How Bioelectronic Interfaces Are Shaping Telemedicine Advances
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of these interfaces has been marked by significant milestones over the past three decades, transitioning from rudimentary signal detection systems to sophisticated bidirectional communication platforms that can both monitor and modulate physiological processes.
In the 1990s, early bioelectronic interfaces primarily focused on non-invasive monitoring through electroencephalography (EEG) and electrocardiography (ECG). These technologies provided limited insights into biological signals but established the foundation for future developments. The early 2000s witnessed the emergence of more advanced implantable devices, such as cochlear implants and deep brain stimulators, which demonstrated the potential for electronic devices to directly interface with neural systems.
The 2010s marked a paradigm shift with the development of flexible and stretchable electronics, enabling more comfortable and less intrusive interfaces. Materials science innovations, including conductive polymers and nanomaterials, facilitated the creation of biocompatible devices that could conform to biological tissues while maintaining electronic functionality. This period also saw significant advancements in signal processing algorithms and wireless communication protocols, enhancing the capabilities of bioelectronic systems.
Current bioelectronic interfaces incorporate sophisticated features such as real-time data analytics, artificial intelligence for pattern recognition, and closed-loop feedback systems. These technologies enable personalized interventions based on individual physiological responses, representing a crucial advancement for telemedicine applications. The miniaturization of components has further expanded deployment possibilities, allowing for less invasive monitoring and treatment options.
The primary objectives of bioelectronic interface development in telemedicine include enhancing remote patient monitoring capabilities, enabling precise therapeutic interventions, and improving accessibility to specialized healthcare services. These interfaces aim to provide continuous, real-time physiological data collection while minimizing patient discomfort and disruption to daily activities. Additionally, they seek to establish reliable bidirectional communication channels between patients and healthcare providers, facilitating timely interventions and adjustments to treatment protocols.
Looking forward, the field is trending toward increased integration with artificial intelligence, improved biocompatibility through novel materials, enhanced energy efficiency, and expanded wireless capabilities. These developments collectively aim to create more seamless, intuitive, and effective connections between electronic systems and biological processes, ultimately transforming the landscape of remote healthcare delivery and patient management in the telemedicine ecosystem.
In the 1990s, early bioelectronic interfaces primarily focused on non-invasive monitoring through electroencephalography (EEG) and electrocardiography (ECG). These technologies provided limited insights into biological signals but established the foundation for future developments. The early 2000s witnessed the emergence of more advanced implantable devices, such as cochlear implants and deep brain stimulators, which demonstrated the potential for electronic devices to directly interface with neural systems.
The 2010s marked a paradigm shift with the development of flexible and stretchable electronics, enabling more comfortable and less intrusive interfaces. Materials science innovations, including conductive polymers and nanomaterials, facilitated the creation of biocompatible devices that could conform to biological tissues while maintaining electronic functionality. This period also saw significant advancements in signal processing algorithms and wireless communication protocols, enhancing the capabilities of bioelectronic systems.
Current bioelectronic interfaces incorporate sophisticated features such as real-time data analytics, artificial intelligence for pattern recognition, and closed-loop feedback systems. These technologies enable personalized interventions based on individual physiological responses, representing a crucial advancement for telemedicine applications. The miniaturization of components has further expanded deployment possibilities, allowing for less invasive monitoring and treatment options.
The primary objectives of bioelectronic interface development in telemedicine include enhancing remote patient monitoring capabilities, enabling precise therapeutic interventions, and improving accessibility to specialized healthcare services. These interfaces aim to provide continuous, real-time physiological data collection while minimizing patient discomfort and disruption to daily activities. Additionally, they seek to establish reliable bidirectional communication channels between patients and healthcare providers, facilitating timely interventions and adjustments to treatment protocols.
Looking forward, the field is trending toward increased integration with artificial intelligence, improved biocompatibility through novel materials, enhanced energy efficiency, and expanded wireless capabilities. These developments collectively aim to create more seamless, intuitive, and effective connections between electronic systems and biological processes, ultimately transforming the landscape of remote healthcare delivery and patient management in the telemedicine ecosystem.
Telemedicine Market Demand Analysis
The telemedicine market has experienced unprecedented growth in recent years, accelerated significantly by the COVID-19 pandemic. According to Fortune Business Insights, the global telemedicine market size was valued at $87.41 billion in 2022 and is projected to grow to $286.21 billion by 2030, with a compound annual growth rate (CAGR) of 16.8%. This remarkable expansion reflects the increasing acceptance of remote healthcare solutions among patients, providers, and payers alike.
Bioelectronic interfaces are emerging as a critical driver in this market evolution. The integration of these technologies addresses several key market demands that traditional telemedicine approaches cannot fulfill. Primary among these is the need for more accurate remote patient monitoring capabilities. Market research by Grand View Research indicates that the remote patient monitoring segment of telemedicine is expected to grow at a CAGR of 19.7% through 2028, outpacing other segments.
Consumer demand for personalized healthcare experiences represents another significant market force. A survey by Accenture revealed that 75% of patients expect personalized healthcare experiences, including those delivered via telemedicine platforms. Bioelectronic interfaces enable this personalization by providing continuous, real-time physiological data that allows for more tailored treatment approaches and interventions.
The aging global population is creating substantial market pressure for innovative telemedicine solutions. The United Nations projects that by 2050, one in six people worldwide will be over 65 years old, up from one in eleven in 2019. This demographic shift is driving demand for remote monitoring technologies that can help manage chronic conditions while allowing seniors to maintain independence. Bioelectronic interfaces that can monitor vital signs, medication adherence, and mobility patterns are particularly valuable in this context.
Healthcare provider organizations are increasingly seeking telemedicine solutions that integrate seamlessly with existing workflows and electronic health record systems. Market analysis by Deloitte shows that 84% of healthcare executives consider interoperability a top priority for telemedicine implementation. Bioelectronic interfaces that can collect, analyze, and transmit patient data directly to clinical systems address this critical market need.
The market also shows strong regional variations in demand patterns. North America currently dominates the telemedicine market with approximately 41% market share, driven by advanced healthcare infrastructure and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the fastest growth rate, with increasing smartphone penetration, rising healthcare expenditure, and government initiatives supporting telemedicine adoption.
Bioelectronic interfaces are emerging as a critical driver in this market evolution. The integration of these technologies addresses several key market demands that traditional telemedicine approaches cannot fulfill. Primary among these is the need for more accurate remote patient monitoring capabilities. Market research by Grand View Research indicates that the remote patient monitoring segment of telemedicine is expected to grow at a CAGR of 19.7% through 2028, outpacing other segments.
Consumer demand for personalized healthcare experiences represents another significant market force. A survey by Accenture revealed that 75% of patients expect personalized healthcare experiences, including those delivered via telemedicine platforms. Bioelectronic interfaces enable this personalization by providing continuous, real-time physiological data that allows for more tailored treatment approaches and interventions.
The aging global population is creating substantial market pressure for innovative telemedicine solutions. The United Nations projects that by 2050, one in six people worldwide will be over 65 years old, up from one in eleven in 2019. This demographic shift is driving demand for remote monitoring technologies that can help manage chronic conditions while allowing seniors to maintain independence. Bioelectronic interfaces that can monitor vital signs, medication adherence, and mobility patterns are particularly valuable in this context.
Healthcare provider organizations are increasingly seeking telemedicine solutions that integrate seamlessly with existing workflows and electronic health record systems. Market analysis by Deloitte shows that 84% of healthcare executives consider interoperability a top priority for telemedicine implementation. Bioelectronic interfaces that can collect, analyze, and transmit patient data directly to clinical systems address this critical market need.
The market also shows strong regional variations in demand patterns. North America currently dominates the telemedicine market with approximately 41% market share, driven by advanced healthcare infrastructure and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the fastest growth rate, with increasing smartphone penetration, rising healthcare expenditure, and government initiatives supporting telemedicine adoption.
Current Bioelectronic Interface Challenges
Despite significant advancements in bioelectronic interfaces for telemedicine, several critical challenges continue to impede widespread adoption and optimal functionality. Signal quality and reliability remain primary concerns, as bioelectronic sensors often struggle with maintaining consistent performance across diverse patient physiologies and environmental conditions. Interference from ambient electromagnetic fields, motion artifacts, and biological processes frequently compromises data integrity, particularly in non-clinical settings where controlled conditions cannot be maintained.
Miniaturization presents another significant hurdle, as developers strive to balance functionality with patient comfort and usability. Current wearable bioelectronic interfaces often face trade-offs between battery life, processing power, and form factor, limiting their practical application for continuous remote monitoring scenarios essential to effective telemedicine.
Power management constitutes a persistent challenge, with many advanced bioelectronic interfaces requiring frequent recharging or battery replacement. This limitation particularly affects implantable devices and continuous monitoring systems where power interruptions can result in critical data gaps. Emerging energy harvesting technologies show promise but remain insufficient for high-demand applications.
Biocompatibility and long-term tissue integration represent crucial barriers, especially for implantable interfaces. Current materials often trigger inflammatory responses or experience performance degradation over time due to biofouling and tissue encapsulation. These issues significantly impact device longevity and reliability in clinical applications.
Data security and privacy vulnerabilities pose increasingly complex challenges as bioelectronic interfaces collect and transmit sensitive health information. Many current systems lack robust encryption protocols or secure authentication mechanisms, creating potential exposure points for patient data. The regulatory landscape surrounding these security requirements remains fragmented globally.
Interoperability between different bioelectronic platforms and existing healthcare information systems presents substantial integration difficulties. The absence of standardized communication protocols and data formats results in isolated technological ecosystems that cannot effectively share critical patient information across the care continuum.
Cost factors continue to limit accessibility, with advanced bioelectronic interfaces often requiring expensive materials, sophisticated manufacturing processes, and specialized expertise for implementation and maintenance. These economic barriers disproportionately affect resource-limited healthcare settings and underserved populations, creating equity concerns in telemedicine advancement.
Miniaturization presents another significant hurdle, as developers strive to balance functionality with patient comfort and usability. Current wearable bioelectronic interfaces often face trade-offs between battery life, processing power, and form factor, limiting their practical application for continuous remote monitoring scenarios essential to effective telemedicine.
Power management constitutes a persistent challenge, with many advanced bioelectronic interfaces requiring frequent recharging or battery replacement. This limitation particularly affects implantable devices and continuous monitoring systems where power interruptions can result in critical data gaps. Emerging energy harvesting technologies show promise but remain insufficient for high-demand applications.
Biocompatibility and long-term tissue integration represent crucial barriers, especially for implantable interfaces. Current materials often trigger inflammatory responses or experience performance degradation over time due to biofouling and tissue encapsulation. These issues significantly impact device longevity and reliability in clinical applications.
Data security and privacy vulnerabilities pose increasingly complex challenges as bioelectronic interfaces collect and transmit sensitive health information. Many current systems lack robust encryption protocols or secure authentication mechanisms, creating potential exposure points for patient data. The regulatory landscape surrounding these security requirements remains fragmented globally.
Interoperability between different bioelectronic platforms and existing healthcare information systems presents substantial integration difficulties. The absence of standardized communication protocols and data formats results in isolated technological ecosystems that cannot effectively share critical patient information across the care continuum.
Cost factors continue to limit accessibility, with advanced bioelectronic interfaces often requiring expensive materials, sophisticated manufacturing processes, and specialized expertise for implementation and maintenance. These economic barriers disproportionately affect resource-limited healthcare settings and underserved populations, creating equity concerns in telemedicine advancement.
Current Bioelectronic Interface Solutions
01 Neural interfaces for bioelectronic applications
Neural interfaces are a key component in bioelectronic systems that establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity or deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create biocompatible neural electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces enable applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders.- Neural interfaces for bioelectronic applications: Neural interfaces are a key component in bioelectronic systems that enable direct communication between electronic devices and the nervous system. These interfaces can record neural activity and deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create flexible, biocompatible neural electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces find applications in neuroprosthetics, brain-computer interfaces, and therapeutic devices for neurological disorders.
- Implantable bioelectronic devices: Implantable bioelectronic devices are designed to function within the body for extended periods, requiring specific considerations for biocompatibility, power supply, and communication capabilities. These devices incorporate miniaturized electronics, sensors, and stimulation components encapsulated in biocompatible materials to prevent rejection by the body. Advanced implantable systems may include wireless power transfer mechanisms, biodegradable components, or closed-loop control systems that can adjust therapy based on physiological feedback, enabling personalized treatment approaches for various medical conditions.
- Biosensing and molecular detection interfaces: Bioelectronic interfaces for biosensing applications incorporate biological recognition elements with electronic transduction mechanisms to detect specific biomolecules, cells, or physiological parameters. These interfaces may utilize electrochemical, optical, or mechanical sensing principles to convert biological interactions into measurable electronic signals. Advanced biosensing platforms integrate nanomaterials, microfluidics, and signal processing to achieve high sensitivity, specificity, and multiplexing capabilities for applications in medical diagnostics, environmental monitoring, and biomedical research.
- Flexible and wearable bioelectronic interfaces: Flexible and wearable bioelectronic interfaces are designed to conform to the body's contours while maintaining reliable electrical performance. These interfaces utilize stretchable substrates, conductive polymers, and novel fabrication techniques to create devices that can withstand mechanical deformation while maintaining functionality. Wearable bioelectronic systems may incorporate multiple sensing modalities, wireless communication capabilities, and energy harvesting components to enable continuous health monitoring and personalized healthcare applications without restricting user mobility or comfort.
- Bioelectronic interfaces for tissue engineering and regenerative medicine: Bioelectronic interfaces for tissue engineering applications combine electronic components with biological scaffolds to guide cell growth, monitor tissue development, and deliver electrical stimulation to enhance regeneration. These interfaces may incorporate conductive biomaterials, electrode arrays, and stimulation protocols designed to mimic natural bioelectrical cues present during development and healing. Advanced systems may feature biodegradable electronics that provide therapeutic stimulation during critical healing phases and then safely degrade, eliminating the need for removal procedures and reducing long-term foreign body responses.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic and curved surfaces of biological tissues. These interfaces incorporate elastic substrates, serpentine interconnects, and thin-film electronics to achieve mechanical compliance with soft tissues while maintaining electronic functionality. The flexibility reduces mechanical mismatch between rigid electronics and soft biological tissues, minimizing inflammation and improving long-term stability for applications such as epidermal electronics, implantable sensors, and wearable health monitoring devices.Expand Specific Solutions03 Biosensing interfaces with molecular recognition elements
Bioelectronic interfaces incorporating molecular recognition elements enable highly specific detection of biological analytes. These interfaces integrate biomolecules such as antibodies, enzymes, DNA aptamers, or synthetic receptors with electronic transducers to convert biological binding events into measurable electrical signals. Advanced surface chemistry techniques are employed to immobilize these recognition elements while maintaining their biological activity. These biosensing platforms offer high sensitivity, selectivity, and potential for miniaturization for applications in medical diagnostics, environmental monitoring, and point-of-care testing.Expand Specific Solutions04 Wireless bioelectronic interfaces for implantable devices
Wireless bioelectronic interfaces enable power and data transmission to implantable medical devices without physical connections through tissue. These interfaces utilize technologies such as radiofrequency communication, inductive coupling, ultrasonic transmission, or optical methods to establish wireless links. Energy harvesting mechanisms may be incorporated to extend device lifetime and reduce battery size. The wireless approach minimizes infection risk associated with transcutaneous wires and allows for deeper implantation while enabling continuous monitoring and control of implanted bioelectronic systems.Expand Specific Solutions05 Organic and biomaterial-based bioelectronic interfaces
Organic and biomaterial-based bioelectronic interfaces bridge the gap between conventional electronics and biological systems by using materials with similar mechanical and chemical properties to living tissues. These interfaces incorporate conducting polymers, hydrogels, protein-based materials, or hybrid composites that can transduce ionic biological signals into electronic signals. The use of soft, biocompatible materials reduces foreign body response and improves integration with host tissues. These interfaces enable applications in tissue engineering, drug delivery systems, bioelectronic medicine, and regenerative therapies.Expand Specific Solutions
Leading Companies in Bioelectronic Telemedicine
Bioelectronic interfaces in telemedicine are evolving rapidly, currently in a growth phase characterized by expanding applications and increasing market adoption. The global market is experiencing significant expansion, projected to reach billions in value as healthcare systems increasingly embrace remote monitoring solutions. Technologically, the field shows varying maturity levels across different applications. Leading players like Medtronic and Johnson & Johnson are advancing clinical-grade solutions, while MIT and University of Rochester drive fundamental research innovations. Teladoc Health is establishing telehealth platforms that integrate these technologies, and companies like 3M and OMRON HEALTHCARE are developing consumer-facing bioelectronic monitoring devices. Emerging players such as Precision Biosensor and ROM Technologies are introducing specialized applications, indicating a competitive landscape that balances established medical device manufacturers with innovative startups and research institutions.
Teladoc Health, Inc.
Technical Solution: Teladoc Health has pioneered a comprehensive bioelectronic interface platform specifically designed for telemedicine applications. Their system integrates multiple non-invasive biometric monitoring devices with their proprietary virtual care delivery platform. The technology utilizes a network of wearable sensors that capture real-time physiological data including ECG, blood pressure, blood glucose, respiratory rate, and temperature. These sensors employ advanced bioelectronic interfaces that maximize signal quality while minimizing patient discomfort. Data is transmitted securely to Teladoc's cloud infrastructure where sophisticated algorithms analyze patterns and flag potential health concerns. Their platform incorporates machine learning capabilities that improve diagnostic accuracy over time by learning from patient outcomes. Teladoc has also developed specialized interfaces for neurological assessment that can measure subtle changes in motor function and cognitive performance remotely. The system features bidirectional communication, allowing physicians to not only receive data but also send commands to therapeutic devices when necessary[2][5].
Strengths: Market leader in telemedicine with extensive provider network and established patient base, allowing rapid scaling of new bioelectronic interfaces. Their platform offers exceptional user experience and integration with existing healthcare systems. Weaknesses: Reliance on third-party sensor manufacturers may limit technical innovation, and their business model faces increasing competition from healthcare systems developing in-house telemedicine capabilities.
The Regents of the University of California
Technical Solution: The University of California has developed groundbreaking bioelectronic interfaces through its multi-campus research initiatives. Their technology focuses on ultra-flexible, biodegradable electronics that conform to biological tissues for enhanced signal quality and patient comfort. UC researchers have pioneered "electronic tattoo" technology—ultrathin devices that adhere directly to skin and can monitor multiple physiological parameters simultaneously. These interfaces utilize advanced materials including graphene and biodegradable polymers that minimize foreign body responses while maintaining excellent electrical properties. The UC system has also developed implantable neural interfaces that can record from and stimulate thousands of individual neurons, enabling unprecedented detail in remote neurological monitoring. Their bioelectronic platforms incorporate energy harvesting from body movement and temperature gradients, eliminating the need for battery replacement in long-term monitoring applications. UC researchers have created closed-loop systems that can detect neurological events such as seizures and automatically deliver appropriate therapeutic stimulation, all while transmitting data to remote clinicians. Their technology includes specialized interfaces for monitoring gastrointestinal electrical activity, enabling remote diagnosis of motility disorders that previously required in-person hospitalization[9][11].
Strengths: Cutting-edge research capabilities across multiple disciplines (materials science, electrical engineering, medicine) enable development of next-generation bioelectronic interfaces. Their academic approach facilitates open innovation and collaboration with industry partners. Weaknesses: Longer commercialization timeline compared to industry players, and potential challenges in scaling laboratory prototypes to mass production while maintaining performance characteristics.
Key Patents in Bioelectronic-Telemedicine Integration
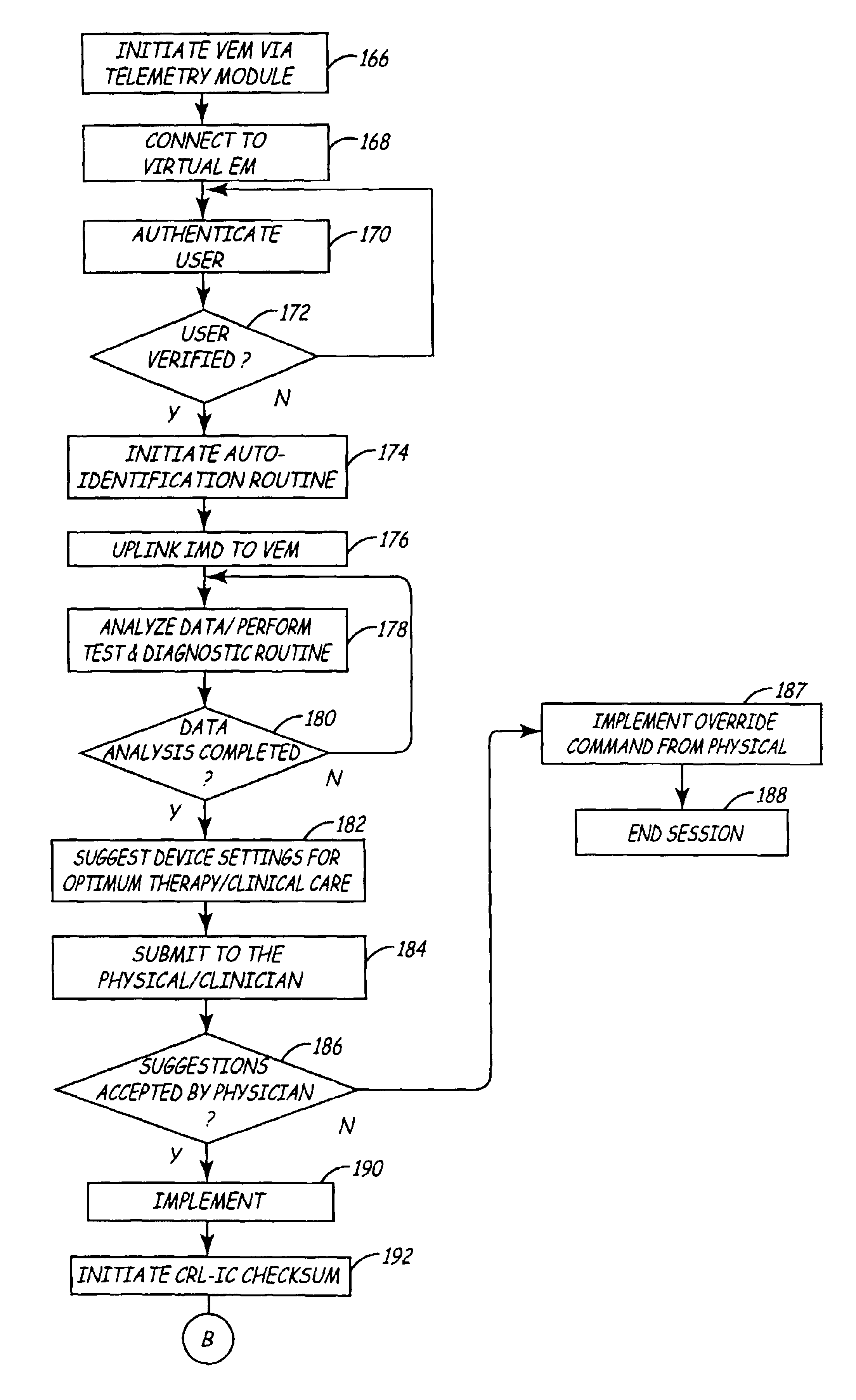
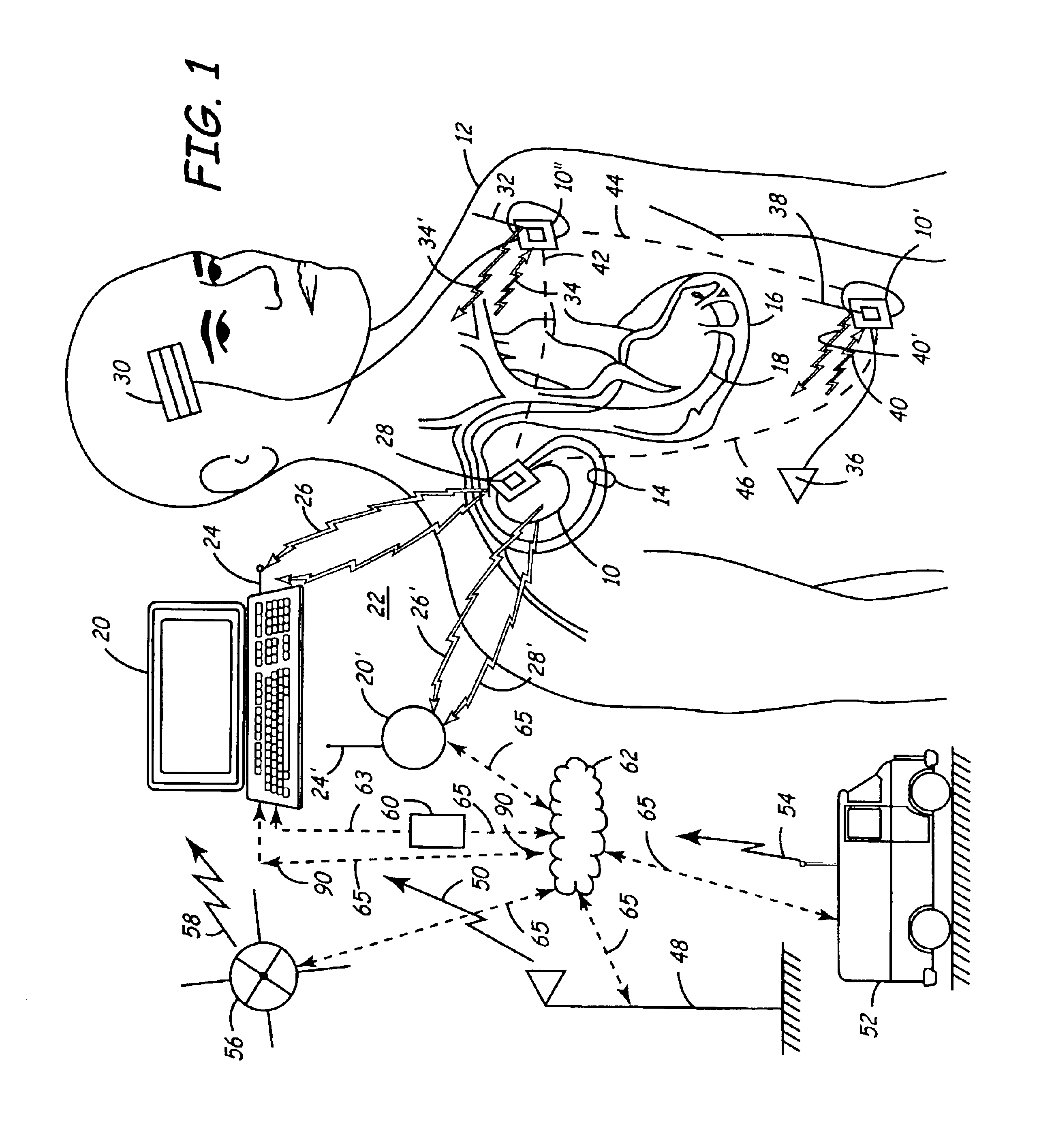
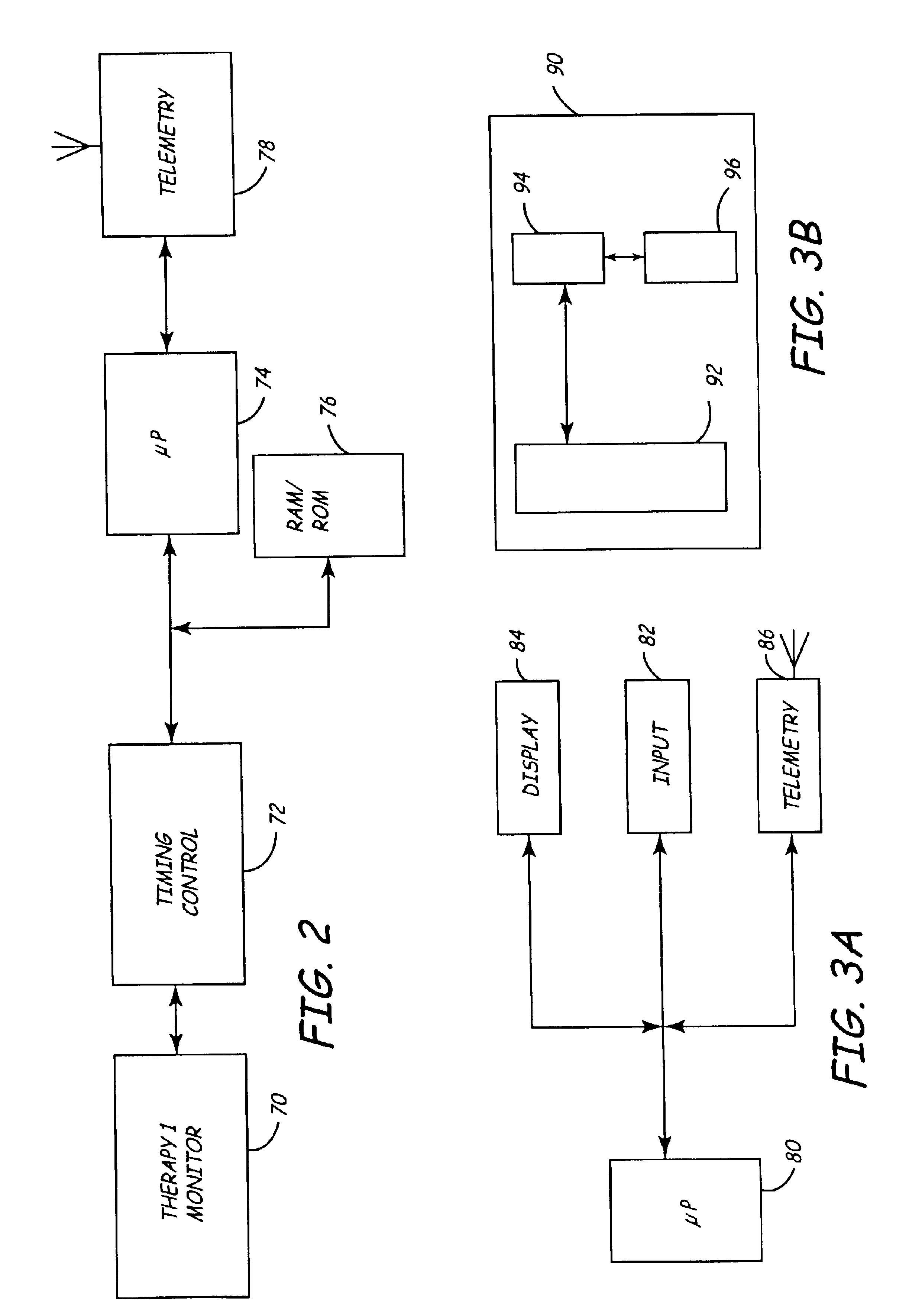
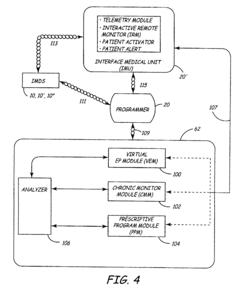
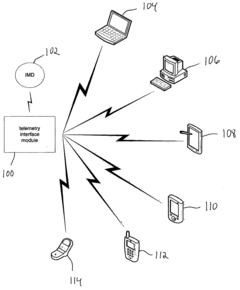
Virtual remote monitor, alert, diagnostics and programming for implantable medical device systems
PatentInactiveUS6878112B2
Innovation
- A web-based expert data center enables bi-directional communication with IMDs via a programmer, allowing real-time remote monitoring, maintenance, and software upgrades using various communication networks, including the Internet, to manage and tune the operational and functional parameters of IMDs, facilitating remote diagnosis, maintenance, and software updates.
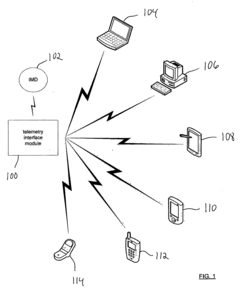
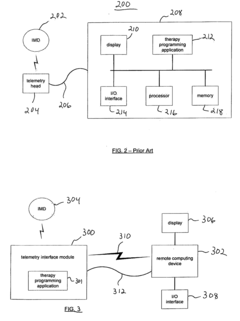
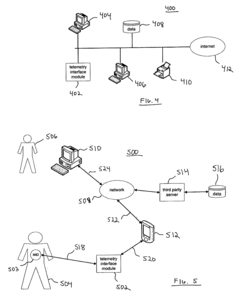
Apparatus and method for serving medical device application content to a remote computing device
PatentInactiveUS20050061336A1
Innovation
- A telemetry interface module that decouples from specific computing devices, using standard communication protocols to communicate with general-purpose computing devices, allowing for hardware-agnostic IMD programming and leveraging existing computing hardware to facilitate enhanced data transfer and remote network-based programming.
Data Security and Privacy Considerations
As bioelectronic interfaces increasingly integrate with telemedicine systems, data security and privacy considerations have become paramount concerns. The sensitive nature of health data collected through these interfaces—ranging from neural signals to physiological parameters—demands robust protection frameworks. Current regulatory landscapes, including HIPAA in the United States and GDPR in Europe, provide baseline requirements but often lag behind the rapid technological advancements in bioelectronic telemedicine.
Encryption technologies represent the first line of defense, with end-to-end encryption becoming standard practice for data transmission between bioelectronic devices and healthcare providers. However, the miniaturization of bioelectronic interfaces presents unique challenges, as these devices often have limited computational resources for implementing sophisticated encryption algorithms. This constraint necessitates innovative approaches to lightweight cryptography that maintain security without compromising device performance.
Authentication mechanisms for bioelectronic interfaces have evolved beyond traditional password systems to include biometric verification, which leverages the unique physiological signatures that these interfaces can detect. This approach enhances security while improving user experience, though it introduces additional privacy considerations regarding the storage and processing of biometric identifiers.
Data minimization principles are increasingly being applied to bioelectronic telemedicine systems, ensuring that only essential health information is collected and transmitted. This approach not only reduces security risks but also addresses privacy concerns by limiting the scope of potential data breaches. Complementary to this, anonymization and pseudonymization techniques are being refined to protect patient identities while preserving the analytical value of collected data for research and system improvement.
Consent management frameworks have become more sophisticated, allowing patients greater control over how their bioelectronic data is used. Dynamic consent models enable individuals to modify their preferences over time, reflecting the ongoing nature of data collection through implanted or wearable bioelectronic interfaces. These frameworks must balance comprehensive information disclosure with usability to ensure meaningful consent.
Cross-border data transfer presents particular challenges for bioelectronic telemedicine, as regulatory requirements vary significantly between jurisdictions. International standards and certification processes are emerging to facilitate secure and compliant data sharing, though harmonization efforts remain incomplete. The development of federated learning approaches offers promising solutions by enabling analysis across distributed datasets without centralizing sensitive information.
As bioelectronic interfaces become more integrated with artificial intelligence systems for telemedicine applications, additional privacy considerations arise regarding algorithmic transparency and the potential for unintended inferences from seemingly innocuous data. Addressing these concerns requires interdisciplinary collaboration between technologists, healthcare professionals, ethicists, and policy makers to establish comprehensive governance frameworks that protect individual rights while enabling beneficial innovation.
Encryption technologies represent the first line of defense, with end-to-end encryption becoming standard practice for data transmission between bioelectronic devices and healthcare providers. However, the miniaturization of bioelectronic interfaces presents unique challenges, as these devices often have limited computational resources for implementing sophisticated encryption algorithms. This constraint necessitates innovative approaches to lightweight cryptography that maintain security without compromising device performance.
Authentication mechanisms for bioelectronic interfaces have evolved beyond traditional password systems to include biometric verification, which leverages the unique physiological signatures that these interfaces can detect. This approach enhances security while improving user experience, though it introduces additional privacy considerations regarding the storage and processing of biometric identifiers.
Data minimization principles are increasingly being applied to bioelectronic telemedicine systems, ensuring that only essential health information is collected and transmitted. This approach not only reduces security risks but also addresses privacy concerns by limiting the scope of potential data breaches. Complementary to this, anonymization and pseudonymization techniques are being refined to protect patient identities while preserving the analytical value of collected data for research and system improvement.
Consent management frameworks have become more sophisticated, allowing patients greater control over how their bioelectronic data is used. Dynamic consent models enable individuals to modify their preferences over time, reflecting the ongoing nature of data collection through implanted or wearable bioelectronic interfaces. These frameworks must balance comprehensive information disclosure with usability to ensure meaningful consent.
Cross-border data transfer presents particular challenges for bioelectronic telemedicine, as regulatory requirements vary significantly between jurisdictions. International standards and certification processes are emerging to facilitate secure and compliant data sharing, though harmonization efforts remain incomplete. The development of federated learning approaches offers promising solutions by enabling analysis across distributed datasets without centralizing sensitive information.
As bioelectronic interfaces become more integrated with artificial intelligence systems for telemedicine applications, additional privacy considerations arise regarding algorithmic transparency and the potential for unintended inferences from seemingly innocuous data. Addressing these concerns requires interdisciplinary collaboration between technologists, healthcare professionals, ethicists, and policy makers to establish comprehensive governance frameworks that protect individual rights while enabling beneficial innovation.
Regulatory Framework for Bioelectronic Telemedicine
The regulatory landscape for bioelectronic telemedicine represents a complex intersection of medical device regulation, telecommunications standards, data privacy laws, and healthcare delivery protocols. Currently, the FDA has established a Digital Health Innovation Action Plan that addresses software as a medical device (SaMD) and provides regulatory pathways for bioelectronic interfaces used in telemedicine. These frameworks include the Pre-Certification Program, which evaluates the quality and organizational excellence of companies rather than focusing solely on individual products.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have created more stringent requirements for bioelectronic devices, particularly regarding clinical evidence, post-market surveillance, and unique device identification. These regulations have significant implications for telemedicine applications utilizing bioelectronic interfaces, as they must demonstrate both safety and performance efficacy before market approval.
Data privacy considerations present another critical regulatory dimension. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe establish strict requirements for handling patient data collected through bioelectronic interfaces. These regulations mandate secure transmission protocols, encryption standards, and explicit patient consent mechanisms for data collection and processing.
Reimbursement policies also significantly impact the adoption of bioelectronic telemedicine solutions. The Centers for Medicare & Medicaid Services (CMS) has expanded coverage for remote patient monitoring and telehealth services, particularly accelerated by the COVID-19 pandemic. However, permanent reimbursement pathways for novel bioelectronic applications remain inconsistent across different healthcare systems and insurance providers.
International standardization efforts are emerging to address interoperability challenges. The International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) have developed standards such as ISO/IEEE 11073 for health informatics and medical device communication, which are increasingly important for bioelectronic telemedicine applications that must function across different platforms and healthcare systems.
Regulatory harmonization initiatives, including the International Medical Device Regulators Forum (IMDRF), are working to align global approaches to bioelectronic device regulation. These efforts aim to reduce redundant testing requirements and streamline approval processes while maintaining safety standards, potentially accelerating innovation in bioelectronic telemedicine technologies.
The evolving nature of bioelectronic interfaces presents ongoing regulatory challenges, particularly regarding artificial intelligence and machine learning components that may change functionality over time. Regulatory bodies are developing frameworks for these "adaptive" technologies, with the FDA's proposed regulatory framework for modifications to AI/ML-based software as a medical device representing a significant step toward addressing these novel regulatory questions.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have created more stringent requirements for bioelectronic devices, particularly regarding clinical evidence, post-market surveillance, and unique device identification. These regulations have significant implications for telemedicine applications utilizing bioelectronic interfaces, as they must demonstrate both safety and performance efficacy before market approval.
Data privacy considerations present another critical regulatory dimension. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe establish strict requirements for handling patient data collected through bioelectronic interfaces. These regulations mandate secure transmission protocols, encryption standards, and explicit patient consent mechanisms for data collection and processing.
Reimbursement policies also significantly impact the adoption of bioelectronic telemedicine solutions. The Centers for Medicare & Medicaid Services (CMS) has expanded coverage for remote patient monitoring and telehealth services, particularly accelerated by the COVID-19 pandemic. However, permanent reimbursement pathways for novel bioelectronic applications remain inconsistent across different healthcare systems and insurance providers.
International standardization efforts are emerging to address interoperability challenges. The International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) have developed standards such as ISO/IEEE 11073 for health informatics and medical device communication, which are increasingly important for bioelectronic telemedicine applications that must function across different platforms and healthcare systems.
Regulatory harmonization initiatives, including the International Medical Device Regulators Forum (IMDRF), are working to align global approaches to bioelectronic device regulation. These efforts aim to reduce redundant testing requirements and streamline approval processes while maintaining safety standards, potentially accelerating innovation in bioelectronic telemedicine technologies.
The evolving nature of bioelectronic interfaces presents ongoing regulatory challenges, particularly regarding artificial intelligence and machine learning components that may change functionality over time. Regulatory bodies are developing frameworks for these "adaptive" technologies, with the FDA's proposed regulatory framework for modifications to AI/ML-based software as a medical device representing a significant step toward addressing these novel regulatory questions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!