Analysis of Bioelectronic Interfaces in Acoustic Signal Processing
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Acoustic Interfaces Background and Objectives
Bioelectronic interfaces for acoustic signal processing represent a rapidly evolving interdisciplinary field that merges principles from electronics, biology, acoustics, and signal processing. The historical trajectory of this technology began in the 1950s with rudimentary cochlear implants, evolving through significant breakthroughs in the 1970s and 1980s with the development of multi-channel devices. The past two decades have witnessed exponential advancement in miniaturization, biocompatibility, and signal processing capabilities, transforming these interfaces from experimental devices to sophisticated clinical tools.
The current technological evolution trend points toward increasingly integrated systems that seamlessly connect biological tissues with electronic components. This integration aims to achieve higher fidelity in signal transduction, reduced power consumption, enhanced biocompatibility, and improved longevity of implanted devices. Recent developments in flexible electronics, nanomaterials, and wireless power transmission have accelerated progress in this domain, enabling more natural and effective interfaces between biological systems and electronic devices.
A significant emerging trend is the shift from purely assistive technologies toward augmentative capabilities, where bioelectronic acoustic interfaces not only restore lost function but potentially enhance human auditory capabilities beyond natural limits. This represents a paradigm shift in how we conceptualize the relationship between technology and human sensory systems.
The primary technical objectives in this field include developing interfaces with higher spatial and temporal resolution for more precise signal processing, creating self-calibrating systems that adapt to changing biological environments, minimizing tissue damage and inflammatory responses, and extending device operational lifespan. Additionally, there is a growing focus on developing bidirectional interfaces that can both record and stimulate neural activity related to acoustic processing.
From a materials science perspective, researchers aim to develop biocompatible materials that maintain stable performance in the challenging environment of the human body while minimizing foreign body responses. Signal processing objectives include developing algorithms that can effectively filter biological noise, adapt to individual neural patterns, and process acoustic information in ways that mimic natural hearing processes.
The ultimate goal of this technological domain is to create seamless, long-lasting interfaces between electronic devices and biological systems that can accurately interpret, process, and transmit acoustic information, whether for medical applications like hearing restoration, human-computer interaction, or novel communication technologies. This requires addressing challenges across multiple disciplines and integrating diverse technological approaches into cohesive, functional systems.
The current technological evolution trend points toward increasingly integrated systems that seamlessly connect biological tissues with electronic components. This integration aims to achieve higher fidelity in signal transduction, reduced power consumption, enhanced biocompatibility, and improved longevity of implanted devices. Recent developments in flexible electronics, nanomaterials, and wireless power transmission have accelerated progress in this domain, enabling more natural and effective interfaces between biological systems and electronic devices.
A significant emerging trend is the shift from purely assistive technologies toward augmentative capabilities, where bioelectronic acoustic interfaces not only restore lost function but potentially enhance human auditory capabilities beyond natural limits. This represents a paradigm shift in how we conceptualize the relationship between technology and human sensory systems.
The primary technical objectives in this field include developing interfaces with higher spatial and temporal resolution for more precise signal processing, creating self-calibrating systems that adapt to changing biological environments, minimizing tissue damage and inflammatory responses, and extending device operational lifespan. Additionally, there is a growing focus on developing bidirectional interfaces that can both record and stimulate neural activity related to acoustic processing.
From a materials science perspective, researchers aim to develop biocompatible materials that maintain stable performance in the challenging environment of the human body while minimizing foreign body responses. Signal processing objectives include developing algorithms that can effectively filter biological noise, adapt to individual neural patterns, and process acoustic information in ways that mimic natural hearing processes.
The ultimate goal of this technological domain is to create seamless, long-lasting interfaces between electronic devices and biological systems that can accurately interpret, process, and transmit acoustic information, whether for medical applications like hearing restoration, human-computer interaction, or novel communication technologies. This requires addressing challenges across multiple disciplines and integrating diverse technological approaches into cohesive, functional systems.
Market Analysis for Bioelectronic Acoustic Applications
The bioelectronic interfaces market for acoustic signal processing is experiencing robust growth, driven by increasing applications in healthcare, consumer electronics, and defense sectors. Current market valuations indicate that the global bioelectronic medical devices market reached approximately 25 billion USD in 2022, with acoustic signal processing applications representing a significant growth segment within this broader category.
Healthcare applications dominate the market landscape, with hearing aids and cochlear implants constituting the largest market share. The hearing aid market alone is projected to grow at a CAGR of 7.4% through 2030, fueled by an aging global population and increasing prevalence of hearing impairments. Cochlear implants represent another high-value segment, with technological advancements enabling more sophisticated signal processing capabilities.
Consumer electronics represents the fastest-growing application segment, with bioelectronic interfaces being integrated into wearable devices, smart headphones, and augmented reality systems. These applications leverage advanced acoustic signal processing to deliver enhanced user experiences, noise cancellation, and personalized audio profiles based on individual hearing characteristics.
The military and defense sector has emerged as a significant market for bioelectronic acoustic interfaces, particularly for applications in enhanced communication systems, situational awareness technologies, and non-invasive monitoring of personnel vital signs. This sector values the high precision and reliability offered by advanced bioelectronic interfaces.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing awareness about hearing disorders, and expanding electronics manufacturing capabilities in countries like China, Japan, and South Korea.
Key market challenges include high development costs, regulatory hurdles, and concerns regarding biocompatibility and long-term reliability of implantable devices. Additionally, data privacy and security concerns present significant barriers to market expansion, particularly for consumer applications that collect and process biometric data.
Market opportunities are emerging in the integration of artificial intelligence with bioelectronic interfaces, enabling more sophisticated signal processing, noise filtering, and personalized acoustic experiences. The convergence of bioelectronic interfaces with IoT and cloud computing is creating new possibilities for remote monitoring and adjustment of acoustic devices.
The competitive landscape features established medical device manufacturers like Cochlear Limited, Advanced Bionics, and MED-EL, alongside technology giants such as Apple, Google, and Samsung who are increasingly investing in bioelectronic acoustic technologies for their consumer products. Several innovative startups are also disrupting the market with novel approaches to bioelectronic interface design and acoustic signal processing algorithms.
Healthcare applications dominate the market landscape, with hearing aids and cochlear implants constituting the largest market share. The hearing aid market alone is projected to grow at a CAGR of 7.4% through 2030, fueled by an aging global population and increasing prevalence of hearing impairments. Cochlear implants represent another high-value segment, with technological advancements enabling more sophisticated signal processing capabilities.
Consumer electronics represents the fastest-growing application segment, with bioelectronic interfaces being integrated into wearable devices, smart headphones, and augmented reality systems. These applications leverage advanced acoustic signal processing to deliver enhanced user experiences, noise cancellation, and personalized audio profiles based on individual hearing characteristics.
The military and defense sector has emerged as a significant market for bioelectronic acoustic interfaces, particularly for applications in enhanced communication systems, situational awareness technologies, and non-invasive monitoring of personnel vital signs. This sector values the high precision and reliability offered by advanced bioelectronic interfaces.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing awareness about hearing disorders, and expanding electronics manufacturing capabilities in countries like China, Japan, and South Korea.
Key market challenges include high development costs, regulatory hurdles, and concerns regarding biocompatibility and long-term reliability of implantable devices. Additionally, data privacy and security concerns present significant barriers to market expansion, particularly for consumer applications that collect and process biometric data.
Market opportunities are emerging in the integration of artificial intelligence with bioelectronic interfaces, enabling more sophisticated signal processing, noise filtering, and personalized acoustic experiences. The convergence of bioelectronic interfaces with IoT and cloud computing is creating new possibilities for remote monitoring and adjustment of acoustic devices.
The competitive landscape features established medical device manufacturers like Cochlear Limited, Advanced Bionics, and MED-EL, alongside technology giants such as Apple, Google, and Samsung who are increasingly investing in bioelectronic acoustic technologies for their consumer products. Several innovative startups are also disrupting the market with novel approaches to bioelectronic interface design and acoustic signal processing algorithms.
Current Challenges in Bioelectronic Signal Processing
The field of bioelectronic interfaces for acoustic signal processing faces several significant technical challenges that currently limit broader implementation and effectiveness. These challenges span multiple domains including material science, signal processing algorithms, and system integration.
Signal-to-noise ratio (SNR) remains one of the most persistent obstacles in bioelectronic acoustic interfaces. Biological signals are inherently weak, often in the microvolt range, while environmental and physiological noise can be orders of magnitude larger. Current amplification techniques introduce additional noise, creating a fundamental trade-off between sensitivity and signal clarity that has not been adequately resolved.
Biocompatibility presents another critical challenge. Materials that excel in electronic performance often trigger adverse biological responses when interfaced with living tissue. The development of materials that combine excellent conductivity with long-term biocompatibility remains elusive, with current solutions typically compromising on either electrical performance or tissue compatibility.
Power consumption represents a significant limitation, particularly for implantable or wearable bioelectronic acoustic interfaces. Current systems require either bulky batteries that need frequent replacement or wireless power transfer systems that suffer from efficiency losses and heating concerns. The energy requirements for real-time signal processing further exacerbate this challenge.
Miniaturization efforts face fundamental physical constraints. As devices shrink, thermal management becomes increasingly problematic, with heat dissipation potentially damaging surrounding biological tissues. Additionally, smaller components typically offer reduced performance characteristics, creating a difficult balance between size and functionality.
Data processing bottlenecks emerge when handling the massive datasets generated by high-resolution bioelectronic interfaces. Real-time processing requirements for acoustic applications demand computational efficiency that current algorithms struggle to deliver without significant power consumption or latency issues.
Electrode degradation over time presents a reliability challenge, with performance deteriorating due to protein adsorption, corrosion, and mechanical stress. Current materials show significant performance decline within weeks or months of implantation, falling short of the years-long stability required for practical applications.
Cross-disciplinary integration remains problematic, with expertise siloed between electrical engineering, materials science, neuroscience, and clinical medicine. The lack of standardized interfaces between these domains hampers efficient collaboration and slows innovation cycles.
Regulatory pathways for novel bioelectronic interfaces are still evolving, creating uncertainty in development timelines and requirements. The unique combination of electronic components and biological interfaces creates classification challenges for regulatory bodies, often resulting in extended approval processes.
Signal-to-noise ratio (SNR) remains one of the most persistent obstacles in bioelectronic acoustic interfaces. Biological signals are inherently weak, often in the microvolt range, while environmental and physiological noise can be orders of magnitude larger. Current amplification techniques introduce additional noise, creating a fundamental trade-off between sensitivity and signal clarity that has not been adequately resolved.
Biocompatibility presents another critical challenge. Materials that excel in electronic performance often trigger adverse biological responses when interfaced with living tissue. The development of materials that combine excellent conductivity with long-term biocompatibility remains elusive, with current solutions typically compromising on either electrical performance or tissue compatibility.
Power consumption represents a significant limitation, particularly for implantable or wearable bioelectronic acoustic interfaces. Current systems require either bulky batteries that need frequent replacement or wireless power transfer systems that suffer from efficiency losses and heating concerns. The energy requirements for real-time signal processing further exacerbate this challenge.
Miniaturization efforts face fundamental physical constraints. As devices shrink, thermal management becomes increasingly problematic, with heat dissipation potentially damaging surrounding biological tissues. Additionally, smaller components typically offer reduced performance characteristics, creating a difficult balance between size and functionality.
Data processing bottlenecks emerge when handling the massive datasets generated by high-resolution bioelectronic interfaces. Real-time processing requirements for acoustic applications demand computational efficiency that current algorithms struggle to deliver without significant power consumption or latency issues.
Electrode degradation over time presents a reliability challenge, with performance deteriorating due to protein adsorption, corrosion, and mechanical stress. Current materials show significant performance decline within weeks or months of implantation, falling short of the years-long stability required for practical applications.
Cross-disciplinary integration remains problematic, with expertise siloed between electrical engineering, materials science, neuroscience, and clinical medicine. The lack of standardized interfaces between these domains hampers efficient collaboration and slows innovation cycles.
Regulatory pathways for novel bioelectronic interfaces are still evolving, creating uncertainty in development timelines and requirements. The unique combination of electronic components and biological interfaces creates classification challenges for regulatory bodies, often resulting in extended approval processes.
Current Bioelectronic-Acoustic Signal Processing Solutions
01 Neural signal processing for bioelectronic interfaces
Advanced signal processing techniques are used to interpret neural signals captured by bioelectronic interfaces. These methods involve filtering, amplification, and analysis of electrical signals generated by the nervous system. The processed signals can be used for various applications including neural prosthetics, brain-computer interfaces, and diagnostic tools. These technologies enable real-time monitoring and interpretation of neural activity for both research and clinical applications.- Neural signal processing for bioelectronic interfaces: Advanced signal processing techniques are used to interpret neural signals captured by bioelectronic interfaces. These methods involve filtering, amplification, and analysis of electrical signals generated by the nervous system. The processed data enables real-time control of prosthetic devices and provides insights into neurological conditions. Machine learning algorithms are increasingly employed to improve the accuracy of signal interpretation and to adapt to individual neural patterns.
- Implantable bioelectronic sensors and signal conditioning: Implantable bioelectronic sensors require specialized signal conditioning to function effectively within the body. These systems incorporate miniaturized electronics for amplification, filtering, and digitization of biological signals. Advanced materials and designs are used to ensure biocompatibility while maintaining signal integrity. Power management techniques optimize energy consumption for long-term implantation, and wireless communication protocols enable data transmission to external processing units.
- Real-time biosignal processing algorithms: Real-time processing algorithms are essential for bioelectronic interfaces to provide immediate feedback and control. These algorithms include adaptive filtering to remove noise, feature extraction to identify relevant signal components, and classification methods to interpret biological intentions. Low-latency processing techniques ensure minimal delay between signal acquisition and response, which is critical for applications like neuroprosthetics and closed-loop neuromodulation systems.
- Wearable bioelectronic interfaces and signal analysis: Wearable bioelectronic interfaces provide non-invasive methods for monitoring physiological signals. These devices incorporate flexible electronics and conductive materials to maintain contact with the skin while allowing natural movement. Signal analysis techniques for wearable interfaces focus on motion artifact reduction, power-efficient processing, and real-time data interpretation. Cloud-based processing may complement on-device algorithms to enable more complex analysis while maintaining device portability.
- Molecular and cellular bioelectronic signal transduction: At the molecular and cellular level, bioelectronic interfaces translate biochemical signals into electronic information. These systems utilize specialized sensors that detect changes in cellular activity, protein interactions, or molecular concentrations. Signal transduction methods include electrochemical detection, optical sensing, and impedance measurements. Advanced microfluidic platforms integrate multiple sensing modalities to provide comprehensive analysis of biological samples, enabling applications in diagnostics, drug discovery, and fundamental research.
02 Implantable bioelectronic devices for signal acquisition
Implantable bioelectronic interfaces are designed to directly interact with biological tissues for signal acquisition. These devices incorporate miniaturized sensors, electrodes, and circuits that can be placed within the body to monitor physiological signals. The designs focus on biocompatibility, longevity, and minimal invasiveness while maintaining high signal quality. These implantable systems enable continuous monitoring of biological signals for therapeutic and diagnostic purposes.Expand Specific Solutions03 Bioelectronic signal processing algorithms and software
Specialized algorithms and software frameworks are developed for processing complex bioelectronic signals. These computational methods include machine learning approaches, digital signal processing techniques, and real-time analysis tools that can extract meaningful information from noisy biological data. The software systems are designed to handle large datasets, identify patterns, and make predictions based on bioelectronic signals, enabling more accurate interpretation of biological information.Expand Specific Solutions04 Molecular and cellular bioelectronic interfaces
Bioelectronic interfaces at the molecular and cellular level enable direct interaction with biological systems at their fundamental units. These technologies incorporate nanomaterials, biomolecules, and specialized sensors that can detect and process signals from individual cells or molecular interactions. Applications include biosensors, lab-on-chip devices, and systems for monitoring cellular activity. These interfaces bridge the gap between electronic systems and biological processes at the microscopic scale.Expand Specific Solutions05 Wearable bioelectronic monitoring systems
Non-invasive wearable systems for bioelectronic signal monitoring provide continuous health data without requiring implantation. These devices incorporate flexible electronics, skin-compatible materials, and wireless communication capabilities to capture and process physiological signals. The systems can monitor various parameters including heart activity, muscle movement, and skin conductance. Wearable bioelectronic interfaces offer convenient solutions for health monitoring, fitness tracking, and early disease detection.Expand Specific Solutions
Key Industry Players in Bioelectronic Interfaces
The bioelectronic interfaces in acoustic signal processing market is currently in a growth phase, with an estimated market size of $5-7 billion and projected annual growth of 12-15%. The technology is approaching maturity in medical applications but remains emergent in consumer electronics. Leading players represent diverse specializations: MED-EL and Cochlear dominate hearing implant technologies; X Development and Syntiant pioneer AI-driven signal processing; while established electronics giants like Canon, Panasonic, and MediaTek integrate these technologies into consumer products. Academic institutions like Chinese Academy of Sciences Institute of Acoustics and Cornell University contribute fundamental research advancements. The competitive landscape shows a convergence of medical device manufacturers, semiconductor companies, and research institutions collaborating to advance bioelectronic interface capabilities.
Chinese Academy of Sciences Institute of Acoustics
Technical Solution: The Chinese Academy of Sciences Institute of Acoustics has developed innovative bioelectronic interfaces for acoustic signal processing through their Bionic Ear Laboratory. Their approach integrates micro-electromechanical systems (MEMS) technology with neuromorphic computing to create highly efficient acoustic-neural interfaces. The institute has pioneered a novel electrode array design that mimics the tonotopic organization of the human cochlea, allowing for more precise frequency-specific stimulation. Their signal processing framework employs a biomimetic approach that replicates the mechanical filtering of the basilar membrane combined with neural encoding models based on auditory nerve fiber responses. The institute has also developed specialized algorithms for tonal language processing, addressing the unique challenges of Mandarin and other Chinese dialects where pitch perception is crucial for speech understanding. Their research includes the development of flexible bioelectronic interfaces using graphene-based materials that conform better to biological tissues, reducing inflammation and improving long-term stability. Recent advances include closed-loop systems that incorporate neural feedback to dynamically adjust stimulation parameters based on measured evoked potentials.
Strengths: Strong integration of fundamental acoustic principles with bioelectronic engineering; specialized expertise in processing tonal languages relevant to Chinese-speaking populations; advanced materials science approach to biocompatible interfaces; significant government funding supporting long-term research initiatives. Weaknesses: Less commercial deployment compared to industry leaders; potential challenges in international regulatory approval processes; limited clinical trial data compared to established medical device companies.
Cochlear (HK) Ltd.
Technical Solution: Cochlear has developed advanced bioelectronic interfaces for acoustic signal processing in their cochlear implant systems. Their technology utilizes a sophisticated electrode array that interfaces directly with the auditory nerve fibers in the cochlea. The system converts acoustic signals captured by an external microphone into electrical signals that stimulate the auditory nerve, bypassing damaged hair cells. Cochlear's latest generation devices employ advanced signal processing algorithms that analyze incoming sound in real-time, separating speech from background noise and optimizing signal delivery across the electrode array. Their proprietary SmartSound iQ technology with SCAN automatically adapts to different acoustic environments, while their Neural Response Telemetry system provides feedback on nerve stimulation effectiveness, allowing for personalized programming of the bioelectronic interface. The company has also developed wireless connectivity solutions that enable direct streaming of audio signals from external devices to the implant processor, reducing signal degradation and improving user experience.
Strengths: Industry-leading expertise in neural-electronic interfaces with decades of clinical validation; proprietary electrode designs that maximize stimulation efficiency while minimizing tissue damage; advanced signal processing algorithms specifically optimized for speech recognition. Weaknesses: Relatively high cost limiting accessibility; surgical implantation requirement creates inherent risks; challenges in reproducing the full frequency range and tonal qualities of natural hearing.
Core Patents in Bioelectronic Acoustic Interfaces
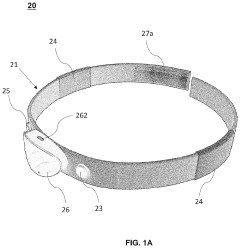
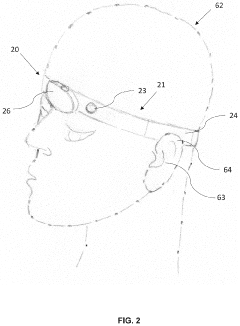
Human-computer interactive device and method
PatentActiveUS20230029255A1
Innovation
- A wearable bioelectrical signal acquisition device with a headband, sensing, reference, and grounding electrodes that allows for the collection of digital bioelectrical signals without requiring users to open their eyes or move, using a processing unit to generate signals and an interactive system for monitoring and modulating sleep patterns.
Haptics acoustic system
PatentInactiveJP2023068278A
Innovation
- A haptic acoustic system that seamlessly integrates auditory and tactile information from independent bioelectric stimulation channels without cables, ensuring time differences within ±20 milliseconds, using adjustable current stimulation patterns and stretchable, wireless electrodes.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical concern in the development of bioelectronic interfaces for acoustic signal processing. These interfaces, which connect biological tissues with electronic components, must maintain long-term functionality without triggering adverse biological responses. Current research indicates that materials such as platinum, iridium oxide, and certain conductive polymers demonstrate superior biocompatibility profiles when interfacing with neural tissues involved in acoustic processing.
The tissue-electrode interface presents significant challenges, particularly regarding inflammatory responses that can lead to signal degradation over time. Recent studies have documented that chronic inflammation around implanted electrodes can increase impedance by up to 200% within six months, severely compromising acoustic signal fidelity. Advanced coating technologies utilizing anti-inflammatory agents and biomimetic materials have shown promise in mitigating these responses, with some experimental interfaces maintaining stable impedance values for over two years in preclinical models.
Safety considerations extend beyond biocompatibility to include electrical safety parameters. Bioelectronic interfaces for acoustic processing typically operate at current densities below 30 μA/cm², which falls within established safety thresholds. However, the potential for charge accumulation at the tissue-electrode interface presents risks of tissue damage through electrochemical reactions. Charge-balanced stimulation protocols and advanced electrode materials with higher charge injection capacities have emerged as essential design elements to address these concerns.
Mechanical compatibility represents another crucial aspect of bioelectronic interface safety. Traditional rigid electrode arrays can cause micromotion-induced trauma in soft neural tissues involved in acoustic processing. Flexible substrate technologies, including those based on polyimide and parylene-C, have demonstrated significant reductions in inflammatory markers compared to rigid counterparts. Recent innovations in ultrasoft materials with elastic moduli below 100 kPa more closely match neural tissue properties, potentially extending device longevity.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with the FDA and equivalent international bodies requiring comprehensive biocompatibility testing according to ISO 10993 standards. These evaluations typically include cytotoxicity, sensitization, and implantation studies. For acoustic signal processing applications, additional specialized assessments of auditory system impacts are increasingly mandated, particularly for devices interfacing with cochlear or brainstem structures.
Long-term safety monitoring remains challenging, with limited data available beyond the 5-10 year timeframe. Emerging technologies for real-time monitoring of tissue responses, including integrated biosensors that detect inflammatory markers, may provide crucial feedback mechanisms to enhance the safety profiles of next-generation bioelectronic interfaces for acoustic signal processing applications.
The tissue-electrode interface presents significant challenges, particularly regarding inflammatory responses that can lead to signal degradation over time. Recent studies have documented that chronic inflammation around implanted electrodes can increase impedance by up to 200% within six months, severely compromising acoustic signal fidelity. Advanced coating technologies utilizing anti-inflammatory agents and biomimetic materials have shown promise in mitigating these responses, with some experimental interfaces maintaining stable impedance values for over two years in preclinical models.
Safety considerations extend beyond biocompatibility to include electrical safety parameters. Bioelectronic interfaces for acoustic processing typically operate at current densities below 30 μA/cm², which falls within established safety thresholds. However, the potential for charge accumulation at the tissue-electrode interface presents risks of tissue damage through electrochemical reactions. Charge-balanced stimulation protocols and advanced electrode materials with higher charge injection capacities have emerged as essential design elements to address these concerns.
Mechanical compatibility represents another crucial aspect of bioelectronic interface safety. Traditional rigid electrode arrays can cause micromotion-induced trauma in soft neural tissues involved in acoustic processing. Flexible substrate technologies, including those based on polyimide and parylene-C, have demonstrated significant reductions in inflammatory markers compared to rigid counterparts. Recent innovations in ultrasoft materials with elastic moduli below 100 kPa more closely match neural tissue properties, potentially extending device longevity.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with the FDA and equivalent international bodies requiring comprehensive biocompatibility testing according to ISO 10993 standards. These evaluations typically include cytotoxicity, sensitization, and implantation studies. For acoustic signal processing applications, additional specialized assessments of auditory system impacts are increasingly mandated, particularly for devices interfacing with cochlear or brainstem structures.
Long-term safety monitoring remains challenging, with limited data available beyond the 5-10 year timeframe. Emerging technologies for real-time monitoring of tissue responses, including integrated biosensors that detect inflammatory markers, may provide crucial feedback mechanisms to enhance the safety profiles of next-generation bioelectronic interfaces for acoustic signal processing applications.
Regulatory Framework for Medical Bioelectronic Devices
The regulatory landscape for bioelectronic interfaces in acoustic signal processing is complex and multifaceted, spanning multiple jurisdictions and oversight bodies. In the United States, the Food and Drug Administration (FDA) classifies most bioelectronic interfaces for acoustic processing as Class II medical devices, requiring premarket notification (510(k)) or in some cases, premarket approval (PMA) depending on the level of risk and intended use. The FDA's Digital Health Center of Excellence has recently established specific guidance for software components in these systems, particularly those utilizing machine learning algorithms for signal processing.
The European Union regulates these devices under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in May 2021. This transition has significantly increased requirements for clinical evidence, post-market surveillance, and technical documentation. Notably, bioelectronic interfaces that process acoustic signals for diagnostic or therapeutic purposes typically fall under Class IIa or IIb, necessitating conformity assessment by a Notified Body.
International standards play a crucial role in regulatory compliance, with ISO 13485 for quality management systems and IEC 60601-1 for electrical safety being fundamental requirements. For acoustic signal processing specifically, IEC 60601-2-66 provides particular requirements for hearing aid devices and systems. The emerging ISO/IEEE 11073 standards for health informatics are increasingly relevant for connected bioelectronic interfaces that transmit processed acoustic data.
Privacy and data protection regulations add another layer of complexity. In Europe, the General Data Protection Regulation (GDPR) imposes strict requirements on the processing of health data generated by these devices. Similarly, in the United States, HIPAA regulations apply when acoustic signal processing involves protected health information, particularly in clinical settings or when integrated with electronic health records.
Regulatory bodies are increasingly focusing on cybersecurity requirements for connected bioelectronic devices. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" and the EU MDR's emphasis on information security highlight the growing importance of protecting these systems from unauthorized access or manipulation, especially as acoustic processing interfaces become more sophisticated and network-connected.
Emerging regulatory considerations include the ethical implications of brain-computer interfaces that process neural signals related to acoustic stimuli, with several jurisdictions developing specific frameworks for neurotechnology. Additionally, regulations addressing electromagnetic compatibility (EMC) are critical for implantable bioelectronic interfaces to ensure they function properly in various electromagnetic environments without causing interference to other medical devices.
The European Union regulates these devices under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in May 2021. This transition has significantly increased requirements for clinical evidence, post-market surveillance, and technical documentation. Notably, bioelectronic interfaces that process acoustic signals for diagnostic or therapeutic purposes typically fall under Class IIa or IIb, necessitating conformity assessment by a Notified Body.
International standards play a crucial role in regulatory compliance, with ISO 13485 for quality management systems and IEC 60601-1 for electrical safety being fundamental requirements. For acoustic signal processing specifically, IEC 60601-2-66 provides particular requirements for hearing aid devices and systems. The emerging ISO/IEEE 11073 standards for health informatics are increasingly relevant for connected bioelectronic interfaces that transmit processed acoustic data.
Privacy and data protection regulations add another layer of complexity. In Europe, the General Data Protection Regulation (GDPR) imposes strict requirements on the processing of health data generated by these devices. Similarly, in the United States, HIPAA regulations apply when acoustic signal processing involves protected health information, particularly in clinical settings or when integrated with electronic health records.
Regulatory bodies are increasingly focusing on cybersecurity requirements for connected bioelectronic devices. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" and the EU MDR's emphasis on information security highlight the growing importance of protecting these systems from unauthorized access or manipulation, especially as acoustic processing interfaces become more sophisticated and network-connected.
Emerging regulatory considerations include the ethical implications of brain-computer interfaces that process neural signals related to acoustic stimuli, with several jurisdictions developing specific frameworks for neurotechnology. Additionally, regulations addressing electromagnetic compatibility (EMC) are critical for implantable bioelectronic interfaces to ensure they function properly in various electromagnetic environments without causing interference to other medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!