How Bioelectronic Interfaces Enable Advanced Diagnostic Platforms
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of biology and electronics, enabling direct communication between biological systems and electronic devices. The evolution of these interfaces traces back to the 1970s with the development of the first biosensors, which could detect specific biological analytes through electrochemical reactions. These early interfaces were rudimentary, offering limited sensitivity and specificity, but laid the foundation for subsequent innovations.
The 1990s witnessed significant advancements with the introduction of microelectronic fabrication techniques, allowing for miniaturization and integration of bioelectronic components. This period marked the transition from macro-scale to micro-scale interfaces, enabling more precise measurements and expanding potential applications in medical diagnostics.
The early 2000s brought nanotechnology into bioelectronic interfaces, dramatically enhancing sensitivity through nanomaterials like carbon nanotubes, quantum dots, and gold nanoparticles. These nanomaterials provided unprecedented surface-to-volume ratios and unique electrical properties, allowing detection of biomarkers at previously unattainable concentrations.
Recent developments have focused on flexible and stretchable bioelectronics, addressing the fundamental mechanical mismatch between rigid electronic components and soft biological tissues. Materials science breakthroughs have enabled the creation of conformable interfaces that maintain electrical performance while adapting to biological surfaces, significantly improving signal quality and user comfort.
The primary objective of modern bioelectronic interfaces in diagnostic platforms is to achieve real-time, highly sensitive, and specific detection of multiple biomarkers simultaneously. This includes developing interfaces capable of detecting disease biomarkers at ultra-low concentrations, potentially enabling earlier diagnosis of conditions like cancer and neurodegenerative diseases.
Another critical goal is creating interfaces that remain stable in complex biological environments, resisting biofouling and maintaining performance over extended periods. This stability is essential for reliable long-term monitoring applications and implantable diagnostic devices.
Miniaturization and power efficiency represent additional objectives, with researchers aiming to develop bioelectronic interfaces that can function with minimal power requirements and potentially harvest energy from the biological environment itself. This would enable truly autonomous diagnostic platforms suitable for continuous monitoring.
The integration of wireless communication capabilities constitutes another key objective, allowing bioelectronic diagnostic platforms to transmit data to healthcare providers in real-time, facilitating remote monitoring and timely interventions. This connectivity aspect is particularly relevant in the context of telemedicine and personalized healthcare.
The 1990s witnessed significant advancements with the introduction of microelectronic fabrication techniques, allowing for miniaturization and integration of bioelectronic components. This period marked the transition from macro-scale to micro-scale interfaces, enabling more precise measurements and expanding potential applications in medical diagnostics.
The early 2000s brought nanotechnology into bioelectronic interfaces, dramatically enhancing sensitivity through nanomaterials like carbon nanotubes, quantum dots, and gold nanoparticles. These nanomaterials provided unprecedented surface-to-volume ratios and unique electrical properties, allowing detection of biomarkers at previously unattainable concentrations.
Recent developments have focused on flexible and stretchable bioelectronics, addressing the fundamental mechanical mismatch between rigid electronic components and soft biological tissues. Materials science breakthroughs have enabled the creation of conformable interfaces that maintain electrical performance while adapting to biological surfaces, significantly improving signal quality and user comfort.
The primary objective of modern bioelectronic interfaces in diagnostic platforms is to achieve real-time, highly sensitive, and specific detection of multiple biomarkers simultaneously. This includes developing interfaces capable of detecting disease biomarkers at ultra-low concentrations, potentially enabling earlier diagnosis of conditions like cancer and neurodegenerative diseases.
Another critical goal is creating interfaces that remain stable in complex biological environments, resisting biofouling and maintaining performance over extended periods. This stability is essential for reliable long-term monitoring applications and implantable diagnostic devices.
Miniaturization and power efficiency represent additional objectives, with researchers aiming to develop bioelectronic interfaces that can function with minimal power requirements and potentially harvest energy from the biological environment itself. This would enable truly autonomous diagnostic platforms suitable for continuous monitoring.
The integration of wireless communication capabilities constitutes another key objective, allowing bioelectronic diagnostic platforms to transmit data to healthcare providers in real-time, facilitating remote monitoring and timely interventions. This connectivity aspect is particularly relevant in the context of telemedicine and personalized healthcare.
Market Analysis for Advanced Diagnostic Platforms
The global market for advanced diagnostic platforms is experiencing robust growth, driven by increasing demand for early disease detection and personalized medicine. The bioelectronic interface segment within this market is projected to grow at a compound annual growth rate of 14.2% from 2023 to 2030, reaching a market value of $18.7 billion by 2030. This growth trajectory is supported by rising healthcare expenditure worldwide and greater emphasis on preventive healthcare approaches.
North America currently dominates the advanced diagnostic platforms market, accounting for approximately 42% of global market share. This dominance stems from well-established healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with anticipated growth rates exceeding 16% annually, fueled by improving healthcare access and rising chronic disease prevalence.
The COVID-19 pandemic has significantly accelerated market development, creating unprecedented demand for rapid, accurate diagnostic solutions. This catalyst effect has compressed innovation timelines and attracted substantial investment capital to the sector. Post-pandemic, the market continues to benefit from heightened awareness of diagnostic importance and increased healthcare system preparedness funding.
Key market segments within bioelectronic diagnostic platforms include point-of-care testing devices, wearable diagnostics, lab-on-chip technologies, and implantable sensors. Point-of-care testing represents the largest segment at 38% market share, while wearable diagnostics demonstrates the highest growth potential with projected 17.5% annual growth through 2030.
Consumer behavior is shifting toward greater engagement with personal health monitoring, creating expanded opportunities for direct-to-consumer diagnostic solutions. This trend is complemented by healthcare providers seeking more efficient diagnostic workflows to manage increasing patient volumes and control costs. The convergence of these demand factors is creating a favorable environment for innovative diagnostic platforms.
Reimbursement landscapes are evolving to accommodate novel diagnostic approaches, though regulatory approval pathways remain a significant market entry barrier. Markets with streamlined regulatory frameworks for digital health and diagnostic technologies are experiencing accelerated innovation cycles and earlier technology adoption.
Strategic partnerships between technology developers, healthcare providers, and payers are emerging as a dominant market access strategy, enabling faster commercialization pathways and broader distribution channels for advanced diagnostic solutions incorporating bioelectronic interfaces.
North America currently dominates the advanced diagnostic platforms market, accounting for approximately 42% of global market share. This dominance stems from well-established healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with anticipated growth rates exceeding 16% annually, fueled by improving healthcare access and rising chronic disease prevalence.
The COVID-19 pandemic has significantly accelerated market development, creating unprecedented demand for rapid, accurate diagnostic solutions. This catalyst effect has compressed innovation timelines and attracted substantial investment capital to the sector. Post-pandemic, the market continues to benefit from heightened awareness of diagnostic importance and increased healthcare system preparedness funding.
Key market segments within bioelectronic diagnostic platforms include point-of-care testing devices, wearable diagnostics, lab-on-chip technologies, and implantable sensors. Point-of-care testing represents the largest segment at 38% market share, while wearable diagnostics demonstrates the highest growth potential with projected 17.5% annual growth through 2030.
Consumer behavior is shifting toward greater engagement with personal health monitoring, creating expanded opportunities for direct-to-consumer diagnostic solutions. This trend is complemented by healthcare providers seeking more efficient diagnostic workflows to manage increasing patient volumes and control costs. The convergence of these demand factors is creating a favorable environment for innovative diagnostic platforms.
Reimbursement landscapes are evolving to accommodate novel diagnostic approaches, though regulatory approval pathways remain a significant market entry barrier. Markets with streamlined regulatory frameworks for digital health and diagnostic technologies are experiencing accelerated innovation cycles and earlier technology adoption.
Strategic partnerships between technology developers, healthcare providers, and payers are emerging as a dominant market access strategy, enabling faster commercialization pathways and broader distribution channels for advanced diagnostic solutions incorporating bioelectronic interfaces.
Current Bioelectronic Interface Challenges
Despite significant advancements in bioelectronic interfaces for diagnostic platforms, several critical challenges continue to impede their widespread adoption and optimal functionality. Signal-to-noise ratio remains a fundamental obstacle, particularly in non-invasive sensing applications where biological signals are often weak and susceptible to environmental interference. This challenge is especially pronounced in continuous monitoring scenarios where signal quality must be maintained across varying physiological states and environmental conditions.
Material biocompatibility presents another significant hurdle. Current electrode materials often trigger foreign body responses when implanted, leading to inflammation, fibrosis, and eventual signal degradation. Even surface-contact electrodes can cause irritation during prolonged use, limiting the duration of effective monitoring. The development of truly biocompatible materials that maintain electrical performance while minimizing biological reactions remains an active research frontier.
Power management constitutes a critical limitation for implantable and wearable diagnostic platforms. Current battery technologies offer insufficient energy density for long-term operation, while wireless power transfer solutions face efficiency and safety concerns. Energy harvesting approaches from the body itself show promise but currently generate insufficient power for many diagnostic applications requiring continuous operation or complex signal processing.
Miniaturization challenges persist as diagnostic platforms strive for less invasive form factors. As devices shrink, maintaining sufficient electrode surface area for reliable signal acquisition becomes problematic. Additionally, miniaturized components must withstand biological environments without performance degradation, requiring novel packaging solutions and circuit designs that can function reliably at micro and nano scales.
Data processing and interpretation represent increasingly complex challenges as bioelectronic interfaces generate massive datasets. Real-time analysis of multimodal biological signals requires sophisticated algorithms that can distinguish clinically relevant information from artifacts. The computational demands often exceed the capabilities of miniaturized, low-power systems, necessitating compromises between processing depth and power consumption.
Standardization across bioelectronic interface technologies remains inadequate, hampering interoperability and clinical validation. Different manufacturers employ proprietary designs and communication protocols, creating fragmented ecosystems that impede comparative studies and regulatory approval pathways. This lack of standardization also complicates data integration across multiple diagnostic modalities, limiting the potential for comprehensive health monitoring systems.
Manufacturing scalability presents a significant barrier to commercialization. Many promising bioelectronic interfaces rely on fabrication techniques that are difficult to scale for mass production while maintaining performance consistency. The integration of biological and electronic components often requires specialized processes that are challenging to automate, resulting in high production costs that limit market penetration.
Material biocompatibility presents another significant hurdle. Current electrode materials often trigger foreign body responses when implanted, leading to inflammation, fibrosis, and eventual signal degradation. Even surface-contact electrodes can cause irritation during prolonged use, limiting the duration of effective monitoring. The development of truly biocompatible materials that maintain electrical performance while minimizing biological reactions remains an active research frontier.
Power management constitutes a critical limitation for implantable and wearable diagnostic platforms. Current battery technologies offer insufficient energy density for long-term operation, while wireless power transfer solutions face efficiency and safety concerns. Energy harvesting approaches from the body itself show promise but currently generate insufficient power for many diagnostic applications requiring continuous operation or complex signal processing.
Miniaturization challenges persist as diagnostic platforms strive for less invasive form factors. As devices shrink, maintaining sufficient electrode surface area for reliable signal acquisition becomes problematic. Additionally, miniaturized components must withstand biological environments without performance degradation, requiring novel packaging solutions and circuit designs that can function reliably at micro and nano scales.
Data processing and interpretation represent increasingly complex challenges as bioelectronic interfaces generate massive datasets. Real-time analysis of multimodal biological signals requires sophisticated algorithms that can distinguish clinically relevant information from artifacts. The computational demands often exceed the capabilities of miniaturized, low-power systems, necessitating compromises between processing depth and power consumption.
Standardization across bioelectronic interface technologies remains inadequate, hampering interoperability and clinical validation. Different manufacturers employ proprietary designs and communication protocols, creating fragmented ecosystems that impede comparative studies and regulatory approval pathways. This lack of standardization also complicates data integration across multiple diagnostic modalities, limiting the potential for comprehensive health monitoring systems.
Manufacturing scalability presents a significant barrier to commercialization. Many promising bioelectronic interfaces rely on fabrication techniques that are difficult to scale for mass production while maintaining performance consistency. The integration of biological and electronic components often requires specialized processes that are challenging to automate, resulting in high production costs that limit market penetration.
Current Bioelectronic Sensing Solutions
01 Bioelectronic sensors for disease detection
Bioelectronic interfaces can be designed with specialized sensors that detect biological markers associated with various diseases. These sensors typically incorporate biorecognition elements such as antibodies, enzymes, or nucleic acids that interact specifically with target analytes. The resulting electrical signals are processed to provide diagnostic information about the presence and concentration of disease markers, enabling early detection and monitoring of health conditions.- Bioelectronic sensors for disease detection: Bioelectronic interfaces can be designed with specialized sensors that detect biological markers associated with various diseases. These sensors typically incorporate biorecognition elements such as antibodies, enzymes, or nucleic acids that interact with specific biomarkers. When the target biomarker binds to the recognition element, the interface generates electrical signals that can be measured and analyzed for diagnostic purposes. This technology enables rapid, sensitive, and potentially point-of-care diagnosis of various conditions.
- Implantable bioelectronic diagnostic devices: Implantable bioelectronic interfaces provide continuous monitoring capabilities for diagnostic purposes. These devices can be inserted under the skin or within specific organs to monitor physiological parameters in real-time. The interfaces typically consist of biocompatible materials and incorporate miniaturized electronics that can detect changes in the body's biochemistry or electrical activity. Data collected from these implantable devices can be wirelessly transmitted to external receivers for analysis, enabling early detection of health issues and personalized treatment approaches.
- Neural interfaces for diagnostic applications: Neural bioelectronic interfaces enable direct communication with the nervous system for diagnostic purposes. These interfaces can record electrical activity from neurons, providing insights into neurological conditions and brain function. The technology typically involves microelectrode arrays or flexible electronic meshes that interface with neural tissue. By monitoring neural signals, these interfaces can help diagnose neurological disorders, assess brain injuries, or evaluate cognitive function. Advanced signal processing algorithms are often employed to interpret the complex neural data collected by these interfaces.
- Wearable bioelectronic diagnostic platforms: Wearable bioelectronic interfaces provide non-invasive diagnostic capabilities through continuous monitoring of physiological parameters. These devices can be integrated into clothing, accessories, or applied directly to the skin as patches. They typically incorporate flexible electronics, conductive materials, and biosensors that can detect various biomarkers in sweat, interstitial fluid, or through skin contact. The collected data can be processed locally or transmitted to smartphones for analysis, enabling early detection of health issues and personalized health management.
- Lab-on-chip bioelectronic diagnostic systems: Lab-on-chip bioelectronic interfaces integrate multiple laboratory functions on a single microchip platform for diagnostic applications. These miniaturized systems typically incorporate microfluidic channels, electrochemical sensors, and signal processing components to perform complex diagnostic tests with minimal sample volumes. The technology enables rapid analysis of biological samples such as blood, saliva, or urine to detect disease biomarkers. These systems offer advantages including portability, reduced reagent consumption, faster analysis times, and potential for point-of-care diagnostics in resource-limited settings.
02 Wearable diagnostic platforms
Wearable bioelectronic interfaces provide continuous monitoring capabilities for diagnostic purposes. These devices integrate flexible electronics with biocompatible materials that can be worn on the skin or incorporated into clothing. They collect physiological data in real-time, allowing for non-invasive monitoring of vital signs, metabolites, and other health indicators. The continuous data collection enables early detection of abnormalities and personalized health management.Expand Specific Solutions03 Neural interface diagnostic systems
Neural bioelectronic interfaces enable direct communication with the nervous system for diagnostic applications. These systems can record electrical activity from neurons to detect neurological disorders, assess brain function, or monitor nerve conduction. Advanced neural interfaces incorporate microelectrode arrays and signal processing algorithms to interpret complex neural patterns, providing insights into neurological conditions and enabling early intervention for disorders such as epilepsy, Parkinson's disease, or peripheral neuropathies.Expand Specific Solutions04 Implantable diagnostic devices
Implantable bioelectronic interfaces provide continuous internal monitoring for diagnostic purposes. These devices are designed to be placed within the body and can directly measure parameters such as glucose levels, cardiac activity, or specific biomarkers. They typically incorporate biocompatible materials, miniaturized electronics, and wireless communication capabilities to transmit diagnostic data to external receivers. The ability to monitor internal physiological parameters continuously enables more accurate diagnosis and personalized treatment approaches.Expand Specific Solutions05 Lab-on-chip diagnostic platforms
Lab-on-chip bioelectronic interfaces integrate multiple diagnostic functions into miniaturized platforms. These systems combine microfluidics with electronic sensing to perform complex diagnostic tests with minimal sample volumes. They can process biological samples, separate components, and detect specific markers through integrated bioelectronic sensors. The integration of multiple analytical steps into a single platform enables rapid, point-of-care diagnostics with high sensitivity and specificity, making sophisticated testing accessible in resource-limited settings.Expand Specific Solutions
Leading Companies in Bioelectronic Diagnostics
Bioelectronic interfaces for advanced diagnostics are evolving rapidly, currently transitioning from early development to commercial growth phase. The market is expanding significantly, projected to reach billions by 2030, driven by increasing demand for point-of-care testing and personalized medicine. Technologically, the field shows varying maturity levels across applications. Leading academic institutions (MIT, University of Michigan, Rice University) are pioneering fundamental research, while established companies (Samsung Electronics, Infineon Technologies) provide essential hardware components. Specialized firms like DexCom, Precision Biosensor, and Magnomics are commercializing innovative diagnostic platforms. Research collaborations between industry players and institutions like IMEC and Katholieke Universiteit Leuven are accelerating development of next-generation bioelectronic sensing technologies, particularly in implantable and wearable diagnostic devices.
Massachusetts Institute of Technology
Technical Solution: MIT has developed groundbreaking bioelectronic interface technology through their "electronic skin" platforms that enable advanced diagnostics without invasive procedures. Their approach combines nanomaterial-based sensors with custom integrated circuits to create conformable, adhesive patches that can detect multiple biomarkers through skin. MIT's platform utilizes graphene-based field-effect transistors (GFETs) that achieve unprecedented sensitivity for detecting protein biomarkers at femtomolar concentrations. The technology incorporates microfluidic channels that can extract and concentrate interstitial fluid without needles, enabling continuous monitoring of metabolites and electrolytes. MIT researchers have also pioneered multiplexed sensing arrays that can simultaneously track inflammatory markers, stress hormones, and metabolic indicators, providing comprehensive health assessment from a single wearable device. Their recent innovations include machine learning algorithms that analyze temporal patterns in biomarker fluctuations to predict disease progression before clinical symptoms appear.
Strengths: Cutting-edge nanofabrication capabilities; strong integration of hardware with advanced AI algorithms for predictive diagnostics; extensive industry partnerships accelerating commercialization. Weaknesses: Some technologies require specialized manufacturing processes limiting mass production; higher initial costs compared to conventional diagnostic methods; potential regulatory hurdles for novel sensing modalities.
Interuniversitair Micro-Electronica Centrum VZW
Technical Solution: IMEC has pioneered next-generation bioelectronic interfaces through their advanced semiconductor and microelectronic technologies specifically adapted for biological sensing. Their platform utilizes silicon nanowire field-effect transistors (SiNW-FETs) with functionalized surfaces that can detect biomolecular binding events with exceptional sensitivity. IMEC has developed proprietary CMOS-compatible fabrication processes that enable integration of thousands of sensing elements on chips smaller than a fingernail, allowing for highly multiplexed detection of disease biomarkers. Their technology incorporates on-chip microfluidics with electronic sensing to create complete lab-on-chip diagnostic solutions that require minimal sample volumes. IMEC researchers have created specialized surface chemistry approaches that maintain biomolecule activity while providing stable attachment to electronic sensing elements, solving a critical challenge in bioelectronic interfaces. Their recent innovations include neural probes with unprecedented channel counts (>10,000 electrodes) that can map brain activity with cellular resolution, demonstrating the extreme miniaturization capabilities of their manufacturing approach.
Strengths: World-class semiconductor fabrication capabilities; strong expertise in system integration combining microfluidics, electronics, and biochemistry; extensive industry partnership network. Weaknesses: Some technologies require advanced fabrication facilities limiting accessibility; higher initial development costs; challenges in transitioning from silicon-based platforms to fully flexible systems.
Key Patents in Bioelectronic Interface Technology
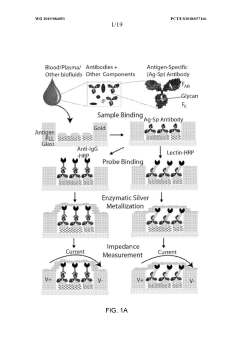
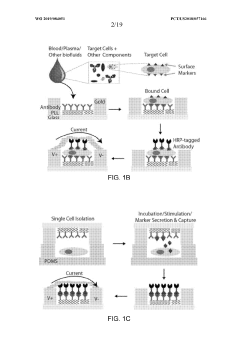
System and method for digital, multiplexed, extracellular vesicle-derived biomarker diagnostic lab-on-a-chip and method of use thereof
PatentWO2024238971A1
Innovation
- A digital, multiplexed lab-on-a-chip system using a filter cartridge with dielectrophoresis (DEP) for label-free, non-mechanical isolation of extracellular vesicle-derived biomarkers from biological samples, enabling sensitive and specific detection through a software-controlled device for processing and analysis.
An integrated microfluidic electrode array system for enzyme-linked immuno-sorbent assay for point- of-care detection of biomarkers
PatentWO2019084051A1
Innovation
- A microfluidic electrode array system that uses direct electrical impedance-based detection and enzymatically-amplified metal nanoparticle deposition for sensitive and quantitative detection of molecular and cellular biomarkers without intermediate optics or complex fluidics, enabling a portable, inexpensive platform for point-of-care diagnostics.
Regulatory Framework for Medical Bioelectronics
The regulatory landscape for bioelectronic medical devices presents a complex framework that manufacturers, researchers, and healthcare providers must navigate. In the United States, the Food and Drug Administration (FDA) classifies bioelectronic diagnostic platforms primarily under Class II or Class III medical devices, depending on their risk profile and intended use. The 510(k) clearance pathway is commonly utilized for devices similar to existing technologies, while novel bioelectronic interfaces often require the more rigorous Premarket Approval (PMA) process, including extensive clinical trials to demonstrate safety and efficacy.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which significantly increased requirements for clinical evidence, post-market surveillance, and technical documentation for bioelectronic diagnostic platforms. These regulations emphasize the importance of risk management throughout the product lifecycle and introduce Unique Device Identification (UDI) systems for improved traceability.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide essential frameworks for manufacturers. Additionally, the IEC 62304 standard specifically addresses software lifecycle requirements, which is particularly relevant for the software components of bioelectronic diagnostic systems.
Data privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient data generated by bioelectronic diagnostic platforms. These regulations mandate secure data storage, transmission protocols, and patient consent mechanisms, adding another layer of compliance complexity for developers.
Emerging regulatory considerations include cybersecurity requirements, as connected bioelectronic devices become increasingly vulnerable to security threats. Regulatory bodies are developing frameworks to address these concerns, such as the FDA's guidance on cybersecurity for medical devices and the EU's Network and Information Systems (NIS) Directive.
Regulatory harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), aim to streamline approval processes across different jurisdictions. However, significant regional variations persist, creating challenges for global deployment of bioelectronic diagnostic technologies.
For rapidly evolving technologies like bioelectronic interfaces, regulatory bodies have introduced adaptive pathways such as the FDA's Breakthrough Devices Program and the EU's IVDR Class D expedited assessment, allowing promising diagnostic innovations to reach patients more quickly while maintaining appropriate oversight.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which significantly increased requirements for clinical evidence, post-market surveillance, and technical documentation for bioelectronic diagnostic platforms. These regulations emphasize the importance of risk management throughout the product lifecycle and introduce Unique Device Identification (UDI) systems for improved traceability.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide essential frameworks for manufacturers. Additionally, the IEC 62304 standard specifically addresses software lifecycle requirements, which is particularly relevant for the software components of bioelectronic diagnostic systems.
Data privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient data generated by bioelectronic diagnostic platforms. These regulations mandate secure data storage, transmission protocols, and patient consent mechanisms, adding another layer of compliance complexity for developers.
Emerging regulatory considerations include cybersecurity requirements, as connected bioelectronic devices become increasingly vulnerable to security threats. Regulatory bodies are developing frameworks to address these concerns, such as the FDA's guidance on cybersecurity for medical devices and the EU's Network and Information Systems (NIS) Directive.
Regulatory harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), aim to streamline approval processes across different jurisdictions. However, significant regional variations persist, creating challenges for global deployment of bioelectronic diagnostic technologies.
For rapidly evolving technologies like bioelectronic interfaces, regulatory bodies have introduced adaptive pathways such as the FDA's Breakthrough Devices Program and the EU's IVDR Class D expedited assessment, allowing promising diagnostic innovations to reach patients more quickly while maintaining appropriate oversight.
Biosensor Materials and Manufacturing Processes
The evolution of bioelectronic interfaces for advanced diagnostic platforms heavily depends on the materials and manufacturing processes used in biosensor development. Traditional biosensor materials include noble metals like gold and platinum, which offer excellent conductivity and biocompatibility. However, recent advancements have introduced nanomaterials such as carbon nanotubes, graphene, and quantum dots that provide enhanced surface-to-volume ratios and unique electrical properties, significantly improving detection sensitivity and response time.
Conductive polymers represent another crucial material category, with polyaniline, polypyrrole, and PEDOT:PSS gaining prominence due to their tunable conductivity and compatibility with biological systems. These polymers can be functionalized with various biorecognition elements including antibodies, enzymes, and nucleic acids to create highly specific sensing platforms.
Manufacturing processes for biosensors have evolved from conventional lithography techniques to more sophisticated approaches. Photolithography remains fundamental for creating precise microelectrode patterns, while soft lithography has emerged as a cost-effective alternative for rapid prototyping. Screen printing technology offers scalable production of disposable biosensors, particularly evident in glucose monitoring strips that dominate the commercial market.
Additive manufacturing technologies, especially 3D printing, have revolutionized biosensor fabrication by enabling complex three-dimensional architectures with precise spatial control. Inkjet printing allows for the deposition of biological materials with minimal waste, while roll-to-roll processing facilitates high-throughput production of flexible biosensor arrays on polymer substrates.
Surface modification techniques play a critical role in biosensor performance. Self-assembled monolayers (SAMs) provide controlled immobilization of biomolecules, while plasma treatment alters surface properties to enhance biocompatibility. Layer-by-layer assembly enables the creation of multilayered functional interfaces with nanometer precision.
Recent innovations include the integration of microfluidic systems with biosensing elements, creating lab-on-a-chip devices that require minimal sample volumes. The emergence of stretchable and flexible electronics based on elastomeric substrates has enabled conformable biosensors that maintain functionality under mechanical deformation, opening new possibilities for wearable diagnostic platforms.
The miniaturization trend continues with advances in nanofabrication techniques such as electron beam lithography and focused ion beam milling, enabling feature sizes below 100 nm. These nanoscale biosensors demonstrate unprecedented sensitivity, potentially reaching single-molecule detection limits in controlled environments.
Conductive polymers represent another crucial material category, with polyaniline, polypyrrole, and PEDOT:PSS gaining prominence due to their tunable conductivity and compatibility with biological systems. These polymers can be functionalized with various biorecognition elements including antibodies, enzymes, and nucleic acids to create highly specific sensing platforms.
Manufacturing processes for biosensors have evolved from conventional lithography techniques to more sophisticated approaches. Photolithography remains fundamental for creating precise microelectrode patterns, while soft lithography has emerged as a cost-effective alternative for rapid prototyping. Screen printing technology offers scalable production of disposable biosensors, particularly evident in glucose monitoring strips that dominate the commercial market.
Additive manufacturing technologies, especially 3D printing, have revolutionized biosensor fabrication by enabling complex three-dimensional architectures with precise spatial control. Inkjet printing allows for the deposition of biological materials with minimal waste, while roll-to-roll processing facilitates high-throughput production of flexible biosensor arrays on polymer substrates.
Surface modification techniques play a critical role in biosensor performance. Self-assembled monolayers (SAMs) provide controlled immobilization of biomolecules, while plasma treatment alters surface properties to enhance biocompatibility. Layer-by-layer assembly enables the creation of multilayered functional interfaces with nanometer precision.
Recent innovations include the integration of microfluidic systems with biosensing elements, creating lab-on-a-chip devices that require minimal sample volumes. The emergence of stretchable and flexible electronics based on elastomeric substrates has enabled conformable biosensors that maintain functionality under mechanical deformation, opening new possibilities for wearable diagnostic platforms.
The miniaturization trend continues with advances in nanofabrication techniques such as electron beam lithography and focused ion beam milling, enabling feature sizes below 100 nm. These nanoscale biosensors demonstrate unprecedented sensitivity, potentially reaching single-molecule detection limits in controlled environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!