Comparative Analysis of Bioelectronic Interfaces in Artificial Organs
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a critical technological frontier in the development of artificial organs, marking the intersection between electronic systems and biological tissues. The evolution of these interfaces has been characterized by significant advancements over the past three decades, transitioning from rudimentary electrode-based systems to sophisticated biocompatible materials that facilitate seamless integration with human physiology.
The initial development phase in the 1990s focused primarily on basic electrode designs utilizing noble metals such as platinum and gold, which offered reasonable conductivity but limited biocompatibility. These early interfaces faced substantial challenges including tissue inflammation, signal degradation, and limited longevity due to biofouling and encapsulation processes.
A paradigm shift occurred in the early 2000s with the introduction of conductive polymers and carbon-based materials, including polypyrrole (PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT). These materials significantly improved charge transfer efficiency while reducing the mechanical mismatch between electronic components and soft biological tissues, addressing a fundamental limitation of earlier rigid metal electrodes.
The past decade has witnessed remarkable progress in nanomaterial-based interfaces, incorporating graphene, carbon nanotubes, and various nanocomposites. These advanced materials offer exceptional electrical properties while providing topographical features that promote cellular adhesion and tissue integration. Concurrently, the development of hydrogel-based interfaces has further enhanced biocompatibility by mimicking the extracellular matrix environment.
Current research objectives in bioelectronic interfaces for artificial organs are multifaceted, focusing on several critical areas. Foremost is the development of self-healing materials capable of maintaining functional integrity despite mechanical stress and biological degradation processes. This capability is essential for long-term implantable devices that must operate reliably within dynamic biological environments.
Another key objective involves the creation of bidirectional communication systems that not only stimulate biological tissues but also accurately sense and interpret biological signals. This feedback mechanism is crucial for developing responsive artificial organs that can adapt to changing physiological conditions and patient needs.
The field is also moving toward wireless power and data transmission technologies to eliminate transcutaneous wiring, thereby reducing infection risks and improving patient mobility and quality of life. Additionally, researchers are exploring bioresorbable electronics that can perform necessary functions during critical healing periods before safely degrading, eliminating the need for removal surgeries.
The ultimate technological goal remains the development of fully integrated, biocompatible interfaces that seamlessly connect artificial organs with the human nervous system, enabling natural control and sensory feedback comparable to native organs.
The initial development phase in the 1990s focused primarily on basic electrode designs utilizing noble metals such as platinum and gold, which offered reasonable conductivity but limited biocompatibility. These early interfaces faced substantial challenges including tissue inflammation, signal degradation, and limited longevity due to biofouling and encapsulation processes.
A paradigm shift occurred in the early 2000s with the introduction of conductive polymers and carbon-based materials, including polypyrrole (PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT). These materials significantly improved charge transfer efficiency while reducing the mechanical mismatch between electronic components and soft biological tissues, addressing a fundamental limitation of earlier rigid metal electrodes.
The past decade has witnessed remarkable progress in nanomaterial-based interfaces, incorporating graphene, carbon nanotubes, and various nanocomposites. These advanced materials offer exceptional electrical properties while providing topographical features that promote cellular adhesion and tissue integration. Concurrently, the development of hydrogel-based interfaces has further enhanced biocompatibility by mimicking the extracellular matrix environment.
Current research objectives in bioelectronic interfaces for artificial organs are multifaceted, focusing on several critical areas. Foremost is the development of self-healing materials capable of maintaining functional integrity despite mechanical stress and biological degradation processes. This capability is essential for long-term implantable devices that must operate reliably within dynamic biological environments.
Another key objective involves the creation of bidirectional communication systems that not only stimulate biological tissues but also accurately sense and interpret biological signals. This feedback mechanism is crucial for developing responsive artificial organs that can adapt to changing physiological conditions and patient needs.
The field is also moving toward wireless power and data transmission technologies to eliminate transcutaneous wiring, thereby reducing infection risks and improving patient mobility and quality of life. Additionally, researchers are exploring bioresorbable electronics that can perform necessary functions during critical healing periods before safely degrading, eliminating the need for removal surgeries.
The ultimate technological goal remains the development of fully integrated, biocompatible interfaces that seamlessly connect artificial organs with the human nervous system, enabling natural control and sensory feedback comparable to native organs.
Market Analysis of Artificial Organ Technologies
The global artificial organ market is experiencing significant growth, valued at approximately $26.5 billion in 2022 and projected to reach $44.2 billion by 2030, representing a compound annual growth rate (CAGR) of 6.7%. This expansion is primarily driven by the increasing prevalence of chronic diseases, organ failure cases, and the persistent shortage of donor organs worldwide. In 2022 alone, over 100,000 patients were on organ transplant waiting lists in the United States, with only about 30% receiving transplants.
Bioelectronic interfaces represent a critical component within the artificial organ technology landscape, serving as the communication bridge between electronic devices and biological systems. The market for these interfaces is growing at an accelerated rate of 9.3% annually, outpacing the broader artificial organ market, indicating their increasing importance in next-generation medical devices.
Regionally, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth trajectory with a 10.1% CAGR, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced medical technologies in countries like China, Japan, and South Korea.
By application segment, artificial hearts and cardiac assist devices represent the largest market share (34%), followed by artificial kidneys (28%), artificial pancreas (15%), and artificial lungs (12%). The remaining 11% encompasses other artificial organs including liver, cornea, and skin. This distribution reflects both the prevalence of cardiovascular and renal diseases globally and the technological maturity of these specific artificial organ systems.
Consumer demand is increasingly focused on biocompatible interfaces that minimize rejection and infection risks while maximizing device longevity. Market research indicates that 78% of healthcare providers prioritize long-term biocompatibility when selecting artificial organ technologies, while 65% emphasize the importance of reduced immunosuppression requirements.
Key market drivers include technological advancements in materials science, miniaturization of electronic components, improved power management systems, and enhanced data processing capabilities. Additionally, favorable reimbursement policies in developed countries and increasing private and public funding for artificial organ research are accelerating market growth.
Challenges limiting market expansion include high development and manufacturing costs, stringent regulatory approval processes, and concerns regarding long-term reliability. The average development timeline for new artificial organ technologies incorporating advanced bioelectronic interfaces spans 7-10 years, with regulatory approval adding an additional 2-4 years before commercialization.
Bioelectronic interfaces represent a critical component within the artificial organ technology landscape, serving as the communication bridge between electronic devices and biological systems. The market for these interfaces is growing at an accelerated rate of 9.3% annually, outpacing the broader artificial organ market, indicating their increasing importance in next-generation medical devices.
Regionally, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth trajectory with a 10.1% CAGR, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced medical technologies in countries like China, Japan, and South Korea.
By application segment, artificial hearts and cardiac assist devices represent the largest market share (34%), followed by artificial kidneys (28%), artificial pancreas (15%), and artificial lungs (12%). The remaining 11% encompasses other artificial organs including liver, cornea, and skin. This distribution reflects both the prevalence of cardiovascular and renal diseases globally and the technological maturity of these specific artificial organ systems.
Consumer demand is increasingly focused on biocompatible interfaces that minimize rejection and infection risks while maximizing device longevity. Market research indicates that 78% of healthcare providers prioritize long-term biocompatibility when selecting artificial organ technologies, while 65% emphasize the importance of reduced immunosuppression requirements.
Key market drivers include technological advancements in materials science, miniaturization of electronic components, improved power management systems, and enhanced data processing capabilities. Additionally, favorable reimbursement policies in developed countries and increasing private and public funding for artificial organ research are accelerating market growth.
Challenges limiting market expansion include high development and manufacturing costs, stringent regulatory approval processes, and concerns regarding long-term reliability. The average development timeline for new artificial organ technologies incorporating advanced bioelectronic interfaces spans 7-10 years, with regulatory approval adding an additional 2-4 years before commercialization.
Current Bioelectronic Interface Challenges
Despite significant advancements in bioelectronic interfaces for artificial organs, several critical challenges continue to impede optimal integration and functionality. The biocompatibility of materials remains a primary concern, as long-term implantation often triggers foreign body responses, inflammation, and fibrous encapsulation that deteriorate signal quality and device performance. Current materials struggle to maintain stable performance while minimizing tissue damage and immune responses over extended periods.
Signal fidelity presents another substantial challenge, particularly in maintaining consistent electrical conductivity at the tissue-electrode interface. Environmental factors such as protein adsorption, cellular migration, and micromotion can significantly degrade signal quality over time. The signal-to-noise ratio deterioration remains problematic for precise monitoring and control of artificial organ functions, especially in dynamic physiological environments.
Power management continues to be a limiting factor in bioelectronic interface development. Existing power solutions either require transcutaneous connections that increase infection risk or rely on implantable batteries with limited lifespans. Wireless power transfer technologies show promise but face efficiency challenges when transmitting through biological tissues, and energy harvesting approaches currently yield insufficient power for many artificial organ applications.
Miniaturization while maintaining functionality represents another significant hurdle. As artificial organs become more sophisticated, the corresponding bioelectronic interfaces must accommodate increasing numbers of sensors and stimulators without expanding in size. Current microfabrication techniques struggle to achieve the necessary density of functional elements while ensuring reliable operation and durability.
Data processing capabilities present limitations in real-time analysis and response. The volume and complexity of physiological data generated by artificial organs often exceed the processing capacity of implantable systems. Edge computing solutions remain constrained by power limitations and heat dissipation concerns, while cloud-based processing introduces latency issues that may be unacceptable for critical organ functions.
Regulatory and standardization frameworks have not kept pace with technological advancements. The lack of standardized testing protocols and performance metrics for bioelectronic interfaces complicates comparative analysis and slows clinical translation. Regulatory pathways remain unclear for novel interface technologies, particularly those incorporating adaptive or learning capabilities.
Addressing these interconnected challenges requires multidisciplinary approaches that span materials science, electrical engineering, data analytics, and regulatory science to develop the next generation of bioelectronic interfaces capable of supporting increasingly sophisticated artificial organ systems.
Signal fidelity presents another substantial challenge, particularly in maintaining consistent electrical conductivity at the tissue-electrode interface. Environmental factors such as protein adsorption, cellular migration, and micromotion can significantly degrade signal quality over time. The signal-to-noise ratio deterioration remains problematic for precise monitoring and control of artificial organ functions, especially in dynamic physiological environments.
Power management continues to be a limiting factor in bioelectronic interface development. Existing power solutions either require transcutaneous connections that increase infection risk or rely on implantable batteries with limited lifespans. Wireless power transfer technologies show promise but face efficiency challenges when transmitting through biological tissues, and energy harvesting approaches currently yield insufficient power for many artificial organ applications.
Miniaturization while maintaining functionality represents another significant hurdle. As artificial organs become more sophisticated, the corresponding bioelectronic interfaces must accommodate increasing numbers of sensors and stimulators without expanding in size. Current microfabrication techniques struggle to achieve the necessary density of functional elements while ensuring reliable operation and durability.
Data processing capabilities present limitations in real-time analysis and response. The volume and complexity of physiological data generated by artificial organs often exceed the processing capacity of implantable systems. Edge computing solutions remain constrained by power limitations and heat dissipation concerns, while cloud-based processing introduces latency issues that may be unacceptable for critical organ functions.
Regulatory and standardization frameworks have not kept pace with technological advancements. The lack of standardized testing protocols and performance metrics for bioelectronic interfaces complicates comparative analysis and slows clinical translation. Regulatory pathways remain unclear for novel interface technologies, particularly those incorporating adaptive or learning capabilities.
Addressing these interconnected challenges requires multidisciplinary approaches that span materials science, electrical engineering, data analytics, and regulatory science to develop the next generation of bioelectronic interfaces capable of supporting increasingly sophisticated artificial organ systems.
Current Bioelectronic Integration Solutions
01 Neural-electronic interfaces for biomedical applications
Neural-electronic interfaces enable direct communication between biological neural systems and electronic devices. These interfaces can be used for various biomedical applications, including neural prosthetics, brain-computer interfaces, and treatment of neurological disorders. The technology typically involves electrodes or sensor arrays that can record neural activity or stimulate neural tissue, facilitating bidirectional communication between biological systems and electronic devices.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neural tissue, or both. They are crucial for applications such as brain-computer interfaces, neural prosthetics, and neuromodulation therapies. Advanced materials and fabrication techniques are employed to create biocompatible interfaces that minimize tissue damage and maintain long-term functionality.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces utilize materials and structures that can bend, stretch, and adapt to tissue movement while maintaining electrical functionality. This approach reduces mechanical mismatch between rigid electronics and soft tissues, minimizing inflammation and improving long-term performance. Applications include wearable health monitors, implantable sensors, and conformable neural interfaces.
- Biosensing interfaces for molecular detection: Biosensing interfaces enable the detection and quantification of biological molecules through electronic transduction mechanisms. These interfaces incorporate recognition elements such as antibodies, enzymes, or nucleic acids that specifically bind to target analytes. The binding events are converted into measurable electronic signals through various transduction methods. Applications include point-of-care diagnostics, environmental monitoring, and continuous health monitoring systems.
- Nanomaterial-based bioelectronic interfaces: Nanomaterials offer unique properties for bioelectronic interfaces, including high surface-to-volume ratios, tunable electronic properties, and dimensions comparable to biological entities. Materials such as carbon nanotubes, graphene, and metal nanoparticles are incorporated into bioelectronic interfaces to enhance sensitivity, improve signal transduction, and enable novel functionalities. These nanomaterial-based interfaces find applications in biosensing, neural recording, and cellular stimulation.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices integrate electronic components with biological systems for therapeutic or diagnostic purposes. These devices include neural stimulators, drug delivery systems, and physiological monitors that can be implanted within the body for extended periods. Advanced materials, hermetic packaging, and wireless communication capabilities are employed to ensure biocompatibility, longevity, and functionality in the physiological environment.
02 Flexible and implantable bioelectronic devices
Flexible and implantable bioelectronic devices are designed to interface with biological tissues with minimal invasiveness and mechanical mismatch. These devices incorporate flexible substrates, stretchable electronics, and biocompatible materials to ensure long-term stability and functionality within the body. Applications include continuous health monitoring, targeted drug delivery, and therapeutic stimulation of tissues and organs.Expand Specific Solutions03 Biosensors and molecular detection interfaces
Bioelectronic interfaces for molecular detection utilize various sensing mechanisms to detect biological molecules, pathogens, or physiological parameters. These biosensors often incorporate nanomaterials, functionalized surfaces, or biological recognition elements to achieve high sensitivity and specificity. The technology enables real-time monitoring of biomarkers, environmental contaminants, or disease indicators through direct electronic readout of biological interactions.Expand Specific Solutions04 Organic and conductive polymer interfaces
Organic and conductive polymer materials are used to create biocompatible electronic interfaces that bridge the gap between conventional rigid electronics and soft biological tissues. These materials offer advantages including flexibility, biocompatibility, and tunable electrical properties. Applications include organic electrochemical transistors, conductive scaffolds for tissue engineering, and polymer-based neural interfaces that minimize foreign body responses.Expand Specific Solutions05 Wireless and energy harvesting bioelectronic systems
Wireless bioelectronic interfaces eliminate the need for transcutaneous connections, reducing infection risk and improving patient mobility. These systems incorporate wireless power transfer, energy harvesting from biological sources, and low-power communication protocols. The technology enables remote monitoring, data transmission, and control of implanted devices without physical connections, enhancing the longevity and functionality of bioelectronic interfaces.Expand Specific Solutions
Leading Artificial Organ Manufacturers
The bioelectronic interfaces market for artificial organs is currently in a growth phase, characterized by significant academic-industry collaboration. The market is projected to expand substantially due to increasing organ failure cases and transplantation needs. Technology maturity varies across applications, with established players like Medtronic and Abbott Cardiovascular Systems leading in cardiac applications, while newer entrants like TransMedics are innovating in organ preservation systems. Academic institutions (MIT, Harvard, Michigan) are driving fundamental research, while companies like Cochlear and MED-EL dominate neural interfaces. Emerging players include Google and IBM, leveraging AI capabilities to enhance bioelectronic systems. The field is witnessing convergence of materials science, electronics, and biotechnology, with significant investment in miniaturization and biocompatibility.
Massachusetts Institute of Technology
Technical Solution: MIT has developed revolutionary bioelectronic interfaces for artificial organs through their Research Laboratory of Electronics and Institute for Medical Engineering and Science. Their approach focuses on nanomaterial-based interfaces that minimize the mechanical and biological mismatch between electronic components and living tissues. MIT researchers have pioneered the use of carbon nanotube fiber electrodes that exhibit exceptional electrical properties while maintaining flexibility comparable to neural tissue. Their interfaces incorporate specialized coatings including conducting polymers and bioactive molecules that promote tissue integration while maintaining excellent charge transfer characteristics. MIT has developed innovative fabrication techniques for creating 3D electrode arrays that can penetrate tissue volumes with minimal damage, enabling more comprehensive monitoring and stimulation capabilities. Their recent work includes "tissue-like" electronics that can be injected as a liquid suspension and self-assemble into functional networks within target tissues. MIT researchers have also created wireless, biodegradable sensors that can monitor tissue health during critical healing periods and then harmlessly dissolve once their function is complete, eliminating the need for removal surgeries.
Strengths: World-leading expertise in materials science and nanofabrication; strong interdisciplinary approach combining engineering, biology, and medicine; innovative solutions for fundamental biocompatibility challenges. Weaknesses: Many technologies remain in early research stages; complex fabrication requirements may limit commercial translation; academic focus sometimes prioritizes novelty over practical implementation.
President & Fellows of Harvard College
Technical Solution: Harvard's research teams have developed groundbreaking bioelectronic interfaces for artificial organs through their Wyss Institute and School of Engineering. Their approach centers on biomimetic materials that closely resemble native tissue properties, reducing foreign body responses and improving long-term stability. Harvard researchers have pioneered stretchable electronics using serpentine metal traces embedded in elastomeric substrates, creating interfaces that match the mechanical properties of target tissues. Their "e-scaffolds" technology combines traditional tissue engineering scaffolds with embedded electronic components, allowing simultaneous tissue regeneration and functional monitoring/stimulation. Harvard has also developed innovative hydrogel-based interfaces that provide a seamless transition between rigid electronics and soft tissues, significantly reducing inflammatory responses at the tissue-electrode interface. Their recent work includes ultra-thin, mesh-like electronic interfaces that can be injected through minimally invasive procedures and unfold within target tissues. These interfaces incorporate multiplexed sensor arrays capable of monitoring multiple physiological parameters simultaneously, including pH, oxygen levels, temperature, and electrical activity.
Strengths: Cutting-edge materials science expertise; strong interdisciplinary collaboration between engineering, medicine, and biology; access to substantial research funding and resources. Weaknesses: Technologies often remain in pre-clinical stages; complex manufacturing requirements may limit scalability; academic focus sometimes prioritizes novelty over practical implementation considerations.
Key Patents in Bioelectronic Interfaces
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
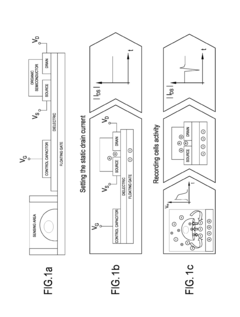
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
Renewable bioelectronic interface for electrobiocatalytic reactor
PatentInactiveUS20160326658A1
Innovation
- A bioelectronic device with a conductive carbon electrode and a bioelectronic interface where the catalytically active material is electrostatically bound, allowing for easy removal and replacement, and a process for reconstituting the interface using pH-dependent bonding and sequential immersion in solutions with soluble reactants, enabling renewable and efficient electron transfer.
Biocompatibility and Safety Standards
Biocompatibility and safety standards for bioelectronic interfaces in artificial organs have evolved significantly over the past decade, establishing crucial frameworks for clinical implementation. The ISO 10993 series remains the cornerstone for evaluating biological responses to medical devices, with specific extensions addressing the unique challenges of bioelectronic components. These standards emphasize comprehensive testing protocols for cytotoxicity, sensitization, irritation, and systemic toxicity when electronic components interface with biological tissues.
The FDA's guidance on implantable electronic devices has recently expanded to include specific provisions for bioelectronic interfaces, requiring rigorous documentation of long-term stability and degradation profiles. European MDR regulations have similarly strengthened requirements for bioelectronic implants, particularly regarding leachable compounds from electrode materials and insulation components that may cause adverse biological responses.
Material selection for bioelectronic interfaces presents unique challenges at the intersection of electronic functionality and biological compatibility. Noble metals like platinum and iridium oxide have demonstrated superior long-term stability in physiological environments, while newer carbon-based materials including graphene and carbon nanotubes show promising biocompatibility profiles with enhanced electrical properties. Polymer coatings such as Parylene-C and polyimide serve as critical insulation materials, though their long-term degradation characteristics remain under investigation.
Safety testing methodologies have advanced beyond traditional in vitro assays to include specialized protocols for electroactive materials. Accelerated aging studies simulating years of implantation under electrical stimulation conditions have become standard practice, with particular attention to electrochemical stability and corrosion resistance. Microelectrode array (MEA) testing platforms now enable real-time monitoring of cellular responses to electrical stimulation, providing crucial data on the biocompatibility of active interfaces.
International harmonization efforts through organizations like IMDRF (International Medical Device Regulators Forum) are working to standardize biocompatibility requirements specifically for bioelectronic interfaces. These initiatives aim to establish consistent safety thresholds for charge injection limits, electrochemical stability, and foreign body responses across global regulatory frameworks.
Recent developments in safety standards increasingly focus on the unique challenges of adaptive bioelectronic systems that modify their properties over time. This includes establishing safety parameters for self-calibrating interfaces and those incorporating machine learning algorithms that adjust stimulation parameters based on physiological feedback. These emerging standards recognize that traditional fixed-parameter safety assessments may be insufficient for dynamic bioelectronic systems in artificial organs.
The FDA's guidance on implantable electronic devices has recently expanded to include specific provisions for bioelectronic interfaces, requiring rigorous documentation of long-term stability and degradation profiles. European MDR regulations have similarly strengthened requirements for bioelectronic implants, particularly regarding leachable compounds from electrode materials and insulation components that may cause adverse biological responses.
Material selection for bioelectronic interfaces presents unique challenges at the intersection of electronic functionality and biological compatibility. Noble metals like platinum and iridium oxide have demonstrated superior long-term stability in physiological environments, while newer carbon-based materials including graphene and carbon nanotubes show promising biocompatibility profiles with enhanced electrical properties. Polymer coatings such as Parylene-C and polyimide serve as critical insulation materials, though their long-term degradation characteristics remain under investigation.
Safety testing methodologies have advanced beyond traditional in vitro assays to include specialized protocols for electroactive materials. Accelerated aging studies simulating years of implantation under electrical stimulation conditions have become standard practice, with particular attention to electrochemical stability and corrosion resistance. Microelectrode array (MEA) testing platforms now enable real-time monitoring of cellular responses to electrical stimulation, providing crucial data on the biocompatibility of active interfaces.
International harmonization efforts through organizations like IMDRF (International Medical Device Regulators Forum) are working to standardize biocompatibility requirements specifically for bioelectronic interfaces. These initiatives aim to establish consistent safety thresholds for charge injection limits, electrochemical stability, and foreign body responses across global regulatory frameworks.
Recent developments in safety standards increasingly focus on the unique challenges of adaptive bioelectronic systems that modify their properties over time. This includes establishing safety parameters for self-calibrating interfaces and those incorporating machine learning algorithms that adjust stimulation parameters based on physiological feedback. These emerging standards recognize that traditional fixed-parameter safety assessments may be insufficient for dynamic bioelectronic systems in artificial organs.
Regulatory Framework for Medical Implants
The regulatory landscape for bioelectronic interfaces in artificial organs represents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies most bioelectronic implants as Class III medical devices, requiring rigorous premarket approval (PMA) processes that include extensive clinical trials demonstrating both safety and efficacy. The FDA's regulatory pathway specifically for bioelectronic interfaces has evolved significantly since 2015, with the introduction of specialized guidance for implantable electronic devices that interface directly with neural tissue.
The European Union operates under the Medical Device Regulation (MDR) framework, which came into full effect in 2021, replacing the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for implantable bioelectronic devices. Notably, the MDR places greater emphasis on biocompatibility testing and long-term safety monitoring than previous regulatory frameworks, directly impacting development timelines for artificial organ interfaces.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established a specialized pathway for emerging bioelectronic technologies through its Sakigake designation system, which can accelerate approval for breakthrough technologies. This contrasts with China's National Medical Products Administration (NMPA) approach, which has recently implemented a dual-track system that distinguishes between conventional medical implants and those incorporating novel bioelectronic interfaces.
International harmonization efforts, particularly through the International Medical Device Regulators Forum (IMDRF), have made progress in standardizing biocompatibility testing protocols and electromagnetic compatibility requirements for implantable bioelectronic devices. However, significant regulatory divergence remains in areas such as data privacy requirements for devices that transmit physiological data and long-term monitoring protocols.
A critical regulatory challenge specific to bioelectronic interfaces in artificial organs involves the classification of hybrid devices that combine biological components with electronic elements. These boundary-crossing technologies often fall between traditional regulatory categories, creating uncertainty in approval pathways. Recent regulatory developments have begun addressing this gap, with the FDA's Digital Health Innovation Action Plan and the EU's guidance on qualification of novel methodologies providing frameworks for evaluating such hybrid technologies.
Reimbursement policies represent another crucial aspect of the regulatory landscape, with significant variation in how national healthcare systems classify and cover bioelectronic implants. This economic dimension of regulation can substantially impact market access and technology adoption rates, with some jurisdictions requiring specialized health technology assessment processes for novel bioelectronic interfaces.
The European Union operates under the Medical Device Regulation (MDR) framework, which came into full effect in 2021, replacing the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for implantable bioelectronic devices. Notably, the MDR places greater emphasis on biocompatibility testing and long-term safety monitoring than previous regulatory frameworks, directly impacting development timelines for artificial organ interfaces.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established a specialized pathway for emerging bioelectronic technologies through its Sakigake designation system, which can accelerate approval for breakthrough technologies. This contrasts with China's National Medical Products Administration (NMPA) approach, which has recently implemented a dual-track system that distinguishes between conventional medical implants and those incorporating novel bioelectronic interfaces.
International harmonization efforts, particularly through the International Medical Device Regulators Forum (IMDRF), have made progress in standardizing biocompatibility testing protocols and electromagnetic compatibility requirements for implantable bioelectronic devices. However, significant regulatory divergence remains in areas such as data privacy requirements for devices that transmit physiological data and long-term monitoring protocols.
A critical regulatory challenge specific to bioelectronic interfaces in artificial organs involves the classification of hybrid devices that combine biological components with electronic elements. These boundary-crossing technologies often fall between traditional regulatory categories, creating uncertainty in approval pathways. Recent regulatory developments have begun addressing this gap, with the FDA's Digital Health Innovation Action Plan and the EU's guidance on qualification of novel methodologies providing frameworks for evaluating such hybrid technologies.
Reimbursement policies represent another crucial aspect of the regulatory landscape, with significant variation in how national healthcare systems classify and cover bioelectronic implants. This economic dimension of regulation can substantially impact market access and technology adoption rates, with some jurisdictions requiring specialized health technology assessment processes for novel bioelectronic interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!