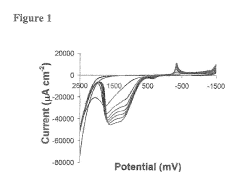
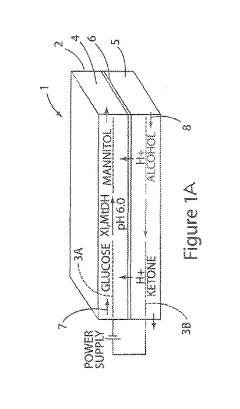
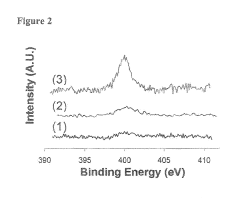
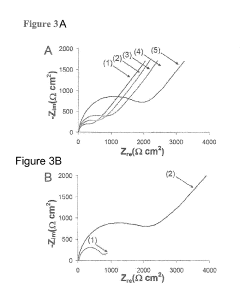
Bioelectronic Interface and Its Application in Personalized Medicine
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, evolving significantly over the past three decades. Initially emerging in the 1990s as rudimentary neural implants, these interfaces have transformed into sophisticated systems capable of bidirectional communication with biological tissues. The evolution trajectory has been marked by miniaturization, enhanced biocompatibility, and increased functionality, moving from rigid, invasive devices to flexible, minimally invasive platforms.
The field gained momentum in the early 2000s with the development of microelectrode arrays capable of recording neural activity. By 2010, researchers had achieved significant breakthroughs in materials science, introducing conductive polymers and nanomaterials that substantially improved signal quality and reduced tissue damage. The past decade has witnessed the integration of wireless capabilities, enabling real-time data transmission and remote control of implanted devices.
Current bioelectronic interfaces incorporate advanced features such as closed-loop systems that can detect specific biological signals and respond with appropriate interventions. These developments have been driven by interdisciplinary collaboration among electrical engineers, materials scientists, neuroscientists, and medical professionals, creating a rich ecosystem of innovation.
The primary objective of bioelectronic interface technology in personalized medicine is to establish seamless integration between electronic devices and biological systems, enabling precise monitoring and modulation of physiological processes. This aims to facilitate individualized treatment approaches based on real-time biological data, moving beyond the traditional one-size-fits-all medical paradigm.
Specific technical goals include developing interfaces with enhanced longevity, minimizing foreign body responses through novel materials and designs, and improving spatial and temporal resolution of biological signal detection. Additionally, researchers are working toward creating self-powered systems that harvest energy from biological processes, eliminating the need for battery replacement surgeries.
The field is increasingly focused on expanding application domains beyond neurological conditions to encompass cardiovascular monitoring, immune system modulation, and metabolic regulation. This broadening scope reflects the technology's potential to revolutionize treatment approaches across multiple medical specialties.
Looking forward, the trajectory of bioelectronic interfaces points toward fully integrated, intelligent systems capable of autonomous operation within the human body, continuously adapting to changing physiological conditions and delivering personalized therapeutic interventions with minimal external intervention.
The field gained momentum in the early 2000s with the development of microelectrode arrays capable of recording neural activity. By 2010, researchers had achieved significant breakthroughs in materials science, introducing conductive polymers and nanomaterials that substantially improved signal quality and reduced tissue damage. The past decade has witnessed the integration of wireless capabilities, enabling real-time data transmission and remote control of implanted devices.
Current bioelectronic interfaces incorporate advanced features such as closed-loop systems that can detect specific biological signals and respond with appropriate interventions. These developments have been driven by interdisciplinary collaboration among electrical engineers, materials scientists, neuroscientists, and medical professionals, creating a rich ecosystem of innovation.
The primary objective of bioelectronic interface technology in personalized medicine is to establish seamless integration between electronic devices and biological systems, enabling precise monitoring and modulation of physiological processes. This aims to facilitate individualized treatment approaches based on real-time biological data, moving beyond the traditional one-size-fits-all medical paradigm.
Specific technical goals include developing interfaces with enhanced longevity, minimizing foreign body responses through novel materials and designs, and improving spatial and temporal resolution of biological signal detection. Additionally, researchers are working toward creating self-powered systems that harvest energy from biological processes, eliminating the need for battery replacement surgeries.
The field is increasingly focused on expanding application domains beyond neurological conditions to encompass cardiovascular monitoring, immune system modulation, and metabolic regulation. This broadening scope reflects the technology's potential to revolutionize treatment approaches across multiple medical specialties.
Looking forward, the trajectory of bioelectronic interfaces points toward fully integrated, intelligent systems capable of autonomous operation within the human body, continuously adapting to changing physiological conditions and delivering personalized therapeutic interventions with minimal external intervention.
Market Analysis for Bioelectronic Solutions in Healthcare
The bioelectronic interface market in healthcare is experiencing unprecedented growth, driven by increasing demand for personalized medicine solutions and advancements in miniaturization technologies. Current market valuations place the global bioelectronic medicine sector at approximately 25 billion USD in 2023, with projections indicating a compound annual growth rate of 13.2% through 2030. This remarkable expansion reflects the convergence of electronics, biology, and medicine in addressing chronic conditions through targeted neuromodulation and real-time physiological monitoring.
Patient demand for non-pharmacological treatment alternatives represents a significant market driver, particularly for conditions like rheumatoid arthritis, Parkinson's disease, and treatment-resistant depression. Healthcare systems worldwide are increasingly recognizing the long-term cost benefits of bioelectronic interventions compared to lifetime medication regimens, with some studies demonstrating 30-40% reductions in total treatment costs over five-year periods for certain conditions.
The personalized medicine segment within bioelectronics is growing at 16.8% annually, outpacing the broader market. This acceleration stems from the ability of bioelectronic interfaces to deliver precisely calibrated therapeutic interventions based on individual patient physiology and response patterns. Closed-loop systems that continuously monitor biomarkers and adjust treatment parameters in real-time represent the fastest-growing product category, with 22.3% annual growth.
Regional market analysis reveals North America currently dominates with 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the highest growth trajectory at 17.5% annually, driven by substantial healthcare infrastructure investments in China, Japan, and South Korea, alongside increasing chronic disease prevalence.
Reimbursement landscapes are evolving favorably, with major insurers in the US and EU expanding coverage for bioelectronic therapies. Medicare's decision to cover certain bioelectronic treatments for chronic pain management in 2022 represents a significant milestone, potentially influencing private insurer policies. The UK's NHS has similarly initiated coverage pathways for select bioelectronic interventions demonstrating cost-effectiveness in managing inflammatory conditions.
Market segmentation analysis indicates implantable devices currently represent 58% of the bioelectronic medicine market, with non-invasive wearable solutions growing rapidly at 19.2% annually. This shift reflects technological improvements in transcutaneous stimulation efficacy and patient preference for non-surgical options when clinically viable.
Patient demand for non-pharmacological treatment alternatives represents a significant market driver, particularly for conditions like rheumatoid arthritis, Parkinson's disease, and treatment-resistant depression. Healthcare systems worldwide are increasingly recognizing the long-term cost benefits of bioelectronic interventions compared to lifetime medication regimens, with some studies demonstrating 30-40% reductions in total treatment costs over five-year periods for certain conditions.
The personalized medicine segment within bioelectronics is growing at 16.8% annually, outpacing the broader market. This acceleration stems from the ability of bioelectronic interfaces to deliver precisely calibrated therapeutic interventions based on individual patient physiology and response patterns. Closed-loop systems that continuously monitor biomarkers and adjust treatment parameters in real-time represent the fastest-growing product category, with 22.3% annual growth.
Regional market analysis reveals North America currently dominates with 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the highest growth trajectory at 17.5% annually, driven by substantial healthcare infrastructure investments in China, Japan, and South Korea, alongside increasing chronic disease prevalence.
Reimbursement landscapes are evolving favorably, with major insurers in the US and EU expanding coverage for bioelectronic therapies. Medicare's decision to cover certain bioelectronic treatments for chronic pain management in 2022 represents a significant milestone, potentially influencing private insurer policies. The UK's NHS has similarly initiated coverage pathways for select bioelectronic interventions demonstrating cost-effectiveness in managing inflammatory conditions.
Market segmentation analysis indicates implantable devices currently represent 58% of the bioelectronic medicine market, with non-invasive wearable solutions growing rapidly at 19.2% annually. This shift reflects technological improvements in transcutaneous stimulation efficacy and patient preference for non-surgical options when clinically viable.
Current Bioelectronic Interface Technologies and Barriers
Bioelectronic interfaces have evolved significantly over the past decade, with current technologies spanning a wide spectrum of applications in personalized medicine. Non-invasive interfaces include electroencephalography (EEG), electrocardiography (ECG), and electromyography (EMG) systems that monitor electrical signals from the brain, heart, and muscles respectively. These technologies have been miniaturized and made wireless, enabling continuous health monitoring without restricting patient mobility.
Minimally invasive interfaces have progressed to include microneedle arrays and transdermal patches that can deliver medications and collect biometric data simultaneously. These systems often incorporate closed-loop feedback mechanisms that adjust treatment parameters based on real-time physiological responses, representing a significant advancement in personalized medicine delivery.
Fully implantable bioelectronic interfaces have seen remarkable innovation, with devices such as neural implants for deep brain stimulation, cochlear implants, and retinal prostheses becoming more sophisticated. Recent developments include biodegradable electronics that dissolve after completing their therapeutic function, reducing the need for removal surgeries and associated risks.
Despite these advancements, significant barriers remain in the widespread adoption of bioelectronic interfaces. Biocompatibility issues continue to challenge long-term implantation, with foreign body responses often leading to device encapsulation and reduced functionality over time. Materials science has yet to fully solve the mechanical mismatch between rigid electronic components and soft biological tissues, leading to inflammation and potential tissue damage.
Power management represents another critical challenge, as implantable devices require long-lasting, safe power sources. While wireless power transfer technologies have improved, they still face limitations in transmission efficiency and depth penetration. Battery technologies that are both miniature and long-lasting remain elusive for many applications.
Signal quality and stability present ongoing technical hurdles. Bioelectrical signals are inherently weak and susceptible to noise, requiring sophisticated signal processing algorithms. The dynamic nature of biological systems means that signal characteristics can change over time, necessitating adaptive algorithms that can maintain performance despite these variations.
Regulatory pathways for bioelectronic medical devices remain complex and time-consuming, with stringent requirements for demonstrating both safety and efficacy. This regulatory landscape significantly impacts development timelines and costs, particularly for novel technologies without predicate devices.
Data security and privacy concerns have emerged as critical barriers, especially as bioelectronic interfaces increasingly connect to external systems for data analysis and treatment adjustments. Protecting sensitive health information while maintaining system functionality requires sophisticated encryption and security protocols that add complexity to device design.
Minimally invasive interfaces have progressed to include microneedle arrays and transdermal patches that can deliver medications and collect biometric data simultaneously. These systems often incorporate closed-loop feedback mechanisms that adjust treatment parameters based on real-time physiological responses, representing a significant advancement in personalized medicine delivery.
Fully implantable bioelectronic interfaces have seen remarkable innovation, with devices such as neural implants for deep brain stimulation, cochlear implants, and retinal prostheses becoming more sophisticated. Recent developments include biodegradable electronics that dissolve after completing their therapeutic function, reducing the need for removal surgeries and associated risks.
Despite these advancements, significant barriers remain in the widespread adoption of bioelectronic interfaces. Biocompatibility issues continue to challenge long-term implantation, with foreign body responses often leading to device encapsulation and reduced functionality over time. Materials science has yet to fully solve the mechanical mismatch between rigid electronic components and soft biological tissues, leading to inflammation and potential tissue damage.
Power management represents another critical challenge, as implantable devices require long-lasting, safe power sources. While wireless power transfer technologies have improved, they still face limitations in transmission efficiency and depth penetration. Battery technologies that are both miniature and long-lasting remain elusive for many applications.
Signal quality and stability present ongoing technical hurdles. Bioelectrical signals are inherently weak and susceptible to noise, requiring sophisticated signal processing algorithms. The dynamic nature of biological systems means that signal characteristics can change over time, necessitating adaptive algorithms that can maintain performance despite these variations.
Regulatory pathways for bioelectronic medical devices remain complex and time-consuming, with stringent requirements for demonstrating both safety and efficacy. This regulatory landscape significantly impacts development timelines and costs, particularly for novel technologies without predicate devices.
Data security and privacy concerns have emerged as critical barriers, especially as bioelectronic interfaces increasingly connect to external systems for data analysis and treatment adjustments. Protecting sensitive health information while maintaining system functionality requires sophisticated encryption and security protocols that add complexity to device design.
Contemporary Bioelectronic Solutions for Personalized Treatment
01 Neural-electronic interfaces for biosensing
Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, facilitating applications in neurological diagnostics and brain-computer interfaces.- Neural-electronic interfaces for biosensing: Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, facilitating applications in neurological diagnostics and brain-computer interfaces.
- Implantable bioelectronic devices: Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices feature biocompatible materials and specialized coatings that minimize immune responses and promote stable integration with surrounding tissues. The technology includes power management systems for sustained operation within the body and wireless communication capabilities for data transmission without physical connections, enabling applications in chronic disease management and therapeutic interventions.
- Molecular bioelectronic interfaces: Interfaces that utilize molecular components to bridge the gap between biological systems and electronic devices. These interfaces incorporate biomolecules such as proteins, enzymes, or DNA as functional elements that can interact with both biological processes and electronic signals. The molecular components serve as transducers that convert biological signals into electronic outputs or vice versa, enabling highly specific detection of biological analytes and targeted modulation of cellular functions.
- Flexible and wearable bioelectronic interfaces: Bioelectronic interfaces designed with flexible, stretchable materials that conform to biological tissues for non-invasive or minimally invasive applications. These interfaces incorporate advanced materials such as conductive polymers and elastomers that maintain electrical functionality while accommodating the mechanical properties of biological tissues. The technology enables continuous monitoring of physiological parameters through skin-mounted sensors and provides platforms for delivering therapeutic stimulation in a comfortable, wearable format.
- Nanomaterial-based bioelectronic interfaces: Bioelectronic interfaces that utilize nanomaterials to enhance the connection between biological systems and electronic devices. These interfaces incorporate nanomaterials such as carbon nanotubes, graphene, or nanoparticles that provide improved electrical conductivity, increased surface area, and enhanced biocompatibility. The nanoscale dimensions of these materials enable interactions with biological systems at the cellular or subcellular level, facilitating high-resolution sensing and precise stimulation capabilities for advanced biomedical applications.
02 Implantable bioelectronic devices
Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices feature biocompatible materials and coatings that minimize immune responses and promote stable tissue integration. The technology includes power management systems for sustained operation within the body and wireless communication capabilities for data transmission without invasive connections, enabling applications in chronic disease management and therapeutic interventions.Expand Specific Solutions03 Molecular bioelectronic interfaces
Interfaces that utilize molecular components to bridge the gap between biological systems and electronic devices. These interfaces incorporate biomolecules such as proteins, enzymes, or DNA as functional elements that can interact with both biological processes and electronic signals. The molecular components serve as transducers that convert biological events into measurable electronic signals, enabling highly specific detection of biological analytes and processes at the molecular level.Expand Specific Solutions04 Flexible and wearable bioelectronic interfaces
Bioelectronic interfaces designed with flexible, stretchable materials that conform to biological tissues and body surfaces. These interfaces incorporate advanced materials such as conductive polymers and elastomeric substrates that maintain functionality during movement and deformation. The technology enables non-invasive monitoring of physiological parameters through skin contact, with applications in wearable health monitoring, athletic performance tracking, and personalized medicine.Expand Specific Solutions05 Microfluidic bioelectronic platforms
Integration of microfluidic systems with electronic components to create comprehensive bioelectronic interfaces. These platforms combine controlled fluid handling with electronic sensing to analyze biological samples with high precision. The technology incorporates microfabricated channels, chambers, and electronic sensors that enable manipulation and analysis of small sample volumes, facilitating applications in point-of-care diagnostics, drug screening, and personalized medicine.Expand Specific Solutions
Leading Organizations in Bioelectronic Medicine
The bioelectronic interface market for personalized medicine is currently in its growth phase, characterized by rapid technological advancement and expanding clinical applications. The market is projected to reach significant scale as integration between biological systems and electronic devices becomes more sophisticated. Leading academic institutions like MIT, University of California, and Rice University are driving fundamental research, while established medical technology companies including Johnson & Johnson Vision Care, Abbott Diabetes Care, and Medtronic (via Covidien) are commercializing applications. Emerging players such as Neuralink and Genetesis are accelerating innovation with novel brain-computer interfaces and cardiac monitoring technologies. The technology maturity varies across applications, with glucose monitoring systems reaching commercial maturity while neural interfaces remain largely experimental, indicating substantial growth potential as technical challenges in biocompatibility, power management, and signal processing are overcome.
Massachusetts Institute of Technology
Technical Solution: MIT has developed cutting-edge bioelectronic interface technologies through its Research Laboratory of Electronics, Media Lab, and Institute for Medical Engineering and Science. Their platforms include conformable electronic systems that can intimately interface with biological tissues for both sensing and stimulation. MIT researchers have pioneered hydrogel-based bioelectronic interfaces that bridge the mechanical and chemical mismatch between conventional electronics and biological tissues, enabling stable long-term recording of physiological signals. For personalized medicine applications, MIT has developed systems that can monitor multiple physiological parameters simultaneously and correlate them with individual health states. Their research includes flexible, stretchable electronic systems that can be worn on the skin or implanted within the body, providing continuous monitoring of vital signs, biochemical markers, and electrophysiological signals. MIT's bioelectronic platforms incorporate advanced machine learning algorithms that can identify individual-specific patterns in physiological data and adapt therapeutic interventions accordingly. Their researchers have also developed biodegradable electronics that can be programmed to dissolve after a specific therapeutic window, eliminating the need for removal surgeries and reducing long-term foreign body responses that vary between individuals.
Strengths: World-class expertise in materials science, electronics, and bioengineering; strong focus on translational research with numerous industry partnerships; innovative approaches to solving biocompatibility challenges at the tissue-electronic interface. Weaknesses: Some technologies remain at early research stages with significant translational hurdles; complex regulatory pathway for novel implantable electronic systems; potential high costs limiting accessibility of advanced personalized bioelectronic therapies.
The Regents of the University of California
Technical Solution: The University of California system has developed pioneering bioelectronic interface technologies across multiple campuses. Their research encompasses both invasive and non-invasive bioelectronic platforms for personalized medicine applications. UC Berkeley and UCSF have collaborated on "neural dust" - ultrasmall (millimeter-scale) wireless implantable devices that can record and stimulate neural activity with minimal tissue disruption. These devices use ultrasonic power and communication to eliminate wired connections. UC San Diego has developed wearable chemical sensors that can continuously monitor biomarkers in sweat, tears, and interstitial fluid, providing real-time biochemical information for personalized health monitoring. Their flexible, stretchable electronic platforms incorporate electrochemical sensors capable of detecting multiple analytes simultaneously. For personalized medicine applications, UC researchers have demonstrated closed-loop systems that combine sensing and therapeutic delivery, such as glucose-responsive insulin delivery systems and neural stimulation platforms that adapt to individual brain activity patterns. Their bioelectronic interfaces incorporate advanced materials including conducting polymers, nanomaterials, and biodegradable electronics that can be tailored to specific physiological environments and therapeutic needs.
Strengths: Extensive interdisciplinary collaboration across engineering, medicine, and basic sciences; strong track record of translating research innovations to clinical applications; diverse portfolio of technologies spanning multiple physiological systems and disease states. Weaknesses: Complex intellectual property landscape due to distributed research across multiple campuses; challenges in standardizing technologies developed by different research groups; varying levels of clinical validation across different platforms.
Key Patents and Innovations in Bioelectronic Interfaces
Renewable bioelectronic interface for electrobiocatalytic reactor
PatentInactiveUS10246786B2
Innovation
- A bioelectronic device with a conductive carbon electrode and a bioelectronic interface where the catalytically active material is electrostatically bound, allowing for easy removal and replacement by changing the pH, and a process for reconstituting the interface using aqueous media with specific pH levels to facilitate bonding and regeneration of the interface.
Stretchable Microelectronic Fibers and Their Assemblies as Multifunctional Bioelectronic Interfaces for Whole Organs
PatentPendingUS20230321431A1
Innovation
- The development of soft and stretchable bioelectronic interfaces using elastic microelectronic fibers with liquid metal-filled channels and microelectronic components, integrated into a low-modulus elastomeric substrate, allowing for scalable fabrication and robust mechanical performance while maintaining electrical functionality.
Regulatory Framework for Bioelectronic Medical Devices
The regulatory landscape for bioelectronic medical devices presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies bioelectronic interfaces into three categories based on risk level, with Class III devices requiring the most rigorous premarket approval process due to their direct interaction with critical physiological systems. The FDA's Digital Health Innovation Action Plan specifically addresses software components in bioelectronic medicine, recognizing the unique challenges posed by devices that combine hardware with sophisticated algorithms.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification for bioelectronic interfaces. Notably, the EU framework places greater emphasis on the entire product lifecycle management compared to the US approach.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake Designation System to expedite approval for innovative medical technologies, including certain bioelectronic devices. China's National Medical Products Administration (NMPA) has recently reformed its regulatory framework to accelerate approval processes while maintaining safety standards for emerging bioelectronic technologies.
A critical regulatory consideration specific to bioelectronic interfaces is the management of data privacy and security. As these devices often collect sensitive physiological data for personalized medicine applications, they must comply with regulations such as HIPAA in the US, GDPR in Europe, and various national data protection laws. The integration of artificial intelligence and machine learning algorithms in these devices introduces additional regulatory challenges regarding algorithm transparency and validation.
International harmonization efforts, led by the International Medical Device Regulators Forum (IMDRF), aim to standardize regulatory approaches across borders. Their guidance documents on Software as a Medical Device (SaMD) are particularly relevant for bioelectronic interfaces that incorporate sophisticated software components for personalized medicine applications.
Regulatory pathways for combination products—devices that incorporate medicinal components or biologics—present unique challenges for bioelectronic interfaces designed for drug delivery or tissue regeneration. These products often require coordinated reviews across multiple regulatory divisions, adding complexity to the approval process and potentially extending development timelines.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification for bioelectronic interfaces. Notably, the EU framework places greater emphasis on the entire product lifecycle management compared to the US approach.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake Designation System to expedite approval for innovative medical technologies, including certain bioelectronic devices. China's National Medical Products Administration (NMPA) has recently reformed its regulatory framework to accelerate approval processes while maintaining safety standards for emerging bioelectronic technologies.
A critical regulatory consideration specific to bioelectronic interfaces is the management of data privacy and security. As these devices often collect sensitive physiological data for personalized medicine applications, they must comply with regulations such as HIPAA in the US, GDPR in Europe, and various national data protection laws. The integration of artificial intelligence and machine learning algorithms in these devices introduces additional regulatory challenges regarding algorithm transparency and validation.
International harmonization efforts, led by the International Medical Device Regulators Forum (IMDRF), aim to standardize regulatory approaches across borders. Their guidance documents on Software as a Medical Device (SaMD) are particularly relevant for bioelectronic interfaces that incorporate sophisticated software components for personalized medicine applications.
Regulatory pathways for combination products—devices that incorporate medicinal components or biologics—present unique challenges for bioelectronic interfaces designed for drug delivery or tissue regeneration. These products often require coordinated reviews across multiple regulatory divisions, adding complexity to the approval process and potentially extending development timelines.
Data Security and Patient Privacy Considerations
The integration of bioelectronic interfaces in personalized medicine introduces significant data security and patient privacy challenges that must be addressed comprehensively. As these interfaces collect unprecedented volumes of sensitive biometric and health data, robust encryption protocols become essential. Current industry standards employ end-to-end encryption with 256-bit AES algorithms, complemented by secure key management systems to protect data both in transit and at rest.
Regulatory frameworks worldwide are evolving to address these emerging technologies. The European Union's GDPR establishes strict requirements for health data processing, while the U.S. HIPAA regulations are being adapted to encompass bioelectronic data streams. These frameworks mandate explicit informed consent mechanisms, requiring patients to understand and approve specific data collection, storage, and sharing practices before device implementation.
Authentication mechanisms represent another critical security layer, with multi-factor authentication becoming standard practice. Advanced systems now incorporate biometric verification (fingerprint, retinal scan, or voice recognition) alongside traditional password protection, significantly reducing unauthorized access risks. Some cutting-edge interfaces implement continuous authentication protocols that monitor usage patterns to detect anomalies indicative of security breaches.
De-identification and anonymization techniques are increasingly sophisticated, allowing valuable research data utilization while protecting individual identities. Techniques such as k-anonymity, differential privacy, and homomorphic encryption enable data analysis without compromising patient confidentiality. These approaches facilitate collaborative research while maintaining strict privacy standards.
The distributed nature of bioelectronic data presents unique challenges, as information flows between implanted devices, mobile applications, cloud storage, and healthcare provider systems. Blockchain technology has emerged as a promising solution, creating immutable audit trails of data access and transfer while enabling patients to maintain granular control over their information through smart contracts.
Ethical considerations extend beyond technical solutions, necessitating transparent data governance policies. Leading institutions have established independent ethics committees specifically focused on bioelectronic data management, ensuring that technological capabilities remain balanced with patient rights. These committees develop frameworks for handling incidental findings, secondary data use, and appropriate data retention periods.
As bioelectronic interfaces become more prevalent in personalized medicine, security and privacy considerations will continue evolving. The industry is moving toward "privacy by design" approaches, where protection measures are integrated into devices from conception rather than added retrospectively, establishing a foundation for trustworthy implementation of these transformative technologies.
Regulatory frameworks worldwide are evolving to address these emerging technologies. The European Union's GDPR establishes strict requirements for health data processing, while the U.S. HIPAA regulations are being adapted to encompass bioelectronic data streams. These frameworks mandate explicit informed consent mechanisms, requiring patients to understand and approve specific data collection, storage, and sharing practices before device implementation.
Authentication mechanisms represent another critical security layer, with multi-factor authentication becoming standard practice. Advanced systems now incorporate biometric verification (fingerprint, retinal scan, or voice recognition) alongside traditional password protection, significantly reducing unauthorized access risks. Some cutting-edge interfaces implement continuous authentication protocols that monitor usage patterns to detect anomalies indicative of security breaches.
De-identification and anonymization techniques are increasingly sophisticated, allowing valuable research data utilization while protecting individual identities. Techniques such as k-anonymity, differential privacy, and homomorphic encryption enable data analysis without compromising patient confidentiality. These approaches facilitate collaborative research while maintaining strict privacy standards.
The distributed nature of bioelectronic data presents unique challenges, as information flows between implanted devices, mobile applications, cloud storage, and healthcare provider systems. Blockchain technology has emerged as a promising solution, creating immutable audit trails of data access and transfer while enabling patients to maintain granular control over their information through smart contracts.
Ethical considerations extend beyond technical solutions, necessitating transparent data governance policies. Leading institutions have established independent ethics committees specifically focused on bioelectronic data management, ensuring that technological capabilities remain balanced with patient rights. These committees develop frameworks for handling incidental findings, secondary data use, and appropriate data retention periods.
As bioelectronic interfaces become more prevalent in personalized medicine, security and privacy considerations will continue evolving. The industry is moving toward "privacy by design" approaches, where protection measures are integrated into devices from conception rather than added retrospectively, establishing a foundation for trustworthy implementation of these transformative technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!