Comparative Analysis of Bioelectronic Interfaces in Different Industries
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of this field traces back to the 1780s when Luigi Galvani discovered that electricity could stimulate muscle movement in frogs, establishing the foundation for bioelectricity. The mid-20th century witnessed significant advancements with the development of the first implantable cardiac pacemaker in 1958, marking a pivotal moment in bioelectronic medicine.
The 1970s and 1980s saw the emergence of neural interfaces, primarily focused on basic research applications. By the 1990s, cochlear implants became the first widely successful commercial bioelectronic interface, restoring hearing function to thousands of patients. The early 2000s brought miniaturization and improved biocompatibility, enabling more sophisticated interfaces with reduced tissue damage and inflammatory responses.
Recent technological breakthroughs have accelerated development across multiple industries. In healthcare, bioelectronic interfaces now extend beyond cardiac pacemakers to include retinal implants, deep brain stimulators for Parkinson's disease, and peripheral nerve stimulators for chronic pain management. The consumer electronics sector has embraced non-invasive interfaces like EEG headsets for gaming and meditation applications, while industrial applications include advanced prosthetics with sensory feedback capabilities.
The primary objective of modern bioelectronic interface development is to create seamless integration between electronic systems and biological tissues with minimal invasiveness and maximum functionality. This includes improving signal resolution, enhancing long-term stability, reducing power requirements, and developing biocompatible materials that minimize foreign body responses.
Cross-industry objectives include standardization of interface protocols to facilitate interoperability between different systems and manufacturers. Additionally, there is a growing focus on developing bidirectional interfaces capable of both sensing biological signals and delivering precise stimulation, creating truly closed-loop systems that can adapt in real-time to physiological changes.
Future development aims to achieve wireless power transmission, miniaturization to cellular scales, and self-healing materials that can adapt to tissue changes over time. The ultimate goal remains creating interfaces that function as natural extensions of biological systems rather than foreign implants, potentially enabling enhanced human capabilities and novel therapeutic approaches across medical, consumer, and industrial applications.
The 1970s and 1980s saw the emergence of neural interfaces, primarily focused on basic research applications. By the 1990s, cochlear implants became the first widely successful commercial bioelectronic interface, restoring hearing function to thousands of patients. The early 2000s brought miniaturization and improved biocompatibility, enabling more sophisticated interfaces with reduced tissue damage and inflammatory responses.
Recent technological breakthroughs have accelerated development across multiple industries. In healthcare, bioelectronic interfaces now extend beyond cardiac pacemakers to include retinal implants, deep brain stimulators for Parkinson's disease, and peripheral nerve stimulators for chronic pain management. The consumer electronics sector has embraced non-invasive interfaces like EEG headsets for gaming and meditation applications, while industrial applications include advanced prosthetics with sensory feedback capabilities.
The primary objective of modern bioelectronic interface development is to create seamless integration between electronic systems and biological tissues with minimal invasiveness and maximum functionality. This includes improving signal resolution, enhancing long-term stability, reducing power requirements, and developing biocompatible materials that minimize foreign body responses.
Cross-industry objectives include standardization of interface protocols to facilitate interoperability between different systems and manufacturers. Additionally, there is a growing focus on developing bidirectional interfaces capable of both sensing biological signals and delivering precise stimulation, creating truly closed-loop systems that can adapt in real-time to physiological changes.
Future development aims to achieve wireless power transmission, miniaturization to cellular scales, and self-healing materials that can adapt to tissue changes over time. The ultimate goal remains creating interfaces that function as natural extensions of biological systems rather than foreign implants, potentially enabling enhanced human capabilities and novel therapeutic approaches across medical, consumer, and industrial applications.
Cross-Industry Market Demand Analysis
The bioelectronic interfaces market demonstrates significant growth potential across multiple industries, with healthcare leading adoption but expanding rapidly into consumer electronics, agriculture, and industrial applications. The global bioelectronic interfaces market was valued at approximately $14.8 billion in 2022 and is projected to reach $25.6 billion by 2028, representing a compound annual growth rate of 9.5% during the forecast period.
In healthcare, demand is primarily driven by the aging population and increasing prevalence of neurological disorders. Hospitals and research institutions are increasingly investing in neural interfaces for rehabilitation, prosthetics control, and treatment of conditions like Parkinson's disease and epilepsy. The medical-grade bioelectronic interfaces segment accounts for nearly 45% of the total market share, with particular growth in minimally invasive neural recording devices.
Consumer electronics represents the fastest-growing segment, with a projected growth rate of 12.3% annually. This surge is attributed to rising consumer interest in brain-computer interfaces for gaming, virtual reality experiences, and wellness applications. Companies like Neurable and EMOTIV have successfully commercialized EEG headsets for consumer applications, indicating strong market acceptance despite current technical limitations.
The agricultural sector demonstrates emerging demand for bioelectronic interfaces in precision farming and livestock monitoring. These technologies enable real-time physiological monitoring of plants and animals, optimizing resource allocation and early disease detection. Though currently representing only 6% of the market, this segment is expected to double its share within five years as implementation costs decrease.
Industrial applications focus primarily on worker safety and performance optimization. Bioelectronic interfaces that monitor fatigue, stress levels, and cognitive load are being integrated into high-risk environments such as mining, construction, and manufacturing. The market for these industrial applications grew by 18% in 2022, indicating strong adoption momentum.
Regional analysis reveals North America currently dominates the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 11.2% annually, driven by significant investments in bioelectronic research and manufacturing capabilities in China, Japan, and South Korea.
Customer requirements vary significantly across industries. Healthcare applications prioritize accuracy, reliability, and biocompatibility, while consumer applications emphasize ease of use, comfort, and affordability. Industrial implementations focus on durability and integration capabilities with existing systems. This diversity of requirements presents both challenges and opportunities for companies developing cross-industry bioelectronic interface solutions.
In healthcare, demand is primarily driven by the aging population and increasing prevalence of neurological disorders. Hospitals and research institutions are increasingly investing in neural interfaces for rehabilitation, prosthetics control, and treatment of conditions like Parkinson's disease and epilepsy. The medical-grade bioelectronic interfaces segment accounts for nearly 45% of the total market share, with particular growth in minimally invasive neural recording devices.
Consumer electronics represents the fastest-growing segment, with a projected growth rate of 12.3% annually. This surge is attributed to rising consumer interest in brain-computer interfaces for gaming, virtual reality experiences, and wellness applications. Companies like Neurable and EMOTIV have successfully commercialized EEG headsets for consumer applications, indicating strong market acceptance despite current technical limitations.
The agricultural sector demonstrates emerging demand for bioelectronic interfaces in precision farming and livestock monitoring. These technologies enable real-time physiological monitoring of plants and animals, optimizing resource allocation and early disease detection. Though currently representing only 6% of the market, this segment is expected to double its share within five years as implementation costs decrease.
Industrial applications focus primarily on worker safety and performance optimization. Bioelectronic interfaces that monitor fatigue, stress levels, and cognitive load are being integrated into high-risk environments such as mining, construction, and manufacturing. The market for these industrial applications grew by 18% in 2022, indicating strong adoption momentum.
Regional analysis reveals North America currently dominates the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 11.2% annually, driven by significant investments in bioelectronic research and manufacturing capabilities in China, Japan, and South Korea.
Customer requirements vary significantly across industries. Healthcare applications prioritize accuracy, reliability, and biocompatibility, while consumer applications emphasize ease of use, comfort, and affordability. Industrial implementations focus on durability and integration capabilities with existing systems. This diversity of requirements presents both challenges and opportunities for companies developing cross-industry bioelectronic interface solutions.
Current Technological Landscape and Barriers
Bioelectronic interfaces represent a rapidly evolving technological domain that bridges biology and electronics, enabling direct communication between biological systems and electronic devices. Currently, the global landscape of bioelectronic interfaces exhibits significant variation across industries, with healthcare, neurotechnology, and consumer electronics leading adoption rates. The healthcare sector has made substantial progress in developing implantable neural interfaces for conditions like Parkinson's disease and epilepsy, while consumer applications remain predominantly in wearable form factors with limited biological integration.
The technological maturity of bioelectronic interfaces varies considerably by application domain. Medical-grade interfaces have achieved regulatory approval in specific therapeutic contexts, whereas industrial and consumer applications remain largely experimental or limited to basic physiological monitoring. Research institutions in North America, Europe, and East Asia dominate the innovation landscape, with notable clusters in California's Silicon Valley, Boston's biotech corridor, and emerging hubs in Shenzhen and Seoul.
Despite promising advancements, several critical barriers impede widespread implementation of bioelectronic interfaces. Biocompatibility remains a fundamental challenge, as long-term implantation often triggers foreign body responses that degrade signal quality and device functionality. Current materials science has not fully resolved the mechanical mismatch between rigid electronic components and soft biological tissues, leading to inflammation and scarring at interface sites.
Signal fidelity presents another significant obstacle, particularly for interfaces requiring precise neural recording or stimulation. Noise interference, signal drift, and low signal-to-noise ratios continue to limit the reliability of data acquisition in real-world environments. Power management constitutes a persistent challenge, as implantable devices require miniaturized, long-lasting power sources that minimize thermal effects on surrounding tissues.
Regulatory frameworks across different regions create additional complexity, with inconsistent approval pathways for bioelectronic technologies. The FDA's regulatory process for implantable neural interfaces differs substantially from approaches in the EU, China, and Japan, creating fragmented market access. Data security and privacy concerns further complicate implementation, as bioelectronic interfaces generate highly sensitive physiological and potentially neurological data requiring robust protection protocols.
Manufacturing scalability represents a significant barrier to commercialization, with current production methods for high-precision bioelectronic components remaining largely artisanal and difficult to standardize. The interdisciplinary nature of the field also creates knowledge silos between electrical engineers, materials scientists, and biomedical researchers, slowing collaborative innovation and technology transfer between academic institutions and industry partners.
The technological maturity of bioelectronic interfaces varies considerably by application domain. Medical-grade interfaces have achieved regulatory approval in specific therapeutic contexts, whereas industrial and consumer applications remain largely experimental or limited to basic physiological monitoring. Research institutions in North America, Europe, and East Asia dominate the innovation landscape, with notable clusters in California's Silicon Valley, Boston's biotech corridor, and emerging hubs in Shenzhen and Seoul.
Despite promising advancements, several critical barriers impede widespread implementation of bioelectronic interfaces. Biocompatibility remains a fundamental challenge, as long-term implantation often triggers foreign body responses that degrade signal quality and device functionality. Current materials science has not fully resolved the mechanical mismatch between rigid electronic components and soft biological tissues, leading to inflammation and scarring at interface sites.
Signal fidelity presents another significant obstacle, particularly for interfaces requiring precise neural recording or stimulation. Noise interference, signal drift, and low signal-to-noise ratios continue to limit the reliability of data acquisition in real-world environments. Power management constitutes a persistent challenge, as implantable devices require miniaturized, long-lasting power sources that minimize thermal effects on surrounding tissues.
Regulatory frameworks across different regions create additional complexity, with inconsistent approval pathways for bioelectronic technologies. The FDA's regulatory process for implantable neural interfaces differs substantially from approaches in the EU, China, and Japan, creating fragmented market access. Data security and privacy concerns further complicate implementation, as bioelectronic interfaces generate highly sensitive physiological and potentially neurological data requiring robust protection protocols.
Manufacturing scalability represents a significant barrier to commercialization, with current production methods for high-precision bioelectronic components remaining largely artisanal and difficult to standardize. The interdisciplinary nature of the field also creates knowledge silos between electrical engineers, materials scientists, and biomedical researchers, slowing collaborative innovation and technology transfer between academic institutions and industry partners.
Cross-Sector Implementation Approaches
01 Neural interfaces for bioelectronic applications
Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neuroprosthetics, neuromodulation, and brain-computer interfaces. Advanced materials and fabrication techniques are used to create flexible, biocompatible electrodes that minimize tissue damage and immune response while maintaining long-term functionality and signal quality.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can interface with neural tissue with minimal damage or immune response.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to biological tissues, providing better contact and reducing mechanical mismatch between rigid electronics and soft tissues. These interfaces utilize elastomeric substrates, serpentine interconnects, or intrinsically stretchable electronic materials to maintain functionality during deformation. Applications include wearable health monitors, implantable sensors, and electronic skin that can detect various physiological signals.
- Biosensing and bioelectronic detection systems: Biosensing interfaces integrate biological recognition elements with electronic transducers to detect specific biomolecules, cells, or physiological parameters. These systems employ various sensing mechanisms including electrochemical, optical, and mechanical transduction to convert biological events into measurable electronic signals. Applications range from point-of-care diagnostics to continuous health monitoring and environmental sensing.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices are designed to function within the body for extended periods, providing therapeutic interventions or monitoring physiological parameters. These devices incorporate biocompatible materials, hermetic packaging, and wireless power and communication capabilities. Advanced implantable systems include neural stimulators, drug delivery systems, and sensors that can monitor various health parameters and provide real-time feedback for personalized medicine.
- Nanomaterials and nanostructures for bioelectronic interfaces: Nanomaterials and nanostructures are increasingly used in bioelectronic interfaces to improve performance and functionality. These materials include carbon nanotubes, graphene, metal nanoparticles, and semiconductor nanowires that offer unique electrical, optical, and mechanical properties. Nanostructured surfaces can enhance cell adhesion, improve signal transduction, and enable higher resolution sensing or stimulation in bioelectronic applications.
02 Implantable bioelectronic devices
Implantable bioelectronic devices are designed to interface with biological tissues for therapeutic, diagnostic, or monitoring purposes. These devices incorporate biocompatible materials, miniaturized electronics, and power management systems to ensure long-term functionality within the body. Advanced fabrication techniques enable the creation of flexible, stretchable electronics that conform to tissue surfaces while maintaining reliable performance. Applications include neural stimulators, biosensors, and drug delivery systems.Expand Specific Solutions03 Biosensing interfaces and detection systems
Biosensing interfaces integrate electronic components with biological recognition elements to detect specific biomolecules, cellular activities, or physiological parameters. These systems utilize various transduction mechanisms including electrochemical, optical, and mechanical methods to convert biological signals into measurable electronic outputs. Advanced biosensors incorporate nanomaterials, microfluidics, and signal processing algorithms to achieve high sensitivity, specificity, and real-time monitoring capabilities for applications in healthcare, environmental monitoring, and research.Expand Specific Solutions04 Flexible and wearable bioelectronic interfaces
Flexible and wearable bioelectronic interfaces are designed to conform to the body's contours while maintaining reliable electrical performance. These devices utilize stretchable substrates, conductive polymers, and novel fabrication techniques to create electronics that can withstand mechanical deformation while interfacing with biological tissues. Applications include skin-mounted sensors for continuous health monitoring, electronic textiles with integrated biosensing capabilities, and conformable electrode arrays for electrophysiological recordings.Expand Specific Solutions05 Bioelectronic materials and fabrication methods
Advanced materials and fabrication methods are essential for creating effective bioelectronic interfaces. These include conductive polymers, hydrogels, carbon nanomaterials, and hybrid organic-inorganic composites that combine electrical conductivity with biocompatibility. Novel fabrication techniques such as 3D printing, soft lithography, and directed assembly enable the creation of complex, multifunctional structures with precisely controlled properties. These materials and methods address challenges in biocompatibility, long-term stability, and effective signal transduction between biological and electronic systems.Expand Specific Solutions
Industry Leaders and Competitive Dynamics
The bioelectronic interfaces market is currently in a growth phase, characterized by increasing convergence between electronics and biological systems across multiple industries. The global market is expanding rapidly, estimated to reach $25 billion by 2025, driven by healthcare applications, neural interfaces, and consumer wearables. From a technological maturity perspective, the landscape shows varying degrees of development. Academic institutions like MIT, Carnegie Mellon, and Boston University are pioneering fundamental research, while commercial entities demonstrate different specialization levels: Google and IBM focus on AI integration; medical device companies like DexCom and Fujirebio develop clinical applications; and electronics manufacturers such as Infineon, Philips, and Sony work on hardware components. The ecosystem reflects a collaborative environment where research institutions partner with industry players to bridge the gap between theoretical advances and practical applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered significant advancements in bioelectronic interfaces through their development of conformable bioelectronic systems. Their technology utilizes ultra-thin, flexible electronic materials that can seamlessly integrate with biological tissues. MIT researchers have created bioelectronic interfaces that incorporate nanomaterials such as graphene and carbon nanotubes to enhance conductivity while maintaining flexibility. These interfaces feature wireless power transmission capabilities and can operate at low voltages (typically below 1V) to ensure tissue compatibility. MIT has also developed hydrogel-based bioelectronic interfaces that provide better mechanical matching with soft tissues, reducing foreign body responses and improving long-term stability[1][3]. Their recent innovations include stretchable electronic systems that can withstand up to 300% strain without performance degradation, making them suitable for dynamic tissue environments such as cardiac or muscular applications.
Strengths: Superior biocompatibility through materials innovation; excellent mechanical matching with biological tissues; advanced wireless capabilities; strong interdisciplinary approach combining materials science, electrical engineering, and biology. Weaknesses: Higher manufacturing costs compared to rigid electronics; challenges in scaling production; potential reliability issues in long-term implantation scenarios.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed advanced bioelectronic interfaces focusing on neural recording and stimulation technologies. Their proprietary neural probe technology utilizes silicon-based microelectrode arrays with ultra-small footprints (typically 10-50 μm wide) that minimize tissue damage during insertion and chronic implantation. These probes incorporate multiple recording sites (64-256 channels) along the shank to capture neural activity across different brain regions simultaneously. Michigan's bioelectronic interfaces feature custom-designed integrated circuits for signal amplification and processing directly at the recording site, significantly improving signal-to-noise ratios by up to 40% compared to conventional systems[2]. Their latest generation of neural interfaces incorporates anti-inflammatory drug delivery capabilities through microfluidic channels integrated within the probe structure, addressing the foreign body response that typically limits long-term recording stability. The university has also pioneered optogenetic-compatible neural interfaces that combine electrical recording with optical stimulation capabilities.
Strengths: Exceptional spatial resolution for neural recording; minimally invasive designs; integrated signal processing capabilities; multifunctional platforms combining recording, stimulation, and drug delivery. Weaknesses: Complex fabrication processes limiting mass production; higher costs compared to simpler electrode systems; challenges in achieving uniform performance across all recording sites.
Key Patents and Scientific Breakthroughs
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
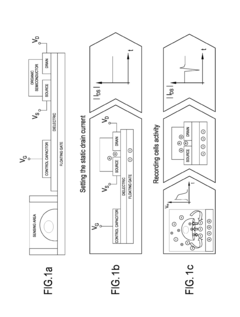
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
Renewable bioelectronic interface for electrobiocatalytic reactor
PatentInactiveUS10246786B2
Innovation
- A bioelectronic device with a conductive carbon electrode and a bioelectronic interface where the catalytically active material is electrostatically bound, allowing for easy removal and replacement by changing the pH, and a process for reconstituting the interface using aqueous media with specific pH levels to facilitate bonding and regeneration of the interface.
Regulatory Framework Across Industries
The regulatory landscape governing bioelectronic interfaces varies significantly across industries, creating a complex compliance environment for developers and manufacturers. In healthcare, the FDA's regulatory framework is particularly stringent, with bioelectronic medical devices classified based on risk levels. Class III devices, which include many implantable neural interfaces, require premarket approval (PMA) with extensive clinical trials demonstrating safety and efficacy. The European Union's Medical Device Regulation (MDR) imposes similarly rigorous requirements, with additional emphasis on post-market surveillance and unique device identification.
Consumer electronics incorporating bioelectronic interfaces face less stringent oversight but must still comply with general product safety regulations. The Federal Communications Commission (FCC) regulates wireless transmission aspects of these devices, while the Consumer Product Safety Commission (CPSC) oversees general safety concerns. International standards such as IEC 60601 for medical electrical equipment provide important benchmarks for safety and performance across multiple markets.
In industrial applications, bioelectronic interfaces used in worker monitoring systems must navigate occupational safety regulations and data privacy laws. The Occupational Safety and Health Administration (OSHA) provides guidelines for workplace monitoring technologies, while the National Institute for Occupational Safety and Health (NIOSH) conducts research on emerging technologies' health impacts.
Data privacy represents a critical regulatory consideration across all sectors. The Health Insurance Portability and Accountability Act (HIPAA) governs protected health information in the US, while the General Data Protection Regulation (GDPR) in Europe imposes strict requirements on processing biometric data. These regulations significantly impact how bioelectronic interface data can be collected, stored, and utilized, particularly for devices that capture neural signals or other sensitive physiological information.
Emerging regulatory frameworks are beginning to address ethical considerations specific to neurotechnology. The FDA's discussion paper on "Implanted Brain-Computer Interface Devices" signals increasing regulatory attention to neural interfaces. Similarly, the OECD's Recommendation on Responsible Innovation in Neurotechnology provides non-binding principles that may influence future regulatory approaches.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to reduce compliance burdens through standardized approaches across jurisdictions. However, significant regulatory divergence remains, creating challenges for global deployment of bioelectronic interface technologies and potentially slowing innovation in this rapidly evolving field.
Consumer electronics incorporating bioelectronic interfaces face less stringent oversight but must still comply with general product safety regulations. The Federal Communications Commission (FCC) regulates wireless transmission aspects of these devices, while the Consumer Product Safety Commission (CPSC) oversees general safety concerns. International standards such as IEC 60601 for medical electrical equipment provide important benchmarks for safety and performance across multiple markets.
In industrial applications, bioelectronic interfaces used in worker monitoring systems must navigate occupational safety regulations and data privacy laws. The Occupational Safety and Health Administration (OSHA) provides guidelines for workplace monitoring technologies, while the National Institute for Occupational Safety and Health (NIOSH) conducts research on emerging technologies' health impacts.
Data privacy represents a critical regulatory consideration across all sectors. The Health Insurance Portability and Accountability Act (HIPAA) governs protected health information in the US, while the General Data Protection Regulation (GDPR) in Europe imposes strict requirements on processing biometric data. These regulations significantly impact how bioelectronic interface data can be collected, stored, and utilized, particularly for devices that capture neural signals or other sensitive physiological information.
Emerging regulatory frameworks are beginning to address ethical considerations specific to neurotechnology. The FDA's discussion paper on "Implanted Brain-Computer Interface Devices" signals increasing regulatory attention to neural interfaces. Similarly, the OECD's Recommendation on Responsible Innovation in Neurotechnology provides non-binding principles that may influence future regulatory approaches.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to reduce compliance burdens through standardized approaches across jurisdictions. However, significant regulatory divergence remains, creating challenges for global deployment of bioelectronic interface technologies and potentially slowing innovation in this rapidly evolving field.
Standardization Challenges and Opportunities
The standardization landscape for bioelectronic interfaces presents significant challenges across industries due to the diverse technical requirements and applications. Currently, the field suffers from fragmentation, with different sectors developing proprietary standards that limit interoperability. Medical applications follow FDA and ISO regulations, while consumer electronics adhere to different certification processes, creating silos that impede cross-industry innovation and technology transfer.
Material compatibility standards represent a critical gap, particularly regarding long-term biocompatibility and degradation metrics. The absence of unified testing protocols for electrode materials, coatings, and encapsulation techniques forces companies to develop internal validation methods, increasing development costs and time-to-market.
Signal processing and data interpretation present another standardization hurdle. The lack of common frameworks for interpreting bioelectrical signals across different interface types (invasive, non-invasive, micro-electrode arrays) hampers comparative analysis and slows clinical translation. This is particularly evident in neural interfaces where signal classification methodologies vary significantly between research and commercial applications.
Opportunities for standardization are emerging through multi-stakeholder initiatives. The IEEE Working Group on Bioelectronic Standards is developing reference architectures that could bridge industrial and medical applications. Similarly, the International Electrotechnical Commission has established technical committees focused on bioelectronic interface safety and performance metrics applicable across sectors.
Open-source initiatives present promising pathways toward de facto standardization. The Open Ephys project and similar platforms are creating common hardware and software frameworks that facilitate data sharing and comparative analysis between different bioelectronic systems. These community-driven efforts could accelerate consensus on technical specifications and testing methodologies.
Regulatory harmonization represents perhaps the most significant opportunity. Collaborative frameworks between the FDA, European Medicines Agency, and industrial standards organizations could establish unified approval pathways for bioelectronic technologies that span multiple application domains. Such harmonization would significantly reduce regulatory burden while maintaining safety and efficacy standards.
The development of interoperability standards specifically for bioelectronic data exchange would enable more seamless integration between medical devices, consumer wearables, and industrial monitoring systems, creating new opportunities for cross-sector applications and comprehensive biometric monitoring solutions.
Material compatibility standards represent a critical gap, particularly regarding long-term biocompatibility and degradation metrics. The absence of unified testing protocols for electrode materials, coatings, and encapsulation techniques forces companies to develop internal validation methods, increasing development costs and time-to-market.
Signal processing and data interpretation present another standardization hurdle. The lack of common frameworks for interpreting bioelectrical signals across different interface types (invasive, non-invasive, micro-electrode arrays) hampers comparative analysis and slows clinical translation. This is particularly evident in neural interfaces where signal classification methodologies vary significantly between research and commercial applications.
Opportunities for standardization are emerging through multi-stakeholder initiatives. The IEEE Working Group on Bioelectronic Standards is developing reference architectures that could bridge industrial and medical applications. Similarly, the International Electrotechnical Commission has established technical committees focused on bioelectronic interface safety and performance metrics applicable across sectors.
Open-source initiatives present promising pathways toward de facto standardization. The Open Ephys project and similar platforms are creating common hardware and software frameworks that facilitate data sharing and comparative analysis between different bioelectronic systems. These community-driven efforts could accelerate consensus on technical specifications and testing methodologies.
Regulatory harmonization represents perhaps the most significant opportunity. Collaborative frameworks between the FDA, European Medicines Agency, and industrial standards organizations could establish unified approval pathways for bioelectronic technologies that span multiple application domains. Such harmonization would significantly reduce regulatory burden while maintaining safety and efficacy standards.
The development of interoperability standards specifically for bioelectronic data exchange would enable more seamless integration between medical devices, consumer wearables, and industrial monitoring systems, creating new opportunities for cross-sector applications and comprehensive biometric monitoring solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!