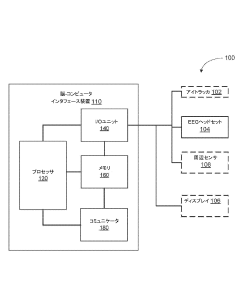
Bioelectronic Interface Advancements in Brain-Computer Interfaces
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Bioelectronic Interface Evolution and Objectives
Brain-Computer Interface (BCI) technology has evolved significantly since its conceptual inception in the 1970s, transitioning from theoretical frameworks to practical applications that bridge the gap between neural activity and computational systems. The field has witnessed accelerated development over the past two decades, driven by advancements in neuroscience, materials engineering, and computational algorithms. Initially focused on basic signal detection, modern BCIs now enable increasingly sophisticated interactions between the human brain and external devices.
The evolution of bioelectronic interfaces in BCI technology can be traced through several distinct phases. Early systems relied on invasive electrodes that required surgical implantation, limiting their application primarily to clinical settings. The subsequent development of non-invasive technologies such as electroencephalography (EEG) expanded the potential user base but faced challenges in signal resolution and accuracy. Recent innovations in semi-invasive approaches and flexible electronics represent a middle ground, offering improved signal quality while minimizing biological disruption.
Material science breakthroughs have been pivotal in this technological progression. Traditional rigid electrodes have given way to flexible, biocompatible materials that conform to neural tissue, reducing inflammatory responses and enabling longer-term implantation. Parallel advances in signal processing algorithms have enhanced the extraction of meaningful data from neural recordings, improving the precision and reliability of BCI systems across various applications.
The primary objectives of current bioelectronic interface research center on several key dimensions. First, enhancing signal fidelity and resolution to capture increasingly nuanced neural activity patterns. Second, improving biocompatibility to minimize tissue damage and enable long-term integration with neural systems. Third, developing wireless capabilities to eliminate physical tethering and associated infection risks. Fourth, miniaturizing components to reduce invasiveness while maintaining functional performance.
Looking forward, the field aims to achieve bidirectional communication systems that not only record neural activity but also deliver precise feedback to neural circuits. This closed-loop capability represents a significant frontier, potentially enabling applications ranging from advanced prosthetic control to therapeutic interventions for neurological conditions. Additionally, researchers are working toward self-calibrating systems that adapt to changes in neural activity patterns over time, addressing the current challenge of signal drift in long-term implementations.
The convergence of nanotechnology, artificial intelligence, and neuroscience is expected to accelerate progress in this domain, potentially leading to transformative applications in healthcare, human-computer interaction, and cognitive enhancement. As these technologies mature, ethical frameworks and regulatory standards will need to evolve in parallel to address questions of privacy, autonomy, and equitable access.
The evolution of bioelectronic interfaces in BCI technology can be traced through several distinct phases. Early systems relied on invasive electrodes that required surgical implantation, limiting their application primarily to clinical settings. The subsequent development of non-invasive technologies such as electroencephalography (EEG) expanded the potential user base but faced challenges in signal resolution and accuracy. Recent innovations in semi-invasive approaches and flexible electronics represent a middle ground, offering improved signal quality while minimizing biological disruption.
Material science breakthroughs have been pivotal in this technological progression. Traditional rigid electrodes have given way to flexible, biocompatible materials that conform to neural tissue, reducing inflammatory responses and enabling longer-term implantation. Parallel advances in signal processing algorithms have enhanced the extraction of meaningful data from neural recordings, improving the precision and reliability of BCI systems across various applications.
The primary objectives of current bioelectronic interface research center on several key dimensions. First, enhancing signal fidelity and resolution to capture increasingly nuanced neural activity patterns. Second, improving biocompatibility to minimize tissue damage and enable long-term integration with neural systems. Third, developing wireless capabilities to eliminate physical tethering and associated infection risks. Fourth, miniaturizing components to reduce invasiveness while maintaining functional performance.
Looking forward, the field aims to achieve bidirectional communication systems that not only record neural activity but also deliver precise feedback to neural circuits. This closed-loop capability represents a significant frontier, potentially enabling applications ranging from advanced prosthetic control to therapeutic interventions for neurological conditions. Additionally, researchers are working toward self-calibrating systems that adapt to changes in neural activity patterns over time, addressing the current challenge of signal drift in long-term implementations.
The convergence of nanotechnology, artificial intelligence, and neuroscience is expected to accelerate progress in this domain, potentially leading to transformative applications in healthcare, human-computer interaction, and cognitive enhancement. As these technologies mature, ethical frameworks and regulatory standards will need to evolve in parallel to address questions of privacy, autonomy, and equitable access.
Market Analysis for Neural Interface Technologies
The global neural interface technology market is experiencing unprecedented growth, driven by advancements in bioelectronic interfaces for brain-computer interaction systems. Current market valuations place this sector at approximately $2.4 billion in 2023, with projections indicating a compound annual growth rate of 15.7% through 2030, potentially reaching $6.8 billion by the end of the decade.
Medical applications currently dominate the market landscape, accounting for roughly 60% of total market share. Within this segment, neurodegenerative disease management represents the fastest-growing application area, particularly for conditions such as Parkinson's disease, epilepsy, and paralysis. The therapeutic potential of neural interfaces has attracted substantial investment from both pharmaceutical companies and medical device manufacturers seeking to develop next-generation treatment modalities.
Consumer applications are emerging as a secondary but rapidly expanding market segment. Companies like Neuralink, Kernel, and CTRL-labs (acquired by Meta) are pioneering consumer-oriented neural interface products, though regulatory hurdles remain significant barriers to widespread adoption. Military and defense applications constitute approximately 15% of the current market, with substantial government funding directed toward enhanced soldier capabilities and rehabilitation technologies.
Regionally, North America leads with approximately 45% of the global market share, followed by Europe (25%) and Asia-Pacific (20%). China has demonstrated the most aggressive growth trajectory, with annual investment in neural interface technologies increasing by over 30% year-over-year since 2019, primarily through state-backed initiatives and academic-industrial partnerships.
Key market drivers include miniaturization of implantable devices, improvements in biocompatibility of materials, enhanced signal processing algorithms, and growing acceptance of invasive technologies for severe medical conditions. The reduction in device size has decreased surgical complications by approximately 40% over the past five years, significantly improving patient outcomes and market acceptance.
Barriers to market expansion include stringent regulatory requirements, with FDA approval processes averaging 4-7 years for invasive neural technologies. Additionally, high development and implementation costs—with advanced BCI systems typically costing between $50,000-$100,000 per unit—limit widespread adoption outside specialized research and medical settings. Ethical concerns regarding data privacy, cognitive liberty, and potential surveillance capabilities also represent significant market constraints that technology developers must address to achieve broader market penetration.
Medical applications currently dominate the market landscape, accounting for roughly 60% of total market share. Within this segment, neurodegenerative disease management represents the fastest-growing application area, particularly for conditions such as Parkinson's disease, epilepsy, and paralysis. The therapeutic potential of neural interfaces has attracted substantial investment from both pharmaceutical companies and medical device manufacturers seeking to develop next-generation treatment modalities.
Consumer applications are emerging as a secondary but rapidly expanding market segment. Companies like Neuralink, Kernel, and CTRL-labs (acquired by Meta) are pioneering consumer-oriented neural interface products, though regulatory hurdles remain significant barriers to widespread adoption. Military and defense applications constitute approximately 15% of the current market, with substantial government funding directed toward enhanced soldier capabilities and rehabilitation technologies.
Regionally, North America leads with approximately 45% of the global market share, followed by Europe (25%) and Asia-Pacific (20%). China has demonstrated the most aggressive growth trajectory, with annual investment in neural interface technologies increasing by over 30% year-over-year since 2019, primarily through state-backed initiatives and academic-industrial partnerships.
Key market drivers include miniaturization of implantable devices, improvements in biocompatibility of materials, enhanced signal processing algorithms, and growing acceptance of invasive technologies for severe medical conditions. The reduction in device size has decreased surgical complications by approximately 40% over the past five years, significantly improving patient outcomes and market acceptance.
Barriers to market expansion include stringent regulatory requirements, with FDA approval processes averaging 4-7 years for invasive neural technologies. Additionally, high development and implementation costs—with advanced BCI systems typically costing between $50,000-$100,000 per unit—limit widespread adoption outside specialized research and medical settings. Ethical concerns regarding data privacy, cognitive liberty, and potential surveillance capabilities also represent significant market constraints that technology developers must address to achieve broader market penetration.
Current Challenges in Bioelectronic Brain Interfaces
Despite significant advancements in bioelectronic brain interfaces, several critical challenges continue to impede widespread clinical adoption and technological maturity. The biocompatibility of implanted materials remains a fundamental obstacle, as foreign body responses trigger inflammation, glial scarring, and eventual signal degradation. Current electrode materials struggle to maintain long-term stability in the biochemically hostile environment of neural tissue, with performance typically deteriorating within months to a few years after implantation.
Signal quality presents another significant hurdle, with low signal-to-noise ratios hampering reliable neural recording. The electrical impedance at the electrode-tissue interface often increases over time, further degrading signal fidelity. Additionally, current systems face limitations in spatial and temporal resolution, making it difficult to precisely target and monitor specific neural populations at the necessary scale for advanced applications.
Miniaturization challenges persist despite advances in microelectronics. Power requirements for wireless data transmission and processing create thermal management issues that can damage surrounding neural tissue. The development of efficient, biocompatible power sources that can operate safely within or adjacent to neural tissue remains an unresolved engineering challenge.
Surgical invasiveness represents a major barrier to widespread adoption, with current high-performance interfaces requiring craniotomy and direct brain tissue contact. Less invasive alternatives typically suffer from reduced signal quality and specificity, creating an unresolved tradeoff between performance and invasiveness.
Data processing and interpretation present computational challenges of enormous scale. Neural signals are inherently complex, non-stationary, and context-dependent, requiring sophisticated algorithms to extract meaningful information. Current systems struggle with real-time processing of high-dimensional neural data while maintaining low latency for closed-loop applications.
Regulatory and ethical frameworks have not kept pace with technological developments. Questions regarding data ownership, privacy, cognitive liberty, and potential for misuse remain inadequately addressed. The lack of standardized safety protocols and performance metrics further complicates clinical translation and comparative assessment of different interface technologies.
Cross-disciplinary integration remains suboptimal, with insufficient collaboration between neuroscientists, materials engineers, computer scientists, and clinicians. This siloed approach has limited the development of holistic solutions that address the multifaceted challenges of bioelectronic brain interfaces.
Signal quality presents another significant hurdle, with low signal-to-noise ratios hampering reliable neural recording. The electrical impedance at the electrode-tissue interface often increases over time, further degrading signal fidelity. Additionally, current systems face limitations in spatial and temporal resolution, making it difficult to precisely target and monitor specific neural populations at the necessary scale for advanced applications.
Miniaturization challenges persist despite advances in microelectronics. Power requirements for wireless data transmission and processing create thermal management issues that can damage surrounding neural tissue. The development of efficient, biocompatible power sources that can operate safely within or adjacent to neural tissue remains an unresolved engineering challenge.
Surgical invasiveness represents a major barrier to widespread adoption, with current high-performance interfaces requiring craniotomy and direct brain tissue contact. Less invasive alternatives typically suffer from reduced signal quality and specificity, creating an unresolved tradeoff between performance and invasiveness.
Data processing and interpretation present computational challenges of enormous scale. Neural signals are inherently complex, non-stationary, and context-dependent, requiring sophisticated algorithms to extract meaningful information. Current systems struggle with real-time processing of high-dimensional neural data while maintaining low latency for closed-loop applications.
Regulatory and ethical frameworks have not kept pace with technological developments. Questions regarding data ownership, privacy, cognitive liberty, and potential for misuse remain inadequately addressed. The lack of standardized safety protocols and performance metrics further complicates clinical translation and comparative assessment of different interface technologies.
Cross-disciplinary integration remains suboptimal, with insufficient collaboration between neuroscientists, materials engineers, computer scientists, and clinicians. This siloed approach has limited the development of holistic solutions that address the multifaceted challenges of bioelectronic brain interfaces.
Contemporary Bioelectronic Interface Solutions
01 Flexible and wearable bioelectronic interfaces
Advancements in flexible and wearable bioelectronic interfaces that can conform to biological tissues for improved signal detection and transmission. These interfaces utilize stretchable materials and novel fabrication techniques to create comfortable, long-term monitoring solutions that maintain stable contact with skin or internal tissues while allowing natural movement. The flexibility enables better integration with curved biological surfaces, enhancing both user comfort and signal quality for medical monitoring and therapeutic applications.- Flexible and wearable bioelectronic interfaces: Advancements in flexible and wearable bioelectronic interfaces that can conform to biological tissues for improved signal detection and transmission. These interfaces utilize stretchable materials and novel fabrication techniques to create comfortable, long-term monitoring solutions that maintain stable contact with skin or internal tissues while allowing natural movement. The flexibility enables better integration with curved biological surfaces, enhancing both user comfort and signal quality for medical monitoring and therapeutic applications.
- Neural interface technologies: Innovative neural interface technologies that establish direct communication pathways between the nervous system and electronic devices. These interfaces incorporate advanced electrode designs, biocompatible materials, and signal processing algorithms to record and stimulate neural activity with high spatial and temporal resolution. Applications include brain-computer interfaces for controlling prosthetics, treating neurological disorders, and enabling bidirectional communication between neural tissues and external devices for both research and therapeutic purposes.
- Nanomaterial-based bioelectronic interfaces: Integration of nanomaterials such as carbon nanotubes, graphene, and nanoparticles into bioelectronic interfaces to enhance performance and functionality. These nanomaterials provide improved electrical conductivity, increased surface area for cellular interaction, and enhanced biocompatibility. The nanoscale dimensions enable more precise interfacing with biological components at the cellular and subcellular levels, resulting in higher sensitivity, lower impedance, and better signal-to-noise ratios for sensing and stimulation applications.
- Implantable bioelectronic systems: Advanced implantable bioelectronic systems designed for long-term integration within the body. These systems incorporate biocompatible materials, hermetic packaging, wireless power transfer, and data communication capabilities to ensure durability and functionality in the physiological environment. Innovations include miniaturization techniques, improved power efficiency, and self-calibrating mechanisms that adapt to tissue changes over time, enabling applications such as neural stimulators, biosensors, and drug delivery systems that can operate autonomously within the body.
- Biohybrid interface technologies: Development of biohybrid interfaces that combine living biological components with electronic systems to create more naturalistic and functional connections. These interfaces incorporate cells, tissues, or biomolecules as integral parts of the electronic system, facilitating better integration with the host biology. Approaches include cell-seeded electrodes, protein-coated surfaces, and tissue-engineered constructs that serve as biological intermediaries between electronic components and native tissues, reducing foreign body responses and improving long-term stability and functionality of the interface.
02 Nanomaterial-based bioelectronic interfaces
Integration of nanomaterials such as carbon nanotubes, graphene, and nanoparticles to enhance the performance of bioelectronic interfaces. These materials provide improved electrical conductivity, increased surface area for biological interaction, and enhanced biocompatibility. Nanomaterial-based interfaces enable higher sensitivity in biosensing applications, more efficient signal transduction, and reduced impedance at the tissue-electrode interface, resulting in better signal quality and more effective stimulation capabilities.Expand Specific Solutions03 Neural interface technologies
Advanced neural interfaces that establish direct communication pathways between electronic devices and the nervous system. These technologies include microelectrode arrays, optogenetic interfaces, and minimally invasive neural probes that can record and stimulate neural activity with high spatial and temporal resolution. Innovations focus on reducing tissue damage, preventing immune responses, and maintaining long-term stability for applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders.Expand Specific Solutions04 Biofunctionalized interface surfaces
Surface modification techniques that enhance biocompatibility and functionality of bioelectronic interfaces through biofunctionalization. These approaches include coating electrodes with bioactive molecules, cell-adhesive proteins, anti-inflammatory agents, or conductive polymers to improve tissue integration and reduce foreign body responses. Biofunctionalized surfaces can promote specific cell attachment, reduce fibrosis, prevent biofouling, and extend the functional lifetime of implanted devices while improving signal quality and stability.Expand Specific Solutions05 Wireless and self-powered bioelectronic systems
Development of wireless power transfer and communication technologies for bioelectronic interfaces, eliminating the need for transcutaneous wires and reducing infection risks. These systems incorporate energy harvesting mechanisms that utilize body motion, temperature gradients, or biochemical energy to generate power for operation. Advancements include miniaturized antennas, efficient power management circuits, and low-power communication protocols that enable continuous monitoring and stimulation without external power sources or physical connections.Expand Specific Solutions
Leading Organizations in BCI Development
Brain-Computer Interface (BCI) technology is currently in a transitional phase from early development to commercial application, with the global market expected to reach $3.7 billion by 2027. The technology maturity varies across applications, with medical uses being more advanced than consumer applications. Leading academic institutions (Washington University, Cornell, Tianjin University) are driving fundamental research, while commercial players are diversifying the competitive landscape. Precision Neuroscience and Neurable are pioneering minimally invasive interfaces, while established corporations like Philips and Nokia are leveraging their healthcare expertise to develop integrated BCI solutions. Chinese universities (Zhejiang, Beijing Institute of Technology) are rapidly advancing in neural signal processing, while specialized startups like MindPortal and Neurolutions focus on specific therapeutic applications, creating a dynamic ecosystem balancing scientific innovation with commercial potential.
Precision Neuroscience Corp.
Technical Solution: Precision Neuroscience has developed an ultra-thin, flexible neural interface called the Layer 7 Cortical Interface. This technology consists of arrays of micron-scale electrodes embedded in a material thinner than human hair, designed for minimally invasive insertion through a cranial slit smaller than 1cm without requiring traditional open-brain surgery. The Layer 7 system uses a specialized insertion mechanism that unfolds the flexible electrode array across the brain surface, allowing for high-resolution neural recording with significantly reduced tissue damage compared to conventional rigid electrode arrays. Their interface captures neural signals with exceptional fidelity while minimizing foreign body response, enabling stable long-term recordings essential for advanced BCI applications in both clinical and research settings.
Strengths: Minimally invasive insertion technique reduces surgical risks; ultra-thin flexible materials minimize tissue damage and immune response; high-resolution neural recording capabilities. Weaknesses: Still requires surgical procedure albeit minimized; long-term biocompatibility challenges may emerge; potential signal degradation over extended periods due to micro-movement or glial scarring.
Neurable, Inc.
Technical Solution: Neurable has pioneered non-invasive BCI technology focusing on EEG-based systems with advanced signal processing algorithms. Their approach combines dry electrode technology with machine learning algorithms that can extract meaningful neural signals from noisy EEG data. Neurable's system employs a proprietary combination of spatial filters, artifact rejection techniques, and deep learning models to decode user intent from brain activity patterns. Their technology has evolved from bulky headsets to more streamlined form factors, with recent developments integrating their BCI technology into everyday wearables like headphones and glasses. This approach prioritizes user comfort and social acceptability while maintaining sufficient signal quality for consumer applications such as attention monitoring, meditation assistance, and basic device control.
Strengths: Non-invasive approach eliminates surgical risks; consumer-friendly form factors increase adoption potential; machine learning algorithms continuously improve with more user data. Weaknesses: Limited signal resolution compared to invasive methods; susceptibility to environmental electrical noise; requires regular calibration for optimal performance.
Critical Patents in Neural Interface Technology
Brain-computer interface with adaptations for high-speed, accurate, and intuitive user interactions
PatentPendingJP2024075573A
Innovation
- A hybrid BCI system that integrates eye movement and brain activity tracking to enable real-time positioning of user gaze and action selection, using a combination of eye trackers and neural recording headsets to process signals for intuitive and accurate human-machine interaction, allowing for hardware-independent operation across various platforms.
Brain-computer interface with adaptations for high-speed, accurate, and intuitive user interactions
PatentPendingUS20250093951A1
Innovation
- A hardware-agnostic integrated oculomotor-neural hybrid BCI platform that combines real-time eye-movement tracking with brain activity tracking to mediate user interactions, using a hybrid BCI system with pointing and action control features to enhance user interface design for high-speed and accurate interactions.
Biocompatibility and Longevity Considerations
Biocompatibility and longevity remain critical challenges in advancing brain-computer interface (BCI) technologies. Current implantable neural interfaces face significant limitations due to the foreign body response, which triggers inflammation, glial scarring, and eventual signal degradation. This immune response typically begins within hours of implantation and can severely compromise device functionality within weeks to months, making long-term neural recording and stimulation unreliable.
Material selection represents a fundamental consideration in improving biocompatibility. Traditional electrode materials like platinum, iridium oxide, and silicon have demonstrated limited success in long-term applications. Recent advances in ultrasoft materials, including hydrogels and elastomers with mechanical properties closer to brain tissue (approximately 1-10 kPa), show promise in reducing mechanical mismatch and subsequent inflammatory responses. Notably, research into conducting polymers like PEDOT:PSS has demonstrated improved charge transfer characteristics while maintaining flexibility.
Surface modification strategies have emerged as effective approaches to enhance biocompatibility. Biomimetic coatings incorporating anti-inflammatory agents, neural adhesion molecules, or extracellular matrix components can significantly reduce foreign body responses. For example, phosphorylcholine-based coatings have demonstrated reduced protein adsorption and cellular adhesion, while neural adhesion molecule L1 coatings promote neuronal attachment and neurite outgrowth around electrodes.
Miniaturization and flexible designs represent another frontier in improving interface longevity. Ultrathin, flexible electrode arrays that conform to brain tissue movement reduce chronic micromotion and subsequent inflammation. Mesh electronics and injectable neural lace technologies developed at Harvard University have demonstrated promising long-term stability with minimal immune response in animal models, maintaining single-neuron recording capabilities for over a year.
Controlled drug delivery systems integrated into neural interfaces offer additional strategies for managing the biological response. Local delivery of anti-inflammatory agents, neurotrophic factors, or immunosuppressants can modulate the tissue response at the neural interface. Microfluidic channels and biodegradable polymeric matrices have been employed to provide sustained release of therapeutic agents directly at the implant site.
Wireless power and data transmission technologies are increasingly important for long-term implantable systems, eliminating transcutaneous wires that increase infection risk and mechanical failure points. Recent advances in resonant inductive coupling, ultrasonic power transfer, and near-field communication have enabled fully implantable systems with reduced hardware complexity and improved reliability.
Hermetic packaging solutions remain essential for protecting electronic components from the corrosive physiological environment. Advanced encapsulation techniques using biocompatible polymers, atomic layer deposition, and ceramic materials have extended device lifespans from months to potentially years, though perfect hermeticity remains elusive for chronic neural interfaces.
Material selection represents a fundamental consideration in improving biocompatibility. Traditional electrode materials like platinum, iridium oxide, and silicon have demonstrated limited success in long-term applications. Recent advances in ultrasoft materials, including hydrogels and elastomers with mechanical properties closer to brain tissue (approximately 1-10 kPa), show promise in reducing mechanical mismatch and subsequent inflammatory responses. Notably, research into conducting polymers like PEDOT:PSS has demonstrated improved charge transfer characteristics while maintaining flexibility.
Surface modification strategies have emerged as effective approaches to enhance biocompatibility. Biomimetic coatings incorporating anti-inflammatory agents, neural adhesion molecules, or extracellular matrix components can significantly reduce foreign body responses. For example, phosphorylcholine-based coatings have demonstrated reduced protein adsorption and cellular adhesion, while neural adhesion molecule L1 coatings promote neuronal attachment and neurite outgrowth around electrodes.
Miniaturization and flexible designs represent another frontier in improving interface longevity. Ultrathin, flexible electrode arrays that conform to brain tissue movement reduce chronic micromotion and subsequent inflammation. Mesh electronics and injectable neural lace technologies developed at Harvard University have demonstrated promising long-term stability with minimal immune response in animal models, maintaining single-neuron recording capabilities for over a year.
Controlled drug delivery systems integrated into neural interfaces offer additional strategies for managing the biological response. Local delivery of anti-inflammatory agents, neurotrophic factors, or immunosuppressants can modulate the tissue response at the neural interface. Microfluidic channels and biodegradable polymeric matrices have been employed to provide sustained release of therapeutic agents directly at the implant site.
Wireless power and data transmission technologies are increasingly important for long-term implantable systems, eliminating transcutaneous wires that increase infection risk and mechanical failure points. Recent advances in resonant inductive coupling, ultrasonic power transfer, and near-field communication have enabled fully implantable systems with reduced hardware complexity and improved reliability.
Hermetic packaging solutions remain essential for protecting electronic components from the corrosive physiological environment. Advanced encapsulation techniques using biocompatible polymers, atomic layer deposition, and ceramic materials have extended device lifespans from months to potentially years, though perfect hermeticity remains elusive for chronic neural interfaces.
Ethical and Regulatory Framework for Neural Technologies
The rapid advancement of brain-computer interfaces (BCIs) necessitates a robust ethical and regulatory framework to guide their development and implementation. Current regulatory landscapes vary significantly across regions, with the United States FDA classifying neural devices under medical device regulations, while the European Union employs the Medical Device Regulation (MDR) framework with specific provisions for implantable devices.
Privacy concerns represent a paramount ethical consideration, as BCIs collect unprecedented amounts of neural data that could reveal sensitive information about cognitive processes, emotional states, and even thoughts. Establishing clear guidelines for neural data ownership, storage, and sharing is essential to protect user privacy while enabling scientific progress.
Informed consent protocols for BCI technologies require significant enhancement beyond traditional medical standards. Users must fully comprehend not only the physical risks but also the potential psychological and identity-related implications of interfacing technology with their neural systems. This is particularly challenging when considering applications for individuals with cognitive impairments or communication difficulties.
The principle of autonomy raises complex questions in BCI development. While these technologies aim to restore function and enhance capabilities, they simultaneously introduce risks of external control or influence over neural processes. Regulatory frameworks must balance the therapeutic benefits against potential compromises to individual agency and decision-making independence.
Equity of access represents another critical ethical dimension. As BCI technologies advance, preventing the emergence of neurological "haves and have-nots" requires deliberate policy interventions. International cooperation on regulatory standards could help ensure that these technologies don't exacerbate existing social disparities.
Several international organizations have begun developing ethical guidelines specifically for neurotechnology, including the OECD's Recommendation on Responsible Innovation in Neurotechnology and the IEEE's standards for neurotechnologies. These frameworks emphasize principles of transparency, accountability, and human dignity.
Future regulatory approaches will likely require adaptive governance models that can evolve alongside rapidly advancing technologies. This includes creating specialized regulatory bodies with neuroscientific expertise, establishing ongoing monitoring systems for long-term neural device safety, and developing international standards for neural data protection.
The integration of neuroethicists into research and development processes from early stages represents a promising approach to embedding ethical considerations into the technological architecture of BCIs, rather than addressing ethical concerns as afterthoughts.
Privacy concerns represent a paramount ethical consideration, as BCIs collect unprecedented amounts of neural data that could reveal sensitive information about cognitive processes, emotional states, and even thoughts. Establishing clear guidelines for neural data ownership, storage, and sharing is essential to protect user privacy while enabling scientific progress.
Informed consent protocols for BCI technologies require significant enhancement beyond traditional medical standards. Users must fully comprehend not only the physical risks but also the potential psychological and identity-related implications of interfacing technology with their neural systems. This is particularly challenging when considering applications for individuals with cognitive impairments or communication difficulties.
The principle of autonomy raises complex questions in BCI development. While these technologies aim to restore function and enhance capabilities, they simultaneously introduce risks of external control or influence over neural processes. Regulatory frameworks must balance the therapeutic benefits against potential compromises to individual agency and decision-making independence.
Equity of access represents another critical ethical dimension. As BCI technologies advance, preventing the emergence of neurological "haves and have-nots" requires deliberate policy interventions. International cooperation on regulatory standards could help ensure that these technologies don't exacerbate existing social disparities.
Several international organizations have begun developing ethical guidelines specifically for neurotechnology, including the OECD's Recommendation on Responsible Innovation in Neurotechnology and the IEEE's standards for neurotechnologies. These frameworks emphasize principles of transparency, accountability, and human dignity.
Future regulatory approaches will likely require adaptive governance models that can evolve alongside rapidly advancing technologies. This includes creating specialized regulatory bodies with neuroscientific expertise, establishing ongoing monitoring systems for long-term neural device safety, and developing international standards for neural data protection.
The integration of neuroethicists into research and development processes from early stages represents a promising approach to embedding ethical considerations into the technological architecture of BCIs, rather than addressing ethical concerns as afterthoughts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!