breaches to potential manipulation of physical and cognitive functions.
International harmonization of regulatory standards remains fragmented, creating challenges for global development and deployment. The WHO and International Medical Device Regulators Forum (IMDRF) have initiated discussions on coordinated approaches, but binding international standards specific to neural interfaces have yet to emerge.
Industry self-regulation through organizations like the Neural Engineering Society has produced ethical guidelines emphasizing reversibility of interventions, transparent data practices, and prioritization of therapeutic applications. However, these voluntary frameworks lack enforcement mechanisms and universal adoption.
Looking forward, regulatory evolution must balance innovation with protection. Adaptive licensing models that allow for phased approval with enhanced post-market surveillance may provide a pathway for responsible advancement. Ethical frameworks will need to address emerging capabilities like brain-to-brain interfaces and cognitive enhancement applications, potentially requiring new categories of oversight beyond traditional medical device regulation.
A significant regulatory gap exists in addressing the unique characteristics of implantable biochips, particularly regarding long-term safety monitoring, data privacy, and the potential for unauthorized access or manipulation. The FDA's recent guidance on "Software as a Medical Device" (SaMD) begins to address some digital aspects, but comprehensive regulations specific to neural interfaces remain underdeveloped.
Ethical considerations surrounding these technologies are multifaceted and evolve alongside technological capabilities. Patient autonomy represents a primary concern, particularly regarding informed consent for devices that may alter neural function or collect sensitive biological data. The potential for cognitive enhancement through neural interfaces raises questions about equitable access and the creation of a "neural divide" between enhanced and non-enhanced individuals.
Data security and privacy frameworks for implantable technologies require substantial strengthening. Current regulations like GDPR in Europe and HIPAA in the US provide general data protection principles but lack specific provisions for the continuous, intimate biological data collection enabled by implantable biochips. The potential for unauthorized access to neural interfaces presents unprecedented security vulnerabilities that extend beyond traditional data breaches to potential manipulation of physical and cognitive functions.
International harmonization of regulatory standards remains fragmented, creating challenges for global development and deployment. The WHO and International Medical Device Regulators Forum (IMDRF) have initiated discussions on coordinated approaches, but binding international standards specific to neural interfaces have yet to emerge.
Industry self-regulation through organizations like the Neural Engineering Society has produced ethical guidelines emphasizing reversibility of interventions, transparent data practices, and prioritization of therapeutic applications. However, these voluntary frameworks lack enforcement mechanisms and universal adoption.
Looking forward, regulatory evolution must balance innovation with protection. Adaptive licensing models that allow for phased approval with enhanced post-market surveillance may provide a pathway for responsible advancement. Ethical frameworks will need to address emerging capabilities like brain-to-brain interfaces and cognitive enhancement applications, potentially requiring new categories of oversight beyond traditional medical device regulation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!