Emerging Applications of Bioelectronic Interface in the IoT Sector
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a convergence of biological systems with electronic devices, enabling unprecedented interaction between living organisms and technological platforms. This field has evolved significantly over the past two decades, transitioning from rudimentary signal detection systems to sophisticated bidirectional communication platforms capable of both sensing biological signals and delivering targeted stimulation.
The evolution of bioelectronic interfaces began in the early 2000s with basic neural recording devices primarily used in laboratory settings. By 2010, significant advancements in materials science, particularly the development of flexible and biocompatible substrates, enabled longer-term implantation with reduced tissue damage. The period between 2015 and 2020 witnessed the miniaturization of these interfaces, alongside improvements in wireless power and data transmission capabilities, making them increasingly suitable for real-world applications.
In the IoT context, bioelectronic interfaces have progressed from simple wearable sensors to integrated systems capable of continuous health monitoring and environmental interaction. This evolution has been driven by parallel advancements in low-power electronics, edge computing, and secure data transmission protocols essential for handling sensitive biological data.
The primary objective of current bioelectronic interface development in the IoT sector is to create seamless, minimally invasive systems that can reliably translate biological signals into digital information while maintaining user comfort and privacy. This includes developing interfaces that can function autonomously for extended periods without maintenance or recalibration, a critical requirement for widespread IoT deployment.
Another key objective is enhancing the specificity and sensitivity of these interfaces to detect subtle biological signals amidst environmental noise, thereby improving the accuracy of health monitoring and human-machine interaction applications. Researchers are also focusing on developing standardized communication protocols to facilitate interoperability between different bioelectronic devices within larger IoT ecosystems.
Looking forward, the field aims to achieve fully integrated bioelectronic systems capable of closed-loop operation, where sensing and stimulation functions work in concert to maintain optimal physiological states or enhance human capabilities. This includes developing interfaces that can adapt to individual users over time, learning from interaction patterns to provide increasingly personalized responses.
The ultimate technological goal remains the development of bioelectronic interfaces that can seamlessly integrate with human biology while maintaining robust connectivity with digital infrastructure, effectively bridging the biological and digital realms in ways that enhance human health, productivity, and quality of life.
The evolution of bioelectronic interfaces began in the early 2000s with basic neural recording devices primarily used in laboratory settings. By 2010, significant advancements in materials science, particularly the development of flexible and biocompatible substrates, enabled longer-term implantation with reduced tissue damage. The period between 2015 and 2020 witnessed the miniaturization of these interfaces, alongside improvements in wireless power and data transmission capabilities, making them increasingly suitable for real-world applications.
In the IoT context, bioelectronic interfaces have progressed from simple wearable sensors to integrated systems capable of continuous health monitoring and environmental interaction. This evolution has been driven by parallel advancements in low-power electronics, edge computing, and secure data transmission protocols essential for handling sensitive biological data.
The primary objective of current bioelectronic interface development in the IoT sector is to create seamless, minimally invasive systems that can reliably translate biological signals into digital information while maintaining user comfort and privacy. This includes developing interfaces that can function autonomously for extended periods without maintenance or recalibration, a critical requirement for widespread IoT deployment.
Another key objective is enhancing the specificity and sensitivity of these interfaces to detect subtle biological signals amidst environmental noise, thereby improving the accuracy of health monitoring and human-machine interaction applications. Researchers are also focusing on developing standardized communication protocols to facilitate interoperability between different bioelectronic devices within larger IoT ecosystems.
Looking forward, the field aims to achieve fully integrated bioelectronic systems capable of closed-loop operation, where sensing and stimulation functions work in concert to maintain optimal physiological states or enhance human capabilities. This includes developing interfaces that can adapt to individual users over time, learning from interaction patterns to provide increasingly personalized responses.
The ultimate technological goal remains the development of bioelectronic interfaces that can seamlessly integrate with human biology while maintaining robust connectivity with digital infrastructure, effectively bridging the biological and digital realms in ways that enhance human health, productivity, and quality of life.
IoT Market Demand for Bioelectronic Solutions
The bioelectronic interface market within the IoT sector is experiencing unprecedented growth, driven by increasing demand for advanced health monitoring solutions and smart wearable technologies. Current market projections indicate that the global bioelectronic medical devices market is expected to reach $25 billion by 2025, with a compound annual growth rate of approximately 11.7% during the forecast period. This growth is primarily fueled by rising healthcare costs, aging populations, and the increasing prevalence of chronic diseases requiring continuous monitoring.
Consumer demand for personalized healthcare solutions has created significant market opportunities for bioelectronic interfaces in IoT applications. The wearable medical device segment, which heavily incorporates bioelectronic interfaces, has seen particularly strong growth, with over 60% of consumers expressing interest in using wearable technology to monitor their health metrics in real-time.
Industrial applications represent another substantial market segment, with manufacturing and logistics companies increasingly adopting bioelectronic interfaces to monitor worker health and safety. This sector is projected to grow at 14.2% annually through 2026, as companies recognize the potential for reduced workplace injuries and improved operational efficiency.
The healthcare sector remains the largest market for bioelectronic IoT solutions, with hospitals and clinics investing heavily in remote patient monitoring systems. These systems enable healthcare providers to track patient vital signs and other health metrics outside traditional clinical settings, reducing hospitalization rates by up to 30% for certain chronic conditions and generating estimated annual savings of $6,500 per patient.
Regional market analysis reveals that North America currently holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing healthcare expenditure, growing awareness about advanced medical technologies, and improving healthcare infrastructure.
Consumer preferences are shifting toward non-invasive, comfortable, and aesthetically pleasing bioelectronic interfaces. Market research indicates that 78% of potential users cite comfort and ease of use as primary factors influencing their purchasing decisions, while 65% express concerns about data privacy and security.
The market is also seeing increased demand for integrated solutions that combine multiple sensing capabilities with advanced analytics. Devices that can monitor various physiological parameters simultaneously while providing actionable insights through AI-powered analytics are commanding premium prices and experiencing faster adoption rates across both consumer and professional markets.
Consumer demand for personalized healthcare solutions has created significant market opportunities for bioelectronic interfaces in IoT applications. The wearable medical device segment, which heavily incorporates bioelectronic interfaces, has seen particularly strong growth, with over 60% of consumers expressing interest in using wearable technology to monitor their health metrics in real-time.
Industrial applications represent another substantial market segment, with manufacturing and logistics companies increasingly adopting bioelectronic interfaces to monitor worker health and safety. This sector is projected to grow at 14.2% annually through 2026, as companies recognize the potential for reduced workplace injuries and improved operational efficiency.
The healthcare sector remains the largest market for bioelectronic IoT solutions, with hospitals and clinics investing heavily in remote patient monitoring systems. These systems enable healthcare providers to track patient vital signs and other health metrics outside traditional clinical settings, reducing hospitalization rates by up to 30% for certain chronic conditions and generating estimated annual savings of $6,500 per patient.
Regional market analysis reveals that North America currently holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing healthcare expenditure, growing awareness about advanced medical technologies, and improving healthcare infrastructure.
Consumer preferences are shifting toward non-invasive, comfortable, and aesthetically pleasing bioelectronic interfaces. Market research indicates that 78% of potential users cite comfort and ease of use as primary factors influencing their purchasing decisions, while 65% express concerns about data privacy and security.
The market is also seeing increased demand for integrated solutions that combine multiple sensing capabilities with advanced analytics. Devices that can monitor various physiological parameters simultaneously while providing actionable insights through AI-powered analytics are commanding premium prices and experiencing faster adoption rates across both consumer and professional markets.
Current Bioelectronic Interface Technologies and Barriers
The bioelectronic interface landscape in IoT applications is currently dominated by several key technologies, each with specific capabilities and limitations. Non-invasive wearable sensors represent the most commercially mature segment, utilizing electroencephalography (EEG), electromyography (EMG), and electrocardiography (ECG) to monitor neural, muscular, and cardiac activities respectively. These technologies offer accessibility and minimal user risk but suffer from signal noise, limited depth of tissue penetration, and relatively low spatial resolution.
Semi-invasive interfaces, including epidermal electronics and microneedle arrays, provide improved signal quality while maintaining reasonable user acceptance. These technologies bridge the gap between fully non-invasive and invasive approaches, offering enhanced data fidelity without requiring surgical implantation. However, challenges persist in long-term skin compatibility, consistent signal quality during movement, and manufacturing scalability.
Fully invasive neural interfaces represent the technological frontier, featuring microelectrode arrays and neural dust that directly interface with neural tissue. While these technologies deliver exceptional signal resolution and bandwidth, they face substantial barriers including surgical risks, biocompatibility issues, long-term stability concerns, and significant regulatory hurdles that limit widespread adoption in consumer IoT applications.
Data processing represents another critical technological challenge across all bioelectronic interfaces. Real-time signal processing demands substantial computational resources, while maintaining low power consumption remains essential for portable and wearable applications. Current algorithms struggle with signal artifact removal, particularly in real-world environments where movement and electromagnetic interference are prevalent.
Power management constitutes a persistent barrier to advancement, with most current solutions requiring frequent recharging or battery replacement. Emerging energy harvesting technologies show promise but remain insufficient for high-bandwidth neural interfaces. Wireless data transmission protocols face similar constraints, balancing bandwidth requirements against power consumption and transmission range.
Standardization across the bioelectronic interface ecosystem presents a significant industry-wide challenge. The fragmentation of protocols, data formats, and connectivity standards impedes interoperability between devices and platforms, limiting the potential for comprehensive bioelectronic IoT ecosystems. This lack of standardization also complicates regulatory approval pathways, particularly for technologies that span multiple regulatory categories.
Security and privacy concerns represent perhaps the most critical non-technical barrier to widespread adoption. Bioelectronic data is inherently sensitive, potentially revealing health conditions, emotional states, and even thought patterns. Current encryption and anonymization techniques often prove inadequate when balanced against the power and processing constraints of wearable and implantable devices.
Semi-invasive interfaces, including epidermal electronics and microneedle arrays, provide improved signal quality while maintaining reasonable user acceptance. These technologies bridge the gap between fully non-invasive and invasive approaches, offering enhanced data fidelity without requiring surgical implantation. However, challenges persist in long-term skin compatibility, consistent signal quality during movement, and manufacturing scalability.
Fully invasive neural interfaces represent the technological frontier, featuring microelectrode arrays and neural dust that directly interface with neural tissue. While these technologies deliver exceptional signal resolution and bandwidth, they face substantial barriers including surgical risks, biocompatibility issues, long-term stability concerns, and significant regulatory hurdles that limit widespread adoption in consumer IoT applications.
Data processing represents another critical technological challenge across all bioelectronic interfaces. Real-time signal processing demands substantial computational resources, while maintaining low power consumption remains essential for portable and wearable applications. Current algorithms struggle with signal artifact removal, particularly in real-world environments where movement and electromagnetic interference are prevalent.
Power management constitutes a persistent barrier to advancement, with most current solutions requiring frequent recharging or battery replacement. Emerging energy harvesting technologies show promise but remain insufficient for high-bandwidth neural interfaces. Wireless data transmission protocols face similar constraints, balancing bandwidth requirements against power consumption and transmission range.
Standardization across the bioelectronic interface ecosystem presents a significant industry-wide challenge. The fragmentation of protocols, data formats, and connectivity standards impedes interoperability between devices and platforms, limiting the potential for comprehensive bioelectronic IoT ecosystems. This lack of standardization also complicates regulatory approval pathways, particularly for technologies that span multiple regulatory categories.
Security and privacy concerns represent perhaps the most critical non-technical barrier to widespread adoption. Bioelectronic data is inherently sensitive, potentially revealing health conditions, emotional states, and even thought patterns. Current encryption and anonymization techniques often prove inadequate when balanced against the power and processing constraints of wearable and implantable devices.
Mainstream Bioelectronic Interface Implementations
01 Neural-electronic interfaces for biosensing
Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect electrical signals from neurons and convert them into electronic data for analysis and interpretation.- Neural-electronic interfaces for biosensing: Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, providing valuable data for medical diagnostics and neural research.
- Implantable bioelectronic medical devices: Implantable bioelectronic interfaces designed for therapeutic and monitoring applications within the body. These devices integrate with biological tissues to deliver targeted treatments or monitor physiological parameters continuously. The technology includes biocompatible materials and designs that minimize immune responses while maintaining long-term functionality. These interfaces can be used for various medical applications including drug delivery, neural stimulation, and chronic disease management.
- Molecular bioelectronic interfaces: Interfaces that operate at the molecular level, connecting biological molecules with electronic components. These systems utilize biomolecules such as proteins, enzymes, or DNA as functional elements within electronic circuits. The technology enables highly specific detection of biological targets through molecular recognition events that generate measurable electronic signals. Applications include advanced diagnostics, environmental monitoring, and biomolecular computing.
- Flexible and wearable bioelectronic interfaces: Bioelectronic interfaces designed with flexibility and wearability for non-invasive biological monitoring. These interfaces incorporate stretchable electronics, conductive polymers, and thin-film technologies to create conformable devices that maintain intimate contact with biological tissues. The technology enables continuous monitoring of physiological parameters through skin contact or minimally invasive methods, suitable for healthcare monitoring, fitness tracking, and personalized medicine applications.
- Advanced materials for bioelectronic interfaces: Novel materials developed specifically for improving the performance and biocompatibility of bioelectronic interfaces. These materials address challenges such as biofouling, immune rejection, and signal degradation at the bio-electronic junction. The technology includes conductive hydrogels, carbon-based nanomaterials, bioactive coatings, and hybrid organic-inorganic composites that enhance signal transduction while maintaining compatibility with biological systems.
02 Implantable bioelectronic medical devices
Bioelectronic interfaces designed for implantation in the human body to monitor health parameters or deliver therapeutic interventions. These devices integrate with biological tissues and can communicate wirelessly with external systems. They often incorporate biocompatible materials to minimize rejection and inflammation while maintaining long-term functionality within the body. Applications include neural stimulation, drug delivery systems, and continuous physiological monitoring.Expand Specific Solutions03 Nanomaterial-based bioelectronic interfaces
Advanced bioelectronic interfaces utilizing nanomaterials such as carbon nanotubes, graphene, or quantum dots to enhance connectivity between biological systems and electronic components. These nanomaterials provide improved electrical conductivity, increased surface area for biological interaction, and enhanced biocompatibility. The nanoscale dimensions allow for more precise interfacing with cellular structures and biomolecules, enabling higher resolution sensing and more targeted stimulation capabilities.Expand Specific Solutions04 Flexible and wearable bioelectronic sensors
Bioelectronic interfaces designed with flexible, stretchable materials that can conform to biological surfaces for non-invasive monitoring. These wearable sensors can detect various physiological signals through the skin and transmit data wirelessly to monitoring systems. The flexibility allows for comfortable, long-term wear while maintaining reliable signal acquisition. Applications include continuous health monitoring, athletic performance tracking, and early disease detection.Expand Specific Solutions05 Microfluidic bioelectronic platforms
Integration of microfluidic systems with electronic components to create bioelectronic interfaces for analyzing biological samples. These platforms combine controlled fluid handling with electronic sensing to detect biomarkers, cells, or other biological analytes. The microfluidic channels enable precise sample preparation and delivery to sensing elements, while integrated electronics provide signal processing and data analysis capabilities. Applications include point-of-care diagnostics, drug screening, and environmental monitoring.Expand Specific Solutions
Leading Companies in Bioelectronic IoT Ecosystem
The bioelectronic interface market in IoT is currently in its early growth phase, characterized by rapid technological advancement and expanding applications. The market size is projected to grow significantly as healthcare, consumer electronics, and industrial sectors increasingly adopt these technologies. In terms of technical maturity, established players like Samsung Electronics, Google, and Infineon Technologies are leading with substantial R&D investments, while specialized companies such as Povini Biotechnology and Hileap Medtech are developing niche applications. Academic institutions including Peking University and Shanghai Jiao Tong University are contributing fundamental research. The competitive landscape features a mix of large technology conglomerates, specialized biotech firms, and research institutions collaborating to overcome technical challenges in biocompatibility, power efficiency, and data security.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced bioelectronic interfaces for IoT applications through their IOFIT platform, which integrates flexible biosensors with wireless connectivity for real-time health monitoring. Their technology utilizes ultra-thin, stretchable electronic patches that can be applied directly to the skin to monitor various physiological parameters including heart rate, muscle activity, and body temperature. Samsung's BioProcessor SoC specifically designed for wearable health devices enables efficient signal processing of bioelectric signals with minimal power consumption. The company has also pioneered graphene-based bioelectronic sensors that offer superior conductivity and flexibility compared to traditional materials, allowing for more accurate detection of bioelectric signals even during movement. Samsung's ARTIK IoT platform provides secure cloud connectivity for these bioelectronic devices, enabling comprehensive health data analysis and integration with other smart home and healthcare systems.
Strengths: Samsung's extensive manufacturing capabilities and semiconductor expertise allow for miniaturization and mass production of bioelectronic interfaces. Their established ecosystem of connected devices provides immediate integration pathways. Weaknesses: Their solutions tend to be proprietary and may lack interoperability with non-Samsung systems, potentially limiting adoption in diverse healthcare environments.
Infineon Technologies AG
Technical Solution: Infineon has developed a comprehensive bioelectronic interface ecosystem for IoT applications centered around their XENSIV sensor family and AURIX microcontroller platform. Their technology integrates highly sensitive biosignal acquisition circuits with advanced signal processing capabilities, enabling accurate detection and analysis of bioelectric signals such as ECG, EMG, and EEG. Infineon's bioelectronic interfaces feature ultra-low power consumption (as low as 200μW during active sensing) and high noise immunity, critical for wearable and implantable applications. The company has pioneered specialized analog front-end (AFE) chips that can detect microvolt-level biosignals while rejecting common-mode interference, achieving a CMRR exceeding 100dB. Their PSoC (Programmable System-on-Chip) architecture allows for adaptive signal processing algorithms that can be customized for specific biomedical applications. Infineon has also developed secure authentication and encryption protocols specifically designed for medical IoT devices, addressing the critical privacy concerns in bioelectronic applications while maintaining energy efficiency.
Strengths: Infineon's solutions excel in power efficiency and signal integrity, making them ideal for long-term biomonitoring applications. Their strong security architecture provides robust protection for sensitive biometric data. Weaknesses: Their systems often require more specialized knowledge to implement compared to consumer-oriented solutions, potentially limiting adoption among smaller developers without biomedical expertise.
Key Patents and Research in Bioelectronic IoT
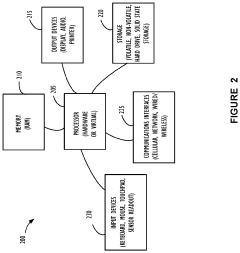
Internet of things (IOT) platform for device configuration management and support
PatentActiveUS11228648B2
Innovation
- A standardized interface, implemented through a software development kit (SDK), is integrated into IoT devices at the manufacturing stage, enabling automatic connection to cloud-based management servers, facilitating communication and control, and allowing for virtual IoT devices to act as proxies for unavailable or future devices.
Method and apparatus for transmitting and receiving uplink reference signal in wireless communication system
PatentWO2020167080A1
Innovation
- The method involves identifying and using sequences with specific PAPR characteristics for pi/2 BPSK modulation in the physical uplink shared channel (PUSCH) and physical uplink control channel (PUCCH) DMRS, based on transform precoding and high-layer signaling, to optimize PAPR and improve coverage, by generating Rel-16 PUSCH/PUCCH DMRS sequences that mimic pi/2 BPSK modulation.
Data Security and Privacy Considerations
The integration of bioelectronic interfaces with IoT systems introduces significant data security and privacy challenges that must be addressed comprehensively. These interfaces collect highly sensitive biometric and health-related data, which falls under special protection categories in many jurisdictions worldwide. The intimate nature of bioelectronic data—potentially revealing medical conditions, emotional states, and cognitive processes—demands unprecedented security measures beyond conventional IoT protections.
Regulatory frameworks governing this intersection remain fragmented, with the EU's GDPR, the US Health Insurance Portability and Accountability Act (HIPAA), and emerging bioethics guidelines creating a complex compliance landscape. Organizations deploying bioelectronic IoT solutions must navigate these regulations while implementing robust technical safeguards.
Encryption requirements for bioelectronic data transmission present unique challenges due to the real-time nature of many applications. Traditional encryption methods may introduce latency that compromises functionality in time-sensitive bioelectronic applications such as neural interfaces or emergency medical monitoring. Advanced encryption protocols optimized for low-latency transmission while maintaining security integrity are being developed specifically for this domain.
Data minimization principles take on heightened importance in bioelectronic contexts. The principle of collecting only necessary data conflicts with the exploratory nature of many bioelectronic applications where the full utility of collected signals may not be immediately apparent. This tension necessitates new approaches to progressive consent and dynamic data governance models.
Authentication mechanisms for bioelectronic interfaces require innovation beyond traditional methods. Continuous authentication based on the bioelectronic signals themselves—effectively using the unique biological signatures as authentication factors—represents a promising direction. However, this approach introduces questions about revocability and changeability of biometric credentials.
The potential for unauthorized inference presents another critical concern. Even with protected primary data, sophisticated algorithms might derive sensitive information from seemingly innocuous bioelectronic signals. For example, neural activity patterns collected for prosthetic control might inadvertently reveal cognitive states or medical conditions beyond the intended application scope.
Long-term data stewardship considerations are particularly relevant as bioelectronic data may retain value and sensitivity throughout an individual's lifetime. Establishing appropriate data retention policies, subject access rights, and mechanisms for data portability between systems will be essential components of responsible bioelectronic IoT deployments.
Regulatory frameworks governing this intersection remain fragmented, with the EU's GDPR, the US Health Insurance Portability and Accountability Act (HIPAA), and emerging bioethics guidelines creating a complex compliance landscape. Organizations deploying bioelectronic IoT solutions must navigate these regulations while implementing robust technical safeguards.
Encryption requirements for bioelectronic data transmission present unique challenges due to the real-time nature of many applications. Traditional encryption methods may introduce latency that compromises functionality in time-sensitive bioelectronic applications such as neural interfaces or emergency medical monitoring. Advanced encryption protocols optimized for low-latency transmission while maintaining security integrity are being developed specifically for this domain.
Data minimization principles take on heightened importance in bioelectronic contexts. The principle of collecting only necessary data conflicts with the exploratory nature of many bioelectronic applications where the full utility of collected signals may not be immediately apparent. This tension necessitates new approaches to progressive consent and dynamic data governance models.
Authentication mechanisms for bioelectronic interfaces require innovation beyond traditional methods. Continuous authentication based on the bioelectronic signals themselves—effectively using the unique biological signatures as authentication factors—represents a promising direction. However, this approach introduces questions about revocability and changeability of biometric credentials.
The potential for unauthorized inference presents another critical concern. Even with protected primary data, sophisticated algorithms might derive sensitive information from seemingly innocuous bioelectronic signals. For example, neural activity patterns collected for prosthetic control might inadvertently reveal cognitive states or medical conditions beyond the intended application scope.
Long-term data stewardship considerations are particularly relevant as bioelectronic data may retain value and sensitivity throughout an individual's lifetime. Establishing appropriate data retention policies, subject access rights, and mechanisms for data portability between systems will be essential components of responsible bioelectronic IoT deployments.
Regulatory Framework for Bioelectronic Devices
The regulatory landscape for bioelectronic interfaces in IoT applications presents a complex and evolving framework that varies significantly across global jurisdictions. In the United States, the FDA has established a multi-tiered classification system for bioelectronic devices based on risk levels, with Class I devices requiring minimal oversight while Class III devices demand rigorous premarket approval processes. The FDA's Digital Health Innovation Action Plan specifically addresses software as a medical device (SaMD), which has direct implications for IoT-connected bioelectronic interfaces.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification systems. Notably, the EU has implemented specific provisions for software and AI-enabled bioelectronic devices, emphasizing data protection compliance with GDPR.
In Asia, regulatory approaches vary substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system to expedite approval for innovative medical technologies. China has recently reformed its regulatory framework through the National Medical Products Administration (NMPA), introducing a special approval pathway for innovative medical devices.
Data privacy regulations represent a critical component of the regulatory framework, with particular relevance to bioelectronic IoT devices that collect sensitive physiological data. Beyond GDPR in Europe, the California Consumer Privacy Act (CCPA) and Brazil's Lei Geral de Proteção de Dados (LGPD) exemplify the global trend toward stricter data protection requirements.
Industry standards further complement formal regulations, with organizations like IEEE, ISO, and IEC developing technical specifications for bioelectronic interfaces. The IEEE 11073 standards family specifically addresses interoperability for personal health devices, while ISO 13485 sets quality management standards for medical device manufacturers.
Emerging regulatory challenges include the classification of hybrid bioelectronic-digital products, establishing appropriate cybersecurity protocols, and addressing ethical considerations around autonomy and informed consent. Regulatory bodies are increasingly adopting adaptive licensing approaches that allow for iterative development while maintaining safety standards.
For companies developing bioelectronic interfaces for IoT applications, early engagement with regulatory authorities through pre-submission consultations has proven beneficial in navigating this complex landscape. Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to reduce regulatory fragmentation and establish more consistent global approaches to bioelectronic device oversight.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification systems. Notably, the EU has implemented specific provisions for software and AI-enabled bioelectronic devices, emphasizing data protection compliance with GDPR.
In Asia, regulatory approaches vary substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system to expedite approval for innovative medical technologies. China has recently reformed its regulatory framework through the National Medical Products Administration (NMPA), introducing a special approval pathway for innovative medical devices.
Data privacy regulations represent a critical component of the regulatory framework, with particular relevance to bioelectronic IoT devices that collect sensitive physiological data. Beyond GDPR in Europe, the California Consumer Privacy Act (CCPA) and Brazil's Lei Geral de Proteção de Dados (LGPD) exemplify the global trend toward stricter data protection requirements.
Industry standards further complement formal regulations, with organizations like IEEE, ISO, and IEC developing technical specifications for bioelectronic interfaces. The IEEE 11073 standards family specifically addresses interoperability for personal health devices, while ISO 13485 sets quality management standards for medical device manufacturers.
Emerging regulatory challenges include the classification of hybrid bioelectronic-digital products, establishing appropriate cybersecurity protocols, and addressing ethical considerations around autonomy and informed consent. Regulatory bodies are increasingly adopting adaptive licensing approaches that allow for iterative development while maintaining safety standards.
For companies developing bioelectronic interfaces for IoT applications, early engagement with regulatory authorities through pre-submission consultations has proven beneficial in navigating this complex landscape. Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to reduce regulatory fragmentation and establish more consistent global approaches to bioelectronic device oversight.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!