Impact of Emerging Technologies on Bioelectronic Interface Applications
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronic engineering and biological systems, enabling direct communication between electronic devices and biological entities. The evolution of these interfaces has been marked by significant technological advancements over the past several decades, transitioning from rudimentary electrode-based systems to sophisticated, miniaturized implantable devices capable of bidirectional communication with neural tissues.
The field originated in the 1970s with basic neural recording techniques, progressing through the development of microelectrode arrays in the 1990s, and advancing to today's flexible, biocompatible interfaces that minimize tissue damage and immune responses. This evolution has been driven by interdisciplinary collaboration among neuroscientists, materials engineers, and electronic specialists, resulting in increasingly sophisticated systems for both recording and stimulation applications.
Current bioelectronic interfaces face several technical challenges, including achieving long-term stability in biological environments, improving spatial and temporal resolution, minimizing power requirements, and enhancing biocompatibility. These challenges have spurred innovation in materials science, with the development of novel conducting polymers, carbon-based materials, and hydrogels that better mimic the mechanical properties of biological tissues.
The primary objectives of contemporary bioelectronic interface research include developing interfaces with enhanced longevity and reduced foreign body responses, improving signal quality and information transfer rates, and miniaturizing components while maintaining functionality. Additionally, researchers aim to create wireless, self-powered systems that eliminate the need for transcutaneous connections and external power sources, thereby reducing infection risks and improving patient mobility.
Emerging technologies such as optogenetics, ultrasonic neural dust, and magnetoelectric materials are expanding the capabilities of bioelectronic interfaces beyond traditional electrical stimulation and recording. These innovations promise to enable more precise control over specific cell populations and provide new modalities for interfacing with biological systems.
The ultimate goal of bioelectronic interface development is to create seamless integration between electronic systems and biological tissues, facilitating applications ranging from neuroprosthetics and brain-computer interfaces to bioelectronic medicine and advanced health monitoring. This vision requires overcoming significant technical hurdles while addressing ethical considerations regarding privacy, autonomy, and the potential for enhancement beyond therapeutic applications.
As these technologies continue to evolve, they are expected to revolutionize healthcare by enabling personalized, minimally invasive treatments for neurological disorders, providing restored function for individuals with disabilities, and offering new insights into fundamental biological processes through high-resolution monitoring capabilities.
The field originated in the 1970s with basic neural recording techniques, progressing through the development of microelectrode arrays in the 1990s, and advancing to today's flexible, biocompatible interfaces that minimize tissue damage and immune responses. This evolution has been driven by interdisciplinary collaboration among neuroscientists, materials engineers, and electronic specialists, resulting in increasingly sophisticated systems for both recording and stimulation applications.
Current bioelectronic interfaces face several technical challenges, including achieving long-term stability in biological environments, improving spatial and temporal resolution, minimizing power requirements, and enhancing biocompatibility. These challenges have spurred innovation in materials science, with the development of novel conducting polymers, carbon-based materials, and hydrogels that better mimic the mechanical properties of biological tissues.
The primary objectives of contemporary bioelectronic interface research include developing interfaces with enhanced longevity and reduced foreign body responses, improving signal quality and information transfer rates, and miniaturizing components while maintaining functionality. Additionally, researchers aim to create wireless, self-powered systems that eliminate the need for transcutaneous connections and external power sources, thereby reducing infection risks and improving patient mobility.
Emerging technologies such as optogenetics, ultrasonic neural dust, and magnetoelectric materials are expanding the capabilities of bioelectronic interfaces beyond traditional electrical stimulation and recording. These innovations promise to enable more precise control over specific cell populations and provide new modalities for interfacing with biological systems.
The ultimate goal of bioelectronic interface development is to create seamless integration between electronic systems and biological tissues, facilitating applications ranging from neuroprosthetics and brain-computer interfaces to bioelectronic medicine and advanced health monitoring. This vision requires overcoming significant technical hurdles while addressing ethical considerations regarding privacy, autonomy, and the potential for enhancement beyond therapeutic applications.
As these technologies continue to evolve, they are expected to revolutionize healthcare by enabling personalized, minimally invasive treatments for neurological disorders, providing restored function for individuals with disabilities, and offering new insights into fundamental biological processes through high-resolution monitoring capabilities.
Market Analysis for Bioelectronic Interface Solutions
The bioelectronic interface market is experiencing unprecedented growth, driven by technological advancements and increasing applications across healthcare, consumer electronics, and industrial sectors. Current market valuations place the global bioelectronic interface industry at approximately 5.7 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 14.3% through 2030, potentially reaching 15.2 billion USD by the end of the decade.
Healthcare applications represent the largest market segment, accounting for nearly 60% of current demand. Within this segment, neural interfaces for treatment of neurological disorders, particularly Parkinson's disease, epilepsy, and chronic pain management, demonstrate the strongest growth trajectory. The aging global population and increasing prevalence of chronic neurological conditions are primary demand drivers, with over 50 million people worldwide suffering from epilepsy and approximately 10 million with Parkinson's disease.
Consumer applications are emerging as the fastest-growing segment, with a projected CAGR of 18.7% through 2028. This growth is primarily fueled by wearable bioelectronic interfaces for fitness tracking, stress management, and sleep monitoring. The integration of bioelectronic interfaces with smartphones and other personal devices has significantly expanded market accessibility, with approximately 320 million wearable health monitoring devices shipped globally in 2023.
Geographically, North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years, driven by increasing healthcare expenditure, rapid technological adoption, and substantial government investments in bioelectronic research in countries like China, Japan, and South Korea.
Key market challenges include high development and production costs, with advanced neural interface systems typically requiring investments of 75-150 million USD from research to commercialization. Regulatory hurdles also present significant barriers, with FDA approval processes for implantable bioelectronic devices averaging 4.5 years. Additionally, concerns regarding data privacy, security, and ethical implications of human-machine interfaces continue to influence market adoption rates.
Despite these challenges, investment in bioelectronic interface technologies has surged, with venture capital funding exceeding 2.1 billion USD in 2023 alone. Strategic partnerships between technology companies and healthcare providers are increasingly common, creating new market opportunities and accelerating commercialization timelines. The convergence of artificial intelligence, miniaturization technologies, and advanced materials science is expected to further expand market applications and drive continued growth in this dynamic sector.
Healthcare applications represent the largest market segment, accounting for nearly 60% of current demand. Within this segment, neural interfaces for treatment of neurological disorders, particularly Parkinson's disease, epilepsy, and chronic pain management, demonstrate the strongest growth trajectory. The aging global population and increasing prevalence of chronic neurological conditions are primary demand drivers, with over 50 million people worldwide suffering from epilepsy and approximately 10 million with Parkinson's disease.
Consumer applications are emerging as the fastest-growing segment, with a projected CAGR of 18.7% through 2028. This growth is primarily fueled by wearable bioelectronic interfaces for fitness tracking, stress management, and sleep monitoring. The integration of bioelectronic interfaces with smartphones and other personal devices has significantly expanded market accessibility, with approximately 320 million wearable health monitoring devices shipped globally in 2023.
Geographically, North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years, driven by increasing healthcare expenditure, rapid technological adoption, and substantial government investments in bioelectronic research in countries like China, Japan, and South Korea.
Key market challenges include high development and production costs, with advanced neural interface systems typically requiring investments of 75-150 million USD from research to commercialization. Regulatory hurdles also present significant barriers, with FDA approval processes for implantable bioelectronic devices averaging 4.5 years. Additionally, concerns regarding data privacy, security, and ethical implications of human-machine interfaces continue to influence market adoption rates.
Despite these challenges, investment in bioelectronic interface technologies has surged, with venture capital funding exceeding 2.1 billion USD in 2023 alone. Strategic partnerships between technology companies and healthcare providers are increasingly common, creating new market opportunities and accelerating commercialization timelines. The convergence of artificial intelligence, miniaturization technologies, and advanced materials science is expected to further expand market applications and drive continued growth in this dynamic sector.
Current Landscape and Technical Barriers
The bioelectronic interface landscape has undergone significant transformation in recent years, driven by advancements in materials science, microelectronics, and computational capabilities. Current bioelectronic interfaces primarily fall into three categories: invasive implantable devices, minimally invasive interfaces, and non-invasive systems. Each category represents different trade-offs between signal quality, longevity, and patient comfort. Implantable neural interfaces such as Utah arrays and Michigan probes have demonstrated remarkable capabilities in direct neural recording but face substantial challenges in long-term stability.
The market is witnessing increased participation from both established medical device manufacturers and technology startups, with notable investments from companies like Neuralink, Paradromics, and CTRL-labs (acquired by Meta). Academic research continues to drive innovation, with institutions like MIT, Stanford, and ETH Zurich publishing groundbreaking research on flexible electronics and wireless power transfer systems for bioelectronic applications.
Despite promising advances, significant technical barriers persist in bioelectronic interface development. Biocompatibility remains a critical challenge, as foreign body responses lead to electrode encapsulation and signal degradation over time. Current materials struggle to match the mechanical properties of biological tissues, creating stress at the interface and causing inflammation. The development of materials that can maintain stable electrical properties while mimicking tissue mechanics represents a major research focus.
Power management presents another substantial hurdle, particularly for implantable devices that must operate autonomously for years. Wireless power transfer technologies show promise but face efficiency limitations and safety concerns regarding tissue heating. Data bandwidth constraints further complicate the development of high-resolution interfaces capable of monitoring thousands of neurons simultaneously.
Signal processing algorithms face challenges in extracting meaningful information from noisy biological signals, particularly in ambulatory conditions. The miniaturization of electronics while maintaining thermal stability presents additional engineering challenges, as heat dissipation must be carefully managed to prevent tissue damage.
Regulatory pathways for novel bioelectronic interfaces remain complex and time-consuming, with FDA and international regulatory bodies still developing frameworks for evaluating the safety and efficacy of these emerging technologies. The lack of standardized testing protocols and long-term safety data creates uncertainty for developers and investors alike.
Geographical distribution of bioelectronic interface research shows concentration in North America, Western Europe, and East Asia, with the United States maintaining leadership in neural interface technologies and Japan excelling in flexible electronics. China has rapidly increased investment in this sector, particularly in applications related to rehabilitation medicine and prosthetics.
The market is witnessing increased participation from both established medical device manufacturers and technology startups, with notable investments from companies like Neuralink, Paradromics, and CTRL-labs (acquired by Meta). Academic research continues to drive innovation, with institutions like MIT, Stanford, and ETH Zurich publishing groundbreaking research on flexible electronics and wireless power transfer systems for bioelectronic applications.
Despite promising advances, significant technical barriers persist in bioelectronic interface development. Biocompatibility remains a critical challenge, as foreign body responses lead to electrode encapsulation and signal degradation over time. Current materials struggle to match the mechanical properties of biological tissues, creating stress at the interface and causing inflammation. The development of materials that can maintain stable electrical properties while mimicking tissue mechanics represents a major research focus.
Power management presents another substantial hurdle, particularly for implantable devices that must operate autonomously for years. Wireless power transfer technologies show promise but face efficiency limitations and safety concerns regarding tissue heating. Data bandwidth constraints further complicate the development of high-resolution interfaces capable of monitoring thousands of neurons simultaneously.
Signal processing algorithms face challenges in extracting meaningful information from noisy biological signals, particularly in ambulatory conditions. The miniaturization of electronics while maintaining thermal stability presents additional engineering challenges, as heat dissipation must be carefully managed to prevent tissue damage.
Regulatory pathways for novel bioelectronic interfaces remain complex and time-consuming, with FDA and international regulatory bodies still developing frameworks for evaluating the safety and efficacy of these emerging technologies. The lack of standardized testing protocols and long-term safety data creates uncertainty for developers and investors alike.
Geographical distribution of bioelectronic interface research shows concentration in North America, Western Europe, and East Asia, with the United States maintaining leadership in neural interface technologies and Japan excelling in flexible electronics. China has rapidly increased investment in this sector, particularly in applications related to rehabilitation medicine and prosthetics.
Contemporary Bioelectronic Interface Implementations
01 Neural interfaces for bioelectronic applications
Neural interfaces are a key component in bioelectronic systems that enable direct communication between electronic devices and the nervous system. These interfaces can record neural activity or deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create biocompatible neural electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces find applications in neuroprosthetics, brain-computer interfaces, and therapeutic devices for neurological disorders.- Neural interfaces for bioelectronic applications: Neural interfaces are a key component in bioelectronic systems that establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity or deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create flexible, biocompatible electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces enable applications in neuroprosthetics, brain-computer interfaces, and therapeutic neuromodulation systems.
- Implantable bioelectronic devices: Implantable bioelectronic devices are designed to interface with biological systems for monitoring, diagnosis, or therapeutic purposes. These devices incorporate biocompatible materials, miniaturized electronics, and power management systems to ensure long-term functionality within the body. Advanced encapsulation techniques protect electronic components from the harsh biological environment while allowing for signal transduction. These implantable systems can be used for continuous health monitoring, drug delivery, or as therapeutic interventions for various medical conditions.
- Biosensors and molecular detection interfaces: Biosensors integrate biological recognition elements with electronic transducers to detect specific biomolecules, pathogens, or physiological parameters. These bioelectronic interfaces employ various sensing mechanisms including electrochemical, optical, and impedance-based detection methods. Functionalized surfaces with antibodies, enzymes, or nucleic acids provide specificity for target analytes. Advanced signal processing techniques enhance sensitivity and reduce interference from biological matrices. These systems enable point-of-care diagnostics, environmental monitoring, and real-time health tracking applications.
- Flexible and wearable bioelectronic interfaces: Flexible and wearable bioelectronic interfaces are designed to conform to biological tissues and body contours while maintaining electronic functionality. These systems utilize stretchable substrates, conductive polymers, and novel fabrication techniques to create devices that can withstand mechanical deformation. Skin-interfacing electronics enable non-invasive monitoring of physiological parameters through sweat, interstitial fluid, or bioelectric signals. These technologies support applications in continuous health monitoring, human-machine interfaces, and personalized medicine.
- Organic and biomaterial-based electronic interfaces: Organic and biomaterial-based electronic interfaces incorporate biological or bioinspired materials to improve biocompatibility and functionality of bioelectronic systems. These interfaces utilize conducting polymers, hydrogels, or naturally derived materials that better match the mechanical and chemical properties of biological tissues. Biomolecules can be integrated into electronic components to enable specific biological interactions or improve tissue integration. These hybrid bioelectronic systems reduce foreign body responses and enhance long-term stability for applications in tissue engineering, regenerative medicine, and advanced prosthetics.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces utilize elastic substrates, serpentine interconnects, and novel materials to achieve mechanical compliance while maintaining electronic functionality. The flexibility allows for better contact with biological surfaces, reduced mechanical mismatch, and improved signal quality. These interfaces are particularly valuable for wearable health monitoring, implantable devices, and epidermal electronics that must accommodate body movement while providing reliable performance.Expand Specific Solutions03 Biosensing interfaces for molecular detection
Biosensing interfaces enable the detection and quantification of biological molecules through various transduction mechanisms. These interfaces incorporate recognition elements such as antibodies, enzymes, or nucleic acids that specifically bind to target analytes. The binding events are converted into measurable signals through electrochemical, optical, or mechanical transduction. Advanced surface chemistry techniques are employed to immobilize biorecognition elements while maintaining their activity. These biosensing interfaces find applications in medical diagnostics, environmental monitoring, and food safety testing.Expand Specific Solutions04 Nanomaterial-based bioelectronic interfaces
Nanomaterials offer unique properties for bioelectronic interfaces, including high surface area, enhanced electrical conductivity, and tunable surface chemistry. Materials such as carbon nanotubes, graphene, and metal nanoparticles are incorporated into bioelectronic interfaces to improve sensitivity, selectivity, and signal-to-noise ratio. Nanomaterial-based interfaces can be engineered to interact with biological systems at the cellular or molecular level. These advanced materials enable miniaturized devices with improved performance for applications in biosensing, neural recording, and drug delivery systems.Expand Specific Solutions05 Implantable bioelectronic interfaces for therapeutic applications
Implantable bioelectronic interfaces are designed for long-term integration with biological tissues to provide therapeutic benefits. These interfaces incorporate biocompatible materials, hermetic packaging, and wireless communication capabilities to ensure functionality within the body. Advanced fabrication techniques enable miniaturization while maintaining power efficiency. Implantable bioelectronic interfaces are used in neuromodulation therapies, cardiac pacing, drug delivery systems, and other medical applications where direct interaction with internal tissues is required for monitoring or treatment purposes.Expand Specific Solutions
Leading Organizations in Bioelectronic Interface Development
The bioelectronic interface technology sector is currently in a growth phase, with an expanding market driven by healthcare applications and wearable devices. The competitive landscape features established industry leaders like Agilent Technologies, Infineon Technologies, and Philips alongside specialized medical device companies such as DexCom. Academic institutions play a crucial role in advancing fundamental research, with MIT, Caltech, and University of California leading innovation through interdisciplinary approaches. The technology maturity varies across applications - glucose monitoring systems (DexCom) have reached commercial maturity, while neural interfaces remain predominantly in research phases. Industry-academic partnerships are accelerating development, with national laboratories like Lawrence Livermore providing additional research infrastructure to bridge the gap between laboratory discoveries and commercial applications.
The Regents of the University of California
Technical Solution: The University of California system has developed groundbreaking bioelectronic interfaces focusing on biodegradable and environmentally responsive materials. Their technology platform incorporates silk fibroin substrates and magnesium-based conductors that can dissolve harmlessly in the body after their functional period[2]. UC researchers have created "transient electronics" that can be programmed to degrade after specific timeframes, eliminating the need for removal surgeries and reducing long-term biocompatibility concerns[4]. Their bioelectronic interfaces feature multilayer architectures that separate sensing, processing, and communication functions while maintaining minimal form factors. A significant innovation is their development of self-powered bioelectronic systems that harvest energy from biological processes such as glucose oxidation or mechanical movement, enabling autonomous operation without external power sources[6]. UC's platforms incorporate advanced microfluidic channels that can simultaneously deliver therapeutics while monitoring biological responses, creating closed-loop treatment systems for conditions like epilepsy and Parkinson's disease. Their latest interfaces utilize biodegradable polymers with controlled drug release capabilities triggered by electrical stimulation.
Strengths: Leading expertise in biodegradable electronics that eliminate removal procedures; innovative energy harvesting solutions for self-powered operation; integration of therapeutic delivery with monitoring capabilities. Weaknesses: Degradable materials typically offer lower performance specifications than permanent alternatives; challenges in precisely controlling degradation rates in variable biological environments; limited long-term data storage capabilities in transient systems.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered significant advancements in bioelectronic interfaces through their development of flexible, ultrathin electronics that conform to biological tissues. Their technology utilizes nanomaterials like graphene and carbon nanotubes to create interfaces with unprecedented signal quality and biocompatibility. MIT researchers have developed a platform that integrates wireless power transmission and data communication capabilities, enabling long-term implantable devices that don't require battery replacement[1]. Their recent innovation includes hydrogel-based electrodes that match the mechanical properties of neural tissue, reducing immune responses while maintaining electrical conductivity[3]. MIT has also created "tissue-like" electronics that can be injected via syringe, minimizing invasiveness for neural recording applications[5]. Their bioelectronic interfaces incorporate machine learning algorithms that adapt to changing biological signals over time, improving long-term performance in applications ranging from neural prosthetics to continuous health monitoring systems.
Strengths: Superior materials science expertise enabling flexible, biocompatible interfaces with minimal tissue damage; strong integration of wireless technologies for practical implementation; advanced signal processing capabilities. Weaknesses: Higher manufacturing costs compared to traditional rigid electronics; challenges in scaling production for commercial applications; potential reliability concerns in long-term implantation scenarios requiring further clinical validation.
Critical Patents and Research Breakthroughs
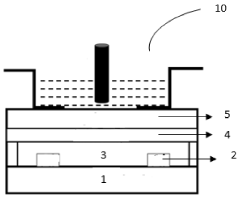
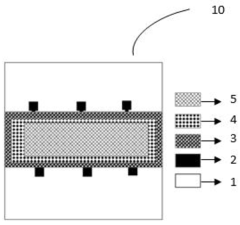
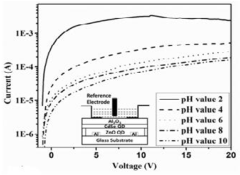
A colloidal quantum dots (QDS) based ion sensitive field effect transistor
PatentActiveIN201911049752A
Innovation
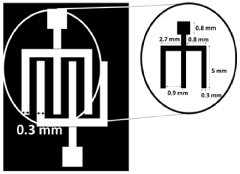
- A colloidal ZnO quantum dots (QDs) based ion sensitive field effect transistor (ISFET) with an interdigitated electrode and a CdSe QDs capping layer, which enhances stability and charge transfer, is developed using a method involving thermal evaporation and colloidal nanosynthesis, where the CdSe QDs protect the ZnO QDs from hydrogenation and improve the device's response to pH variations.
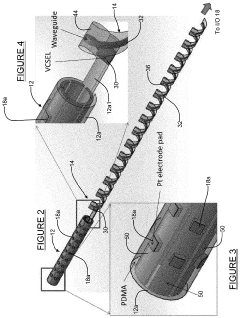
Systems and methods for flexible, high-density opto-electronic arrays
PatentActiveUS20210101013A1
Innovation
- The development of an opto-electronic probe system with integrated microelectrodes and an optical interface that converts electrical signals to optical signals for transmission via an optical waveguide, reducing the need for point-to-point wiring and enabling more compact, high-density electrode arrays.
Regulatory Framework for Bioelectronic Devices
The regulatory landscape for bioelectronic devices represents a complex and evolving framework that significantly impacts the development, approval, and commercialization of these innovative technologies. Currently, most bioelectronic interfaces fall under the jurisdiction of major regulatory bodies such as the FDA in the United States, the EMA in Europe, and the NMPA in China, each with distinct approaches to classification and approval processes.
In the United States, the FDA categorizes bioelectronic devices primarily under Class II or Class III medical devices, depending on their invasiveness and risk profile. Implantable neural interfaces typically require the more stringent Premarket Approval (PMA) pathway, while non-invasive interfaces may qualify for the less demanding 510(k) clearance process. The FDA has recently established the Digital Health Center of Excellence, which provides specialized oversight for software-enabled bioelectronic technologies.
European regulations have undergone significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, introducing more rigorous clinical evidence requirements and post-market surveillance for bioelectronic interfaces. The MDR specifically addresses software as a medical device (SaMD) and active implantable medical devices, both critical categories for bioelectronic applications.
Regulatory frameworks are increasingly acknowledging the hybrid nature of bioelectronic devices that combine hardware, software, and biological components. This has led to the development of novel regulatory pathways such as the FDA's Breakthrough Devices Program, which has accelerated the approval process for several innovative neuromodulation technologies in recent years.
Data privacy and security regulations present additional compliance challenges for bioelectronic interfaces that collect and transmit physiological data. The GDPR in Europe and HIPAA in the United States impose strict requirements on data handling practices, particularly relevant for cloud-connected bioelectronic systems that process neural or physiological information.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing approaches to bioelectronic device regulation globally, though significant regional variations persist. The IMDRF has developed specific guidance for software as a medical device that applies to many bioelectronic interface applications.
Emerging technologies such as AI-enabled bioelectronic interfaces present novel regulatory challenges, as current frameworks struggle to address systems with adaptive algorithms that may change functionality over time. Regulatory bodies are developing new approaches for these technologies, including the FDA's proposed regulatory framework for AI/ML-based software as a medical device.
In the United States, the FDA categorizes bioelectronic devices primarily under Class II or Class III medical devices, depending on their invasiveness and risk profile. Implantable neural interfaces typically require the more stringent Premarket Approval (PMA) pathway, while non-invasive interfaces may qualify for the less demanding 510(k) clearance process. The FDA has recently established the Digital Health Center of Excellence, which provides specialized oversight for software-enabled bioelectronic technologies.
European regulations have undergone significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, introducing more rigorous clinical evidence requirements and post-market surveillance for bioelectronic interfaces. The MDR specifically addresses software as a medical device (SaMD) and active implantable medical devices, both critical categories for bioelectronic applications.
Regulatory frameworks are increasingly acknowledging the hybrid nature of bioelectronic devices that combine hardware, software, and biological components. This has led to the development of novel regulatory pathways such as the FDA's Breakthrough Devices Program, which has accelerated the approval process for several innovative neuromodulation technologies in recent years.
Data privacy and security regulations present additional compliance challenges for bioelectronic interfaces that collect and transmit physiological data. The GDPR in Europe and HIPAA in the United States impose strict requirements on data handling practices, particularly relevant for cloud-connected bioelectronic systems that process neural or physiological information.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing approaches to bioelectronic device regulation globally, though significant regional variations persist. The IMDRF has developed specific guidance for software as a medical device that applies to many bioelectronic interface applications.
Emerging technologies such as AI-enabled bioelectronic interfaces present novel regulatory challenges, as current frameworks struggle to address systems with adaptive algorithms that may change functionality over time. Regulatory bodies are developing new approaches for these technologies, including the FDA's proposed regulatory framework for AI/ML-based software as a medical device.
Ethical Implications of Human-Machine Integration
The integration of bioelectronic interfaces with human biology raises profound ethical questions that society must address as these technologies advance. The blurring boundaries between human and machine challenge our traditional understanding of personhood and autonomy. When neural implants can directly influence brain function or when prosthetics provide capabilities beyond normal human limits, we must reconsider what constitutes human identity and whether enhancement creates new forms of inequality.
Privacy concerns become particularly acute with bioelectronic interfaces that can monitor, record, or potentially manipulate neural activity. The intimate nature of data collected from brain-computer interfaces represents an unprecedented level of access to human thought processes. Questions arise regarding who owns this neural data, how it may be used, and what protections should be established to prevent misuse by governments, corporations, or malicious actors.
Informed consent presents another critical ethical dimension. As bioelectronic interfaces grow more sophisticated, ensuring users fully understand the implications of these technologies becomes increasingly complex. The potential for dependency on these technologies raises questions about reversibility and long-term consequences that may not be apparent at implementation.
The potential for creating a technological divide between enhanced and non-enhanced humans presents significant social justice concerns. If bioelectronic interfaces that significantly enhance cognitive or physical capabilities become available only to privileged segments of society, they could exacerbate existing inequalities and create new forms of discrimination based on technological access.
Medical ethics frameworks must evolve to address the unique challenges of bioelectronic interfaces. The distinction between treatment and enhancement becomes blurred, requiring nuanced approaches to determine appropriate applications. Additionally, questions of agency arise when devices can potentially influence decision-making processes or when algorithms make autonomous adjustments to neural stimulation parameters.
Regulatory frameworks currently lag behind technological developments in this field. International standards for safety, efficacy, and ethical implementation of bioelectronic interfaces remain inconsistent or absent in many jurisdictions. The development of comprehensive governance structures that balance innovation with protection of human rights and dignity represents one of the most pressing challenges in this domain.
As these technologies progress toward more seamless integration with human biology, society must engage in broad, inclusive discussions about the kind of future we wish to create. Proactive ethical frameworks that anticipate challenges rather than merely responding to them will be essential to ensuring that bioelectronic interfaces enhance human flourishing while respecting fundamental values of autonomy, justice, and human dignity.
Privacy concerns become particularly acute with bioelectronic interfaces that can monitor, record, or potentially manipulate neural activity. The intimate nature of data collected from brain-computer interfaces represents an unprecedented level of access to human thought processes. Questions arise regarding who owns this neural data, how it may be used, and what protections should be established to prevent misuse by governments, corporations, or malicious actors.
Informed consent presents another critical ethical dimension. As bioelectronic interfaces grow more sophisticated, ensuring users fully understand the implications of these technologies becomes increasingly complex. The potential for dependency on these technologies raises questions about reversibility and long-term consequences that may not be apparent at implementation.
The potential for creating a technological divide between enhanced and non-enhanced humans presents significant social justice concerns. If bioelectronic interfaces that significantly enhance cognitive or physical capabilities become available only to privileged segments of society, they could exacerbate existing inequalities and create new forms of discrimination based on technological access.
Medical ethics frameworks must evolve to address the unique challenges of bioelectronic interfaces. The distinction between treatment and enhancement becomes blurred, requiring nuanced approaches to determine appropriate applications. Additionally, questions of agency arise when devices can potentially influence decision-making processes or when algorithms make autonomous adjustments to neural stimulation parameters.
Regulatory frameworks currently lag behind technological developments in this field. International standards for safety, efficacy, and ethical implementation of bioelectronic interfaces remain inconsistent or absent in many jurisdictions. The development of comprehensive governance structures that balance innovation with protection of human rights and dignity represents one of the most pressing challenges in this domain.
As these technologies progress toward more seamless integration with human biology, society must engage in broad, inclusive discussions about the kind of future we wish to create. Proactive ethical frameworks that anticipate challenges rather than merely responding to them will be essential to ensuring that bioelectronic interfaces enhance human flourishing while respecting fundamental values of autonomy, justice, and human dignity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!