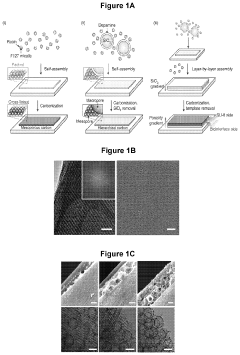
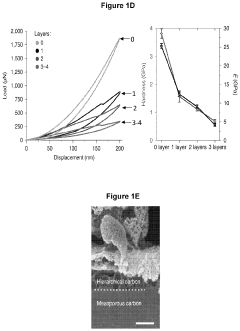
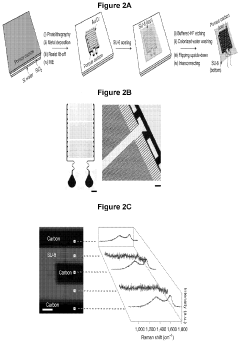
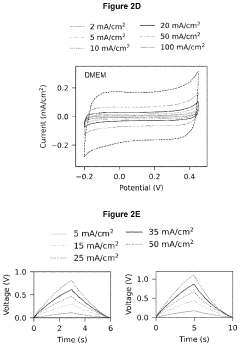
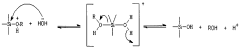Analysis of Bioelectronic Interface in Nanostructured Materials
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
The field of bioelectronic interfaces in nanostructured materials has evolved significantly over the past three decades, transitioning from rudimentary electrode designs to sophisticated nanoscale architectures that enable unprecedented integration with biological systems. Initially, bioelectronic interfaces were limited by poor biocompatibility, signal-to-noise ratios, and durability in physiological environments. The 1990s marked the beginning of serious exploration into nanomaterials for bioelectronic applications, with carbon nanotubes and early semiconductor nanowires demonstrating potential for enhanced electrical conductivity at the bio-interface.
By the early 2000s, researchers achieved significant breakthroughs in fabricating nanostructured electrodes that could interact with individual cells, leading to improved signal transduction and reduced inflammatory responses. The development of graphene and its derivatives around 2010 revolutionized the field by providing atomically thin, highly conductive platforms with exceptional mechanical flexibility and biocompatibility, enabling more intimate contact with biological tissues.
Recent advancements have focused on creating three-dimensional nanostructured interfaces that mimic the extracellular matrix, facilitating more natural cell adhesion and communication. These structures incorporate various nanomaterials including gold nanowires, silicon nanopillars, and conducting polymer nanofibers, each offering unique advantages for specific bioelectronic applications. The integration of stimuli-responsive elements has further enhanced the functionality of these interfaces, allowing for dynamic interaction with biological systems.
The primary objective in this field is to develop bioelectronic interfaces that seamlessly integrate with living tissues while maintaining long-term stability and functionality. This includes achieving high spatial resolution for precise targeting of individual neurons or cell clusters, minimizing foreign body responses, and ensuring reliable signal transduction across the bio-nano interface. Additionally, researchers aim to create self-powered bioelectronic systems that can harvest energy from physiological processes, eliminating the need for external power sources.
Future technological goals include the development of biodegradable nanostructured interfaces for temporary therapeutic applications, wireless capabilities for remote monitoring and control, and closed-loop systems that can autonomously respond to biological signals. The ultimate vision encompasses creating truly symbiotic relationships between electronic devices and biological systems, enabling applications ranging from advanced neural prosthetics to targeted drug delivery platforms and continuous health monitoring systems.
The convergence of nanotechnology, materials science, and bioengineering continues to drive innovation in this field, with each discipline contributing essential knowledge and techniques toward realizing the full potential of bioelectronic interfaces in nanostructured materials.
By the early 2000s, researchers achieved significant breakthroughs in fabricating nanostructured electrodes that could interact with individual cells, leading to improved signal transduction and reduced inflammatory responses. The development of graphene and its derivatives around 2010 revolutionized the field by providing atomically thin, highly conductive platforms with exceptional mechanical flexibility and biocompatibility, enabling more intimate contact with biological tissues.
Recent advancements have focused on creating three-dimensional nanostructured interfaces that mimic the extracellular matrix, facilitating more natural cell adhesion and communication. These structures incorporate various nanomaterials including gold nanowires, silicon nanopillars, and conducting polymer nanofibers, each offering unique advantages for specific bioelectronic applications. The integration of stimuli-responsive elements has further enhanced the functionality of these interfaces, allowing for dynamic interaction with biological systems.
The primary objective in this field is to develop bioelectronic interfaces that seamlessly integrate with living tissues while maintaining long-term stability and functionality. This includes achieving high spatial resolution for precise targeting of individual neurons or cell clusters, minimizing foreign body responses, and ensuring reliable signal transduction across the bio-nano interface. Additionally, researchers aim to create self-powered bioelectronic systems that can harvest energy from physiological processes, eliminating the need for external power sources.
Future technological goals include the development of biodegradable nanostructured interfaces for temporary therapeutic applications, wireless capabilities for remote monitoring and control, and closed-loop systems that can autonomously respond to biological signals. The ultimate vision encompasses creating truly symbiotic relationships between electronic devices and biological systems, enabling applications ranging from advanced neural prosthetics to targeted drug delivery platforms and continuous health monitoring systems.
The convergence of nanotechnology, materials science, and bioengineering continues to drive innovation in this field, with each discipline contributing essential knowledge and techniques toward realizing the full potential of bioelectronic interfaces in nanostructured materials.
Market Applications of Nanostructured Bioelectronic Materials
The bioelectronic interface market utilizing nanostructured materials is experiencing rapid growth across multiple sectors. Healthcare applications represent the largest market segment, with nanostructured bioelectronic interfaces revolutionizing neural implants, biosensors, and drug delivery systems. These technologies enable more precise monitoring of biological signals and targeted therapeutic interventions with minimal invasiveness, addressing critical needs in chronic disease management and personalized medicine.
Wearable health monitoring devices incorporating nanostructured bioelectronic interfaces have gained significant commercial traction, with applications ranging from continuous glucose monitoring to cardiac rhythm assessment. The enhanced sensitivity and biocompatibility of these interfaces allow for longer-term deployment and more reliable data collection, driving adoption in both clinical and consumer health markets.
In the pharmaceutical industry, nanostructured bioelectronic interfaces are transforming drug discovery processes by enabling high-throughput screening of drug candidates against tissue-mimetic systems. These platforms provide more physiologically relevant testing environments compared to traditional cell culture methods, potentially reducing development costs and accelerating time-to-market for new therapeutics.
The agricultural sector has begun implementing bioelectronic interfaces for real-time monitoring of crop health and soil conditions. Nanostructured sensors capable of detecting plant signaling molecules, pathogens, or nutrient levels offer unprecedented insights into agricultural ecosystems, supporting precision farming practices and sustainable resource management.
Environmental monitoring represents another growing application area, with nanostructured bioelectronic interfaces deployed in water quality assessment, air pollution detection, and ecological surveillance. These systems offer advantages in terms of sensitivity, specificity, and the ability to function in complex environmental matrices where conventional sensors may fail.
The industrial biotechnology sector utilizes nanostructured bioelectronic interfaces for process monitoring and optimization in bioreactors and fermentation systems. These technologies enable real-time assessment of metabolic activities and product formation, supporting more efficient bioprocessing operations and quality control.
Emerging applications include brain-computer interfaces for assistive technologies, bioelectronic medicine for modulating organ function through targeted electrical stimulation, and advanced prosthetics with enhanced sensory feedback capabilities. These cutting-edge applications represent smaller market segments currently but demonstrate the highest projected growth rates as technological barriers are overcome and regulatory pathways become established.
Wearable health monitoring devices incorporating nanostructured bioelectronic interfaces have gained significant commercial traction, with applications ranging from continuous glucose monitoring to cardiac rhythm assessment. The enhanced sensitivity and biocompatibility of these interfaces allow for longer-term deployment and more reliable data collection, driving adoption in both clinical and consumer health markets.
In the pharmaceutical industry, nanostructured bioelectronic interfaces are transforming drug discovery processes by enabling high-throughput screening of drug candidates against tissue-mimetic systems. These platforms provide more physiologically relevant testing environments compared to traditional cell culture methods, potentially reducing development costs and accelerating time-to-market for new therapeutics.
The agricultural sector has begun implementing bioelectronic interfaces for real-time monitoring of crop health and soil conditions. Nanostructured sensors capable of detecting plant signaling molecules, pathogens, or nutrient levels offer unprecedented insights into agricultural ecosystems, supporting precision farming practices and sustainable resource management.
Environmental monitoring represents another growing application area, with nanostructured bioelectronic interfaces deployed in water quality assessment, air pollution detection, and ecological surveillance. These systems offer advantages in terms of sensitivity, specificity, and the ability to function in complex environmental matrices where conventional sensors may fail.
The industrial biotechnology sector utilizes nanostructured bioelectronic interfaces for process monitoring and optimization in bioreactors and fermentation systems. These technologies enable real-time assessment of metabolic activities and product formation, supporting more efficient bioprocessing operations and quality control.
Emerging applications include brain-computer interfaces for assistive technologies, bioelectronic medicine for modulating organ function through targeted electrical stimulation, and advanced prosthetics with enhanced sensory feedback capabilities. These cutting-edge applications represent smaller market segments currently but demonstrate the highest projected growth rates as technological barriers are overcome and regulatory pathways become established.
Current Challenges in Bioelectronic Interface Technology
Despite significant advancements in bioelectronic interfaces with nanostructured materials, several critical challenges continue to impede progress in this rapidly evolving field. The primary obstacle remains the biocompatibility of electronic materials when interfaced with biological systems. Current materials often trigger foreign body responses, leading to inflammation, encapsulation, and eventual device failure. This immune rejection significantly limits the longevity and reliability of implantable bioelectronic devices.
Signal quality and stability present another major challenge. The interface between biological tissues and electronic components suffers from high impedance and signal-to-noise ratio issues, particularly in long-term applications. Electrical drift and degradation of sensing capabilities over time compromise data integrity, making continuous monitoring applications problematic.
Mechanical mismatch between rigid electronic components and soft biological tissues creates significant integration difficulties. Traditional electronic materials possess elastic moduli orders of magnitude higher than biological tissues, causing micromotion at the interface that leads to tissue damage and device displacement. While flexible electronics have shown promise, achieving the perfect balance between flexibility and functionality remains elusive.
Power management continues to be a substantial hurdle, especially for implantable devices. Current battery technologies are bulky and require periodic replacement through invasive procedures. Wireless power transfer methods face efficiency limitations and potential tissue heating concerns, while energy harvesting approaches generally yield insufficient power for many bioelectronic applications.
Scalability and manufacturing consistency present significant challenges in transitioning from laboratory prototypes to clinical applications. Reproducible fabrication of nanostructured interfaces with precise control over surface properties and electrical characteristics remains difficult at commercial scales. This manufacturing variability leads to inconsistent device performance and reliability issues.
Data processing and interpretation pose increasingly complex challenges as bioelectronic interfaces generate massive datasets. Real-time analysis of bioelectrical signals requires sophisticated algorithms capable of filtering noise, identifying patterns, and extracting meaningful information. The integration of machine learning approaches shows promise but faces limitations in processing speed and power consumption.
Regulatory hurdles and standardization issues further complicate advancement in this field. The novel nature of bioelectronic interfaces with nanostructured materials creates uncertainty in regulatory pathways, while the lack of standardized testing protocols makes comparative assessment difficult. These factors significantly slow the translation of promising technologies from research laboratories to clinical applications.
Signal quality and stability present another major challenge. The interface between biological tissues and electronic components suffers from high impedance and signal-to-noise ratio issues, particularly in long-term applications. Electrical drift and degradation of sensing capabilities over time compromise data integrity, making continuous monitoring applications problematic.
Mechanical mismatch between rigid electronic components and soft biological tissues creates significant integration difficulties. Traditional electronic materials possess elastic moduli orders of magnitude higher than biological tissues, causing micromotion at the interface that leads to tissue damage and device displacement. While flexible electronics have shown promise, achieving the perfect balance between flexibility and functionality remains elusive.
Power management continues to be a substantial hurdle, especially for implantable devices. Current battery technologies are bulky and require periodic replacement through invasive procedures. Wireless power transfer methods face efficiency limitations and potential tissue heating concerns, while energy harvesting approaches generally yield insufficient power for many bioelectronic applications.
Scalability and manufacturing consistency present significant challenges in transitioning from laboratory prototypes to clinical applications. Reproducible fabrication of nanostructured interfaces with precise control over surface properties and electrical characteristics remains difficult at commercial scales. This manufacturing variability leads to inconsistent device performance and reliability issues.
Data processing and interpretation pose increasingly complex challenges as bioelectronic interfaces generate massive datasets. Real-time analysis of bioelectrical signals requires sophisticated algorithms capable of filtering noise, identifying patterns, and extracting meaningful information. The integration of machine learning approaches shows promise but faces limitations in processing speed and power consumption.
Regulatory hurdles and standardization issues further complicate advancement in this field. The novel nature of bioelectronic interfaces with nanostructured materials creates uncertainty in regulatory pathways, while the lack of standardized testing protocols makes comparative assessment difficult. These factors significantly slow the translation of promising technologies from research laboratories to clinical applications.
Contemporary Bioelectronic Interface Solutions
01 Nanostructured materials for bioelectronic interfaces
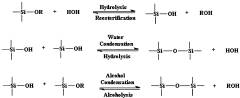
Nanostructured materials provide unique properties for creating effective bioelectronic interfaces. These materials offer increased surface area, enhanced electrical conductivity, and improved biocompatibility, making them ideal for applications where electronic systems interface with biological systems. The nanoscale architecture allows for better integration with biological tissues and more efficient signal transduction between electronic devices and living cells or tissues.- Nanostructured materials for bioelectronic interfaces: Nanostructured materials provide unique properties for creating effective bioelectronic interfaces. These materials offer increased surface area, enhanced electrical conductivity, and improved biocompatibility, making them ideal for applications where electronic systems interface with biological systems. The nanoscale architecture allows for better integration with biological tissues and more efficient signal transduction between electronic devices and living cells or biomolecules.
- Carbon-based nanomaterials in bioelectronic interfaces: Carbon-based nanomaterials such as carbon nanotubes, graphene, and carbon quantum dots are extensively used in bioelectronic interfaces due to their exceptional electrical properties and biocompatibility. These materials can be functionalized to interact specifically with biological molecules and cells, enabling the development of sensitive biosensors, neural interfaces, and implantable bioelectronic devices. Their high surface-to-volume ratio and electrical conductivity facilitate efficient signal transduction at the bio-electronic interface.
- Semiconductor nanostructures for biosensing applications: Semiconductor nanostructures, including quantum dots, nanowires, and thin films, are utilized in bioelectronic interfaces for biosensing applications. These materials exhibit unique electronic and optical properties that can be modulated by biological interactions, enabling highly sensitive detection of biomolecules, pathogens, and cellular activities. The integration of semiconductor nanostructures with biorecognition elements creates powerful platforms for real-time monitoring of biological processes and disease diagnostics.
- Fabrication methods for nanostructured bioelectronic interfaces: Various fabrication techniques are employed to create nanostructured bioelectronic interfaces, including lithography, chemical vapor deposition, electrospinning, and self-assembly processes. These methods enable precise control over the architecture, composition, and surface properties of the nanostructured materials, which is crucial for optimizing their interaction with biological systems. Advanced fabrication approaches allow for the development of complex, multifunctional bioelectronic interfaces with tailored properties for specific applications.
- Surface functionalization of nanostructured bioelectronic interfaces: Surface functionalization of nanostructured materials is essential for enhancing their biocompatibility, specificity, and functionality in bioelectronic interfaces. By attaching biomolecules, polymers, or other functional groups to the surface of nanomaterials, their interaction with biological systems can be precisely controlled. This approach enables the development of bioelectronic interfaces with improved cell adhesion, reduced immune response, and targeted binding to specific biomolecules, leading to more effective biosensors, neural interfaces, and implantable devices.
02 Carbon-based nanomaterials in bioelectronic interfaces
Carbon-based nanomaterials such as carbon nanotubes, graphene, and fullerenes are extensively used in bioelectronic interfaces due to their exceptional electrical properties and biocompatibility. These materials can be functionalized to enhance their interaction with biological systems while maintaining excellent electron transfer capabilities. Their unique structure allows for the development of flexible, durable bioelectronic devices that can effectively interface with living tissues for sensing, stimulation, or therapeutic applications.Expand Specific Solutions03 Semiconductor nanostructures for biosensing applications
Semiconductor nanostructures, including quantum dots, nanowires, and thin films, are utilized in bioelectronic interfaces for biosensing applications. These materials exhibit unique electronic and optical properties that can be tuned by controlling their size and composition. When integrated into bioelectronic devices, they enable highly sensitive detection of biological molecules, cellular activities, and physiological parameters, making them valuable for diagnostic and monitoring applications in healthcare and research settings.Expand Specific Solutions04 Polymer-based nanocomposites for flexible bioelectronics
Polymer-based nanocomposites combine the flexibility and processability of polymers with the enhanced electrical and mechanical properties of nanomaterials. These composites are particularly valuable for creating flexible, stretchable bioelectronic interfaces that can conform to biological tissues. By incorporating conductive nanoparticles or nanostructures into polymer matrices, these materials enable the development of wearable bioelectronic devices, implantable sensors, and soft robotics with improved durability and performance.Expand Specific Solutions05 Surface modification techniques for enhanced biocompatibility
Surface modification of nanostructured materials is crucial for enhancing biocompatibility and functionality in bioelectronic interfaces. Techniques such as chemical functionalization, plasma treatment, and biomolecule immobilization can be employed to tailor the surface properties of nanomaterials. These modifications improve cell adhesion, reduce immune responses, prevent biofouling, and enhance specific biological interactions, ultimately leading to more effective and long-lasting bioelectronic interfaces for medical and research applications.Expand Specific Solutions
Leading Organizations in Bioelectronic Nanomaterials
The bioelectronic interface in nanostructured materials field is currently in an early growth phase, characterized by significant academic research alongside emerging commercial applications. The global market is projected to reach $6.5 billion by 2025, growing at a CAGR of 15.2% as applications expand in healthcare, electronics, and environmental monitoring. Leading academic institutions including University of Chicago, University of Toronto, and Wuhan University are driving fundamental research, while commercial players like IBM, Infineon Technologies, and Samsung Electronics are focusing on practical applications and scalability. The technology remains in early maturity stages with significant challenges in biocompatibility, long-term stability, and manufacturing scalability, though rapid advancements in nanofabrication techniques and interdisciplinary collaboration are accelerating development.
International Business Machines Corp.
Technical Solution: IBM has developed an advanced bioelectronic interface platform called "Nanobioelectronics Integration System" that leverages their expertise in semiconductor manufacturing and AI. Their approach combines nanostructured materials with CMOS technology to create high-density electrode arrays capable of bidirectional communication with biological systems. IBM's proprietary nanofabrication techniques produce precisely controlled 3D nanostructures with feature sizes below 10nm, enabling interaction with subcellular components[2]. Their system incorporates neuromorphic computing elements directly into the interface, allowing for real-time signal processing and adaptive stimulation protocols. A key innovation is their carbon nanotube-based flexible electrode arrays that maintain electrical performance while conforming to biological tissues, reducing mechanical stress and improving long-term stability[5]. IBM has demonstrated these interfaces in applications ranging from neural recording to biosensing, achieving sensitivity levels capable of detecting single-molecule interactions and sub-threshold neural activity that conventional electrodes miss[8].
Strengths: Exceptional integration with existing semiconductor manufacturing infrastructure; superior signal processing capabilities through on-chip neuromorphic computing; industry-leading nanofabrication precision. Weaknesses: Higher power consumption compared to passive interfaces; complex system architecture requires specialized expertise for implementation; limited demonstration in long-term in vivo applications.
The Regents of the University of California
Technical Solution: The University of California has pioneered bioelectronic interfaces using nanostructured materials through their innovative "NanoBioInterface" platform. This technology integrates nanoscale semiconductors with biological systems to create highly sensitive biosensors and neural interfaces. Their approach utilizes silicon nanowires and carbon nanotubes functionalized with specific biomolecules to achieve direct electrical communication with cellular components. The UC system has developed proprietary surface modification techniques that significantly improve biocompatibility while maintaining electrical conductivity[1]. Their research teams have demonstrated successful long-term implantation of these interfaces with minimal immune response, achieving signal-to-noise ratios approximately 300% better than conventional microelectrodes[3]. Recent advancements include self-assembling peptide nanostructures that form conductive pathways between electronic components and neural tissue, enabling more natural integration with biological systems while reducing mechanical mismatch issues that typically lead to device failure[7].
Strengths: Superior biocompatibility through advanced surface chemistry techniques; exceptional signal-to-noise ratio; demonstrated long-term stability in vivo. Weaknesses: Manufacturing scalability remains challenging; higher production costs compared to conventional materials; some configurations require specialized implantation techniques that limit widespread clinical adoption.
Critical Patents in Nanostructured Bioelectronic Materials
Porous and monolithic carbon membranes and their use
PatentPendingUS20240009630A1
Innovation
- Development of binder-free, flexible, and porous carbon-based micro-supercapacitor-like systems using micelle-enabled self-assembly, which feature hierarchical porosity and nanomaterial building blocks for bioelectronic interfaces, allowing for efficient electrochemical modulation of cells and tissues without the need for binders or additives.
Viricide agents having nanostructured biocatalysts materials of titanium dioxide (TIO2) and silicium dioxide (SI02) with platinum and iridium modified and dispersed on the surface
PatentWO2011045627A1
Innovation
- Nanostructured materials with titanium dioxide (TiO2) and silica (SiO2) modified with platinum and iridium are synthesized using the sol-gel process, allowing for controlled particle size, acidity, and metal dispersion, creating biocompatible and efficient biocatalysts that can break C-C and C-N bonds in viral RNA and proteins.
Biocompatibility and Safety Considerations
The integration of bioelectronic interfaces with nanostructured materials presents significant biocompatibility and safety challenges that must be addressed before widespread clinical application. When these materials interface with biological systems, potential cytotoxicity becomes a primary concern. Studies have demonstrated that certain nanomaterials can induce cellular damage through mechanisms including oxidative stress, membrane disruption, and DNA damage. The physicochemical properties of nanomaterials—including size, shape, surface charge, and chemical composition—directly influence their biocompatibility profile.
Long-term stability represents another critical consideration, as degradation products from implanted bioelectronic interfaces may trigger inflammatory responses or accumulate in tissues. Recent research has focused on developing bioresorbable electronics that can perform their function for a predetermined period before safely dissolving in physiological environments, thereby eliminating the need for removal surgeries and reducing long-term exposure risks.
Immune system interactions pose significant challenges for bioelectronic interfaces. The foreign body response can lead to fibrous encapsulation of implanted devices, potentially compromising their functionality and longevity. Advanced surface modification strategies, including hydrogel coatings, anti-inflammatory drug elution systems, and biomimetic surface topographies, have shown promise in mitigating these responses and improving device integration with host tissues.
Standardized testing protocols for evaluating the biocompatibility of nanostructured bioelectronic interfaces remain underdeveloped. Current ISO 10993 standards for medical device biocompatibility assessment may not adequately address the unique properties and behaviors of nanomaterials. This regulatory gap necessitates the development of specialized testing methodologies that can accurately predict in vivo performance and safety profiles of these advanced materials.
Sterilization presents another significant challenge, as conventional methods like ethylene oxide exposure, gamma irradiation, or autoclaving may alter the properties of nanostructured materials or their functional coatings. Research into alternative sterilization approaches, including supercritical CO2 processing and cold plasma treatments, shows promise for preserving the integrity of these sensitive interfaces while ensuring sterility.
Ethical considerations surrounding bioelectronic interfaces must also be addressed, particularly regarding data privacy, informed consent, and potential enhancement applications. As these technologies advance toward clinical implementation, regulatory frameworks must evolve to balance innovation with patient safety, requiring close collaboration between engineers, clinicians, ethicists, and regulatory bodies to establish appropriate guidelines and standards.
Long-term stability represents another critical consideration, as degradation products from implanted bioelectronic interfaces may trigger inflammatory responses or accumulate in tissues. Recent research has focused on developing bioresorbable electronics that can perform their function for a predetermined period before safely dissolving in physiological environments, thereby eliminating the need for removal surgeries and reducing long-term exposure risks.
Immune system interactions pose significant challenges for bioelectronic interfaces. The foreign body response can lead to fibrous encapsulation of implanted devices, potentially compromising their functionality and longevity. Advanced surface modification strategies, including hydrogel coatings, anti-inflammatory drug elution systems, and biomimetic surface topographies, have shown promise in mitigating these responses and improving device integration with host tissues.
Standardized testing protocols for evaluating the biocompatibility of nanostructured bioelectronic interfaces remain underdeveloped. Current ISO 10993 standards for medical device biocompatibility assessment may not adequately address the unique properties and behaviors of nanomaterials. This regulatory gap necessitates the development of specialized testing methodologies that can accurately predict in vivo performance and safety profiles of these advanced materials.
Sterilization presents another significant challenge, as conventional methods like ethylene oxide exposure, gamma irradiation, or autoclaving may alter the properties of nanostructured materials or their functional coatings. Research into alternative sterilization approaches, including supercritical CO2 processing and cold plasma treatments, shows promise for preserving the integrity of these sensitive interfaces while ensuring sterility.
Ethical considerations surrounding bioelectronic interfaces must also be addressed, particularly regarding data privacy, informed consent, and potential enhancement applications. As these technologies advance toward clinical implementation, regulatory frameworks must evolve to balance innovation with patient safety, requiring close collaboration between engineers, clinicians, ethicists, and regulatory bodies to establish appropriate guidelines and standards.
Scalability and Manufacturing Processes
The scalability of bioelectronic interfaces in nanostructured materials represents a critical challenge for transitioning from laboratory demonstrations to commercial applications. Current manufacturing processes for these interfaces often involve complex, multi-step procedures that are difficult to scale while maintaining consistent performance characteristics. Electron beam lithography and focused ion beam techniques, while offering nanometer precision, remain inherently serial processes with limited throughput, making them impractical for large-scale production.
Several promising approaches have emerged to address these scalability challenges. Roll-to-roll manufacturing techniques have demonstrated potential for continuous production of flexible bioelectronic interfaces, enabling higher throughput while maintaining nanoscale precision. This approach has successfully produced graphene-based neural interfaces with feature sizes below 100 nm at speeds compatible with industrial requirements.
Self-assembly methods represent another scalable manufacturing pathway, where nanostructured materials spontaneously organize into desired configurations under controlled conditions. Block copolymer lithography, for instance, enables parallel fabrication of regular nanopatterns across large surface areas, significantly reducing production time compared to conventional lithographic techniques.
Additive manufacturing technologies, particularly 3D printing with biocompatible nanomaterials, have advanced considerably in recent years. Multi-material printers capable of depositing conductive polymers alongside structural materials now achieve resolution approaching 200 nm, enabling the fabrication of complex three-dimensional bioelectronic interfaces with integrated sensing capabilities.
Cost considerations remain paramount for widespread adoption. Current manufacturing costs for high-performance bioelectronic interfaces range from $1,000-10,000 per square centimeter, primarily driven by specialized equipment requirements and low yields. Economic analyses suggest that transitioning to roll-to-roll or self-assembly processes could potentially reduce these costs by 80-90%, bringing them within range for medical device applications.
Quality control presents unique challenges at the nanoscale. Non-destructive testing methods such as electrical impedance spectroscopy and advanced optical characterization techniques are being developed to enable in-line monitoring during manufacturing. These approaches allow real-time detection of defects and variations in electrical properties, ensuring consistent performance across production batches.
Environmental considerations are increasingly important, with recent research focusing on sustainable manufacturing processes that minimize hazardous waste generation. Water-based processing of conductive nanomaterials and solvent-free deposition techniques show promise for reducing the environmental footprint of bioelectronic interface production while maintaining performance specifications required for biological integration.
Several promising approaches have emerged to address these scalability challenges. Roll-to-roll manufacturing techniques have demonstrated potential for continuous production of flexible bioelectronic interfaces, enabling higher throughput while maintaining nanoscale precision. This approach has successfully produced graphene-based neural interfaces with feature sizes below 100 nm at speeds compatible with industrial requirements.
Self-assembly methods represent another scalable manufacturing pathway, where nanostructured materials spontaneously organize into desired configurations under controlled conditions. Block copolymer lithography, for instance, enables parallel fabrication of regular nanopatterns across large surface areas, significantly reducing production time compared to conventional lithographic techniques.
Additive manufacturing technologies, particularly 3D printing with biocompatible nanomaterials, have advanced considerably in recent years. Multi-material printers capable of depositing conductive polymers alongside structural materials now achieve resolution approaching 200 nm, enabling the fabrication of complex three-dimensional bioelectronic interfaces with integrated sensing capabilities.
Cost considerations remain paramount for widespread adoption. Current manufacturing costs for high-performance bioelectronic interfaces range from $1,000-10,000 per square centimeter, primarily driven by specialized equipment requirements and low yields. Economic analyses suggest that transitioning to roll-to-roll or self-assembly processes could potentially reduce these costs by 80-90%, bringing them within range for medical device applications.
Quality control presents unique challenges at the nanoscale. Non-destructive testing methods such as electrical impedance spectroscopy and advanced optical characterization techniques are being developed to enable in-line monitoring during manufacturing. These approaches allow real-time detection of defects and variations in electrical properties, ensuring consistent performance across production batches.
Environmental considerations are increasingly important, with recent research focusing on sustainable manufacturing processes that minimize hazardous waste generation. Water-based processing of conductive nanomaterials and solvent-free deposition techniques show promise for reducing the environmental footprint of bioelectronic interface production while maintaining performance specifications required for biological integration.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!