Bioelectronic Interfaces in the Context of International Patents
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of this field traces back to the 1780s when Luigi Galvani discovered that electricity could stimulate muscle movement in frogs, establishing the foundation for bioelectricity. However, significant technological advancements only emerged in the mid-20th century with the development of the first implantable cardiac pacemakers in the 1950s, marking the beginning of practical bioelectronic applications.
The 1970s witnessed the emergence of cochlear implants, while the 1990s saw the development of deep brain stimulation technologies for treating neurological disorders. The early 2000s brought miniaturization and wireless capabilities to bioelectronic devices, dramatically expanding their potential applications. Recent years have seen exponential growth in patent filings related to bioelectronic interfaces, reflecting the accelerating pace of innovation in this domain.
Current technological objectives in bioelectronic interfaces focus on several key areas. Improving biocompatibility remains paramount, as long-term integration with biological tissues without adverse reactions continues to challenge researchers. Enhanced signal resolution and sensitivity are critical for applications requiring precise monitoring or stimulation of biological processes. Miniaturization efforts aim to develop increasingly smaller devices that minimize invasiveness while maintaining functionality.
Power management represents another significant objective, with research focused on developing energy-efficient devices and novel power sources, including wireless power transfer and biofuel cells that harvest energy from the body itself. Wireless communication capabilities are being refined to enable seamless data transmission between implanted devices and external systems, crucial for real-time monitoring and adjustment of therapeutic interventions.
The integration of artificial intelligence and machine learning algorithms with bioelectronic interfaces aims to create adaptive systems capable of responding dynamically to changing physiological conditions. This evolution toward "smart" bioelectronic interfaces represents a significant paradigm shift from static to responsive therapeutic approaches.
Looking forward, the field is moving toward closed-loop systems that can both sense biological signals and deliver appropriate responses automatically. The ultimate objective is to develop fully integrated bioelectronic ecosystems that can restore or enhance biological functions across multiple physiological systems simultaneously, potentially revolutionizing treatment approaches for conditions ranging from neurological disorders to cardiovascular diseases and beyond.
The 1970s witnessed the emergence of cochlear implants, while the 1990s saw the development of deep brain stimulation technologies for treating neurological disorders. The early 2000s brought miniaturization and wireless capabilities to bioelectronic devices, dramatically expanding their potential applications. Recent years have seen exponential growth in patent filings related to bioelectronic interfaces, reflecting the accelerating pace of innovation in this domain.
Current technological objectives in bioelectronic interfaces focus on several key areas. Improving biocompatibility remains paramount, as long-term integration with biological tissues without adverse reactions continues to challenge researchers. Enhanced signal resolution and sensitivity are critical for applications requiring precise monitoring or stimulation of biological processes. Miniaturization efforts aim to develop increasingly smaller devices that minimize invasiveness while maintaining functionality.
Power management represents another significant objective, with research focused on developing energy-efficient devices and novel power sources, including wireless power transfer and biofuel cells that harvest energy from the body itself. Wireless communication capabilities are being refined to enable seamless data transmission between implanted devices and external systems, crucial for real-time monitoring and adjustment of therapeutic interventions.
The integration of artificial intelligence and machine learning algorithms with bioelectronic interfaces aims to create adaptive systems capable of responding dynamically to changing physiological conditions. This evolution toward "smart" bioelectronic interfaces represents a significant paradigm shift from static to responsive therapeutic approaches.
Looking forward, the field is moving toward closed-loop systems that can both sense biological signals and deliver appropriate responses automatically. The ultimate objective is to develop fully integrated bioelectronic ecosystems that can restore or enhance biological functions across multiple physiological systems simultaneously, potentially revolutionizing treatment approaches for conditions ranging from neurological disorders to cardiovascular diseases and beyond.
Market Analysis for Bioelectronic Interface Applications
The bioelectronic interfaces market is experiencing robust growth, driven by increasing applications in healthcare, neuroscience, and consumer electronics. Current market valuations place the global bioelectronic medicine sector at approximately 25 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 13.2% through 2030. This growth trajectory is supported by substantial investments from both private and public sectors, with venture capital funding in bioelectronic startups exceeding 4.7 billion USD in the past five years.
Healthcare applications represent the largest market segment, accounting for over 60% of current bioelectronic interface deployments. Within this segment, neuromodulation devices for chronic pain management and neurological disorders lead revenue generation, followed by cochlear implants and retinal prostheses. The therapeutic applications market is expected to maintain dominance due to aging populations and increasing prevalence of chronic conditions in developed economies.
Emerging consumer applications, particularly in human-computer interaction and wellness monitoring, represent the fastest-growing segment with a CAGR of 18.7%. This expansion is fueled by miniaturization of components, improved biocompatibility of materials, and enhanced signal processing capabilities that enable non-invasive or minimally invasive solutions.
Geographically, North America holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the most aggressive growth rate, particularly in China, Japan, and South Korea, where government initiatives supporting bioelectronic research have increased significantly since 2018.
Patent analysis reveals concentrated innovation clusters in the United States (primarily California and Massachusetts), Germany, Japan, and increasingly China. The patent landscape shows a shift from predominantly academic institutions holding foundational patents to corporate entities developing application-specific technologies, indicating market maturation.
Key market barriers include regulatory hurdles, with FDA and CE approval processes averaging 3-5 years for implantable devices, reimbursement challenges in healthcare systems, and technical limitations in long-term biocompatibility and power requirements. These factors have created a bifurcated market where non-invasive consumer applications advance rapidly while therapeutic medical applications progress more deliberately through regulatory pathways.
Customer adoption patterns demonstrate increasing acceptance of bioelectronic solutions, with patient satisfaction rates for implantable neuromodulation devices exceeding 75% in recent clinical studies. This positive reception, coupled with decreasing production costs through manufacturing scale, suggests accelerating market penetration in the coming decade.
Healthcare applications represent the largest market segment, accounting for over 60% of current bioelectronic interface deployments. Within this segment, neuromodulation devices for chronic pain management and neurological disorders lead revenue generation, followed by cochlear implants and retinal prostheses. The therapeutic applications market is expected to maintain dominance due to aging populations and increasing prevalence of chronic conditions in developed economies.
Emerging consumer applications, particularly in human-computer interaction and wellness monitoring, represent the fastest-growing segment with a CAGR of 18.7%. This expansion is fueled by miniaturization of components, improved biocompatibility of materials, and enhanced signal processing capabilities that enable non-invasive or minimally invasive solutions.
Geographically, North America holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the most aggressive growth rate, particularly in China, Japan, and South Korea, where government initiatives supporting bioelectronic research have increased significantly since 2018.
Patent analysis reveals concentrated innovation clusters in the United States (primarily California and Massachusetts), Germany, Japan, and increasingly China. The patent landscape shows a shift from predominantly academic institutions holding foundational patents to corporate entities developing application-specific technologies, indicating market maturation.
Key market barriers include regulatory hurdles, with FDA and CE approval processes averaging 3-5 years for implantable devices, reimbursement challenges in healthcare systems, and technical limitations in long-term biocompatibility and power requirements. These factors have created a bifurcated market where non-invasive consumer applications advance rapidly while therapeutic medical applications progress more deliberately through regulatory pathways.
Customer adoption patterns demonstrate increasing acceptance of bioelectronic solutions, with patient satisfaction rates for implantable neuromodulation devices exceeding 75% in recent clinical studies. This positive reception, coupled with decreasing production costs through manufacturing scale, suggests accelerating market penetration in the coming decade.
Global Bioelectronic Interface Development Status and Barriers
The global bioelectronic interface landscape presents a complex tapestry of technological advancement and regulatory challenges. Currently, North America leads in research output and patent filings, with the United States accounting for approximately 40% of global bioelectronic interface patents. The European Union follows with roughly 25%, while Asia-Pacific countries, particularly Japan, South Korea, and China, collectively represent about 30% of patent activities in this domain.
Key technological barriers persist across multiple dimensions. At the material science level, biocompatibility remains a significant challenge, as long-term implantation often triggers foreign body responses that compromise device functionality. Current electrode materials struggle to maintain stable performance beyond 1-2 years in vivo, necessitating repeated surgical interventions that limit clinical adoption.
Signal processing represents another critical bottleneck. The translation of complex biological signals into meaningful digital information requires sophisticated algorithms capable of filtering physiological noise while preserving essential data. Current systems achieve only 70-85% accuracy in signal interpretation under optimal conditions, with performance degrading significantly in dynamic environments or during physical activity.
Power management constitutes a persistent limitation, with most implantable bioelectronic interfaces requiring either transcutaneous charging or periodic battery replacement. Energy harvesting technologies have demonstrated theoretical promise but currently generate only 10-15% of the power required for continuous operation of comprehensive neural interfaces.
Miniaturization challenges further constrain development, as reducing device dimensions while maintaining functionality necessitates advanced fabrication techniques. Current state-of-the-art interfaces achieve functional densities of approximately 100-200 electrodes per square centimeter, whereas theoretical models suggest requirements of 1000+ electrodes for high-fidelity neural recording and stimulation.
Regulatory frameworks vary significantly across regions, creating a fragmented approval landscape. The FDA's regulatory pathway for bioelectronic interfaces remains evolving, with devices often classified under multiple categories. European MDR implementation has introduced additional compliance requirements, while Asian markets maintain distinct approval processes that necessitate market-specific development strategies.
Standardization efforts remain nascent, with limited consensus on testing protocols, performance metrics, or interoperability standards. The IEEE Working Group on Bioelectronic Interfaces has proposed preliminary standards, but widespread adoption remains limited, hampering cross-platform compatibility and technology transfer between research institutions and commercial entities.
Key technological barriers persist across multiple dimensions. At the material science level, biocompatibility remains a significant challenge, as long-term implantation often triggers foreign body responses that compromise device functionality. Current electrode materials struggle to maintain stable performance beyond 1-2 years in vivo, necessitating repeated surgical interventions that limit clinical adoption.
Signal processing represents another critical bottleneck. The translation of complex biological signals into meaningful digital information requires sophisticated algorithms capable of filtering physiological noise while preserving essential data. Current systems achieve only 70-85% accuracy in signal interpretation under optimal conditions, with performance degrading significantly in dynamic environments or during physical activity.
Power management constitutes a persistent limitation, with most implantable bioelectronic interfaces requiring either transcutaneous charging or periodic battery replacement. Energy harvesting technologies have demonstrated theoretical promise but currently generate only 10-15% of the power required for continuous operation of comprehensive neural interfaces.
Miniaturization challenges further constrain development, as reducing device dimensions while maintaining functionality necessitates advanced fabrication techniques. Current state-of-the-art interfaces achieve functional densities of approximately 100-200 electrodes per square centimeter, whereas theoretical models suggest requirements of 1000+ electrodes for high-fidelity neural recording and stimulation.
Regulatory frameworks vary significantly across regions, creating a fragmented approval landscape. The FDA's regulatory pathway for bioelectronic interfaces remains evolving, with devices often classified under multiple categories. European MDR implementation has introduced additional compliance requirements, while Asian markets maintain distinct approval processes that necessitate market-specific development strategies.
Standardization efforts remain nascent, with limited consensus on testing protocols, performance metrics, or interoperability standards. The IEEE Working Group on Bioelectronic Interfaces has proposed preliminary standards, but widespread adoption remains limited, hampering cross-platform compatibility and technology transfer between research institutions and commercial entities.
Current Bioelectronic Interface Patent Solutions
01 Neural interfaces for bioelectronic applications
Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and immune response.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neural prosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and inflammatory responses.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the complex geometries of biological tissues and organs. These interfaces incorporate elastic materials, serpentine structures, or mesh designs to achieve mechanical compliance while maintaining electronic functionality. Such interfaces can be used for long-term monitoring of physiological signals, as they reduce mechanical mismatch with soft tissues and minimize foreign body responses, leading to improved signal quality and device longevity.
- Biosensing and molecular detection interfaces: Bioelectronic interfaces for biosensing applications incorporate biological recognition elements with electronic transduction mechanisms to detect specific biomolecules or cellular activities. These interfaces may utilize enzymes, antibodies, aptamers, or other biological receptors coupled with electrochemical, optical, or field-effect sensing modalities. Such systems enable rapid, sensitive detection of biomarkers for applications in medical diagnostics, environmental monitoring, and bioprocess control.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices integrate electronic components with biological systems for therapeutic or diagnostic purposes. These devices include neural stimulators, cardiac pacemakers, drug delivery systems, and continuous glucose monitors. Advanced encapsulation techniques and biocompatible materials are employed to protect electronic components from the harsh biological environment while ensuring long-term functionality and minimal immune response. Wireless power transfer and communication capabilities enable external control and data retrieval without transcutaneous connections.
- Nanomaterial-based bioelectronic interfaces: Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles are incorporated into bioelectronic interfaces to enhance their performance. These nanomaterials offer unique electrical, mechanical, and surface properties that improve signal transduction, increase sensitivity, and enable miniaturization of devices. Nanomaterial-based interfaces can be functionalized with biomolecules to increase specificity and biocompatibility, making them suitable for applications ranging from biosensing to tissue engineering and neural interfaces.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic surfaces of biological tissues, providing stable long-term connections. These interfaces utilize elastic materials, serpentine structures, or mesh designs to accommodate movement while maintaining electrical functionality. Such interfaces are particularly valuable for applications requiring integration with soft tissues or organs that undergo regular movement, such as skin, heart, or brain tissue.Expand Specific Solutions03 Biosensing and molecular detection interfaces
Bioelectronic interfaces for biosensing applications incorporate biological recognition elements with electronic transduction mechanisms to detect specific biomolecules, pathogens, or physiological parameters. These systems may utilize enzymes, antibodies, nucleic acids, or other biomolecules as recognition elements coupled with electrochemical, optical, or mechanical transducers. Such interfaces enable rapid, sensitive detection for applications in medical diagnostics, environmental monitoring, and biodefense.Expand Specific Solutions04 Implantable bioelectronic medical devices
Implantable bioelectronic interfaces are designed for long-term integration within the body to monitor health parameters or deliver therapeutic interventions. These devices incorporate biocompatible materials, hermetic packaging, and wireless communication capabilities to ensure functionality while minimizing foreign body response. Applications include cardiac pacemakers, neural stimulators, glucose monitors, and drug delivery systems that can operate autonomously within the body for extended periods.Expand Specific Solutions05 Nanomaterial-based bioelectronic interfaces
Nanomaterials such as carbon nanotubes, graphene, quantum dots, and metal nanoparticles are incorporated into bioelectronic interfaces to enhance performance characteristics. These materials offer advantages including increased surface area, improved electrical conductivity, enhanced sensitivity, and unique optical properties. Nanomaterial-based interfaces enable miniaturization of devices while improving signal-to-noise ratios and enabling novel functionalities such as targeted drug delivery or photothermal therapy.Expand Specific Solutions
Leading Companies and Research Institutions in Bioelectronics
Bioelectronic interfaces represent an emerging field at the intersection of biology and electronics, currently in its early growth phase. The market is expanding rapidly, projected to reach approximately $25 billion by 2030, driven by healthcare applications and consumer wearables. Technologically, the field shows varying maturity levels across different applications. Leading players include established technology giants like Apple, Samsung, and Huawei focusing on consumer applications; healthcare specialists such as Edwards Lifesciences and Roche Diagnostics developing medical implementations; and research institutions like University of Michigan and Harvard College advancing fundamental science. Academic-industry partnerships are accelerating innovation, with companies like NextMind and X-Body representing specialized startups bringing novel approaches to market. The competitive landscape reflects a diverse ecosystem where cross-sector collaboration is increasingly important for technological advancement.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed innovative bioelectronic interfaces focusing on minimally invasive neural probes and implantable biosensors. Their patented technologies include ultra-small, high-density electrode arrays manufactured using advanced microfabrication techniques that minimize tissue damage while maximizing recording capabilities. Michigan's neural interfaces feature unique shuttle delivery systems that allow precise placement of flexible probes deep within neural tissue without buckling during insertion. Their patents cover specialized coating technologies that incorporate anti-inflammatory and neurotrophic factors to improve long-term biocompatibility and reduce glial scarring around implanted devices. Michigan researchers have also pioneered closed-loop neural interface systems that can simultaneously record neural activity and deliver targeted electrical or pharmacological stimulation based on detected signals. Additionally, they've developed novel wireless power harvesting systems specifically designed for neural implants that can operate at extremely low power levels, extending device lifetime for chronic applications.
Strengths: Exceptional miniaturization capabilities; innovative delivery mechanisms for flexible probes; advanced bioactive coatings that improve long-term performance; integrated sensing and stimulation capabilities. Weaknesses: Potential limitations in recording channel count compared to larger arrays; challenges in maintaining stable recordings during micromotion; complex fabrication processes may increase production costs.
NextMind SAS
Technical Solution: NextMind has developed non-invasive bioelectronic interface technology focused on real-time brain-computer interfaces for consumer applications. Their patented system utilizes advanced dry electrodes that can acquire high-quality EEG signals without conductive gel, significantly improving user experience and reducing setup time. NextMind's technology incorporates proprietary signal processing algorithms that can extract meaningful neural signals even in noisy environments, enabling reliable operation outside laboratory settings. Their patents cover specialized machine learning approaches that decode visual attention directly from neural signals, allowing users to control digital interfaces through visual focus alone. The company has developed compact, wearable hardware that integrates electrodes, amplification, digitization, and wireless transmission in a single device that can be easily attached to the back of the head. Additionally, NextMind has created a comprehensive software development kit that allows third-party developers to integrate brain-computer interface capabilities into various applications, from gaming to productivity tools, expanding the potential use cases for their technology.
Strengths: Highly accessible non-invasive approach; user-friendly design requiring minimal setup; real-time decoding capabilities suitable for interactive applications; comprehensive developer ecosystem. Weaknesses: Limited spatial resolution compared to invasive approaches; susceptibility to environmental electrical noise; potential challenges in signal quality across different users with varying head shapes and hair types.
Key Patent Analysis and Technical Innovations
Renewable bioelectronic interface for electrobiocatalytic reactor
PatentInactiveUS10246786B2
Innovation
- A bioelectronic device with a conductive carbon electrode and a bioelectronic interface where the catalytically active material is electrostatically bound, allowing for easy removal and replacement by changing the pH, and a process for reconstituting the interface using aqueous media with specific pH levels to facilitate bonding and regeneration of the interface.
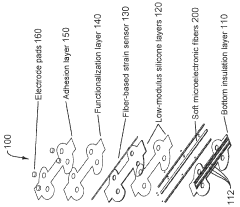
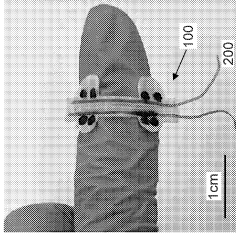
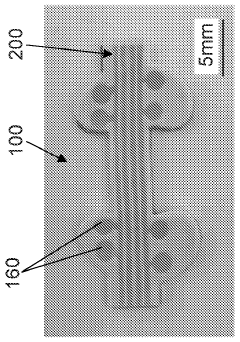
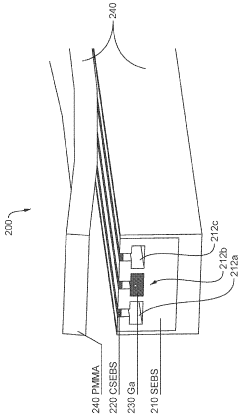
Stretchable microelectronic fibers and assemblies as multifunctional bioelectronic interfaces for whole organs
PatentWO2023201131A1
Innovation
- Development of soft and stretchable bioelectronic interfaces using elastic microelectronic fibers with liquid metal conductors and integrated microelectronic components, such as micro LEDs and sensors, which are fabricated through thermal drawing and integrated into a low-modulus elastomeric substrate for scalable, multifunctional bioelectronic therapies.
International Patent Law and Cross-Border Protection
The global landscape of bioelectronic interface patents presents significant challenges in cross-border protection. Patent laws vary considerably across jurisdictions, creating a complex environment for innovators seeking international protection. The Patent Cooperation Treaty (PCT) offers a streamlined application process for multiple countries, but substantive examination still occurs at the national level, where different standards may apply to bioelectronic innovations.
In major bioelectronic markets, protection strategies must account for regional differences. The United States Patent and Trademark Office (USPTO) generally provides broader protection for bioelectronic interfaces, particularly following the 2013 America Invents Act which shifted to a first-to-file system. The European Patent Office (EPO) maintains stricter requirements regarding medical applications, often requiring more extensive clinical validation data.
Asian markets present distinct challenges: China has strengthened its intellectual property regime but enforcement remains inconsistent; Japan employs stringent examination standards for bioelectronic patents; South Korea has emerged as a strategic filing location due to its growing bioelectronics industry.
Bioelectronic interfaces face unique cross-border protection issues due to their interdisciplinary nature. Patent examiners in different countries may classify these inventions differently—as medical devices, electronic components, or biotechnology—leading to inconsistent examination standards. This classification variability significantly impacts protection scope and enforcement capabilities.
Term harmonization represents another critical challenge. While most jurisdictions offer 20-year protection from filing date, the effective commercial protection period for bioelectronic interfaces is often shortened by regulatory approval processes. Some regions offer patent term extensions for medical technologies, but eligibility criteria and extension periods vary substantially.
Strategic international filing requires careful consideration of market potential, manufacturing locations, and competitor activity. Companies must balance comprehensive protection against prohibitive costs of maintaining patents across multiple jurisdictions. For bioelectronic interfaces specifically, protection strategies often prioritize markets with advanced healthcare systems, strong electronics manufacturing capabilities, and robust enforcement mechanisms.
In major bioelectronic markets, protection strategies must account for regional differences. The United States Patent and Trademark Office (USPTO) generally provides broader protection for bioelectronic interfaces, particularly following the 2013 America Invents Act which shifted to a first-to-file system. The European Patent Office (EPO) maintains stricter requirements regarding medical applications, often requiring more extensive clinical validation data.
Asian markets present distinct challenges: China has strengthened its intellectual property regime but enforcement remains inconsistent; Japan employs stringent examination standards for bioelectronic patents; South Korea has emerged as a strategic filing location due to its growing bioelectronics industry.
Bioelectronic interfaces face unique cross-border protection issues due to their interdisciplinary nature. Patent examiners in different countries may classify these inventions differently—as medical devices, electronic components, or biotechnology—leading to inconsistent examination standards. This classification variability significantly impacts protection scope and enforcement capabilities.
Term harmonization represents another critical challenge. While most jurisdictions offer 20-year protection from filing date, the effective commercial protection period for bioelectronic interfaces is often shortened by regulatory approval processes. Some regions offer patent term extensions for medical technologies, but eligibility criteria and extension periods vary substantially.
Strategic international filing requires careful consideration of market potential, manufacturing locations, and competitor activity. Companies must balance comprehensive protection against prohibitive costs of maintaining patents across multiple jurisdictions. For bioelectronic interfaces specifically, protection strategies often prioritize markets with advanced healthcare systems, strong electronics manufacturing capabilities, and robust enforcement mechanisms.
Bioethical Considerations and Regulatory Compliance
The rapid advancement of bioelectronic interfaces has raised significant ethical concerns and regulatory challenges that must be addressed to ensure responsible innovation. Patient autonomy represents a fundamental bioethical principle that requires careful consideration in the development and implementation of bioelectronic devices. Informed consent processes must be robust, ensuring that patients fully understand the implications of having electronic devices integrated with their biological systems, particularly for implantable technologies that may collect sensitive health data or modify physiological functions.
Privacy and data security present another critical dimension of bioethical concern. Bioelectronic interfaces often generate continuous streams of highly personal health information, raising questions about data ownership, storage security, and potential unauthorized access. International patent frameworks must incorporate provisions that protect patient data while enabling necessary innovation, creating a delicate balance between intellectual property rights and individual privacy protections.
The regulatory landscape for bioelectronic interfaces varies significantly across jurisdictions, creating challenges for global development and deployment. The European Union's Medical Device Regulation (MDR) and the U.S. Food and Drug Administration (FDA) regulatory frameworks represent two distinct approaches to bioelectronic device approval, with different requirements for clinical evidence, risk assessment, and post-market surveillance. Patent applications must navigate these diverse regulatory environments, often requiring strategic adaptations to meet varying compliance standards.
Risk-benefit assessment methodologies constitute another crucial aspect of bioethical consideration. The potential therapeutic benefits of bioelectronic interfaces must be weighed against possible risks, including device malfunction, biological rejection, or unintended physiological effects. International patent applications increasingly require robust evidence of such assessments, reflecting growing regulatory emphasis on safety and efficacy demonstration prior to market approval.
Equitable access to bioelectronic technologies represents a significant ethical challenge that intersects with patent protection. While patents incentivize innovation by providing temporary market exclusivity, they can also create barriers to access, particularly in resource-limited settings. Various mechanisms, including compulsory licensing provisions and humanitarian use exemptions, have emerged within international patent frameworks to address these tensions, though their implementation remains inconsistent across global markets.
Looking forward, harmonization of bioethical standards and regulatory requirements across international boundaries represents a critical need for advancing bioelectronic interface technologies. Patent systems will likely evolve to incorporate more explicit ethical considerations, potentially including mandatory ethics impact assessments as part of the application process for bioelectronic interface technologies.
Privacy and data security present another critical dimension of bioethical concern. Bioelectronic interfaces often generate continuous streams of highly personal health information, raising questions about data ownership, storage security, and potential unauthorized access. International patent frameworks must incorporate provisions that protect patient data while enabling necessary innovation, creating a delicate balance between intellectual property rights and individual privacy protections.
The regulatory landscape for bioelectronic interfaces varies significantly across jurisdictions, creating challenges for global development and deployment. The European Union's Medical Device Regulation (MDR) and the U.S. Food and Drug Administration (FDA) regulatory frameworks represent two distinct approaches to bioelectronic device approval, with different requirements for clinical evidence, risk assessment, and post-market surveillance. Patent applications must navigate these diverse regulatory environments, often requiring strategic adaptations to meet varying compliance standards.
Risk-benefit assessment methodologies constitute another crucial aspect of bioethical consideration. The potential therapeutic benefits of bioelectronic interfaces must be weighed against possible risks, including device malfunction, biological rejection, or unintended physiological effects. International patent applications increasingly require robust evidence of such assessments, reflecting growing regulatory emphasis on safety and efficacy demonstration prior to market approval.
Equitable access to bioelectronic technologies represents a significant ethical challenge that intersects with patent protection. While patents incentivize innovation by providing temporary market exclusivity, they can also create barriers to access, particularly in resource-limited settings. Various mechanisms, including compulsory licensing provisions and humanitarian use exemptions, have emerged within international patent frameworks to address these tensions, though their implementation remains inconsistent across global markets.
Looking forward, harmonization of bioethical standards and regulatory requirements across international boundaries represents a critical need for advancing bioelectronic interface technologies. Patent systems will likely evolve to incorporate more explicit ethical considerations, potentially including mandatory ethics impact assessments as part of the application process for bioelectronic interface technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!