Material Innovations Driving Bioelectronic Interface Advances
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Materials Background and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The field has evolved significantly over the past three decades, transitioning from rudimentary neural implants to sophisticated, minimally invasive interfaces capable of bidirectional communication with living tissues. This evolution has been primarily driven by innovations in materials science, which have addressed critical challenges related to biocompatibility, flexibility, durability, and signal fidelity.
The historical trajectory of bioelectronic materials began with rigid metal electrodes in the 1970s, progressing through silicon-based microelectronics in the 1990s, and more recently advancing to flexible organic electronics and nanomaterials. Each generation has brought improvements in spatial resolution, signal-to-noise ratio, and reduced tissue damage, gradually closing the gap between electronic and biological systems.
Current technological trends indicate a shift toward ultra-thin, flexible, and biodegradable materials that can conform to the dynamic nature of biological tissues. Emerging approaches include the development of conducting polymers with tunable properties, carbon-based nanomaterials with exceptional electrical conductivity, and hydrogel composites that mimic the mechanical properties of native tissues while maintaining electrical functionality.
The primary objective of current bioelectronic material research is to develop interfaces that can seamlessly integrate with biological systems without triggering foreign body responses or causing tissue damage over extended periods. This includes creating materials that can withstand the harsh biochemical environment of the body while maintaining stable electrical properties and minimizing mechanical mismatch with surrounding tissues.
Secondary objectives include developing materials that enable higher spatial and temporal resolution in neural recording and stimulation, creating self-healing interfaces that can adapt to tissue remodeling, and designing biodegradable components that eliminate the need for removal surgeries. Additionally, there is growing interest in materials that can facilitate wireless power and data transmission, reducing the need for transcutaneous connections.
The ultimate goal is to establish a new generation of bioelectronic interfaces that can function as seamless extensions of biological systems, enabling applications ranging from advanced neural prosthetics and brain-machine interfaces to targeted drug delivery systems and bioelectronic medicine. These technologies hold the potential to revolutionize healthcare by providing unprecedented access to physiological processes at the cellular and molecular levels, allowing for more precise diagnosis and treatment of neurological disorders, cardiovascular diseases, and other medical conditions.
The historical trajectory of bioelectronic materials began with rigid metal electrodes in the 1970s, progressing through silicon-based microelectronics in the 1990s, and more recently advancing to flexible organic electronics and nanomaterials. Each generation has brought improvements in spatial resolution, signal-to-noise ratio, and reduced tissue damage, gradually closing the gap between electronic and biological systems.
Current technological trends indicate a shift toward ultra-thin, flexible, and biodegradable materials that can conform to the dynamic nature of biological tissues. Emerging approaches include the development of conducting polymers with tunable properties, carbon-based nanomaterials with exceptional electrical conductivity, and hydrogel composites that mimic the mechanical properties of native tissues while maintaining electrical functionality.
The primary objective of current bioelectronic material research is to develop interfaces that can seamlessly integrate with biological systems without triggering foreign body responses or causing tissue damage over extended periods. This includes creating materials that can withstand the harsh biochemical environment of the body while maintaining stable electrical properties and minimizing mechanical mismatch with surrounding tissues.
Secondary objectives include developing materials that enable higher spatial and temporal resolution in neural recording and stimulation, creating self-healing interfaces that can adapt to tissue remodeling, and designing biodegradable components that eliminate the need for removal surgeries. Additionally, there is growing interest in materials that can facilitate wireless power and data transmission, reducing the need for transcutaneous connections.
The ultimate goal is to establish a new generation of bioelectronic interfaces that can function as seamless extensions of biological systems, enabling applications ranging from advanced neural prosthetics and brain-machine interfaces to targeted drug delivery systems and bioelectronic medicine. These technologies hold the potential to revolutionize healthcare by providing unprecedented access to physiological processes at the cellular and molecular levels, allowing for more precise diagnosis and treatment of neurological disorders, cardiovascular diseases, and other medical conditions.
Market Analysis for Bioelectronic Interfaces
The bioelectronic interfaces market is experiencing robust growth, driven by increasing applications in healthcare, neuroscience research, and human-machine interaction. Current market valuations place the global bioelectronic medicine sector at approximately 25 billion USD in 2023, with projections indicating a compound annual growth rate of 13-15% through 2030. This growth trajectory is supported by substantial investments from both private and public sectors, with venture capital funding for bioelectronic startups exceeding 3 billion USD in the past three years alone.
Healthcare applications represent the largest market segment, accounting for nearly 60% of the total market share. Within this segment, neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management are showing particularly strong demand. The therapeutic devices subsector has demonstrated consistent annual growth rates of 17-20%, outpacing diagnostic applications which grow at 12-14% annually.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 18-20% annually, primarily driven by increasing healthcare expenditure in China, Japan, and South Korea, along with growing research activities in these regions.
Consumer demand patterns reveal increasing acceptance of minimally invasive bioelectronic solutions, with patient surveys indicating that over 70% of respondents would consider bioelectronic therapies as alternatives to conventional pharmaceutical treatments. This shift in consumer preference is particularly pronounced in chronic disease management, where traditional medications often present significant side effects and variable efficacy.
Key market drivers include technological advancements in material science, miniaturization of electronic components, and improved biocompatibility of implantable devices. The convergence of artificial intelligence with bioelectronic interfaces is creating new market opportunities, particularly in closed-loop systems that can adapt to physiological changes in real-time.
Market barriers include stringent regulatory requirements, with approval timelines averaging 3-5 years for novel bioelectronic devices in major markets. Reimbursement challenges also persist, with inconsistent coverage policies across different healthcare systems limiting market penetration. Additionally, high development and manufacturing costs contribute to elevated product pricing, restricting broader adoption in price-sensitive markets.
The competitive landscape features established medical device manufacturers increasingly competing with technology companies and specialized bioelectronic startups. Strategic partnerships between material science companies and medical device manufacturers are becoming more prevalent, highlighting the critical role of advanced materials in driving next-generation bioelectronic interface development.
Healthcare applications represent the largest market segment, accounting for nearly 60% of the total market share. Within this segment, neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management are showing particularly strong demand. The therapeutic devices subsector has demonstrated consistent annual growth rates of 17-20%, outpacing diagnostic applications which grow at 12-14% annually.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 18-20% annually, primarily driven by increasing healthcare expenditure in China, Japan, and South Korea, along with growing research activities in these regions.
Consumer demand patterns reveal increasing acceptance of minimally invasive bioelectronic solutions, with patient surveys indicating that over 70% of respondents would consider bioelectronic therapies as alternatives to conventional pharmaceutical treatments. This shift in consumer preference is particularly pronounced in chronic disease management, where traditional medications often present significant side effects and variable efficacy.
Key market drivers include technological advancements in material science, miniaturization of electronic components, and improved biocompatibility of implantable devices. The convergence of artificial intelligence with bioelectronic interfaces is creating new market opportunities, particularly in closed-loop systems that can adapt to physiological changes in real-time.
Market barriers include stringent regulatory requirements, with approval timelines averaging 3-5 years for novel bioelectronic devices in major markets. Reimbursement challenges also persist, with inconsistent coverage policies across different healthcare systems limiting market penetration. Additionally, high development and manufacturing costs contribute to elevated product pricing, restricting broader adoption in price-sensitive markets.
The competitive landscape features established medical device manufacturers increasingly competing with technology companies and specialized bioelectronic startups. Strategic partnerships between material science companies and medical device manufacturers are becoming more prevalent, highlighting the critical role of advanced materials in driving next-generation bioelectronic interface development.
Current Material Challenges in Bioelectronics
Despite significant advancements in bioelectronic interfaces, current materials present substantial limitations that impede further progress in this rapidly evolving field. Traditional metallic electrodes, while offering excellent conductivity, suffer from mechanical mismatch with biological tissues, creating inflammation and scar tissue formation at the interface. This mechanical incompatibility results in signal degradation over time and ultimately leads to device failure in long-term implantable applications.
Polymeric materials, though more mechanically compatible with biological tissues, face challenges in achieving sufficient electrical conductivity without compromising biocompatibility. Many conductive polymers require dopants that may leach into surrounding tissues, potentially causing toxicity concerns in long-term applications. Additionally, these materials often demonstrate limited stability in physiological environments, with performance degradation occurring through hydrolysis, oxidation, or enzymatic breakdown.
Encapsulation materials represent another critical challenge, as they must simultaneously provide protection for electronic components while remaining permeable to specific biological signals. Current encapsulants struggle to maintain hermeticity while allowing selective molecular transport, particularly for applications requiring biochemical sensing or drug delivery capabilities alongside electrical recording or stimulation.
Biofouling remains a persistent issue across all bioelectronic interfaces, with protein adsorption and cellular encapsulation progressively diminishing device performance. Anti-fouling strategies often involve surface modifications or coatings that have limited durability, with efficacy declining over extended implantation periods. The trade-off between anti-fouling properties and maintaining functional electronic interfaces presents a significant materials science challenge.
The integration of multiple functionalities into a single material system poses additional complexity. Modern bioelectronic applications increasingly demand materials that can simultaneously conduct electricity, sense biochemical markers, deliver therapeutic agents, and adapt to tissue microenvironments. Current material platforms typically excel in one or two of these properties but fail to integrate all required functionalities without compromising performance in at least one critical area.
Manufacturing scalability presents yet another hurdle, as many promising laboratory-developed materials utilize fabrication techniques that are difficult to translate to industrial production. Complex synthesis procedures, stringent purity requirements, and challenges in quality control limit the commercial viability of many advanced bioelectronic materials, creating a significant gap between research innovations and clinical implementation.
Polymeric materials, though more mechanically compatible with biological tissues, face challenges in achieving sufficient electrical conductivity without compromising biocompatibility. Many conductive polymers require dopants that may leach into surrounding tissues, potentially causing toxicity concerns in long-term applications. Additionally, these materials often demonstrate limited stability in physiological environments, with performance degradation occurring through hydrolysis, oxidation, or enzymatic breakdown.
Encapsulation materials represent another critical challenge, as they must simultaneously provide protection for electronic components while remaining permeable to specific biological signals. Current encapsulants struggle to maintain hermeticity while allowing selective molecular transport, particularly for applications requiring biochemical sensing or drug delivery capabilities alongside electrical recording or stimulation.
Biofouling remains a persistent issue across all bioelectronic interfaces, with protein adsorption and cellular encapsulation progressively diminishing device performance. Anti-fouling strategies often involve surface modifications or coatings that have limited durability, with efficacy declining over extended implantation periods. The trade-off between anti-fouling properties and maintaining functional electronic interfaces presents a significant materials science challenge.
The integration of multiple functionalities into a single material system poses additional complexity. Modern bioelectronic applications increasingly demand materials that can simultaneously conduct electricity, sense biochemical markers, deliver therapeutic agents, and adapt to tissue microenvironments. Current material platforms typically excel in one or two of these properties but fail to integrate all required functionalities without compromising performance in at least one critical area.
Manufacturing scalability presents yet another hurdle, as many promising laboratory-developed materials utilize fabrication techniques that are difficult to translate to industrial production. Complex synthesis procedures, stringent purity requirements, and challenges in quality control limit the commercial viability of many advanced bioelectronic materials, creating a significant gap between research innovations and clinical implementation.
State-of-the-Art Material Solutions for Biointerfaces
01 Flexible and stretchable materials for bioelectronic interfaces
Advanced flexible and stretchable materials are being developed for bioelectronic interfaces to improve compatibility with biological tissues. These materials can conform to the irregular surfaces of organs and tissues, providing better signal acquisition and stimulation. The flexibility allows for reduced mechanical mismatch between electronic devices and soft biological tissues, minimizing inflammation and improving long-term stability of implanted devices.- Flexible and stretchable materials for bioelectronic interfaces: Advanced flexible and stretchable materials are being developed for bioelectronic interfaces to improve compatibility with biological tissues. These materials can conform to the irregular surfaces of organs and tissues, providing better contact and signal transmission. The flexibility allows for movement without disrupting the interface, which is crucial for long-term implantable devices. These innovations include conductive polymers, elastomeric substrates, and composite materials that maintain electrical properties under mechanical deformation.
- Nanomaterial-based bioelectronic interfaces: Nanomaterials are revolutionizing bioelectronic interfaces by providing enhanced electrical conductivity, increased surface area, and improved biocompatibility. These materials include carbon nanotubes, graphene, and metallic nanoparticles that can form direct connections with biological systems at the cellular level. Nanomaterial-based interfaces offer higher sensitivity for biosensing applications and can facilitate more precise stimulation of neural tissues, making them valuable for both diagnostic and therapeutic bioelectronic devices.
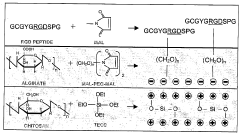
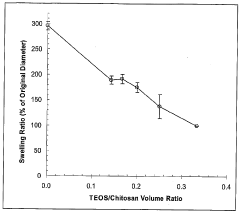
- Biodegradable and biocompatible interface materials: Research is focusing on developing biodegradable and biocompatible materials for bioelectronic interfaces that can safely integrate with biological systems and potentially dissolve after serving their purpose. These materials reduce foreign body responses and inflammation, minimizing scar tissue formation that can degrade signal quality over time. Natural polymers, silk fibroin, and certain metal alloys are being engineered to provide temporary electronic functionality before being metabolized or excreted by the body, eliminating the need for removal surgeries.
- Hydrogel-based bioelectronic interfaces: Hydrogels are emerging as promising materials for bioelectronic interfaces due to their tissue-like mechanical properties and high water content. These soft, hydrated polymers can be engineered to match the mechanical properties of target tissues, reducing mechanical mismatch and associated inflammatory responses. Conductive hydrogels incorporate electronic components while maintaining biocompatibility and can be loaded with bioactive molecules to promote tissue integration or deliver therapeutic agents. Their tunable properties allow for customization based on specific application requirements.
- Surface modification techniques for enhanced biointerfaces: Surface modification techniques are being employed to enhance the performance of bioelectronic interfaces by improving cell adhesion, reducing biofouling, and increasing long-term stability. These techniques include chemical functionalization, plasma treatment, and biomolecule immobilization to create surfaces that actively interact with biological environments. Anti-fouling coatings prevent protein adsorption and cell attachment in undesired areas, while bioactive coatings can promote specific cellular interactions. These surface engineering approaches extend device lifetime and improve signal quality without changing the bulk material properties.
02 Conductive polymers and composites for neural interfaces
Conductive polymers and composite materials are being utilized in bioelectronic interfaces to enhance electrical conductivity while maintaining biocompatibility. These materials can be engineered to have specific properties such as controlled drug release, anti-inflammatory characteristics, and improved charge transfer capabilities. The integration of conductive polymers with other biomaterials creates hybrid interfaces that combine the advantages of both organic and inorganic components.Expand Specific Solutions03 Nanomaterial-based bioelectronic interfaces
Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles are being incorporated into bioelectronic interfaces to improve electrical properties and reduce device dimensions. These nanomaterials provide high surface area-to-volume ratios, enhancing the interface between electronic components and biological systems. The nanoscale dimensions allow for more precise interaction with individual cells and subcellular structures, enabling higher resolution sensing and stimulation.Expand Specific Solutions04 Biodegradable and bioresorbable electronic materials
Biodegradable and bioresorbable materials are being developed for temporary bioelectronic interfaces that can dissolve or be absorbed by the body after their functional lifetime. These materials eliminate the need for secondary surgeries to remove implanted devices and reduce long-term foreign body responses. The degradation rates can be engineered to match specific therapeutic timelines, allowing for controlled dissolution after the intended treatment period.Expand Specific Solutions05 Surface modification techniques for improved biocompatibility
Various surface modification techniques are being employed to enhance the biocompatibility of materials used in bioelectronic interfaces. These include chemical functionalization, protein coating, and topographical patterning to control cellular adhesion and reduce foreign body responses. Modified surfaces can promote specific cell attachment, prevent biofouling, and improve the long-term stability and functionality of implanted bioelectronic devices.Expand Specific Solutions
Leading Organizations in Bioelectronic Material Development
The bioelectronic interface market is currently in a growth phase, characterized by rapid technological advancement and expanding applications in healthcare and neural engineering. The global market size is projected to reach significant value in the coming years, driven by increasing demand for implantable medical devices and neural interfaces. In terms of technical maturity, the field shows varied development stages across different applications. Leading research institutions like MIT, University of California, and Nanyang Technological University are pioneering fundamental material innovations, while companies such as Infineon Technologies and Honeywell are focusing on commercialization aspects. Academic-industry partnerships, exemplified by collaborations involving Yissum Research Development and the Agency for Science, Technology & Research, are accelerating translation of novel biocompatible materials from laboratory to clinical applications, particularly in neural interfaces and implantable sensors.
Agency for Science, Technology & Research
Technical Solution: A*STAR has developed pioneering bioelectronic interfaces using bioresorbable materials and advanced fabrication techniques. Their approach centers on silk fibroin-based flexible electronics that offer exceptional biocompatibility while providing controlled degradation profiles. A*STAR researchers have created composite materials combining conductive polymers (PEDOT:PSS) with natural hydrogels derived from marine organisms, resulting in interfaces with tissue-like mechanical properties and excellent charge transfer characteristics. Their innovations include microfluidic-integrated bioelectronic platforms that enable simultaneous electrical recording and precise delivery of biochemical agents. A*STAR has also pioneered 3D-printed bioelectronic scaffolds with spatially controlled electrical properties that guide tissue regeneration while monitoring healing progress through embedded sensors, representing a significant advance in bioelectronic medicine for regenerative applications.
Strengths: Exceptional integration of biological and electronic components; advanced fabrication capabilities including 3D bioprinting; strong focus on translational medicine applications; excellent biocompatibility profiles. Weaknesses: Some approaches require specialized manufacturing facilities limiting widespread adoption; certain biodegradable electronics face challenges in maintaining consistent performance throughout their functional lifetime.
Infineon Technologies AG
Technical Solution: Infineon has developed advanced semiconductor-based bioelectronic interfaces leveraging their expertise in microelectronics. Their approach centers on silicon carbide (SiC) and gallium nitride (GaN) materials that offer superior biocompatibility compared to traditional silicon. Infineon's bioelectronic platforms feature hermetically sealed packaging technologies that protect sensitive electronics while allowing selective ionic exchange with surrounding tissues. Their innovations include ultra-low power biosensing circuits that extend implant battery life from months to years, addressing a critical limitation in current bioelectronic medicine. Infineon has pioneered diamond-based electrode materials with exceptional electrochemical properties and bioinertness, enabling stable long-term neural interfaces with minimal tissue reaction. Their integrated systems combine sensing, signal processing, and wireless communication in miniaturized packages suitable for minimally invasive implantation procedures.
Strengths: Industry-leading miniaturization capabilities; robust manufacturing infrastructure enabling scale-up; exceptional hermeticity for long-term implantation; advanced power management extending device longevity. Weaknesses: Less experience with soft, flexible materials compared to academic competitors; higher production costs for specialized biocompatible semiconductors; more conservative approach to radical material innovations.
Key Material Innovations and Patent Landscape
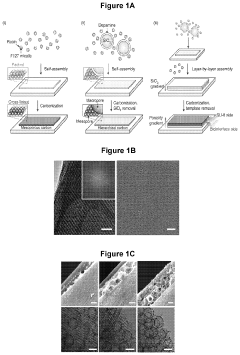
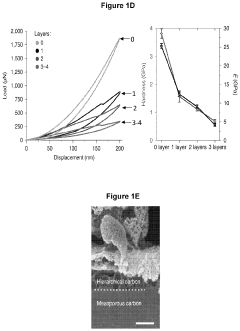
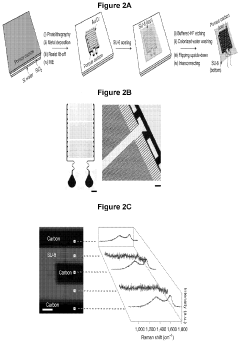
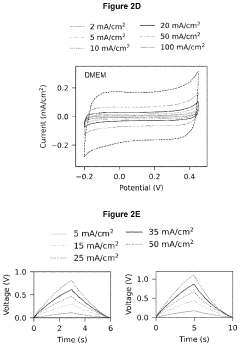
Porous and monolithic carbon membranes and their use
PatentPendingUS20240009630A1
Innovation
- Development of binder-free, flexible, and porous carbon-based micro-supercapacitor-like systems using micelle-enabled self-assembly, which feature hierarchical porosity and nanomaterial building blocks for bioelectronic interfaces, allowing for efficient electrochemical modulation of cells and tissues without the need for binders or additives.
Cell-adhesive polyelectrolyte material for use as membrane and coating
PatentWO2007108775A1
Innovation
- A cell-adhesive polyelectrolyte material comprising alternating layers of polyelectrolytes with opposite charges, where a polyelectrolyte-polyethylene glycol layer with specific functional groups reduces non-specific interactions and a ligand is conjugated to direct cell or protein attachment, using non-carboxyl and non-amino group reactions to minimize cross-reactions with proteins.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical challenge in bioelectronic interface development, as materials must maintain functionality while minimizing adverse biological responses. The host immune system typically recognizes implanted devices as foreign objects, triggering inflammatory responses that can lead to fibrous encapsulation, degrading device performance and longevity. Recent material innovations have focused on mitigating these responses through both passive and active strategies.
Passive approaches include the development of ultrasoft materials that better match tissue mechanical properties, reducing mechanical mismatch and subsequent inflammation. Polymers such as PEDOT:PSS and elastomeric substrates have demonstrated improved compatibility by minimizing micromotion at the tissue interface. Additionally, surface modifications using hydrophilic coatings, anti-fouling materials, and biomimetic structures have shown promise in reducing protein adsorption and subsequent immune cell recruitment.
Active biocompatibility strategies incorporate bioactive components that modulate the biological environment. Controlled release systems delivering anti-inflammatory agents directly at the implant site have demonstrated effectiveness in managing acute inflammatory responses. More sophisticated approaches include materials functionalized with cell-adhesion peptides that promote selective cell attachment and tissue integration while discouraging inflammatory cell activity.
Safety considerations extend beyond biocompatibility to include long-term stability of materials in the physiological environment. Degradation products must be non-toxic and safely cleared by the body. Recent research has focused on understanding the chemical stability of conductive polymers and electrode materials under chronic implantation conditions, with particular attention to potential leaching of dopants or breakdown products that could cause delayed toxicity.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with ISO 10993 standards providing guidelines for biological evaluation. However, the unique nature of bioelectronic interfaces—combining electrical functionality with biological interaction—presents challenges for comprehensive safety assessment. Emerging evaluation methodologies include accelerated aging tests, advanced in vitro models, and computational simulations to predict long-term material behavior.
The development of standardized testing protocols specific to bioelectronic materials represents a significant opportunity for advancing the field. Current efforts focus on establishing correlations between in vitro testing results and in vivo performance, particularly for novel materials without extensive clinical history. This includes evaluating both acute responses and chronic effects that may emerge only after extended implantation periods.
Passive approaches include the development of ultrasoft materials that better match tissue mechanical properties, reducing mechanical mismatch and subsequent inflammation. Polymers such as PEDOT:PSS and elastomeric substrates have demonstrated improved compatibility by minimizing micromotion at the tissue interface. Additionally, surface modifications using hydrophilic coatings, anti-fouling materials, and biomimetic structures have shown promise in reducing protein adsorption and subsequent immune cell recruitment.
Active biocompatibility strategies incorporate bioactive components that modulate the biological environment. Controlled release systems delivering anti-inflammatory agents directly at the implant site have demonstrated effectiveness in managing acute inflammatory responses. More sophisticated approaches include materials functionalized with cell-adhesion peptides that promote selective cell attachment and tissue integration while discouraging inflammatory cell activity.
Safety considerations extend beyond biocompatibility to include long-term stability of materials in the physiological environment. Degradation products must be non-toxic and safely cleared by the body. Recent research has focused on understanding the chemical stability of conductive polymers and electrode materials under chronic implantation conditions, with particular attention to potential leaching of dopants or breakdown products that could cause delayed toxicity.
Regulatory frameworks for bioelectronic interfaces continue to evolve, with ISO 10993 standards providing guidelines for biological evaluation. However, the unique nature of bioelectronic interfaces—combining electrical functionality with biological interaction—presents challenges for comprehensive safety assessment. Emerging evaluation methodologies include accelerated aging tests, advanced in vitro models, and computational simulations to predict long-term material behavior.
The development of standardized testing protocols specific to bioelectronic materials represents a significant opportunity for advancing the field. Current efforts focus on establishing correlations between in vitro testing results and in vivo performance, particularly for novel materials without extensive clinical history. This includes evaluating both acute responses and chronic effects that may emerge only after extended implantation periods.
Manufacturing Scalability of Advanced Biomaterials
The scalability of manufacturing processes for advanced biomaterials represents a critical challenge in translating bioelectronic interface innovations from laboratory prototypes to commercially viable products. Current manufacturing approaches for high-performance biomaterials often rely on complex, multi-step processes that are difficult to standardize and scale. These limitations significantly impact production costs and ultimately restrict market penetration of novel bioelectronic devices.
Traditional manufacturing methods such as chemical vapor deposition and photolithography, while effective for conventional electronics, face substantial challenges when adapted for biomaterials that require preservation of biocompatibility and functionality. The intricate micro and nanostructures essential for optimal tissue-electrode interfaces demand precision manufacturing techniques that can maintain consistency across large production volumes.
Recent advances in additive manufacturing technologies offer promising solutions to these scalability challenges. 3D bioprinting techniques have evolved to enable the fabrication of complex biomaterial structures with precise control over spatial organization and material composition. These approaches allow for customization while maintaining the potential for scaled production. However, significant hurdles remain in achieving the resolution and throughput necessary for commercial viability.
Roll-to-roll processing represents another emerging manufacturing paradigm with substantial potential for scaling biomaterial production. This continuous fabrication approach enables high-throughput production of flexible bioelectronic interfaces with consistent quality. Companies pioneering this technology have demonstrated the ability to produce conductive polymer-based interfaces at scales previously unattainable with batch processing methods.
Material formulation standardization presents an additional challenge to manufacturing scalability. The complex compositions of advanced biomaterials often include multiple components with strict purity requirements and precise mixing ratios. Establishing robust quality control protocols and sourcing reliable raw materials at scale requires significant investment in process engineering and supplier development.
Regulatory considerations further complicate the manufacturing scale-up process. Biomaterials for medical applications must adhere to stringent quality standards, with documented consistency in production processes. The establishment of validated manufacturing protocols that satisfy regulatory requirements while maintaining economic viability represents a significant hurdle for commercialization.
Strategic partnerships between academic institutions, where many biomaterial innovations originate, and established manufacturing entities offer a pathway to overcome these challenges. Such collaborations can leverage complementary expertise in material science and production engineering to develop scalable manufacturing solutions tailored to the unique requirements of advanced biomaterials for bioelectronic interfaces.
Traditional manufacturing methods such as chemical vapor deposition and photolithography, while effective for conventional electronics, face substantial challenges when adapted for biomaterials that require preservation of biocompatibility and functionality. The intricate micro and nanostructures essential for optimal tissue-electrode interfaces demand precision manufacturing techniques that can maintain consistency across large production volumes.
Recent advances in additive manufacturing technologies offer promising solutions to these scalability challenges. 3D bioprinting techniques have evolved to enable the fabrication of complex biomaterial structures with precise control over spatial organization and material composition. These approaches allow for customization while maintaining the potential for scaled production. However, significant hurdles remain in achieving the resolution and throughput necessary for commercial viability.
Roll-to-roll processing represents another emerging manufacturing paradigm with substantial potential for scaling biomaterial production. This continuous fabrication approach enables high-throughput production of flexible bioelectronic interfaces with consistent quality. Companies pioneering this technology have demonstrated the ability to produce conductive polymer-based interfaces at scales previously unattainable with batch processing methods.
Material formulation standardization presents an additional challenge to manufacturing scalability. The complex compositions of advanced biomaterials often include multiple components with strict purity requirements and precise mixing ratios. Establishing robust quality control protocols and sourcing reliable raw materials at scale requires significant investment in process engineering and supplier development.
Regulatory considerations further complicate the manufacturing scale-up process. Biomaterials for medical applications must adhere to stringent quality standards, with documented consistency in production processes. The establishment of validated manufacturing protocols that satisfy regulatory requirements while maintaining economic viability represents a significant hurdle for commercialization.
Strategic partnerships between academic institutions, where many biomaterial innovations originate, and established manufacturing entities offer a pathway to overcome these challenges. Such collaborations can leverage complementary expertise in material science and production engineering to develop scalable manufacturing solutions tailored to the unique requirements of advanced biomaterials for bioelectronic interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!